
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Energy Res. , 23 April 2020
Sec. Bioenergy and Biofuels
Volume 8 - 2020 | https://doi.org/10.3389/fenrg.2020.00055
This article is part of the Research Topic Energy and Resource Valorisation of Biomass and Waste towards Sustainable Environment via Thermochemical and Biological Application View all 11 articles
Bioprocesses based on (ligno-)cellulosic biomass are highly prone to batch-to-batch variations. Varying raw material compositions and enzyme activities hamper the prediction of process yields, economic feasibility and environmental impacts. Commonly, these performance indicators are averaged over several experiments to select suitable process designs. The variabilities in performance indicators resulting from variable process inputs are often neglected, causing a risk for faulty performance predictions and poor process design choices during scale-up. In this paper, a multi-scale variability analysis framework is presented that quantifies the effects of process input variations on performance indicators. Using the framework, a kinetic model describing simultaneous saccharification and ethanol fermentation was integrated with a flowsheet process model, techno-economic analysis and life cycle assessment in order to evaluate a wheat straw-based ethanol biorefinery. Hydrolytic activities reported in the literature for the enzyme cocktail Cellic® CTec2, ranging from 62 to 266 FPU·mL−1, were used as inputs to the multi-scale model to compare the variability in performance indicators under batch and multi-feed operation for simultaneous saccharification and fermentation. Bioprocess simulations were stopped at ethanol productivities ≤0.1 g·L−1·h−1. The resulting spreads in process times, hydrolysis yields, and fermentation yields were incorporated into flowsheet, techno-economic and life cycle scales. At median enzymatic activities the payback time was 7%, equal to 0.6 years, shorter under multi-feed conditions. All other performance indicators showed insignificant differences. However, batch operation is simpler to control and well-established in industry. Thus, an analysis at median conditions might favor batch conditions despite the disadvantage in payback time. Contrary to median conditions, analyzing the input variability favored multi-feed operation due to a lower variability in all performance indicators. Variabilities in performance indicators were at least 50% lower under multi-feed operation. Counteracting the variability in enzymatic activities by adjusting the amount of added enzyme instead resulted in higher uncertainties in environmental impacts. The results show that the robustness of performance indicators against input variations must be considered during process development. Based on the multi-scale variability analysis process designs can be selected which deliver more precise performance indicators at multiple system levels.
Ethanol derived from lignocellulosic biomass is regarded to be a sustainable replacement of fossil fuels in the transport sector. Compared to gasoline, bioethanol has the potential to significantly reduce greenhouse gas emissions (Muñoz et al., 2014) and provide energy security (Uría-Martínez et al., 2018). Lignocellulosic ethanol production utilizes agricultural and forestry waste, amongst others wheat straw (Erdei et al., 2013; Westman et al., 2017), corn (Öhgren et al., 2006; Uppugundla et al., 2014), and wood (Wang et al., 2014).
The utilization of lignocellulosic materials and hydrolytic enzymes poses several challenges at different system scales, e.g., the optimal choice of products, seasonal availability of raw materials with their inherent compositional variation (Collins et al., 2014), and the inhibitory action of pretreated raw materials on fermentation (Horváth et al., 2003; Bellido et al., 2011). During process development, variations in raw material types and compositions or enzymatic activities can influence decisions on the process design. In simultaneous saccharification and fermentation (SSF) processes for example, the hydrolysis rate, with which polysaccharides are broken down into fermentable sugars, is typically the overall rate limiting step. Changes in enzymatic activities result in different saccharification yields and rates which determine the amount of sugars that can undergo ethanol fermentation, with subsequent impacts on techno-economic analysis (TEA) and life cycle assessment (LCA) performance indicators. Thereby, uncertainties in process inputs can result in process designs that eventually fail to meet set production and cost targets.
TEAs and LCAs commonly incorporate uncertainties in performance indicators based on rough estimates of increasing or decreasing performance (Olofsson et al., 2017). Very few studies have based their uncertainty estimates on laboratory data (Vicari et al., 2012). Still, these studies investigate the impacts of uncertainties on single system level. However, data presented in these studies indicate that changes in process inputs affect process indicators at multiple system levels. Changing the amount of added enzyme will for example influence hydrolysis yields. Moreover, the energy-intense off-site production of hydrolytic enzymes, especially the concentration and purification of the enzyme product which is typically driven by fossil energy, contributes significantly to the climate impact of the overall ethanol production process (MacLean and Spatari, 2009; Janssen et al., 2014, 2016). Hence, changing the amount of added enzyme will affect both the bioprocess and the LCA as increased hydrolysis yields will likely result in improved ethanol yields while the climate impact might also increase. In this example, a single level analysis will not enable prediction of the multi-level, diverging outcomes of the overall process. It is therefore necessary to assess the impacts of the variability in process inputs on all important performance indicators across multiple system scales at once.
For a systematic analysis of variations across system scales, multi-scale models are inevitable. Multi-scale models integrate several models at different scales and allow precise descriptions and analyses of each scale by collaborating experts. Multiscale models have been established in various disciplines, e.g., in fluid phase and wastewater treatment research (Deen et al., 2004; Xavier et al., 2007; Ofiteru et al., 2014), metabolic engineering (Dada and Mendes, 2011; Bogart and Myers, 2016), and biomass pretreatment (Hosseini and Shah, 2009). Zhuang and Herrgård (2015) proposed a biorefinery multi-scale model to describe the impacts different microbial strains, products and raw materials have on process economics and environmental impacts by connecting dynamic flux balance analysis to TEA and LCA. The authors used the model to guide strain development from an economic and environmental perspective in early process development stages. However, a systematic assessment of the effects of process input variations across system scales in early process development stages is still missing in the field of lignocellulosic biorefineries.
In this study, we developed a multi-scale variability analysis framework which integrates bioprocess, flowsheet, TEA and LCA scales. The framework was developed with the objective to systematically assess the effects of input variations on the performance indicators of a wheat straw-based ethanol biorefinery. A previously published bioprocess model describing simultaneous saccharification and fermentation to ethanol (Wang et al., 2016) was integrated with flowsheet, TEA and LCA models to form a multi-scale model describing the biorefinery. The validity of the simulated results at bioprocess scale was analyzed by local sensitivity and uncertainty analyses. In a case study the variability in reported hydrolytic, specifically cellulolytic, enzymatic activities was propagated through the multi-scale model to compare two alternative modes of operation, batch and multi-feed. With the help of the multi-scale model the effects of the input variability on the performance indicators were determined and compared between the two modes of operation, and the mode most robust against the input variations was identified.
The developed multi-scale variability analysis framework covers a multi-scale model describing a wheat straw-based ethanol biorefinery. The biorefinery was assumed to be situated in the south of Sweden. Wheat is cultivated and harvested (Figure 1). It was assumed that wheat straw is available at an average distance of 45 km from the biorefinery plant. The straw is transported to the biorefinery and pretreated by H2SO4-catalyzed steam explosion. The resulting slurry is pH-adjusted with NaOH and separated by a filter press into a solid fraction and a liquid fraction, referred to as hydrolysate. The hydrolysate is added to molasses medium used in the on-site propagation of Saccharomyces cerevisiae to adapt the yeast cells to the lignocellulose-derived inhibitory compounds. The yeast and the solid fraction are added to the simultaneous saccharification and fermentation (SSF) process in which hydrolytic enzymes depolymerize polysaccharides into fermentable sugars. The sugars are taken up by the yeast which convert them into ethanol. After the SSF, ethanol is purified from the bioreactor content through distillation and molecular sieves. The residual slurry is filtered, and the solids are burned in a boiler to generate process steam. Electricity is produced from the excess steam. The process has a net surplus of produced electricity. The liquid fraction of the distillation residues is mixed with residual streams from pretreatment and propagation and sent to anaerobic digestion (AD) to produce biogas from remaining sugar and protein sources. After AD, the liquid is sent to a waste water treatment plant including aerobic bio-oxidation and filtration. Solid residues from AD are dewatered and sent to the boiler for steam production. A detailed description of the modeled biorefinery can be found in section The Flowsheet Model. In the LCA model, upstream activities include the production of H2SO4 for pretreatment, NaOH for pH adjustment after pretreatment, glucose as carbon source in yeast propagation, hydrolytic enzymes for the SSF, non-ionic surfactant used in waste water treatment, and all fuel and electricity requirements. The three products of the biorefinery system are ethanol, biogas and electricity.
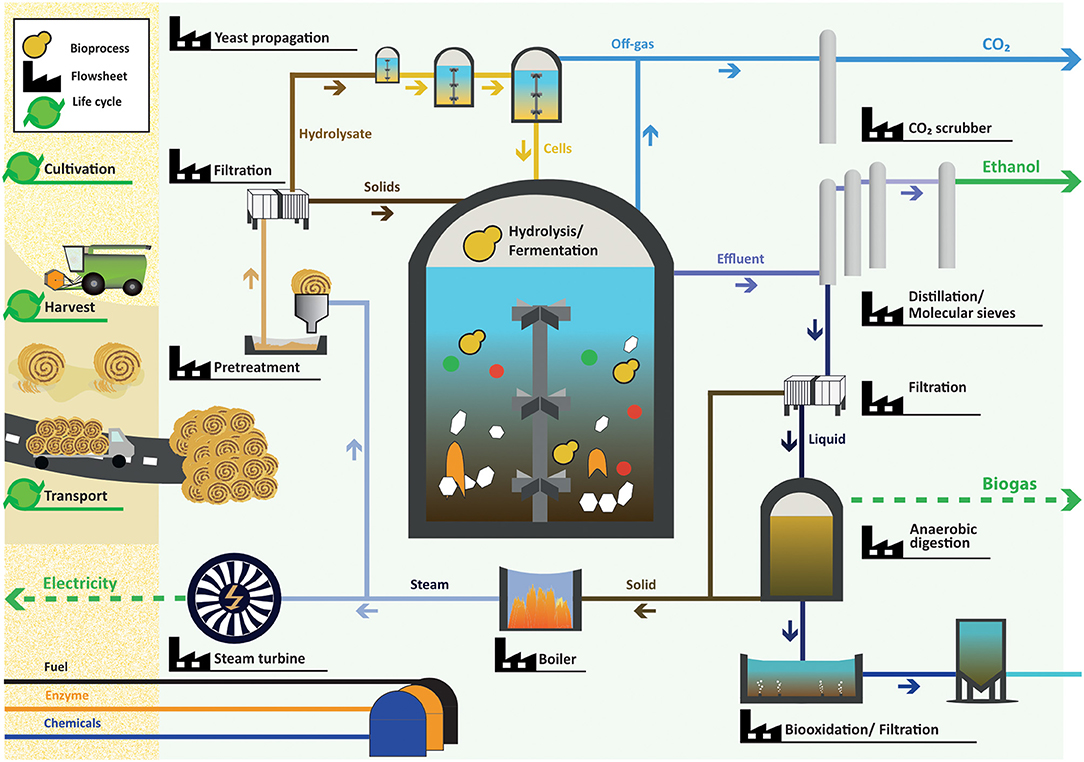
Figure 1. The wheat straw-based ethanol biorefinery system. Wheat straw is cultivated, harvested and transported to the factory. The pretreatment of wheat straw is followed by filtration. The hydrolysate is used for cell propagation while the solid phase is added together with the propagated cells into the bioreactor in which the hydrolysis of the solids into monosaccharides and fermentation to ethanol take place. CO2 is extracted from the exhaust gas of all bioreactors. After fermentation the bioreactor content is sent to distillation columns and molecular sieves to reach ethanol concentrations ≥ 99%. The remainder of the first distillation column is filtered. The liquid fraction undergoes AD, aerobic bio-oxidation and filtration while the solids are burned together with the solid leftovers after AD in a boiler, generating steam for pretreatment. The excess steam is used in a backpressure turbine to produce power. The biorefinery products are ethanol, biogas, and electricity.
The multi-scale variability analysis framework was developed with the objective to quantify the variation of performance indicators in response to varying process inputs. The framework consists of three phases: (1) The collection of variable process input data, (2) the use of these data in a multi-scale model, and (3) a statistical analysis of the resulting variability in performance indicators (Figure 2).
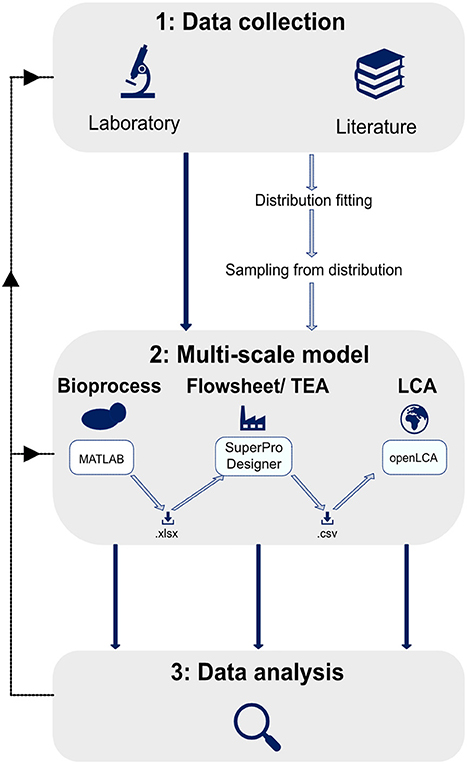
Figure 2. Schematic description of multi-scale variability analysis framework. First, data on the variability of a process input are collected in laboratories or through literature search. The data can be used as direct inputs to the multi-scale model. If enough datapoints are collected, a distribution can be fitted to the data. Samples from the distribution are then inputs to the multi-scale model, following the Monte-Carlo approach. In step 2 the collected data are inputs to the multi-scale model which consists of a bioprocess model in MATLAB, a flowsheet model and TEA in SuperPro Designer, and an LCA in openLCA. The different models are connected via .xlsx- and .csv-files. The resulting variabilities in performance indicators are analyzed statistically in a third step.
Within the framework, variable process inputs are defined based on laboratory measurement or literature data. These data can either be direct inputs to the developed multi-scale model, or, in case of a sufficiently large dataset, serve as basis for fitting distributions to be used in Monte-Carlo simulations.
The established multi-scale model simulates a wheat straw-based ethanol biorefinery. A macro-kinetic bioprocess model was connected to flowsheet, TEA and LCA models. The multi-scale model is characterized as a serial-integrated, scale-connecting multi-scale model (Yang and Marquardt, 2009). The data collected in phase (1) are inputs to a bioprocess model covering simultaneous saccharification and ethanol fermentation. The differential equation system of the bioprocess model is solved with the ode15s solver for stiff problems in MATLAB R2016b (The MathWorks Inc., Nattick, USA). The resulting spreads in the bioprocess model outputs hydrolysis yield, fermentation yield and process time are, after a statistical analysis, automatically stored in a .xlsx-file using a component object model. To ensure a feasible number of simulations at flowsheet scale, the 5th, 25th, 50th, 75th, and 95th percentile of each bioprocess model output are automatically retrieved and used as inputs to flowsheet simulations in SuperPro Designer (Intelligen Inc., Scotch Plains, USA). Iterations over the varying inputs are run through an MS Excel-based dashboard. The flowsheet model solves the plant-wide mass and energy balances. The economic performance of each iteration of the biorefinery is determined using the TEA model as defined in SuperPro Designer. Relevant flows and emission data are exported to a .csv-file and transferred to the LCA software openLCA version 1.7 (Ciroth, 2007). To enable a feasible amount of simulations, variabilities in the LCA input data were restricted to the 5th, 50th, and 95th percentiles. At LCA scale the environmental impacts of the produced ethanol are assessed from the cultivation and harvesting of the wheat straw to the gate of the biorefinery (cradle-to-gate). The performance indicators analyzed in this study can be found in Table 1.
A macro-kinetic model was used to describe the bioprocess. The model was developed and validated by Wang et al. (2016) to simulate batch and multi-feed SSF processes based on steam pretreated wheat straw at 5–15 filter paper units (FPU)· and 0.02 gCells· at maximally 13% water insoluble solids (WIS). Multi-feed SSF processes enable efficient mixing and high cell viabilities as solids and cells are added at discrete times with sufficient liquefaction in between. Due to the solid additions and their subsequent hydrolysis, the cumulative WIS is higher than the 13% maximum operating WIS. In the case study the simulated multi-feed process conditions resembled the experimental conditions of a demo plant run at an enzyme activity of 9.3 FPU·, a cell load of 0.02 gCells· and a final cumulative WIS of 21.85%, with cell additions at 0, 12, 24, 48, and 72 h and solid additions at 0, 4, 12, 24, 48, and 72 h (Wang et al., 2016, Figure 6). Batch processes were simulated at identical relative cell and enzymatic activity loadings, but at 13% final WIS, all of which was added initially. The total added enzyme activity was relative to the final cumulative WIS of the process.
The bioprocess model covers the progress of the concentrations of solids, cellulose, xylan, adsorbed hydrolytic enzymes, glucose, xylose, ethanol, cells and the volume in the SSF process. A boundary condition was added to the model to avoid further enzyme adsorption after reaching equilibrium state. Multi-feed SSF processes were modeled as repeated, piece-wise batch processes.
Uncertainty and sensitivity analyses were performed based on the methods described by Sin et al. (Sin et al., 2009; Sin and Gernaey, 2016). The uncertainty analysis comprised the following steps:
(1) Process and model definition
The process was defined to be a 96 h SSF batch process under previously described conditions. The model structure was represented by:
where x(t) ∈ ℝn are the state variables, t ∈ [t0 tend] is the process time, θ ∈ ℝn the model parameters, and y(t) ∈ ℝn the model outputs.
(2) Uncertainty definition
The input uncertainties for the estimated parameters k, kG, γ, kad, and KiEtOH were defined based on published confidence intervals (Table 2). As the parameters k, kG, KiEtOH, and γ were log-transformed before parameter estimation, they were as well log-transformed before sampling from the input domain. Based on experience, uncorrelated uniform distributions with a low uncertainty of ± 10% for YEtOH and a medium one of ± 25% for KS, qG, α, and β around the mean parameter value were assumed (Table 2).
(3) Sampling from uncertainty input domain
For each of the 10 parameters, 1,000 probabilistic samples were taken generating pseudo-random numbers from the specified normal and uniform distributions.
(4) Propagation of uncertainties through Monte-Carlo simulations
The parameter uncertainties were propagated through the bioprocess model, obtaining 1,000 simulation results for each simulated state variable and time point.
(5) Statistical analysis of simulation results
The obtained cumulative distribution functions were analyzed with respect to their mean, 5 and 95% confidence intervals.
Local sensitivity analysis was performed using the finite difference method, specifically the central difference between backward and forward perturbations, to analyze the effect of a change in each parameter θi on the model output yj(t) under the same process conditions as for the uncertainty analysis. The relative sensitivity function rsi, j (Equation 2) was computed by multiplying the first order derivative of yj with regards to θi with the ratio of scaling factors sci and scj.
To assess the importance of each parameter on the variance in model outputs, parameters were ranked as proposed by Sin and Gernaey (2016) according to the δmsqr measure (Equation 3) which summarizes the influence of each parameter θi on a model output yj over process time.
The flowsheet model was developed in SuperPro Designer with the objective to assess the preliminary design in early process development of a typical wheat straw-based biorefinery with ethanol, biogas and electricity as products. Variations of process inputs were analyzed at a fixed flowrate of wheat straw into the process. The flowrate was determined by setting the ethanol output to 100,000 m3 per year for a 96 h multi-feed process under conditions described in 3.2.1. The flowsheet model was fitted to data obtained from lab- (1–2.5 L), pilot-, and demo scale (10 m3) experiments and to the bioprocess model outputs: hydrolysis yield, ethanol yield, and process time.
The flowsheet starts with a one-step steam explosion pretreatment. Wheat straw is first heated by recycled flash steam followed by an addition of 0.2% H2SO4 and pretreatment at 188°C for 7 min. The pretreatment temperature is reached by direct steam injection with steam at 12.3 bar. After pretreatment one flash drum lowers the pressure. Thereby, water and a fraction of the volatile compounds generated during pretreatment, mainly furfural and acetic acid, are removed. The flash steam was assumed to be recycled to the pre-steaming reactor, thus reducing energy and water demands. The pretreatment and the concentrations of total organic carbon, total sulfur and furfural in the flash steam were based on experiments conducted in the Biorefinery Demo Plant at RISE Processum (Örnsköldsvik, Sweden) (Fornell et al., 2016).
The slurry resulting from pretreatment is cooled and separated in a filter press into a solid fraction and a hydrolysate. The hydrolysate with a total (soluble) solids concentration of 85 g·L−1 is sent to the SSF bioreactors, the yeast propagation and AD. The solid fraction with a total solids concentration of 440 g·L−1 (WIS: 385 g·L−1) is sent to the SSF bioreactor. The solid fraction is diluted to a WIS concentration resulting in the same final ethanol concentrations and yields (based on the solid fraction after pretreatment and filtration) as in the bioprocess model. The WIS contents differ between the bioprocess and flowsheet model due to additional reactions and literature estimates included in the flowsheet model for the SSF description in order to close the mass balances.
The yeast propagation is designed as a series of five propagation reactors, and two parallel trains (Humbird et al., 2011). The carbon sources for yeast propagation consist of 50% molasses (containing sucrose, modeled as glucose) and 50% hydrolysate (containing glucose and xylose). The stoichiometries for glucose and xylose conversion were experimentally determined and 100% conversion was assumed:
After cell propagation the yeast cells are separated by centrifugation.
To start the SSF, hydrolytic enzymes and propagated yeast cells are added to the solids in the SSF bioreactor. The SSF reactor system was designed as a set of 12 parallel reactors (Humbird et al., 2011). The stoichiometries of the SSF reactions are based on moles except for the conversion of cellulose to glucose. The conversion yields from cellulose to glucose and glucose to ethanol varies based on the outputs from the bioprocess model (Appendix 1), while other yields did not change. The reactions defined in SuperPro Designer are the following:
The hydrolysis of galactan, mannan, and arabinan is excluded from the model due to their low content in the solids added to the SSF. The flowsheet model excludes xylose to ethanol conversion based on experimental data and in accordance with the bioprocess model.
In the distillation sequence three heat-integrated columns are used. The first two columns are parallel strippers. The crude ethanol from the strippers is purified in a rectification column to near azeotropic concentrations while the bottom slurry is separated in a filter press into solids mainly consisting of lignin and a filtrate. To minimize potential fouling in the strippers, the highest pressure is applied to the rectification column. After distillation the crude ethanol at >90% purity is dehydrated in a set of molecular sieves. The molecular sieves and the filter press are designed according to Humbird et al. (2011).
The modeled biorefinery has its own waste water treatment. The filtrate from the strippers, the centrifuge water from yeast propagation, and excess hydrolysate are mixed and cooled to 32°C. In subsequent AD organic compounds are converted to CH4. Conversions are simulated based on chemical oxygen demands according to reference literature values (Barta et al., 2010; Humbird et al., 2011; Petersson, 2012). The waste water from AD is treated by aerobic bio-oxidation and filtration to decrease the concentration of relevant substances to acceptable levels before being released to the recipient.
The solids from the AD are centrifuged and combined with the solids from the filtration of the stripper bottoms. These solids are sent to the steam boiler which produces steam at 90 bar and 470°C with an excess oxygen input of 10% and overall heat losses of 5%. The boiler was designed based on reference literature (Humbird et al., 2011; Fornell and Berntsson, 2012; Fornell et al., 2012). The blow-down of boiler feed water was set to 5% of the total steam production. The 90 bar pressure steam is sent to a backpressure turbine with steam extractions at 12.3 bar for the pretreatment reactor and 4.5 bar for other process demands, e.g., distillation and preheating, and a condensing tail with an exhaust pressure of 0.1 bar. The cold utility in the process is based on the cooling demand as calculated by SuperPro Designer. A cooling tower is used for the cooling water system. The cooling water system is designed based on information found in Perry's Chemical Engineers' Handbook (Humbird et al., 2011; Ahmetović et al., 2014).
The heat exchangers in the process simulation model were integrated based on a pinch analysis. The analysis is an important part of the assessment since the energy integration might affect both TEA and LCA. The heat exchanger network was designed in order to be well-integrated and practically feasible, and the different heat exchangers needed were designed and included in the simulation model. In this heat exchanger network the distillation section is internally integrated, i.e., the distillation bottoms are used to preheat the column feeds. The 4.5 bar steam condensate in the boiler section is heat exchanged with the makeup boiler water and the condensate from the turbine while the flash steam from pretreatment is directly recycled in that section.
The flowsheet modeling software SuperPro Designer includes the capacity to conduct TEAs based on data and information generated by the performed flowsheet simulations. Different costs for design and operation of a lignocellulosic ethanol biorefinery were manually adjusted in the software, and a TEA was performed in order to study the propagation of variability from the bioprocess model to the flowsheet model and TEA. Since the focus of this TEA was to assess the effect of variations in input data to the bioprocess model on the economic performance, no statistical analysis of the input data specific for the TEA (e.g., costs for raw material and market price of products) was performed. In the TEA the yearly revenues, operating cost, internal rate of return (IRR), and payback time (PBT) were calculated. All equipment cost correlations, operating costs, and product values (including references), and model inputs and outputs are presented in Appendix 1.
The LCA was defined as a cradle-to-gate system, from the cultivation of wheat (to obtain the wheat straw) until the gate of the ethanol biorefinery (Figure 3). The functional unit in this LCA was the amount of ethanol produced from a fixed input of wheat straw into the biorefinery. This functional unit was chosen because the primary function of the analyzed system was to produce bioethanol and not to process wheat straw. Furthermore, the biorefinery flowsheet model was simulated with a fixed input of wheat straw. The LCA results are, however, given per liter of ethanol produced.
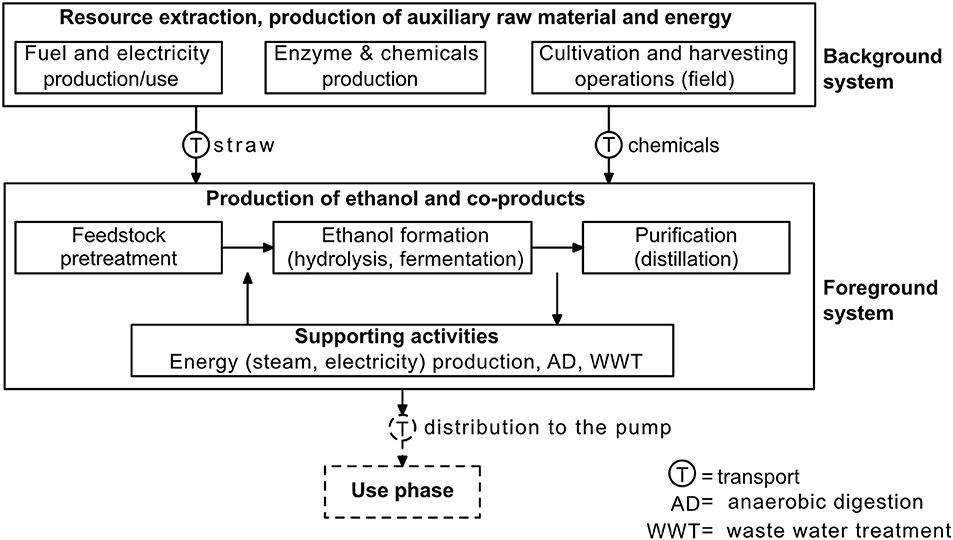
Figure 3. System boundaries of the life cycle assessment. The dashed lines indicate processes and flows that are outside of the scope of this study.
The LCA was carried out using an attributional approach (Baumann and Tillman, 2004) since the focus of this study lay on the variability of process inputs and parameters in the production process, and its impact on the LCA results. The life cycle impact assessment was conducted using the CML characterization method (Gorrée et al., 2002). The climate impact and eutrophication potential (EP) were used as impact categories for the evaluation of the system. These two impact categories were chosen since a reduction of fossil-based fuel use is the main goal of biofuel production, and fertilizer and nutrients are used in the production of straw-based ethanol, respectively. The need for allocation of the environmental burden of the system to the main product (ethanol) and its by-products (biogas and electricity) was avoided, thanks to the available data and information, and sufficient detail from the process flowsheet model that was used to define the LCA model.
The LCA model was implemented and run in openLCA version 1.7 for batch and multi-feed processes at variable process times, with median inventory values, and with 5 and 95% confidence interval inventory values for the different process flows (both foreground and background) that were part of the model. These values, except for those for the cultivation and harvesting of the wheat straw and the straw preparation, were delivered by the process simulations done in SuperPro Designer. The following choices were made for modeling the wheat cultivation and harvesting, straw preparation, and enzymes and chemicals needed in the process:
1. Cultivation and harvesting were modeled using the inventory data for wheat production compiled by Röös, Sundberg, and Hansson (Röös et al., 2011). It was assumed that 15% of the harvested wheat straw was lost due to its transportation and preparation for the pretreatment.
2. In the straw preparation to cut the straw in sufficiently small chips, 3.6·10−3 kWhelectricity·kg−1 of wheat straw was needed. Electricity use was modeled with the ecoinvent process “market for electricity, medium voltage” for Sweden (Frischknecht et al., 2007).
3. Enzyme production was modeled using inventory data compiled by Liptow et al. (2013) and was assumed to take place in Kalundborg, Denmark.
4. Use of NaOH, H2SO4, glucose and non-ionic surfactant were modeled with the ecoinvent processes “market for sodium hydroxide, without water, in 50% solution state,” “market for sulfuric acid,” “glucose production,” and “market for non-ionic surfactant,” respectively (Althaus et al., 2007).
Lignocellulosic bioprocesses rely on saccharification to obtain monosaccharides for the microbial conversion into desired products. Therefore, SSF process development focuses both on hydrolysability and fermentability of pretreated materials. This case study has the objective to analyze with the help of the developed multi-scale variability analysis framework, how robust performance indicators of a projected wheat straw-based biorefinery are against variations in enzymatic activities under batch and multi-feed operation. The case study specifically reflects conditions at early process development stages to illustrate the use of the multiscale variability analysis as a help for stakeholders to decide on projected process designs given the reliability of the performance indicators.
The Cellic® CTec2 enzyme cocktail (Novozymes A/S, Denmark) has been widely used in research. Hence, many publications report enzymatic activity measurements for Cellic® CTec2, allowing for a systematic assessment of enzymatic variability from literature data. To obtain an unbiased selection of publications, a literature search with the search terms “Cellic,” “CTec2,” and “CTec 2” was performed in SciFinder (CAS Chemical Abstracts Service, Columbus, USA) on 2018-01-23. The results (Appendix 1) were statistically analyzed as described in Appendix 2 and a final dataset for the variability analysis was retrieved.
The impact of the variability in enzymatic activities on the choice of process design based on metrics obtained from the multi-scale model was evaluated for batch and multi-feed SSF processes under previously described conditions at variable process times. Two scenarios were simulated:
(1) The variability in enzymatic activities was unknown a priori and a fixed amount of Cellic® CTec2 was added to the reactor. The scenario was simulated for batch and multi-feed operation. The scenario reflects a single activity measurement which is then used as input for several experiments with one or several batches of the enzyme cocktail.
(2) The variability in enzymatic activity was known a priori. To compensate for the variability in generated products, the amount of added Cellic® CTec2 was adjusted to reach the same enzymatic activity in the reactor. This scenario was simulated for multi-feed operation at all system levels and for batch processes at TEA level. The scenario reflects the unbiased measurement of enzymatic activities before each laboratory experiment.
For simulations of scenario (1) the dataset on the variability in enzymatic activities was directly used as input to the bioprocess model. For scenario (2), the amount of enzyme to be added to reach the nominal activity was calculated and used as input to the simulations. The total volume was balanced by modulating the addition of process water.
Basic statistical entities including mean, and 5th, 25th, 50th, 75th, and 95th percentiles were determined for all performance indicators at bioprocess, flowsheet and TEA scales. At the LCA scale, the 5th, 50th, and 95th percentiles were determined for all performance indicators.
The developed multi-scale model relies on a serial connection of models at different scales. To assess the validity of the bioprocess model under the chosen process conditions prior to its integration into the multi-scale model, an uncertainty and sensitivity analysis was performed on a 96 h batch process. Hydrolytic enzymes were simulated to adsorb to the solids, leading to cellulose and xylan degradation and a release of glucose and xylose. As glucose uptake was limited by the maximal glucose uptake rate of the yeast cells, glucose accumulated (Figure 4). Eventually, the hydrolysis rate decreased due to glucose feed-back inhibition and depletion of available substrate, after which an equilibrium between glucose release and uptake was established, reflected in glucose concentrations close to 0 g·L−1. According to the model, the yeast cells converted the released glucose directly into ethanol. When both the solid cellulose and the dissolved glucose were depleted, the ethanol production stopped due to a lack of substrate.
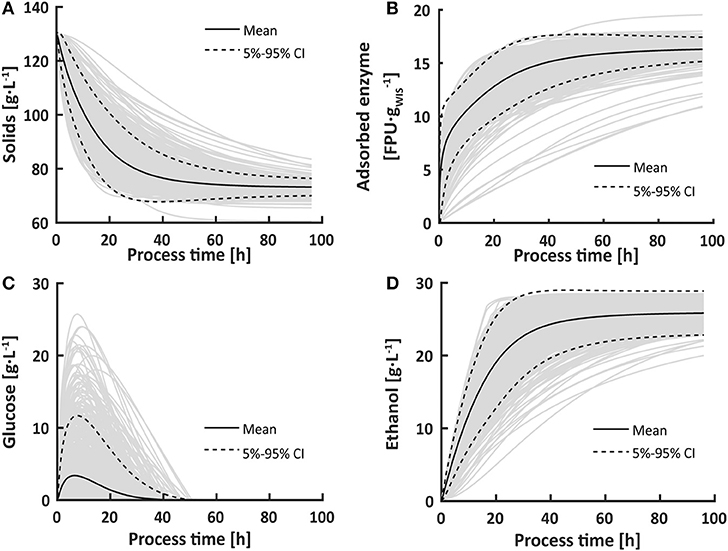
Figure 4. Uncertainty in the modeled solids (A), adsorbed enzyme (B), glucose (C) and ethanol (D) concentrations for a 96 h SSF batch process. The uncertainty in model parameters, evaluated in 1,000 Monte-Carlo simulations, lead to a high spread in glucose concentration in the first 40 h of the bioprocess due to a shifting balance between hydrolysis and fermentation. The dashed lines indicate the 5 to 95% confidence intervals in each set of 1,000 results.
In the first 40 h, uncertainties in model parameter estimates led to a spread in glucose concentrations of up to 26 g·L−1 (Figure 4). The large spread indicates that the bioprocess model has difficulties to precisely predict the shift in the overall rate limiting step from fermentation to hydrolysis. Limitations in glucose uptake and ethanol conversion capacities result in glucose accumulation, whereas a limited hydrolysis capacity results in low glucose concentrations. Accordingly, sensitivity analysis showed that glucose concentrations were most influenced by the hydrolysis rate constant k, the glucose uptake rate qG, and the cell death rate α (Figure 5). Increases in qG led to lower glucose concentrations, thereby relieving feed-back inhibition of hydrolysis. In contrast, increases in k or α caused glucose accumulation because the glucose consumption rate became the overall limiting step. Under glucose-limited conditions after 40 h, the influence of qG and k declined.
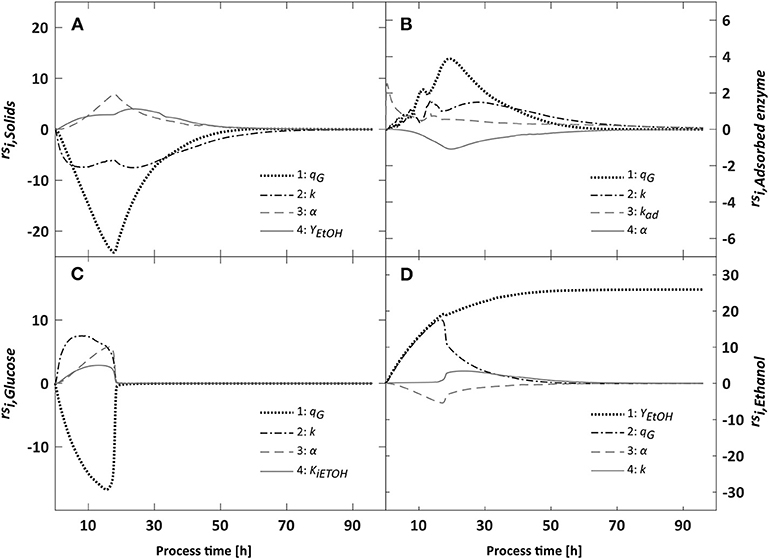
Figure 5. Relative sensitivities of the modeled solids (A), adsorbed enzyme (B), glucose (C) and ethanol (D) concentrations toward changes in their four most influential model parameters. The overall influence of parameters on each output was computed using the δmsqr measure for a 96 h batch process. Overall, the hydrolysis rate k and the glucose uptake rate qG influenced the model most, especially during the first 40 h of the process.
Final ethanol concentrations were mainly influenced by the ethanol yield on glucose, YEtOH. Ultimately, variations in final ethanol concentrations would only stem from YEtOH if operating the process infinitely long time. The other model parameters affected the dynamic changes in ethanol concentrations. The parameter-related uncertainty was low when stopping the process at ethanol productivities lower than 0.1 g·L−1·h−1.
A literature search on the enzymatic activity of Cellic® CTec2 resulted in 476 hits. Forty nine hits contained measurements of the protein content, and 81 presented the cellulose hydrolysis activity expressed in FPU·mL−1. Because the protein content had a higher standard deviation of 72 mg·mL−1 around a mean of 150 mg·mL−1, and the lower number of hits, the cellulose hydrolysis activity was used as a direct measure for enzymatic activity. After statistical analysis 70 hits representing the variability in the enzymatic activity of Cellic® CTec2 remained. The average activity was 146 FPU·mL−1, the median activity 139 FPU·mL−1, and the standard deviation was 41 FPU·mL−1. The distribution of enzymatic activities was negatively skewed, reflected in a maximum activity of 266 FPU·mL−1 vs. a minimum of 62 FPU·mL−1.
The high spread in measured activities is very problematic during process development at laboratory scale and scale-up. Commonly, the amount of added enzyme is stated as hydrolytic activity relative to the WIS, total solid or cellulose content to enable comparisons of experiments across scales and laboratories. Failures to measure enzymatic activities accurately and precisely could therefore lead to suboptimal process designs and the exclusion of better process alternatives.
The collected enzyme activity data include both measurement variability and the actual enzyme product variability. It is likely that measurement variability contributes more to the overall variability, as the FPU assay has been reported to be difficult to reproduce (Dashtban et al., 2010).
In the first case study scenario batch and multi-feed operations were analyzed under the assumption that actual enzymatic activities were unknown. Thus, in simulations a fixed amount of enzyme, calculated from the median activity, was added to the bioreactors, resulting in variations in the actual added enzymatic activity per WIS.
Variations in enzymatic activities had no effect on final ethanol concentrations when running batch processes for 10,000 h. Instead, the variability affected the time it took to reach the final ethanol concentrations. These findings are in agreement with uncertainty analysis and confirm that variation or improvements in enzymatic activities affect process dynamics but not final titers. To further investigate these dynamic effects a stop criterion was applied. The stop criterion resulted in a spread in final process times from 31.6 h (5th percentile) to 46.2 h (95th percentile) at a median of 36.6 h. Ethanol concentrations ranged from 23.6 to 25.4 g·L−1 (Figure 6).
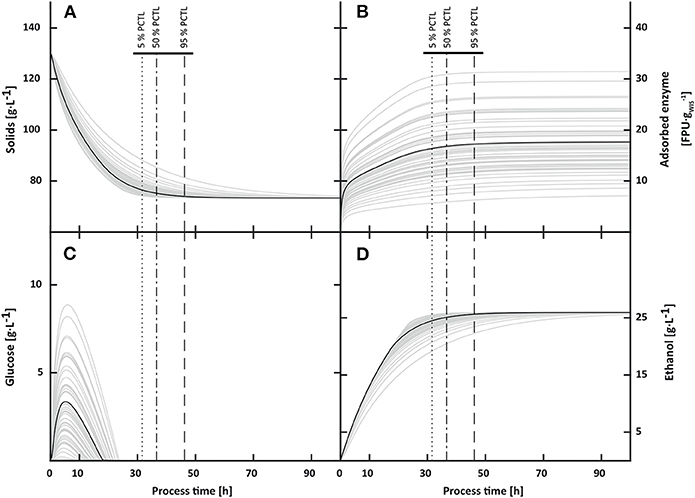
Figure 6. Impact of variability in enzymatic activities on solids (A), adsorbed enzyme (B), glucose (C) and ethanol (D) concentrations in batch process. Final ethanol concentrations were not affected by the variable input. Instead, the variability in enzymatic activities affected the time to completely deplete glucose and thereby the final process time. When applying a process stop criterion at ethanol productivities ≤ 0.1 g·L−1, the final process time varied between 31.6 h (5th percentile) and 46.2 h (95th percentile).
Variations in process times were highly dependent on the inoculum size. Large inocula led to immediate glucose consumption. Therefore, hydrolysis became rate-limiting and variations in hydrolysis rates had a direct effect on the process time. With small inocula instead, glucose accumulated as the hydrolysis rate exceeded the fermentative capacity of the cells. Hence, these conditions minimized process time variations at the expense of overall longer process times. Given the high model uncertainty around the shift between hydrolysis and fermentation as rate-limiting steps, the predictive quality of the model would need improvements to infer exact inoculum sizes to restrict process time variations under batch operation.
Under multi-feed operation, frequent solid additions resulted in an accumulated solid load of 21.85% WIS, 68% higher than under batch operation. In the simulations, the higher solid loadings led on average to 2.6 times longer process times than in the batch operation (5th percentile: 89.7 h; 50th percentile: 95.1 h; 95th percentile: 102.7 h) and final ethanol concentrations between 41.9 and 43.6 g·L−1. The spread in final process times relative to the median was 65% lower than in batch operation. The standard deviation in final ethanol concentrations and the variation in the ethanol yield [gEtOH·] decreased compared to batch operation.
The simulations indicate that the multi-feed operation could be further improved. As Figure 7 shows, ethanol concentrations stabilized after each solids and cell addition while maintaining enough cell viability for ethanol production, indicating cellulose depletion. With the proposed stop criterion and online ethanol measurements, feeding events could be triggered, thereby achieving a higher productivity while decreasing the process time. However, as feeding events would be triggered by decrements in fermentation rates caused by decreasing hydrolysis rates due to cellulose depletion, the hydrolysis rate would ultimately be the overall rate limiting step. Hence, such an adaptive strategy might be highly susceptible to varying enzymatic activities.
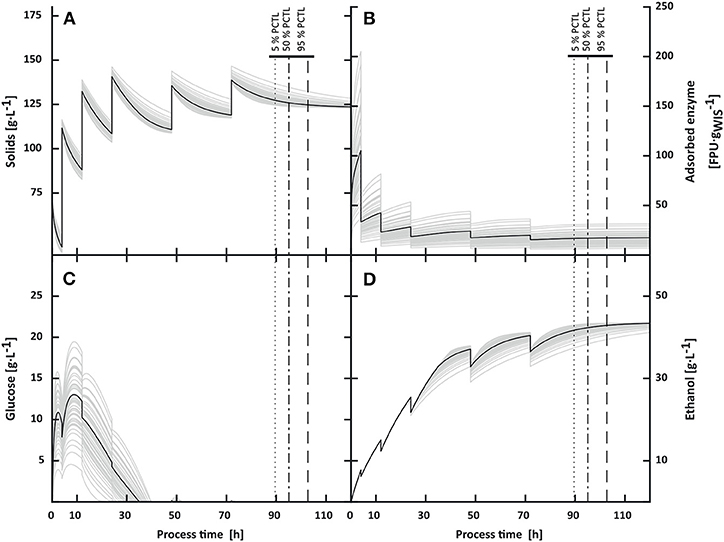
Figure 7. Impact of variability in enzymatic activities on solids (A), adsorbed enzyme (B), glucose (C) and ethanol (D) concentrations in multi-feed process. At solid additions ethanol was diluted and increased afterwards due to cellulose hydrolysis and fermentation. The final process time ranged from 89.7 h (5th percentile) to 102.7 h (95th percentile). Final ethanol concentrations varied by 2%.
The performance indicator most affected by varying enzymatic activities at bioprocess scale was the final process time. Hydrolysis and fermentation yields showed low variation. Therefore, the variation in final process times was integrated into higher system scales. Batch and multi-feed operations were analyzed when 5, 25, 50, 75, and 95% of all bioprocesses were stopped according to the stop criterion.
Variation in enzymatic activities affected ethanol, methane and electricity production in the biorefinery under batch and multi-feed operation. At median enzymatic activities and median process times approximately 72 MWLHV (LHV = lower heating value) of the main product ethanol were produced in both modes of operation. However, at median final process times, the variability in produced ethanol was 65% higher under batch than under multi-feed conditions (Figure 8). The difference in variabilities decreased with increasing final process times as maximum final ethanol concentrations were reached even at lower enzymatic activities. Under batch operation, ethanol production ranged from 70 to 74 MWLHV across the analyzed process times at median enzymatic activities, under multi-feed operation from 71 to 73 MWLHV.
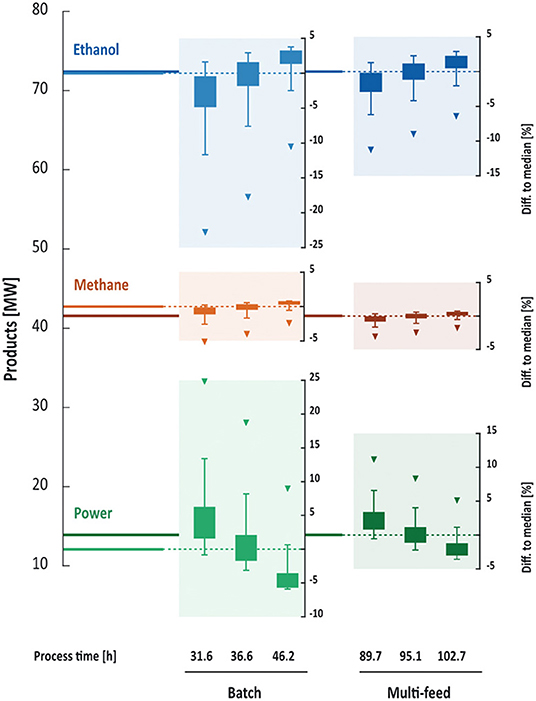
Figure 8. Median product generation and variability in products. Under batch conditions less power and more methane are produced due to a less complete cellulose conversion compared to multi-feed conditions. The variability in generated products as response to variance in enzymatic activities is larger under batch conditions. The least affected product is methane. Longer process times reduce the variability as more processes at different hydrolysis efficiency reach full conversion of cellulose to ethanol. The triangles indicate the minimum enzymatic activity, box limits 25th and 75th percentiles, and error bar limits the 95th and 5th percentiles in enzymatic activities. The left axis relates to the median values under investigated conditions, the right axes to the variability expressed as percentage of the median.
At median final process times and nominal enzyme activity approximately 42 MWLHV of methane were produced in AD under batch and multi-feed operation. The AD process seemed to be nearly independent of the variation in enzymatic activities. The small variations in the range of 1–3% of the solids content sent to AD, caused by different hydrolysis and fermentation yields, were smaller than the variations inherent in model calculations. To investigate these differences, a more detailed model would be required. The low variability in biogas production can on the one hand be attributed to the constant flow of hydrolysate from pretreatment to AD, in all cases corresponding to >50% of the total feed, which is independent of the hydrolysis efficiency. On the other hand, differences in the hydrolysis efficiency affected ethanol production rather than AD as >90% of the sugars released during hydrolysis were converted to ethanol. Therefore, the feed from the distillation columns to AD varied only by a few percent under all investigated conditions.
Electric power generation, however, was affected by enzymatic variabilities to the same extent as ethanol production, suggesting that there is a counterbalance between ethanol production and electric power generation via the left-over cellulose that instead can be burnt for steam generation. This hypothesis was substantiated by the observation that the minimum enzymatic activity caused the highest electric power generation in both process configurations. In short, lower hydrolysis yields increased the amounts of solids (cellulose) remaining after SSF and thereby the amount of fuel sent to the boiler. Accordingly, less electricity was produced at longer process times as more cellulose was hydrolyzed and subsequently converted into ethanol.
The amount of electricity produced from steam generated in the boiler was higher under multi-feed operation (Figure 8). The underlying reasons for the lower electricity production under batch operation were the higher flow rates and the substantially lower final ethanol concentrations after SSF. The lower ethanol concentrations in batch processes increased the steam demand for distillation, thereby lowering the capacity in the steam turbine for electricity production.
With the assumptions made in the TEA, the variability in enzymatic activities caused no significant changes (<2%) in the equipment costs under both modes of operation. However, the costs for the bioreactors varied by 20% across the different process times under batch and by 8% under multi-feed operation (Figure 9A). The lower variation in bioreactor costs under multi-feed operation was a result of the lower variation in final bioprocess times. The cost for the SSF bioreactors accounted for 10% of the total equipment cost in multi-feed processes. Due to longer residence times in response to higher cumulative WIS content, the bioreactor equipment costs were higher in multi-feed processes, whereas the equipment costs for product upgrading and waste water treatment were lower due to decreased flow rates in response to the higher cumulative WIS contents.

Figure 9. Impact of variability in enzymatic activity on techno-economic parameters under batch and multi-feed conditions. The variability in enzymatic activities had no effect on equipment cost (A). Batch and multi-feed conditions affected the cost for the bioreaction, product upgrading and waste water treatment section. The yearly revenues were significantly influenced by the variability in enzymatic activities, with a higher variability under batch conditions (B). The variability in yearly revenues propagated to the IRR (C) and PBT (D). The triangles indicate the minimum enzymatic activity, box limits 25th and 75th percentiles, and error bar limits the 95th and 5th percentiles in enzymatic activities. The left axis relates to the median values under investigated conditions, the right axes to the variability expressed as percentage of the median.
Contrary to the equipment and operating costs which were almost constant under all investigated conditions given the assumptions made in the TEA model, the yearly revenues were significantly affected by variations in enzymatic activities (Figure 9B). The responses to the variation followed the same trend as the ethanol production, with higher variances caused under batch operation. As ethanol production increased across process times, the yearly revenues increased. The median yearly revenues of 79.3 MEUR·year−1 under batch and 80 MEUR·year−1 under multi-feed operation differed only by 0.7 MEUR·year−1. Since the economic results followed the trend of produced ethanol, the data indicate that ethanol production affects the economic profitability of the biorefinery system the most even though increased ethanol production is counterbalanced by reduced electricity production and, under batch operation, by increased equipment cost.
The variance in the internal rate of return (IRR) was at median process times 56% higher under batch operation (Figure 9C), and in the payback time (PBT) 81% higher (Figure 9D). Furthermore, the median IRR was lower under batch operation, leading to 7% longer median PBT. Across process times the IRRs increased, following the trend in ethanol production, and the payback times decreased. The TEA results indicate that the TEA performance indicators are more robust under multi-feed operation against variations in enzymatic activities. Taking the IRR and PBT into account, the multi-feed process was economically more favorable than batch operation.
In order to draw conclusions regarding the economic feasibility of batch and multi-feed operation, more detailed assessments are required. These assessments include the scheduling of the bioreactor system including yeast propagation and SSF, and different utilizations of excess hydrolysate after pretreatment. For example, sending the hydrolysate to the SSF and yeast propagation bioreactors instead of the biogas plant directly might affect both the TEA results and the sensitivity to variations in process parameters.
At median enzyme activities and median process times the climate impact under batch operation was equal to that of the multi-feed operation (0.869 and 0.864 kgCO2eq·, respectively). However, as observed for the economic measures, the variation under batch operation was 70% higher (Figure 10A). The climate impact decreased with increasing final process times due to higher ethanol yields relating to a more complete raw material utilization. The contribution of enzyme production to the climate impact was stable at 43% for all investigated cases as the same amount of enzyme was added. The EP followed the same trends as the climate impact (Appendix 1). As these findings also follow the trends for the ethanol production, it supports the hypothesis that the variability in climate impact is directly related to the variability in produced ethanol as a response to variances in enzymatic activities.
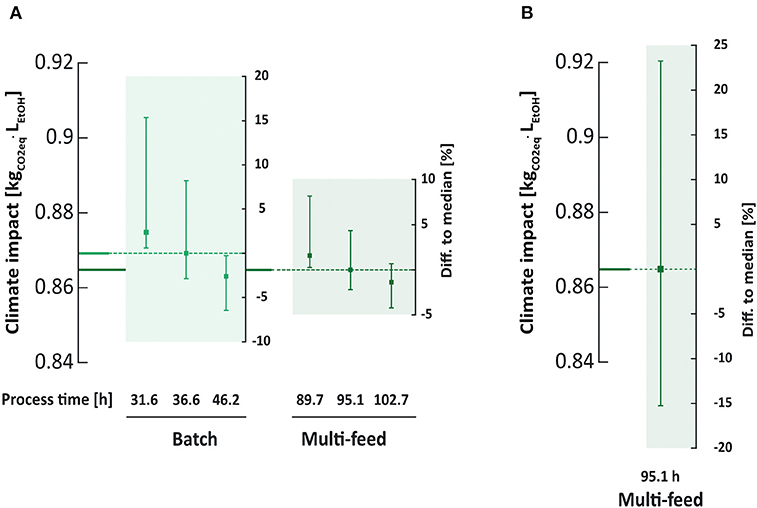
Figure 10. Variation in climate impact as response to variation in enzymatic activities. In scenario 1 the variability in enzymatic activities affects the global warming potential significantly more under batch than under multi-feed conditions as response to changing saccharification efficiency (A). Changing the amount of added enzyme as response to variation in enzymatic activities (scenario 2) results in a spread between the 5th and 95th percentile of 39% around the median for the multi-feed process running 95.1 h (B), almost six times higher than in scenario 1. The error bar limits indicate the 95th and 5th percentiles in enzymatic activities. The left axis relates to the median values under investigated conditions, the right axes to the variability expressed as percentage of the median.
Scenario 1 shows that multi-scale variability analysis can have a strong influence on the process design of projected biorefineries. Considering only the mean or median values for enzymatic activity, batch and multi-feed operations have the same yearly revenues. Although performance indicators such as the IRR and PBT indicate slight advantages of multi-feed operations, process designers might tend more toward choosing batch operation due to its wide application and simpler operability and controllability, contrary to multi-feed operation. However, multi-scale variability analysis shows that under multi-feed operation, all performance indicators are more robust against variations in enzymatic activities. Therefore, performance indicators retrieved from multi-feed experiments at laboratory and demo plant scale are more reliable when coping with variations in hydrolysis rates. Variations in hydrolysis rates could also stem from variations in the amount of added enzyme due to the high viscosity of enzyme preparations. The analyzed scenario shows that the developed multi-scale variability analysis framework can pinpoint bottlenecks in the process which are not robust against input variations. In contrast to an analysis at median conditions, the framework delivers a measure of the reliability in performance indicators and provides stakeholders with valuable information on how to proceed in process development. Compared to Vicari et al. (2012), the framework not only considers the lower uncertainties in yields but also takes the process dynamics into account. Specifically, the spread in process times at bioprocess scale is transformed in a spread in yearly material flows at flowsheet, TEA and LCA scales. The performed analysis shows that this spread in process times affects performance indicators at higher system scales more than variabilities in hydrolysis or fermentation yields.
Another advantage of the variability analysis approach shown by scenario 1 is the consistent evaluation of variability effects on all system levels. Thereby, process dynamics of the biorefinery can be reflected in greater detail and be introduced to non-dynamic models at flowsheet, TEA and LCA levels. Furthermore, the variabilities derived from measurement data could potentially be used as inputs to uncertainty and sensitivity analysis at the TEA and LCA levels. The variabilities could improve assumptions on step changes or distributions in input variables and flowsheet model or LCA parameters, and their dynamic effects on the bioethanol plant.
The second scenario covered an a priori knowledge of the actual enzyme activity and adjustment of the added amount of enzyme to reach the target activity of 9.3 FPU· under multi-feed operation. Since the amount of added enzyme changed, the same activity was added to the bioreactors, resulting in identical final ethanol concentrations and process times for all simulations. The differences in the added amount of enzyme resulted in differing process costs, emissions and resources related to enzyme production.
Under the assumptions made in this case study, for multi-feed operation a constant addition of enzyme (scenario 1) would be preferable over adjusting the amount of added enzyme to varying activities (scenario 2). Variations in the enzyme prices due to changed amounts of added volume would have a larger effect on the process economics (scenario 2) than the variation in process performance (scenario 1). Under batch conditions adjusting the amount of added enzyme to varying activities (scenario 2) would be preferable except for process times longer than 46.2 h (95th percentile). In the case of longer process times the variation in process performance is decreased in scenario 1 to an extent that the variation in enzyme prices due to varying amounts of added enzyme (scenario 2) is higher.
The climate impact was significantly affected by changes in the added enzyme volume. Contrary to the situation in scenario 1, the contribution of the enzymes on the climate impact and EP varied in scenario 2 for multi-feed operation. The contribution of enzyme addition to the climate impact ranged from 33 to 54%, and to the EP from 20 to 37%. The high contribution of enzymes produced off-site to the climate impact is in agreement with other LCA studies on second generation bioethanol production (Janssen et al., 2014, 2016; Olofsson et al., 2017). Moreover, the variability in climate impact was 588% higher in scenario 2 compared to scenario 1 (Figure 10B).
For batch operation scenario 2 seems favorable due to a high stability in generated products and techno-economic parameters. However, the improvements in process robustness are counterbalanced by the increased variability in climate impact. For multi-feed operation both the techno-economic parameters and the climate impact show higher variation in scenario 2 compared to scenario 1, favoring the addition of a defined amount of enzyme regardless of varying activities. This indicates that there might be potential for aligning environmental and economic goals with design strategies for ethanol-based biorefineries, if the process is designed to be robust against variations in performance. In case of a process sensitive to variations in process inputs, such as the batch process in this study, the environmental and economic goals will be contradictive.
A multi-scale variability analysis framework was developed to quantify variances in performance indicators resulting from process input variations. The framework was applied to evaluate a wheat straw-based ethanol biorefinery. An application to different raw materials and products requires the integration of other validated bioprocess models into the framework. With the existing serial structure, new models can be easily integrated at bioprocess scale. Therefore, the framework could also be extended to compare the predictions based on different models describing the same bioprocess at different system scales. Another extension to the developed framework could include the analysis of one or more process inputs different to the enzymatic activity. Therefore, the model structure at flowsheet scale might need adjustments to call the necessary variables from bioprocess scale. Furthermore, future work should aim to directly link the different model environments to reduce the complexity in data transfer and remove potential error sources.
In the presented multi-scale variability analysis framework input variations were propagated through a multi-scale model describing a wheat straw-based biorefinery. Thereby, the robustness of performance indicators against these variations could be quantified at multiple system scales for different process operations and designs.
Case study scenario 1 illustrated that analyses of median or average process inputs lead to different conclusions than using the full variability in process inputs. The developed framework enables the assessment of the reliability of performance indicators whereas an analysis at median levels provides single values without an uncertainty estimate. The results of scenario 1 showed that performance indicators are more reliable under multi-feed than under batch operation. Furthermore, the analysis revealed that only a specific group of performance indicators is affected by variations in enzymatic activities. While equipment and operating costs remained almost constant, the yearly revenues, for example, were significantly affected within this analysis.
A key advantage over single-scale assessments is the integration of process dynamics into static TEA and LCA models. Until now, uncertainty and variability assessments at TEA scale were restricted to changes in yields or raw material compositions. As the uncertainty analysis of the bioprocess model showed, the variation in process times is higher than the variation in hydrolysis and fermentation yields, unless a large part of the cellulose remains unconverted, which in any case would result in a non-viable process. The results of case study scenario 1 illustrated that process time evaluation is key to predict process designs with favorable economics and environmental impact as time-dependent trends can be identified. Furthermore, the multi-scale approach allows for the inclusion of multiple criteria for process evaluation. As such, multi-scale variability analysis could be used for multi-objective optimization under variable process inputs.
The uncertainty estimates of performance indicators are very important in a biorefinery context as process development is confronted with multiple design choices under varying process inputs, e.g., varying raw material compositions, supply chains or process portfolios. The framework developed in this study offers a methodology for performing such investigations.
The original literature dataset, the output datasets from each model scale as well as the statistical analysis of raw data are available in Appendix 1. The models in MATLAB, SuperPro Designer, and openLCA are available from the corresponding authors on reasonable request.
DN performed the search and statistical analyses of literature data, the mathematical modeling, and uncertainty analysis of the fermentation process. RF designed and performed the process simulations and techno-economic analysis. MJ performed the life cycle assessment. DN and RF designed and programmed the automated data exchange between fermentation simulations, process system simulations, and techno-economic analyses. DN, RF, and MJ drafted the manuscript. All authors analyzed, interpreted, integrated the results, edited the manuscript, read and approved the final manuscript, and conceived and designed the study.
This research was funded by the Swedish Energy Agency (P41272-1) and the Area of Advance Energy at Chalmers University of Technology. The funding bodies had no influence on the design of the study and were involved neither in the collection, analysis and interpretation of data, nor in the writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Ruifei Wang for providing raw data from laboratory and demo scale experiments.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2020.00055/full#supplementary-material
AD, Anaerobic digestion; EP, Eutrophication potential; FPU, Filter paper units; IRR, Internal rate of return; LCA, Life cycle assessment; LHV, Lower heating value; PBT, Payback time; SSF, Simultaneous Saccharification and Fermentation; TEA, Techno-Economic Analysis; WIS, Water Insoluble Solids.
Ahmetović, E., Ibrić, N., and Kravanja, Z. (2014). Optimal design for heat-integrated water-using and wastewater treatment networks. Appl. Energy 135, 791–808. doi: 10.1016/j.apenergy.2014.04.063
Althaus, H.-J., Chudacoff, M., Hischier, R., Jungbluth, N., Osses, M., and Primas, A. (2007). Life Cycle Inventories of Chemicals. Ecoinvent Report No 8., v2.0. Dübendorf: EMPA Dübendorf, Swiss Centre for Life Cycle Inventories.
Barta, Z., Reczey, K., and Zacchi, G. (2010). Techno-economic evaluation of stillage treatment with anaerobic digestion in a softwood-to-ethanol process. Biotechnol. Biofuels 3:21. doi: 10.1186/1754-6834-3-21
Baumann, H., and Tillman, A.-M. (2004). The Hitch Hiker's Guide to LCA: An Orientation in Life Cycle Assessment Methodology and Application. Lund: Studentlitteratur AB.
Bellido, C., Bolado, S., Coca, M., Lucas, S., González-Benito, G., and García-Cubero, M. T. (2011). Effect of inhibitors formed during wheat straw pretreatment on ethanol fermentation by Pichia stipitis. Bioresour. Technol. 102, 10868–10874. doi: 10.1016/j.biortech.2011.08.128
Bogart, E., and Myers, C. R. (2016). Multiscale metabolic modeling of C4 plants: connecting nonlinear genome-scale models to leaf-scale metabolism in developing maize leaves. PLoS ONE 11:e0151722. doi: 10.1371/journal.pone.0151722
Ciroth, A. (2007). ICT for environment in life cycle applications openLCA – a new open source software for life cycle assessment. Int. J. Life Cycle Assess. 12:209. doi: 10.1065/lca2007.06.337
Collins, S. R., Wellner, N., Bordonado, I. M., Harper, A. L., Miller, C. N., Bancroft, I., et al. (2014). Variation in the chemical composition of wheat straw: the role of tissue ratio and composition. Biotechnol. Biofuels 7:121. doi: 10.1186/s13068-014-0121-y
Dada, J. O., and Mendes, P. (2011). Multi-scale modelling and simulation in systems biology. Integr. Biol. 3, 86–96. doi: 10.1039/c0ib00075b
Dashtban, M., Maki, M., Leung, K. T., Mao, C., and Qin, W. (2010). Cellulase activities in biomass conversion: measurement methods and comparison. Crit. Rev. Biotechnol. 30, 302–309. doi: 10.3109/07388551.2010.490938
Deen, N. G., van Sint Annaland, M., and Kuipers, J. A. M. (2004). Multi-scale modeling of dispersed gas–liquid two-phase flow. Chem. Eng. Sci. 59, 1853–1861. doi: 10.1016/j.ces.2004.01.038
Erdei, B., Hancz, D., Galbe, M., and Zacchi, G. (2013). SSF of steam-pretreated wheat straw with the addition of saccharified or fermented wheat meal in integrated bioethanol production. Biotech. Biofuels 6:169. doi: 10.1186/1754-6834-6-169
Fornell, R., and Berntsson, T. (2012). Process integration study of a kraft pulp mill converted to an ethanol production plant – Part A: Potential for heat integration of thermal separation units. Appl. Therm. Eng. 35, 81–90. doi: 10.1016/j.applthermaleng.2011.10.010
Fornell, R., Berntsson, T., and Åsblad, A. (2012). Process integration study of a kraft pulp mill converted to an ethanol production plant – part B: Techno-economic analysis. Appl. Therm. Eng. 42, 179–190. doi: 10.1016/j.applthermaleng.2012.02.043
Fornell, R., Willquist, K., Petersson, A., and Franzén, C. J. (2016). Water management in lignocellulosic ethanol production – a case study and comparative analysis from a Swedish perspective. Chem. Eng. Trans. 52, 703–708. doi: 10.3303/CET1652118
Frischknecht, R., Tuchschmid, M., Faist Emmenegger, M., Bauer, C., and Dones, R. (2007). Strommix und Stromnetz. Sachbilanzen von Energiesystemen: Grundlagen für den Ökologischen Vergleich von Energiesystemen und den Einbezug von Energiesystemen in Ökobilanzen für die Schweiz. Dübendorf: Swiss Centre for Life Cycle Inventories.
Gorrée, M., Heijungs, R., Huppes, G., Kleijn, R., de Koning, A., Van Oers, L., et al. (2002). Handbook on Life Cycle Assessment: Operational Guide to the ISO Standards. Dordrecht: Kluwer Academic Publishers.
Horváth, I. S., Franzén, C. J., Taherzadeh, M. J., Niklasson, C., and Lidén, G. (2003). Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl. Environ. Microbiol. 69, 4076–4086. doi: 10.1128/AEM.69.7.4076-4086.2003
Hosseini, S. A., and Shah, N. (2009). Multiscale modelling of hydrothermal biomass pretreatment for chip size optimization. Bioresour. Technol. 100, 2621–2628. doi: 10.1016/j.biortech.2008.11.030
Humbird, D., Davis, R., Tao, L., Kinchin, C., Hsu, D., Aden, A., et al. (2011). Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Technical Report NREL/TP-5100-477642011. Golden: National Renewable Energy Laboratory (NREL).
Janssen, M., Tillman, A.-M., Cannella, D., and Jørgensen, H. (2014). Influence of high gravity process conditions on the environmental impact of ethanol production from wheat straw. Bioresour. Technol. 173, 148–158. doi: 10.1016/j.biortech.2014.09.044
Janssen, M., Xiros, C., and Tillman, A.-M. (2016). Life cycle impacts of ethanol production from spruce wood chips under high-gravity conditions. Biotechnol. Biofuels 9:53. doi: 10.1186/s13068-016-0468-3
Liptow, C., Tillman, A.-M., Janssen, M., Wallberg, O., and Taylor, G. A. (2013). Ethylene based on woody biomass – what are environmental key issues of a possible future Swedish production on industrial scale. Int. J. Life Cycle Assess. 18, 1071–1081. doi: 10.1007/s11367-013-0564-6
MacLean, H. L., and Spatari, S. (2009). The contribution of enzymes and process chemicals to the life cycle of ethanol. Environ. Res. Lett. 4:014001. doi: 10.1088/1748-9326/4/1/014001
Muñoz, I., Flury, K., Jungbluth, N., Rigarlsford, G., i Canals, L. M., and King, H. (2014). Life cycle assessment of bio-based ethanol produced from different agricultural feedstocks. Int. J. Life Cycle Assess. 19, 109–119. doi: 10.1007/s11367-013-0613-1
Ofiteru, I. D., Bellucci, M., Picioreanu, C., Lavric, V., and Curtis, T. P. (2014). Multi-scale modelling of bioreactor–separator system for wastewater treatment with two-dimensional activated sludge floc dynamics. Water Res. 50, 382–395. doi: 10.1016/j.watres.2013.10.053
Öhgren, K., Bengtsson, O., Gorwa-Grauslund, M. F., Galbe, M., Hahn-Hägerdal, B., and Zacchi, G. (2006). Simultaneous saccharification and co-fermentation of glucose and xylose in steam-pretreated corn stover at high fiber content with Saccharomyces cerevisiae TMB3400. J. Biotechnol. 126, 488–498. doi: 10.1016/j.jbiotec.2006.05.001
Olofsson, J., Barta, Z., Börjesson, P., and Wallberg, O. (2017). Integrating enzyme fermentation in lignocellulosic ethanol production: life-cycle assessment and techno-economic analysis. Biotechnol. Biofuels 10:51. doi: 10.1186/s13068-017-0733-0
Röös, E., Sundberg, C., and Hansson, P.-A. (2011). Uncertainties in the carbon footprint of refined wheat products: a case study on Swedish pasta. Int. J. Life Cycle Assess. 16, 338–250. doi: 10.1007/s11367-011-0270-1
Sin, G., and Gernaey, K. (2016). “Data handling and parameter estimation”, in Experimental Methods in Wastewater Treatment, eds M. C. M. van Loosdrecht, P.H. Nielsen, C.M. Lopez-Vazquez, and D. Brdjanovic (London: IWA Publishing, 201–234.
Sin, G., Gernaey, K. V., and Lantz, A. E. (2009). Good modeling practice for PAT applications: propagation of input uncertainty and sensitivity analysis. Biotechnol. Prog. 25, 1043–1053. doi: 10.1002/btpr.166
Uppugundla, N., da Costa Sousa, L., Chundawat, S. P., Yu, X., Simmons, B., Singh, S., et al. (2014). A comparative study of ethanol production using dilute acid, ionic liquid and AFEX™ pretreated corn stover. Biotechnol. Biofuels 7:72. doi: 10.1186/1754-6834-7-72
Uría-Martínez, R., Leiby, P. N., and Brown, M. L. (2018). Energy security role of biofuels in evolving liquid fuel markets. Biofuel. Bioprod. Bioref. 12, 802–814. doi: 10.1002/bbb.1891
Vicari, K. J., Tallam, S. S., Shatova, T., Joo, K. K., Scarlata, C. J., Humbird, D., et al. (2012). Uncertainty in techno-economic estimates of cellulosic ethanol production due to experimental measurement uncertainty. Biotechnol. Biofuels 5:23. doi: 10.1186/1754-6834-5-23
Wang, R., Koppram, R., Olsson, L., and Franzén, C. J. (2014). Kinetic modeling of multi-feed simultaneous saccharification and co-fermentation of pretreated birch to ethanol. Bioresour. Technol. 172, 303–311. doi: 10.1016/j.biortech.2014.09.028
Wang, R., Unrean, P., and Franzén, C. J. (2016). Model-based optimization and scale-up of multi-feed simultaneous saccharification and co-fermentation of steam pre-treated lignocellulose enables high gravity ethanol production. Biotechnol. Biofuels 9:88. doi: 10.1186/s13068-016-0500-7
Westman, J. O., Wang, R., Novy, V., and Franzén, C. J. (2017). Sustaining fermentation in high-gravity ethanol production by feeding yeast to a temperature-profiled multifeed simultaneous saccharification and co-fermentation of wheat straw. Biotechnol. Biofuels 10:213. doi: 10.1186/s13068-017-0893-y
Xavier, J. B., De Kreuk, M. K., Picioreanu, C., and van Loosdrecht, M. C. (2007). Multi-scale individual-based model of microbial and bioconversion dynamics in aerobic granular sludge. Environ. Sci. Technol. 41, 6410–6417. doi: 10.1021/es070264m
Yang, A., and Marquardt, W. (2009). An ontological conceptualization of multiscale models. Comput. Chem. Eng. 33, 822–837. doi: 10.1016/j.compchemeng.2008.11.015
Keywords: multi-scale model, variability analysis, biorefinery, bioethanol, uncertainty analysis, techno-economic analysis, life cycle assessment, system analysis
Citation: Nickel DB, Fornell R, Janssen M and Franzén CJ (2020) Multi-Scale Variability Analysis of Wheat Straw-Based Ethanol Biorefineries Identifies Bioprocess Designs Robust Against Process Input Variations. Front. Energy Res. 8:55. doi: 10.3389/fenrg.2020.00055
Received: 26 September 2019; Accepted: 17 March 2020;
Published: 23 April 2020.
Edited by:
Su Shiung Lam, University of Malaysia Terengganu, MalaysiaReviewed by:
Zhi-Hua Liu, Texas A&M University, United StatesCopyright © 2020 Nickel, Fornell, Janssen and Franzén. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rickard Fornell, cmlja2FyZC5mb3JuZWxsQHJpLnNl; Carl Johan Franzén, ZnJhbnplbkBjaGFsbWVycy5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.