- 1Department of Biochemistry and Molecular Biology, University of Nevada Reno, Reno, NV, USA
- 2Department of Civil and Environmental Engineering, University of Nevada Reno, Reno, NV, USA
Microalgae offer great potential as a third-generation biofuel feedstock, especially when grown on wastewater, as they have the dual application for wastewater treatment and as a biomass feedstock for biofuel production. The potential for growth on wastewater centrate was evaluated for forty microalgae strains from fresh (11), brackish (11), or saltwater (18) genera. Generally, freshwater strains were able to grow at high concentrations of centrate, with two strains, Neochloris pseudostigmata and Neochloris conjuncta, demonstrating growth at up to 40% v/v centrate. Fourteen of 18 salt water Dunaliella strains also demonstrated growth in centrate concentrations at or above 40% v/v. Lipid profiles of freshwater strains with high-centrate tolerance were determined using gas chromatography–mass spectrometry and compared against those obtained on cells grown on defined maintenance media. The major lipid compounds were found to be palmitic (16:0), oleic (18:1), and linoleic (18:2) acids for all freshwater strains grown on either centrate or their respective maintenance medium. These results demonstrate the highly concentrated wastewater can be used to grow microalgae, which limits the need to dilute wastewater prior to algal production. In addition, the algae produced generate lipids suitable for biodiesel or green diesel production.
Introduction
The world’s demand for petroleum fuels continues to grow even as supplies dwindle. In recent years, there has been a strong push to develop alternative energy sources to help supplement or potentially replace fossil fuels. Wind turbines and photovoltaic technologies offer renewable sources of electric power; however, liquid fuels for the transportation sector make up more than 70% of the energy consumed in US (Forsberg, 2009). Biofuels have great potential to help fill this need, and there has been significant research in this area. First- and second-generation liquid biofuels, such as corn ethanol and soy biodiesel, have received considerable interest and resources in the last 20 years, and are considered technically mature. Third-generation biofuels, such as lignocellulosics and microalgae, although not as technologically advanced, hold great promise as sustainable biofuels because they avoid the food versus fuel debate that has plagued corn and soy-based biofuels. As part of the Renewable Fuel Standard II, the US government has tapped the third-generation biofuels for 21 × 109 barrels/year of fuel by 2022, with 4 × 109 barrels/year of that coming from non-cellulosic and non-corn-based biodiesel fuels, such as those derived from microalgae (Schnepf and Yacobucci, 2010).
As with many third-generation fuels, microalgae have great potential as a biofuel feedstock source. They are among the most rapidly growing photosynthetic organisms on the planet (Chisti, 2007), and they can be cultured year-round in even cold climates if growth is coupled to a low-cost heat source (i.e., waste or low-grade geothermal). Large-scale production of algae biomass has been demonstrated, but several technical hurdles remain that must be addressed before microalgae can become a viable biofuel feedstock. Currently, biomass harvesting and oil extraction are key processing steps that are energy intensive and cost prohibitive. From the algal growth perspective, a sustainable water source and nutrient supply are paramount to economic microalgae production. Regardless of the strain used (salt or fresh water), a non-saline water supply will be required to make up for evaporative loss in an open-pond cultivation system. Lastly, off-setting part or all of the nutrient supply required to grow microalgae with non-petroleum-derived fertilizer such as municipal wastewater can help improve the carbon budget of microalgal-based biofuel production systems (Chisti, 2007; Wang et al., 2010; Bhatt et al., 2014; Dong et al., 2014; Mu et al., 2014).
Wastewater offers the possibility of serving as both a freshwater and a nutrient source, with most municipalities having a continuous supply. Ideally, the wastewater could be used in its raw state or with minimal treatment so as to reduce the costs of the wastewater treatment plant (WWTP) (Pittman et al., 2011; Bhatt et al., 2014; Mu et al., 2014). Centrate, the liquid fraction after anaerobic digestion, offers a feed stream high in nitrogen and phosphorous, which are two of the main nutrients required for microalgae and also two compounds that cause high removal costs for most municipal WWTPs (Wang et al., 2010; Li et al., 2011a; Bhatt et al., 2014; Dong et al., 2014; Mu et al., 2014). In addition to decreasing the nutrient load to the plant, diverting centrate to algae production also decreases the treatment volume, thereby decreasing treatment costs of other chemical constituents (Li et al., 2011a; Mu et al., 2014). Metal content of wastewaters or centrate are unlikely to inhibit microalgal growth (Wang et al., 2010; Dong et al., 2014).
In this work, centrate was used as a water and nutrient source for the growth of green algae as a biofuel feedstock. A total of 40 microalgae strains comprised of isolates from freshwater, brackish water, and saltwater strains were evaluated for tolerance to and growth in centrate. The growth characteristics, biomass and lipid yields, and lipid profiles were determined for the two most tolerant freshwater strains, Neochloris conjuncta and Neochloris pseudostigmata, to evaluate the potential of utilizing municipal wastewater centrate to grow microalgae as a biofuel feedstock.
Materials and Methods
Strains and Media
Eleven freshwater Neochloris strains, 11 brackish water Nannochloropsis strains, and 18 saltwater Dunaliella strains were evaluated for their ability to grow on municipal wastewater centrate (Table 1). The cultures were obtained from the Culture Collection of Algae at The University of Texas at Austin (UTEX), the Culture Collection of Algae and Protozoa (CCAP), the Canadian Phycological Culture Centre [CPCC; formerly known as the University of Toronto Culture Collection of Algae and Cyanobacteria (UTCC)], or the Culture Collection of Algae at the University of Göttingen, Germany (SAG). All cultures were grown at room temperature under an 18/6 h light/dark cycle on their respective maintenance media. The freshwater Neochloris strains were maintained on Bold’s modified Bristol medium (Bold, 1949): 2.94 mM NaNO3, 0.17 mM CaCl2 (2H2O), 0.3 mM MgSO4 (7H2O), 0.43 mM K2HPO4, 1.29 mM KH2PO4, and 0.43 mM NaCl at pH = 7.7. Saltwater Dunaliella strains were maintained on a modified 2ASW (artificial seawater) medium (Gomord et al., 2010); 33.6 g/L of sea salts were used in place of the NaCl. The brackish water Nannochloropsis strains were maintained on slightly modified f/2 Medium of Guillard and Ryther (1962) in which the vitamin solution from the 2ASW medium (Gomord et al., 2010), which contained additional vitamin components, was used in place of that described for the original f/2 medium.
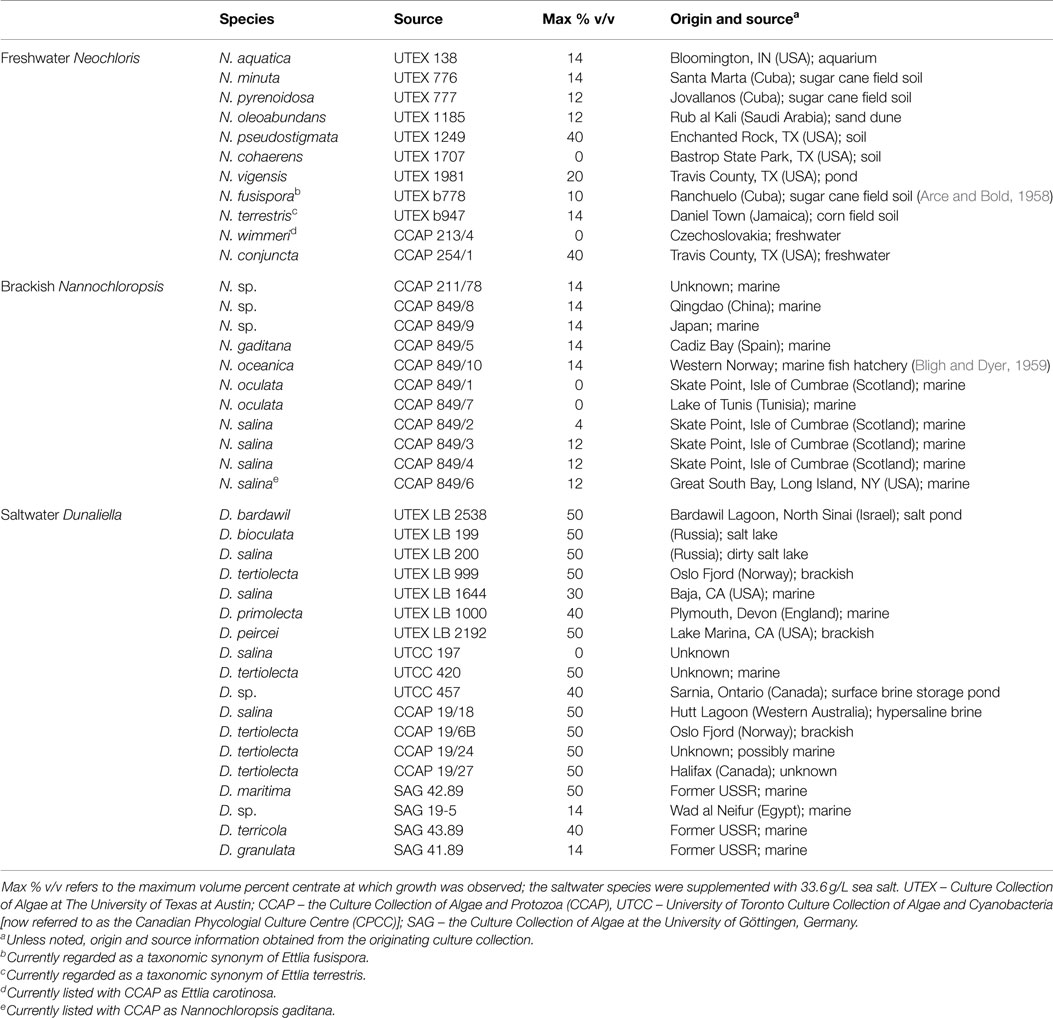
Table 1. The 40 strains evaluated, their original culture collection source, and the maximum centrate concentration at which growth was observed for each strain in preliminary plate screening tests.
Centrate Characteristics
Centrate was collected from the Truckee Meadows Water Authority Reclamation Facility, the local municipal WWTP facility located in Sparks, Nevada. Centrate was filtered through Miracloth (typical pore size 22–25 μm, EMD Millipore), then autoclaved and stored at 4°C until use (<3 days). Average nitrogen, phosphorus, and potassium content of centrate from this facility have been previously reported as 1003.0 ± 174.6 mg/L NH4-N, 244.5 ± 34.5 mg/L ortho-P, and 202 ± 54 mg/L K+, respectively (Herrera, 2009). The raw centrate had an average pH = 7.8 and contained 1.284 mg/L TDS and 726.2 mg/L bicarbonate, and the major ions by concentration were sodium (77.0 ± 36.5 mg/L) and chloride (189.2 ± 132.4 mg/L). A detailed characterization of the centrate can be found in Herrera (2009).
Preliminary Screening
All strains were screened initially for their tolerance to grow on centrate using a 96-well titer plate format. Plates were loaded with 200 μL of medium and 25 μL of inoculum culture per well and then incubated at 22°C and a 18/6 h light/dark cycle under 100 μmol/m2 s on an orbital shaking table rotating at 100 rpm. For the Neochloris and Nannochloropsis strains, the medium consisted of centrate diluted with nanopure water to 0, 2, 4, 6, 8, 10, 12, 14, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, and 100% v/v. The Dunaliella strains were screened with the same centrate concentrations, but dilutions were made with a 33.6 g/L sea-salt solution, as no growth was observed without the addition of sea salts (data not shown). The appropriate maintenance media and sterile water were used as positive and negative growth medium controls, respectively. Each culture was inoculated in duplicate on two plates (four total replicates) to allow for statistical testing power. The 96-well titer plates were monitored visually to monitor growth (Figure S1 in Supplementary Material).
Microalgae Growth
The two freshwater strains with the highest centrate tolerance, N. pseudostigmata (UTEX 1249) and N. conjuncta (CCAP 254/1), were grown in 2-L Erlenmeyer flasks to determine growth characteristics. Optical density (measured as absorbance at 600 nm) was used to monitor the cell density of the cultures and to determine the growth phase of the culture. Maximum growth rates were determined by plotting the A600 values versus time (Figures S2 and S3 in Supplementary Material) and taking the slope of growth rate plot during the exponential growth phase. Each medium was prepared by mixing the filtered and autoclaved centrate with nanopure water to 10, 25, or 40% v/v centrate, then the pH adjusted to 7.7 with 0.1N HCl or NaOH as required. Bold’s modified Bristol medium (Bold, 1949) at pH = 7.7 was also prepared and used as a baseline. Each batch of medium was divided into 1.5 L aliquots, transferred to the flasks, and then autoclaved. After sterilization, flasks were inoculated with 30 mL of mid-log phase culture and then stoppered with a bubbler apparatus (sterile two-holed stopper with two glass tubes with a small amount of cotton batting). Cultures were grown in a growth chamber (Conviron PGR15) with a 18/6 h light/dark cycle at 26/20°C and 140 μmol/m2 s light supplied by a mixture of fluorescent and incandescent lamps. Using an aquarium pump, atmospheric air was pumped through a 2-L flask with sterile water, which served to both filter and humidify the air, and then was split into eight flasks using a manifold. The air bubbles served as both a CO2 source and agitation for the flasks.
Centrate Acclimation Testing
In a separate experiment, cells were grown to mid-log phase in Bold’s modified Bristol medium (Sandnes et al., 2006) or in 10, 25, or 40% v/v centrate. To evaluate the effects of pre-acclimation, 30 mL of each of the liquid cultures were transferred to 2-L Erlenmeyer flasks with their respective fresh media. The cultures were grown and monitored as described above.
Harvesting, Drying, and Lipid Extraction
The algae were harvested via centrifugation (6000 × g for 5 min) in early stationary phase (3–5 days after log phase) and the algae paste was then stored at −80°C. The samples were lyophilized to remove all moisture and dry weights were determined. The dried samples were then re-suspended in water (1 mL/g dry algae) overnight at 4°C. A modified Bligh and Dyer (1959) extraction method was used to extract the lipids (and other chloroform-soluble components). Modifications include centrifugation-assisted phase separation (2800 × g for 10 min) and a second extraction on the non-lipid phase to ensure complete extraction. Chloroform was evaporated under a stream of nitrogen and dry weights of the lipids were determined.
FAME Preparation and GC/MS
Fatty acid profiles were determined via GC/MS analysis of fatty acid methyl esters (FAMEs). Briefly, the dried lipid extracts were esterified to FAMEs using the rapid BF3-methanol esterification procedure (Metcalfe et al., 1966). After esterification, samples were dried completely under nitrogen then re-suspended in 1 mL carbon disulfide. FAME profiles were obtained by the Nevada Proteomics Center using GC/MS (Thermo Polaris Q) on an Agilent HP – INNOWAX column (P/N 19091N-136; 60 m × 0.250 mm, 0.25 μm film thickness) using helium as the carrier gas at 1.0 mL/min constant flow and a split ratio of 1:10 (split flow 10 mL/min) with 1 μL injection volumes. The GC was operated with an inlet temperature of 225°C and a column temperature starting at 180°C and ramping at 5°C/min to 240°C with a 30 min hold. The transfer line temperature between the GC and the MS was maintained at 250°C. The MS was operated in Full Scan mode with a mass range of 40–450 m/z at 70 eV and an ion source temperature of 200°C. Chromatograms and spectra were analyzed using XCalibur (Thermo Fisher Scientific, v1.3).
Results and Discussion
Preliminary Centrate Screening
Preliminary screening of the various microalgae strains revealed a wide range of tolerance to centrate (Table 1). Autoclaved centrate was used to avoid the possible complicating influence of undefined microbial flora on algal growth. Sterile centrate has been used in several other investigations (Wang et al., 2010; Li et al., 2011a; Zhu et al., 2013; Dong et al., 2014). Of the 11 freshwater Neochloris strains, two (N. cohaerens and N. wimmeri) were unable to grow in the presence of any centrate, while two strains (N. conjuncta and N. pseudostigmata) were tolerant of concentrations up to 40% v/v. Growth was observed at maximum centrate concentrations between 10 and 20% v/v for the remaining seven strains. The brackish water Nannochloropsis strains had a lower centrate tolerance, with growth observed at a maximum of 14% v/v centrate for five strains and two strains unable to grow in the presence of any centrate. This low centrate tolerance might possibly be due to a lack of salts in the growth medium (centrate diluted with nanopure water) rather than as a function of the centrate concentration itself. As expected, saltwater Dunaliella strains were unable to grow when the centrate lacked salt and was diluted using nanopure water alone (data not shown). However, when sea salts were added to match the salt concentration of the 2ASW (33.6 g/L) to the diluting water, all but one of the saltwater strains (D. salina UTC 197) were able to grow at 14% v/v or greater centrate (Table 1). Of the 18 species or strains tested, 11 grew at 50% v/v centrate, and an additional 3 grew at 40% v/v centrate levels. Interestingly, of four different D. salina strains (e.g., UTEX LB 200, UTEX LB 1644, UTCC 197, and CCAP 19/18) evaluated, two UTEX strains (LB 200 and LB 1644) and CCAP 19/18 all demonstrated high-centrate tolerance, while UTCC 197 was the only Dunaliella strain unable to grow in centrate. Recent work suggests that UTEX 200 should not be designated as D. salina based on physiological and molecular markers (Ben-Amotz et al., 2009).
These tolerances to centrate were lower than those reported previously for several other algal species and strains (Wang et al., 2010; Li et al., 2011a; Zhu et al., 2013). However, such differences might be due to differences in centrate characteristics arising from the unique inputs and processing steps of a particular WWTP. Wang and Lan (2011) reported growth with a wild-type Chlorella sp. on a 100% centrate medium, which contained 72 mg/L total N, and would correspond to ~7% v/v centrate used in this work on a total N basis. Further evaluation with this same centrate source found a total of fourteen strains from genera Chlorella, Haematococcus, Scenedesmus, Chlamydomonas, and Chloroccum that were able to grow on the pure centrate (Li et al., 2011b). From the present work, the two freshwater strains (N. conjuncta and N. pseudostigmata) were considered for further evaluation because of their high-centrate tolerance and no need for additional salt supplementation.
Microalgal Growth
The N. conjuncta and N. pseudostigmata were grown in 2-L flasks with modified Bristol medium and various concentrations of centrate. No significant differences (p > 0.05) were observed in the maximum growth rates for either species (Table 2). For both species, no significant difference in the acclimation period was observed between modified Bristol medium and 10% v/v centrate. However, significant time lag periods in growth were observed for both species when the centrate concentration was increased to 25% v/v (p < 0.01), and additional lags were observed when the concentration was increased to 40% (p < 0.01 for N. conjuncta; p < 0.05 for N. pseudostigmata). At the highest centrate concentrations, a delay of more than 40 days was observed for both species. This increase in the lag time with increasing centrate concentration suggested the presence of a compound(s) that inhibit microalgal growth in the centrate, although the cells were able to grow given enough time. The identity of the putative inhibitor(s) is unknown, but likely candidates might include ammonia or urea (Moazeni, 2013). Additional studies are needed to verify the presence of ammonia or urea in centrate and to test this possibility in future studies. However, neither metals nor ammonia ions were reported to be likely candidates for toxicity responses to centrate (Dong et al., 2014).
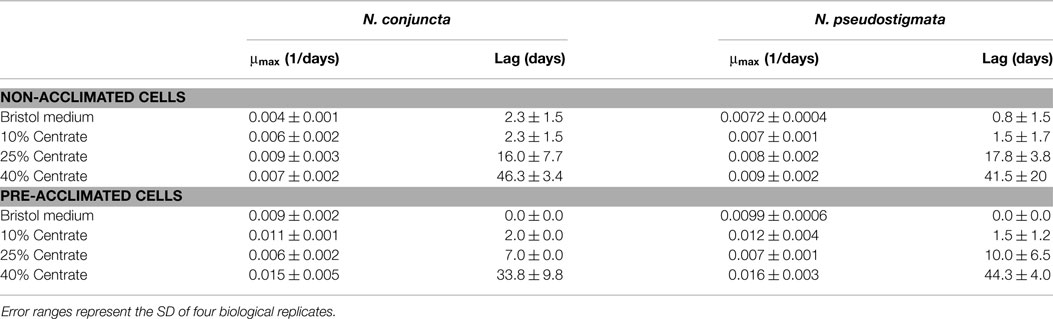
Table 2. Maximum growth rate (μmax) and observed lag time of N. conjuncta and N. pseudostigmata grown in 10, 25, and 40% v/v centrate and in Bold’s modified Bristol medium (Bold, 1949).
The long lag period in growth at elevated centrate concentrations would be problematic for large-scale cultivation, especially in open-pond systems, due to the prolonged opportunities for contamination of the cultures by other species (Chisti, 2007; Bhatt et al., 2014). To evaluate whether the cells could be acclimating to the inhibiting compound(s), and thereby decrease the observed lag time, cells exposed to centrate were used as inoculum for serial growth trials. For these tests, N. conjuncta and N. pseudostigmata grown in either Bold’s modified Bristol medium (Bold, 1949) or in 10, 25, and 40% v/v centrate were inoculated into fresh batches of their respective medium and monitored (Table 2; Figures S2 and S3 in Supplementary Material). In general, the maximum growth rates of the pre-acclimated cells increased relative to the non-acclimated cells. As expected, no difference in the lag time was observed with the cells grown in Bristol medium and the 10% v/v centrate for either species. However, the average lag times for the pre-acclimated N. conjuncta decreased by 9 and 12.5 days when grown on 25 and 40% v/v centrate, respectively. The trend was not as strong with the pre-acclimated N. pseudostigmata cells, although the average lag time of those grown on 25% v/v centrate decreased 7.8 days (Table 2; Figures S2 and S3 in Supplementary Material). These results suggest that the inhibitory compound found in centrate is one that the freshwater Neochloris species may be able to acclimate to, and a further reduction in the lag time might be achieved with additional acclimation cycles.
Biomass Production and Productivity
The N. conjuncta and N. pseudostigmata cultures were harvested via centrifugation ~5 days after reaching stationary phase, as determined by A600 measurements. After harvesting, the cell pellets were frozen and then water removed via lyophilization. For both species, the dry biomass obtained (normalized to the culture volume harvested) increased with increasing centrate concentrations (Figure 1A). Biomass concentrations ranged over nearly an order of magnitude, from 0.045 to 0.404 g dry biomass/L for N. conjuncta and from 0.107 to 0.544 g dry biomass/L for N. pseudostigmata. The N. conjuncta cultures grown on centrate all had significantly (p = 0.0026) more biomass than the Bristol culture, whereas only the N. pseudostigmata cultures grown on the two higher centrate concentrations had significantly (p < 0.05 for 25% v/v; p < 0.01 for 40% v/v) more biomass. Because centrate has a very high nutrient content (e.g., ~1000 mg/L N and ~200 mg/L P), the greater biomass amounts were expected.
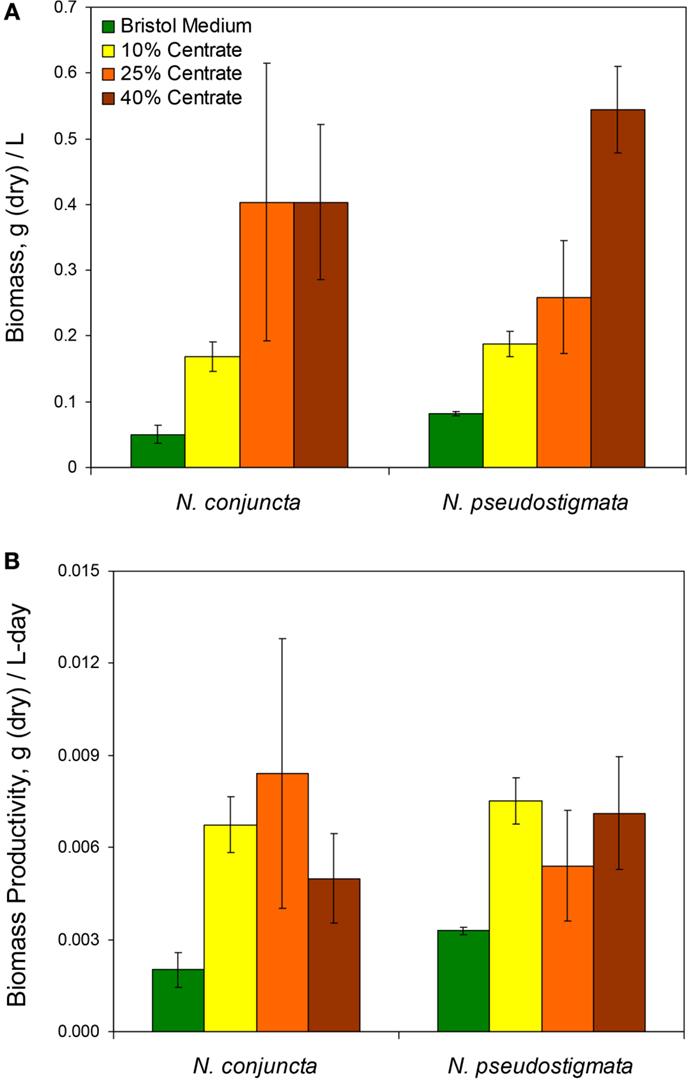
Figure 1. (A) Measured total biomass at harvest and (B) calculated biomass productivity of N. conjuncta and N. pseudostigmata grown in 10, 25, and 40% v/v centrate and in Bold’s modified Bristol medium (Bold, 1949). Error bars represent the SD of four biological replicates.
Despite the greater biomass amounts with increasing centrate concentrations, the amount of dry biomass obtained per day did not appear to be affected by the centrate concentration (Figure 1B). There was no significant difference (p > 0.05) for either species across the various centrate concentrations, with productivity values ranging from 0.0050 to 0.0084 g dry biomass/L-day. However, biomass productivity of N. conjuncta was significantly (p = 0.012) lower at 0.0018 g dry biomass/L-day when grown on Bristol medium. These values were lower than those obtained by Wang and Lan (2011) who reported a biomass productivity of 0.233 g dry biomass/L-day with N. oleoabundans grown on secondary municipal wastewater effluents enriched with nitrogen. Sun et al. (2014) also reported higher biomass productivities of 0.113 g dry biomass/L-day for N. oleoabundans grown in basal SE medium. Although biomass was not measured for the cultures inoculated with pre-acclimated cells, the reduction in lag times with the higher centrate concentrations would be expected to result in higher biomass productivities due to the shorter cultivation times. However, biomass productivities can vary as a result of other influences, such as mixing of the cultures, CO2 supplementation, light intensity, and carbon source.
Lipid Production and Productivity
Unlike the biomass, the mass of lipids produced (per mass of dry biomass) was found to decrease with increasing centrate concentrations for both Neochloris species (Figure 2A). The N. conjuncta grown in Bristol medium was found to have a lipid content (1.07 + ∕− 0.4 g lipid/g dry biomass) significantly (p = 0.0004) higher than any other species/medium combination, which ranged from 0.5 to 50 g lipid/g dry biomass. These values agree with the 0.27 (Sun et al., 2014), 0.50 (Griffiths et al., 2012), and 0.52 (Gouveia et al., 2009) g lipid/g dry biomass reported for N. oleoabundans.
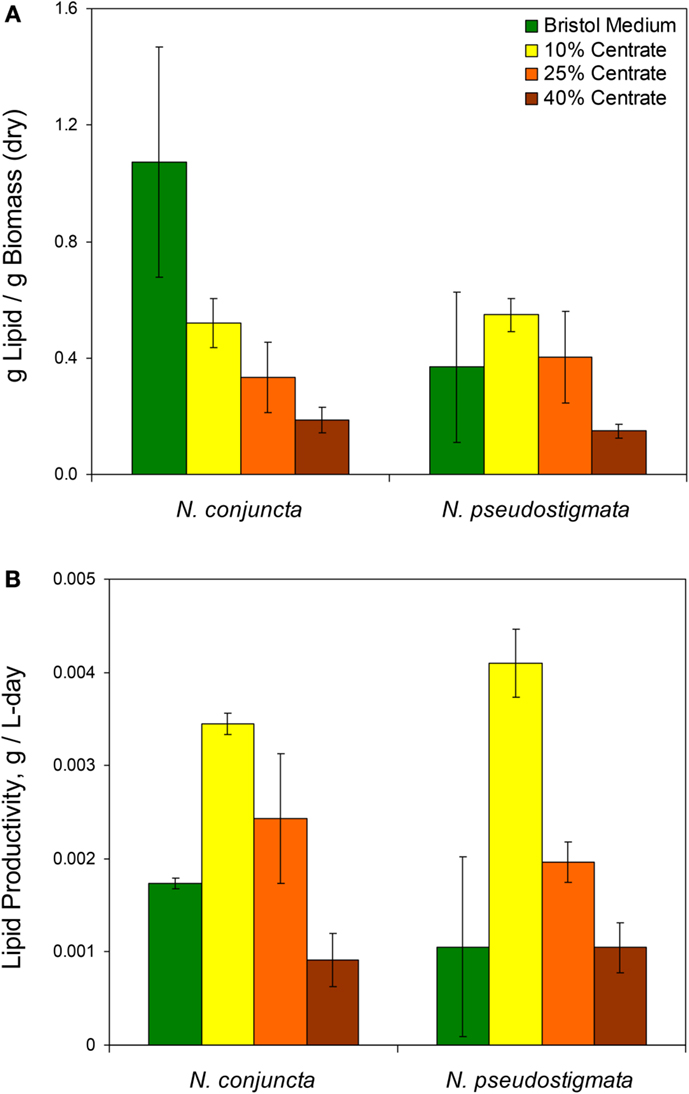
Figure 2. (A) Lipid content and (B) calculated lipid productivity of N. conjuncta and N. pseudostigmata grown in 10, 25, and 40% v/v centrate and in Bold’s modified Bristol medium (Bold, 1949). Error bars represent the SD of four biological replicates.
The lipid productivity also decreased with increasing centrate concentrations for both species (Figure 2B). Interestingly, the highest lipid productivity (0.0041 g lipid/L-day, N. pseudostigmata) was observed with 10% v/v centrate rather than on the Bristol medium with both species. The lowest productivities (0.001 g lipid/g dry biomass) were observed with 40% v/v centrate. This range is similar to the 0.003 g lipid/L-day (Sun et al., 2014) and 0.0029 g lipid/L-day (Griffiths et al., 2012) reported for N. oleoabundans under nitrogen-limited conditions. As mentioned previously, the lipid productivities would be expected to increase with the higher centrate concentrations when pre-acclimated cells are used. Therefore, centrate-grown cultures all would likely have greater lipid productivities than those grown in Bristol medium.
In addition to the total lipid measurements, fatty acid profiles were obtained for the two species grown with Bristol medium and various centrate concentrations with the most prevalent fatty acids presented in Figure 3. The dominant fatty acids for both Neochloris species were palmitic (C16:0), oleic (C18:1), linoleic (C18:2), and linolenic (C18:3). Although the lipid profiles varied by species, the profiles in Figure 3 agree generally with what others have observed for various Neochloris species (Griffiths et al., 2012; Sun et al., 2014) and other green algae (Islam et al., 2013). The most notable differences between the lipid profiles of N. conjuncta was the significant increase in C18:1 (p < 0.01) and decrease in C18:3 (p < 0.05) when changing from Bristol medium to centrate (Figure 3A). There were no significant differences in any of the other fatty acids with any growth media for the N. conjuncta. Interestingly, very similar changes (e.g., C18:1 increase, C18:3 decrease) were observed when going from nitrogen-replete to nitrogen-limited conditions using N. oleoabundans (Griffiths et al., 2012). Lipid accumulation in microalgae is known to occur as a result of stress events, including nutrient starvation, temperature or pH shock, or light limitation (Wang et al., 2010; Li et al., 2011a; Sharma et al., 2012). With these experiments, the cultures were grown until early stationary phase (based on A600) before harvesting, which corresponds to ~5 days of growth when at least one nutrient was limiting. The differences in lipid productivity observed between the cells grown in Bristol medium and the centrate solutions are possibly due to a different nutrient becoming limited. Xin et al. (2010) demonstrated that Scenedesmus subjected to nitrogen starvation had a 30% increase in lipids, whereas the same cells subjected to phosphorus starvation had a 53% increase, suggesting that nutrient stress affects the degree of lipid accumulation (Xin et al., 2010).
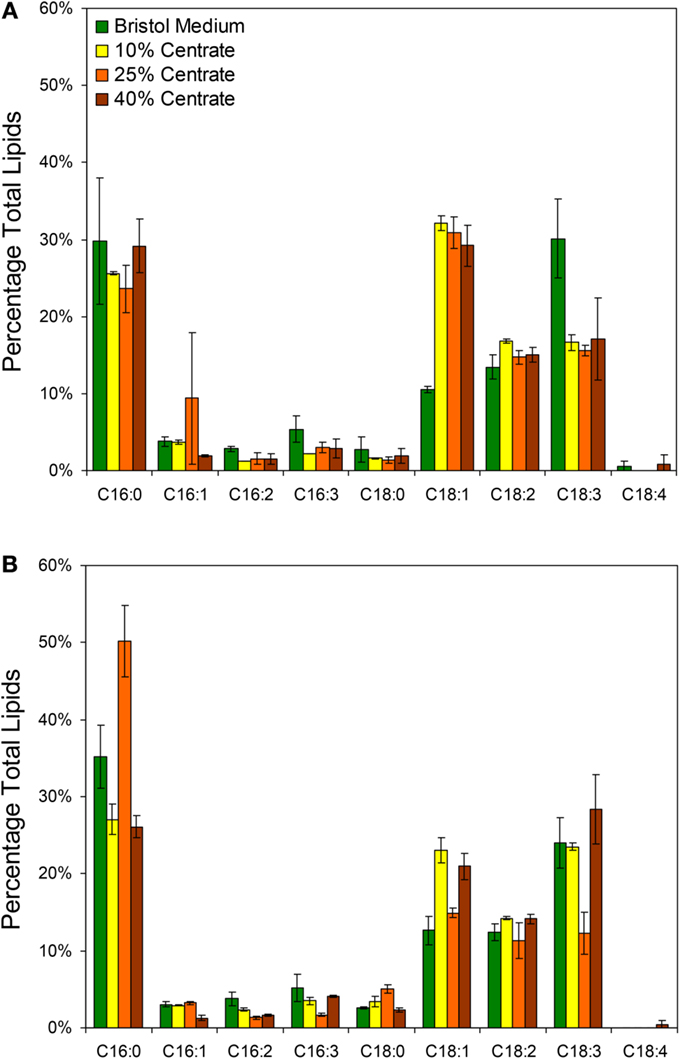
Figure 3. Lipid profiles based on GC/MS analysis of FAMEs from (A) N. conjuncta and (B) N. pseudostigmata grown in 10, 25, and 40% v/v centrate and in Bold’s modified Bristol medium (Bold, 1949). Error bars represent the SD of two biological replicates.
The N. pseudostigmata profiles had more variability (Figure 3B), but a significant decrease in C18:1 (p < 0.05) was observed in the Bristol medium compared to the 10 and 40% v/v centrate cultures. The increase in C18:3 in Bristol was not observed, as was the case with N. conjuncta. Instead, a decrease in C18:3 with corresponding increases in C16:0 and C16:3 were observed for the N. pseudostigmata culture grown in 25% v/v centrate. Sun et al. (2014) also observed increases in C16:0 accumulation with increasing nutrient stress duration for N. oleoabundans; however, they also found an increase in C18:1, which is in contradiction of what was observed here for N. conjuncta.
Conclusion
Wastewater centrate offers a promising alternative water source for the cultivation of microalgae for biofuels production. Forty microalgae species were screened for tolerance; two freshwater species were found to grow in up to 40% v/v centrate and multiple saltwater species could grow in up to 50% v/v centrate when supplemented with sea salt. Despite these promising results, many of the strains evaluated were very sensitive to centrate and failed to grow at even low concentrations, and even those strains with high tolerance to centrate had increased lag times with increasing centrate concentration. This lag time could be partially reduced by pre-acclimating the cells to the centrate. Using the two freshwater Neochloris strains as models, improvements in both biomass productivity and lipid productivity were observed when the cells were grown on centrate, relative to defined maintenance medium. The lipid profiles of the microalgae grown with centrate and with the maintenance medium were similar, although a significant increase in C18:1 and significant decrease in C18:3 were observed in N. conjuncta. Screening of additional microalgae strains should be continued, especially of environmental strains with pre-exposure to the centrate, to identify those strains that can be grown with higher centrate concentrations and also those strains with high lipid content to be used for biofuel feedstock production. The identification of microalgal species or strains adapted to the nutrient profile of a particular waste stream would likely be less expensive than the potential costs associated with supplementing the nutrient profile of a particular waste stream in order to attain optimal growth. Lastly, high performing strains should be evaluated in larger volumes and in raceway ponds or photobioreactors to further evaluate their applicability for full-scale use.
Author Contributions
SRH conducted the research, designed experiments, and wrote the bulk of the manuscript. MSL assisted with experimental design and experimentation and assisted with the preparation of the manuscript. BPK assisted with centrate testing studies, contributed to interpretation of results, and assisted with the preparation of the manuscript. JCC initiated research on wastewater algae, conceived of the study, helped design experiments, and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Financial support was provided by Department of Energy’s Nevada Renewable Energy Consortium (Grant DE-EE0000272). The Nevada Proteomics Center receives financial support from Nevada INBRE, which is funded by NIH Grant number P20 RR-016464 from the INBRE Program of the National Center for Research Resources. The authors would also like to thank Rebecca Albion for all of her assistance in the lab and Rebekah Woolsey for performing the GC-MS analyses.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fenrg.2015.00020
References
Arce, G., and Bold, H. (1958). Some Chlorophyceae from Cuban soils. Am. J. Bot. 45, 492–503. doi: 10.2307/2439186
Ben-Amotz, A., Polle, J., and Rao, D. (2009). The Alga Dunaliella: Biodiversity, Physiology, Genomics, and Biotechnology. Enfield, NH: Science Publishers, Inc.
Bhatt, N., Panwar, A., Bisht, T., and Tamta, S. (2014). Coupling of algal biofuel production with wastewater. ScientificWorldJournal Article ID 210504, 10. doi:10.1155/2014/210504
Bligh, E., and Dyer, W. (1959). A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharmacol. 37, 911–917. doi:10.1139/y59-099
Bold, H. (1949). The morphology of Chlamydomonas chlamydogama sp. nov. Bull. Torrey Botanical Club 76, 101–108. doi:10.2307/2482218
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306. doi:10.1016/j.biotechadv.2007.02.001
Dong, B., Ho, N., Ogden, K., and Arnold, R. (2014). Cultivation of Nannochloropsis salina in municipal wastewater or digester centrate. Ecotoxicol. Environ. Saf. 103, 45–53. doi:10.1016/j.ecoenv.2014.02.001
Forsberg, C. (2009). Sustainability by combining nuclear, fossil, and renewable energy sources. Prog. Nucl. Energy 51, 192–200. doi:10.1016/j.pnucene.2008.04.002
Gomord, V., Fitchette, A., Menu-Bouaouiche, L., Saint-Jore-Dupas, C., Plasson, C., Michaud, D., et al. (2010). Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 8, 564–587. doi:10.1111/J.1467-7652.2009.00497.X
Gouveia, L., Marques, A., Da Silva, T., and Reis, A. (2009). Neochloris oleoabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J. Ind. Microbiol. Biotechnol. 36, 821–826. doi:10.1007/s10295-009-0559-2
Griffiths, M., Van Hille, R., and Harrison, T. (2012). Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 24, 989–1001. doi:10.1007/s10811-011-9723-y
Guillard, R., and Ryther, J. (1962). Studies of marine planktonic diatoms. Cyclotella nana Hustedt and Detonula confervaceae (Cleve) Gran. Can. J. Microbiol. 8, 229–239. doi:10.1139/m62-029
Herrera, N. (2009). Analysis of Centrate Composition and Evaluation of its Applicability as a Nutrient Supplement to Irrigation Water. M.S. Reno: University of Nevada.
Islam, M., Magnusson, M., Brown, R., Ayoko, G., Nabi, M., and Heimann, K. (2013). Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 6, 5676–5702. doi:10.3390/en6115676
Xin, L., Hong-ying, H., Ke, G., and Ying-xue, S. (2010). Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 101, 5494–5500. doi:10.1016/j.biortech.2010.02.016
Li, Y., Chen, Y.-F., Chen, P., Min, M., Zhou, W., Martinez, B., et al. (2011a). Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 102, 5138–5144. doi:10.1016/j.biortech.2011.01.091
Li, Y., Zhou, W., Hu, B., Min, M., Chen, P., and Ruan, R. (2011b). Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: strains screening and significance evaluation of environmental factors. Bioresour. Technol. 102, 10861–10867. doi:10.1016/j.biortech.2011.09.064
Metcalfe, L., Schmitz, A., and Pelka, J. (1966). Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 38, 514–515. doi:10.1021/ac60235a044
Moazeni, F. (2013). Investigating the Feasibility of Growing Algae for Fuel in Southern Nevada. Ph.D. Dissertation Las Vegas: University of Nevada.
Mu, D., Min, M., Krohn, B., Mullins, K., Ruan, R., and Hill, J. (2014). Life cycle environmental impacts of wastewater-based algal biofuels. Environ. Sci. Technol. 48, 11696–11704. doi:10.1021/es5027689
Pittman, J., Dean, A., and Osundeko, O. (2011). The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 102, 17–25. doi:10.1016/j.biortech.2010.06.035
Sandnes, J., Ringstad, T., Wenner, D., Heyerdahl, P., Kallqvist, I., and Gislerod, H. (2006). Real-time monitoring and automatic density control of large-scale microalgal cultures using near infrared (NIR) optical density sensors. J. Biotechnol. 122, 209–215. doi:10.1016/j.jbiotec.2005.08.034
Schnepf, R., and Yacobucci, B. (2010). Renewable Fuel Standard (RFS): Overview and Issues. Washington, DC: Congressional Research Service.
Sharma, K., Schuhmann, H., and Schenk, P. (2012). High lipid induction in microalgae for biodiesel production. Energies 5, 1532–1553. doi:10.3390/en5051532
Sun, X., Cao, Y., Xu, H., Liu, Y., Sun, J., Qiao, D., et al. (2014). Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour. Technol. 155, 204–212. doi:10.1016/j.biortech.2013.12.109
Wang, B., and Lan, C. (2011). Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour. Technol. 102, 5639–5644. doi:10.1016/j.biortech.2011.02.054
Wang, L., Min, M., Li, Y., Chen, P., Chen, Y., Liu, Y., et al. (2010). Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 162, 1174–1186. doi:10.1007/s12010-009-8866-7
Keywords: microalgae, fresh water, brackish water, salt water, biofuel, municipal wastewater, centrate
Citation: Hiibel SR, Lemos MS, Kelly BP and Cushman JC (2015) Evaluation of diverse microalgal species as potential biofuel feedstocks grown using municipal wastewater. Front. Energy Res. 3:20. doi: 10.3389/fenrg.2015.00020
Received: 16 February 2015; Accepted: 17 April 2015;
Published: 11 May 2015
Edited by:
Umakanta Jena, Desert Research Institute, USAReviewed by:
Arumugam Muthu, Council of Scientific and Industrial Research, IndiaProbir Das, Qatar University, Qatar
Sivasubramanian Velusamy, Phycospectrum Environmental Research Centre, India
Copyright: © 2015 Hiibel, Lemos, Kelly and Cushman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John C. Cushman, Department of Biochemistry and Molecular Biology, University of Nevada Reno, MS 330, 1664 N. Virginia Street, Reno, NV 89557-0330, USA,amN1c2htYW5AdW5yLmVkdQ==
 Sage R. Hiibel
Sage R. Hiibel Mark S. Lemos
Mark S. Lemos Brian P. Kelly
Brian P. Kelly John C. Cushman
John C. Cushman