- 1Department of Molecular Biology and Biotechnology, Tezpur University, Tezpur, India
- 2Department of Chemical Sciences, Tezpur University, Tezpur, India
- 3Biochemical Conversion Division, Sardar Swaran Singh National Institute of Bio-Energy, Kapurthala, India
- 4Department of Energy, Tezpur University, Tezpur, India
Lignocellulosic biomass (LCB) is the most abundantly available bioresource amounting to about a global yield of up to 1. 3 billion tons per year. The hydrolysis of LCB results in the release of various reducing sugars which are highly valued in the production of biofuels such as bioethanol and biogas, various organic acids, phenols, and aldehydes. The majority of LCB is composed of biological polymers such as cellulose, hemicellulose, and lignin, which are strongly associated with each other by covalent and hydrogen bonds thus forming a highly recalcitrant structure. The presence of lignin renders the bio-polymeric structure highly resistant to solubilization thereby inhibiting the hydrolysis of cellulose and hemicellulose which presents a significant challenge for the isolation of the respective bio-polymeric components. This has led to extensive research in the development of various pretreatment techniques utilizing various physical, chemical, physicochemical, and biological approaches which are specifically tailored toward the source biomaterial and its application. The objective of this review is to discuss the various pretreatment strategies currently in use and provide an overview of their utilization for the isolation of high-value bio-polymeric components. The article further discusses the advantages and disadvantages of the various pretreatment methodologies as well as addresses the role of various key factors that are likely to have a significant impact on the pretreatment and digestibility of LCB.
Introduction
The population of the world is projected to reach 8.5 billion by 2030 and 9.7 billion in 2050. Commensurate with the increasing population, the global energy consumption is expected to rise from 575 British thermal units (BTU), as estimated in 2015, to about 736 quadrillion BTU in 2040, which is a 28% increase over a period of 25 years (EIA, 2017). The global dependence on non-renewable fossil fuels for meeting the current energy needs, cannot be sustained for long in the face of the depleting fuel reserves. The effects of this excessive dependence are already evident in the escalation of fuel prices, over the past decade and the severe environmental impacts like climate change. With a mere 23.7% utilization of renewable energy sources for energy needs, it is vital that world switches over to renewable and sustainable energy alternatives, on an urgent basis (Hussain et al., 2017; Oliva et al., 2017). Amidst the existing alternative sources for energy, bioenergy derived from the renewable and sustainable resources, LCB for the production of biofuels like biodiesel/ biogasoline, high value chemicals such as ethanol fuels, acids, saccharides, phenols, aldehydes, xylitol, and cellulose acetate with low carbon emissions are the most promising (Behera et al., 2014; Chen et al., 2017). LCB involves organic materials including agricultural crops and agrowastes, algae, grass, wood all of which are abundant and renewable resources.
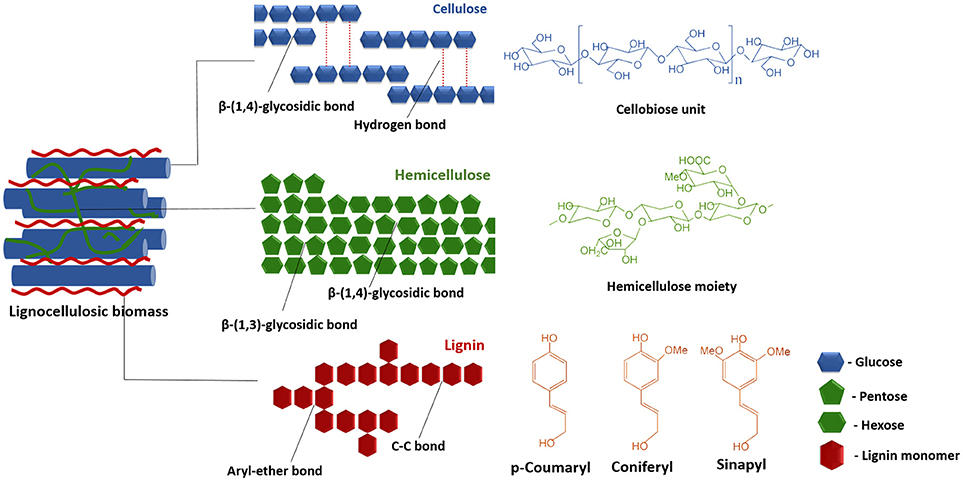
In the past two decades, LCB such as wheat straw, rice straw, sugarcane bagasse, barley, and timothy grass, woody raw materials, forest residues, softwoods and paper pulps have been extensively researched for biofuel production (Loow et al., 2015; Akhtar et al., 2016). Lignocelluloses are composed of cellulose (40–50%), hemicelluloses (25–35%) and lignin (15–20%) in an intricate structure where the components are rigidly associated through non-covalent bonds and covalent cross-linkages (Figure 1) (Sun S. et al., 2016). Crystallized cellulose and hemicellulose polymer matrix are encrusted by the highly polymerized phenolic lignin that leads to the difficulties in the conversion processes. The complex, hierarchical and recalcitrant nature of the LCB is the primary bottleneck in the utilizing of these resources for bioenergy production (Akhtar et al., 2016). This problem has so far been addressed by increasing the digestibility and availability of cellulosic and hemicellulosic fractions, together with the removal of the lignin fractions, through a series of targeted pretreatments steps (Kumari and Singh, 2018). The pretreatment techniques currently in use, may be broadly classified as physical, chemical, physicochemical, and biological processes. It is worthwhile to mention that the pretreatment phase contributes to a minimum of 20% of the total cost of conversion for different products and is one area where the energy inputs can be significantly lowered (Mafe et al., 2015; Seidl and Goulart, 2016). This urges further study on the understanding of the different techniques and is imperative for the improvement upon the pretreatment phase so as to make the process of conversion of biomass to biofuels and value-added chemicals economical and adaptable to sustainable biorefinery based approaches. This paper reviews the currently pretreatment techniques for production of biofuels and value-added chemicals, highlights their pros and cons, their recent advancements and effect on different LCBs.
Structure of LCB and Its Components
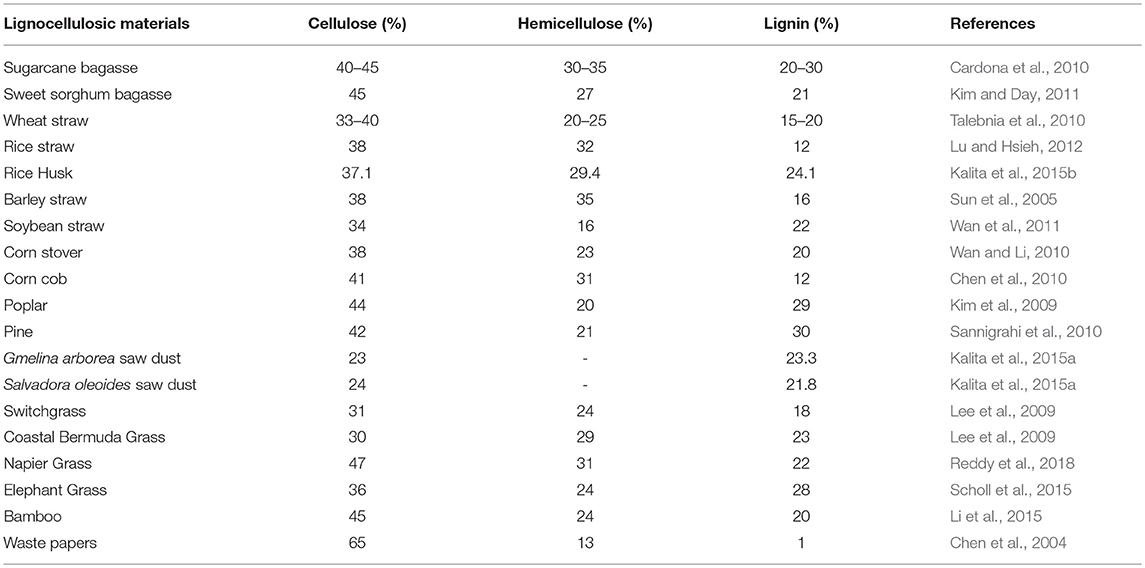
LCB is mainly composed of three polymers: cellulose (C6H10O5)n, hemicellulose (C5H8O4)m, and lignin [C9H10O3(OCH3)0.9−1.7]x along with minor amounts of other compounds such as proteins, ash, and pectin (Akhtar et al., 2016). In general, the cellulose, hemicellulose and lignin contents in a typical LCB fall within the range of 30–60, 20–40, and 15–25%, respectively (Dahadha et al., 2017). However, the composition of these major components varies depending on the source as depicted in Table 1. Cellulose is a main structural and integral part of LCB which is a linear polysaccharide and consists of D-glucose subunits linked by β-(1,4)-glycosidic bonds (Kumar et al., 2017). This polymer is insoluble in water unless at extremely low or high pH levels. However, it is soluble in solvents like ionic liquids (ILs) and N-methylmorpholine-N-oxide (NMMO) (Liu et al., 2016). Cellulose possesses the advantageous properties such as biocompatibility, stereoregularity, hydrophilicity, and reactive hydroxyl groups and serves as a versatile resource for derivatized materials such as fibers, films, composites as well as fuels and chemicals (Jedvert and Heinze, 2017). Hemicellulose is a second major component of LCB that consists of short chains of different polysaccharides such as xylan, galactomannan, glucuronoxylan, arabinoxylan, glucomannan, and xyloglucan that are held together by β-(1,4)- and/or β-(1,3)-glycosidic bonds (Zhou S. et al., 2017). In contrast to cellulose, hemicellulose is readily degradable into monosaccharides due to low degree of polymerization and non-crystalline nature and thereby widely used in industrial applications such as drug carriers, hydrogels, and cosmetics (Farhat et al., 2017). Lignin forms a protecting boundary by covalently linking to the cellulose and hemicellulose which enhances the recalcitrance of the lignocellulose. It is a complex, three-dimensional cross-linked polymer that consists of phenyl propane structural units and vary depending on the substitute of the methoxyl groups present in the aromatic rings and are linked to each other by aryl ether linkages e.g., β-O-4, α-O-4 and carbon-carbon bonds e.g., 5–5, β-β. Three basic units that constitute the lignin polymer are p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) (Xu and Ferdosian, 2017).
Pretreatment of LCB: Necessity
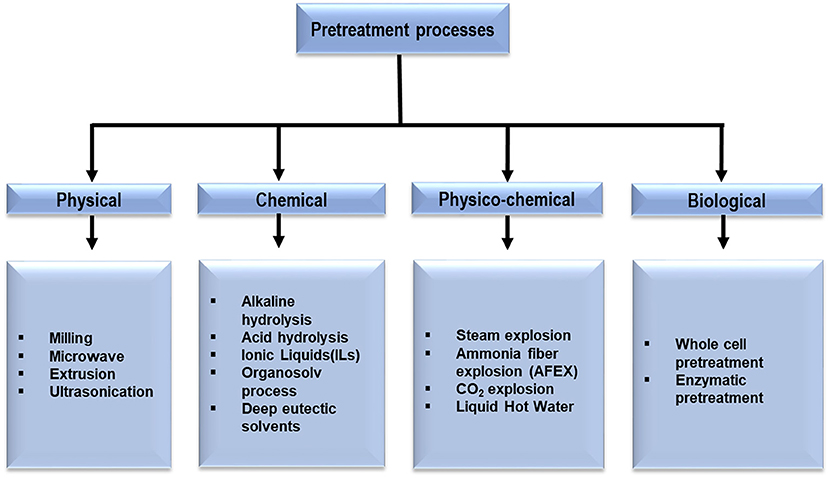
LCBs are resistant to chemical and biological breakdown, known as biomass recalcitrance. Several factors such as the crystalline structure of cellulose, the degree of lignification and the structural heterogeneity and complexity of cell-wall constituents are responsible for the biomass recalcitrance that must be overcome for valuable utilization of lignocellulosic feedstocks (Guerriero et al., 2016). In this context, the pretreatment is a significant step in the biorefinery process. Throughout the pretreatment process, the recalcitrant structure of lignocellulose is disrupted resulting in breakage of lignin sheath, degradation of hemicellulose and reduction in crystallinity and degree of polymerization of cellulose (Loow et al., 2015; Chen et al., 2017). A multitude of pretreatment techniques have been developed in the last few decades to improve the deconstruction of LCBs and can be categorized as shown in Figure 2. Among the several pretreatment processes the preference of the convenient one depends on the type of LCBs used as the composition of cellulose, hemicellulose and lignin vary (Dahadha et al., 2017). In the following sections, the major pretreatment techniques and their effects on the separation of the complex components of various lignocellulosic sources are discussed.
Physical Pretreatment
Physical pretreatment of LCB is a prerequisite prior to any other pretreatment methods. It is primarily carried out to reduce the particle size that results in the increase in surface area, and decrease in degree of polymerization and crystallinity (Rajendran et al., 2017). Consequently, the subsequent processes become more effective and easier (Chen et al., 2017). These methods are eco-friendly and seldom produce any toxic material (Shirkavand et al., 2016). However, one major disadvantage of physical pretreatment is its high energy consumption. Generally, energy consumption depends on the type of LCB used. It was reported that size reduction of softwoods such as corn stover and switchgrass requires 11.0 and 27.6 kWh/metric ton, respectively, while hardwoods such as pine and poplar chips require 85.4 and 118.5 kWh/metric ton, respectively (Rajendran et al., 2017). The commonly prevalent physical pretreatment methods include milling, extrusion, microwave treatment, and ultrasonication. These approaches are described in detail in the following sections.
Milling
Milling is employed to reduce the crystallinity and particle size of LCB. Milling can reduce the particle size upto 0.2 mm. However, Chang et al. (1997) unveiled that biomass particle of size < 0.4 mm has no remarkable effect on the rate and yield of hydrolysis. Depending upon the type of motorized equipment operated the different milling methods are two-roll milling, ball milling, rod milling, hammer milling, vibratory milling, colloid milling, and wet disk milling. The reduction in particle size and crystallinity is determined by the type of milling method adopted, processing time and also the type of biomass used (Kumar and Sharma, 2017). Bai et al. (2018) studied the efficiency of pyrolysis using rod-milling and hammer-milling pretreatment in wheat straw wherein they have found significant size reduction and a decrease in crystallinity using rod-milling at an optimum duration of 60 min. The effective size reduction and the decrease in crystallinity lead to high surface contact and pore volume of wheat straw. Also, the kinetic analysis showed that wheat straw pretreated through rod milling has lower thermal degradation temperature as compared to the hammer-milled wheat straw that enhanced the pyrolysis efficiency. However, one of the biggest shortcomings of milling pretreatment is its high-energy requirement and the capital cost of mechanical equipment. Gu et al. (2018) studied the effect of planetary ball milling on pre-milled wood fiber and found an improved energy consumption efficiency of 0.50–2.15 kWh/kg for 7–30 min of milling at 270 rpm. The process produced high glucose and xylose in the range of 24.45–59.67% and 11.92–23.82%, respectively through enzymatic hydrolysis. Also, mild acid hydrolysis of pretreated cellulose paper and cellulose powder using ball milling resulted in a high yield of nanocellulose with high crystallinity and high thermal stability (Phanthong et al., 2016). Milling pretreatment of raw LCB for solid-state anaerobic digestion process showed that reduction in particle size greatly increases the substrate solubility thereby increasing the reaction kinetics (Motte et al., 2014).
Wet disk milling has been a well-known physical pretreatment owing to its low energy consumption. A study of combined pretreatment using hydrothermal and wet disk milling on oil palm mesocarp fiber (OPMF) demonstrated that hydrothermal pretreatment of OPMF followed by wet disk milling reduces the power consumption upto 9.6 MJ/kg with more than 98% glucose yield (Zakaria et al., 2015b). Another combined pretreatment of hot compressed water (HCW) and wet disk milling on oil palm biomass produced 85.5 and 100% of total sugar yields from oil palm empty fruit branch and oil palm frond fiber, respectively (Zakaria et al., 2015a). Kim et al. (2013) compared three different methods of milling; ball, attrition and planetary milling. It was found that attrition and planetary milling effectively reduced biomass particle size as compared to ball milling and the highest yield of glucose and galactose was obtained by planetary milling. Since milling pretreatment does not result in any toxic or inhibitory compounds it is a preferred preliminary pretreatment method for a wide variety of lignocellulosic feedstocks.
Microwave Assisted Size Reduction
Microwave irradiation is a non-conventional heating method that has long been used for the pretreatment of LCB under an applied electromagnetic field. The first study of microwave irradiation pretreatment was carried out by Ooshima et al. (1984) and since then this method has been retained a convenient one owing to its several advantages including easy operation, energy efficient, minimum inhibitors formation and high heating capacity in short period of time (Tayyab et al., 2018). In this method, the dielectric polarization causes molecular collisions and generates thermal energy that results in the disruption of the complex lignocellulosic structure (Aguilar-Reynosa et al., 2017). Microwave irradiation was categorized into atmospheric and high-pressure treatment. High-pressure microwave pretreatments are operated in closed reactors within the temperature range from 150 to 250°C (Li H. et al., 2016). A study on the microwave pretreatment of Panicum spp. and Miscanthus spp. showed 7–10% higher solubility of the materials in subcritical water as compared to the untreated materials. The samples were pretreated at different temperatures and the optimum conditions obtained are 60°C and 120°C for Miscanthus spp. and Panicum spp., respectively at 1,600 W treatment power (Irmak et al., 2018). In another study, the microwave pretreatment of Hyacinthus spp. was examined to enhance the methane production from anaerobic digestion and obtained the highest methane yield of 221 mL·g-sub−1 which was 38.3% higher than the substrate pretreated with water-heating (Zhao et al., 2017). In recent researches, microwave pretreatment is often added up with other treatment methods as an upgraded attempt. Liu et al. (2018) studied the effect of microwave irradiation during alkaline treatment for the separation of cellulose and hemicellulose from a delignified hardwood kraft pulp. They have found that under microwave treatment the complex fiber structure fractured effectively and the alkaline solution penetrated into the inner fiber structure that resulted in the significant removal of hemicelluloses and high cellulose yield of 93.05%. Microwave-assisted ionic liquid treatment of Crotalaria juncea fibers produced 78.7% glucose at 160°C in only 46 min processing time (Paul and Dutta, 2018). The studies of the microwave-assisted acid pretreatment of lignin and moso bamboo sawdust have been reported that microwave temperature is the most significant factor in determining the structure of the pretreated samples. The microwave temperature facilitated decarboxylation and dehydration during the process. Different analysis showed that the pretreated samples have lower O, H, and ash contents and higher C contents, while in raw bamboo sawdust the C, H, and O are predominant (Wang et al., 2017; Duan et al., 2018). Likewise, microwave-assisted acid hydrolysis of jabon kraft pulp produced 49.2% reducing sugars with 0% lignin content at 190°C. The lignin removal promoted the production of high sugar yield (Fatriasari et al., 2018). Moodley and Kana, 2017 investigated the effects of microwave-assisted inorganic salt pretreatment on sugarcane leaf wastes to enhance enzymatic saccharification. When pretreated with 2 M FeCl3 at 700 W and 3.5 min irradiation time, the maximum reducing sugar yield obtained was 0.406 g/g. Similarly, Germec et al. (2017) have studied extensively the microwave-assisted dilute acid pretreatment of different agricultural bioresources viz. barley husk, oat husk, wheat bran, and rye bran. The results established the microwave system as an assuring technique for fermentable sugar production from lignocellulosic materials.
Extrusion
Extrusion is one of the most commonly used physical pretreatment method applied to LCBs. The action of one or two screws that spin into a tight barrel, which is furnished with temperature control forms the basis of this method (Duque et al., 2017). The raw materials are passed through the barrel under high temperature (>300°C) where the recalcitrant structure of the lignocellulose disrupts due to the combined effects of high temperature and the shear forces caused by the rotating screw blades in the barrel (Kumar and Sharma, 2017). Extrusion machines are mainly classified into single-screw extruders (made of one single solid piece) and twin-screw extruders (made of small pieces called screw elements arranged cylindrically). The screw configuration is an important factor affecting the decomposition of LCB. Wahid et al. (2015) carried a study on the effect of different screw configurations during extrusion pretreatment on wheat straw and deep litter to enhance biogas production. They analyzed pretreatment using five screw configurations namely, mild kneading, long kneading, reverse, kneading, and reverse and kneading with reverse. The results showed effective sugar yield with each configurations that consequently led to significant methane production. However, high energy consumption (226–324 kWh t−1) is the main bottleneck in this study that requires further investigation to make the pretreatment economical.
Various parameters such as screw design, screw speed and barrel temperature also control the extrusion pretreatment (Duque et al., 2017). Moro et al. (2017) studied the pretreatment of sugarcane bagasse and straw using twin-screw extruder and optimized the variables viz. type of additives, biomass:additive ratio, number of extrusion passes, barrel temperature, screw speed, and screw configurations. The pretreatment was carried out using different additives such as water, glycerol, ethylene glycol and Tween® 80 with different loading amounts. Experiments showed glycerol as the suitable additive when pretreatment was conducted using a bagasse:glycerol and straw:glycerol ratios of 1:0.75 and 1:0.5 ratios, respectively. Also, the effect of extrusion was controlled by introducing two new screw configurations in the extruder and found glucose yield of 68.2% on enzymatic hydrolysis. This report proves extrusion pretreatment alone as an efficient technique over extrusion assisted alkaline pretreatment of olive tree pruning that yields 69% glucose (Negro et al., 2015). Heredia-Olea et al. (2015) investigated the different process parameters of extrusion pretreatment on sweet sorghum bagasse (SSB) and its successive enzymatic hydrolysis and bioethanol production. They operated the process at different conditions using surface response methodology and found 100°C barrel temperature, 200 rpm screw speed and 30% feedstock moisture content to be the optimal extrusion conditions and generated 70% of the total sugars on enzymatic hydrolysis. This study explains the effect of interaction between temperature and moisture content and its effect on extruder shear stress which consequently enhanced the disintegration of cellulose fibers and its porosity and contact surface to enzymes. Furthermore, subsequent fermentation of the extruded hydrolyzed SSB yielded ethanol of about 200 ml.kg−1 of bagasse.
Ultrasonication
Ultrasonication pretreatment is based on the principle of cavitation through the employment of ultrasonic radiation. The cavitation generates shear forces that cleaves the complex network structure of LCB and promotes the extraction of desired compounds such as, cellulose, hemicellulose, and/or lignin (Ravindran and Jaiswal, 2016). A study on the effects of ultrasound pretreatment on the structural changes of eucalyptus wood reveales that the crystallinity of the pretreated wood increased from 34.7 to 35.3% in aqueous soda solution, 32.6–35.5% in distilled water and 33.4–35.5% in aqueous acetic acid solvent. The increased crystallinity is due to the effective removal of the amorphous hemicellulose and lignin fractions that was established by the FTIR analysis (He et al., 2017). It has been seen that the choice of solvents (dilute aqueous solutions of inorganic acids or alkalis, organic solvents or ionic liquids) is critical in determining the optimum conditions for ultrasonication pretreatments (Koutsianitis et al., 2015). Several factors influencing the sonication treatment includes ultrasound frequency, sonication duration, sonication power and temperature Liyakathali et al. (2016) have found that the enzymatic digestibility of energy cane bagasse increases with increase in the sonication time and temperature while ultrasonic frequencies had no effect on enzymatic digestibility. Cherpozat et al. (2017) studied the use of ultrasonic pretreatment on wood chips for bio-oil production. The experiments were carried out at different conditions regarding frequency (40, 68, and 170 kHz), treatment time (0.5, 1 and 1.5 h) and applied power (125, 250, 500, and 1,000 W). They found the combination of 170 kHz for 0.5 h and 40 kHz for 1.5 h and a power of 1,000 W as adequate, resulting 12% increased yield of bio-oil as compared to untreated wood. However, ultrasonication for a prolonged period might cause adverse effect due to collision and aggregation between the particles (Ivetić et al., 2017). Li,kewise, high sonication power leads to cavitation near the ultrasound transducer tip that prohibits the energy transfer to the liquid medium (Subhedar and Gogate, 2014). Another study on the ultrasound pretreatment on sugar beet shreds followed by enzymatic hydrolysis resulted in a yield of 780 mg/g cellulose, which was 3.7 times higher than that of achieved with untreated samples (Ivetić et al., 2017). Luzzi et al. (2017) represented an interesting work on pretreatment of LCB where they performed the ultrasonication treatment and enzymatic hydrolysis in a single step. The activity of cellulase enzyme was examined in terms of temperature, Ph, and ultrasound power and found the maximum yield of 15.5 UPFml−1 at 40°C, pH 4.6 and applied power of 44 W. The optimum conditions further showed effective sugar production on enzymatic hydrolysis of filter paper and bagasse malt. Moreover, the impact of LCB characteristics, reactor geometry and kinetics was also observed during ultrasonic pretreatment (Nakashima et al., 2016).
The above studies clearly demonstrate that ultrasonication is a viable pretreatment technique owing to its potential to facilitate the disruption of various lignocellulosic materials. In fact, the use of ultrasound can scale down the hydrolysis time of biomass up to 80% aiding benefit to bio-fuel production (Luo et al., 2014). However, the process is energy intensive and detailed investigations are necessary to optimize the process parameters for high-scale applications.
Chemical Pretreatment
Alkali Pretreatment
Alkali pretreatment is a widely studied chemical pretreatment method which is based on the solubilization of lignin in the alkali solution. The various alkaline reagents used commonly for alkali pretreatment are the hydroxides of sodium, potassium, calcium and ammonium. Among these sodium hydroxide was found to be the most effective (Kim et al., 2016). A saponification reaction takes place throughout the alkali pretreatment process which causes cleavage of the intermolecular ester linkages between hemicelluloses and lignin. This results in solubilisation of lignin and hemicellulose fragments in the alkali solution and brings the cellulose in the interaction of enzymes (Sun S. et al., 2016). Also, alkali pretreatment changes the lignocellulosic structure via cellulose swelling that leads to reduction in crystallinity and degree of polymerization thereby increasing internal surface area (Behera et al., 2014). In addition, removal of acetyl groups and uronic acid substitutions in hemicelluloses during alkali pretreatment also increases the accessibility of the carbohydrates to enzymatic hydrolysis (Maurya et al., 2015).
Various studies reflect many advantages of the alkali pretreatment technique. A study on the alkaline pretreatment of rice straw for biomethane production reported that 1% NaOH at room temperature for 3 h significantly reduces the hemicellulose and lignin contents while the cellulose content remains unaltered. This led to an increase of methane yield by more than 34% compared to untreated rice straw (Shetty et al., 2017). Likewise, Talha et al. (2016) optimized the conditions of alkaline pretreatment of sugarcane bagasse and filter mud to enhance biomethanation. The results showed 86.27% lignin removal using 1% NaOH at 100°C for 3 h and increase of 82.20% methane yield. Shen et al. (2017) proved sodium hydroxide pretreatment as an effective method to enhance the process of anaerobic digestion. They optimized the pretreatment conditions on vinegar residue (VR) and found an increased methane yield of 205.86 mL g−1vs at 3% NaOH concentration which was 53.99% higher than the untreated VR. Alkaline pretreatment using calcium hydroxide (also called lime) has also been studied and found simple and effective since Ca(OH)2 is very inexpensive and safe to handle. A study on the lime pretreatment of corn cob residue to enhance biogas production revealed that the pretreatment accelerates the digestion process by removing lignin and obtained biogas 2 times higher than untreated corn cob (Shah and Tabassum, 2018). Rabelo et al. (2011) compared the effect of alkali pretreatment on sugarcane bagasse with lime and alkaline peroxide and found maximum glucose yield of 200 mg g−1 on lime loading of 0.04 g g−1 at 70°C for 37 h. However, lime pretreatment takes long residence time and high temperature compared to peroxide pretreatment. Ghorbani et al. (2017) investigated the lime pretreatment effects on date palm leaves under aerated and non-aerated conditions and found oxidative treatment at 40°C with 0.2 g g−1 Ca(OH)2 loading more suitable for delignification than the non-oxidative treatment. Extensive researches have also been conducted on ammonia-based alkali pretreatment because of non-corrosivity, non-toxicity and easy recovery of ammonia. Sakuragi et al. (2018) studied the effects of ammonia pretreatment on six different hardwood species and reported that species with high xylan and low lignin contents promotes enzymatic hydrolysis more effectively. (Oladi and Aita, 2017) optimized the ammonia pretreatment conditions for greater yield of sugar from energy cane bagasse. The optimal conditions obtained for the highest glucose and xylose yields were 208°C, 36 min and ammonium hydroxide to biomass ratio of 0.4:1 for glucose and 160°C, 60 min and ammonium hydroxide to biomass ratio of 0.31:1 for xylose. However, the pretreatment conditions yielded only 23.34 g glucose/100 g untreated biomass which was much low than the predicted value. Low xylose yield was also observed which can be ascribed to solubilization.
From the above studies we can summarize that the alkali pretreatment is an effective technique in removing lignin and makes carbohydrates more exposed to use for the downstream processes. However, a major disadvantage of the technique is the recovery of the added alkalis which requires further investigations. Furthermore, alkali pretreatment is more favorable for low lignin content biomass such as herbaceous crops and agricultural residues and less productive for hardwoods.
Acid Pretreatment
Acid pretreatment of LCBs is based on the susceptibility of the glucosidic bonds between hemicellulose and cellulose to acid. Hydronium ions which originates from the acid catalyst cause breakdown of the long cellulose and hemicellulose chains into sugar monomers (Lloyd and Wyman, 2005). Both, inorganic acids such as sulfuric acid (Kärcher et al., 2015), phosphoric acid (Nair et al., 2015), nitric acid (Kim et al., 2015), and hydrochloric acid (Zu et al., 2014), and organic acids such as formic acid (Du et al., 2016), maleic acid (Jung et al., 2015), and oxalic acid (Jeong and Lee, 2016) are used. Acid pretreatment can be used either as concentrated acids (30–70%) at low temperature (< 100°C) or as dilute acids (0.1–10%) at high temperatures (100–250°C). Although, the concentrated acid pretreatment can highly accelerate the sugar conversion rate (higher than 90%), most of the concentrated acids are very toxic and corrosive and hence require high operational and maintenance costs. Moreover, they cause undesired cellulose degradation leading to generation of inhibitory compounds viz. furfurals, 5-hydroxy methyl furfural, phenolic acids and aldehydes. There are several approaches which include chemical and biological detoxification methods to overcome the effect of these inhibitors (Jönsson and Martín, 2016). Chemical detoxification involves ion exchange resins, activated charcoal or tin oxides, calcium hydroxide overliming and neutralization techniques that either convert the inhibitor compounds into inert substances or reduce their concentration (Ko et al., 2015; Li et al., 2018). On the other hand, the biological methods are based on using microbes like Rhodococcus sp. YHY01, Streptomyces coelicolor etc. to bring about the same changes on the inhibitors (Bhatia et al., 2016, 2017a). However, it has to be kept in mind that the selection of feedstocks that are amenable to breakdown under milder pretreatment conditions and generate lesser inhibitors during the process is the simples and most effective way to counterpoise the problems relating to inhibition. As such, pretreatments with the dilute acids are most suitable at industrial scale as they bring about the conversions in an economical and environmentally friendly manner (Zheng et al., 2014; Kumar and Sharma, 2017).
Studies reveal that among the acids, dilute sulphuric acid (H2SO4) is the most extensively used to pretreat LCBs. Sahoo et al. (2018) compared the effect of dilute acid and alkali pretreatment on wild rice grass (Zizania latifolia) for enzymatic hydrolysis. Results showed that 0.4% H2SO4 with 10% biomass loading releases 163 mg sugar g−1 biomass, while only 92 mg sugar g−1 biomass was obtained on treated with 1 % NaOH. This proves dilute acid pretreatment a more feasible method for this grass compared to alkali pretreatment. Sindhu et al. (2014) studied dilute acid pretreatment of Indian bamboo (Bambusa spp.) varieties for bioethanol production. The pretreatment process with various mineral and organic acids revealed H2SO4 as the best pretreatment agent. Experiments were carried out with different biomass loadings, acid concentrations and pretreatment time. An optimized conditions of 15% (w/w) biomass loading, 5% acid concentration and 30 min pretreatment time yielded 0.319 g/g of reducing sugar which produced 1.76% (v/v) ethanol on subsequent enzymatic saccharification and fermentation. Acid pretreatment also depends on the characteristics of the LCB used. Research studies have been done on the effects of acid pretreatment on different parts of corn stalk for bioethanol production. They pretreated the stem, leaf, flower, cob, and husk by 2% H2SO4 at 121°C under 10% biomass loadings for 60 min and found that the cob had the highest sugar content resulting in 94.2% glucose and 24.0 gL−1 ethanol (Li P. et al., 2016). Furthermore, addition of surfactants like Tween-80 before or after the pretreatment with dilute sulphuric acid have shown significant increase in glucose concentrations on enzymatic hydrolysis, when used on waste-paper feedstocks (Alencar et al., 2017). Here, the role of Tween-80 was also believed to prevent thermal denaturation of the enzymes used for hydrolysis, even at higher temperatures. It has also been observed that the surfactants may get adsorbed on the lignin fraction of LCB, through hydrophobic interactions during the conversion of LCB to glucose. This prevents the cross-binding of enzymes to lignin and results in the improved turnover of glucose (Li K. et al., 2016). Nonetheless, dilute acid pretreatments also cause sugar degradation leading to the formation of inhibitory byproducts that needs to be removed. Moreover, neutralization of the pretreated slurry adds another negative effect on the downstream processes.
Amnuaycheewa et al. (2016) investigated rice straw pretreatment with organic acids for the improvement of enzymatic hydrolysis and biogas production. Using a response surface method they optimized the pretreatment conditions with acetic acid (C2H4O2), citric acid (C6H8O7), oxalic acid (C2H2O4) and hydrochloric acid (HCl) and compared their effects. Results showed that oxalic acid pretreatment under optimal conditions of 5%, 135.91°C and 30.86 min led to the highest sugar production of 213.4 mg/ 500 mg of pretreated sample on enzymatic saccharification. Organic acids such as maleic acid and formic acid are also more efficient than dilute mineral acid for LCB pretreatment (Qing et al., 2015). In addition, organic acids catalyze negligible sugar degradation. However, a study on the comparative effect of citric acid (C6H8O−7) pretreated rice husk and inorganic acids (HCl, H2SO4 and HNO3) pretreated rice husk on the adsorption of phenol from wastewater showed less adsorption by the citric acid treated rice husk (Daffalla et al., 2012). Therefore, detailed investigations are still awaited to explore the advantages of organic acids over mineral acids for the pretreatment of LCBs.
Ionic Liquids
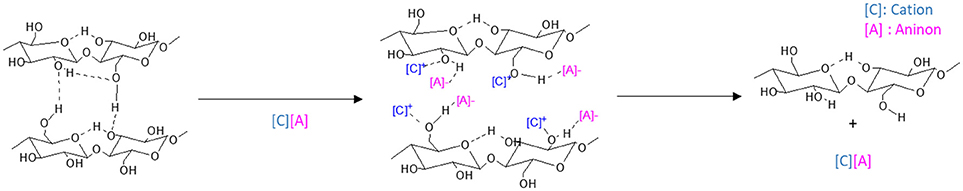
Swatloski et al. (2002) put forward the use of ionic liquids (ILs) as cellulose solvent and ever since pretreatment of LCB by ILs has become a promising prospect. ILs are relatively new class of solvents with melting point < 100°C which are comprised of cations and anions. The cations, in general, are organic viz. imidazolium, pyridinium, aliphatic ammonium, alkylated phosphonium, and sulfonium ions, while the anions include both organic and inorganic ions (Yoo et al., 2017). During the pretreatment process both the cations and anions play a significant role in solubilizing the cellulose and lignin. Figure 3 represents the interruption of the intra- and intermolecular hydrogen bonding in cellulose by IL ions. Also, the cellulose dissolution increases in presence of electron-withdrawing groups in the alkyl chains of IL cations (Yoo et al., 2017). Most of the ILs are recoverable and reusable. They possess the striking advantages of negligible vapor pressure, non-volatility, non-toxicity, large thermal, and chemical stability and most importantly the adjustable nature of their cations and anions on which the properties of the ILs depend (Chen et al., 2017). These are the reasons why ILs have often been described as green solvents. Several types of ILs include imidazolium-based ([(C3N2)Xn]+), pyridinium based ([(C5N)Xn]+), pyrrolidinium-based ([C4N)Xn]+, ammonium-based [NX4]+, phosphonium-based [PX4]+, sulfonium-based [SO3]+, and others such as cholin. Among these, imidazolium salts are the most commonly used ILs (Zhang et al., 2017).
Raj et al. (2018) investigated the effect of various imidazolium-based ILs pretreatment on enzymatic hydrolysis of mustard stalk. It was found that ILs containing acetate ion enhanced specific surface area and porosity accessible for enzymes and improved the yield of reducing sugar significantly. The effect of pretreatment temperature on acetate ion based ILs was also studied. Differential scanning calorimetry analysis showed an increased porosity (108.9 mg/g) at 130°C/2h compared to 100°C/5h (107.8 mg/g) and produced 97.7 and 78.7% glucose, respectively, on subsequent hydrolysis. Stanton et al. (2018) also studied the effect of different imidazolium-based ILs on the structure and properties of microcrystalline cellulose and silk blended biocomposite films. The results revealed that the intermolecular interactions in the films are directly correlated to the anion structure of the ILs. Smuga-Kogut et al. (2017) carried out the pretreatment of rye straw using 1-ethyl-3methylimidazonium chloride and found an optimal condition of 2 h, 120°C and 1 ml g−1. DM rye straw using response surface methodology for highest sugar yield. It was found that pretreatment led to threefold increase in the yields of reducing sugars on enzymatic hydrolysis in comparison with the untreated straw. Use of ILs other than imidazolium salts also showed strong influence on the pretreatment of lignocellulosic materials. For example, pretreatment of bagasse powder using choline acetate (ChOAc) enhanced the tensile strength of the propionylated bagasse composites from 35 MPa to 40 MPa and the elastic moduli from 2.0 GPa to 2.6 GPa compared to the composites made of untreated bagasse powder (Ninomiya et al., 2017). Likewise, Ma et al. (2016) compared the pretreatment of corn stalk with a series of pyrrolidonium-based ILs at 90°C for 30 min and observed 85.94% lignin removal and a reducing sugar yield of 91.81% after enzymatic hydrolysis.
Regardless of their distinctive chemical properties, ILs present the major shortcomings of being expensive and toxic to microorganisms and enzymes. Brandt-Talbot et al. (2017) investigated for the first time the application of the low-cost IL triethylammonium hydrogen sulfate for the pretreatment of Miscanthus x giganteus grass. They found up to 75% lignin and 100% hemicellulose solubilization in the IL solution and a yield of 77% glucose by enzymatic saccharification. Also the IL was reused four times with 99% recovery at each time. Nevertheless, further studies on these aspects with low-cost recovery technology and its toxicity to enzymes are still needed for economically-viable large-scale applications.
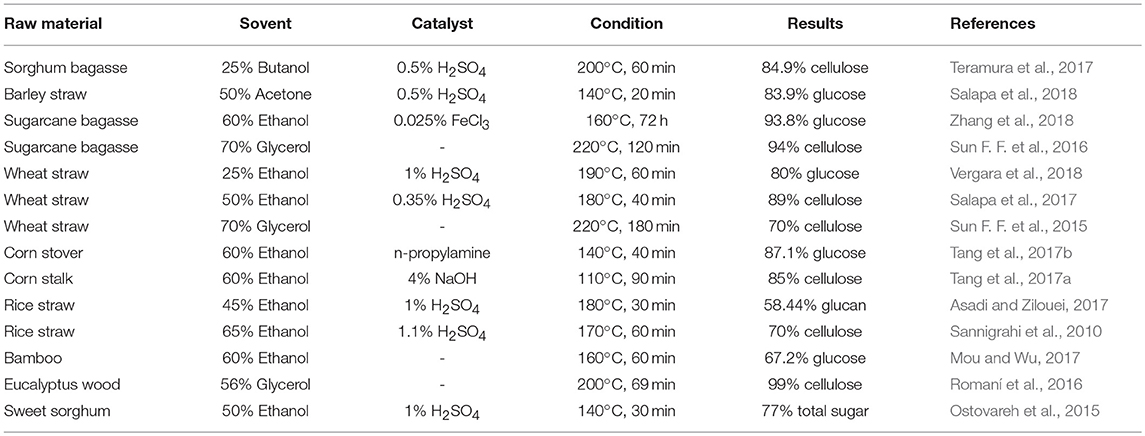
Organosolv Process
In this process, LCBs are pretreated with organic solvents or their aqueous solutions that causes break down of the internal bonds between lignin and hemicellulose thereby leaving a relatively pure cellulose residue. During the process delignification and solubilization of hemicellulose lead to increase of pore volume and surface area of cellulose and enhances the accessibility of enzymatic hydrolysis and saccharification (Zhang K. et al., 2016). A wide range of organic solvents such as, ethanol, methanol, acetone, organic acid, organic peracid, and ethylene glycol or their mixture with water have been employed to pretreat various LCBs. The process is often accompanied by the addition of a catalyst to either lower the pretreatment temperature or improve the delignification rate. Usually mineral acids (hydrochloric acid, sulfuric acid, phosphoric acid), bases (lime, sodium hydroxide, ammonia) and some salts are used as catalysts (Borand and Karaosmanoglu, 2018).
Organosolv presents itself as an emerging pretreatment process owing to its inherent advantages such as, easy recovery of the solvents by distillation, recycles the solvents back to pretreatment and utilization of high-quality lignin isolated from this process as value-added byproducts for industrial applications. However, there also lies a few major disadvantages to the organosolv pretreatment. Most of the organic solvents are too expensive and need to be recovered as much as possible which is an energy-intensive process. In addition, high flammability and volatility of the organic solvents make the pretreatment to be carried out under especially controlled conditions (Borand and Karaosmanoglu, 2018). The organosolv pretreatment process for various feedstocks has been summarized in Table 2.
Deep Eutectic Solvents
Pretreatment of LCB using deep eutectic solvents (DES) has attracted much interest in recent years. DESs are a new generation of ionic fluids comprising of two or three components, often interlinked through hydrogen bonding and form a eutectic mixture with a lower melting point than each individual components. They are usually liquids at temperature < 100°C. DESs and ILs are much alike in terms of their physicochemical properties but their low-cost synthetic technology and biodegradability make them versatile alternatives to ILs (Zdanowicz et al., 2018). DESs are mostly derived by mixing a quaternary ammonium salt with a metal salt or hydrogen bond donor (HBD) which is capable of forming a complex with the halide ion of the quaternary ammonium salt (Smith et al., 2014). The decrease in melting point of the eutectic mixture is ascribed to the charge delocalization occurring between the halide ion and the hydrogen-donor moiety (Wagle et al., 2014).
DESs can be represented by the general formula
where Cat+ is basically any ammonium, phosphonium or sulfonium cation, X− is a Lewis base, commonly a halide anion, Y is Lewis or Brønsted acid, and z is the number of Y molecules that interact with the anion. The complexation between X− and Y forms different anionic species (Smith et al., 2014). Being a biodegradable, non-toxic and cheap organic salt, cholin chloride (ChCl) are used in most of the DESs in combination with the low-risk HBDs like, urea, glycerol, carboxylic acids, and polyols (Zdanowicz et al., 2018). A study on the corn stover pretreatment by different DESs having the same halide salt i.e., ChCl found ChCl:Formic acid to be the ideal mixture for butanol fermentation. The findings implied that DES with acidic hydrogen donors could enhance the removal of lignin and hemicellulose more efficiently and are superior to ILs (Xu et al., 2016). The results are further proved by Zhang and his coworkers where they investigated the pretreatment of corn cob by using three DESs; monocarboxylic acid: ChCl, dicarboxylic acid: ChCl and polyalcohol: ChCl. They found that delignification and cellulose accessibility greatly depends on the strength and amount of acid and the nature of hydrogen bond acceptors. The free hydroxyl groups of polyalcohol interacts with the free and etherified hydroxyl groups of lignin thereby making the polyalcohol: ChCl to be an adequate mixture for pretreatment (Zhang C. W. et al., 2016).
Physicochemical Pretreatments
Steam Explosion
Steam explosion is the most commonly employed and effective pretreatment method, which is typically a combination of both mechanical forces and chemical effects applied to LCBs. In this technique, the biomass is subjected to high-pressure saturated steam (0.69–4.83 MPa) at a temperature of 160–260°C to let water molecules penetrate the substrate structure. The pressure is then suddenly reduced to let the water molecules escape in an explosive way. This rapid release of pressure causes explosion of the bulk LCB into splitted fibers. Besides, the high temperature and pressure enhance the breakdown of the glycosidic bonds in cellulose and hemicellulose and cleavage of hemicellulose-lignin bonds (Chen and Liu, 2015). During this treatment, the hydrolysis of hemicellulose into glucose and xylose monomers liberate acetic acid which catalyze the further hydrolysis of hemicelluloses, and; hence the process is also termed as autohydrolysis (Singh et al., 2015).
Steam explosion has several advantages such as low environmental effect, limited chemicals use, high energy efficiency, no recycling costs and total sugar recovery compared with other pretreatment methods (Pielhop et al., 2016). It was found that almost 70% more energy is needed by the conventional mechanical methods than steam explosion to attain the same particle size reduction (Kim, 2018). This pretreatment is affected by several factors such as steam temperature, residence time, the size of biomass and moisture content (Rabemanolontsoa and Saka, 2016). Steam explosion can be employed directly to milled LCB without employing any chemicals. Vivekanand et al. (2013) pretreated birch samples with steam at 170–230°C to investigate their enzymatic hydrolysis performance. Results showed that steam explosion at 220°C for 10 min generates the maximum enzymatic hydrolysis yield. However, under harsh steam explosion conditions, the production of inhibitors such as aromatic compounds and dehydration byproducts (weak acids and furan derivatives) influenced the subsequent hydrolysis process (Vivekanand et al., 2013; Sun Y. G. et al., 2015; Verardi et al., 2018). Similar results were also observed when corn stover was pretreated with steam explosion at 140–220°C for biogas production. Pretreatment at 160 °C for 2 min increased the methane yield by 22% while harsh pretreatment conditions led to the formation of inhibitors (Lizasoain et al., 2017). Besides, many phenolic compounds are produced when the lignin is broken down. Therefore, some detoxification methods are required to reduce the hindrances caused by these compounds for the benefit of the downstream processes. Steam explosion was found to be an effective pretreatment technique in enhancing the microbial digestion of pretreated corn stover (Zhao et al., 2018). The efficiency of this pretreatment can be adequately improved by the use of catalysts such as H2SO4, H3PO4, SO2, or CO2. Addition of these catalysts in steam explosion can reduce the residence time and temperature, minimize the generation of inhibitory compounds, improve the enzymatic hydrolysis significantly and lead to complete hemicellulose sugar recovery (Negro et al., 2014; Neves et al., 2016). Guerrero et al. (2017) investigated the effect of temperature and residence time of acid-impregnated steam explosion of banana stem with response surface methodology. The optimal conditions were found at 170°C, 5 min and 2.2% H2SO4 (v/v) producing a high glucose yield of 91%. Wang et al. (2018) explored the enzymatic hydrolysis and fermentation of SO2 and H2SO4 impregnated spruce separately pretreated by steam explosion at 195–215 °C for the same time period. Higher yields of glucose and total sugar were obtained for SO2- impregnated spruce, while lower hemicellulosic sugar yield was observed for H2SO4- impregnated spruce. This is because H2SO4 accelerated the hydrolysis of polysaccharides thereby catalyzing the formation of inhibitors. However, comparatively low inhibitor contents were observed in the hydrolysates of SO2-impregnated spruce. Recently, a study on deacetylation of corn stover prior to steam explosion showed that deacetlyation eliminated over 85% inhibitor formation. However, an incorporation of a catalyst was required for better hydrolysis of the deacetylated feedstock (Tang et al., 2018).
Ammonia Fiber Explosion (AFEX)
In AFEX process, the LCB is heated with liquid ammonia (in 1:1 ratio) in a closed vessel at temperature around 60–100°C under high pressure for 5–30 min, and then the pressure is suddenly released (Shirkavand et al., 2016). The high pressure and given temperature causes swelling of lignocellulose and the rapid release of pressure disrupts the fibrous structure of biomass, reduces the crystallinity of cellulose and thereby improves the accessibility of enzyme. Optimization of AFEX pretreatment can be done by varying the four parameters including temperature, blowdown pressure, water loading and ammonia loading (El-Naggar et al., 2014). Silvergrass (Miscanthus spp.) when presoaked in water prior to AFEX pretreatment showed up to 10% increase in glucan conversion indicating that moisture content plays a major role in AFEX (Lee and Kuan, 2015). AFEX pretreatment partly removes the lignin and hemicellulose from lignocellulosic materials, but shows better enzymatic hydrolysis results at low enzyme loadings compared to other pretreatment processes. When oil palm empty fruit bunch fiber were pretreated with AFEX at 135°C for 45 min, the lignin-carbohydrate linkages changed along with some relocalization of lignin and resulted in 90% glucan conversion at lower enzyme loading of 13.8 FPU_g−1 glucan as compared to 25.5% conversion for untreated biomass (Abdul et al., 2016). Therefore, AFEX pretreatment is more suitable for low lignin-content LCB such as agricultural wastes and herbaceous plants like switch grass, rice straw, corn stover etc. Several investigations have been carried out to determine the optimal AFEX conditions for different LCBs. Zhao et al. (2014) reported ammonia to biomass loading of 5:1, 70% moisture content and 170°C as optimal for enhanced enzymatic digestibility of corn stover. They further demonstrated that presoaking of corn stover prior to AFEX pretreatment enhances the delignification of corn stover from 15.74 to 24.07% and thereby increases glucan digestibility from 82.13 to 87.78%. Mathew et al. (2016) when compared the effect of AFEX and dilute acid pretreatment on corn stover, found that AFEX treated hydrolysate of corn stover was superior to dilute acid treated hydrolysate for ethanol production.
The main advantage of AFEX lies in the negligible formation of inhibitors as compared to other pretreatment methods. Nonetheless, ammonia should be recovered and recycled due to its high cost and volatility to reduce the overall operating cost and minimize environmental damage.
CO2 Explosion Pretreatment
A key drawback in the application of steam explosion pretreatment lies in its use of high thermal energy which is essential for the decomposition of the LCB. The AFEX pretreatments on the other hand use ammonia, which is highly corrosive with detrimental environmental effects, thereby limiting its scope. In this context, supercritical CO2 explosion appears to be a viable alternative owing to its lower energy requirements and use of the greener alternative compared to ammonia (Bharathiraja et al., 2018). Because of its characteristics of mass transfer of a “gaslike” with a “liquidlike” solvating power, supercritical CO2 can diffuse through interspaces like gas and dissolve materials like liquid (Rostagno et al., 2015). Under high pressure the CO2 molecules penetrate into the biomass and shattered the higher level of structures comprising hemicellulose and lignin. Once dissolved in water, CO2 will form carbonic acid, which catalyzes the hydrolysis of hemicellulose. This is the reason why this pretreatment process is not satisfactory to biomass with no moisture content. On the other hand, when the pressurized gas is released, it breaks the compact matrix structure of biomass thereby improving the accessibility of cellulose fibers (Capolupo and Faraco, 2016). The penetrating rate of the CO2 molecules into the cellulosic pores increases with pressure and leads to high glucose yield. Also, it was reported that addition of water-ethanol as co-solvents during supercritical CO2 explosion pretreatment can significantly remove the lignin and enhance enzymatic hydrolysis of corn stover (Morais et al., 2014; Serna et al., 2016). Benazzi et al. (2013) reported a single-step process for the hydrolysis of sugarcane bagasse using supercritical CO2 and obtained 60% yield of fermentable sugars. Soybean hull pretreated with supercritical CO2 showed a high glucose yield of 97% after enzymatic hydrolysis. This high glucose yield was obtained under optimal conditions of 8 MPa pretreatment pressure and 130°C temperature for 30 min. Thus the pretreatment pressure and temperature also plays a significant role in supercritical-CO2 treatment (Islam et al., 2017). Likewise, Narayanaswamy et al. (2011) found that supercritical-CO2 pretreatment of corn stover (75% moisture content) under optimal conditions of 24 MPa and 150°C for 60 min, increased the glucose yield by 2.5 fold compared with native corn stove. A combined pretreatment of corn cob and corn stalk by supercritical CO2 and ultrasonic pretreatment improved the enzymatic hydrolysis by 75 and 13.4%, respectively under optimal conditions of 20 MPa and 170°C (Yin et al., 2014).
Low cost of CO2, low environmental impact, non-flammability, no toxins formation and easy recovery makes the employment of supercritical-CO2 in the pretreatment of LCB an attractive approach. However, the economic input for the experimental set up that can withstand high-pressure conditions of CO2 pretreatment is significantly high and is a great obstacle in its application in industrial scale.
Liquid Hot Water (LHW)
LHW pretreatment is very much alike to steam explosion but as the name suggests, LHW uses water at elevated temperature (170–230°C) and pressure (up to 5 MPa) in place of steam. Unlike steam explosion rapid release of pressure is not required in LHW and the application of pressure is only to prevent evaporation of water. LHW hydrolyzes hemicellulose by liberating its acetyl groups and removes lignin thereby making the cellulose fibers more exposable (Zhuang et al., 2016). The detached hemicellulose remains in the liquid fraction of the pretreated slurry and formation of monomeric sugars during the process is minimal. However, in order to avoid the sugar degradation and inhibitors formation, the LHW pretreatment is carried out at controlled pH between 4 and 7 (Li et al., 2014). It was reported that Miscanthus X giganteus pretreated with LHW under controlled pH at 200°C for 15 min showed high ethanol yield of 71.8% of theoretical on fermentation (Boakye-Boaten et al., 2015). Hongdan et al. (2013) optimized the process variables (temperature and residence time) in LHW pretreatment of sugarcane bagasse and achieved 90% glucose recovery on enzymatic hydrolysis at 180°C for 30 min. LHW pretreatment of corn cobs at 160°C for 10 min provided maximum hemicellulose-derived sugar recovery of 58.8% and enzymatic hydrolysis yield of 73.1% with more than 60% lignin removal (Imman et al., 2018). Furthermore, Imman et al. (2015) investigated the effect of the alkaline catalyst on LHW pretreatment of rice straw and demonstrated that rice straw pretreated with LHW in presence of NaOH showed remarkably higher glucose yield compared with LHW pretreatment in the absence of NaOH. Likewise, another study on the effects of acid and alkali promoters during LHW pretreatment of rice straw revealed that the presence of such promoters changes the physical structure of the pretreated biomass and thus lowered the required LHW temperature and improved the enzymatic digestibility (Imman et al., 2014).
LHW pretreatment possesses several advantages as it does not require any catalyst or chemicals, the formation of toxic materials is almost absent and has low-cost of the solvent for large-scale applications. Furthermore, biomass size has no influence since the particles are broken down during the treatment which makes the process more striking for large scale (Bhutto et al., 2017). However, the process is very energy intensive due to a large amount of water involved.
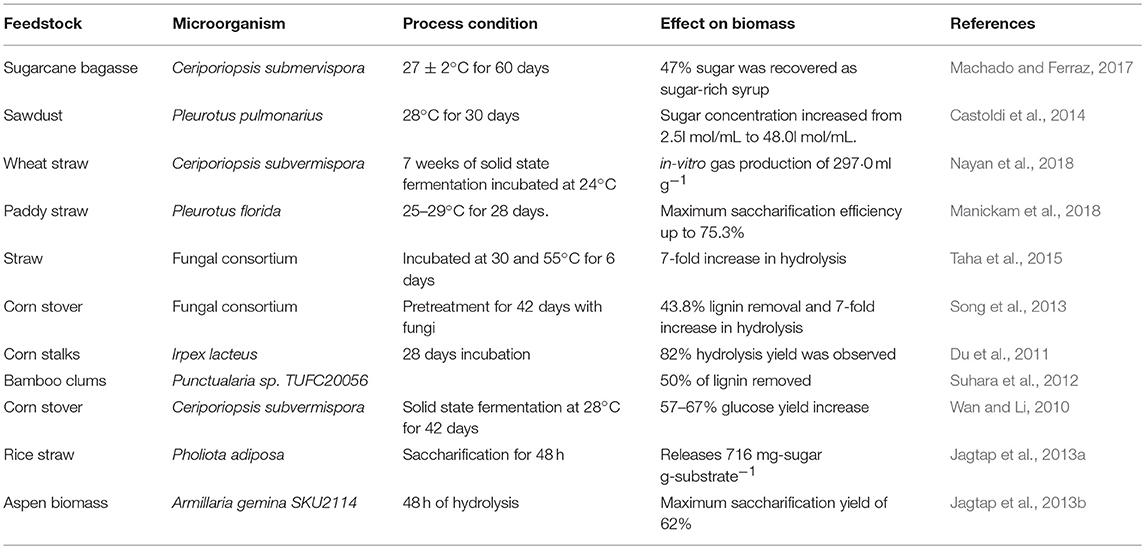
Biological Pretreatment
Biological pretreatment is a low cost and eco-friendly technique to treat LCB prior to enzymatic saccharification. This technique is promising as there is no inhibitor formation during the process, requires lesser energy consuming and is eco-friendly (Sindhu et al., 2016; Bhatia et al., 2017b). Through this method, lignin degrading bacteria or fungi, as whole cell or enzymes, are used to pretreat LCB. The enzymes used in degradation of lignin are laccases, lignin peroxidase, manganese peroxidase, and versatile peroxidase. Fungi are the best suited for such applications as they are capable of degrading cellulose, hemicelluloses, and lignin. Biological pretreatment is not only used for lignin removal, but also for removal of specific components such as antimicrobial substances (Wan and Li, 2012). White-rot, brown-rot and soft-rot fungi are used for degradation of lignin and hemicelluloses present in LCB (Chen et al., 2010). However, mostly white-rot fungi are involved in biological pretreatment due to high sugar yield associated with enzymatic saccharification (Yesilada et al., 2018). Some white-rot fungi can simultaneously degrade lignin and polysaccharides, resulting in the loss of carbohydrates, while other white-rot fungi can selectively degrade lignin. There are two extracellular enzymatic systems involved in microorganisms, one hydrolytic and another ligninolytic system (Wagner et al., 2018). Hydrolytic system is responsible for degradation of cellulose and hemicelluloses, while ligninolytic system depolymerizes the lignin. Lignin can be degraded by enzymes produced by various organisms, among which white-rot fungus has been found the most effective. Fungi are usually isolated from the soil, living plants, or agricultural waste materials (Vats et al., 2013).
Whole Cell Pretreatment
White-rot fungi are generally used as whole cell microorganism since it is less potent for the degradation of the cellulosic fraction of the LCB. The ligninolytic system in these fungi secretes one or more extracellular enzymes which are responsible for the degradation of lignin (aromatic polymer) and aliphatic fragments (Hammell, 1997). White-rot fungi commonly employed for ligninolytic pretreatment are Phanerochaete chrysosporium, Ceriporiopsis subvermispora, Ceriporia lacerata, Cyathus stercolerus, Pycnoporus cinnarbarinus, Pleurotus ostreaus, Phlebia subserialis, Pleurotus streatus, Postia placenta, Gloeophyllum trabeum, and Echindodontium taxodii (Kumar and Sharma, 2017) are capable of efficiently metabolizing lignin in a variety of LCBs. Among these, Phanerochaete chrysosporium is a model organism for lignin degradation. However, white-rot fungi may face challenges in lignin degradation due to presence of carbon-carbon bonds within the large lignin polymers (Martinez et al., 2004).
Table 3 summarizes the different biological pretreatment conditions that have been used in the recent past for treatment of various biomass feedstocks using whole cell biocatalyst. The major disadvantages of biological pretreatment methods are low efficiency and long residence periods, when used in isolation (Tian et al., 2012; Maurya et al., 2015). Saritha et al. (2012) studied biological pretreatment of paddy straw using Trametes hirsute, which enhanced the sugar recovery followed by enzymatic saccharification. Trametes hirsuta showed the high ligninase but low cellulase activity under solid state fermentation conditions and enhanced carbohydrate content recovery by 11.1% within 10 days of incubation time. Suhara et al. (2012) reported the biological pretreatment of bamboo culms using Ceriporiopsis subvermispora and showed lignin degradation bat an efficiency of 50%. Taha et al. (2015) reported the straw saccharification through co-culturing of lignocellulose degrading microorganisms and showed that enzyme activities of fungal isolates were 2-fold higher than those from bacteria. Co-culturing resulted in 7- fold increase in saccharification rate. Du et al. (2011) studied the white-rot fungi Irpex lacteus, which produces a variety of extracellular hydrolytic and oxidative enzymes and reported the hydrolysis yield of 82% after 28 days of biological pretreatment of corn stalks.
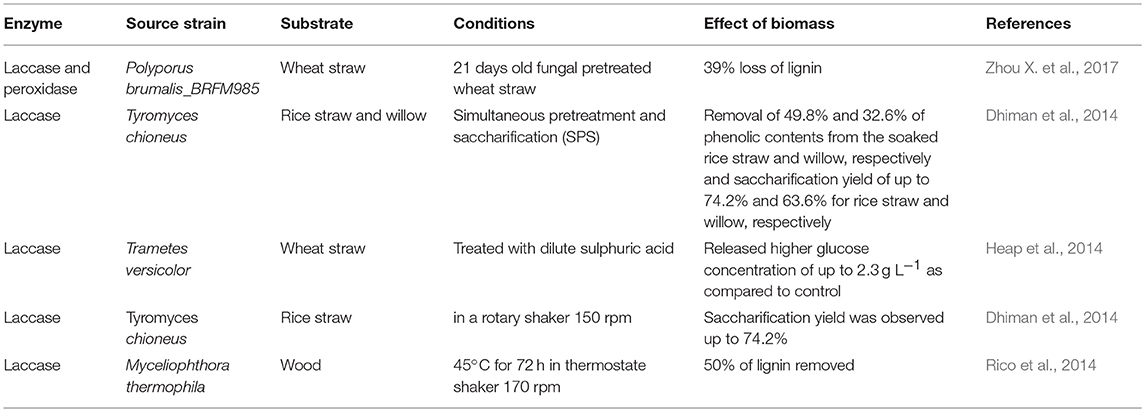
Enzymatic Pretreatment
Lignin is the most abundant aromatic polymer consisting of phenolic and non-phenolic compounds. Some fungi, bacteria, and insects are capable of producing enzymes which digest lignin. There are two families of ligninolytic enzymes which play an important role in enzymatic degradation: phenol oxidase (laccase) (Lac) and peroxidases (lignin peroxidase (LiP), versatile peroxidase (VP), and manganese peroxidase (MnP)) (Zamocky et al., 2014). These are heme-containing glycoproteins which require hydrogen peroxide (H2O2) as oxidant. The role of other enzymes has not yet been fully elucidated including glyoxal oxidase (GLOX; EC 1.2.3.5), glucose oxidase (EC 1.1.3.4), cellobiose dehydrogenase (CDH; EC 1.1.99.18) oxido-reductase and methanol oxidase (Janusz et al., 2017). There are different microorganisms i.e. bacteria and fungi, which produce celluloytic, hemicellulolytic and ligninolytic enzymes. Lignin degrading enzymes are directly employed to pretreat the biomass. These are employed in a group of one or more. Table 4 shows the effect of enzymatic pretreatment on different lignocellulosic feedstocks.
Lignin Peroxidase
Lignin peroxidase (LiP) (EC 1.11.1.4) was first discovered in Phanerochaete chrysosporium grown in nitrogen-limited medium. LiP is H2O2 dependent glycoprotein, which contains heme and has a molecular mass ranges from 35 to 40 kDa (Hammel and Cullen, 2008). LiP has been reported to produce by white-rot fungi like Phlebia flavido-alba, Bjerkandera sp. strains BOS55, Trametes trogii, Phlebia tremesllosa, Gloeophyllum trabeum, Trametes versicolor, Phanerochaete chrysosporium, etc. (Houtman et al., 2018).
Laccases
Laccases (Lac) (EC 1.10.3.2) or benzenediol oxygen oxidoreductase is a multicopper enzyme that belongs to a group of blue copper containing oxidase. White-rot fungi produced laccase, catalyzes the oxidation of aromatic amines and phenolic compounds such as phenolic substructure of lignin (Wong, 2009; Heap et al., 2014). Lac is glycoprotein and its molecular weight ranges between 60 and 80 kDa. It is also able to oxidize non-phenolic substructure of lignin in the presence of low molecular weight compounds hydroxylbenzotriazole. Laccase producing microorganisms are both fungi (Trametes versicolor, Tremetes trogii, Phlebia floridensis), and bacteria (Citrobacter spp., Straphylococcus saprophlticus, Bacillus subtilis). Lac enzyme plays an important role in lignin degradation and modification processes which increase the yield of both hydrolysis and fermentation process (Piscitelli et al., 2011; Fillat et al., 2017). Laccase is an important enzyme since it oxidizes both toxic and non-toxic substrates. It is used in textile, food processing, wood processing, pharmaceutical, and chemical industries. Laccase enzyme is very specific, ecologically sustainable and a proficient catalyst (Giacobbea et al., 2018). Several bacterial and fungal laccases have been used for detoxification of various agrowaste (pretreated and unpretreated) feedstocks in the presence of mediator system. Rico et al. (2014) studied the ability of an industrial laccase-mediator system to modify and to remove the lignin during pretreatment of wood (Eucalyptus globulus) feedstock, to improve the saccharification, and to analyze the chemical modifications. Up to 50% lignin removal from ground eucalyptus wood was attained when pretreated with recombinant Myceliophthora thermophila (50 U·g−1) laccase.
Manganese Peroxidase
Manganese peroxidase (MnP) (EC 1.11.1.13), Mn (II) hydrogen peroxide oxidoreductase, catalyzes Mn dependent reactions. Similar to LiP, MnP is also heme glycoprotein. Major difference in MnP and LiP is: LiP generally oxidizes non-phenolic lignin, while MnP oxidizes phenolic ring of lignin and plays an important role in the initial stage of lignin degradation. Manganese peroxidase oxidizes phenolic and non-phenolic lignin units through lipid peroxidation reactions and forms various phenolic compounds i.e., 3-ethylthiazoline-6-sulfonate, 2, 6-dimethyloxyphenol syringol, guaiacol, and non-phenol compound i.e., alcohol (Brown and Chang, 2014). It oxidizes Mn2+ to Mn3+ which further oxidizes phenol rings to phenoxy radicals leading to decomposition of compounds.
Versatile Peroxidase (VP)
Versatile peroxidase (VP) (EC 1.11.1.16) oxidizes phenolic and non-phenolic aromatic compounds. VP enzymes have the catalytic activities of both MnP and LiP, and are able to oxidize Mn2+ like MnP as well as high-redox potential non-phenolic compounds like LiP (Abdel-Hamid et al., 2013; Zavarzina et al., 2018). VP is employed together with MnP, LiP and other microbial peroxidases in degradation of non-phenolic aromatic compounds such as veratrylglycerol β-guaiacyl ether to veratraldehyde. VP also oxidize Mn2+ to Mn3+, veratyl alcohol to veratraldehyde and p-dimethoxybenzene to p-benzoquinone (Narayanaswamy et al., 2013). VP comprises the ligninolytic heme peroxidase gene family of Pleutotus ostreatus (Fernandez-Fueyo et al., 2014). It is found in various Bjerkandera species and Pleurotus species (Chen et al., 2010). It catalyzes oxidation of wide range of substrates from plant peroxidase hydroquinones, substituted phenols to bulky recalcitrant lignin directly, without redox mediators. It is used in textile, bleaching, paper and pulp industries, production of biofuels, bioremediation of xenobiotic compounds, degradation of endocrine disrupting chemicals (Ravichandran and Sridhar, 2016).
Conclusion
The high crystallinity of cellulose and its sheathing by hemicellulose-lignin matrix furnish the resilient structure of LCB. Therefore, pretreatment is an extremely important step for conversion of LCB to sugars and further processing for industrially important bioproducts and biofuels. Extensive investigations have been carried out on the effects of different pretreatment methods on lignocellulosic composition and sugar yield. Analysis of various methods brings us to the conclusion that each method has its pros and cons. Therefore, assessing these methods straight through the test data is not accurate. Until now, a cost-effective and environmentally benign pretreatment method that can completely delignify biomass is yet to be established. Moreover, the reaction mechanisms of the different pretreatment technologies have not been explored in details to improve the existing methods and optimize the process conditions. In addition, the LCB properties and features greatly influence in choosing the correct pretreatment method. Hence, the challenges to pretreat biomass with high efficiency comprise of cost-effectivity, energy-effectivity, environmental sustainability, which are the existing bottlenecks for its integration as feedstocks in biorefinery approaches. On the basis of this review, we can propose that an improved chemical production and industrial lignocellulose applications can be achieved by the development of an economically feasible, extremely productive and ecofriendly pretreatment method based on the evaluation standard.
Author Contributions
JB and BN drafted the portions on physical, chemical and physicochemical pretreatments. RS and SK drafted the portion on biological pretreatments. RD and DB edited the portions on chemical and physicochemical pretreatments. EK planned the outlay of the review, supervised the development of topics and edited the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
EK, JB, SK, and RS wish to acknowledge DBT, Government of India, for the Twinning Research Grant (Grant No. BT/PR16008/NER/95/47/2015).
References
Abdel-Hamid, A. M., Solbiati, J. O., and Cann, I. K. O. (2013). Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 82, 1–28. doi: 10.1016/B978-0-12-407679-2.00001-6
Abdul, P. M., Jahim, J. M., Harun, S., Markom, M., Lutpi, N. A., Hassan, O., et al. (2016). Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour. Technol. 211, 200–208. doi: 10.1016/j.biortech.2016.02.135
Aguilar-Reynosa, A., Romaní, A., Rodríguez-Jasso, R. M., Aguilar, C. N., Garrote, G., and Ruiz, H. A. (2017). Microwave heating processing as alternative of pretreatment in second-generation biorefinery: an overview. Energy Conver. Manage. 136, 50–65. doi: 10.1016/j.enconman.2017.01.004
Akhtar, N., Gupta, K., Goyal, D., and Goyal, A. (2016). Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ. Prog. Sustain Energy. 35, 489–511. doi: 10.1002/ep.12257
Alencar, B. R. A., Rocha, J. M. T. S., Rocha, G. J. M., and Gouveia, E. R. (2017). Effect of tween-80 addition in dilute acid pretreatment of waste office paper on enzymatic hydrolysis for bioethanol production by SHF and SSF processes. Cellulose Chem. Technol. 51, 121–126. doi: 10.17648/sinaferm-2015-33868
Amnuaycheewa, P., Hengaroonprasan, R., Rattanaporn, K., Kirdponpattara, S., Cheenkachorn, K., and Sriariyanun, M. (2016). Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind. Crops Prod. 87, 247–254. doi: 10.1016/j.indcrop.2016.04.069
Asadi, N., and Zilouei, H. (2017). Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol. 227, 335–344. doi: 10.1016/j.biortech.2016.12.073
Bai, X., Wang, G., Yu, Y., Wang, D., and Wang, Z. (2018). Changes in the physicochemical structure and pyrolysis characteristics of wheat straw after rod-milling pretreatment. Bioresour. Technol. 250, 770–776. doi: 10.1016/j.biortech.2017.11.085
Behera, S., Arora, R., Nandhagopal, N., and Kumar, S. (2014). Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sust. Energ. Rev. 36, 91–106. doi: 10.1016/j.rser.2014.04.047
Benazzi, T., Calgaroto, S., Dalla Rosa, C., Vladimir Oliveira, J., and Mazutti, M. A. (2013). Hydrolysis of sugarcane bagasse using supercritical carbon dioxide to obtain fermentable sugars. J. Chem. Technol. Biotechnol. 88, 1766–1768. doi: 10.1002/jctb.4002
Bharathiraja, B., Jayamuthunagai, J., Chakravarthy, M., and Kumar, R. P. (2018). Bioprocessing of Biofuels for Green and Clean Environment. Florida: CRC Press
Bhatia, S. K., Kim, J., Song, H. S., Kim, H. J., Jeon, J. M., Sathiyanarayanan, G., et al. (2017a). Microbial biodiesel production from oil palm biomass hydrolysate using marine Rhodococcus sp. YHY01. Bioresour. Technol. 233, 99-109. doi: 10.1016/j.biortech.2017.02.061
Bhatia, S. K., Kim, S. H., Yoon, J. J., and Yang, Y. H. (2017b). Current status and strategies for second generation biofuel production using microbial systems. Energy Conver. Manag. 148, 1142–1156. doi: 10.1016/j.enconman.2017.06.073
Bhatia, S. K., Lee, B. R., Sathiyanarayanan, G., Song, H. S., Kim, J., Jeon, J. M., et al. (2016). Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour. Technol. 217, 141–149. doi: 10.1016/j.biortech.2016.02.055
Bhutto, A. W., Qureshi, K., Harijan, K., Abro, R., Abbas, T., Bazmi, A. A., et al. (2017). Insight into progress in pre-treatment of lignocellulosic biomass. Energy. 122, 724–745. doi: 10.1016/j.energy.2017.01.005
Boakye-Boaten, N. A., Xiu, S., Shahbazi, A., and Fabish, J. (2015). Liquid hot water pretreatment of Miscanthus × giganteus for the sustainable production of bioethanol. Bioresources 10, 5890–5905. doi: 10.15376/biores.10.3.5890-5905
Borand, M. N., and Karaosmanoglu, F. (2018). Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: a review. J. Renew. Sustain Ener. 10, 033104. doi: 10.1063/1.5025876
Brandt-Talbot, A., Gschwend, F. J., Fennell, P. S., Lammens, T. M., Tan, B., Weale, J., et al. (2017). An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 19, 3078–3102. doi: 10.1039/C7GC00705A
Brown, M. E., and Chang, M. C. (2014). Exploring bacterial lignin degradation. Curr. Opin Cheml Biol. 19, 1–7. doi: 10.1016/j.cbpa.2013.11.015
Capolupo, L., and Faraco, V. (2016). Green methods of lignocellulose pretreatment for biorefinery development. Appl. Microbiol. Biotechnol. 100, 9451–9467. doi: 10.1007/s00253-016-7884-y
Cardona, C. A., Quintero, J. A., and Paz, I. C. (2010). Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. 101, 4754–4766. doi: 10.1016/j.biortech.2009.10.097
Castoldi, R., Bracht, A., Morais, G. D., Baesso, M. L., Correa, R. C. G., Peralta, R. A., et al. (2014). Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 258, 240–246. doi: 10.1016/j.cej.2014.07.090
Chang, V. S., Burr, B., and Holtzapple, M. T. (1997). Biotechnology for Fuels and Chemicals. New York, NY: Humana Press.
Chen, H., Liu, J., Chang, X., Chen, D., Xue, Y., Liu, P., et al. (2017). A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 160, 196–206. doi: 10.1016/j.fuproc.2016.12.007
Chen, H. Z., and Liu, Z. H. (2015). Steam explosion and its combinatorial pretreatment refining technology of plant biomass to bio-based products. Biotechnol. J. 10, 866–885. doi: 10.1002/biot.201400705
Chen, S., Zhang, X., Singh, D., Yu, H., and Yang, X. (2010). Biological pretreatment of lignocellulosics: potential, progress and challenges. Biofuels 1, 177–199. doi: 10.4155/bfs.09.13
Chen, Y., Knappe, D. R., and Barlaz, M. A. (2004). Effect of cellulose/hemicellulose and lignin on the bioavailability of toluene sorbed to waste paper. Environ. Sci. Technol. 38, 3731–3736. doi: 10.1021/es035286x
Cherpozat, L., Loranger, E., and Daneault, C. (2017). Ultrasonic pretreatment effects on the bio-oil yield of a laboratory-scale slow wood pyrolysis. J. Anal. Appl. Pyrolysis. 126, 31–38. doi: 10.1016/j.jaap.2017.06.027
Daffalla, S. B., Hilmi, M., and Maizatul, S. S. (2012). Effect of organic and inorganic acid pretreatment on structural properties of rice husk and adsorption mechanism of phenol. Int. J. Chem. Environ. Eng. 3, 192–200.
Dahadha, S., Amin, Z., Bazyar Lakeh, A. A., and Elbeshbishy, E. (2017). Evaluation of different pretreatment processes of lignocellulosic biomass for enhanced biomethane production. Energy Fuels. 31, 10335–10347. doi: 10.1021/acs.energyfuels.7b02045
Dhiman, S. S., Haw, J. R., Kalyani, D., Kalia, V. C., Kang, Y. C., and Lee, J. K. (2014). Simultaneous pretreatment and saccharification: green technology for enhanced sugar yields from biomass using a fungal consortium. Bioresour. Technol. 179, 50–57. doi: 10.1016/j.biortech.2014.11.059
Du, H., Liu, C., Zhang, Y., Yu, G., Si, C., and Li, B. (2016). Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind. Crops Prod. 94, 736–745. doi: 10.1016/j.indcrop.2016.09.059
Du, W., Yu, H., Song, L., Zhang, J., Weng, C., Ma, F., et al. (2011). The promising effects of by-products from Irpex lacteus on subsequent enzymatic hydrolysis of bio-pretreated corn stalks. Biotechnol. Biofuels. 4:37. doi: 10.1186/1754-6834-4-37
Duan, D., Ruan, R., Wang, Y., Liu, Y., Dai, L., Zhao, Y., et al. (2018). Microwave-assisted acid pretreatment of alkali lignin: Effect on characteristics and pyrolysis behavior. Bioresour. Technol. 251, 57–62. doi: 10.1016/j.biortech.2017.12.022
Duque, A., Manzanares, P., and Ballesteros, M. (2017). Extrusion as a pretreatment for lignocellulosic biomass: fundamentals and applications. Renew Energy. 114, 1427–1441. doi: 10.1016/j.renene.2017.06.050
EIA. (2017). International Energy Outlook. Washington, DC: US Department of Energy, Energy Information administration
El-Naggar, N. E., Deraz, S., and Khalil, A. (2014). Bioethanol production from lignocellulosic feedstocks based on enzymatic hydrolysis: current status and recent developments. Biotechnology 13, 1–21. doi: 10.3923/biotech.2014.1.21
Farhat, W., Venditti, R. A., Hubbe, M., Taha, M., Becquart, F., and Ayoub, A. (2017). A review of water-resistant hemicellulose-based materials: processing and applications. ChemSusChem. 10, 305–323. doi: 10.1002/cssc.201601047
Fatriasari, W., Fajriutami, T., Laksana, R. B., and andWistara, N. J. (2018). Microwave assisted-acid hydrolysis of jabon kraft pulp. Waste Biomass Valorization. 9, 1–15. doi: 10.1007/s12649-017-0182-9
Fernandez-Fueyo, E., Ruiz-Duenas, F. J., JesusMartinez, M., Romero, A., Hammel, K. E., Javier Medrano, F., et al. (2014). Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol. Biofuels 7:2. doi: 10.1186/1754-6834-7-2
Fillat, U., Ibarra, D., Eugenio, M. E., Moreno, A. D., Pejo, E. T., and Sampedro, M. R. (2017). Laccases as a potential tool for the efficient conversion of lignocellulosic biomass: a review. Fermentation 3:17. doi: 10.3390/fermentation3020017
Germec, M., Demirel, F., Tas, N., Ozcan, A., Yilmazer, C., Onuk, Z., et al. (2017). Microwave-assisted dilute acid pretreatment of different agricultural bioresources for fermentable sugar production. Cellulose 24, 4337–4353. doi: 10.1007/s10570-017-1408-5
Ghorbani, M., Ahmadi, F., Rad, A. R., Zamiri, M. J., Cone, J. W., and Polikarpov, I. (2017). The effect of lime pre-treatments of date palm leaves on delignification and in vitro rumen degradability. J Agric Sci. 155, 184–190. doi: 10.1017/S0021859616000666
Giacobbea, S., Pezzellaa, C., Letteraa, V., Sanniaa, G., and Piscitellia, A. (2018). Laccase pretreatment for agrofood wastes valorization. Bioresour. Technol. 265, 59–65. doi: 10.1016/j.biortech.2018.05.108
Gu, B. J., Wang, J., Wolcott, M. P., and Ganjyal, G. M. (2018). Increased sugar yield from pre-milled Douglas-fir forest residuals with lower energy consumption by using planetary ball milling. Bioresour. Technol. 251, 93–98. doi: 10.1016/j.biortech.2017.11.103
Guerrero, A. B., Ballesteros, I., and Ballesteros, M. (2017). Optimal conditions of acid-catalysed steam explosion pretreatment of banana lignocellulosic biomass for fermentable sugar production. J. Chem. Technol. Biotechnol. 92, 2351–2359. doi: 10.1002/jctb.5239
Guerriero, G., Hausman, J. F., Strauss, J., Ertan, H., and Siddiqui, K. S. (2016). Lignocellulosic biomass: biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 16, 1–16. doi: 10.1002/elsc.201400196
Hammel, K. E., and Cullen, D. (2008). Role of fungal peroxidases in biological ligninolysis. Curr. Opin. Plant Biol. 11, 349–355. doi: 10.1016/j.pbi.2008.02.003
Hammell, K. E. (1997). ”Fungal degradation of lignin,” in Driven by Nature: Plant Litter Quality and Decomposition, ed. G. Cadisch (Madison, WI: CAB International), 33–45.
He, Z., Wang, Z., Zhao, Z., Yi, S., Mu, J., and Wang, X. (2017). Influence of ultrasound pretreatment on wood physiochemical structure. Ultrason. Sonochem. 34, 136–141. doi: 10.1016/j.ultsonch.2016.05.035
Heap, L., Green, A., Brown, D., van Dongen, B., and Turner, N. (2014). Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Cat. Sci. Technol. 4, 2251–2259. doi: 10.1039/C4CY00046C
Heredia-Olea, E., Pérez-Carrillo, E., Montoya-Chiw, M., and Serna-Saldívar, S. O. (2015). Effects of extrusion pretreatment parameters on sweet sorghum bagasse enzymatic hydrolysis and its subsequent conversion into bioethanol. Biomed Res Int. 2015:325905. doi: 10.1155/2015/325905
Hongdan, Z., Shaohua, X., and Shubin, W. (2013). Enhancement of enzymatic saccharification of sugarcane bagasse by liquid hot water pretreatment. Bioresour. Technol. 143, 391–396. doi: 10.1016/j.biortech.2013.05.103
Houtman, C. J., Maligaspe, E., Hunt, C. G., Fernández-Fueyo, E., Martínez, A. T., and Hammel, K. E. (2018). Fungal lignin peroxidase does not produce the veratryl alcohol cation radical as a diffusible ligninolytic oxidant. J. Biolog. Chem. 293, 4702–4712. doi: 10.1074/jbc.RA117.001153
Hussain, A., Arif, S. M., and Aslam, M. (2017). Emerging renewable and sustainable energy technologies: state of the art. Renew Sust Energ Rev. 71, 12–28. doi: 10.1016/j.rser.2016.12.033
Imman, S., Arnthong, J., Burapatana, V., Champreda, V., and Laosiripojana, N. (2014). Effects of acid and alkali promoters on compressed liquid hot water pretreatment of rice straw. Bioresour. Technol. 171, 29–36. doi: 10.1016/j.biortech.2014.08.022
Imman, S., Arnthong, J., Burapatana, V., Champreda, V., and Laosiripojana, N. (2015). Influence of alkaline catalyst addition on compressed liquid hot water pretreatment of rice straw. Chem. Eng. J. 278, 85–91. doi: 10.1016/j.cej.2014.12.032
Imman, S., Laosiripojana, N., and Champreda, V. (2018). Effects of liquid hot water pretreatment on enzymatic hydrolysis and physicochemical changes of corncobs. Appl. Biochem. Biotechnol. 184, 432–443. doi: 10.1007/s12010-017-2541-1
Irmak, S., Meryemoglu, B., Sandip, A., Subbiah, J., Mitchell, R. B., and Sarath, G. (2018). Microwave pretreatment effects on switchgrass and miscanthus solubilization in subcritical water and hydrolysate utilization for hydrogen production. Biomass Bioenergy. 108, 48–54. doi: 10.1016/j.biombioe.2017.10.039
Islam, S. M., Li, Q., Al Loman, A., and Ju, L. K. (2017). CO2-H2O based pretreatment and enzyme hydrolysis of soybean hulls. Enzyme Microb. Technol. 106, 18–27. doi: 10.1016/j.enzmictec.2017.06.011
Ivetić, D. Ž., Omorjan, R. P., ordevi,ć, T. R., and Antov, M. G. (2017). The impact of ultrasound pretreatment on the enzymatic hydrolysis of cellulose from sugar beet shreds: modeling of the experimental results. Environ. Prog. Sustain Energy. 36, 1164–1172. doi: 10.1002/ep.12544
Jagtap, S. S., Dhiman, S. S., Kim, T. S., Li, J., Kang, Y. C., and Lee, J. K. (2013a). Characterization of a β-1, 4-glucosidase from a newly isolated strain of Pholiota adiposa and its application to the hydrolysis of biomass. Biomass Bioenergy 54, 181–190. doi: 10.1016/j.biombioe.2013.03.032
Jagtap, S. S., Dhiman, S. S., Kim, T. S., Li, J., Lee, J. K., and Kang, Y. C. (2013b). Enzymatic hydrolysis of aspen biomass into fermentable sugars by using lignocellulases from Armillaria gemina. Bioresour. Technol. 133, 307–314. doi: 10.1016/j.biortech.2013.01.118
Janusz, G., Pawlik, A., Sulej, J., Urszula, S., Anna, S. B., Andrzej, J. W., et al. (2017). Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 41, 941–962. doi: 10.1093/femsre/fux049
Jedvert, K., and Heinze, T. (2017). Cellulose modification and shaping–a review. J. Polym. Eng. 37, 845–860. doi: 10.1515/polyeng-2016-0272
Jeong, S. Y., and Lee, J. W. (2016). Optimization of pretreatment condition for ethanol production from oxalic acid pretreated biomass by response surface methodology. Ind. Crops Prod. 79, 1–6. doi: 10.1016/j.indcrop.2015.10.036
Jönsson, L. J., and Martín, C. (2016). Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 199, 103–112. doi: 10.1016/j.biortech.2015.10.009
Jung, Y. H., Park, H. M., and Kim, K. H. (2015). Whole slurry saccharification and fermentation of maleic acid-pretreated rice straw for ethanol production. Bioprocess Biosyst Eng. 38, 1639–1644. doi: 10.1007/s00449-015-1405-8
Kalita, E., Nath, B. K., Agan, F., More, V., and Deb, P. (2015a). Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crops Prod. 65, 550–555. doi: 10.1016/j.indcrop.2014.10.004
Kalita, E., Nath, B. K., Deb, P., Agan, F., Islam, M. R., and Saikia, K. (2015b). High quality fluorescent cellulose nanofibers from endemic rice husk: isolation and characterization. Carbohydr Polym. 122, 308–313. doi: 10.1016/j.carbpol.2014.12.075
Kärcher, M. A., Iqbal, Y., Lewandowski, I., and Senn, T. (2015). Comparing the performance of Miscanthus x giganteus and wheat straw biomass in sulfuric acid based pretreatment. Bioresour. Technol. 180, 360–364. doi: 10.1016/j.biortech.2014.12.107
Kim, D. (2018). Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: a mini review. Molecules 23:309. doi: 10.3390/molecules23020309
Kim, H. J., Lee, S., Kim, J., Mitchell, R. J., and Lee, J. H. (2013). Environmentally friendly pretreatment of plant biomass by planetary and attrition milling. Bioresour. Technol. 144, 50–56. doi: 10.1016/j.biortech.2013.06.090
Kim, I., Seo, Y. H., Kim, G. Y., and Han, J. I. (2015). Co-production of bioethanol and biodiesel from corn stover pretreated with nitric acid. Fuel 143, 285–289. doi: 10.1016/j.fuel.2014.11.031
Kim, J. S., Lee, Y. Y., and Kim, T. H. (2016). A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 199, 42–48. doi: 10.1016/j.biortech.2015.08.085
Kim, M., and Day, D. F. (2011). Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 38, 803–807. doi: 10.1007/s10295-010-0812-8
Kim, Y., Mosier, N. S., and Ladisch, M. R. (2009). Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol Prog. 25, 340–348. doi: 10.1002/btpr.137
Ko, J. K., Um, Y., Park, Y. C., Seo, J. H., and Kim, K. H. (2015). Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl. Microbiol. Biotechnol. 99, 4201–4212. doi: 10.1007/s00253-015-6595-0
Koutsianitis, D., Mitani, C., Giagli, K., Tsalagkas, D., Halász, K., Kolonics, O., et al. (2015). Properties of ultrasound extracted bicomponent lignocellulose thin films. Ultrason. Sonochem. 23, 148–155. doi: 10.1016/j.ultsonch.2014.10.014
Kumar, A. K., and Sharma, S. (2017). Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess. 4, 7. doi: 10.1186/s40643-017-0137-9
Kumar, R., Sharma, R. K., and Singh, A. P. (2017). Cellulose based grafted biosorbents-Journey from lignocellulose biomass to toxic metal ions sorption applications-a review. J. Mol. Liq. 232, 62–93. doi: 10.1016/j.molliq.2017.02.050
Kumari, D., and Singh, R. (2018). Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 90, 877–891. doi: 10.1016/j.rser.2018.03.111
Lee, J. M., Shi, J., Venditti, R. A., and Jameel, H. (2009). Autohydrolysis pretreatment of coastal bermuda grass for increased enzyme hydrolysis. Bioresour. Technol. 100, 6434–6441. doi: 10.1016/j.biortech.2008.12.068
Lee, W. C., and Kuan, W. C. (2015). Miscanthus as cellulosic biomass for bioethanol production. Biotechnol. J. 10, 840–854. doi: 10.1002/biot.201400704
Li, H., Qu, Y., Yang, Y., Chang, S., and Xu, J. (2016). Microwave irradiation–A green and efficient way to pretreat biomass. Bioresour. Technol. 199, 34–41. doi: 10.1016/j.biortech.2015.08.099
Li, H. Q., Jiang, W., Jia, J. X., and Xu, J. (2014). pH pre-corrected liquid hot water pretreatment on corn stover with high hemicellulose recovery and low inhibitors formation. Bioresour. Technol. 153, 292–299. doi: 10.1016/j.biortech.2013.11.089
Li, J., Shi, S., Tu, M., Via, B., Sun, F. F., and Adhikari, S. (2018). Detoxification of Organosolv-Pretreated Pine Prehydrolysates with Anion Resin and Cysteine for Butanol Fermentation. Appl. Biochem. Biotechnol. 1-19. doi: 10.1007/s12010-018-2769-4
Li, K., Wan, J., Wang, X., Wang, J., and Zhang, J. (2016). Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: effect of Tween 80. Ind. Crops Prod. 83, 414–422. doi: 10.1016/j.indcrop.2016.01.003
Li, P., Cai, D., Luo, Z., Qin, P., Chen, C., Wang, Y., et al. (2016). Effect of acid pretreatment on different parts of corn stalk for second generation ethanol production. Bioresour. Technol. 206, 86–92. doi: 10.1016/j.biortech.2016.01.077
Li, X., Sun, C., Zhou, B., and He, Y. (2015). Determination of hemicellulose, cellulose and lignin in moso bamboo by near infrared spectroscopy. Sci Rep. 5, 17210. doi: 10.1038/srep17210
Liu, Y., Sun, B., Zheng, X., Yu, L., and Li, J. (2018). Integrated microwave and alkaline treatment for the separation between hemicelluloses and cellulose from cellulosic fibers. Bioresour. Technol. 247, 859–863. doi: 10.1016/j.biortech.2017.08.059
Liu, Y. R., Thomsen, K., Nie, Y., Zhang, S. J., and Meyer, A. S. (2016). Predictive screening of ionic liquids for dissolving cellulose and experimental verification. Green Chem. 18, 6246–6254. doi: 10.1039/C6GC01827K
Liyakathali, N. A. M., Muley, P. D., Aita, G., and Boldor, D. (2016). Effect of frequency and reaction time in focused ultrasonic pretreatment of energy cane bagasse for bioethanol production. Bioresour. Technol. 200, 262–271. doi: 10.1016/j.biortech.2015.10.028
Lizasoain, J., Trulea, A., Gittinger, J., Kral, I., Piringer, G., Schedl, A., et al. (2017). Corn stover for biogas production: effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 244, 949–956. doi: 10.1016/j.biortech.2017.08.042
Lloyd, T. A., and Wyman, C. E. (2005). Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 96, 1967–1977. doi: 10.1016/j.biortech.2005.01.011
Loow, Y. L., Wu, T. Y., Tan, K. A., Lim, Y. S., Siow, L. F., Md Jahim, J., et al. (2015). Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 63, 8349–8363. doi: 10.1021/acs.jafc.5b01813
Lu, P., and Hsieh, Y. L. (2012). Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 87, 564–573. doi: 10.1016/j.carbpol.2011.08.022
Luo, J., Fang, Z., and Smith Jr, R. L. (2014). Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 41, 56–93. doi: 10.1016/j.pecs.2013.11.001
Luzzi, S. C., Artifon, W., Piovesan, B., Tozetto, E., Mulinari, J., Kuhn, G. D. O., et al. (2017). Pretreatment of lignocellulosic biomass using ultrasound aiming at obtaining fermentable sugar. Biocatal. Biotransform. 35, 161–167. doi: 10.1080/10242422.2017.1310206
Ma, H. H., Zhang, B. X., Zhang, P., Li, S., Gao, Y. F., and Hu, X. M. (2016). An efficient process for lignin extraction and enzymatic hydrolysis of corn stalk by pyrrolidonium ionic liquids. Fuel Process. Technol. 148, 138–145. doi: 10.1016/j.fuproc.2016.02.038
Machado, A. D. S., and Ferraz, A. (2017). Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 225, 17–22. doi: 10.1016/j.biortech.2016.11.053
Mafe, O. A., Davies, S. M., Hancock, J., and Du, C. (2015). Development of an estimation model for the evaluation of the energy requirement of dilute acid pretreatments of biomass. Biomass Bioenergy. 72, 28–38. doi: 10.1016/j.biombioe.2014.11.024
Manickam, N. K., Rajarathinam, R., Muthuvelu, K. S., and Senniyappan, T. (2018). New insight into the effect of fungal mycelia present in the bio-pretreated paddy straw on their enzymatic saccharification and optimization of process parameters. Bioresour. Technol. 267, 291–302. doi: 10.1016/j.biortech.2018.07.003
Martinez, D., Larrondo, L. F., Putnam, N., Sollewijn Gelpke, M. D., Huang, K., Chapman, J., et al. (2004). Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat. Biotechnol. 22, 695–700. doi: 10.1038/nbt967
Mathew, A. K., Parameshwaran, B., Sukumaran, R. K., and Pandey, A. (2016). An evaluation of dilute acid and ammonia fiber explosion pretreatment for cellulosic ethanol production. Bioresour. Technol. 199, 13–20. doi: 10.1016/j.biortech.2015.08.121
Maurya, D. P., Singla, A., and Negi, S. (2015). An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 5, 597–609. doi: 10.1007/s13205-015-0279-4
Moodley, P., and Kana, E. G. (2017). Microwave-assisted inorganic salt pretreatment of sugarcane leaf waste: Effect on physiochemical structure and enzymatic saccharification. Bioresour. Technol. 235, 35–42. doi: 10.1016/j.biortech.2017.03.031
Morais, A. R., da Costa Lopes, A. M., and Bogel-Łukasik, R. (2014). Carbon dioxide in biomass processing: contributions to the green biorefinery concept. Chem. Rev. 115, 3–27. doi: 10.1021/cr500330z
Moro, M. K., Teixeira, R. S. S., da Silva, A. S. A., Fujimoto, M. D., Melo, P. A., Secchi, A. R., et al. (2017). Continuous pretreatment of sugarcane biomass using a twin-screw extruder. Ind Crops Prod. 97, 509–517. doi: 10.1016/j.indcrop.2016.12.051
Motte, J. C., Escudi,é, R., Hamelin, J., Steyer, J. P., Bernet, N., Delgenes, J. P., et al. (2014). Substrate milling pretreatment as a key parameter for solid-state anaerobic digestion optimization. Bioresour. Technol.173, 185–192. doi: 10.1016/j.biortech.2014.09.015
Mou, H., and Wu, S. (2017). Comparison of hydrothermal, hydrotropic and organosolv pretreatment for improving the enzymatic digestibility of bamboo. Cellulose. 24, 85–94. doi: 10.1007/s10570-016-1117-5
Nair, R. B., Lundin, M., Brandberg, T., Lennartsson, P. R., and Taherzadeh, M. J. (2015). Dilute phosphoric acid pretreatment of wheat bran for enzymatic hydrolysis and subsequent ethanol production by edible fungi Neurospora intermedia. Ind. Crops Prod. 69, 314–323. doi: 10.1016/j.indcrop.2015.02.038
Nakashima, K., Ebi, Y., Shibasaki-Kitakawa, N., Soyama, H., and Yonemoto, T. (2016). Hydrodynamic cavitation reactor for efficient pretreatment of lignocellulosic biomass. Ind. Eng. Chem. Res. 55, 1866–1871. doi: 10.1021/acs.iecr.5b04375
Narayanaswamy, N., Dheeran, P., Verma, S., and Kumar, S. (2013). “Biological pretreatment of lignocellulosic biomass for enzymatic saccharification,” in Pretreatment Techniques for Biofuels and Biorefineries. Green Energy and Technology, ed Z. Fang (Springer-Verlag Berlin Heidelberg), 3–34.
Narayanaswamy, N., Faik, A., Goetz, D. J., and Gu, T. (2011). Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour. Technol. 102, 6995–7000. doi: 10.1016/j.biortech.2011.04.052
Nayan, N., Sonnenberg, A. S. M., Hendriks, W. H., and Cone, J. W. (2018). Screening of white-rot fungi for bioprocessing of wheat straw into ruminant feed. J. Appl. Microbiol. 125, 468–479. doi: 10.1111/jam.13894
Negro, M. J., Alvarez, C., Ballesteros, I., Romero, I., Ballesteros, M., Castro, E., et al. (2014). Ethanol production from glucose and xylose obtained from steam exploded water-extracted olive tree pruning using phosphoric acid as catalyst. Bioresour. Technol. 153, 101–107. doi: 10.1016/j.biortech.2013.11.079
Negro, M. J., Duque, A., Manzanares, P., Sáez, F., Oliva, J. M., Ballesteros, I., et al. (2015). Alkaline twin-screw extrusion fractionation of olive-tree pruning biomass. Ind. Crops Prod. 74, 336–341. doi: 10.1016/j.indcrop.2015.05.018
Neves, P. V., Pitarelo, A. P., and Ramos, L. P. (2016). Production of cellulosic ethanol from sugarcane bagasse by steam explosion: Effect of extractives content, acid catalysis and different fermentation technologies. Bioresour. Technol. 208, 184–194. doi: 10.1016/j.biortech.2016.02.085
Ninomiya, K., Abe, M., Tsukegi, T., Kuroda, K., Omichi, M., Takada, K., et al. (2017). Ionic liquid pretreatment of bagasse improves mechanical property of bagasse/polypropylene composites. Ind. Crops Prod. 109, 158–162. doi: 10.1016/j.indcrop.2017.08.019
Oladi, S., and Aita, G. M. (2017). Optimization of liquid ammonia pretreatment variables for maximum enzymatic hydrolysis yield of energy cane bagasse. Ind. Crops Prod. 103, 122–132. doi: 10.1016/j.indcrop.2017.02.023
Oliva, J. M., Negro, M. J., Manzanares, P., Ballesteros, I., Chamorro, M. Á., Sáez, F., et al. (2017). A sequential steam explosion and reactive extrusion pretreatment for lignocellulosic biomass conversion within a fermentation-based biorefinery perspective. Fermentation 3:15. doi: 10.3390/fermentation3020015
Ooshima, H., Aso, K., Harano, Y., and Yamamoto, T. (1984). Microwave treatment of cellulosic materials for their enzymatic hydrolysis. Biotechnol. Lett. 6, 289–294. doi: 10.1007/BF00129056
Ostovareh, S., Karimi, K., and Zamani, A. (2015). Efficient conversion of sweet sorghum stalks to biogas and ethanol using organosolv pretreatment. Ind. Crops Prod. 66, 170–177. doi: 10.1016/j.indcrop.2014.12.023
Paul, S., and Dutta, A. (2018). Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recy. 130, 164–174. doi: 10.1016/j.resconrec.2017.12.005
Phanthong, P., Guan, G., Ma, Y., Hao, X., and Abudula, A. (2016). Effect of ball milling on the production of nanocellulose using mild acid hydrolysis method. J. Taiwan Inst. Chem. Eng. 60, 617–622. doi: 10.1016/j.jtice.2015.11.001
Pielhop, T., Amgarten, J., Rohr, P. R., and Studer, M. H. (2016). Steam explosion pretreatment of softwood: the effect of the explosive decompression on enzymatic digestibility. Biotechnol. Biofuels. 9:152. doi: 10.1186/s13068-016-0567-1
Piscitelli, A., Del Vecchio, C., Faraco, V., Giardina, P., MacEllaro, G., Miele, A., et al. (2011). Fungal laccases: versatile tools for lignocellulose transformation. C. R. Biol. 334, 789–794. doi: 10.1016/j.crvi.2011.06.007
Qing, Q., Huang, M., He, Y., Wang, L., and Zhang, Y. (2015). Dilute oxalic acid pretreatment for high total sugar recovery in pretreatment and subsequent enzymatic hydrolysis. Appl. Biochem. Biotechnol. 177, 1493–1507. doi: 10.1007/s12010-015-1829-2
Rabelo, S. C., Fonseca, N. A., Andrade, R. R., Maciel Filho, R., and Costa, A. C. (2011). Ethanol production from enzymatic hydrolysis of sugarcane bagasse pretreated with lime and alkaline hydrogen peroxide. Biomass Bioenergy. 35, 2600–2607. doi: 10.1016/j.biombioe.2011.02.042
Rabemanolontsoa, H., and Saka, S. (2016). Various pretreatments of lignocellulosics. Bioresour. Technol. 199, 83–91. doi: 10.1016/j.biortech.2015.08.029
Raj, T., Gaur, R., Lamba, B. Y., Singh, N., Gupta, R. P., Kumar, R., et al. (2018). Characterization of ionic liquid pretreated plant cell wall for improved enzymatic digestibility. Bioresour. Technol. 249, 139–145. doi: 10.1016/j.biortech.2017.09.202
Rajendran, K., Drielak, E., Varma, V. S., Muthusamy, S., and Kumar, G. (2017). Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production–a review. Biomass Conver. Biorefin. 8, 471–483. doi: 10.1007/s13399-017-0269-3
Ravichandran, A., and Sridhar, M. (2016). Versatile peroxidases: super peroxidases with potential biotechnological applications-a mini review. J. Dairy Vet. Anim. Res. 4, 277–280. doi: 10.15406/jdvar.2016.04.00116
Ravindran, R., and Jaiswal, A. K. (2016). A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour. Technol. 199, 92–102. doi: 10.1016/j.biortech.2015.07.106
Reddy, K. O., Maheswari, C. U., Dhlamini, M. S., Mothudi, B. M., Kommula, V. P., Zhang, J., et al. (2018). Extraction and characterization of cellulose single fibers from native african napier grass. Carbohydr Polym. 188, 85–91. doi: 10.1016/j.carbpol.2018.01.110
Rico, A., Rencoret, J., Río, J. C. D., Martínez, A., and Gutiérrez, A. (2014). Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol. Biofuels 7:6. doi: 10.1186/1754-6834-7-6
Romaní, A., Ruiz, H. A., Teixeira, J. A., and Domingues, L. (2016). Valorization of Eucalyptus wood by glycerol-organosolv pretreatment within the biorefinery concept: an integrated and intensified approach. Renew Energy. 95, 1–9. doi: 10.1016/j.renene.2016.03.106
Rostagno, M. A., Prado, J. M., Mudhoo, A., Santos, D. T., Forster–Carneiro, T., and Meireles, M. A. A. (2015). Subcritical and supercritical technology for the production of second generation bioethanol. Crit. Rev. Biotechnol. 35, 302–312. doi: 10.3109/07388551.2013.843155
Sahoo, D., Ummalyma, S. B., Okram, A. K., Pandey, A., Sankar, M., and Sukumaran, R. K. (2018). Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 253, 252–255. doi: 10.1016/j.biortech.2018.01.048
Sakuragi, K., Igarashi, K., and Samejima, M. (2018). Application of ammonia pretreatment to enable enzymatic hydrolysis of hardwood biomass. Polym. Degrad. Stab. 148, 19–25. doi: 10.1016/j.polymdegradstab.2017.12.008
Salapa, I., Katsimpouras, C., Topakas, E., and Sidiras, D. (2017). Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy. 100, 10–16. doi: 10.1016/j.biombioe.2017.03.011
Salapa, I., Topakas, E., and Sidiras, D. (2018). Simulation and optimization of barley straw organosolv pretreatment. Ind. Crops Prod. 113, 80–88. doi: 10.1016/j.indcrop.2018.01.018
Sannigrahi, P., Miller, S. J., and Ragauskas, A. J. (2010). Effects of organosolv pretreatment and enzymatic hydrolysis on cellulose structure and crystallinity in Loblolly pine. Carbohydr. Res. 345, 965–970. doi: 10.1016/j.carres.2010.02.010
Saritha, M., Arora, A., and Nain, L. (2012). Pretreatment of paddy straw with Trametes hirsuta for improved enzymatic saccharification. Bioresour. Technol. 104, 459–465. doi: 10.1016/j.biortech.2011.10.043
Scholl, A. L., Menegol, D., Pitarelo, A. P., Fontana, R. C., Zandoná Filho, A., Ramos, L. P., et al. (2015). Elephant grass pretreated by steam explosion for inducing secretion of cellulases and xylanases by Penicillium echinulatum S1M29 solid-state cultivation. Ind. Crops Prod. 77, 97–107. doi: 10.1016/j.indcrop.2015.08.051
Seidl, P. R., and Goulart, A. K. (2016). Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr Opin Green Sustain Chem. 2, 48–53. doi: 10.1016/j.cogsc.2016.09.003
Serna, L. D., Alzate, C. O., and Alzate, C. C. (2016). Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 199, 113–120. doi: 10.1016/j.biortech.2015.09.078
Shah, T. A., and Tabassum, R. (2018). Enhancing biogas production from lime soaked corn cob residue. Int. J. Renew. Energy Res. 8, 761–766.
Shen, J., Zhao, C., Liu, G., and Chen, C. (2017). Enhancing the performance on anaerobic digestion of vinegar residue by sodium hydroxide pretreatment. Waste Biomass Valorizat. 8, 1119–1126. doi: 10.1007/s12649-016-9666-2
Shetty, D. J., Kshirsagar, P., Tapadia-Maheshwari, S., Lanjekar, V., Singh, S. K., and Dhakephalkar, P. K. (2017). Alkali pretreatment at ambient temperature: a promising method to enhance biomethanation of rice straw. Bioresour. Technol. 226, 80–88. doi: 10.1016/j.biortech.2016.12.003
Shirkavand, E., Baroutian, S., Gapes, D. J., and Young, B. R. (2016). Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment–a review. Renew. Sust. Energ. Rev. 54, 217–234. doi: 10.1016/j.rser.2015.10.003
Sindhu, R., Binod, P., and Pandey, A. (2016). Biological pretreatment of lignocellulosic biomass – an overview. Bioresour. Technol. 199,76–82. doi: 10.1016/j.biortech.2015.08.030
Sindhu, R., Kuttiraja, M., Binod, P., Sukumaran, R. K., and Pandey, A. (2014). Bioethanol production from dilute acid pretreated Indian bamboo variety (Dendrocalamus sp.) by separate hydrolysis and fermentation. Ind. Crops Prod. 52, 169–176. doi: 10.1016/j.indcrop.2013.10.021
Singh, J., Suhag, M., and Dhaka, A. (2015). Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohydr. Polym. 117, 624–631. doi: 10.1016/j.carbpol.2014.10.012
Smith, E. L., Abbott, A. P., and Ryder, K. S. (2014). Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114, 11060–11082. doi: 10.1021/cr300162p
Smuga-Kogut, M., Zgórska, K., Kogut, T., Kukiełka, K., Wojdalski, J., Kupczyk, A., et al. (2017). The use of ionic liquid pretreatment of rye straw for bioethanol production. Fuel 191, 266–274. doi: 10.1016/j.fuel.2016.11.066
Song, L., Yu, H., Ma, F., and Zhang, X. (2013). Biological pretreatment under nonsterile conditions for enzymatic hydrolysis of corn stover. Bioresour. Com 8, 3802–3816. doi: 10.15376/biores.8.3.3802-3816
Stanton, J., Xue, Y., Pandher, P., Malek, L., Brown, T., Hu, X., et al. (2018). Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials. Int. J. Biol. Macromol. 108, 333–341. doi: 10.1016/j.ijbiomac.2017.11.137
Subhedar, P. B., and Gogate, P. R. (2014). Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochem. 21, 216–225. doi: 10.1016/j.ultsonch.2013.08.001
Suhara, H., Kodama, S., Kamei, I., Maekawa, N., and Meguro, S. (2012). Screening of selective lignin-degrading basidiomycetes and biological pretreatment for enzymatic hydrolysis of bamboo culms. Int. Biodeter. Biodegr. 75, 176–180. doi: 10.1016/j.ibiod.2012.05.042
Sun, F. F., Wang, L., Hong, J., Ren, J., Du, F., Hu, J., et al. (2015). The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour. Technol. 187, 354–361. doi: 10.1016/j.biortech.2015.03.051
Sun, F. F., Zhao, X., Hong, J., Tang, Y., Wang, L., Sun, H., et al. (2016). Industrially relevant hydrolyzability and fermentability of sugarcane bagasse improved effectively by glycerol organosolv pretreatment. Biotechnol Biofuels. 9:59. doi: 10.1186/s13068-016-0472-7
Sun, J. X., Xu, F., Sun, X. F., Xiao, B., and Sun, R. C. (2005). Physico-chemical and thermal characterization of cellulose from barley straw. Polym. Degrad. Stab. 88, 521–531. doi: 10.1016/j.polymdegradstab.2004.12.013
Sun, S., Sun, S., Cao, X., and Sun, R. (2016). The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 199, 49–58. doi: 10.1016/j.biortech.2015.08.061
Sun, Y. G., Ma, Y. L., Wang, L. Q., Wang, F. Z., Wu, Q. Q., and Pan, G. Y. (2015). Physicochemical properties of corn stalk after treatment using steam explosion coupled with acid or alkali. Carbohydr. Polym. 117, 486–493. doi: 10.1016/j.carbpol.2014.09.066
Swatloski, R. P., Spear, S. K., Holbrey, J. D., and Rogers, R. D. (2002). Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 124, 4974–4975. doi: 10.1021/ja025790m
Taha, M., Shahsavari, E., Hothaly, K. A., Mouradov, A., Smith, A. T., Ball, A. S., et al. (2015). Enhanced biological straw saccharification through coculturing oflignocellulose-degrading microorganisms. Appl. Biochem. Biotechnol. 7:6. doi: 10.1007/s12010-015-1539-9
Talebnia, F., Karakashev, D., and Angelidaki, I. (2010). Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 101, 4744–4753. doi: 10.1016/j.biortech.2009.11.080
Talha, Z., Ding, W., Mehryar, E., Hassan, M., and Bi, J. (2016). Alkaline pretreatment of sugarcane bagasse and filter mud codigested to improve biomethane production. Biomed Res Int. 2016. doi: 10.1155/2016/8650597
Tang, C., Chen, Y., Liu, J., Shen, T., Cao, Z., Shan, J., et al. (2017a). Sustainable biobutanol production using alkali-catalyzed organosolv pretreated cornstalks. Ind. Crops Prod. 95, 383–392. doi: 10.1016/j.indcrop.2016.10.048
Tang, C., Shan, J., Chen, Y., Zhong, L., Shen, T., Zhu, C., et al. (2017b). Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin. Bioresour. Technol. 232, 222–228. doi: 10.1016/j.biortech.2017.02.041
Tang, Y., Dou, X., Hu, J., Jiang, J., and Saddler, J. N. (2018). Lignin sulfonation and SO2 addition enhance the hydrolyzability of deacetylated and then steam-pretreated poplar with reduced inhibitor formation. Appl. Biochem. Biotechnol. 184, 264–277. doi: 10.1007/s12010-017-2545-x
Tayyab, M., Noman, A., Islam, W., Waheed, S., Arafat, Y., Ali, F., et al. (2018). Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: a review. Appl. Ecol. Env. Res. 16, 225–249. doi: 10.15666/aeer/1601_225249
Teramura, H., Sasaki, K., Oshima, T., Kawaguchi, H., Ogino, C., Sazuka, T., et al. (2017). Effective usage of sorghum bagasse: optimization of organosolv pretreatment using 25% 1-butanol and subsequent nanofiltration membrane separation. Bioresour. Technol. 252, 157–164. doi: 10.1016/j.biortech.2017.12.100
Tian, L., Branford-White, C., Wang, W., Nie, H., and Zhu, L. (2012). Laccase-mediated system pretreatment to enhance the effect of hydrogen peroxide bleaching of cotton fabric. Int. J. Biol. Macromol. 50, 782–787. doi: 10.1016/j.ijbiomac.2011.11.025
Vats, S., Maurya, D. P., Shaimoon, M., Agarwal, A., and Negi, S. (2013). Development of a microbial consortium for the production of blend enzymes for the hydrolysis of agricultural waste into sugars. J. Sci. Ind. Res. 72, 585–590.
Verardi, A., Blasi, A., Marino, T., Molino, A., and Calabr,ò, V. (2018). Effect of steam-pretreatment combined with hydrogen peroxide on lignocellulosic agricultural wastes for bioethanol production: Analysis of derived sugars and other by-products. J. Energy Chem. 27, 535–543. doi: 10.1016/j.jechem.2017.11.007
Vergara, P., Wojtusik, M., Revilla, E., Ladero, M., Garcia-Ochoa, F., and Villar, J. C. (2018). Wheat straw fractionation by ethanol-water mixture: Optimization of operating conditions and comparison with diluted sulfuric acid pre-treatment. Bioresour. Technol. 256, 178–186. doi: 10.1016/j.biortech.2018.01.137
Vivekanand, V., Olsen, E. F., Eijsink, V. G., and Horn, S. J. (2013). Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour. Technol. 127, 343–349. doi: 10.1016/j.biortech.2012.09.118
Wagle, D. V., Zhao, H., and Baker, G. A. (2014). Deep eutectic solvents: sustainable media for nanoscale and functional materials. Acc. Chem. Res. 47, 2299–2308. doi: 10.1021/ar5000488
Wagner, A. O., Lackner, N., Mutschlechner, M., Prem, E. V., Markt, R., and Illmer, P. (2018). Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 11:1797. doi: 10.3390/en11071797
Wahid, R., Hjorth, M., Kristensen, S., and Møller, H. B. (2015). Extrusion as pretreatment for boosting methane production: effect of screw configurations. Energy Fuels. 29, 4030–4037. doi: 10.1021/acs.energyfuels.5b00191
Wan, C., and Li, Y. (2010). Microbial delignificant of corn strover with Ceriporiopsis subvermispora for improving cellulose digestibility. Enzyme Microb. Technol. 47, 31–36. doi: 10.1016/j.biortech.2010.03.070
Wan, C., and Li, Y. (2012). Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv. 30, 1447–1457. doi: 10.1016/j.biotechadv.2012.03.003
Wan, C., Zhou, Y., and Li, Y. (2011). Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 102, 6254–6259. doi: 10.1016/j.biortech.2011.02.075
Wang, Y., Duan, D., Liu, Y., Ruan, R., Fu, G., Dai, L., et al. (2017). Properties and pyrolysis behavior of moso bamboo sawdust after microwave-assisted acid pretreatment. J. Anal. Appl. Pyrolysis. 129, 86–92. doi: 10.1016/j.jaap.2017.11.024
Wang, Z., Wu, G., and Jönsson, L. J. (2018). Effects of impregnation of softwood with sulfuric acid and sulfur dioxide on chemical and physical characteristics, enzymatic digestibility, and fermentability. Bioresour. Technol. 247, 200–208. doi: 10.1016/j.biortech.2017.09.081
Wong, D. W. (2009). Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 157, 174–209. doi: 10.1007/s12010-008-8279-z
Xu, C., and Ferdosian, F. (2017). Conversion of Lignin into Bio-Based Chemicals and Materials. Berlin: Springer Heidelberg
Xu, G. C., Ding, J. C., Han, R. Z., Dong, J. J., and Ni, Y. (2016). Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 203, 364–369. doi: 10.1016/j.biortech.2015.11.002
Yesilada, O., Birhanli, E., and Geckil, H. (2018). “Bioremediation and decolorization of textile dyes by white rot fungi and laccase enzymes”, in Mycoremediation and Environmental Sustainability, Fungal Biology, ed R. Prasad (Cham: Springer), 121–153
Yin, J., Hao, L., Yu, W., Wang, E., Zhao, M., Xu, Q., et al. (2014). Enzymatic hydrolysis enhancement of corn lignocellulose by supercritical CO2 combined with ultrasound pretreatment. Chinese J. Catal. 35, 763–769. doi: 10.1016/S1872-2067(14)60040-1
Yoo, C. G., Pu, Y., and Ragauskas, A. J. (2017). Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain Chem. 5, 5–11. doi: 10.1016/j.cogsc.2017.03.003
Zakaria, M. R., Hirata, S., Fujimoto, S., and Hassan, M. A. (2015a). Combined pretreatment with hot compressed water and wet disk milling opened up oil palm biomass structure resulting in enhanced enzymatic digestibility. Bioresour. Technol. 193, 128–134. doi: 10.1016/j.biortech.2015.06.074
Zakaria, M. R., Norrrahim, M. N. F., Hirata, S., and Hassan, M. A. (2015b). Hydrothermal and wet disk milling pretreatment for high conversion of biosugars from oil palm mesocarp fiber. Bioresour. Technol. 181, 263–269. doi: 10.1016/j.biortech.2015.01.072
Zamocky, M., Gasselhuber, B., Furtmüller, P. G., and Obinger, C. (2014). Turning points in the evolution of peroxidase-catalase superfamily: molecular phylogeny of hybrid heme peroxidises. Cell Mol. Life Sci. 71, 4681–4696. doi: 10.1007/s00018-014-1643-y
Zavarzina, A. G., Lisov, A. V., and Leontievsky, A. A. (2018). The role of ligninolytic enzymes laccase and a versatile peroxidase of the white-rot fungus Lentinus tigrinus in biotransformation of soil humic matter: comparative in vivo study. J. Geophys. Res Biogeosci. 123, 2727–2742. doi: 10.1029/2017jg004309
Zdanowicz, M., Wilpiszewska, K., and Spychaj, T. (2018). Deep eutectic solvents for polysaccharides processing. a review. Carbohydr. Polym. 200, 361–380. doi: 10.1016/j.carbpol.2018.07.078
Zhang, C. W., Xia, S. Q., and Ma, P. S. (2016). Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 219, 1–5. doi: 10.1016/j.biortech.2016.07.026
Zhang, H., Zhang, S., Yuan, H., Lyu, G., and Xie, J. (2018). FeCl3-catalyzed ethanol pretreatment of sugarcane bagasse boosts sugar yields with low enzyme loadings and short hydrolysis time. Bioresour. Technol. 249, 395–401. doi: 10.1016/j.biortech.2017.10.053
Zhang, K., Pei, Z., and Wang, D. (2016). Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour. Technol. 199, 21–33. doi: 10.1016/j.biortech.2015.08.102
Zhang, Q., Hu, J., and Lee, D. J. (2017). Pretreatment of biomass using ionic liquids: research updates. Renew Energy. 111, 77–84. doi: 10.1016/j.renene.2017.03.093
Zhao, B. H., Chen, J., Yu, H. Q., Hu, Z. H., Yue, Z. B., and Li, J. (2017). Optimization of microwave pretreatment of lignocellulosic waste for enhancing methane production: Hyacinth as an example. Front. Environ. Sci. Eng. 11, 17. doi: 10.1007/s11783-017-0965-z
Zhao, C., Ding, W., Chen, F., Cheng, C., and Shao, Q. (2014). Effects of compositional changes of AFEX-treated and H-AFEX-treated corn stover on enzymatic digestibility. Bioresour. Technol. 155, 34–40. doi: 10.1016/j.biortech.2013.12.091
Zhao, S., Li, G., Zheng, N., Wang, J., and Yu, Z. (2018). Steam explosion enhances digestibility and fermentation of corn stover by facilitating ruminal microbial colonization. Bioresour. Technol. 253, 244–251. doi: 10.1016/j.biortech.2018.01.024
Zheng, Y., Zhao, J., Xu, F., and Li, Y. (2014). Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 42, 35–53. doi: 10.1016/j.pecs.2014.01.001
Zhou, S., Raouche, S., Grisel, S., Sigoillot, J., and Herpoël-Gimbert, I. (2017). Efficient biomass pretreatment using the white-rot fungus polyporus brumalis. Fungal. Genom. Biol. 7:2. doi: 10.4172/2165-8056.1000150
Zhou, X., Li, W., Mabon, R., and Broadbelt, L. J. (2017). A critical review on hemicellulose pyrolysis. Energy Technol. 5, 52–79. doi: 10.1002/ente.201600327
Zhuang, X., Wang, W., Yu, Q., Qi, W., Wang, Q., Tan, X., et al. (2016). Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour. Technol. 199, 68–75. doi: 10.1016/j.biortech.2015.08.051
Keywords: lignocellulosic biomass, pretreatment, hydrolysis, enzymatic breakdown, cellulose extraction, delignification, biorefinery
Citation: Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC and Kalita E (2018) Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 6:141. doi: 10.3389/fenrg.2018.00141
Received: 18 August 2018; Accepted: 03 December 2018;
Published: 18 December 2018.
Edited by:
Sujit Jagtap, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Saurabh Dhiman, South Dakota School of Mines and Technology, United StatesShashi Kant Bhatia, Konkuk University, South Korea
Copyright © 2018 Baruah, Nath, Sharma, Kumar, Deka, Baruah and Kalita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eeshan Kalita, ZWthbGl0YUB0ZXp1LmVybmV0Lmlu
 Julie Baruah
Julie Baruah Bikash Kar Nath
Bikash Kar Nath Ritika Sharma
Ritika Sharma Sachin Kumar
Sachin Kumar Ramesh Chandra Deka2
Ramesh Chandra Deka2 Deben Chandra Baruah
Deben Chandra Baruah Eeshan Kalita
Eeshan Kalita