
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Energy Res. , 23 July 2019
Sec. Bioenergy and Biofuels
Volume 7 - 2019 | https://doi.org/10.3389/fenrg.2019.00063
This present study aims in understanding the influence of dominant fatty acid esters of waste animal fat biodiesel on its emission characteristics in CI engine. Biodiesel was produced from waste animal fat by means of base catalyzed transesterification; and Ethyl oleate (40.21%), ethyl palmitate (25.36%), and ethyl stearate (16.87%) were characterized as dominant fatty acid esters using GC spectra. Test samples were prepared for these ester molecules based on their availability, in addition to biodiesel blend and plain diesel and were tested for their emission levels in single cylinder four stroke CI engine using flue gas analyser. High exhaust gas temperature was contributed by Ethyl oleate (1.15% lesser than biodiesel), as a result of low cetane number due to unsaturation; and high viscosity. Likewise, the increased carbon chain length and unsaturation of ethyl oleate (2.55% lesser than biodiesel) resulted in high concentration of CO emission for biodiesel whereas high CO2 emission concentration was because of ester molecules with increased carbon chain length (stearate and Oleate esters). Reduced NOX emission for biodiesel was as a result of higher cetane number from ethyl stearate (CN = 86.83) and ethyl palmitate (CN = 86.55), which reduced its ignition delay thereby moderating the heat release rate. In addition, long carbon chained ester molecules (oleate and stearate esters) in biodiesel consumed more oxygen content for improving overall rate of combustion while increased HC emission was explained by unsaturation in biodiesel because of ethyl oleate (on average, 50 PPM).
Increase in use of biodiesel as a viable supplement and alternate for fossil diesel fuel to meet the energy demand has made many researchers to focus on improvising its properties and engine characteristics with minimal energy and capital consumption. This renewable biofuel is highly regarded for combustion based applications because of its reduced emission characteristics owing to zero sulfur and aromatic content; and improvised engine performance due to higher oxygen content and cetane number (Srinivasan and Jambulingam, 2018).
Traditionally, Biodiesel is processed from different feedstocks like waste vegetable oil (Ray and Prakash, 2019), animal fat (Kirubakaran and Selvan, 2018), non-edible seeds oil (Hosseini and Wahid, 2012; Palani et al., 2017), waste oils and greases (Tran et al., 2018) and is converted into low viscous biofuel by means of catalyst assisted transesterification reaction using short chain primary alcohols like methanol, ethanol as solvent. Among these, waste animal fats are regarded as an ideal feedstock for low cost biodiesel production on account of its easy availability as discarded wastes, no “food over fuel” conflicts; in fact, using these wastes for energy production serves as an effective technique for reducing the environment threats caused by them. Apart from environmental concern, these waste fats are widely chosen as most promising feedstock because of its high energy density. In general, these waste animal fats are rendered from discarded fleshing wastes in leather tanneries and discarded fat wastes in slaughter houses and meat processing industries (Srinivasan et al., 2018a); and was found to be unsuitable for edibility but rich in triglyceride content, thereby making it as an ideal option for biodiesel production. Indeed, numerous studies related to its optimized production (Aboelazayem et al., 2018), properties study and their prediction using mathematical modeling (Jambulingam and Srinivasan, 2019), comprehensive engine characteristics (Nabi et al., 2019) and economic analysis (Subramanian et al., 2005) have been carried out to commercialize this biofuel in global market. However, for being a viable replacement for existing fossil fuel, which causes higher pollution, these biofuels must be evaluated thoroughly for its engine characteristics to determine their maximum range of efficiencies and emissions in exhaust gas and factors influencing them. In like manner, numerous experimental works have studied the performance and emission characteristics of biodiesel in compression ignition engine.
In relevance to that, Srinivasan et al. (2017) carried out the preliminary engine characteristics study on waste suet biodiesel and found higher brake-specific fuel consumption (BSFC) for biodiesel and its blends when compared to plain diesel as a result of low calorific value and high viscosity. In addition, the reduced thermal efficiency of biodiesel than plain diesel was because of high viscosity which reduced its atomization, vaporization and combustion. Interestingly, reduction in CO and NOX emission for biodiesel and its blends was noticed by reason of high oxygen content and reduced ignition delay.
Similarly, Emiroğlu et al. (2018) carried out engine characteristics study on turkey fat biodiesel in CI engine by preparing 3 different blends in 10, 20, and 50% (v/v) with plain diesel. The maximum Heat Release rate and cylinder pressure for biodiesel blends was because of low cetane number which prolonged the ignition delay thereby making the accumulated fuel to undergo rapid combustion. Along with lower cetane number, higher rate of density and viscosity of biodiesel increased its NOX emissions and was found to be higher for B50 upon accounting its lowest cetane number. However, lower heating value of biodiesel blend resulted in reduced brake thermal efficiency (BTE) than compared to diesel despite its higher oxygen content.
Following this, Jayaprabakar et al. (2019) carried out the process optimization and engine characteristics study of biodiesel produced from sheep skin in CI engine by blending with plain diesel in varying concentrations: 5, 10, 15, and 20%. Here, CO2 emission increased with blend% for biodiesel because of high oxygen and carbon content whereas high NOX emission from biodiesel was the outcome of its high Cetane number and degree of unsaturation. However, high C/H ratio and low oxygen content resulted in higher hydrocarbon (HC) emission for diesel.
Apart from conventional CI engine, engine characteristics study was carried out in Common Rail Direct Injection (CRDI) engine for animal fat biodiesel by preparing five different blends in varying concentrations of 10–50% with plain diesel and concluded 30% blend as the most optimal blend. Interestingly, lower HC emission was noted for B30 blend because of abundant availability of oxygen and optimum blend which also helped in reducing its BSFC. Apparently, NOX emission was found to be reducing for oxidized biodiesel as compared with unoxidized biodiesel owing to higher cetane number in case of former; and the same was reported by Monyem and Van Gerpen (2001) whose study concluded that both oxidized and unoxidized biodiesel demonstrated identical engine performance (Shahir et al., 2017).
Furthermore, Hazrat et al. (2019) carried out a significant study of emission characteristics in CI engine by employing a heterogeneous blend of waste tallow and cooking oil biodiesel blended with extra low sulfur diesel for varying biodiesel in concentration of 5, 10, and 15% and found that higher CO2 emission concentration for biodiesel blends was on account of high oxygen content and elevated temperature inside combustion chamber. Similarly, NOX emission was found reduced for plain diesel whereas increased for biodiesel, owing to surplus oxygen content from the binary biodiesel itself, which reacted with nitrogen present in the intake air; however, this oxygen content was responsible in reducing the HC emission as it helped in proper mixing and combustion of fuel. In contrast, CO emission was found to be very low for all the blends but higher emission was noted during the start of combustion owing to rapid acceleration resulting in improper oxidation of fuel.
Even though, previous literatures explained the possibility of reduced emission upon using biodiesel in CI engine; the causes and effects of these reduced emission can be explained by studying the role of fatty acid esters in deciding the emission characteristics for biodiesel on combustion. Inspite of its importance, only few studies related to the influence of ester molecules in emission characteristics have been carried out and reported.
Following that, Gopinath et al. (2010) compared the engine characteristics of 13 different biodiesels using single cylinder DI diesel engine and numerous conclusions were derived from this study. Preliminary results concluded that biodiesel with high unsaturated fatty acid content resulted in higher NOX emission and reduced thermal efficiency. The study indicated that the ignition delay increases with fuel density, degree of unsaturation and its iodine value but deceases with high cetane number whereas ignitability of fuel was based on cetane number, fatty acid ester composition, residual methanol, glycerides, and water content. Also, increase in unsaturation and density decreased maximum heat release rate, BTE but increased specific fuel consumption, maximum gas pressure, and exhaust gas temperature, CO and HC (since unsaturation decreases oxygen content) and NOX emission. Interestingly, the dynamic injection timing was found to be faster for high density and high unsaturated fuels.
Similarly, Srinivasan et al. (2019) studied the role of fatty acid esters in influencing the engine characteristics of waste beef tallow biodiesel. Based on characterization, ethyl oleate, ethyl palmitate, and ethyl stearate were characterized as the dominant fatty acid ethyl esters and played a significant role in deciding the engine characteristics of biodiesel. Increased fuel consumption, reduced thermal efficiencies and elevated exhaust gas temperature, for biodiesel blends, were on account of higher viscosity for long carbon chained oleate and stearate esters. Even more, the presence of long chained stearate and oleate molecules in biodiesel blend resulted in high CO2 emission concentration while the unsaturated double bond in fatty acid moiety of oleate molecule led to high CO emission concentration. Despite the fact, NOX emission concentration reduced on account of higher cetane number from palmitate and stearate ester molecules which reduced its ignition delay. Furthermore, high cetane number along with surplus oxygen content contributed by individual fatty acid esters accounted for maximum cylinder pressure and high instantaneous heat release rate.
Moreover, Hellier et al. (2012) investigated the influence of alcohol moiety present in fatty acid ester molecules on engine characteristics. This study proposed that ester molecules released larger proportion of energy upon combustion owing to longer combustion duration and the ignition delay was decided based upon the chain length of fatty acid moiety instead of alcohol moiety. Even so, increase in carbon at alcohol moiety reduced the ignition delay but also increased the mass of particulates by double upon adding single carbon atom to chain of alcohol moiety. Consequently, increased NOX emission was a consequence of unsaturation which increased the adiabatic flame temperature.
In addition, Schönborn et al. (2008) studied the influence of molecular structure of fatty acid esters in NOX and particulate matter (PM) formation during combustion. This study proposed that ignition quality and early ignition increased with length of fatty acid chain length and helped in achieving better HRR. Longer chained molecules had longer combustion duration along with lower peak HRR and peak pressure. Also, long chained fatty acid esters ignited quickly thereby moderating the HRR resulting in peak temperature followed by reduced NOX. Likewise, saturated fatty acid esters had reduced NOX emissions where the emission was directly proportional to FA chain length but increases with addition of double bond in carbon chain. Moreover, increase in carbon atom at alcohol moiety by 1 or 2 atoms has the ability to reduce NOX but increases PM slightly.
In this present work, a novel approach has been attempted in understanding the influence of dominant fatty acid esters in deciding the exhaust gas concentration of waste animal fat biodiesel by comparing emission characteristics of biodiesel from animal fat with characterized dominant fatty acid esters. Upon understanding their substantial role in engine characteristics, this work might act as a platform for redefining the combustion of biodiesel and achieve desired power output along with reduced emission by modifying the ester molecules in biodiesel through suitable techniques.
Waste animal fat was rendered from discarded leather fleshing and meat processing wastes collected from tanneries and animal slaughterhouses; and were refrigerated to evade microbial infection and oxidation (rancidification). Rendering of waste fat was carried out using autoclave operated at 120°C and 2 Bar and; were filtered and degummed using orthophosphoric acid (Srinivasan et al., 2018b). The refined fat was subjected for ethanol based transesterification at stoichiometric molar ratio (1:6) and optimal catalyst: (0.5% wt. of fat) KOH concentration and reaction temperature of 60°C under continuous stirring. The reaction products were separated into biodiesel and glycerol by decantation under the influence of gravity and the resultant biodiesel was water washed successively followed by drying. All the chemicals used for biodiesel production and emission testing were procured from Sigma Aldrich Chemicals Company.
Fatty acid esters in produced biodiesel were characterized from Gas chromatography spectra based on their respective peaks obtained at their corresponding retention time, whereas the area % occupied by the obtained peaks signified their composition. Accordingly, ethyl oleate, ethyl palmitate, and ethyl stearate were regarded as dominant fatty acid esters based on their availability % and are summarized in Table 1 along with their retention time and molecular weight. Figure 1 illustrates the GC spectra of waste animal fat biodiesel.

Table 1. Availability of characterized dominant fatty acid esters along with their retention time and molecular weight.
For emission characteristics study, a set of test samples namely: Biodiesel Blend, Oleate Blend, palmitate blends, and stearate blend, were prepared by blending waste fat biodiesel, ethyl oleate, ethyl palmitate, ethyl stearate with plain diesel, which was also taken as reference blend. The volume of ester/biodiesel sample for preparing the necessary blend was calculated using Equation 1(Srinivasan et al., 2019):
Where, Voverall-Required Sample Volume, ψB-Blend Factor, φE -Ester Factor and φE = 1, in case of biodiesel.
The suitability of waste animal fat biodiesel for commercialization and engine applications were analyzed by studying their thermal and physico-chemical properties determined as per ASTM standards and was found to be a viable supplement upon comparing with plain diesel. Properties like density, kinematic viscosity were determined to understand its physical behavior whereas flash point, cetane number, calorific value defined its thermal behavior. In addition, acid value, saponification, and iodine value explained its chemical behavior. Table 2 consolidates the physico-chemical properties of waste animal fat biodiesel.
The exhaust gas was analyzed for oxides and dioxides of carbon and nitrogen along with residual oxygen content and particulate matter concentration for test samples using a four stroke single cylinder Diesel engine to understand the role of fatty acid esters in producing these emissions while undergoing combustion. Besides, engine performance characteristics like specific fuel consumption and thermal efficiencies were also studied to understand the fuel behavior in operating engine. The emission levels were determined based on undiluted measurement by passing the engine exhaust gas inside the flue gas analyser directly from exhaust manifold, where IR sensors sensed the concentration of oxides of carbon, nitrogen and Hydrocarbon emissions in it as electrical signals and were manipulated into numerical data using suitable software. Table 3 outlines the technical specifications of Kirloskar Engine TV1 and AVL DI GAS 444 N (five gas analyser) (Srinivasan et al., 2019). Figure 2 portrays the schematic diagram of test engine setup.
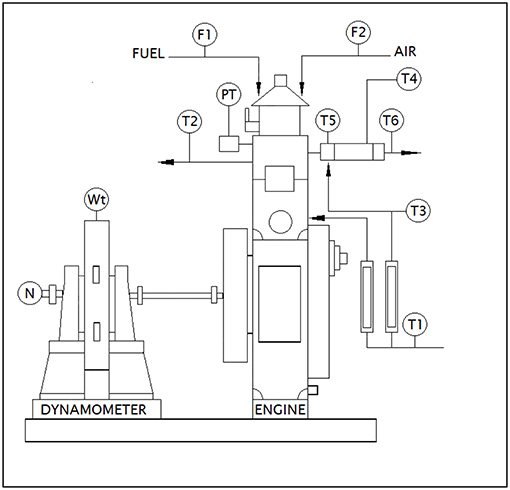
Figure 2. Schematic diagram of test engine setup. Source: Apex innovations (Research equipment supplier in India).
Waste Animal Fat biodiesel was produced by means of ethanol based transesterification using optimized parameters as follows: molar ratio- 1:6 (Ethanol: waste animal fat); potassium hydroxide (KOH) as base catalyst for 0.5% wt. of fat; reaction temperature −60°C; reaction time-2 h; and maximum yield of 94% was achieved using these parameters. Especially, KOH was chosen for this study owing to its effectiveness as homogeneous catalyst for transesterification of animal fats (Carraretto et al., 2004). Besides, moderate reaction temperature was maintained throughout the process to avoid any thermal decomposition of fatty acids present in the waste fats as well as processed biodiesel.
From Equation (1), it was clearly noted that volume of ester samples was a function of blend factor (ψB) and ester factor (φE); and was entirely based on the % availability of fatty acid esters. In particular, the ester factor was maintained as 1, in case of biodiesel blend sample, indicating the summation of all characterized fatty acid esters. Since, the maximum permissible blend for commercial usage of biodiesel and enhanced performance exhibited by animal fat is for 20% blend, the blend factor was maintained as 0.2 for all the ester blends (Öner and Altun, 2009). Table 4 tabulates the blend factor and ester factor for prepared samples.
Based on availability of characterized fatty acid esters, the numbers of carbon, hydrogen and oxygen molecules were calculated and molecular formula of waste fat biodiesel was formulated as C19H37O2. Following that, Stoichiometric air-fuel ratio was calculated from the quantity of air, NAIR (in terms of mols), required for completely combusting of one mol of biodiesel, determined using Equation (3) (Srinivasan et al., 2019), derived from balanced equation (Equation 2) of Carbon, hydrogen and oxygen molecules available in the fuel (Sarkar, 1974):
Using Equation (3), the required air quantity was calculated as 27.25 mols and Stoichiometric air-fuel ratio for the formulated molecular formula was computed and found to be 12.86:1. Since, a surplus amount of air (excess by 1.5 times than the calculated amount), is required for fuel to get completely combusted in engine, the actual stoichiometric air to fuel ratio was calculated as 19.29:1.
Performance characteristics brief about the behavior of fuel during engine operation and propose the maximum work that can be derived from the fuel for any given running condition. In general, it explains the amount of fuel consumed for delivering the required work and maximum work that can be derived for that load condition. Figure 3 portrays the specific fuel consumption, brake and indicated thermal efficiencies of various blends at various load conditions. Table 5 tabulates the average specific fuel consumption, brake and indicated thermal efficiencies of various blends at various load.
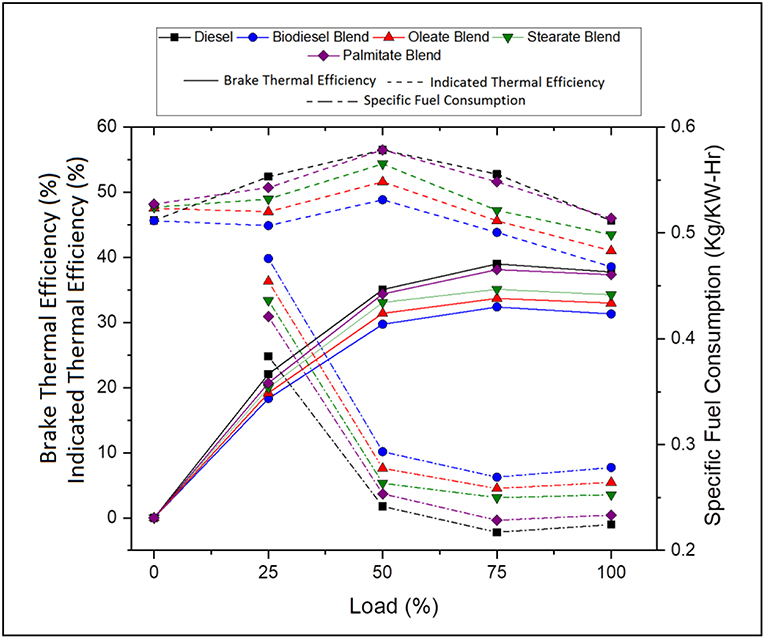
Figure 3. Specific fuel consumption, brake and indicated thermal efficiency of diesel and blend samples for varying load conditions.
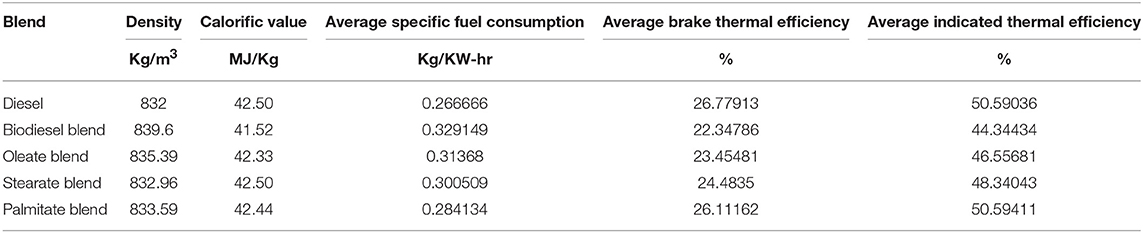
Table 5. Average specific fuel consumption, brake and indicated thermal efficiency of diesel and blend samples.
Accordingly, Specific fuel consumption (SFC) specifies the fuel requirement for producing 1 unit of power and decreases with increasing load owing to increasing brake power (Ray and Prakash, 2019). The SFC was found to be 18.93% lesser, in case of neat diesel and 4.71, 8.73, and 14.07% lesser, in case of oleate, stearate, and palmitate, respectively, compared to biodiesel blend. To emphasize, increase in SFC for biodiesel and its blends was on account of its low calorific value which increased its volumetric fuel consumption so as to sustain the energy demand of the engine. Infact, SFC was found to be higher for oleate and stearate blends because of their high viscosity on account of increased carbon chain length and; hence, the presence of these esters influenced the increased SFC for biodiesel blend.
On the contrary, Brake thermal efficiency (BTE) increased with load due to its high power demand and; on average; it was found to be 15.9% greater, in case of neat diesel and 3.96, 7.63, and 13.14% greater, in case of oleate, stearate, and palmitate, respectively, compared to biodiesel blend. Indeed, the reduced BTE for ester and biodiesel blends were because of their low calorific value and high viscosity (Hasan and Rahman, 2017; Prabu, 2018); however they tend to produce high BTE using the fuel bound oxygen in them at higher loads (Debbarma and Misra, 2017). Relative to SFC, the BTE of waste animal fat biodiesel was on account of stearate and oleate molecules present in it.
Similarly, Indicated thermal efficiency (ITE) defines the ratio between power developed to energy given as input through fuel injection and on average, was found to be 14.26% greater, in case of neat diesel and 5.02, 9.09, and 14.25% greater, in case of oleate, stearate, and palmitate blends, respectively, on comparison with biodiesel blend. Low ITE for biodiesel blend was a consequence of high viscosity (imparted by long carbon chain length) which reduced its atomization and vaporization inspite of its increased Heat Release Rate. In fact, the marginal difference in the ITE between biodiesel and oleate was because their higher rate of viscosity and the influence of latter were reflected in ITE of biodiesel blend.
Exhaust gas temperature helps in reporting the progress of combustion in engine and is influenced by the thermal properties of fuel used and operating parameters of the engine. In general, it tends to be higher for any long duration combustion process with a delayed start and subsequently results in higher concentration of NOX emission. On average, the exhaust gas temperature was found to be 1.74% lesser, in case of neat diesel and 0.87, 0.78, and 1.71% lesser, in case of oleate, stearate, and palmitate, respectively, on comparing with biodiesel blend. Increase in EGT for biodiesel blend counter to diesel was accounted by its high cetane number, which also reduced the pre-mixing time (Abu-Hamdeh and Alnefaie, 2015). Besides, the marginal difference in the EGT for biodiesel blend and oleate and stearate blends were because of high oxidation rate of long chain ester molecules and abundant availability of fuel bound oxygen in it (Hellier et al., 2018) in addition to their high cetane number. The similarity in EGT for biodiesel and oleate blend was because of its unsaturation content which increased its ignition delay and viscosity, thereby producing higher NOX emission upon long combustion duration due to high viscosity. Figure 4 illustrates the exhaust gas temperature of diesel and blend samples for varying load conditions.
Carbon monoxide is a resultant by-product produced during incomplete combustion of fuel and is greatly influenced by nature of fuel and its degree of unsaturation and C/H ratio and engine cylinder temperature. On average, the CO emission was found to be 38.89% lesser, in case of neat diesel and 6.15, 20.04, and 28.54% lesser, in case of oleate, stearate, and palmitate, respectively, as compared with biodiesel blend. The reduction in the level of CO emission for biodiesel and ester samples was because of molecular oxygen available in the structure of ester molecules, which favors the complete oxidation of carbon and hydrogen molecules in the fuel (Hellier et al., 2018); however, their higher CO emission came by a reason of their high viscosity which reduced their atomization and hindered complete combustion. Also, the increased CO emission for biodiesel blend and oleate blend was due to presence of unsaturated double bond (C = C bond) in their carbon chain (Chukwuezie et al., 2017). Above all, higher CO emission at full load condition for all test samples was on account of higher fuel consumption rate to satisfy the higher energy demand. Hence, it can be concluded that concentration of CO emission will be higher for biodiesel with higher concentration of ester molecules having both long carbon chain and unsaturation. Figure 5 shows the CO emission concentration of diesel and blend samples for varying load conditions.
Carbon dioxide is regarded as principal product obtained on complete combustion of fuel and is also deeply affected by type of fuel used and its degree of unsaturation and C/H ratio and engine cylinder temperature. On average, the CO2 emission was 17.83% lesser, in case of neat diesel and 5.36, 3, and 12.34% lesser, in case of oleate, stearate, and palmitate, respectively, than biodiesel blend. Here, the closer margin between CO2 emission concentration for diesel sample and palmitate blend is explained by their reduced carbon chain length and lower C/H ratio which tends to produce less CO2 emission than compared to other blends (Adler and Bandhauer, 2017). Highest CO2 emission concentration was noted for biodiesel blend owing to its adequate oxygen content and increased rate of viscosity that endows sufficient time to complete the combustion during expansion stroke (Abdul Malik et al., 2017). Moreover, increase in CO2 emission concentration for oleate and stearate blend was because of their long carbon chain length corresponding to the fatty acids in their structure. On the other hand, drastic reduction in CO emission was noticed for increasing concentration of CO2 emission. It can be concluded that biodiesel having long chained fatty acid esters tends to produce higher concentration of CO2 emission. Figure 6 depicts the CO2 emission concentration of diesel and blend samples for varying load conditions.
In general, NOX emission is a temperature dependent phenomenon governed by the peak combustion temperature and is directly influenced by fuel with high cetane number and fuel bound oxygen content apart from longer residence time. Besides, higher NOX emission rate for biodiesel fuel at higher temperature is because of the oxidation of hydrocarbons at fuel rich zones by means of fuel bound oxygen present in it (Prabu, 2018). Further, NOX emission increased with availability of biodiesel owing to their high viscosity and oxygen content along with increased cetane number. In contrast, the NOX emission of neat diesel was found to be 7.76% greater than biodiesel blend; and because of its increased ignition delay and reduced air-fuel mixing time that resulted in high cylinder pressure and combustion temperature on account of rapid combustion of fuel that were accumulated during premixed combustion phase. In the same way, the NOX emissions of oleate and stearate blend were found to be 2.4 and 0.8% greater than biodiesel blend due to their reduced cetane number that tends to produce better combustion heat release rate. On the contrary, reduction in NOX emission for palmitate blend was because of reduced Cumulative Heat Release Rate (CHRR) during combustion owing to its reduced carbon chain length which increased its ignition delay slightly (Coniglio et al., 2013) in addition to its high cetane number. Despite the fact, the NOX emission of biodiesel blend remained below that of diesel because of its reduced degree of unsaturation and also slightly higher viscosity rate which hinders perfect atomization. Figure 7 pictures the NOX emission concentration of diesel and blend samples for varying load conditions.
Generally, the oxygen in exhaust gas describes about the completeness of the combustion reaction and explains the amount of oxygen consumed for oxidation of the fuel. On average, the exhaust Oxygen concentration was found to be 14.11% greater, in case of neat diesel and 2.6, 4, and 13.66% greater, in case of oleate, stearate, and palmitate, respectively, comparative to biodiesel blend. Reduced O2 emission for biodiesel blend was due to presence of long carbon chained ester molecules (oleate, stearate, and palmitate) which requires large amount of oxygen for complete combustion and extract maximum work from it (Hellier et al., 2012). In contrast, palmitate blend exhibited higher concentration of O2 by reason of reduced carbon chain length which consumed less amount of oxygen than compared to other ester samples. To emphasize, the oxygen concentration reduced drastically with increase in load for all samples owing to higher consumption of oxygen for combusting the large amount of fuel to meet the power demand. In conclusion, biodiesel with long chained ester molecules consumed more oxygen content which improves the overall rate of combustion and also reduces the concentration of CO emissions. Figure 8 represents the exhaust oxygen concentration of diesel and blend samples for varying load conditions.
Hydrocarbon emission arises due to the unavailability of sufficient temperature for the air-fuel mixture found closer to the cylinder wall to undergo complete combustion thereby leading to unburnt fuel in exhaust gas (Correa and Arbilla, 2008). On average, the hydrocarbon emission was found to be 43.69% lesser, in case of neat diesel and 9.28, 26.6, and 29.27% lesser, in case of oleate, stearate, and palmitate, respectively, when compared to biodiesel blend. Above all, the lower concentration of HC emission for diesel blend was because of its high calorific value and reduced viscosity rate, which upon combustion provided enough temperature for fuel to get combusted at wall surface. Besides, reduced HC emission for stearate, and palmitate blend was because of its higher oxygen content in its molecular structure; however, slight variation in the HC emission was result of difference in carbon chain length (Benjumea et al., 2010). Conversely, for oleate blend, HC emission was found to be higher than compared to other two ester blends owing to its unsaturation Inspite of its higher carbon chain length and good fuel bound oxygen content; and was reflected in the HC emission of biodiesel blend. Very likely, the HC emission inside cylinder is always greater than in exhaust tail pipe as there is a possibility for these unburnt fuels to undergo combustion, if sufficient oxygen and high temperature is available (Faiz et al., 1996). Figure 9 outlines the hydrocarbon emission of diesel and blend samples for varying load conditions.
From these results, it was clearly evident that the emission characteristics of waste animal fat biodiesel were deeply influenced by the dominant fatty acid esters present in it. Properties like density, viscosity, cetane number, and calorific value played a substantial role in deciding the emission concentrations which are in turn depends on molecular weight (length of carbon chain) of the ester molecule along with its unsaturation content. Hence, this study provides sufficient evidence that biodiesel emissions and their concentration are dependent on the fatty acid ester molecules and their availability in it. Table 6 summarizes the key conclusion deduced from this present study along with other similar studies.
Thus, the effect of fatty acid esters of waste animal fat biodiesel in deciding its emission characteristics have been carried by comparing their emission concentration of waste animal fat biodiesel, diesel along with fatty acid esters. Following this, major conclusions deduced from this experimental work related to comparative study in emission characteristics are as follows:
(i) Ethyl oleate (40.21%), ethyl palmitate (25.36%), and ethyl stearate (16.87%) were characterized as dominant fatty acid esters present in waste animal fat biodiesel.
(ii) High exhaust gas temperature was caused by ethyl oleate due to its low cetane number and high viscosity leading to prolonged ignition delay.
(iii) High CO and CO2 emission concentration was on account of increased carbon chain length (stearate and Oleate esters); however, unsaturation in fatty acid moiety (Oleate ester) had an adverse effect in CO emission.
(iv) Reduced NOx emission was because of high cetane number from saturated fatty acid esters (stearate and Palmitate esters), which in turn reduced its ignition delay thereby moderating the HRR.
(v) Likewise, long carbon chain fatty acid esters (stearate and Oleate esters) consumed more oxygen content for undergoing complete combustion whereas increased HC emission was owing to its increased unsaturation content (Oleate ester).
On the whole, the emission characteristics of any biodiesel are intensely based on carbon chain length (C/H ratio), cetane number, degree of unsaturation and density and viscosity of individual fatty acid esters constituting it. In conclusion, this experimental work serves as a precursor for understanding the influence of fatty acid esters on emission characteristics during combustion of biodiesel, which could help in developing modified biofuel aimed for reduced emissions to develop sustainably toward future.
The datasets generated for this study are available on request to the corresponding author.
GS: proposal of idea, literature survey, sample and chemical collection, fat extraction and biodiesel production, engine testing data collection and calculation, drafting of article, final approval. SP: literature survey, sample preparation for spectral analysis, GC testing data collection and interpretation, chemical equation calculations, drafting of article, and verification of typo errors. RJ: project supervision, evaluation of spectral data and chemical equations, critical review of finalized article, final approval, administrative, and technical support. VS: project supervision, fat extraction and handling, biodiesel preparation, Evaluation of spectral data and chemical equations, review of finalized article, final approval, administrative, and technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors wish to thanks VIT University, Vellore, India; St. Peter's University, Chennai, India, and Bharath Institute of Higher Education and Research, Chennai, India for providing their lab facilities for carrying out the experimental works. Authors also wish to express their special thanks to Sri Venkateswara Engineering Consultancy Services, Kanchipuram, India for extending their help in carrying out engine analysis in their testing facility.
BSFC, Brake Specific Fuel Consumption; BTE, Brake Thermal Efficiency; C/H, Carbon-Hydrogen Ratio; CI, Compression Ignition; CO, Carbon monoxide; CO2, Carbon dioxide; CRDI, Common Rail Direct Injection; EGT, Exhaust Gas Temperature; HC, Hydrocarbon; ITE, Indicated Thermal Efficiency; Nair, Air Quantity (in mols); NOx, Nitrogen Oxide; PM, Particulate Matter; SFC, Specific Fuel Consumption; Voverall, Overall volume of sample (in ml); ϕE, Ester Factor; ψB, Blend Factor.
Abdul Malik, M., Shaiful, A., Mohd Jaafar, M., and Mohamad Sahar, A. (2017). Combustion and emission characteristics of coconut-based biodiesel in a liquid fuel burner. Energies 10:458. doi: 10.3390/en10040458
Aboelazayem, O., El-Gendy, N. S., Abdel-Rehim, A. A., Ashour, F., and Sadek, M. A. (2018). Biodiesel production from castor oil in Egypt: process optimisation, kinetic study, diesel engine performance and exhaust emissions analysis. Energy 157, 843–852. doi: 10.1016/j.energy.2018.05.202
Abu-Hamdeh, N. H., and Alnefaie, K. A. (2015). A comparative study of almond biodiesel-diesel blends for diesel engine in terms of performance and emissions. BioMed. Res. Int. 2015:529808. doi: 10.1155/2015/529808
Adler, J., and Bandhauer, T. (2017). Performance of a diesel engine at high coolant temperatures. J. Energ. Resour. ASME. 139:062203. doi: 10.1115/1.4036771
Benjumea, P., Agudelo, J. R., and Agudelo, A. F. (2010). Effect of the degree of unsaturation of biodiesel fuels on engine performance, combustion characteristics, and emissions. Energ. Fuel. 25, 77–85. doi: 10.1021/ef101096x
Carraretto, C., Macor, A., Mirandola, A., Stoppato, A., and Tonon, S. (2004). Biodiesel as alternative fuel: experimental analysis and energetic evaluations. Energy. 29, 2195–2211. doi: 10.1016/j.energy.2004.03.042
Chukwuezie, O. C., Nwakuba, N. R., Asoegwu, S. N., and Nwaigwe, K. N. (2017). Cetane number effect on engine performance and gas emission: a review. Am. J. Eng. Res. 6, 56–67. Available online at: http://www.ajer.org/papers/v6(01)/I06015667.pdf
Coniglio, L., Bennadji, H., Glaude, P. A., Herbinet, O., and Billaud, F. (2013). Combustion chemical kinetics of biodiesel and related compounds (methyl and ethyl esters): experiments and modeling–advances and future refinements. Pr. Energ. Combust. Sci. 39, 340–382. doi: 10.1016/j.pecs.2013.03.002
Correa, S. M., and Arbilla, G. (2008). Carbonyl emissions in diesel and biodiesel exhaust. Atmos Environ. 42, 769–775. doi: 10.1016/j.atmosenv.2007.09.073
Debbarma, S., and Misra, R. D. (2017). Effects of iron nanoparticles blended biodiesel on the performance and emission characteristics of a diesel engine. J. Energ. Resour. ASME. 139:042212. doi: 10.1115/1.4036543
Emiroğlu, A. O., Keskin, A., and Şen, M. (2018). Experimental investigation of the effects of turkey rendering fat biodiesel on combustion, performance and exhaust emissions of a diesel engine. Fuel 216, 266–273. doi: 10.1016/j.fuel.2017.12.026
Faiz, A., Weaver, C. S., and Walsh, M. P. (1996). Air Pollution From Motor Vehicles: Standards and Technologies for Controlling Emissions. Washington, DC: The World Bank.
Gopinath, A., Puhan, S., and Nagarajan, G. (2010). Effect of unsaturated fatty acid esters of biodiesel fuels on combustion, performance and emission characteristics of a DI diesel engine. Int. J. Energ. Environ. 1, 411–430. doi: 10.1016/j.serj.2017.06.004
Hasan, M. M., and Rahman, M. M. (2017). Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: a review. Renew. Sustain. Energy Rev. 74, 938–948. doi: 10.1016/j.rser.2017.03.045
Hazrat, M. A., Rasul, M. G., Khan, M. M. K., Ashwath, N., and Rufford, T. E. (2019). Emission characteristics of waste tallow and waste cooking oil based ternary biodiesel fuels. Enrgy Proced. 160, 842–847. doi: 10.1016/j.egypro.2019.02.149
Hellier, P., Ladommatos, N., Allan, R., and Rogerson, J. (2012). The influence of fatty acid ester alcohol moiety molecular structure on diesel combustion and emissions. Energ Fuel. 26, 1912–1927. doi: 10.1021/ef2017545
Hellier, P., Talibi, M., Eveleigh, A., and Ladommatos, N. (2018). An overview of the effects of fuel molecular structure on the combustion and emissions characteristics of compression ignition engines. P. Inst. Mech. Eng. Part. D. 232, 90–105. doi: 10.1177/0954407016687453
Hosseini, S. E., and Wahid, M. A. (2012). Necessity of biodiesel utilization as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 16, 5732–5740. doi: 10.1016/j.rser.2012.05.025
Jambulingam, R., and Srinivasan, G. R. (2019). “Theoretical prediction of thermophysical properties of waste beef tallow biodiesel,” in ICRDME 2K19 Conference Proceedings (Chennai).
Jayaprabakar, J., Dawn, S. S., Ranjan, A., Priyadharsini, P., George, R. J., Sadaf, S., et al. (2019). Process optimization for biodiesel production from sheep skin and its performance, emission and combustion characterization in CI engine. Energy 174, 54–68. doi: 10.1016/j.energy.2019.02.140
Kirubakaran, M., and Selvan, V. A. M. (2018). A comprehensive review of low cost biodiesel production from waste chicken fat. Renew. Sus. Ener. Rev. 82, 390–401. doi: 10.1016/j.rser.2017.09.039
Monyem, A., and Van Gerpen, J. H. (2001). The effect of biodiesel oxidation on engine performance and emissions. Biomass Bioenerg. 20, 317–325. doi: 10.1016/S0961-9534(00)00095-7
Nabi, M. N., Rasul, M. G., Anwar, M., and Mullins, B. J. (2019). Energy, exergy, performance, emission and combustion characteristics of diesel engine using new series of non-edible biodiesels. Renew. Energy 140, 647–657. doi: 10.1016/j.renene.2019.03.066
Öner, C., and Altun, Ş. (2009). Biodiesel production from inedible animal tallow and an experimental investigation of its use as alternative fuel in a direct injection diesel engine. Appl. Energ. 86, 2114–2120. doi: 10.1016/j.apenergy.2009.01.005
Palani, S., Srinivasan, G. R., and Ranjitha, J. (2017). Biodiesel production from the seeds of Mimusops elengi using potassium aluminium silicate as novel catalyst. Inno. Ener. Res. 6:165. doi: 10.4172/2576-1463.1000165
Prabu, A. (2018). Engine characteristic studies by application of antioxidants and nanoparticles as additives in biodiesel diesel blends. J. Energ. Resour. ASME. 140:082203. doi: 10.1115/1.4039736
Ray, S. K., and Prakash, O. (2019). Biodiesel Extracted from Waste Vegetable Oil as an Alternative Fuel for Diesel Engine: Performance Evaluation of Kirlosker 5 kW Engine. Singapore: Springer.
Schönborn, A., Ladommatos, N., Allan, R., Williams, J., and Rogerson, J. (2008). Effect of the molecular structure of individual fatty acid alcohol esters (biodiesel) on the formation of NOx and particulate matter in the diesel combustion process. SAE. Int. J. Fuel Lubr. 1, 849–872. doi: 10.4271/2008-01-1578
Shahir, V. K., Jawahar, C. P., Suresh, P. R., and Vinod, V. (2017). Experimental investigation on performance and emission characteristics of a common rail direct injection engine using animal fat biodiesel blends. Enrgy Proced. 117, 283–290. doi: 10.1016/j.egypro.2017.05.133
Srinivasan, G. R., and Jambulingam, R. (2018). Comprehensive study on biodiesel produced from waste animal fats–a review. J. Environ. Sci. Tech. 11, 157–166. doi: 10.3923/jest.2018.157.166
Srinivasan, G. R., Palani, S., and Jambulingam, R. (2018a). Biodiesel production from waste animal fat using a novel catalyst HCA immobilized AuNPS amine grafted SBA-15. J. Eng. Sci. Tech. 13, 2632–2643. Available online at: http://jestec.taylors.edu.my/Vol%2013%20issue%208%20August%202018/13_8_25.pdf
Srinivasan, G. R., Palani, S., and Jambulingam, R. (2018b). Optimised production of biodiesel synthesised from waste animal fat. J. Biofuels. 9, 17–24. doi: 10.5958/0976-4763.2018.00003.X
Srinivasan, G. R., Shankar, V., and Jambulingam, R. (2019). Experimental study on influence of dominant fatty acid esters in engine characteristics of waste beef tallow biodiesel. Energ. Explor. Exploit. 37, 1098–1124. doi: 10.1177/0144598718821791
Srinivasan, G. R., Srinivasan, S. R., Venkatachalapathy, D., and Venkatachalapathy, N. (2017). Production, performance, combustion, emission characteristics of biodiesel synthesized from mutton suet. Int. J. Eng. Technol. 9, 3512–3518. doi: 10.21817/ijet/2017/v9i5/170905038
Subramanian, K. A., Singal, S. K., Saxena, M., and Singhal, S. (2005). Utilization of liquid biofuels in automotive diesel engines: an Indian perspective. Biomass Bioenergy 29, 65–72. doi: 10.1016/j.biombioe.2005.02.001
Keywords: animal fat biodiesel, fatty acid esters, cetane number, unsaturated double bonds, emission characteristics
Citation: Jambulingam R, Shankar V, Palani S and Srinivasan GR (2019) Effect of Dominant Fatty Acid Esters on Emission Characteristics of Waste Animal Fat Biodiesel in CI Engine. Front. Energy Res. 7:63. doi: 10.3389/fenrg.2019.00063
Received: 06 May 2019; Accepted: 24 June 2019;
Published: 23 July 2019.
Edited by:
Su Shiung Lam, Universiti Malaysia Terengganu, MalaysiaReviewed by:
C. K. Cheng, Universiti Malaysia Pahang, MalaysiaCopyright © 2019 Jambulingam, Shankar, Palani and Srinivasan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gokul Raghavendra Srinivasan, Z29rdXNyaW5pdmFzYW5AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.