
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 25 March 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1545272
This article is part of the Research TopicOxidative Stress and Male FertilityView all 11 articles
 Yang Xu1,2
Yang Xu1,2 Shuofeng Li3*
Shuofeng Li3*Background: Erectile dysfunction (ED) is a prevalent condition closely associated with systemic inflammation and metabolic disorders. The red cell distribution width to albumin ratio (RAR) is an emerging inflammatory marker; however, its relationship with ED remains poorly understood.
Methods: This study conducted a cross-sectional analysis of data from 3,950 participants in the National Health and Nutrition Examination Survey (NHANES) 2001–2004 cycle to evaluate the association between RAR and ED risk. A Multivariable logistic regression model was employed to assess the relationship between RAR and ED, while a generalized additive model (GAM) and dose-response analysis were utilized to explore potential nonlinear associations. Subgroup analyses were performed to investigate interactions with demographic and lifestyle factors.
Results: Among the study population, 1,157 individuals reported a history of ED. The prevalence of ED was significantly higher in individuals aged 50 years and older (86.78%) and was associated with increased rates of hypertension, diabetes mellitus, and cardiovascular disease (P < 0.001). A J-shaped relationship was identified between RAR and ED risk. Specifically, the risk of ED significantly increased below the RAR threshold of 3.42 (OR = 3.01, 95% CI: 2.08–4.36, P < 0.001), while the risk plateaued at higher RAR values. Subgroup analyses revealed significant interactions with ethnicity (P = 0.018) and moderate-intensity physical activity (P = 0.004). Non-Hispanic whites (OR = 2.85) and individuals engaging in moderate-intensity activity (OR = 3.83) exhibited a heightened risk of ED. No significant interactions were observed for other variables, including age and BMI.
Conclusion: The results demonstrated that RAR was independently associated with ED risk, exhibiting a J-shaped relationship. There was a significant increase in risk below RAR = 3.42, with saturation occurring after exceeding this threshold.
Erectile dysfunction (ED) is a prevalent chronic condition defined by the inability to obtain or maintain an erection sufficient for the completion of satisfactory sexual activity (1). As the population continues to age, the incidence of ED is rising, with a notable impact on the quality of life and mental health of men (2). Concurrently, ED is not merely an expression of a local pathological process; it is also regarded as a comprehensive manifestation of systemic diseases, with a strong correlation to systemic inflammation and metabolic disorders (3). Prior research has demonstrated a significant association between ED and chronic illnesses such as cardiovascular disease and diabetes, underscoring its potential as a marker of systemic diseases (4, 5).
In recent years, there has been a notable increase in the number of studies examining the role of inflammatory markers in the context of chronic diseases (6, 7). Red cell distribution width (RDW), a sensitive indicator of blood inflammation and oxidative stress, has been demonstrated to be significantly associated with the risk of cardiovascular disease, diabetes, and metabolic syndrome (8–10). Concurrently, serum albumin level, as a crucial indicator of nutritional status and anti-inflammatory function, also plays a pivotal role in the investigation of inflammation-related diseases (11, 12). However, the predictive capacity of these individual indicators may be constrained (13). The RDW to Albumin Ratio (RAR) has recently emerged as a promising composite marker, integrating the advantages of RDW and albumin to provide a more comprehensive reflection of systemic inflammatory levels and nutritional status (14, 15). The predictive value of RAR in the risk assessment of cardiovascular disease and diabetes has been demonstrated in numerous studies (16, 17). However, its potential association with ED remains to be elucidated.
To address this gap, we conducted a nationally representative cross-sectional study using NHANES 2001–2004 data. Our analysis focused on adult males, leveraging standardized laboratory measurements and validated questionnaires to examine the RAR-ED relationship while adjusting for sociodemographic, metabolic, and lifestyle confounders.
The data for this study were derived from the NHANES, conducted by the National Center for Health Statistics (NCHS). NHANES is a nationwide population-based cross-sectional study of non-institutionalized U.S. citizens, the objective of which is to assess their nutritional status and potential health risk factors. In order to ensure the representativeness of the sample, a complex hierarchical and multi-stage probabilistic cluster sampling design was adopted. The study protocol for NHANES has been approved by the NCHS Research Ethics Review Committee, and all participants have provided written informed consent. The specific study design and data for NHANES are accessible via the CDC website (www.cdc.gov/nchs/nhanes/).
We analyzed data from the NHANES 2001–2004 cycles (2001–2002 and 2003–2004), which were the only surveys including ED-related questionnaires. Initially, 21,161 participants were screened. After applying exclusion criteria—(1) female sex (n = 10,860), (2) age <20 years (n = 5,347), (3) missing ED data (n = 838), (4) missing RDW values (n = 128), and (5) missing serum albumin levels (n = 38)—a final cohort of 3,950 participants was included (Figure 1).
Blood samples are collected at Mobile Health Examination Centres (MECs) as part of the NHANES. The RDW is determined by a Beckman Coulter MAXXM instrument as part of the complete blood count. The instrument employs the Beckman Coulter method for counting, measuring size, and automating dilution and mixing to process samples (18). Serum albumin concentrations were determined using the DcX800 method, which employs a two-color ratio digital endpoint method to form a complex with serum albumin utilising bromocresol violet (BCP) reagent (19). The RAR is calculated as the ratio of RDW to albumin. In the NHANES database, the question that assesses erectile function is as follows: “During this period, how would you rate your ability to achieve and maintain an erection that is sufficient to make a satisfactory erection?” The response options are as follows: (1) “Never able to get and keep an erection,” (2) “Sometimes able to get and keep an erection,” (3) “Usually able to get and keep an erection,” and (4) “Always or almost always able to get and keep an erection.” Participants selecting options (1) or (2) were classified as having ED, while those choosing options (3) or (4) were categorized as non-ED. Those who selected the first two options were diagnosed with ED, while those who selected the latter two were defined as having no ED. Furthermore, the validity of self-reported methods for diagnosing ED has been validated (20).
In the present study, the covariates included the following: (1) Basic demographic variables, including age, ethnicity, education level, marital status, poverty-to-income ratio (PIR), and (Body Mass index) BMI, were also considered. (2) Lifestyle variables, including smoking, alcohol intake, and physical activity, The ethnic backgrounds were divided into the following categories: Mexican-American, Non-Hispanic White, Non-Hispanic Black, Other Hispanic, and Other Races. The educational levels are classified into three categories: below high school, high school, and above. The marital status is classified as never married, widowed/divorced/separated, and married/living with a partner. The PIR, which serves as an indicator of economic status, is divided into three categories. PIR ≤ 1.3, 1.3–3.5, and >3.5. The term “non-smoker” is used to describe men who have never smoked more than 100 cigarettes in their lifetime. The term “alcohol intake” is defined as the consumption of at least 12 alcoholic beverages over the past 12 months. The diagnosis of medical complications, including diabetes, cardiovascular disease (CVD), and hypertension, is based on self-reported medical history as outlined in the NHANES questionnaire (21).
All statistical analyses were conducted in accordance with the guidelines set forth by the Centers for Disease Control and Prevention (CDC), with the appropriate NHANES weights employed to account for the intricate multi-stage stratified sampling designs. Missing values in covariates were addressed using imputation. In the baseline feature table, categorical variables are expressed as percentages, and continuous variables are summarised as the mean and standard error (SE). The differences between the ED and non-ED groups were assessed using a weighted chi-square test (for categorical variables) or a weighted Student’s t-test (for continuous variables). To investigate the relationship between RAR and the incidence of ED, three logistic regression models were employed. Model 1 is an unadjusted model. Model 2 was adjusted for age, ethnicity, education level, marital status, and PIR. Model 3 was further adjusted for additional variables, including BMI, alcohol intake, hypertension, diabetes, CVD, vigorous activity, moderate activity, smoking status, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting glucose, triglycerides, and total cholesterol (TC), in accordance with the adjustments made in Model 2. The relationship between RAR and ED was described by means of a weighted Multivariable logistic regression, which was analysed separately as a continuous variable and a categorical variable (tertiary classification).
Subsequently, we conducted a further analysis of the nonlinear relationship between RAR and the prevalence of ED using a generalized additive model (GAM) and smooth curve fitting. In instances where a nonlinear relationship was identified, a two-segment linear regression model (piecewise regression model) was applied to each interval. This was then compared with the unilinear model (non-segmented model) to ascertain the threshold effect, which was calculated using the log-likelihood ratio test. Subsequently, subgroup analyses were performed using stratified logistic regression models. The modifications and interactions of subgroups were inspected by likelihood ration tests.
All analyses were conducted using R software, version 3.4.3 (http://www.r-project.org, The R Foundation), and EmpowerStats software (http://www.empowerstats.com; X&Y solutions, Inc., Boston, MA). The level of statistical significance was set at P < 0.05. The p-value of the interaction is employed to indicate the degree of significance of the interaction between the covariate and the primary exposure variable on the outcome variable.
Table 1 presents the baseline characteristics of the 3,950 participants, of whom 1,157 reported experiencing ED. The majority of patients with ED were aged 50 years or above (86.78%), while only a third of patients without ED fell into this age category (P < 0.001). The prevalence of hypertension (52.29% vs. 22.84%), diabetes (22.73% vs. 5.33%) and CVD (28.61% vs. 6.12%) was significantly higher in patients with erectile dysfunction (P < 0.001). Furthermore, they were less likely to engage in vigorous activity (16.34% vs. 40.49%) and exhibited higher fasting glucose levels (119.69 ± 1.58 vs. 104.45 ± 0.69 mg/dL, P < 0.001). Furthermore, patients with ED exhibited lower levels of education and socioeconomic status, a higher proportion of individuals with less than a high school education (40.19% vs. 23.17%), and a higher proportion of individuals with PIR ≤1.3 (28.26% vs. 22.70%, P < 0.001).
As illustrated in Table 2, there is a notable correlation between RAR and ED. In all models, a higher RAR was significantly associated with an increased risk of ED. A stratified analysis by RAR quintile demonstrated a dose-response relationship between the high group and a significantly increased risk of ED (P for trend < 0.001). Furthermore, we investigated the non-linear relationship between RAR and the prevalence of ED utilising GAM and smooth curve fitting (Figure 2). The analysis demonstrated a nonlinear positive correlation between RAR and ED prevalence, with a significantly enhanced association between RAR and ED below the threshold (RAR < 3.42) (OR = 3.01, 95% CI: 2.08–4.36, P < 0.001), and a log-likelihood ratio test P-value < 0.05 (Table 3).
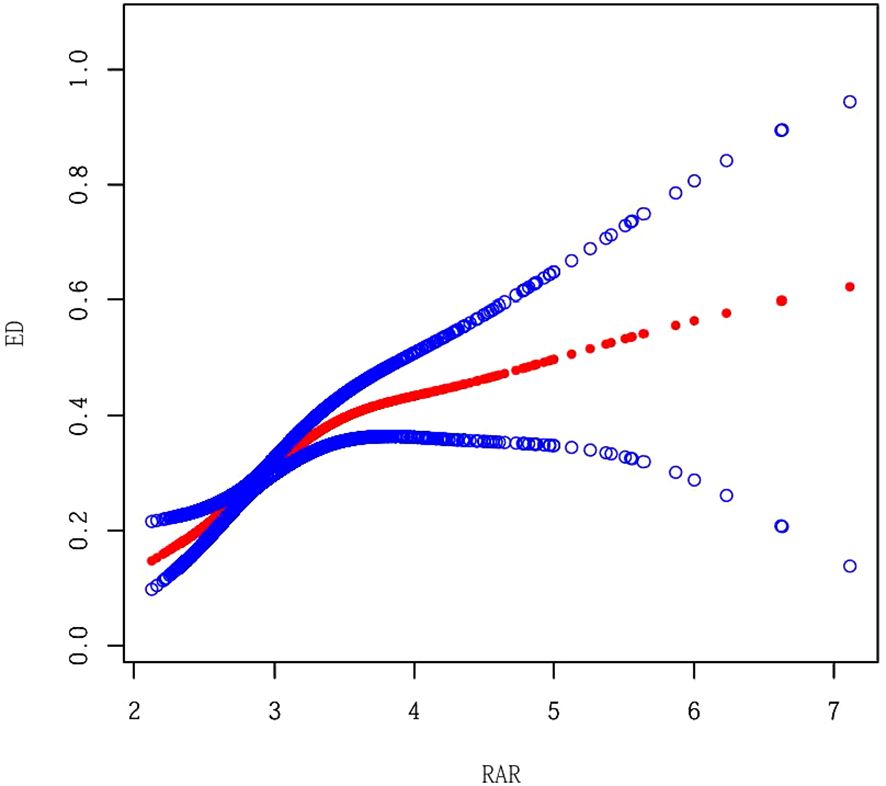
Figure 2. Smooth Curve Fitting between RAR and ED. Adjusted for Age, Race, Marital Status, Education level, PIR, BMI, Alcohol intake, Hypertension, Diabetes, CVD, Vigorous activity, Moderate activity, Smoking status, LDL_C, TC, Triglycerides, DAsting glucose and HDL-C.
In order to evaluate the consistency of the relationship between RAR and the prevalence of ED in different populations, we conducted subgroup analyses (Figure 3). These analyses demonstrated that race exhibited significant interaction effects (P = 0.018), with a higher risk observed among non-Hispanic whites (OR = 2.85) and non-Hispanic blacks (OR = 1.91). The interaction effect of moderate-intensity activity was statistically significant (P = 0.004), indicating that the risk of moderate activity was increased (OR = 3.83). No significant interactions were observed for other factors, including age, marital status, education level, PIR, vigorous activity, alcohol intake, BMI, hypertension, CVD, diabetes, and smoking status.
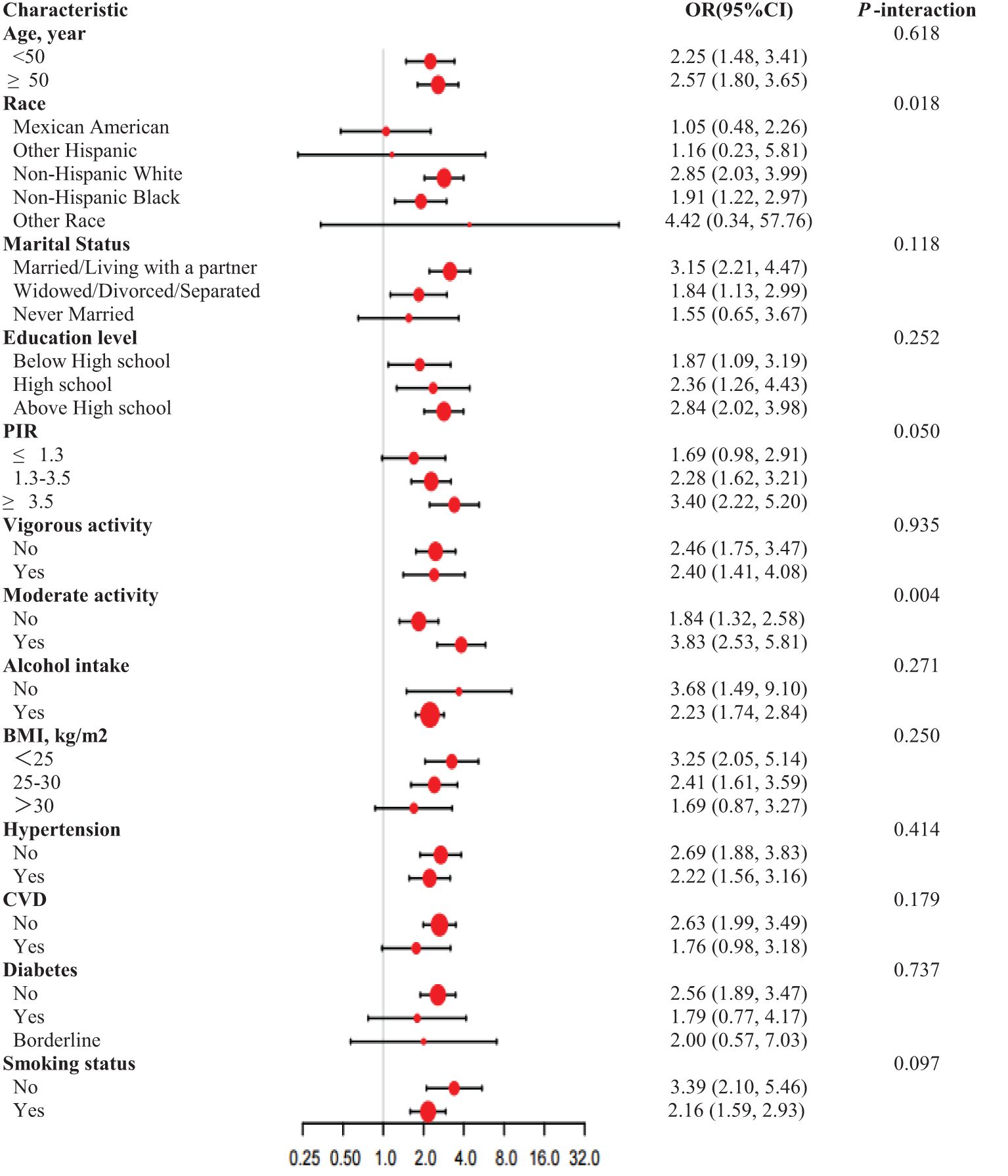
Figure 3. Subgroup analysis for RAR and ED, weighted. Adjusted for Age, Marital status, Education level. PIR, BMI, Alcohol intake, Hypertension, Diabetes, CVD, Vigorous activity, Moderate activity, Smoking status, LDL-C, TC, Triglycerides, fasting glucose and HDL-C. In each case, the model is not adjusted for the stratification variable.
This study revealed, for the first time, a nonlinear J-shaped relationship between RAR and ED, offering novel insights into the interplay of systemic inflammation, metabolic dysregulation, and vascular dysfunction in ED pathogenesis. Below the threshold of RAR = 3.42, ED risk increased sharply (OR = 3.01), plateauing beyond this point. This biphasic association suggests distinct pathophysiological mechanisms dominate at different RAR ranges, mediated by the dual roles of RDW and albumin in inflammatory and vascular homeostasis.
At lower RAR levels (<3.42), the heightened ED risk likely stems from synergistic effects of hypoalbuminemia and elevated RDW. Hypoalbuminemia reflects malnutrition or chronic inflammation, impairing endothelial nitric oxide (NO) synthesis—a cornerstone of erectile function—and reducing testosterone bioavailability due to disrupted steroid hormone transport (22, 23). Albumin’s antioxidant properties mitigate oxidative stress; its deficiency exacerbates endothelial dysfunction by permitting unchecked reactive oxygen species (ROS) accumulation (24). Concurrently, elevated RDW signifies erythrocyte heterogeneity driven by inflammation or oxidative stress, which correlates with endothelial damage and impaired microvascular perfusion (25). Studies demonstrate that RDW elevation is linked to reduced NO-mediated vasodilation, a critical defect in ED pathophysiology (26). These pathways collectively create a “perfect storm” for ED at low RAR values, where inflammation and nutritional deficits converge to disrupt vascular and hormonal homeostasis.
Above the threshold (RAR >3.42), the risk plateau may reflect compensatory adaptations to chronic inflammation. Prolonged inflammatory states activate counter-regulatory mechanisms, such as upregulation of anti-inflammatory cytokines (e.g., IL-10) and endogenous antioxidant systems, which partially offset endothelial damage (27). Additionally, chronic hypoxia may stimulate angiogenesis via VEGF, improving collateral blood flow to penile tissues despite systemic inflammation (28, 29). However, this compensatory capacity likely has limits, explaining the attenuated risk escalation at higher RAR levels. Notably, unmeasured confounders, such as subclinical liver disease or micronutrient deficiencies (e.g., iron, vitamin B12), could influence both RDW and albumin, potentially confounding the observed nonlinearity (30). Future studies should address these factors to refine mechanistic understanding.
RAR serves as a composite biomarker that integrates both inflammatory (RDW) and nutritional (albumin) components, providing a more comprehensive assessment of systemic health compared to using these markers individually. Unlike RDW alone, which primarily reflects erythrocyte variability, or albumin, which indicates nutritional status, RAR captures the interplay between inflammation, oxidative stress, and vascular health (31, 32). This dual-component nature enhances its sensitivity in identifying ED risk, particularly in populations with metabolic disorders (33).
Furthermore, the J-shaped association identified in this study suggests that RAR provides superior predictive value over conventional inflammatory markers such as C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR), which typically show linear associations with disease risk (34). The nonlinear relationship implies that RAR not only identifies high-risk individuals at extreme values but also highlights a potential threshold for early intervention (35). This is particularly relevant for ED, where early detection of vascular dysfunction can facilitate timely lifestyle or pharmacological interventions.
The complexity of RAR as a composite biomarker is further underscored by subgroup analyses. The heightened ED risk among non-Hispanic Whites and physically active individuals suggests that genetic polymorphisms in inflammatory mediators (e.g., CRP, IL-6) may predispose certain ethnic groups to RAR-driven ED (36). Paradoxically, moderate exercise—though generally protective—may transiently exacerbate oxidative stress in susceptible individuals, amplifying ED risk in high-RAR subgroups (37). These interactions highlight the need for personalized approaches, integrating genetic, lifestyle, and inflammatory profiling into ED management.
The J-shaped RAR-ED association has substantial clinical relevance. Since RAR is calculated from routine blood tests (RDW and albumin), it provides a cost-efficient biomarker for ED risk stratification, especially in aging populations or those with metabolic syndrome. Compared to other inflammatory markers, RAR’s accessibility and reliability make it a practical addition to standard ED screening tools. Integrating RAR with the Sexual Health Inventory for Men (SHIM) questionnaire could enhance early detection and improve patient outcomes. For patients with RAR <3.42, targeted strategies—such as anti-inflammatory diets, albumin supplementation, or lifestyle modifications—may mitigate ED progression.
Beyond its localized symptoms, ED is increasingly recognized as a harbinger of systemic pathologies, including cancer and accelerated aging. Recent studies associate ED with cancer progression, possibly via shared pathways of chronic inflammation and oxidative stress, which drive both endothelial dysfunction and genomic instability (38). Furthermore, ED correlates with aging biomarkers such as telomere shortening and senescent cell accumulation, suggesting a role in biological aging (39). These findings position ED not merely as a urological condition but as a multisystem indicator, necessitating holistic management to address underlying comorbidities.
While this study demonstrates a nonlinear relationship between RAR and ED, several limitations must be acknowledged. Firstly, the cross-sectional design precludes causal inference; longitudinal cohorts are needed to validate RAR’s predictive value and establish intervention thresholds. Secondly, missing data in some cases may have introduced potential biases despite statistical adjustments. Additionally, the sample predominantly comprised US individuals, limiting generalizability to other racial and regional groups. Future studies should investigate the molecular mechanisms linking RAR to inflammatory markers, vascular endothelial function, and testosterone metabolism, while evaluating RAR’s clinical utility in diverse populations.
This study identifies a J-shaped relationship between RAR and ED, implicating inflammation, vascular dysfunction, and compensatory mechanisms in its etiology. RAR’s simplicity and affordability position it as a promising biomarker for ED risk assessment, particularly when combined with aging and systemic disease markers. Future research should prioritize mechanistic studies to unravel RAR’s role in endothelial and hormonal pathways, alongside randomized trials testing RAR-guided interventions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All participants provided written informed consent before their involvement in the NHANES study. Furthermore, the research protocol was reviewed and approved by the NCHS Research Ethics Review Board, ensuring compliance with ethical standards for studies involving human subjects.
YX: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. SL: Conceptualization, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang Y, Yong S, Li F, Chang D. The effect of nebivolol on erectile function in the cases with coronary artery bypass surgery. Medicine. (2020) 99. doi: 10.1097/md.0000000000021588
2. Langer R, Gupta R, Thaker KS, Kumari R, Gupta RK, Langer B. Quality of life in patients of erectile dysfunction: A cross-sectional study in tertiary care settings. Int Surg J. (2019) 6:1922. doi: 10.18203/2349-2902.isj20192144
3. Banks E, Joshy G, Abhayaratna WP, Kritharides L, Macdonald PS, Korda RJ, et al. Erectile dysfunction severity as a risk marker for cardiovascular disease hospitalisation and all-cause mortality: A prospective cohort study. PloS Med. (2013) 10. doi: 10.1371/journal.pmed.1001372
4. Zambon JP, Mendonça RRD, Wroclawski ML, Karam A Jr, Santos RD, Carvalho JAM, et al. Cardiovascular and metabolic syndrome risk among men with and without erectile dysfunction: Case-control study. São Paulo Med J. (2010) 128:137–40. doi: 10.1590/s1516-31802010000300006
5. Kouidrat Y, Pizzol D, Cosco TD, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: A systematic review and meta-analysis of 145 studies. Diabetologia. (2017) 34:1185–92. doi: 10.1111/dme.13403
6. Tutan D, Doğan M. Evaluation of neutrophil/lymphocyte ratio, low-density lipoprotein/albumin ratio, and red cell distribution width/albumin ratio in the estimation of proteinuria in uncontrolled diabetic patients. Cureus. (2023) 15. doi: 10.7759/cureus.44497
7. Horta-Baas G, Figueroa MDSR. Clinical utility of red blood cell distribution width in inflammatory and non-inflammatory joint diseases. Int J Rheumatic Dis. (2018) 22:47–54. doi: 10.1111/1756-185x.13332
8. Kengne AP, Patel A, Marre M, Travert F, Lievre M, Zoungas S, et al. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. (2011) 18:393–8. doi: 10.1177/1741826710394270
9. Huang M, Liu F, Li Z, Liu Y, Su J, Ma M, et al. Relationship between red cell distribution width/albumin ratio and carotid plaque in different glucose metabolic states in patients with coronary heart disease: A RCSCD-TCM study in China. Cardiovasc Diabetol. (2023) 22:17. doi: 10.1186/s12933-023-01768-w
10. Engström G, Smith J, Persson M, Nilsson PM, Melander O, Hedblad B, et al. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Internal Med. (2014) 276:174–83. doi: 10.1111/joim.12188
11. Li W, Song Y. Red cell distribution width-to-albumin ratio is a risk factor for atrial fibrillation in the general population. Res Square. (2023). doi: 10.21203/rs.3.rs-3184223/v1
12. Nah EH, Cho S, Kim SY, Cho HI. Comparison of urine albumin-to-creatinine ratio (ACR) between ACR strip test and quantitative test in prediabetes and diabetes. Ann Lab Med. (2017) 37:28–33. doi: 10.3343/alm.2017.37.1.28
13. Liu P, Luo S, Duan X, Chen X, Zhou Q, Jiang Y, et al. RDW-to-ALB ratio is an independent predictor for 30-day all-cause mortality in patients with acute ischemic stroke: A retrospective analysis from the MIMIC-IV database. Behav Neurol. (2022) 2022:1–11. doi: 10.1155/2022/3979213
14. Al-Dahr MHS. Fibrinolytic, platelets and endothelial microparticles abnormalities among obese type 2 diabetic patients. Adv Res Gastroenterol Hepatol. (2017) 3. doi: 10.19080/argh.2017.03.555620
15. Blanc-Bisson C, Vélayoudom-Céphise F, Cougnard Grégoire A, Helmer C, Rajaobelina K, Delcourt C, et al. Skin autofluorescence predicts major adverse cardiovascular events in patients with type 1 diabetes: A 7-year follow-up study. Cardiovasc Diabetol. (2018) 17:71. doi: 10.1186/s12933-018-0718-8
16. Chana KK, Fenwick P, Nicholson AG, Barnes PJ, Donnelly LE, et al. Identification of a distinct glucocorticosteroid-insensitive pulmonary macrophage phenotype in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. (2014) 133:207–16. doi: 10.1016/j.jaci.2013.08.044
17. Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status. Diabetes Care. (2012) 35:396–403. doi: 10.2337/dc11-1588
18. Ainiwaer A, Kadier K, Abulizi A, Hou WQ, Rehemuding R, Maimaiti H, et al. Association of red cell distribution width (RDW) and the RDW to platelet count ratio with cardiovascular disease among US adults: A cross-sectional study based on the National Health and Nutrition Examination Survey 1999–2020. BMJ Open. (2023) 13. doi: 10.1136/bmjopen-2022-068148
19. Hu S, Guo Q, Wang S, Zhang W, Ye J, Su L, et al. Supplementation of serum albumin is associated with improved pulmonary function: NHANES 2013–2014. Front Physiol. (2022) 13:948370. doi: 10.3389/fphys.2022.948370
20. Li L, Yao H, Dai W, Chen Y, Liu H, Ding W, et al. A higher TyG index is related with a higher prevalence of erectile dysfunction in males between the ages 20–70 in the United States, according to a cross-sectional research. Front Endocrinol (Lausanne). (2022) 13:988257. doi: 10.3389/fendo.2022.988257
21. Kadier K, Abulizi A, Ainiwaer A, Rehemuding R, Ma X, Ma YT. Unravelling the link between periodontitis and abdominal aortic calcification in the US adult population: A cross-sectional study based on the NHANES 2013-2014. BMJ Open. (2023) 13:e068931. doi: 10.1136/bmjopen-2022-068931
22. Yin M, Liu S, Qin W, Li C, Zhang J, Yang H, et al. Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: A prospective cohort study. J Intensive Care Med. (2016) 33:687–94. doi: 10.1177/0885066616685300
23. Xu J, Ma H, Shi L, Zhou H, Cheng Y, Tong J, et al. Inflammatory cell–derived MYDGF attenuates endothelial LDL transcytosis to protect against atherogenesis. Arteriosclerosis Thrombosis Vasc Biol. (2023) 43. doi: 10.1161/atvbaha.123.319905
24. Amano H, Yoshimura K, Iijima R, Waki K, Matsumoto K, Ueda H, et al. A slight decrease in the serum albumin level is associated with the rapid progression of kidney dysfunction, even within the normal range. Internal Med. (2020) 59:2679–85. doi: 10.2169/internalmedicine.4466-20
25. Li D, Ruan Z, Wu B. Association of red blood cell distribution width-albumin ratio for acute myocardial infarction patients with mortality: A retrospective cohort study. Clin Appl Thrombosis/Hemostasis. (2022) 28:21286. doi: 10.1177/10760296221121286
26. Suzuki S, Hashizume N, Kanzaki Y, Maruyama T, Kozuka A, Yahikozawa K, et al. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PloS One. (2019) 14. doi: 10.1371/journal.pone.0219044
27. Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PloS One. (2013) 8. doi: 10.1371/journal.pone.0061944
28. Sahara M, Sata M, Morita T, Nakajima T, Hirata Y, Nagai R, et al. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G–dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arteriosclerosis Thrombosis Vasc Biol. (2010) 30:1315–24. doi: 10.1161/atvbaha.109.201327
29. Lu ML, Liu Y, Xian Z, Yu X, Chen J, Tan S, et al. VEGF to CITED2 ratio predicts the collateral circulation of acute ischemic stroke. Front Neurol. (2022) 13:1000992. doi: 10.3389/fneur.2022.1000992
30. Kimura H, Tanaka K, Saito H, Iwasaki T, Kazama S, Shimabukuro M, et al. Impact of red blood cell distribution width–albumin ratio on prognosis of patients with CKD. Sci Rep. (2023) 13:42986. doi: 10.1038/s41598-023-42986-2
31. Wu L, Zhang Y, Chen D, Chen W, Wu Y, Yin B, et al. Association of red blood cell distribution width to albumin ratio with the prevalence of kidney stones among the general adult population. Immunity Inflammation Dis. (2024) 12. doi: 10.1002/iid3.70070
32. Long J, Xie X, Xu D, Huang C, Liu Y, Meng X, et al. Association between red blood cell distribution width-to-albumin ratio and prognosis of patients with aortic aneurysms. Int J Gen Med. (2021) 14:6287–94. doi: 10.2147/ijgm.s328035
33. Zhao F, Liu M, Kong L. Association between red blood cell distribution width-to-albumin ratio and diabetic retinopathy. J Clin Lab Anal. (2022) 36. doi: 10.1002/jcla.24351
34. Li H, Xu Y. Association between red blood cell distribution width-to-albumin ratio and prognosis of patients with acute myocardial infarction. BMC Cardiovasc Disord. (2023) 23. doi: 10.1186/s12872-023-03094-1
35. Zhu M, Han M, Xiao X, Lu S, Guan Z, Song Y, et al. Dynamic differences of red cell distribution width levels contribute to the differential diagnosis of hepatitis B virus-related chronic liver diseases: A case-control study. Int J Med Sci. (2019) 16:720–8. doi: 10.7150/ijms.31826
36. Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, et al. Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function. Am J Physiology-Heart Circulatory Physiol. (2017) 313(5):313–5. doi: 10.1152/ajpheart.00416.2017
37. Kim K. Effects of exercise training on vascular inflammatory markers in high-fat diet-induced obese rats. Asian J Kinesiology. (2020) 22:30–8. doi: 10.15758/ajk.2020.22.2.30
38. Dilixiati D, Kadier K, LaiHaiti D, Lu J-D, Azhati B, Rexiati M, et al. The association between sexual dysfunction and prostate cancer: A systematic review and meta-analysis. J Sexual Med. (2023) 20:184–93. doi: 10.1093/jsxmed/qdac025
Keywords: erectile dysfunction, red cell distribution width, J-shaped association, NHANES, cross-sectional study
Citation: Xu Y and Li S (2025) J-Shaped relationship between the red cell distribution width to albumin ratio and erectile dysfunction: a cross-sectional study from NHANES 2001-2004. Front. Endocrinol. 16:1545272. doi: 10.3389/fendo.2025.1545272
Received: 14 December 2024; Accepted: 10 March 2025;
Published: 25 March 2025.
Edited by:
Roland Eghoghosoa Akhigbe, Ladoke Akintola University of Technology, NigeriaReviewed by:
Aikeliyaer Ainiwaer, Maastricht University, NetherlandsCopyright © 2025 Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuofeng Li, MTkwNzkxNTYxQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.