- 1EndoLab Laboratory, Centre of Postgraduate Medical Education, Warsaw, Poland
- 2Department of Endocrinology, Centre of Postgraduate Medical Education, Warsaw, Poland
- 3Doctoral School of Translational Medicine, Centre of Postgraduate Medical Education, Warsaw, Poland
- 4Department of Endocrinology, Karolinska University Hospital, Stockholm, Sweden
- 5Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden
Primary aldosteronism is the most common cause of secondary hypertension, yet most cases remain unrecognized and left without optimal treatment. The diagnostic inertia may be attributed to the lack of specific symptoms, insufficient awareness among physicians, still conflicting indications for screening for primary aldosteronism and first and foremost challenging diagnostics. This review describes the current challenges of biochemical diagnostics of primary aldosteronism, including screening, case confirmation and subtyping. It also discusses immunoassays widely used in assessment of suspected autonomous aldosterone secretion – recent advances in the field and limitations of the method in comparison to the gold standard - liquid chromatography –tandem mass spectrometry. The review focuses on the application of novel “omics” strategies in the diagnostics of primary aldosteronism. Steroidomics and proteomics offer a possibility to simultaneously assess steroids and protein/peptides on a large scale. This multianalyte approach in comparison to the selective quantification of a chosen compound has been proved useful in the diagnostics of primary aldosteronism. It also offers a unique insight into the individual characteristics, underlying mechanisms and even reflects the genetic alterations of primary aldosteronism cases. The “omics” techniques are associated with large amounts of generated data, the interpretation of which may be troublesome and often necessitates the use of artificial intelligence. The novel advances in the biochemical diagnostics of primary aldosteronism, including “omics” techniques, presented in this review may help to address the most emerging problems, increase the number of diagnosed patients and facilitate the choice of an optimal treatment.
1 Introduction
Primary aldosteronism (PA) is caused by autonomous aldosterone secretion from the zona glomerulosa of adrenal cortex (1). Aldosterone excess leads to increased sodium and water resorption in the distal tubule and collecting duct of the nephron (1, 2). Water and sodium overload suppresses renin release from the renal juxtaglomerular cells (1). Thus, increased aldosterone-to-renin ratio, together with hypertension and hypokalemia, is the hallmark of PA. The aldosterone-to-renin ratio is widely used in screening for PA.
PA is the most common curable form of secondary hypertension, yet it remains widely unrecognized, with fewer than 2% of patients at-risk ever tested and half of those patients diagnosed and treated (1, 3, 4). The reasons why PA is widely overlooked include lack of awareness among clinicians, absence of characteristic symptoms and often burdensome multi-step diagnostics and subtyping process (1). Patients at younger age, with high systolic and diastolic blood pressure (BP), and hypokalemia are more likely to be screened for PA than older patients with relevant comorbidities (5).
The problem of PA underdetection is even more prominent when taken into consideration that the detrimental effects of aldosterone excess are partially independent from the influence of the hypertension caused by PA (1, 6, 7). Patients with PA are more prone to develop left ventricle hypertrophy, increased aortic stiffness, dysfunction of endothelium, albuminuria and hyperfiltration, when compared to BP-matched controls (7–10). PA is also associated with higher prevalence of metabolic syndrome and type 2 diabetes mellitus (7). Furthermore, aldosterone excess contributes to increased urinary calcium loss and hypocalcemia, which translates into higher prevalence of bone fractures and osteoporosis in patients with PA (11, 12).
The established diagnostic workup for PA is a costly, complex process that usually requires multiple visits before the diagnosis is finally confirmed. Thus, mainly patients with high probability of PA and unequivocal diagnostic results undergo screening. Further diagnostics (including confirmatory testing and adrenal venous sampling (AVS) to differentiate between uni- and bilateral subtype) is often burdensome and usually limited to large tertiary centres. As a result, the majority of patients with PA never receive the correct diagnosis (13). Therefore, novel tools and techniques are needed to improve diagnostic stratification and subtyping for PA.
This review provides an overview of recent advances in biochemical diagnostics of PA, including cutting-edge methods of steroidomics and proteomics, with a focus on targeted approaches. These techniques provide “a snapshot” of large numbers of released steroids, metabolites and proteins/peptides at the given time. The global analysis of compound group enables a comprehensive understanding of the underlying mechanisms of PA, but also unveils novel diagnostic options.
2 Diagnostics of primary aldosteronism: current approaches and challenges
According to the Endocrine Society Guidelines, indications of PA screening include patients with: (1) BP above 150/100 mmHg on repeated (at least three) measurements, (2) resistant hypertension, (3) hypertension controlled on 4 or more hypertensive agents, (4) hypertension with hypokalemia (both spontaneous and diuretic-induced), (5) hypertension and adrenal mass, (6) hypertension and obstructive sleep apnea (OSA), (7) hypertension and family history of cerebral vascular event (CVA) or hypertension at the age younger than 40 years, and (8) all patients with hypertension and a first-degree relative diagnosed with PA (14). The authors of a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension recommend that the screening for PA should also include patients with hypertension and atrial fibrillation (AF) not associated with structural heart disease, since the prevalence of AF among patients with PA is nearly four times higher than among subjects with resistant hypertension (15, 16). However, the indication for PA screening in patients with hypertension and OSA has been questioned based on the results of the multiethnic, cross-sectional HYPNOS study (Hyperglycemic Profiles in Obstructive Sleep Apnea) (15, 17).
Recommendations published by the national endocrinology societies also apply non-uniform criteria for PA screening. French Endocrinology Society (SFE), French Hypertension Society (SFHTA) and Francophone Endocrine Surgery Association (AFCE) highlight the need for PA screening in patients with hypertension and disproportionate target organ damage (18). The recently published British and Irish Hypertension Society (BIHS) statement on diagnosis and management of PA recommends facilitated diagnostic strategy for screening for PA (19). According to BIHS, clinical situations in which PA screening should be introduced include resistant hypertension, hypertension with hypokalemia, hypertension with adrenal mass, and hypertension in adults below 40 years (19). However, there are some investigators advocating for screening for PA of all hypertensive patients (20). The rationale behind this approach includes considerable health benefits for diagnosed patients with PA after tailored treatment and the possibility to avoid the confounding effect of antihypertensive medications on aldosterone and renin measurements (20).
Alongside the lack of uniform indications for PA screening, recommended aldosterone-to-renin ratio (ARR) as a screening test is subject to several limitations (14). Renin may be assessed as plasma renin activity (PRA) evaluating renin enzymatic activity to generate angiotensin I from angiotensinogen under controlled conditions over time, or direct renin concentration (DRC) based on the measurement of renin and active prorenin concentration in plasma. Measurement of renin may be challenging, mainly due to low concentration and instability in refrigerated temperature, which leads to cryoactivation of prorenin to renin and falsely elevated results (21, 22). Thus, it is recommended to transport the probes (usually EDTA plasma sample collection tubes are used) at room temperature to the laboratory up to 30 min after blood collection, centrifugate before the immediate automated DRC determination (CLIA, Chemiluminescent immunoassays) or centrifugate, quickly freeze and store frozen plasma prior to the postponed manual DRC measurement (IRMA, Immunoradiometric assay and ELISA, enzyme-linked immunosorbent assay) (14). For the PRA determination, routinely EDTA plasma sample collection tubes are used, samples should be transported on ice to the laboratory within 30 min from the blood collection, centrifugated (4°C), frozen and stored till the manual PRA measurement (commonly radioimmunoassay (RIA) or enzyme immunoassay (EIA, ELISA) methods are used). Immunoassays are widely used for determination of DRC and PRA. Despite convenience and short turnaround time, DRC and PRA immunoassays may lack sensitivity and exhibit low antibody storage stability and cross-reactivity with multiple structurally similar angiotensin-like peptides (21, 23). However, angiotensin I for PRA calculation may also be measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS), which offers high accuracy of angiotensin I measurement at low concentrations (often observed in PA) and allows for determination of metabolites (e.g., angiotensin II, angiotensin III, angiotensin IV) for the broader assessment of the renin‐angiotensin‐aldosterone system (RAAS) (21, 23). Aldosterone can also be measured in serum or plasma. For the serum aldosterone concentration determination clot activator serum sample collection tubes are routinely used, while EDTA plasma sample collection tubes are used when plasma is analysed. Contrary to DRC, the probes for aldosterone determination are not sensitive to temperature drop. Moreover, differences depend on the type of biological material (serum/plasma) used to determine blood aldosterone levels. Aldosterone concentration can be determined by various immunoassays or LC-MS/MS technique. However, the results obtained using LC-MS/MS are 30% lower when compared to radioimmunoassay aldosterone measurement (24, 25). Since the results of aldosterone determination differ significantly depending on the used method (RIA, EIA/ELISA, chemiluminescence immunoassay (CLIA, ECLIA), LC-MS/MS technique), assay-specific thresholds should be used when ARR (or ADRR (aldosterone-to-direct-renin ratio) if DRC is used) is calculated (25, 26).
Since the advantage of ARR in the screening for PA was shown by Hiramatsu et al., ARR screening has been used widely (27). Most commonly used cutoff values are 30 for ARR (ng/dL/ng/mL/h) and 3.7 for ADRR (ng/dL/mU/L) (14). Other proposed screening tests for PA, e.g., the aldosterone-to-angiotensin II ratio using commercial ELISA set showed worse diagnostic performance (28). However, the interpretation of ARR should include various factors and limitations. Firstly, as presented in the meta-analysis by Hung et al., assessing ARR diagnostic performance, no single ARR threshold for PA screening could be recommended (29). Furthermore, nearly all commonly used antihypertensive drugs interfere with RAAS, leading to false positive or false negative results of ARR (14). Not only do antihypertensive agents influence ARR, but also non-steroidal anti-inflammatory drugs (NSAIDs), estrogen-containing contraceptives, hormone replacement therapy (HRT) and selective serotonin reuptake inhibitors (SSRIs) may alter the result (14, 30). False positive results of ARR may also be observed in older age, impaired kidney function, increased dietary salt intake, luteal phase of menstrual cycle and in patients with the extremely rare disorder Gordon syndrome (or pseudohypoaldosteronism type 2) (31). False negative results may be a consequence of pregnancy, hypokalemia, salt intake restriction, vomiting, diarrhoea, malignant or renovascular hypertension (31). In other conditions such as renal pseudohypoaldosteronism type 1 the ARR can be high with very high aldosterone concentrations, but renin concentrations are not suppressed, and the patients do not have hypertension (32).
Bloods for aldosterone and renin measurement should be drawn in the morning, after 2hr in upright position, then permitted to settle (sit for 5 to 15 minutes) before the blood collection (14, 33). Suboptimal screening conditions may, together with other pre-analytical errors (e.g., during specimen collection, handling and transportation to the laboratory), markedly influence the results. Despite measurement in optimal conditions, ARR shows high within-patient variability (34). Thus, in patients with high pretest probability of PA and not elevated ARR, it should be repeated at least twice (34, 35). Suppressed renin concentration often leads to false positives, even if the aldosterone concentration is low. Hence, it has been suggested to use a minimum aldosterone concentration of >15 ng/dL (>415 pmol/L) to be able to interpret a positive test (14). However, it may lead to underdiagnosing some individuals with PA, especially patients with PA and bilateral adrenal hyperplasia (BAH) (14, 21). Thus, some investigators suggest a cut-off of 5 ng/dL (138.7 pmol/L) for aldosterone concentration, especially when assessed by LC-MS/MS (36).
Once a positive ARR is confirmed, lack of aldosterone suppression should be demonstrated in one of four tests (saline infusion test, oral sodium loading test, fludrocortisone suppression test or captopril challenge test) (14). All these tests can be done in an outpatient setting, also fludrocortisone suppression test, even though the latter in most centres is done as an inpatient test (37). In patients with suppressed renin concentration, spontaneous hypokalemia, and plasma/serum aldosterone concentration ≥ 20 ng/dL (≥ 555 pmol/L) confirmatory test is not needed (14). Similarly to the previous stages of PA diagnostics, there is a remarkable heterogeneity in confirmatory testing protocols and interpretation of the results (38). Notably, the use of confirmatory tests is not evidence-based, since none of the studies advocating their use in confirmation of PA diagnosis has met state-of-the-art criteria used for validation of diagnostic tests (19, 39–42).
After the diagnosis of PA, optimal treatment (unilateral adrenalectomy or pharmacotherapy with mineralocorticoid receptor antagonist) should be initiated, depending on the source (unilateral/bilateral) of autonomous aldosterone secretion, surgical candidacy, and patient preference (14, 43). Unenhanced adrenal computed tomography (CT) may assist in treatment planning, although it is associated with substantial number of false positive (identifying non-functional adrenal adenomas incorrectly linked to autonomous aldosterone secretion) or false negative results (underdetection of small lesions), except in patients below 35 years old, with spontaneous hypokalemia, aldosterone concentration >30 ng/dL (>831 pmol/L), and unilateral adrenal lesion measuring ≥ 10 mm, in whom diagnostic accuracy of CT is high and AVS is not necessary (14, 43). Overall, the concordance rate of CT and AVS in patients with unilateral disease was 50% (44). Thus, the majority of patients need to undergo AVS for subtyping. Although AVS is characterized by high sensitivity (95%) and specificity (100%) in detecting unilateral aldosterone hypersecretion, it is a costly, invasive, highly specialized procedure that should be performed only in experienced centres (14, 45). It should be noted that canulating the right adrenal vein is very difficult with low rate of success in non-experienced hands.
Thus, all the listed limitations of routine diagnostics of PA (Figure 1) indicate the necessity to establish novel, improved diagnostic strategies.
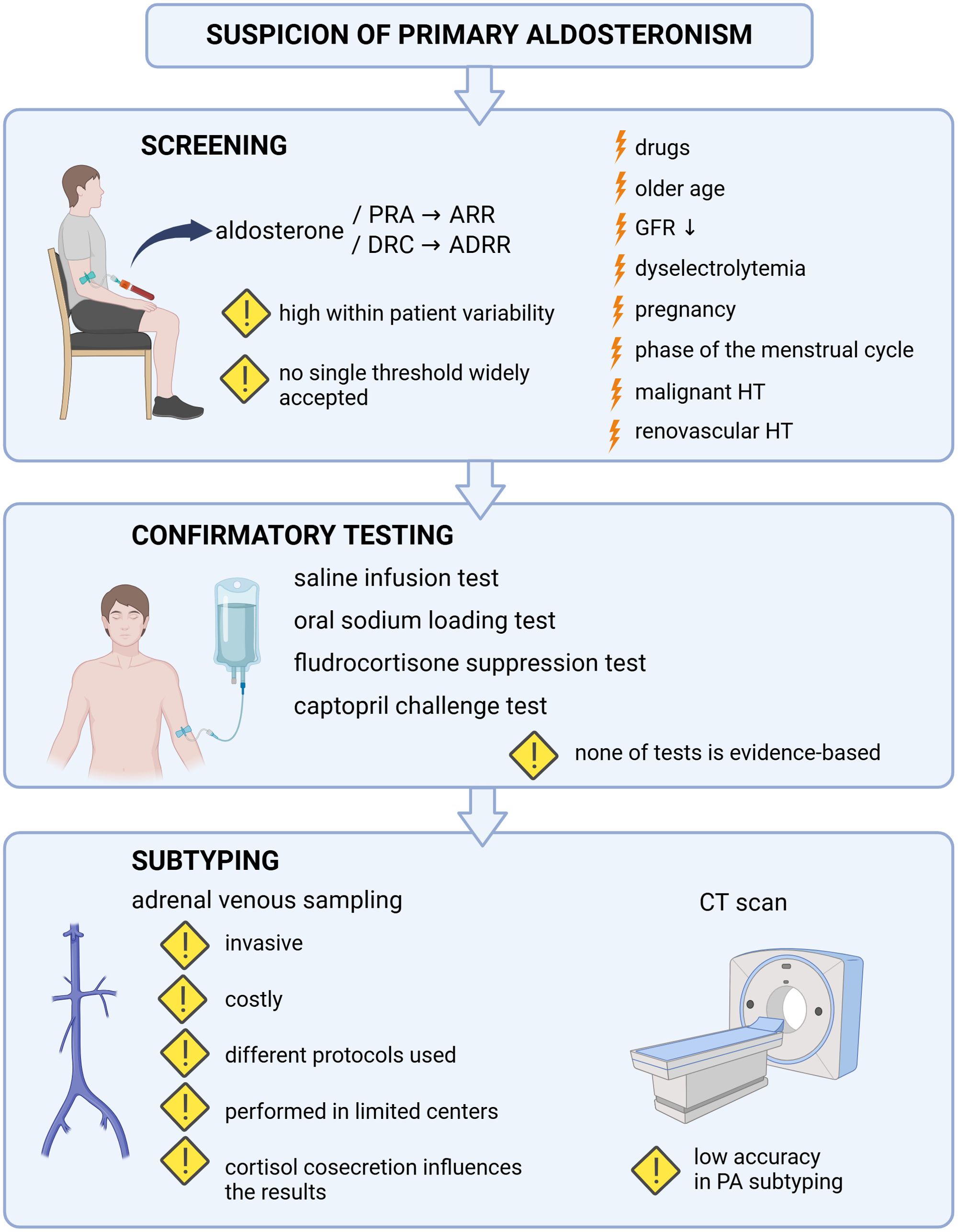
Figure 1. The limitations of routine diagnostics of primary aldosteronism. Factors which may influence the result of primary aldosteronism screening were marked with the lightning sign. Exclamation mark was used to underline the limitations of screening, confirmatory tests and subtyping of primary aldosteronism. PRA, plasma renin activity; ARR, aldosterone-to-renin ratio; DRC, direct renin concentration; ADRR, aldosterone-to-direct-renin ratio; GFR, glomerular filtration rate; HT, hypertension; CT, computed tomography; PA, primary aldosteronism. Created in BioRender. BioRender.com/n46h901
3 From immunoassays to mass-spectrometry
For many years, immunoassays, in particular RIA and CLIA, have been the primary method used for measuring selected steroids in plasma/serum and urine. Nonetheless, immunoassay, which is based on the reaction of an antigen with a specific antibody, has multiple limitations. These include limited sensitivity and reproducibility, especially at lower concentrations. Currently, mass-spectrometry based techniques (including gas chromatography – mass spectrometry (GC/MS) and LC–MS/MS) are considered the gold standard for quantification of steroids in biological fluids (46, 47). LC–MS/MS combines liquid chromatography separation of the particles followed by their mass-based detection. Its high sensitivity and specificity result from minimizing interference from cross-reactivity and non-specific reactions. Its relevant diagnostic value in PA has been reported in a meta-analysis (46).
Furthermore, the LC-MS/MS technique allows simultaneous quantification of multiple compounds and is a time-efficient analysis. Noh et al. demonstrated successful measurements of adrenocortical steroids, catecholamines and plasma free metanephrines in a single run (48). Derivatization with alkyl chloroformates allowed the protection of polar and hydrophilic groups of medullary amines and resulted in a satisfactory signal-to-noise ratio and peak shape, while maintaining effective steroid quantifications (48).
One of the limitations of the LC-MS/MS technique includes laborious sample preparation. Over the past few years, this issue has been addressed in multiple studies and increasingly faster measurement methods using LC-MS/MS have been developed. By improving the efficiency of acid hydrolysis, Yin et al. developed an assay of rapid aldosterone measurement in samples from 24hr urine collections (49). Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) has also been tested in aldosterone quantification in plasma – this method enables rapid aldosterone measurement, while maintaining high accuracy (50).
Nevertheless, given the limited availability of LC-MS/MS in many centres and countries, there has arisen a need to improve existing, widely accessible methods to ensure better diagnostics and the ability to compare research results with those obtained using the LC-MS/MS technique. A new, two-site sandwich chemiluminescent enzyme immunoassay (CLEIA) to automatically measure both plasma/serum aldosterone concentration and active renin concentration (ARC), using monoclonal antibodies immobilized onto ferrite particles, has been implemented into clinical practice (51, 52). Nishikawa et al. compared LC-MS/MS with CLEIA and conventional RIA immunoassays for aldosterone measurement in the blood (53). The median aldosterone concentration of the LC-MS/MS corresponding to RIA established PA criterion was almost 2.5 times lower for LC-MS/MS technique, while CLEIA values were similar to LC-MS/MS. A study conducted by Kobayashi et al. (54) showed that the plasma aldosterone concentration measured by CLEIA was significantly lower than the value obtained with RIA, and new cut-offs for screening and confirmatory tests using CLEIA for the diagnosis of PA were suggested. Thus, the good linearity over a wide range of concentrations and accuracy comparable to LC-MS/MS may establish CLEIA as an alternative testing method in centres and countries without access to LC-MS/MS (51).
Despite the undeniable advantages of LC-MS/MS technique, its application is limited to selected centres and thus the overwhelming majority of routine diagnostics of PA still relies on the use of immunoassays. In the future, LC-MS/MS will not likely replace immunoassays in the PA diagnostics in many centres, thus ways to optimize the simultaneous use of both, diagnostic cut-offs, comparability and reproducibility will be necessary.
4 Targeted steroidomics
The rapidly evolving field of “omics” techniques allows for characterization of an entire set of chosen compounds produced by cell, tissue, organ or organism at the same time. Thus, profound assessment of multiple disorders at a resolution that has never been possible is now available. One of them, targeted steroidomics (the terminology was introduced by Sjovall in 2004), involves mass-spectrometric assessment of predefined steroids on a multianalyte approach, measured in blood (serum/plasma) or urine (24-hour urine collection) (55–57). Although the use of steroid profiling in the diagnostics of adrenocortical diseases has a history of more than half a century, only a recent development of liquid chromatography or gas chromatography coupled with mass spectrometry has transformed the investigation of adrenocortical conditions, unveiling unknown and underappreciated steroid players (56–60).
The application of steroidomics has proven useful in evaluation of adrenocortical cancer (ACC) and autonomous cortisol secretion (CS), but also PA (61–75). In the study of Berke et al., investigating the profile of 19 plasma steroids in 577 patients with adrenal incidentaloma, patients with PA were distinguished by high plasma concentrations of 18-oxocortisol, 18-hydroxycortisol, 18-hydroxycorticosterone and aldosterone (64). The utility of 18-hydroxycorticosterone in plasma and urinary “hybrid steroids” (combining the structural characteristics of aldosterone and cortisol): 18-hydroxycortisol and 18-oxocortisol were demonstrated to differentiate patients with PA from those with primary hypertension and normotensive subjects (65). A threshold for 24hr urinary 18-hydroxycortisol excretion greater than 330 μg/d together with positive ARR confirmed the diagnosis of PA without the need for further confirmatory/exclusion tests (65). A 24hr urinary 18-hydroxycortisol excretion greater than 510 μg/d distinguished the patients with aldosterone producing adenoma (APA), which together with identification of unilateral adrenal tumour on a CT scan may allow to proceed directly to unilateral adrenalectomy without AVS (65). Peripheral plasma 18-oxocortisol concentration measured by LC-MS/MS was also proved useful in discrimination of patients with APA from BHA (a cutoff value of 4.7 ng/dL was used with a sensitivity of 83% and specificity of 99%) (66).
The results of one study by Eisenhofer et al. demonstrated that the use of a 8 steroid panel in plasma (aldosterone, 18-hydroxycortisol, 18-oxocortisol, 11-deoxycoticosterone, cortisol, cortisone, dehydroepiandrosterone, and androstenedione) together with ARR was more effective than ARR alone for discriminating patients with PA from those with primary hypertension (67). Among assessed steroids, aldosterone, 18-oxocortisol, and 18-hydroxycortisol were characterized by the highest discriminatory power (67). This approach combined with machine learning also allowed to differentiate the patients with APA associated with KCNJ5 variants with the sensitivity of 85% and the specificity of 97% (67). In another study published by Eisenhofer et al. on 216 patients with PA, concentrations of 18-oxocortisol in plasma was 8.5 times higher in patients with APA, than in those with BHA (68). However, the area under the curve (AUC) of 0.659 showed limited utility of that steroid in subtyping PA (68). The accuracy was remarkably improved to AUC 0.889 by analysing the whole panel of 15 steroids, emphasizing the potential utility of mass-spectrometry-based measurement of multiple steroids rather than analysing them individually (68). The diagnostic application of the steroid profile of peripheral venous plasma measured by LC-MS/MS was also the subject of the study by Yang et al. (69). The investigators targeted distinguishing the patients with BHA from those with macro-APA (diameter of adrenal lesion ≥ 10 mm) and micro-APA (diameter < 10 mm), the latter often undetectable on CT scans (69). The obtained results revealed distinct differences in steroid profiles between compared groups, with aldosterone, 18-oxocortisol, 18-hydroxycortisol and dehydroepiandrosterone sulphate (DHEAS) having the highest differentiating value (69). Interestingly, the diagnostic performance of steroid probability score for PA (based on mass-spectrometric assessment of steroids in plasma integrated by machine-learning tools) was not influenced significantly by the interfering antihypertensive drugs (AUC 0.848 with the use of antihypertensive medications; AUC 0.893 without antihypertensive medications with the proven impact on the RAAS), as it was observed for ARR (AUC 0.765 with and AUC 0.845 without the interfering with RAAS antihypertensive drugs) (70). Plasma steroids which showed the highest discriminatory power in primary aldosteronism screening and subtyping are presented in Figure 2.
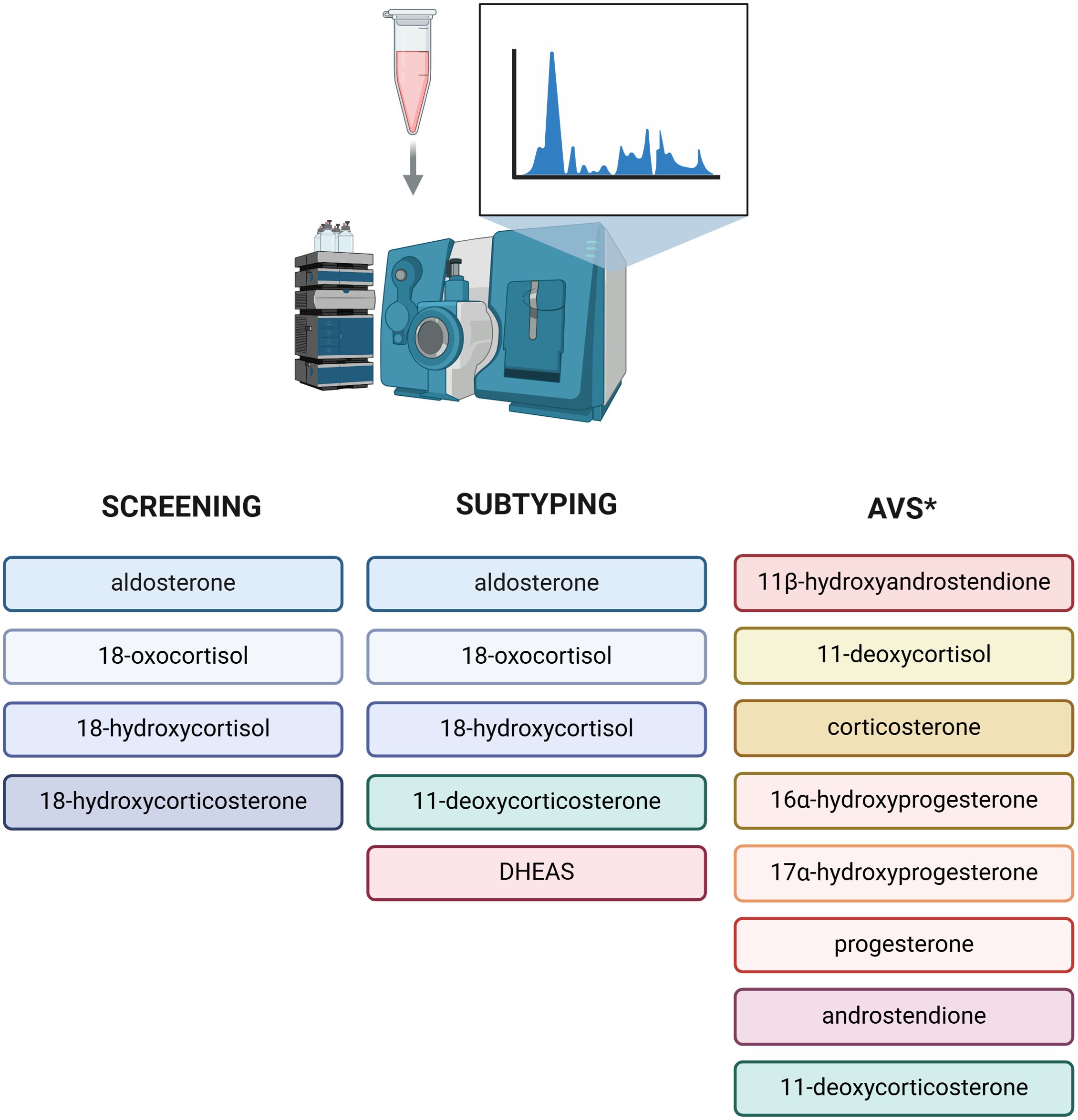
Figure 2. Plasma steroidomics in diagnostics of primary aldosteronism. Steroids demonstrating the highest discriminatory power in primary aldosteronism screening and subtyping. *Panel of 8 steroids characterized by higher selectivity index than cortisol both at baseline and after adrenocorticotropic hormone, which may improve the proportion of successful adrenal venous sampling [70]. DHEAS, dehydroepiandrosterone sulfate; AVS, adrenal venous sampling. Created in BioRender. BioRender.com/u15i195
Apart from the assessment of plasma steroidomics, quantification of steroid metabolites excretion in 24hr urine collection was proven useful in identification and subtyping of PA (71). The choice of urine as a sample matrix has advantages over plasma/serum: non-invasive sample collection, not requiring any medical professionals, thus lower cost, unaffected by alterations in supine vs. seated blood sampling, and in case of 24hr urine collection: comprehensive measurement of steroid output, independent from diurnal steroid differences (56, 67, 71). However, 24hr urine collection can be inconvenient and difficult for some patients, and in those blood samples could be preferred. There are also substantial differences in sample preparation, depending on the matrix used: serum/plasma or urine. Contrary to blood (plasma), in urine steroids are present as conjugates (glucuronides and sulphates). Thus, after extraction and prior to e.g., derivatization and analysis using GC/MS, conjugated steroids need to be hydrolysed (71). To remove those charged moieties different strategies are applied resulting in significant alterations in desired metabolites concentrations varying between the studies (72).
In the study of Prete et al., the assessment of 34 steroids (including mineralocorticoid, glucocorticoid, androgen, hybrid steroid metabolites and their precursors) in 24hr urine sample measured by GC/MS and analysed by machine-learning methods had an excellent accuracy (AUC 0.970) for the distinguishing of patients with PA from controls with normotension (73). Among assessed metabolites, 3α, 5β-tetrahydroaldosterone, tetrahydro-11-deoxycortisol, and 18-hydroxy-tetrahydro-11-dehydrocorticosterone had the highest discriminative value (73). Interestingly, their 24hr urinary output interquartile ranges did not overlap between patients with PA and controls (73). Conversely, computational analysis of urine steroidomics showed suboptimal diagnostic performance (AUC 0.650) in distinguishing patients with APA from those with BHA, probably due to high heterogeneity of the groups (73). However, analysis of 34 steroids integrated by machine learning approach with generalised matrix relevance learning vector quantization, allowed to distinguish patients with APA harbouring KCNJ5 variants from other patients with PA with a high diagnostic accuracy (AUC 0.830) (73).
Multisteroid approach was tested as a tool not only to identify and subtype PA based on the analysis of serum/plasma or urine, but also to improve the diagnostic performance of AVS. Cortisol, used in interpretation of AVS results, has several disadvantages including longer half-life than aldosterone and fluctuations of cortisol concentration during AVS (74). Additionally, mild autonomous cortisol secretion (MACS) is often found in patients with PA which may influence AVS result (74, 75). In a study by Turcu et al., among 17 measured steroids, 8 steroids showed significantly higher selectivity index (SI, calculated as adrenal vein/inferior vena cava steroid concentration) than cortisol, both at baseline and after adrenocorticotropic hormone (ACTH) simulation (Figure 2) (74). Importantly, the use of 11ß-hydroxyandrostendione, corticosterone and 11-deoxycortisol allowed to rescue the majority of unsuccessful baseline catheterizations (with SI <2 for cortisol) (74). In a study by Chang et al., the application of steroid profiling measured by LC-MS/MS rescued 45% unstimulated and 66% ACTH stimulated unsuccessful cases of AVS based on immunoassay assessment (76). Furthermore, steroid profiling using LC-MS/MS allowed to identify 31% more cases of unilateral PA in comparison to widely used immunoassay (76). Thus, the application of steroid profiling may significantly improve the number of successful AVS and diagnostic accuracy of that procedure (77). Nevertheless, the obtained results should be interpreted cautiously, while the application of LC/MS-MS is only being tested in context of AVS, in which the concentrations of steroids are significantly higher than those measured in the blood.
Overall, mass-spectrometric assessment of steroid profiling has improved the understanding of the role of steroids in PA, going far beyond aldosterone. The recently published studies have revisited the importance of “hybrid steroids” as PA biomarkers. The use of targeted steroidomics integrated by machine learning tools may streamline the identification and subtyping of patients with PA. The application of novel methods such as high-resolution matrix-assisted laser desorption/ionization mass-spectrometry (MALDI-MS) was proven useful to integrate metabolomic data with spatial information obtained from standard histology, resulted in better understanding of functional anatomy of APA, defining genotype-phenotype correlations and discovering new biomarkers (78). Furthermore, the application of untargeted metabolomics may open a new chapter in understanding the pathophysiology of PA unveiling alterations in metabolic pathways and defining novel diagnostic biomarkers (79).
5 Targeted proteins and peptides analysis methods, untargeted proteomics
Simultaneously with the quantification of multiple steroids using mass spectrometry in the diagnostics of PA, extensive research has been conducted in the field of proteomics. Targeted proteomics allows for precise quantification of preselected proteins and peptides, while untargeted proteomics aims to quantify all detectable proteins and is associated with laborious work of identifying them. Proteomics examines the structure and function of peptides and proteins involved in the physiological and pathophysiological processes of RAAS, which could be an alternative to PRA/DRC. Recent studies evaluated RAAS equilibrium using LC-MS/MS to quantify the angiotensin peptidome (including angiotensin I, angiotensin II, angiotensin III, angiotensin IV, angiotensin (1–7), and angiotensin (1–5)) simultaneously from a single sample (80–82). The main principle of RAAS equilibrium analysis is based on the incubation of prestored frozen serum at the temperature of 37°C, controlled pH 7.4 for 1 hour without addition of any substances interfering with angiotensin production or degradation, which leads to establishment of equilibrated status of RAAS (82). Thus, the equilibrium angiotensin II (eqAngII) concentration is the resultant of the activity of all angiotensin processing enzymes in the probe, once the equilibrium is established (82). The assessment of eqAngII and calculation of aldosterone-to-angiotensin II ratio may be a promising tool in PA diagnostics, allowing to bypass often cumbersome PRA or DRC determination (82).
Prorenin, a precursor of renin, has been suggested to be valuable in the diagnostics of PA. Compared with active renin, prorenin is characterized by stable release, not affected by multiple stimuli including change of body position (83). The (pro)renin receptor ((P)RR) levels were found to be positively correlated with aldosterone synthase (CYP11B2) concentrations in APA tissues, plasma aldosterone concentrations and urinary aldosterone excretion, suggesting its role in aldosterone synthesis (84). Nevertheless, in the same study, serum (P)RR was neither associated with plasma aldosterone concentration nor adrenal (P)RR expression level (84).
Beyond the direct assessment of RAAS components, other peptides and proteins, primarily associated with, e.g., inflammation or transmembrane transport, were investigated as potential biomarkers of PA. Proteins and peptides from the granin family involved in the regulated secretory pathway (packaging, storage, and release of peptide hormones and neurotransmitters) are currently being studied as a potential biomarkers of PA. The results of the study by Glinicki et al. on 10 patients with PA and 22 patients with non-functional adrenal adenoma (NFAA) demonstrated that among 10 proteins and peptides from the granin family, pancreastatin (pancreastatin/chromogranin A (250-301aa) -amide peptide) and secretoneurin (a small 33aa peptide of secretogranin II (SgII)/chromogranin C (CgC) (1-617aa)) were differentiating those two groups (85). Recently, extracellular vesicles (EVs), biological nanostructures released from all cells, have also become a compelling subject of interest for researchers worldwide (86). Given that EVs transport various biomolecules including multiple proteins, lipids, nucleic acids derived from parent cells, they hold the potential to serve as a source of biomarkers for various diseases, including PA (87).
Recent studies investigated the role in PA diagnosis of serum and urinary alpha-1-acid-glycoprotein (AGP1 or A1G1), also known as orosomucoid protein 1 (ORM1), an acute-phase protein associated with inflammation (87–90). The notable upregulation of AGP1 in urinary extracellular vesicles (uEVs) in PA was studied by Barros et al., who investigated the proteome of patients with PA, searching for mediators associated with renal and extrarenal damage induced by chronic elevated aldosterone concentration (89). Sequential ultracentrifugation was applied for isolation of uEVs, then the International Society for Extracellular Vesicles guidelines were used to describe isolated EVs, using transmission electron microscopy (TEM), immunoblotting, and nanoparticle tracking analysis (NTA) (89, 91). A considerable upregulation of AGP1 in patients with PA (2.43-fold increase) was observed in the comparison to the control group, however, the limited size of a study (7 patients with PA and 8 healthy controls) necessitates interpreting the results with caution (89). Elevated concentration of serum AGP1 in patients with PA compared with individuals with essential hypertension and normotensive controls was also demonstrated by Carvajal et al. (90). Interestingly, the concentrations of other inflammatory markers: high sensitive C-reactive protein (hs-CRP), plasminogen inhibitor activator-1 (PAI-1), matrix metallopeptidase 9 (MMP-9) and malondialdehyde (MDA), free cystatin-C (CysC), neutrophil gelatinase associated lipocalin (NGAL or LCN2), and interleukin 6 (IL-6) did not differ between the groups (90).
Regarding the influence of aldosterone on NaCl-transporting proteins in renal tubules, Ochiai-Homma et al. analysed the quantitative changes in pendrin, a Cl−/HCO3– exchanger protein, in uEVs isolated from patients with PA and from a rat model of aldosterone excess (92). Pendrin was found in uEVs in humans and rats (92). In a rodent model, its levels, as well as epithelial Na+ channel (ENaC) and Na+Cl–cotransporter or thiazide-sensitive sodium chloride cotransporter (NCC) levels in uEVs, were correlated with renal abundance (92). Interestingly, pendrin levels in uEVs were reduced by 49% after adrenalectomy or pharmacological mineral receptor blockade (92). The role of abovementioned uEVs NCC in PA was also studied by Kong et al., who aimed to identify biomarkers helping to distinguish PA subtypes without AVS procedure (92, 93). The promising use of phosphorylated form of NCC (pNCC) in non-invasive PA subtyping was noted (93). In this study, spot urine samples from 50 patients with PA who underwent AVS were compared within the low lateralization index (l-LI) group and high lateralization (h-LI) index group (93). NCC and pNCC were more abundant in the h-LI group (93). Furthermore, somatic KCNJ5 variants were detected in 65.4% of the APA cases, and carriers of somatic KCNJ5 variants compared with non-carriers had a higher abundance of pNCC in uEVs (93). Positive correlation between pNCC abundance and plasma aldosterone concentration was demonstrated (93). However, the results should be confirmed in larger studies and evaluation of NCC as well as pNCC concentrations before and after treatment of PA would also be beneficial (93). The correlation between PAC and NCC adjusted by CD9 protein level in uEVs was found by Hayakawa et al. as well (94). Nevertheless, γ-epithelial sodium channel (ENaC) adjusted by CD9 protein level in uEVs was found to better correlate with plasma aldosterone concentration in patients with PA (94). Of note, ENaC decreased during treatment with mineralocorticoid receptor antagonists and after adrenalectomy, while plasma aldosterone concentration diminished only after surgery treatment, indicating that ENaC reflects mineralocorticoid receptor activity during PA therapy (94).
Ma et al. studied the role of wolframin, a transmembrane protein, which maintains calcium homeostasis by promoting calcium transport from endoplasmic reticulum to cytoplasm, in PA (95). The investigators assessed the proteome and phosphoproteome of tumour tissues from 15 patients with APA and 10 patients with nonfunctioning adrenocortical tumours, applying a 4D label-free quantification approach by high-resolution liquid chromatography-mass spectrometry (95). The results of this study led to the generation of proteome and phosphoproteome signalling network maps of APA and identification of wolframin as a relevant regulatory protein in PA (95).
Another proteomic analysis comparing uEVs derived from patients with essential hypertension or PA revealed six proteins (putative glutathione hydrolase 3 proenzyme, aminopeptidase N, CD63 antigen, aquaporin-1, IST1 homolog and aquaporin-2) distinguishing these two groups (88). Interestingly, a reduced abundance of membrane aquaporins involved in water reabsorption mechanisms in PA was observed, which might be explained by chronic water and sodium retention in PA due to the aldosterone excess, when compared with the patients with essential hypertension (88). The statistical analysis also showed usefulness of the following markers: histone H4, serine palmitoyltransferase 3 (SPTC3), mannosyl-oligosaccharide 1,2-alpha-mannosidase IA (MA1A1), lysozyme C (LYSC), heat shock protein beta-1 (HSPB1) and immunoglobulin lambda variable 3-25 (IGLV3-25), in differentiating BPA from APA (88). Proteins and peptides assessed in blood and in urinary EVs in diagnostics of PA are presented in Figure 3.
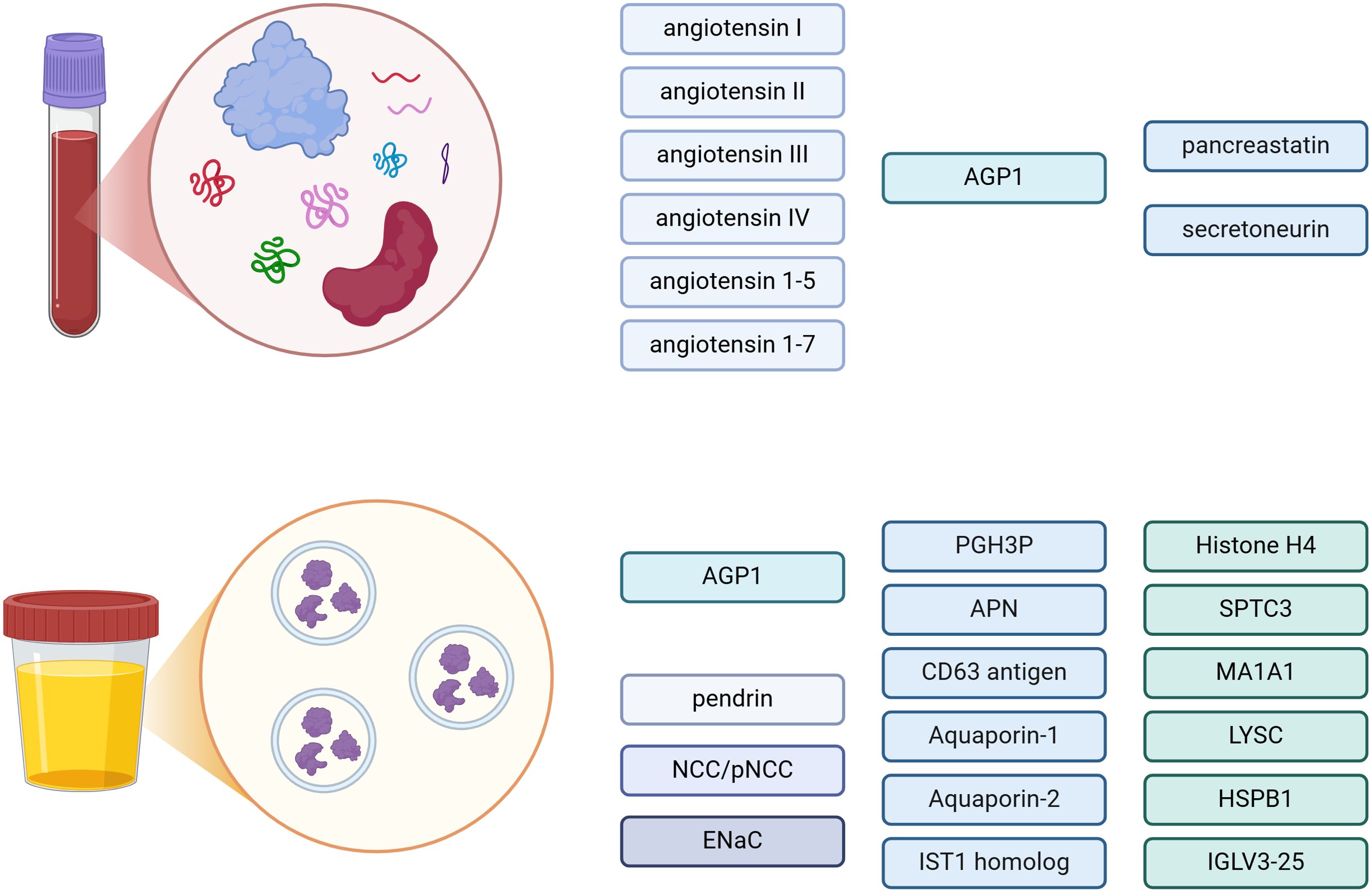
Figure 3. Proteins and peptides in blood and urinary extracellular vesicles (EVs) – potential biomarkers in diagnostics of primary aldosteronism. AGP1, alpha-1-acid-glycoprotein; NCC, Na+Cl- cotransporter; pNCC, phosphorylated Na+Cl- cotransporter; ENaC, γ-epithelial sodium channel; PGH3P, putative glutathione hydrolase 3 proenzyme; APN, aminopeptidase N; SPTC3, serine palmitoyltransferase 3; MA1A1, mannosyl-oligosaccharide 1,2-alpha-mannosidase IA; LYSC, lysozyme C; HSPB1, heat shock protein beta-1; IGLV3-25, immunoglobulin lambda variable 3-25. Created in BioRender. BioRender.com/e86w482
Proteomics research has allowed us to analyze not only a large number of proteins at the given time, but also their properties, abundance and structures. In PA, proteomic studies may help to reliably assess RAAS function, disease subtype, complications and response to the treatment.
6 Conclusions
Despite huge advances in the diagnosis and treatment of adrenal disease, PA still lags behind as the most common cause of secondary hypertension with still only a small percentage of patients diagnosed and successfully treated. The complex biochemical assessment of PA may be challenging since it abounds in pitfalls from the screening, confirmatory testing to disease subtyping. The limitations of routine diagnostics of PA necessitates establishing novel diagnostic strategies. Recent development in LC-MS/MS technique and “omics” techniques, including steroidomics and proteomics, offers unique insights into PA mechanism, subtype, molecular background, and even complications. However, these techniques have limitations to be regarded such as limited availability, high cost, often laborious sample preparation and large amount of generated data that must be interpreted cautiously.
Author contributions
AS: Conceptualization, Data curation, Investigation, Project administration, Visualization, Writing – original draft. AT: Data curation, Investigation, Writing – original draft. HF: Validation, Writing – review & editing. WZ: Validation, Writing – review & editing. PG: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. (2021) 9:876–92. doi: 10.1016/S2213-8587(21)00210-2
2. Loffing J, Summa V, Zecevic M, Verrey F. Mediators of aldosterone action in the renal tubule. Curr Opin Nephrol Hypertens. (2001) 10:667–75. doi: 10.1097/00041552-200109000-00019
3. Araujo-Castro M, Pascual-Corrales E, Martin Rojas P, Parra Ramirez P. Epidemiology and diagnosis of primary aldosteronism. What Have We Learned from the Spain-Aldo Registry? Endocrine. (2024) 83:527–36. doi: 10.1007/s12020-023-03573-7
4. Liu YY, King J, Kline GA, Padwal RS, Pasieka JL, Chen G, et al. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surg. (2021) 156:541–9. doi: 10.1001/jamasurg.2021.0254
5. Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, et al. Screening rates for primary aldosteronism in resistant hypertension: A cohort study. Hypertension. (2020) 75:650–9. doi: 10.1161/HYPERTENSIONAHA.119.14359
6. Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens. (2005) 14:235–41. doi: 10.1097/01.mnh.0000165889.60254.98
7. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2018) 6:41–50. doi: 10.1016/S2213-8587(17)30319-4
8. Ambrosino P, Lupoli R, Tortora A, Cacciapuoti M, Lupoli GA, Tarantino P, et al. Cardiovascular risk markers in patients with primary aldosteronism: A systematic review and meta-analysis of literature studies. Int J Cardiol. (2016) 208:46–55. doi: 10.1016/j.ijcard.2016.01.200
9. Demirkiran A, Everaars H, Elitok A, van de Ven PM, Smulders YM, Dreijerink KM, et al. Hypertension with primary aldosteronism is associated with increased carotid intima-media thickness and endothelial dysfunction. J Clin Hypertens (Greenwich). (2019) 21:932–41. doi: 10.1111/jch.13585
10. Monticone S, Sconfienza E, D’Ascenzo F, Buffolo F, Satoh F, Sechi LA, et al. Renal damage in primary aldosteronism: A systematic review and meta-analysis. J Hypertens. (2020) 38:3–12. doi: 10.1097/HJH.0000000000002216
11. Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. (2014) 63:20–31. doi: 10.1016/j.metabol.2013.08.016
12. Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, et al. Risk of fracture in primary aldosteronism: A population-based cohort study. J Bone Miner Res. (2017) 32:743–52. doi: 10.1002/jbmr.3033
13. Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: A cross-sectional study. Ann Intern Med. (2020) 173:10–20. doi: 10.7326/M20-0065
14. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
15. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the working group on endocrine hypertension of the european society of hypertension. J Hypertens. (2020) 38:1919–28. doi: 10.1097/HJH.0000000000002510
16. Seccia TM, Letizia C, Muiesan ML, Lerco S, Cesari M, Bisogni V, et al. Atrial fibrillation as presenting sign of primary aldosteronism: results of the prospective appraisal on the prevalence of primary aldosteronism in hypertensive (Papphy) study. J Hypertens. (2020) 38:332–9. doi: 10.1097/HJH.0000000000002250
17. Buffolo F, Li Q, Monticone S, Heinrich DA, Mattei A, Pieroni J, et al. Primary aldosteronism and obstructive sleep apnea: A cross-sectional multi-ethnic study. Hypertension. (2019) 74:1532–40. doi: 10.1161/HYPERTENSIONAHA.119.13833
18. Amar L, Baguet JP, Bardet S, Chaffanjon P, Chamontin B, Douillard C, et al. Sfe/sfhta/afce primary aldosteronism consensus: introduction and handbook. Ann Endocrinol (Paris). (2016) 77:179–86. doi: 10.1016/j.ando.2016.05.001
19. Faconti L, Kulkarni S, Delles C, Kapil V, Lewis P, Glover M, et al. Diagnosis and management of primary hyperaldosteronism in patients with hypertension: A practical approach endorsed by the british and irish hypertension society. J Hum Hypertens. (2024) 38:8–18. doi: 10.1038/s41371-023-00875-1
20. Gordon RD, Stowasser M. Primary aldosteronism: the case for screening. Nat Clin Pract Nephrol. (2007) 3:582–3. doi: 10.1038/ncpneph0626
21. Courcelles L, Stoenoiu M, Haufroid V, Lopez-Sublet M, Boland L, Wauthier L, et al. Laboratory testing for endocrine hypertension: current and future perspectives. Clin Chem. (2024) 70:709–26. doi: 10.1093/clinchem/hvae022
22. Hepburn S, Munday C, Taylor K, Halsall DJ. Stability of direct renin concentration and plasma renin activity in edta whole blood and plasma at ambient and refrigerated temperatures from 0 to 72 hours. Clin Chem Lab Med. (2022) 60:1384–92. doi: 10.1515/cclm-2022-0375
23. Liu Z, Jin L, Zhou W, Zhang C. The spectrum of plasma renin activity and hypertension diseases: utility, outlook, and suggestions. J Clin Lab Anal. (2022) 36:e24738. doi: 10.1002/jcla.24738
24. Baron S, Amar L, Faucon AL, Blanchard A, Baffalie L, Faucard C, et al. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. (2018) 36:1592–601. doi: 10.1097/HJH.0000000000001735
25. Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, et al. Aldosterone lc-ms/ms assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. (2018) 103:3965–73. doi: 10.1210/jc.2018-01041
26. Glinicki P, Jeske W, Bednarek-Papierska L, Kruszynska A, Gietka-Czernel M, Roslonowska E, et al. The ratios of aldosterone/plasma renin activity (ARR) versus aldosterone/direct renin concentration (ADRR). J Renin Angiotensin Aldosterone Syst. (2015) 16:1298–305. doi: 10.1177/1470320313519487
27. Hiramatsu K, Yamada T, Yukimura Y, Komiya I, Ichikawa K, Ishihara M, et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Results in Hypertensive Patients. Arch Intern Med. (1981) 141:1589–93. doi: 10.1001/archinte.1981.00340130033011
28. Lebek-Szatanska A, Papierska L, Glinicki P, Zgliczynski W. Poor performance of angiotensin ii enzyme-linked immuno-sorbent assays in mostly hypertensive cohort routinely screened for primary aldosteronism. Diagnostics (Basel). (2022) 12(5):1124. doi: 10.3390/diagnostics12051124
29. Hung A, Ahmed S, Gupta A, Davis A, Kline GA, Leung AA, et al. Performance of the aldosterone to renin ratio as a screening test for primary aldosteronism. J Clin Endocrinol Metab. (2021) 106:2423–35. doi: 10.1210/clinem/dgab348
30. Ahmed AH, Calvird M, Gordon RD, Taylor PJ, Ward G, Pimenta E, et al. Effects of two selective serotonin reuptake inhibitor antidepressants, sertraline and escitalopram, on aldosterone/renin ratio in normotensive depressed male patients. J Clin Endocrinol Metab. (2011) 96:1039–45. doi: 10.1210/jc.2010-2603
31. Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. (2012) 44:170–6. doi: 10.1055/s-0031-1295460
32. Calissendorff J, Falhammar H. Renal pseudohypoaldosteronism type 1-an adult case series including a novel gene variant. Endocrine. (2024) 87(3):1285–90. doi: 10.1007/s12020-024-04120-8
33. Glinicki P, Jeske W, Gietka-Czernel M, Bednarek-Papierska L, Kruszynska A, Slowinska-Srzednicka J, et al. The effect of blood collection procedure on plasma renin activity (PRA) and concentrations of direct renin (DRC) and aldosterone. J Renin Angiotensin Aldosterone Syst. (2015) 16:339–43. doi: 10.1177/1470320313494434
34. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, et al. Intraindividual variability of aldosterone concentrations in primary aldosteronism: implications for case detection. Hypertension. (2021) 77:891–9. doi: 10.1161/HYPERTENSIONAHA.120.16429
35. Ariens J, Horvath AR, Yang J, Choy KW. Performance of the aldosterone-to-renin ratio as a screening test for primary aldosteronism in primary care. Endocrine. (2022) 77:11–20. doi: 10.1007/s12020-022-03084-x
36. Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: updating the approach. J Clin Endocrinol Metab. (2020) 105:3771–83. doi: 10.1210/clinem/dgaa606
37. Carasel A, Calissendorff J, Avander K, Shabo I, Volpe C, Falhammar H. Ambulatory fludrocortisone suppression test in the diagnosis of primary aldosteronism: Safety, accuracy and cost-effectiveness. Clin Endocrinol (Oxf). (2022) 97:730–9. doi: 10.1111/cen.14793
38. Leung AA, Symonds CJ, Hundemer GL, Ronksley PE, Lorenzetti DL, Pasieka JL, et al. Performance of confirmatory tests for diagnosing primary aldosteronism: A systematic review and meta-analysis. Hypertension. (2022) 79:1835–44. doi: 10.1161/HYPERTENSIONAHA.122.19377
39. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. (2006) 48:2293–300. doi: 10.1016/j.jacc.2006.07.059
40. Phillips JL, Walther MM, Pezzullo JC, Rayford W, Choyke PL, Berman AA, et al. Predictive value of preoperative tests in discriminating bilateral adrenal hyperplasia from an aldosterone-producing adrenal adenoma. J Clin Endocrinol Metab. (2000) 85:4526–33. doi: 10.1210/jcem.85.12.7086
41. Gordon RD, Gomez-Sanchez CE, Hamlet SM, Tunny TJ, Klemm SA. Angiotensin-responsive aldosterone-producing adenoma masquerades as idiopathic hyperaldosteronism (Iha: adrenal hyperplasia) or low-renin essential hypertension. J Hypertens Suppl. (1987) 5:S103–6.
42. Irony I, Kater CE, Biglieri EG, Shackleton CH. Correctabl e subsets of primary aldosteronism. Primary adrenal hyperplasia and renin responsive adenoma. Am J Hypertens. (1990) 3:576–82. doi: 10.1093/ajh/3.7.576
43. Dogra P, Bancos I, Young WF Jr. Primary aldosteronism: A pragmatic approach to diagnosis and management. Mayo Clin Proc. (2023) 98:1207–15. doi: 10.1016/j.mayocp.2023.04.023
44. Zhu L, Zhang Y, Zhang H, Zhou W, Shen Z, Zheng F, et al. Comparison between adrenal venous sampling and computed tomography in the diagnosis of primary aldosteronism and in the guidance of adrenalectomy. Med (Baltimore). (2016) 95:e4986. doi: 10.1097/MD.0000000000004986
45. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. (2004) 136:1227–35. doi: 10.1016/j.surg.2004.06.051
46. Hua KF, Wu YH, Zhang ST. Clinical diagnostic value of liquid chromatography-tandem mass spectrometry method for primary aldosteronism in patients with hypertension: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2022) 13:1032070. doi: 10.3389/fendo.2022.1032070
47. Araujo-Castro M, Valderrabano P, Escobar-Morreale HF, Hanzu FA, Casals G. Urine steroid profile as a new promising tool for the evaluation of adrenal tumors. Literature Review. Endocrine. (2021) 72:40–8. doi: 10.1007/s12020-020-02544-6
48. Noh J, Lee C, Kim JH, Myung SW, Choi MH. Lc-ms based simultaneous profiling of adrenal hormones of steroids, catecholamines, and metanephrines. J Lipid Res. (2023) 64:100453. doi: 10.1016/j.jlr.2023.100453
49. Yin Y, Yu S, Qiu L, Wang X, Wang D, Ma C, et al. Establishment of a rapid and simple liquid chromatography tandem mass spectrometry method for measuring aldosterone in urine. J Chromatogr B Analyt Technol BioMed Life Sci. (2019) 1113:84–90. doi: 10.1016/j.jchromb.2019.03.012
50. Lin W, Yao Z, Li Y, Liao Z, Xiao J, Chen Y, et al. Developing an ultra-performance liquid chromatography-tandem mass spectrometry for detecting aldosterone in human plasma. J Clin Lab Anal. (2021) 35:e24029. doi: 10.1002/jcla.24029
51. Teruyama K, Naruse M, Tsuiki M, Kobayashi H. Novel chemiluminescent immunoassay to measure plasma aldosterone and plasma active renin concentrations for the diagnosis of primary aldosteronism. J Hum Hypertens. (2022) 36:77–85. doi: 10.1038/s41371-020-00465-5
52. Ozeki Y, Tanimura Y, Nagai S, Nomura T, Kinoshita M, Shibuta K, et al. Development of a new chemiluminescent enzyme immunoassay using a two-step sandwich method for measuring aldosterone concentrations. Diagnostics (Basel). (2021) 11(3):433. doi: 10.3390/diagnostics11030433
53. Nishikawa T, Satoh F, Takashi Y, Yanase T, Itoh H, Kurihara I, et al. Comparison and commutability study between standardized liquid chromatography-mass spectrometry/mass spectrometry (Lc-ms/ms) and chemiluminescent enzyme immunoassay for aldosterone measurement in blood. Endocr J. (2022) 69:45–54. doi: 10.1507/endocrj.EJ21-0278
54. Kobayashi H, Nakamura Y, Abe M, Tanabe A, Sone M, Katabami T, et al. Impact of a change to a novel chemiluminescent immunoassay for measuring plasma aldosterone on the diagnosis of primary aldosteronism. Endocr J. (2023) 70:489–500. doi: 10.1507/endocrj.EJ22-0585
55. Sjovall J. Fifty years with bile acids and steroids in health and disease. Lipids. (2004) 39:703–22. doi: 10.1007/s11745-004-1288-1
56. Eisenhofer G, Fassnacht M. Steroid profiling for adrenocortical disorders: A pathway for omics-based diagnostics. Clin Chem. (2017) 63:1787–9. doi: 10.1373/clinchem.2017.281048
57. Fanelli F, Di Dalmazi G. Serum steroid profiling by mass spectrometry in adrenocortical tumors: diagnostic implications. Curr Opin Endocrinol Diabetes Obes. (2019) 26:160–5. doi: 10.1097/MED.0000000000000475
58. Martin MM, Hamman BL. Patterns of urinary excretion of steroids in cushing’s syndrome. J Clin Endocrinol Metab. (1966) 26:257–67. doi: 10.1210/jcem-26-3-257
59. Ueshiba H, Segawa M, Hayashi T, Miyachi Y, Irie M. Serum profiles of steroid hormones in patients with cushing’s syndrome determined by a new hplc/ria method. Clin Chem. (1991) 37:1329–33. doi: 10.1093/clinchem/37.8.1329
60. Schoneshofer M, Weber B, Oelkers W, Nahoul K, Mantero F. Urinary excretion rates of 15 free steroids: potential utility in differential diagnosis of cushing’s syndrome. Clin Chem. (1986) 32:93–6.
61. Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, et al. Urine steroid metabolomics as a biomarker tool for detecting Malignancy in adrenal tumors. J Clin Endocrinol Metab. (2011) 96:3775–84. doi: 10.1210/jc.2011-1565
62. Bancos I, Taylor AE, Chortis V, Sitch AJ, Jenkinson C, Davidge-Pitts CJ, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the eurine-act study: A prospective test validation study. Lancet Diabetes Endocrinol. (2020) 8:773–81. doi: 10.1016/S2213-8587(20)30218-7
63. Eisenhofer G, Masjkur J, Peitzsch M, Di Dalmazi G, Bidlingmaier M, Gruber M, et al. Plasma steroid metabolome profiling for diagnosis and subtyping patients with cushing syndrome. Clin Chem. (2018) 64:586–96. doi: 10.1373/clinchem.2017.282582
64. Berke K, Constantinescu G, Masjkur J, Kimpel O, Dischinger U, Peitzsch M, et al. Plasma steroid profiling in patients with adrenal incidentaloma. J Clin Endocrinol Metab. (2022) 107:e1181–e92. doi: 10.1210/clinem/dgab751
65. Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. (2012) 97:881–9. doi: 10.1210/jc.2011-2384
66. Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. (2015) 65:1096–102. doi: 10.1161/HYPERTENSIONAHA.114.04453
67. Eisenhofer G, Duran C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open. (2020) 3:e2016209. doi: 10.1001/jamanetworkopen.2020.16209
68. Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. (2016) 62:514–24. doi: 10.1373/clinchem.2015.251199
69. Yang Y, Burrello J, Burrello A, Eisenhofer G, Peitzsch M, Tetti M, et al. Classification of microadenomas in patients with primary aldosteronism by steroid profiling. J Steroid Biochem Mol Biol. (2019) 189:274–82. doi: 10.1016/j.jsbmb.2019.01.008
70. Constantinescu G, Gruber S, Fuld S, Peitzsch M, Schulze M, Remde H, et al. Steroidomics-based screening for primary aldosteronism: impact of antihypertensive drugs. Hypertension. (2024) 81(10):2060–71. doi: 10.1161/HYPERTENSIONAHA.124.23029
71. Shackleton C, Pozo OJ, Marcos J. GC/MS in recent years has defined the normal and clinically disordered steroidome: will it soon be surpassed by LC/tandem MS in this role? J Endocr Soc. (2018) 2:974–96. doi: 10.1210/js.2018-00135
72. McDonald JG, Matthew S, Auchus RJ. Steroid profiling by gas chromatography-mass spectrometry and high-performance liquid chromatography-mass spectrometry for adrenal diseases. Horm Cancer. (2011) 2:324–32. doi: 10.1007/s12672-011-0099-x
73. Prete A, Lang K, Pavlov D, Rhayem Y, Sitch AJ, Franke AS, et al. Urine steroid metabolomics as a diagnostic tool in primary aldosteronism. J Steroid Biochem Mol Biol. (2024) 237:106445. doi: 10.1016/j.jsbmb.2023.106445
74. Turcu AF, Wannachalee T, Tsodikov A, Nanba AT, Ren J, Shields JJ, et al. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension. (2020) 75:183–92. doi: 10.1161/HYPERTENSIONAHA.119.13866
75. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. (2017) 2(8):e93136. doi: 10.1172/jci.insight.93136
76. Chang YL, Chen GY, Lee BC, Chen PT, Liu KL, Chang CC, et al. Optimizing adrenal vein sampling in primary aldosteronism subtyping through lc-ms/ms and secretion ratios of aldosterone, 18-oxocortisol, and 18-hydroxycortisol. Hypertens Res. (2023) 46:1983–94. doi: 10.1038/s41440-023-01347-2
77. Constantinescu G, Bidlingmaier M, Gruber M, Peitzsch M, Poitz DM, van Herwaarden AE, et al. Mass spectrometry reveals misdiagnosis of primary aldosteronism with scheduling for adrenalectomy due to immunoassay interference. Clin Chim Acta. (2020) 507:98–103. doi: 10.1016/j.cca.2020.04.019
78. Murakami M, Rhayem Y, Kunzke T, Sun N, Feuchtinger A, Ludwig P, et al. In situ metabolomics of aldosterone-producing adenomas. JCI Insight. (2019) 4(17):e130356. doi: 10.1172/jci.insight.130356
79. Song JJ, Cai J, Ma WJ, Lou Y, Bian J, Zhao B, et al. Untargeted metabolomics reveals potential plasma biomarkers for diagnosis of primary aldosteronism using liquid chromatography-mass spectrometry. BioMed Chromatogr. (2024) 38:e5855. doi: 10.1002/bmc.5855
80. van Rooyen JM, Poglitsch M, Huisman HW, Mels C, Kruger R, Malan L, et al. Quantification of systemic renin-angiotensin system peptides of hypertensive black and white african men established from the ras-fingerprint(R). J Renin Angiotensin Aldosterone Syst. (2016) 17(4):1470320316669880. doi: 10.1177/1470320316669880
81. Binder C, Poglitsch M, Agibetov A, Duca F, Zotter-Tufaro C, Nitsche C, et al. Angs (Angiotensins) of the alternative renin-angiotensin system predict outcome in patients with heart failure and preserved ejection fraction. Hypertension. (2019) 74:285–94. doi: 10.1161/HYPERTENSIONAHA.119.12786
82. Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, et al. Measurement of equilibrium angiotensin ii in the diagnosis of primary aldosteronism. Clin Chem. (2020) 66:483–92. doi: 10.1093/clinchem/hvaa001
83. Burdman I, Burckhardt BB. Human prorenin determination by hybrid immunocapture liquid chromatography/mass spectrometry: A mixed-solvent-triggered digestion utilizing D-optimal design. Rapid Commun Mass Spectrom. (2020) 34:e8932. doi: 10.1002/rcm.8932
84. Watanabe D, Morimoto S, Morishima N, Kato Y, Nagashima Y, Shibata N, et al. Adrenal (Pro)Renin receptor expression and serum soluble (Pro)Renin receptor concentration in primary aldosteronism. Int J Endocrinol. (2020) 2020:9640103. doi: 10.1155/2020/9640103
85. Glinicki P, Szatko A, Leszczyńska D, Papierska L, Zgliczyński W, Reincke M. PIPA 8 progress in primary aldosteronism. In: From basic research to clinical evidence. Conference programme and Abstracts. Munich, Germany: Medizinische Klinik und Poliklinik IV Ludwig-Maximilians-Universität Department of Internal Medicine (2024). p. 89–90.
86. Neves KB, Touyz RM. Extracellular vesicles as biomarkers and biovectors in primary aldosteronism. Hypertension. (2019) 74:250–2. doi: 10.1161/HYPERTENSIONAHA.119.13088
87. Carvajal CA, Tapia-Castillo A, Perez JA, Fardella CE. Primary aldosteronism, aldosterone, and extracellular vesicles. Endocrinology. (2022) 163(1):bqab240. doi: 10.1210/endocr/bqab240
88. Bertolone L, Castagna A, Manfredi M, De Santis D, Ambrosani F, Antinori E, et al. Proteomic analysis of urinary extracellular vesicles highlights specific signatures for patients with primary aldosteronism. Front Endocrinol (Lausanne). (2023) 14:1096441. doi: 10.3389/fendo.2023.1096441
89. Barros ER, Rigalli JP, Tapia-Castillo A, Vecchiola A, Young MJ, Hoenderop JGJ, et al. Proteomic profile of urinary extracellular vesicles identifies agp1 as a potential biomarker of primary aldosteronism. Endocrinology. (2021) 162(4):bqab032. doi: 10.1210/endocr/bqab032
90. Carvajal CA, Tapia-Castillo A, Perez JA, Fardella CE. Serum alpha-1-acid glycoprotein-1 and urinary extracellular vesicle mir-21-5p as potential biomarkers of primary aldosteronism. Front Immunol. (2021) 12:768734. doi: 10.3389/fimmu.2021.768734
91. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (Misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
92. Ochiai-Homma F, Kuribayashi-Okuma E, Tsurutani Y, Ishizawa K, Fujii W, Odajima K, et al. Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism. Hypertens Res. (2021) 44:1557–67. doi: 10.1038/s41440-021-00710-5
93. Kong L, Tang X, Kang Y, Dong L, Tong J, Xu J, et al. The role of urinary extracellular vesicles sodium chloride cotransporter in subtyping primary aldosteronism. Front Endocrinol (Lausanne). (2022) 13:834409. doi: 10.3389/fendo.2022.834409
94. Hayakawa T, Fukuhara A, Saiki A, Otsuki M, Shimomura I. Gammaenac/cd9 in urinary extracellular vesicles as a potential biomarker of mr activity. J Endocrinol. (2021) 252:81–90. doi: 10.1530/JOE-21-0228
Keywords: primary aldosteronism, hypertension, adrenal tumor, aldosterone, steroidomics, proteomics, immunoassays, biomarkers
Citation: Szatko A, Toboła A, Falhammar H, Zgliczyński W and Glinicki P (2025) Advances in the biochemical diagnostics of primary aldosteronism: from immunoassays to steroidomics and proteomics. Front. Endocrinol. 16:1548344. doi: 10.3389/fendo.2025.1548344
Received: 19 December 2024; Accepted: 07 March 2025;
Published: 16 April 2025.
Edited by:
Mirko Parasiliti-Caprino, University of Turin, ItalyReviewed by:
Federico Ponzetto, University of Turin, ItalySergei Tevosian, University of Florida, United States
Copyright © 2025 Szatko, Toboła, Falhammar, Zgliczyński and Glinicki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alicja Szatko, alicja.szatko@gmail.com; Piotr Glinicki, piotr.glinicki@bielanski.med.pl
 Alicja Szatko
Alicja Szatko