- 1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Department of General Practice, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Institute of Cardiovascular Disease Research, Xuzhou Medical University, Xuzhou, Jiangsu, China
Background: Upper gastrointestinal bleeding (UGIB) is a common complication in patients with non-ST-segment elevation myocardial infarction (NSTEMI) after percutaneous coronary intervention (PCI), and the aim of our study is to construct a nomogram for predicting the occurrence of UGIB within 1 year after PCI in NSTEMI patients.
Methods: In this study, 784 patients with NSTEMI after PCI in the Affiliated Hospital of Xuzhou Medical University between September 1, 2017 and August 31, 2019 were included as the training group, and 336 patients from the East Affiliated Hospital of Xuzhou Medical University were included as the external validation group. Classical regression methods were combined with a machine learning model to identify the independent risk factors. These factors based on multivariate logistic regression analysis were then utilized to develop a nomogram. The performance of the nomogram was evaluated using the area under the receiver operating characteristic curve (AUC), calibration plots, and decision curve analysis (DCA).
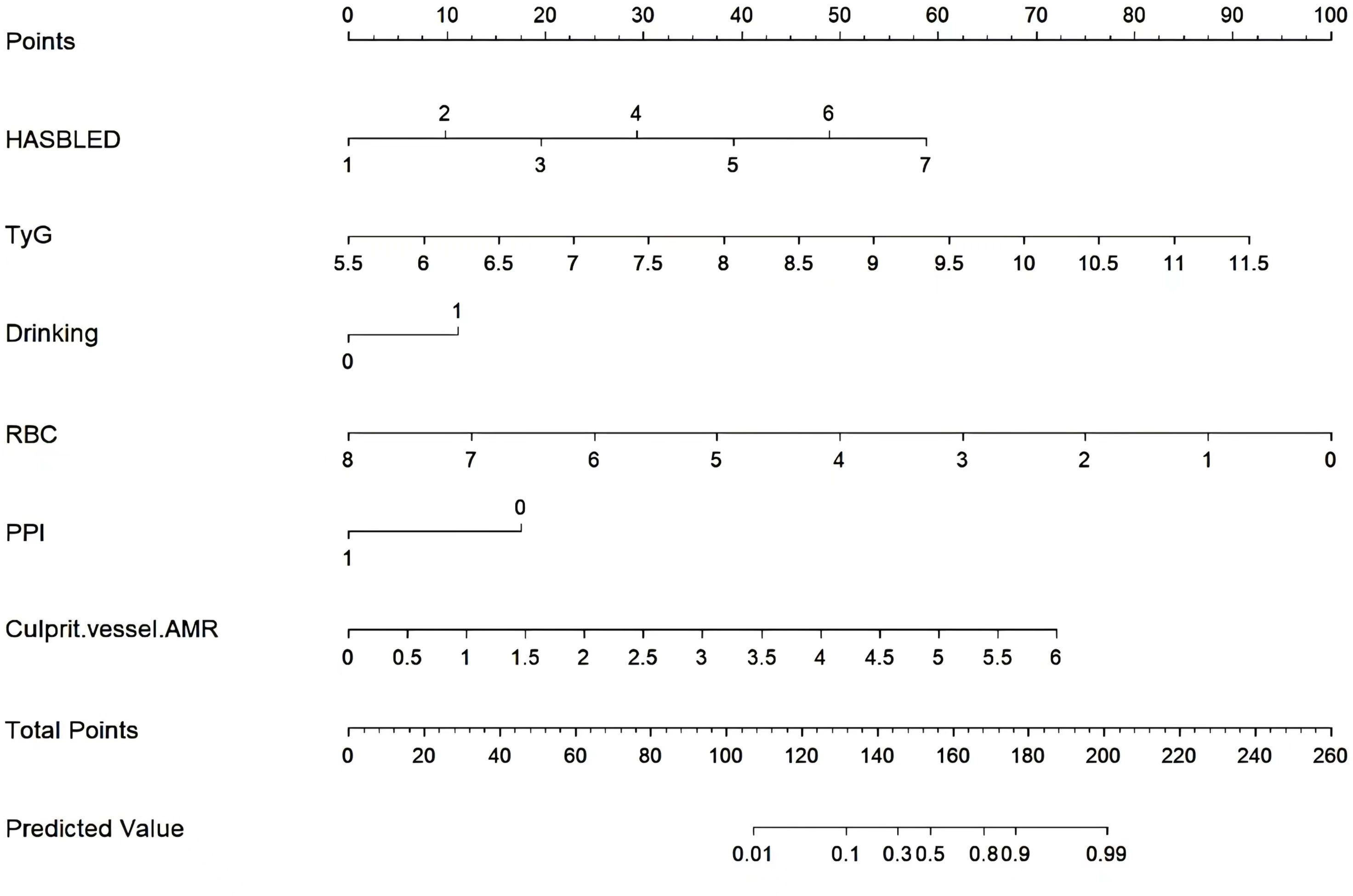
Results: The nomogram consisted of six independent predictors, including HASBLED, triglyceride glucose index, alcohol drinking, red blood cell count, use of proton pump inhibitor, and angiographic microvascular resistance of culprit vessel. Training and validation groups accurately predicted the occurrence of UGIB (AUC, 0.936 and 0.910). The calibration curves showed that the nomogram agreed with the actual observations and the DCA also demonstrated that the nomogram was applicable in the clinic.
Conclusion: We developed a simple and effective nomogram for predicting the occurrence of UGIB within 1 year in NSTEMI patients after PCI based on angiographic microvascular resistance.
Introduction
Coronary artery disease (CAD) is the leading major cause of death and loss of healthy life in society, both in terms of morbidity, mortality, and disease progression (1). Non-ST-segment elevation myocardial infarction (NSTEMI) is a severe type of CAD and in NSTEMI, coronary atherosclerotic plaque erosion, rupture or subsequent thrombosis causes partial obstruction of the coronary arteries, which can lead to myocardial necrosis (2). In myocardial infarction, NSTEMI accounted for 63.1% and 4.2% of NSTEMI patients died in the hospital (3).
With advances in antiplatelet and percutaneous coronary intervention (PCI) therapies, cardiovascular morbidity and mortality in patients with NSTEMI have decreased significantly (4). However, the occurrence of gastrointestinal bleeding (GIB) after PCI remains a major problem. The most common bleeding after PCI was GIB, which accounted for 61.7% of all bleeding (5). A study indicated that the incidence of upper gastrointestinal bleeding (UGIB) in patients with ACS was 8.9% within 30 days of PCI, 4.7% within 1 year, and rose to 10.1% beyond 1 year (6). Yasuda et al. found that the incidence of UGIB at 1 and 2 years after PCI was 2.5% and 5%, respectively, whereas the incidence was higher in patients who did not use the proton pump inhibitor (PPI), at 4.5% and 9.2%, respectively (7). And patients with acute coronary syndrome (ACS) had a 62% mortality rate for UGIB compared to patients with UGIB only (8). This caused a certain burden on both the cost of hospitalization and medical insurance. Thus, early screening for UGIB in ACS patients is necessary.
Coronary microcirculatory disorders (CMD) is disease that affect the structure and function of the coronary microcirculation. There were various methods of assessing CMD, categorized as invasive and non-invasive. Positron emission computed tomography (PET) was considered the gold standard for noninvasive assessment of CMD by quantifying myocardial blood flow (MBF) and assessing myocardial perfusion reserve (9). Cardiac magnetic resonance imaging (CMR) and myocardial contrast echocardiography could also noninvasively quantify MBF (10). Invasive assessment methods such as coronary angiography could assess microvascular dilatation by coronary flow reserve (CFR) (10). The index of microcirculatory resistance (IMR) was the gold standard for measuring CMD and was obtained by measuring the product of distal coronary artery pressure and the mean passage time of saline push during maximal congestion induced by adenosine or opioid (11). In recent years, angiographic microvascular resistance (AMR) has been proposed for the assessment of CMD and was mainly calculated by quantitative flow ratio (QFR) (12). Compared with invasive methods, AMR did not rely on drug induction such as adenosine and require additional operations in clinical applications, providing simplicity and maneuverability. And it enabled accurate numerical assessment of microvascular function at a lower cost than non-invasive methods. Hence, it was considered an emerging method to CMD assessment.
Some chronic diseases (e.g., heart failure, hypertension, diabetes, metabolic syndrome, cardiac hypertrophy, and CAD) and post-PCI were risk factors for CMD (13–16). We pondered whether CMD affected systemic microcirculation and would increase the risk of having UGIB. Therefore, we constructed an AMR-based nomogram to predict UGIB within 1 year in NSTEMI patients after PCI.
Materials and methods
Study population and design
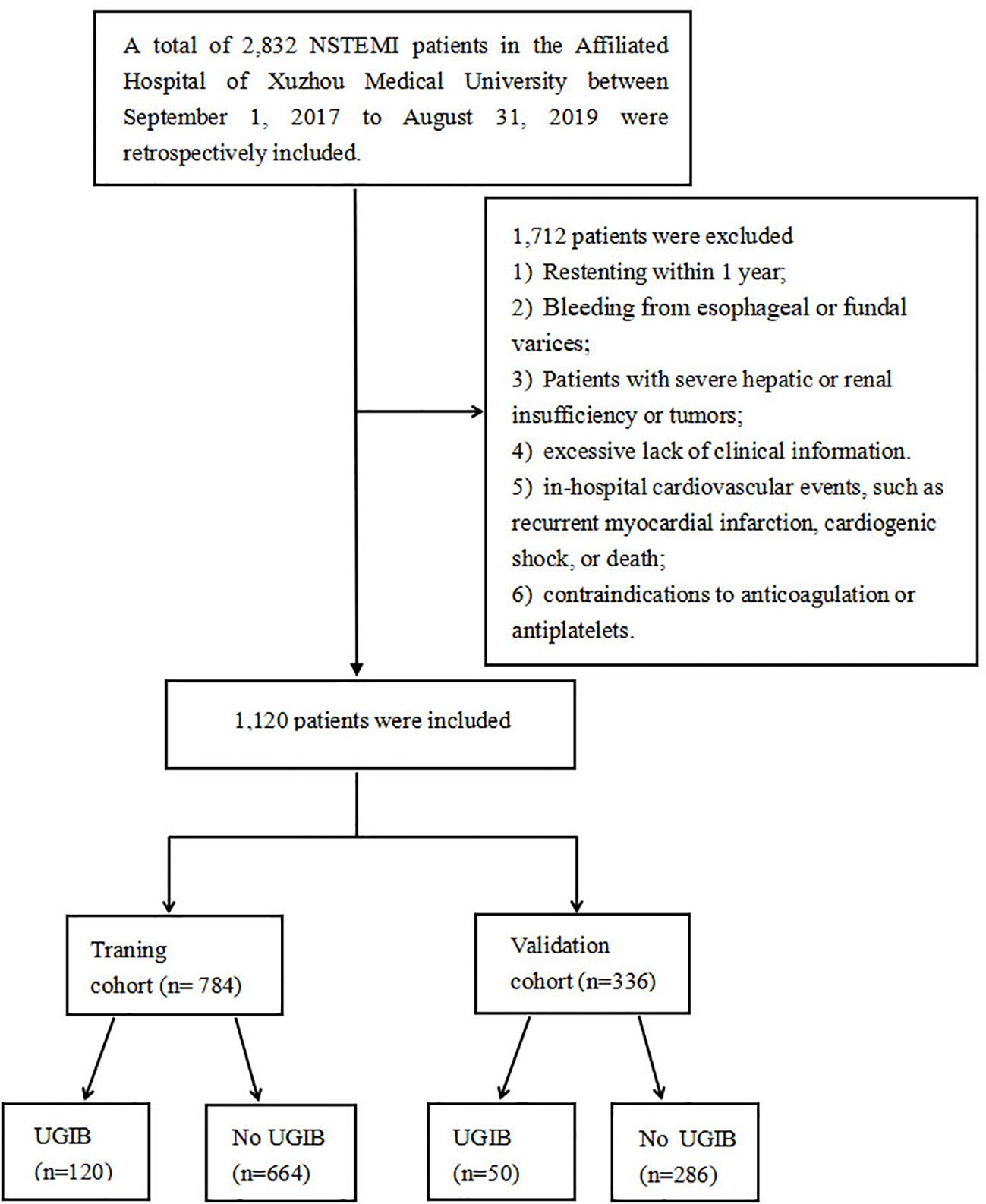
This study was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2023-KL043-01), which was conducted following the Declaration of Helsinki. Because our study was retrospective, the committee waived the requirement for written informed consent. Based on inclusion and exclusion criteria, we included patients with NSTEMI who underwent PCI from September 1, 2017, to August 31, 2019 in the Affiliated Hospital of Xuzhou Medical University (training group, n=784), and the East Hospital of Xuzhou Medical University (validation group, n=336). The flow chart of the study is shown in Figure 1.
Inclusion criteria: (1) Diagnosis of NSTEMI; (2) Successful implantation of the drug-eluting stent; (3) Readmission for UGIB within 1 year after PCI; and (4) over 18 years old.
Meanwhile, exclusion criteria: (1) Restenting within 1 year; (2) Bleeding from esophageal or fundal varices ; (3) Patients with severe hepatic or renal insufficiency or tumors; (4) excessive lack of clinical information; (5) in-hospital cardiovascular events, such as recurrent myocardial infarction, cardiogenic shock, or death; (6) contraindications to anticoagulation or antiplatelets.
Clinical treatment process
All patients with NSTEMI in this study underwent PCI in the digital subtraction angiography (DSA) suite, and patients were anticoagulated with heparin subcutaneously and given 300 mg of aspirin, 300 mg of clopidogrel, or 180 mg of ticagrelor orally as a loading dose before PCI. The various devices, instruments, and adjunctive medications (nitroglycerin, sodium nitroprusside, tirofiban, atropine, etc.) used during the procedure were determined by the operator based on the patient’s intraoperative condition.
Postoperatively, medications were prescribed according to guidelines. These therapies included (1) dual antiplatelet therapy (DAPT), including aspirin (100 mg once daily) in combination with ticagrelor (90 mg twice daily) or clopidogrel (75 mg once daily) (2); statin (3); beta-blocker; and (4) angiotensin-converting enzyme inhibitor/angiotensin receptor antagonist (ACEI/ARB).
Collection of variables
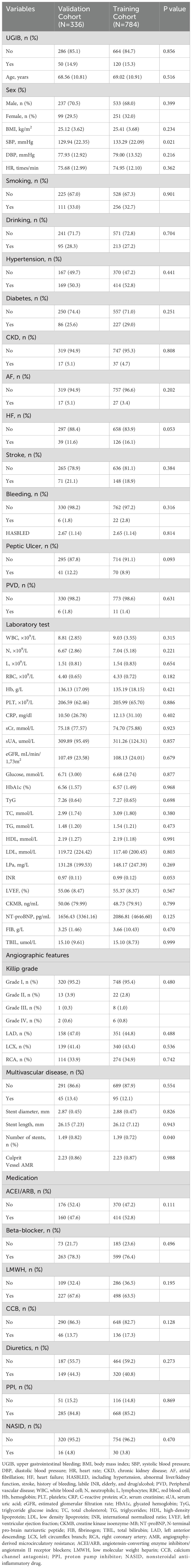
Demographic data included age, sex, smoking, and drinking status. Previous history contained heart failure (HF), diabetes, stroke, hypertension, chronic kidney disease (CKD), bleeding, peripheral vascular disease (PVD), atrial fibrillation (AF), and peptic ulcer. In the history of bleeding, 16 patients had nosebleeds and 12 patients had cerebral hemorrhage before PCI. Physical examination contained body mass index (BMI), diastolic blood pressure (DBP), heart rate (HR), and systolic blood pressure (SBP). Laboratory tests contained white blood cell (WBC) count, hemoglobin (Hb), lymphocyte count, red blood cell (RBC) count, platelet (PLT) count, neutrophil, serum uric acid (sUA), C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), glucose, glycated hemoglobin (HbA1c), serum creatinine (sCr), low-density lipoprotein (LDL), total cholesterol (TC), high-density lipoprotein (HDL), lipoprotein a (LPa), triglycerides (TG), N-terminal pro-brain natriuretic peptide (NT-proBNP), international normalized ratio (INR), left ventricular ejection fraction (LVEF), creatinekinase-MB (CKMB), fibrinogen (FIB), and total bilirubin(TBIL). Image data during PCI contained the culprit vessels (right coronary artery (RCA), left anterior descending branch (LAD), left circumflex branch (LCX)), multivessel disease, the number of stents, stent length, stent diameter, and Killip grade. Postoperative medications comprised of angiotensin-converting enzyme inhibitor/angiotensin receptor antagonist (ACEI/ARB), calcium antagonist (CCB), Low molecular weight heparin (LMWH), diuretics, proton pump inhibitor (PPI), beta-blockers, and nonsteroidal anti-inflammatory drug (NSAID).
Triglyceride glucose index (TyG) was calculated as Ln[TG(mg/dL) × FBG(mg/dL)/2]. HAS-BLED scores up to 9 points including hypertension, stroke, history of bleeding, abnormal liver/kidney function, labile INR, elderly, and drug/alcohol.
Computation of AMR
AMR analysis was performed independently by certified technicians using commercial software (AngioPlus Core, Pulse Medical Imaging Technology Co., Ltd., Shanghai, China), who were blinded to the clinical data. Coronary artery image analysis was performed using the above system. The blood flow velocity was derived by dividing the vessel centerline length by the contrast fill time. Using high blood flow as a boundary condition, the pressure drop was calculated from the hydrodynamic equations. Distal coronary pressure (Pd) was calculated from the pressure drop, and μQFR was calculated by dividing Pd by mean aortic pressure (Pa). Angiography microvascular resistance (AMR) is computed as Pd divided by the hyperaemic flow velocity Velocityhyp (17).
Clinical endpoints and definitions
The clinical endpoint was NSTEMI patients who were readmitted with UGIB symptoms within 1 year after the PCI. The definition of UGIB was clinical signs of coffee-ground vomiting, hematemesis, melena, or endoscopic findings of active bleeding from upper gastrointestinal sites.
Statistical analysis
Categorical variables were expressed as frequencies and percentages and compared using the chi-square test or Fisher’s exact test. Continuous variables are expressed as mean ± standard deviation. Normally distributed variables were compared using the t-test for comparison, while non-normally distributed variables were compared using the Mann-Whitney U-test. The percentage of missing values were less than 20%, and multiple imputation was used to impute the missing data for the covariates. We first used univariate analysis and random forest to predict independent risk factors. The random forest algorithm generated the mean decreased Gini (MDG) that was used to reflect the contribution of each independent variable to the dependent variable. In the random forest algorithm, Ntree specifies the number of decision trees in the random forest and Brenneman suggests that the optimal number of decision trees is 500. Optimal mtry parameter was selected by grid search method, and then combined with different ntree to find the lowest out-of-bag error (OOB) rate. Finally, we included independent risk factors that were statistically significant in the univariate analysis (P< 0.01) and the top ten variables of the random forest MDG in the analysis. The variables were screened and used to perform the multivariate logistic regression model. The least absolute shrinkage and selection operator (LASSO) regression was then performed to ensure that the model was not overfitted. Finally, a nomogram of the multivariate model based on optimal predictors was developed to predict the probability of UGIB within 1 year after PCI. The consistency index (C-index) is the area under the curve (AUC) of the receiver operating characteristic curve (ROC) in logistic regression analysis for the discrimination capacity of the nomogram. The nomogram’s predictive accuracy was evaluated using calibration plots and the consistency between predicted and actual probabilities was assessed by the Hosmer-Lemeshow test. Clinical efficacy was evaluated using decision curve analysis (DCA). Data were analyzed using R Studio (Version 4.2.3, https://www.Rproject.org). P values < 0.05 were significant for all statistical tests.
Ethics approval and consent to participate
This study was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2023-KL043-01), which was conducted following the Declaration of Helsinki. Because our study was retrospective, the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University waived the requirement for written informed consent.
Results
Participants characteristics
Our study included 1,120 NSTEMI patients after PCI from September 1, 2017, to August 31, 2019, from the Affiliated Hospital of Xuzhou Medical University and the East Affiliated Hospital of Xuzhou Medical University. Baseline characteristics were shown in Table 1. The average age of this study was 68.88 years old and most of the patients (68.8%) were male. There were 120 and 50 UGIB participants in the training and validation groups, respectively.
Potential predictors of UGIB and construction of the nomogram
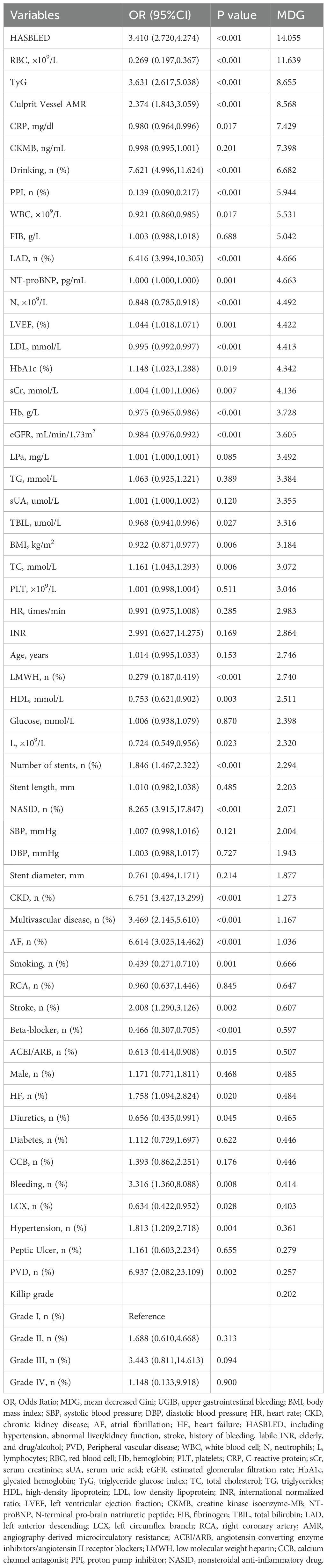
The results of the univariate analyses and random forest were displayed in Table 2. The default value of Ntree was 500. After testing and adjusting, mtry=6 and ntree=300 had the lowest rate of OOB (2.81%). These variables (HASBLED, TyG index, Alcohol drinking, RBC count, PPI use, and AMR of culprit vessel) not only had a significant difference in the univariate analysis (p< 0.01) but also obtained high MDG (top ten) in the random forest. Therefore, these six variables were included in further multivariate logistic regression model.
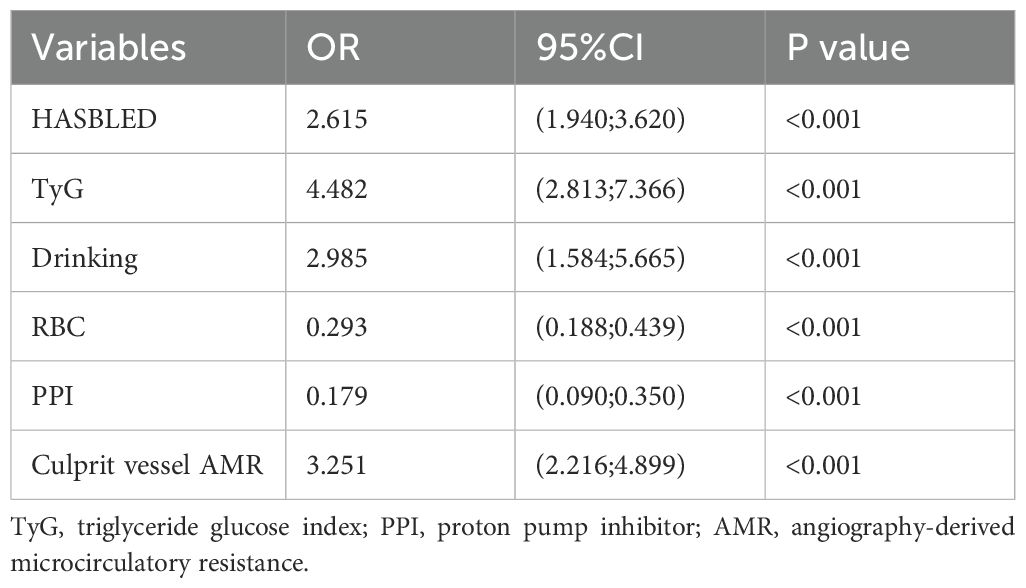
Six potential risk factors were included in the multivariate logistic regression model (Table 3). In the training set model, a high HASBLED, TyG index, and AMR of the culprit vessel were correlated with developing the risk of UGIB (OR: 2.615, 95% CI: 1.940-3.620, P< 0.001; OR: 4.482, 95% CI: 2.813-7.366, P< 0.001; OR: 3.251, 95% CI: 2.216-4.899, P< 0. 001). Alcohol drinking was positively associated with the occurrence of UGIB (OR: 2.985, 95% CI: 1.584-5.665, P< 0.001). While taking PPI and higher RBC count played a protective role (OR: 0.179, 95% CI: 0.090-0.350, P< 0. 001; OR: 0.293, 95% CI: 0.188-0.439, P< 0. 001).
Subsequently, these predictors were determined using the least absolute shrinkage and selection operator (LASSO) method for screening non-zero coefficient characteristics (Figures 2A, B). We used tenfold cross-validation to select the appropriate tuning parameters (λ) for the LASSO model. The optimal reconciliation coefficients λ.min at the minimum MSE and λ.1se at one standard MSE error were 0.004 and 0.031, respectively. The advantages of the Lasso regression were highly predictive and robust and minimized the effects of multicollinearity. Finally, the nomogram model was constructed using these six independent factors to predict the occurrence of UGIB within 1 year after PCI (Figure 3).
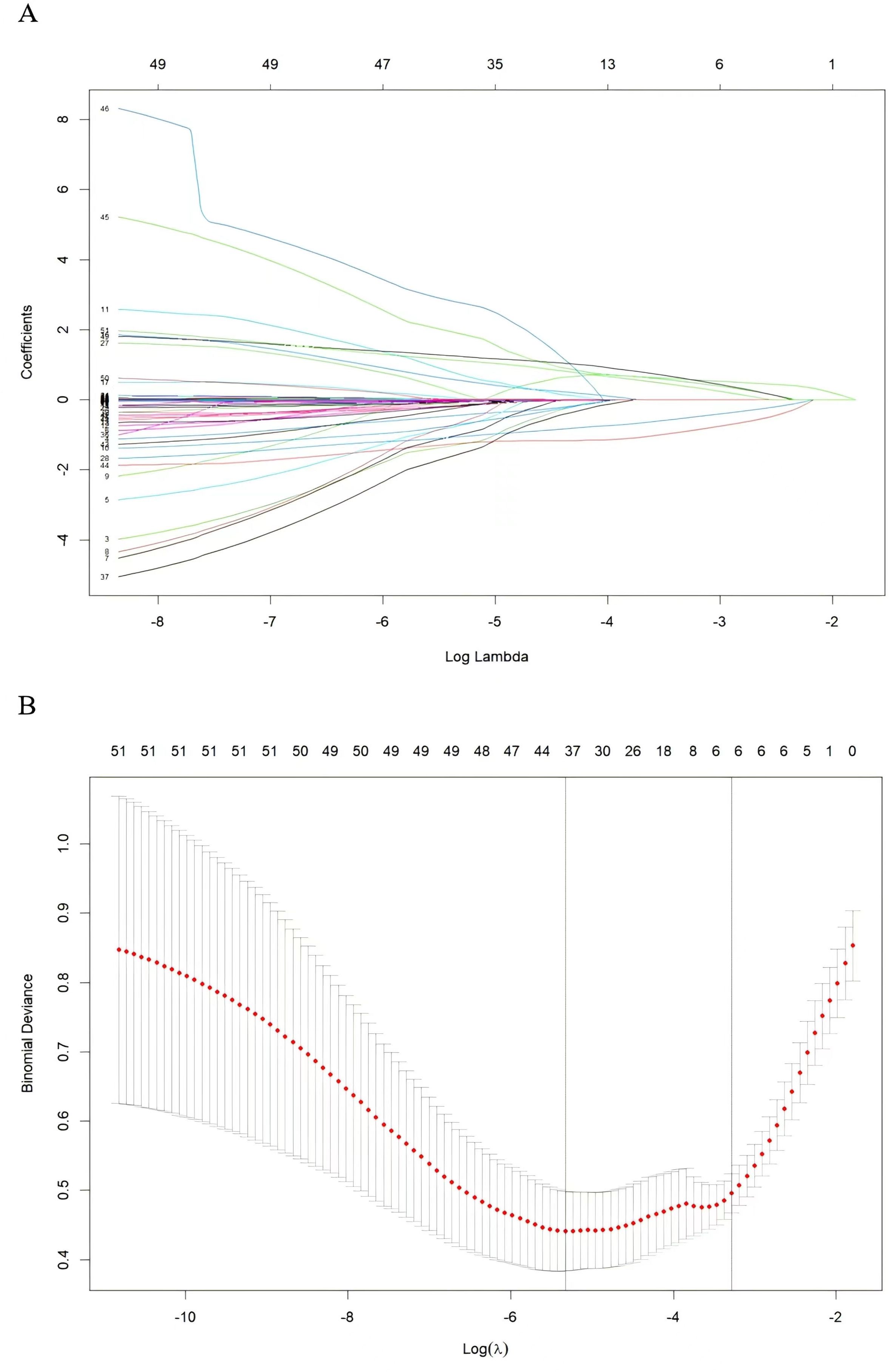
Figure 2. Variable screening based on lasso regression. (A) Characterization of the variation of variable coefficients; (B) The process of selecting the optimal value of the parameter λ in the Lasso regression model by the cross-validation method.
Validation of the nomogram
The AUC of training and external validation groups was 0.936 and 0.910 in the ROC curve (Figures 4A, B), which demonstrated an outstanding discrimination of the nomogram. Calibration plots identified good consistency between the nomogram (Figure 5A) and the validation cohort (Figure 5B). The Hosmer-Lemeshow test indicated that the probability of UGIB after PCI predicted by the nomogram was consistent between the training set (P = 0.728) and the validation set (P = 0.269).
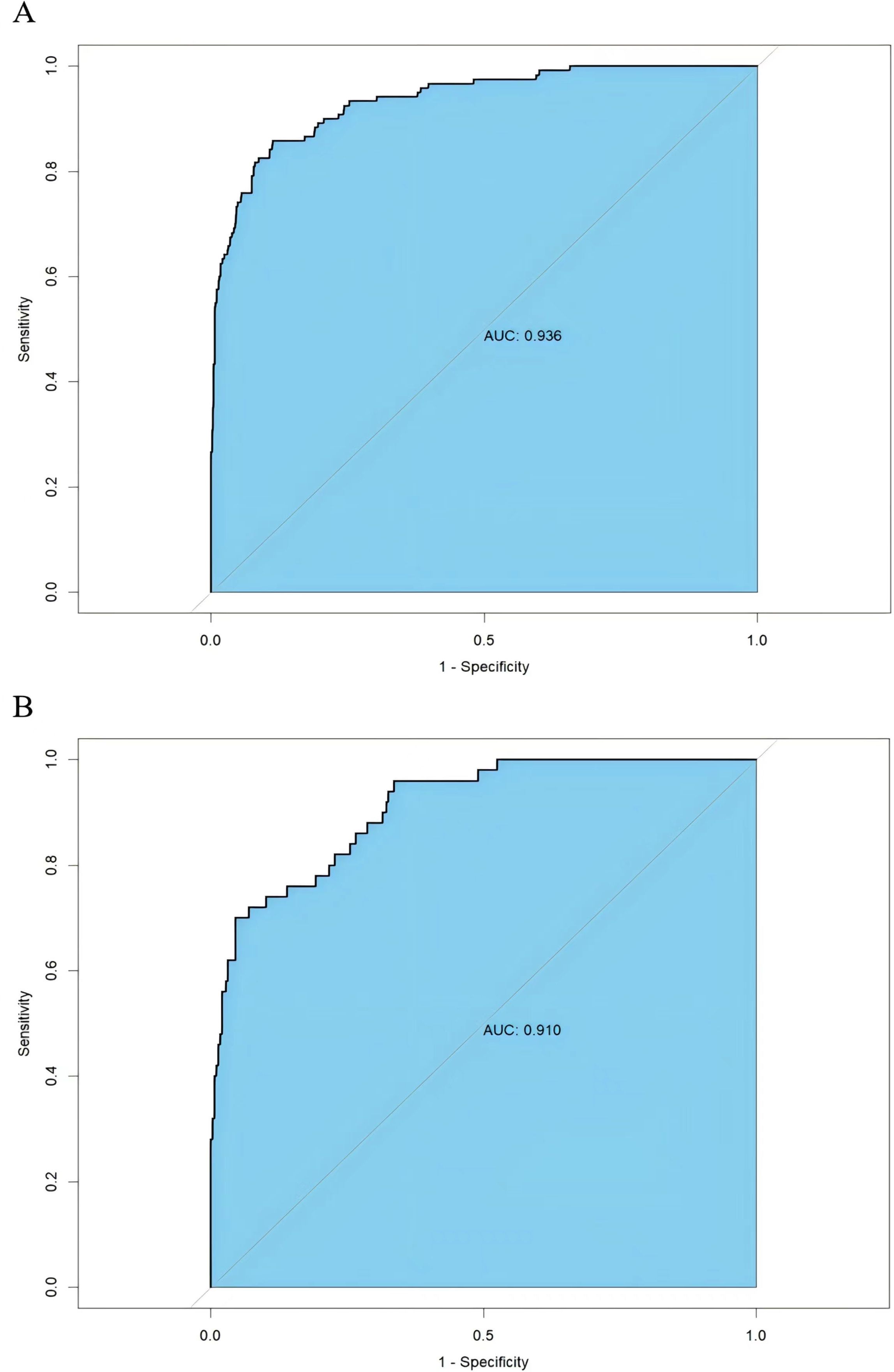
Figure 4. Receiver operating characteristics curve of the nomogram in the training group (A) and the validation group (B).
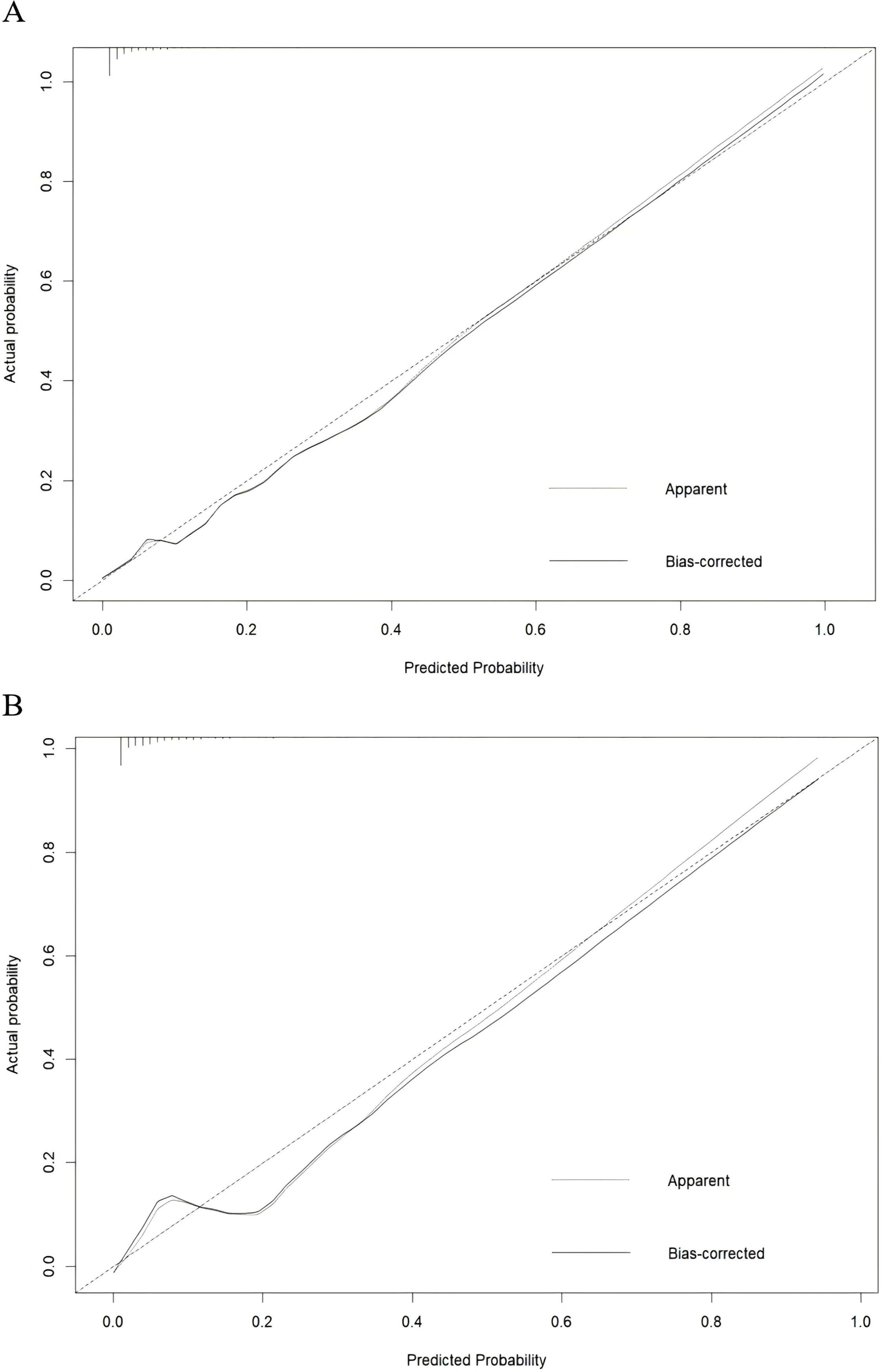
Figure 5. Calibration curve for the training group (A) and the validation group (B), the horizontal axis denotes the overall predicted probability of UGIB in NSTEMI patients after percutaneous coronary intervention, and the vertical axis displays the actual probability.
Clinical use
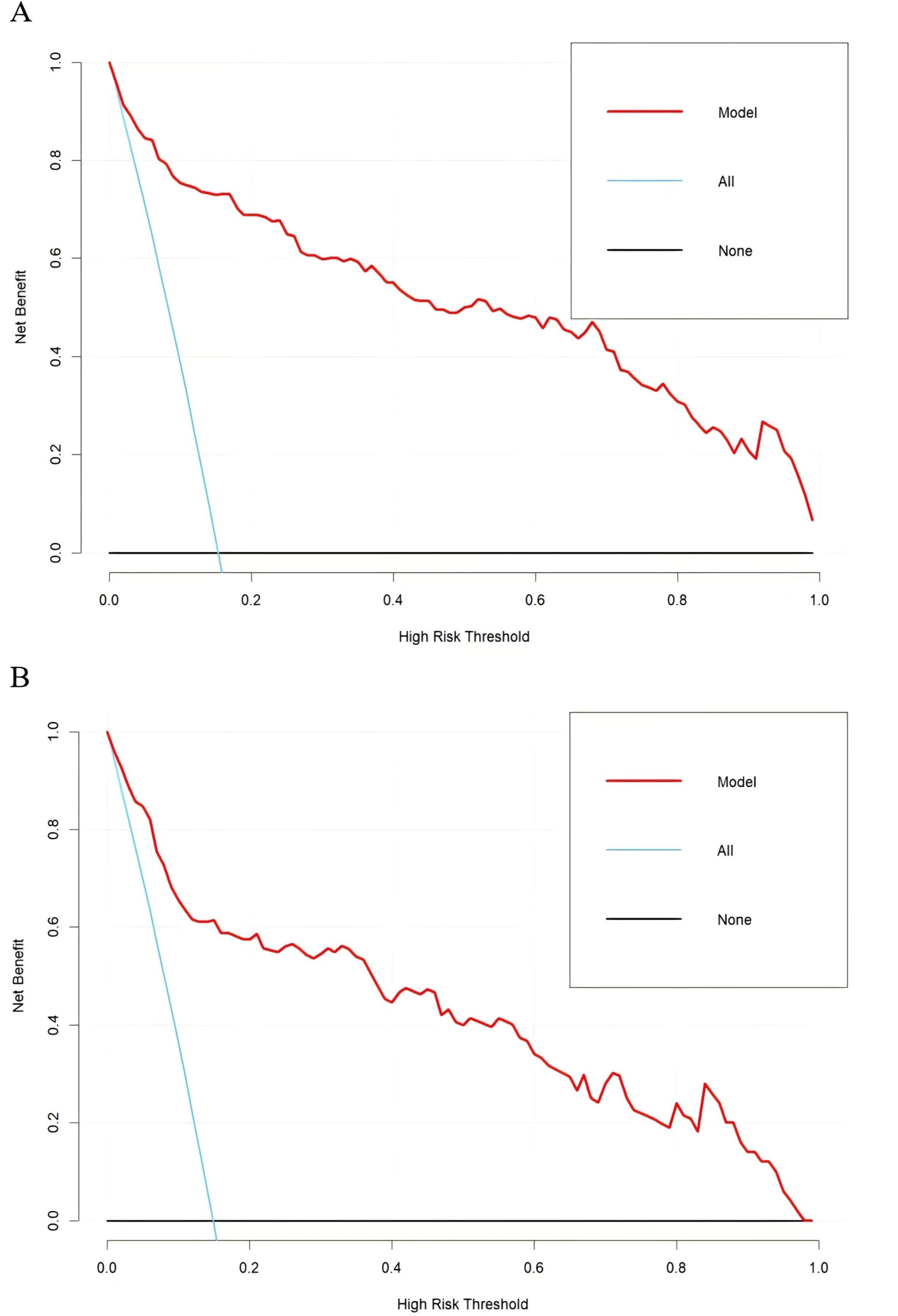
The applicability and utility of the model in the training and external validation sets were evaluated by DCA. The DCA curves for both sets showed a significantly higher net benefit than the two extremes, indicating that the nomogram had good clinical benefits (Figures 6A, B).
Discussion
UGIB is a common cause of bleeding after PCI, and in-hospital occurrence of UGIB has a high mortality rate (18). Some patients are more susceptible to have UGIB in one year of dual antiplatelet therapy applied after PCI (19). Consequently, a prediction model that accurately predicts UGIB is beneficial in obtaining a better clinical significance in patients with NSTEMI after PCI. In this study, 1,120 NSTEMI patients with post-PCI were included for analysis and AMR of culprit vessel (≥ 2.5 mmHg*s/cm) were diagnosed with CMD. The results showed that AMR of culprit vessel was an independent predictor of UGIB. In addition, the nomogram constructed by six factors, including HASBLED, TyG index, Alcohol drinking,RBC count, PPI use, and AMR of culprit vessel, can be used to assess the likelihood of UGIB within 1 year in NSTEMI patients undergoing primary PCI.
The mechanisms of CMD may be closely related to inflammation, microvascular spasm, or endothelial dysfunction (20). There is growing evidence that patients with chronic inflammatory diseases (without clinically evident CVD), including psoriasis, psoriatic arthritis, rheumatoid arthritis, and inflammatory bowel disease, have a higher incidence of endothelial and coronary microcirculatory dysfunction (21). In contrast, presence of CMD in NSTEMI patients after PCI may be related to inflammation. In patients with early CAD, coronary segments with macrophage infiltration and vascular proliferation showed a greater response to acetylcholine (ACh) than those without macrophage infiltration and vascular proliferation, suggesting an important role for inflammation and vascular proliferation in the pathogenesis of CAD (22). In addition, the complexity of CMD is further compounded by the possibility of microvascular obstruction (MVO) in patients with acute coronary syndromes (ACS) (23). The causative mechanisms of MVO include distal atherosclerotic thromboembolism, ischemia-reperfusion injury with endothelial cell death along the myocardial cell death, myocardial edema and/or inflammation, which may ultimately result in persistent angina symptoms, despite the fact that the patient has undergone PCI or coronary artery bypass grafting (CABG) (23).
AMR has recently been proposed as a simple way to measure CMD (12). Fan et al. showed a good correlation between AMR and IMR (r=0.83) and found that the accuracy of AMR (≥ 2.5 mmHg*s/cm) for the diagnosis of CMD was high (87.2%) using IMR (≥ 25 U) as the standardized reference (12). In addition, several studies have shown that AMR is not only strongly associated with CMD, but also has a significant association with the prognosis of cardiovascular events. Ma et al. found that AMR was a valid indicator for assessing CMD in patients with obstructive hypertrophic cardiomyopathy, and high microvascular resistance (3 vessel AMR ≥ 7.04) was correlated with poor prognosis (24). And a retrospective study found that AMR could predict the risk of all-cause death or heart failure readmission after PCI in STEMI patients (25). Therefore, AMR, as a surrogate measurement tool for CMD, has an equally important clinical value in the assessment of patient prognosis.
It has been proposed that patients with CMD may not only manifest in the heart, but rather a systemic microvascular disease that may manifest in multi-system disorders including dementia, renal dysfunction, and retinopathy (26). Ohura-Kajitani et al. demonstrated that, in the absence of inhibitors, patients with MVA (Microvascular Angina) and those with both VSA (Vasospastic Angina) and MVA had little or no significant response in resistance arteries to vasodilator drugs compared with patients with VSA alone (27). Overall, the findings support the idea that MVA is not only a microvascular disease limited to the heart, but may also be a systemic microvascular dysfunction, and that MVA can be considered a cardiac manifestation of systemic microvascular disease (27). In patients with CMD, the problem is not limited to the coronary microcirculation and microvascular function can be abnormal throughout the body. Particularly in patients with NSTEMI, one of the possible causes of UGIB after PCI is dysfunction of the GI microcirculation, which may weaken the defenses of the GI mucosa against the acidic environment, thereby increasing the risk of bleeding. Secondly, dysfunction of the heart as a pumping organ may lead to reduced peripheral blood flow, which in turn exacerbates microcirculatory disturbances in the GI tract, leading to decreased mucosal defenses against acidic environments, and ultimately to bleeding. This mechanism provides a potential pathologic explanation for AMR as an independent predictor of UGIB.
Recently, the TyG index has attracted a lot of attention. Several studies have found that the TyG index was an indicator of insulin resistance (IR) and also reflected systemic metabolism (28, 29). A meta-analysis showed an association between a higher TyG index and the risk of developing CAD (30). Shi et al. found a linear positive correlation between TyG index and the occurrence of ischemic stroke (31). Zhao et al. found that the risk of arterial stiffness and renal microcirculatory injury increased with the TyG index (32). In conclusion, a high TyG index was not only a cardiovascular risk factor, but was also associated with cerebrovascular and renal vascular diseases. Although the exact biological mechanisms linking TyG index to disease were unknown, key pathways may be associated with IR. Chronic hyperglycemia and dyslipidemia induced by IR triggers oxidative stress, exacerbates inflammatory responses, promotes foam cell formation, impairs endothelial function, and contributes to smooth muscle cell proliferation (28, 29). In addition, persistent IR increases sympathetic nervous system activity, renal sodium retention, and elevated blood pressure, which increases cardiac load and leads to vascular and renal injury (33). Finally, IR may affect coronary microcirculation, and is strongly associated with myocardial injury and myocardial reperfusion (34). However, few studies have explored the association between TyG index and gastrointestinal circulation. In our study, we found that the TyG index was a predictor of UGIB in NSTEMI patients after PCI, which implied that gastrointestinal circulation was similarly affected in high TyG populations.
Secondly, Feit et al. found that patients with anemia had almost double the risk of all types of bleeding, including a fourfold increase in the risk of GIB, compared to patients without anemia (35). In a two-center study of patients with atrial fibrillation treated with PCI, severe GIB occurred in 12.4% of patients with anemia compared with 3.1% of patients without anemia (36). Similarly, we found that low red blood cell count had a high risk of developing GIB, probably because patients with low red blood cell count were anemic. Patients with anemia often have poor systemic microcirculation, which may affect gastrointestinal microcirculation. In the context of the above discussion, we believe that it is not just damage to the circulation of one organ, but more likely some degree of damage to the systemic microcirculation, of which gastrointestinal hemorrhage is a manifestation.
The HASBLED score was a tool used to assess the risk of bleeding in patients with atrial fibrillation who were receiving anticoagulation therapy (37). In recent years, the application of the HASBLED score has gradually expanded beyond assessing bleeding risk in patients with atrial fibrillation. Konishi et al. and Castini et al. found that the HASBLED score could predict bleeding risk (including GIB) and mortality in patients without atrial fibrillation after PCI (38, 39). HASBLED score incorporated multiple risk factors for GIB (e.g., gender, age, hepatic and renal insufficiency) and we also found that the HASBLED score could be used to predict the occurrence of UGIB after PCI in patients with NSTEMI. A study of patients with CAD treated with dual antiplatelet therapy showed that the higher incidence of UGIB was due to non-administration of PPI and found proton pump inhibitor (PPI) to be more protective than H2 receptor antagonists (H2RA) (40). And in our study, the protective effect of PPI after PCI in patients with NSTEMI was consistent with previous studies. The reason may be that PPI use could selectively inhibit the H+/K+ ATP enzyme in gastric wall cells, significantly reducing gastric acid secretion. This reduction in acid alleviates gastric mucosal damage caused by antiplatelet drugs, as excessive acid secretion could exacerbate mucosal irritation and damage (41).
In summary, we utilized the combination of the classical regression methods and machine learning model to found that HASBLED, TyG index, alcohol drinking, RBC count, PPI use, and AMR of culprit vessel were independent indicators for having UGIB. The model about the relationship between independent factors and UGIB was presented graphically, which had a simple and intuitive effect and the results of this study showed that our prediction model had good performance. While model simplicity reduced the risk of overfitting and improved generalization, the good performance of our current model may not only be a result of the simplicity of the model, but also of the insufficient sample size.
Our study had several limitations. Firstly, this study was a retrospective study and the external data were from the branch hospital. Although AMR is a predictor of UGIB, future prospective studies with large samples are needed to investigate the association between AMR and UGIB and its potential mechanisms. Secondly, not all patients presented to the hospital promptly when signs or tendencies related to bleeding were detected. Thirdly, the collection of UGIB-related risk variables was not comprehensive enough.
Conclusion
The nomogram clinical prediction model constructed by six factors, including HASBLED, TyG index, alcohol drinking, RBC count, PPI use, and AMR of culprit vessel, can be used to assess the likelihood of UGIB within 1 year in NSTEMI patients undergoing primary PCI, and the nomogram could help clinicians to stratify risk and individualize management for postoperative patients. Secondly this nomogram could also reflect the state of microcirculation throughout the body.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2023-KL043-01), which was conducted following the Declaration of Helsinki. Because our study was retrospective, the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University waived the requirement for written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because our study was retrospective, the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University waived the requirement for written informed consent.
Author contributions
ZW: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. SY: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. CZ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. CL: Data curation, Formal Analysis, Writing – original draft. CC: Data curation, Formal Analysis, Writing – original draft. JC: Writing – review & editing. DL: Writing – review & editing. LL: Writing – review & editing. TX: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Jiangsu Provincial Health Commission Project Fund (Grant number: M2020015), Jiangsu Graduate Student Innovation and Practice Project (Grant number: SJCX231389, KYCX243080, KYCX243081), The Affiliated Hospital of Xuzhou Medical University Clinical Research Special Project (LCZX202405).
Acknowledgments
The authors would like to express our gratitude to the patients for their contribution to this study. The Clinical Research Institute was consulted on some of the statistical issues involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. La Sala L, Pontiroli AE. Prevention of diabetes and cardiovascular disease in obesity. Int J Mol Sci. (2020) 21:8178. doi: 10.3390/ijms21218178
2. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
3. Balzi D, Di Bari M, Barchielli A, Ballo P, Carrabba N, Cordisco A, et al. Should we improve the management of NSTEMI? Results from the population-based “acute myocardial infarction in Florence 2” (AMI-Florence 2) registry. Intern Emerg Med. (2013) 8:725–33. doi: 10.1007/s11739-012-0817-6
4. Shin D, Lee SH, Hong D, Choi KH, Lee JM. Physiologic assessment after percutaneous coronary interventions and functionally optimized revascularization. Interv Cardiol Clin. (2023) 12:55–69. doi: 10.1016/j.iccl.2022.09.006
5. Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. (2015) 66:1036–45. doi: 10.1016/j.jacc.2015.06.1323
6. Tong MS, Sung PH, Liu CF, Chen KH, Chung SY, Chua S, et al. Impact of double loading regimen of clopidogrel on final angiographic results, incidence of upper gastrointestinal bleeding and clinical outcomes in patients with STEMI undergoing primary coronary intervention. Int Heart J. (2017) 58:686–94. doi: 10.1536/ihj.16-325
7. Yasuda H, Yamada M, Sawada S, Endo Y, Inoue K, Asano F, et al. Upper gastrointestinal bleeding in patients receiving dual antiplatelet therapy after coronary stenting. Intern Med. (2009) 48:1725–30. doi: 10.2169/internalmedicine.48.2031
8. Blocksom JM, Tokioka S, Sugawa C. Current therapy for nonvariceal upper gastrointestinal bleeding. Surg Endosc. (2004) 18:186–92. doi: 10.1007/s00464-003-8155-4
9. Bhandiwad AR, Valenta I, Jain S, Schindler TH. PET-determined prevalence of coronary microvascular dysfunction and different types in a cardio-metabolic risk population. Int J Cardiol Heart Vasc. (2023) 46:101206. doi: 10.1016/j.ijcha.2023.101206
10. Ong P, Safdar B, Seitz A, Hubert A, Beltrame JF, Prescott E. Diagnosis of coronary microvascular dysfunction in the clinic. Cardiovasc Res. (2020) 116:841–55. doi: 10.1093/cvr/cvz339
11. El Farissi M, Zimmermann FM, De Maria GL, van Royen N, van Leeuwen MAH, Carrick D, et al. The index of microcirculatory resistance after primary PCI: A pooled analysis of individual patient data. JACC Cardiovasc Interv. (2023) 16:2383–92. doi: 10.1016/j.jcin.2023.08.030
12. Fan Y, Fezzi S, Sun P, Ding N, Li X, Hu X, et al. In vivo validation of a novel computational approach to assess microcirculatory resistance based on a single angiographic view. J Pers Med. (2022) 12. doi: 10.3390/jpm12111798
13. Lin X, Wu G, Wang S, Huang J. The prevalence of coronary microvascular dysfunction (CMD) in heart failure with preserved ejection fraction (HFpEF): a systematic review and meta-analysis. Heart Fail Rev. (2024) 29:405–16. doi: 10.1007/s10741-023-10362-x
14. Crea F, Montone RA, Rinaldi R. Pathophysiology of coronary microvascular dysfunction. Circ J. (2022) 86:1319–28. doi: 10.1253/circj.CJ-21-0848
15. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res. (2020) 116:806–16. doi: 10.1093/cvr/cvaa023
16. Nishi T, Murai T, Waseda K, Hirohata A, Yong ASC, Ng MKC, et al. Association of microvascular dysfunction with clinical outcomes in patients with non-flow limiting fractional flow reserve after percutaneous coronary intervention. Int J Cardiol Heart Vasc. (2021) 35:100833. doi: 10.1016/j.ijcha.2021.100833
17. Gong H, Hsieh SS, Holmes DR 3rd, Cook DA, Inoue A, Bartlett DJ, et al. An interactive eye-tracking system for measuring radiologists’ visual fixations in volumetric CT images: Implementation and initial eye-tracking accuracy validation. Med Phys. (2021) 48:6710–23. doi: 10.1002/mp.v48.11
18. Patel NJ, Pau D, Nalluri N, Bhatt P, Thakkar B, Kanotra R, et al. Temporal trends, predictors, and outcomes of in-hospital gastrointestinal bleeding associated with percutaneous coronary intervention. Am J Cardiol. (2016) 118:1150–7. doi: 10.1016/j.amjcard.2016.07.025
19. Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol. (2016) 68:1851–64. doi: 10.1016/j.jacc.2016.07.760
20. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). (2017) 219:22–96. doi: 10.1111/apha.2017.219.issue-1
21. Piaserico S, Papadavid E, Cecere A, Orlando G, Theodoropoulos K, Katsimbri P, et al. Coronary microvascular dysfunction in asymptomatic patients with severe psoriasis. J Invest Dermatol. (2023) 143:1929–1936.e1922. doi: 10.1016/j.jid.2023.02.037
22. Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, et al. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. (2014) 34:2473–7. doi: 10.1161/ATVBAHA.114.304445
23. Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 78:1352–71. doi: 10.1016/j.jacc.2021.07.042
24. Ma J, Xia R, Lan Y, Wang A, Zhang Y, Ma L. Angiographic microvascular resistance in patients with obstructive hypertrophic cardiomyopathy. Microvasc Res. (2024) 153:104656. doi: 10.1016/j.mvr.2024.104656
25. Qian G, Qin H, Deng D, Feng Y, Zhang C, Qu X, et al. Prognostic value of angiographic microvascular resistance in patients with ST-segment elevation myocardial infarction. Clinics (Sao Paulo). (2024) 79:100429. doi: 10.1016/j.clinsp.2024.100429
26. Berry C, Sidik N, Pereira AC, Ford TJ, Touyz RM, Kaski JC, et al. Small-vessel disease in the heart and brain: current knowledge, unmet therapeutic need, and future directions. J Am Heart Assoc. (2019) 8:e011104. doi: 10.1161/JAHA.118.011104
27. Ohura-Kajitani S, Shiroto T, Godo S, Ikumi Y, Ito A, Tanaka S, et al. Marked impairment of endothelium-dependent digital vasodilatations in patients with microvascular angina: evidence for systemic small artery disease. Arterioscler Thromb Vasc Biol. (2020) 40:1400–12. doi: 10.1161/ATVBAHA.119.313704
28. Nam KW, Kwon HM, Lee YS. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci Rep. (2021) 11:15335. doi: 10.1038/s41598-021-94631-5
29. Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. (2022) 21:68. doi: 10.1186/s12933-022-01511-x
30. Jiang M, Li X, Wu H, Su F, Cao L, Ren X, et al. Triglyceride-glucose index for the diagnosis of metabolic syndrome: A cross-sectional study of 298,652 individuals receiving a health check-up in China. Int J Endocrinol. (2022) 2022:3583603. doi: 10.1155/2022/3583603
31. Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr Metab Cardiovasc Dis. (2020) 30:245–53. doi: 10.1016/j.numecd.2019.09.015
32. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. (2019) 18:95. doi: 10.1186/s12933-019-0898-x
33. Gao S, Ma W, Huang S, Lin X, Yu M. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab Cardiovasc Dis. (2021) 31:3184–92. doi: 10.1016/j.numecd.2021.07.027
34. Trifunovic D, Stankovic S, Sobic-Saranovic D, Marinkovic J, Petrovic M, Orlic D, et al. Acute insulin resistance in ST-segment elevation myocardial infarction in non-diabetic patients is associated with incomplete myocardial reperfusion and impaired coronary microcirculatory function. Cardiovasc Diabetol. (2014) 13:73. doi: 10.1186/1475-2840-13-73
35. Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. (2007) 100:1364–9. doi: 10.1016/j.amjcard.2007.06.026
36. Manzano-Fernández S, Marin F, Martinez JA, Cambronero F, Valdés M, Ruiz-Nodar JM, et al. Anaemia as predictor of gastrointestinal bleeding in atrial fibrillation patients undergoing percutaneous coronary artery stenting. Qjm. (2008) 101:749–51. doi: 10.1093/qjmed/hcn082
37. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. 4-year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. (2022) 79:1–14. doi: 10.1016/j.jacc.2021.10.023
38. Konishi H, Miyauchi K, Tsuboi S, Ogita M, Naito R, Dohi T, et al. Impact of the HAS-BLED score on long-term outcomes after percutaneous coronary intervention. Am J Cardiol. (2015) 116:527–31. doi: 10.1016/j.amjcard.2015.05.015
39. Castini D, Persampieri S, Sabatelli L, Erba M, Ferrante G, Valli F, et al. Utility of the HAS-BLED score for risk stratification of patients with acute coronary syndrome. Heart Vessels. (2019) 34:1621–30. doi: 10.1007/s00380-019-01405-1
40. Ng FH, Lam KF, Wong SY, Chang CM, Lau YK, Yuen WC, et al. Upper gastrointestinal bleeding in patients with aspirin and clopidogrel co-therapy. Digestion. (2008) 77:173–7. doi: 10.1159/000141264
Keywords: angiographic microvascular resistance of culprit vessel, upper gastrointestinal bleeding, non-ST-segment elevation myocardial infarction, nomogram, percutaneous coronary intervention
Citation: Wang Z, Yang S, Zhou C, Li C, Chen C, Chen J, Li D, Li L and Xu T (2025) Development and validation of an AMR-based predictive model for post-PCI upper gastrointestinal bleeding in NSTEMI patients. Front. Endocrinol. 16:1545462. doi: 10.3389/fendo.2025.1545462
Received: 15 December 2024; Accepted: 28 March 2025;
Published: 16 April 2025.
Edited by:
Carmine Izzo, University of Salerno, ItalyReviewed by:
Lingzhang Meng, Guangxi Academy of Medical Sciences, ChinaSiddharth Gosavi, Sai Gosavi Speciality Clinic, India
Copyright © 2025 Wang, Yang, Zhou, Li, Chen, Chen, Li, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Li, bGlncm91cC05OTlAMTYzLmNvbQ==; Tongda Xu, eHV0b25nZGEzMDA0QDE2My5jb20=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
 Zhaokai Wang
Zhaokai Wang Shuping Yang
Shuping Yang Chunxue Zhou1†
Chunxue Zhou1† Junhong Chen
Junhong Chen Dongye Li
Dongye Li Lei Li
Lei Li Tongda Xu
Tongda Xu