- 1School of Public Health, North China University of Science and Technology, Tangshan, China
- 2Department of Cardiology, Kailuan General Hospital, Tangshan, China
- 3Department of Neurosurgery, Affiliated Hospital of North China University of Science and Technology, Tangshan, China
- 4Tangshan Key Laboratory of Clinical Epidemiology, Tangshan, China
- 5Hebei Key Laboratory of Occupational Health and Safety for Coal Industry, Tangshan, China
Background: Metabolic syndrome (MetS) and elevated high-sensitivity C-reactive protein (hs-CRP) have been identified as risk factors for heart failure (HF) in some studies. However, little was known about the co-exposure of MetS and inflammation to HF. We aimed to investigate the combined effect of MetS and high hs-CRP levels on the risk of incident HF.
Methods: The study included 94,841 participants without HF selected from the Kailuan cohort in 2006 (the baseline) and then followed up until 31 December 2020. Participants were divided into four groups based on the presence of MetS and high hs-CRP levels (>3mg/L) at baseline: MetS-CRP- (n=53,937), MetS-CRP+ (n=10,338), MetS+CRP- (n=23,521), MetS+CRP+ (n=7,045). Cox regression models were used to analyze the association of MetS and inflammation with the risk of HF. Statistical significance was defined as a two-tailed P value < 0.05.
Results: The mean age of the participants was 51.5 ± 12.5 years, and 75,976 (80.0%) were male. During 13.1 years of follow-up, 3,058 participants were diagnosed with HF. The HF incidence rate of four groups were 1.69/1000pys, 2.95/1000pys, 3.27/1000pys, 5.33/1000pys. The HR for MetS-CRP+, MetS+CRP-, and MetS+CRP+ were 1.29 (95% CI, 1.15-1.45), 1.40 (95% CI, 1.29-1.53), and 1.85 (95% CI, 1.65-2.06), respectively, compared with MetS-CRP-. After stratification by age (p for interaction < 0.01), compared with the MetS-CRP- group, the HR of the MetS+CRP+ group was 2.17 (95% CI, 1.83-2.57) in participants with < 60 years and 1.53 (95% CI, 1.32-1.78) in participants with ≥ 60 years. There was an interaction between groups and ues of antihypertension medication (p for interaction <0.01). Compared with MetS-CRP-, the risk of HF in the MetS+CRP+ group was increased 1.38-fold (95% CI, 1.12-1.70) in participants with antihypertension medication use and 2.00-fold (95% CI, 1.75-2.27) in participants without antihypertension medication use.
Conclusions: The combination of MetS and elevated hs-CRP was associated with increased risk of HF in the Chinese population.
Clinical trial registration: https://www.chictr.org.cn, identifier ChiCTR-TNRC-11001489.
Introduction
Heart failure (HF) is the end-stage of cardiac dysfunction. According to the Global Burden of Disease (GBD), the global number of HF cases increased from 33.5 million in 1990 to 64.3 million in 2017 (1). In China, the number of people with HF was approximately 12.1 million, and this number was expected to increase in the future (2). A large cohort study reported that all-cause mortality after hospital discharge in Chinese patients with HF was 28.2% at 3 years (3). Many studies suggested an association between the increasing prevalence of HF and factors such as hypertension, diabetes mellitus, cigarette smoking and other unhealthy lifestyle factors (4, 5). However, the causes of HF were not fully understood. The development of the disease is usually influenced by the co-occurrence of multiple adverse factors, and little research has been conducted on the co-occurrence of these factors. Therefore, a joint evaluation of risk factors for HF explores the pathogenesis of HF, and provides crucial evidence for the prevention of HF onset.
Metabolic syndrome (MetS) is a cluster of metabolic disorders including obesity, hypertension, hyperlipidemia and hyperglycemia. It has a high prevalence in both developed and developing countries (6). The pathogenesis of HF caused by MetS was related to visceral fat accumulation, insulin resistance, and neuroendocrine system activation. For example, activation of the neuroendocrine system caused an increase in blood pressure and heart rate, and the heart used compensatory contractions to meet the blood supply (7). However, prolonged cardiac compensation caused hypertrophy of cardiomyocytes, leading to ventricular remodelling and progression to HF. A large cohort study in Korea found an association between MetS and an increased risk of HF (8). High-sensitivity C-reactive protein (hs-CRP) is a widely used clinical marker to assess inflammation, particularly in the assessment of cardiovascular disease risk and prognosis. Inflammation plays a key role in the development of HF. According to Pearson TA et al, hyperactivation of inflammatory factors led to an imbalance in calcium metabolism in cardiomyocytes, resulting in cardiomyocyte apoptosis and necrosis (9). Recent research found that the interaction between metabolic disorders and inflammation created a vicious circle. For example, the release of inflammatory cytokines interfered with peripheral insulin signaling pathways, causing the body to become less sensitive and responsive to insulin, which led to insulin resistance and impaired glucose metabolism (10). Adipocyte expansion caused by metabolic disorders led to reduced levels of anti-inflammatory factors, resulting in systemic inflammation (11). Several studies showed that the combination of MetS and inflammation increases the risk of atrial fibrillation (AF) and gastrointestinal tumours (10, 12–14). However, little was known about the co-exposure of MetS and inflammation to HF. Therefore, we used data from the Kailuan study cohort to systematically analyze the association of MetS and elevated hs-CRP levels with the incident HF.
Methods
Study design and population
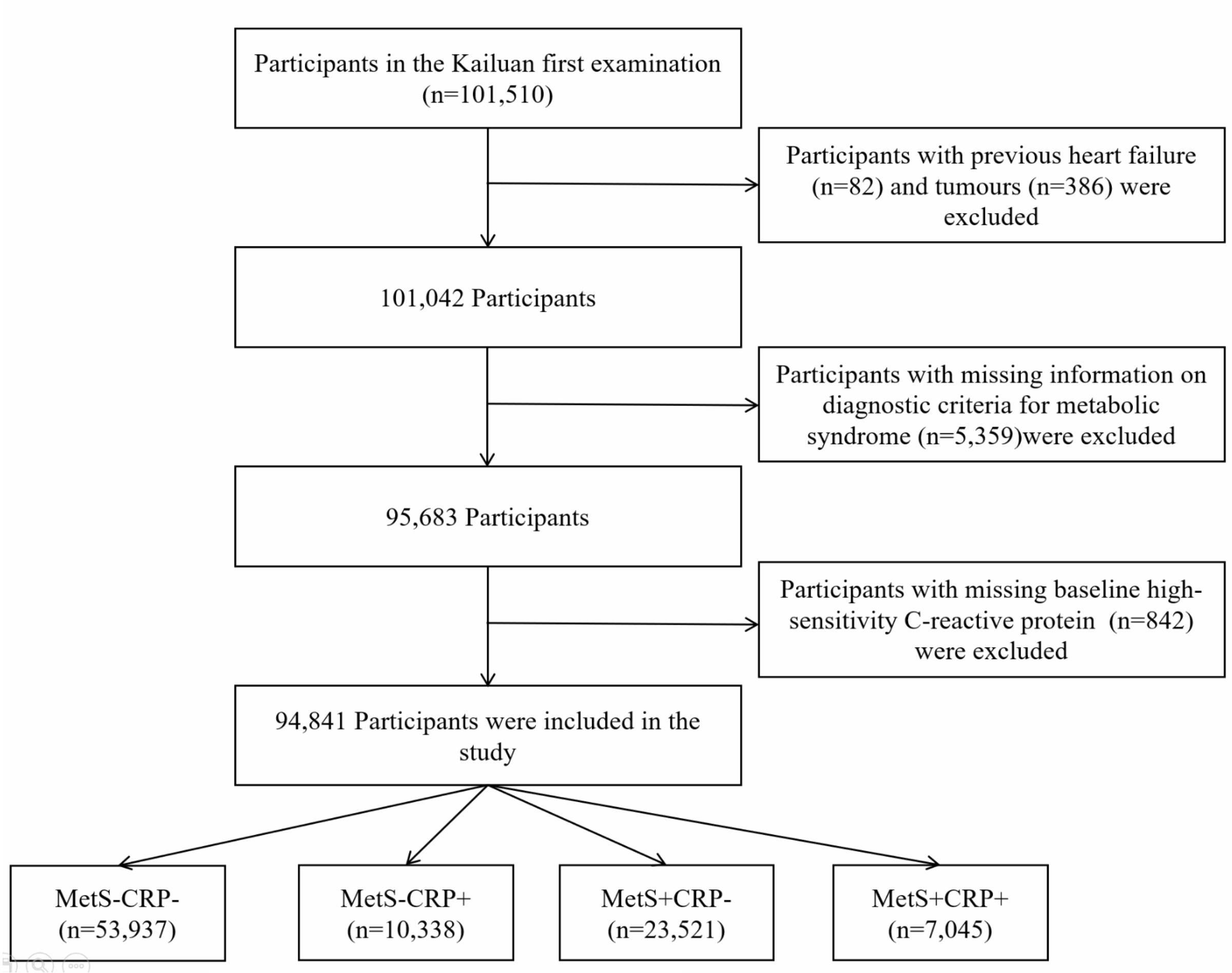
The Kailuan cohort (registration number: ChiCTR-TNRC-11001489) was a large prospective cohort study conducted in the Kailuan group, in Tangshan City, Hebei Province, China. Detailed information on the study design and methods has been previously documented in published reports (15, 16). The initial examination of 101,150 adult participants from the active and retired population of Kailuan Group took place between 2006 and October 2007. They were then followed up every two years with standardized questionnaires, clinical examinations and laboratory tests. Figure 1 showed the study procedure. Participants with a history of HF (n=82), a history of cancer (n=386), missing information on diagnostic criteria for MetS (n=5,359), missing baseline information on hs-CRP (n=842) were excluded. In the end, 94,841 participants were included in the study.
All participants provided written informed consent. The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan Hospital (Ethics approval number:200605).
Data collection
The baseline data were obtained at the first physical examination between 2006 and 2007. Height was measured using a tape measure to an accuracy of 0.1 cm, and weight was measured using a calibrated weight scale to an accuracy of 0.1 kg. Waist circumference (WC) was measured with a tape measure, using the midpoint between the lower edge of the ribs and the upper edge of the hips as the reference point. Blood pressure (BP) was measured twice consecutively with a mercury sphygmomanometer with the participant in an upright sitting position after a 5-minute rest. The mean of these two BP readings was recorded for subsequent analysis. If the difference between the two readings exceeded 5 mm Hg, a third reading was taken and the average of all three readings was used for data analysis. A standardized questionnaire was used to collect information on various baseline characteristics, including sex and date of birth, and lifestyle factors such as smoking status, alcohol consumption, salt intake and physical activity. In addition, personal or family medical history was documented, including conditions such as hypertension, diabetes mellitus and cardiovascular disease. Information on medication use, including antihypertensive, hypoglycemic and lipid-lowering medications, was also recorded.
Laboratory examinations
Blood samples were collected from the antecubital vein in the morning after an overnight fast. Fasting blood glucose (FBG) was measured by the hexokinase/glucose-6-phosphate dehydrogenase method. Triglycerides (TG) were measured by an enzymatic method, and high-density lipoprotein cholesterol (HDL-C) concentration was measured by a direct method. Uric acid (UA) was measured using a commercial kit from Kewa Bioengineering, Shanghai, China. Hs-CRP levels were measured using a highly sensitive immunoturbidimetric assay (Cias Latex CRP-H, Kanto Chemical Co. Inc, Tokyo, Japan) with a detection limit of 0.1 mg/L. Biochemical indices were measured in the central laboratory of Kailuan General Hospital using a Hitachi 7600 autoanalyzer.
Metabolic syndrome definition and subgroups
MetS was defined according to the criteria established by the ATP III criteria, as follows: The presence of three or more of the following factors: 1) SBP≥ 130 mm Hg or DBP ≥ 85 mm Hg or use of antihypertensive medication, 2) FBG ≥ 5.6 mmol/L or use of hypoglycemic medication, 3) TG ≥ 1.69 mmol/L, 4) HDL-C < 1.04 mmol/L in men and < 1.29 mmol/L in women, or using lipid-lowering medication, and 5) WC ≥ 85 cm in men, WC ≥ 80 cm in women (12, 17). High hs-CRP levels were defined as serum hs-CRP > 3 mg/L (9).
Participants were divided into four groups according to the presence or absence of MetS and hs-CRP levels: 1)MetS-CRP-, participants without MetS and with hs-CRP levels ≤ 3 mg/L. 2) MetS-CRP+, participants without MetS and with hs-CRP levels > 3 mg/L. 3) MetS+CRP-, participants with MetS and hs-CRP levels ≤ 3 mg/L. 4) MetS+CRP+, participants with MetS and hs-CRP levels > 3 mg/L.
Definition of study outcomes
The study started with the first physical examination in 2006, and the primary outcome was the first diagnosis of HF. The follow-up end point was 31 December 2020 for individuals not experiencing the event. The end point was defined as the time of death if death occurred during follow-up. Discharge records from 11 local hospitals were collected and reviewed annually by specialized teams to identify patients with suspected HF. The definition of HF was based on the Chinese Guidelines for the Diagnosis and Treatment of HF 2018 (18). Diagnostic parameters included clinical presentation and laboratory tests. The diagnosis of HF was confirmed by the presence of (1) and any of (2) or (3): (1) symptoms of HF, including dyspnea, fatigue, and fluid retention, and a discharge diagnosis of central function classified as NewYork Heart Association cardiac function classes II, III, IV or Killip II, III, IV; (2) left ventricular ejection fraction ≤ 50%, as measured by Simpson’s method modified by 2-dimensional and Doppler echo cardiography; and (3) elevated plasma NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels.
Assessment of covariates
Smoking was defined as an average of at least 1 cigarette per day in the past year, divided into non-smokers and current smokers, with non-smokers including ex-smokers. Alcohol consumption was defined as drinking ≥ 100 ml of liquor (with an alcohol content of 50% or more) per day on average in the past year, divided into those who still drink alcohol and those who do not, and those who do not include those who have stopped drinking. Active physical activity was defined as exercising at least 3 times a week for at least 30 min each time, and a high-salt diet was defined as a salt intake of 10 g or more per day. Educational level was categorized as junior high school and below, and high school and above. Pre-diabetes was defined as FBG at 5.6-6.9 mmol/L (19). Hypertension was defined as self-reported history of hypertension, current treatment with an antihypertensive agent or a measured SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg. Diabetes was defined as a self-reported history of diabetes, current treatment with a hypoglycemic agent or a FBG ≥ 7.0 mmol/L. Dyslipidemia was defined as meeting any of the following criteria: total cholesterol (TC) ≥ 6.22 mmol/L, TG ≥ 2.26 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥ 4.14 mmol/L, HDL-C < 1.04 mmol/L, or self-reported history of hyperlipidemia. The estimated glomerular filtration rate (eGFR) was calculated according to the formula of the Chronic Kidney Disease Epidemiology Cooperation (CKD-EPI) (20).
Statistical analyses
Normally distributed continuous variables were expressed as mean ± SD and compared using the ANOVA. Skewed distribution continuous variables were expressed as median (P25-P75) and compared using the Kruskal-Wallis test. Categorical variables were expressed as percentages and compared using the chi-squared test. Cox proportional hazards models were used to analyze the association of MetS and components with HF and the association of hs-CRP (grouped by 3 mg/L) with HF and the association of the combination of MetS and hs-CRP with HF. The model was adjusted for age, sex, smoking, alcohol consumption, physical activity, education, salt intake, family history of cardiovascular disease, eGFR, UA, use of antihypertensive medication, use of hypoglycemic medication, and use of lipid-lowering medication. Subgroup analyses were stratified by age (< 60 years and ≥ 60 years) and gender. To examine the effect of antihypertensive medication, stratification was performed according to the use and intensity of antihypertensive medication.The incident rate of HF was calculated using the number of HF occurrence divided by 1000 person-years. The cumulative incidence of HF in the different groups was calculated using the Kaplan-Meier method. The log-rank test was used to compare groups. To explore the effect of MetS and hs-CRP interaction on HF, we included MetS, hs-CRP and their multiplicative interaction terms in the Cox model after adjustment for covariates and calculated the excess relative risk (RERI), attributable proportion (AP) and synergy index (SI) to estimate the additive interaction. Data were analyzed using SAS 9.4 (SAS Institute, Cary, North Carolina) statistical software, and statistical significance was defined as a two-tailed P value < 0.05.
Sensitivity analysis
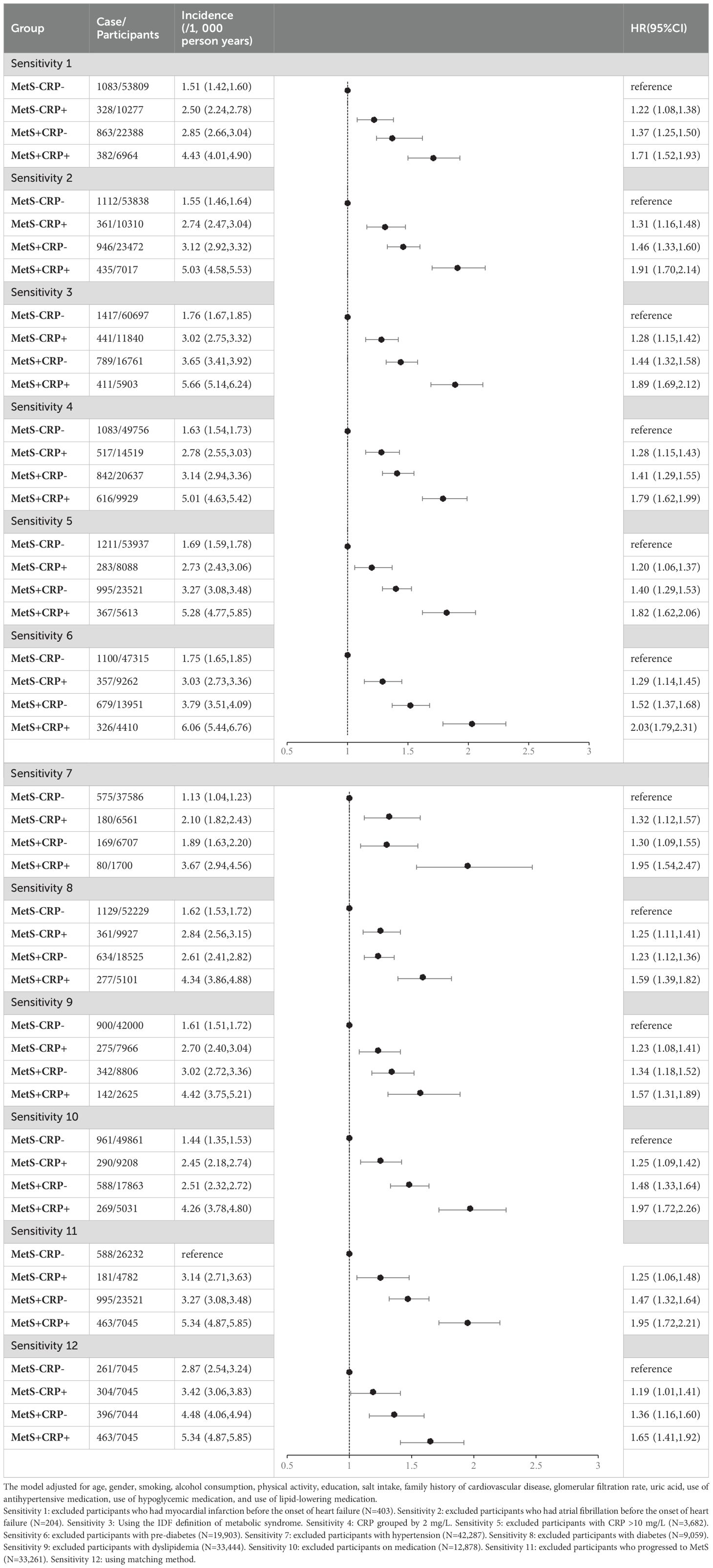
To ensure the robustness of the results, we separately excluded participants who had myocardial infarction (MI), AF before the onset of HF. To avoid the possibility of different definitions of MetS affecting the results of the study, we repeated the analyses using the definition of MetS from the IDF criteria (21). To assess the effect of different inflammation degrees, the sensitivity analysis was performed by re-grouping with CRP 2 mg/L as the cut-off point to verify the reliability of the results (22). To avoid the effects of acute inflammation and infectious diseases, we excluded individuals with hs-CRP > 10 mg/L and repeated analyses (9, 23). Considering that patients with hypertension, diabetes and dyslipidemia may contribute to differences in results, we excluded patients with pre-diabetes, hypertension, diabetes and dyslipidemia from the baseline. Taking into account the effect of treatment on outcome, we excluded participants who received pharmacological treatment (antihypertensive, hypoglycemic, or lipid-lowering drugs). Considering that the progression of non-MetS participants to the MetS population at follow-up had an impact on the results, we excluded participants without MetS at baseline who developed MetS at follow-up (24). Considering the effects of age on outcome, we used participants in the MetS+CRP+ group as the exposure group, and matched three control groups by age (± 1 year) among participants in the MetS-CRP- group, MetS-CRP+ group, and MetS+CRP- group, respectively.
Results
Participant characteristics
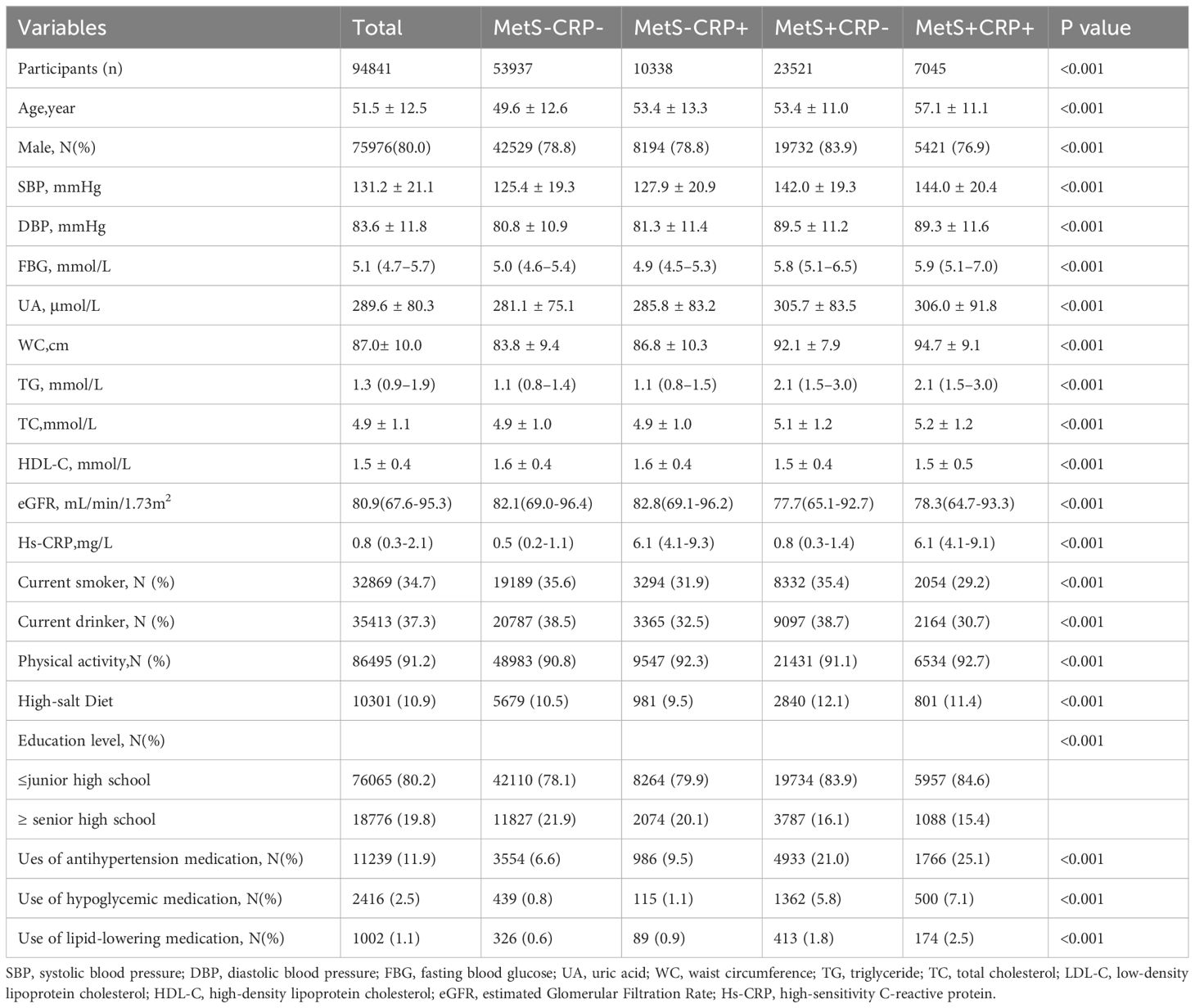
A total of 94,841 participants (mean age 51.5 ± 12.5 years) were recruited, of whom 75,976 (80.0%) were male. The baseline characteristics of the four groups were shown in Table 1. Statistical differences between the four groups were found for sex, age, SBP, DBP, UA, WC, Tg, Tc, LDL-C, HDL-C, eGFR, hs-CRP, smoking, alcohol consumption, educational level, physical activity, use of antihypertensive medication, use of hypoglycemic medication, and use of lipid-lowering medication. Participants in the MetS+CRP+ group were more likely to be older, less educated, had central obesity, higher UA, lower eGFR and higher hs-CRP than those in the MetS-CRP- group.
Association of the combination of MetS and hs-CRP levels with the incidence of HF
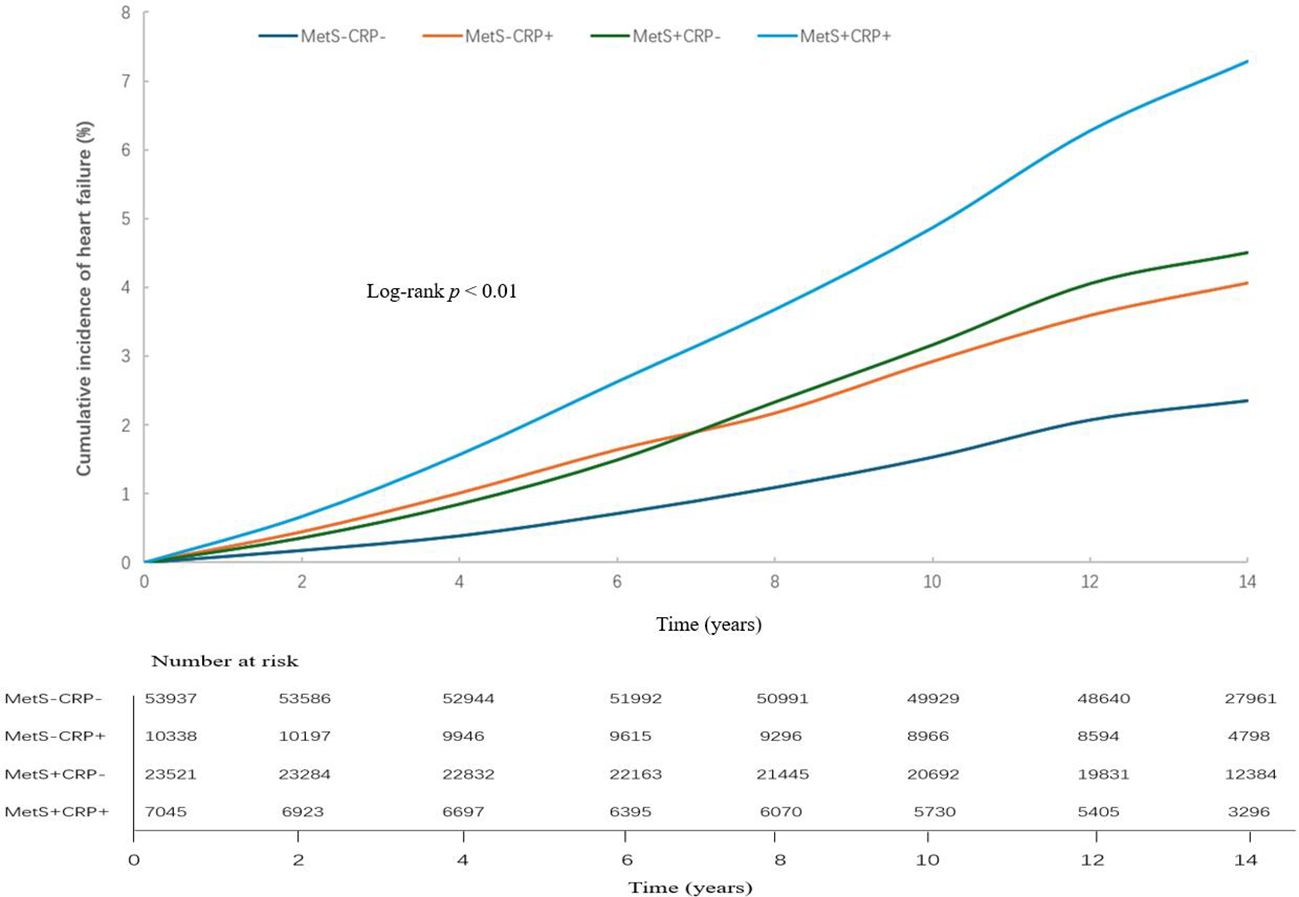
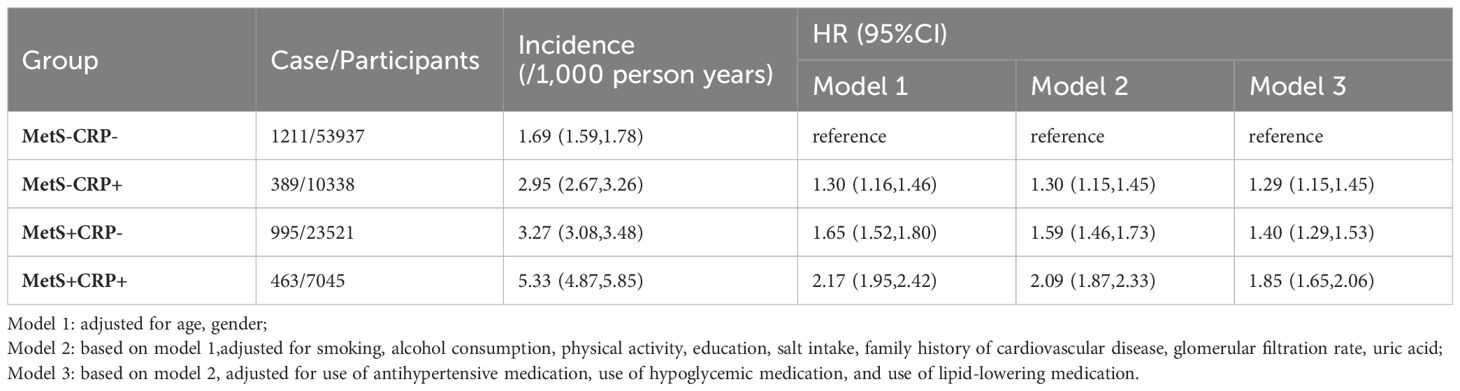
In studies with a mean follow-up of 13.1 ± 2.60 years, 3,058 cases of HF occurred. The cumulative incidence of HF shown in Figure 2. The HF incidence rate of four groups were 1.69/1000pys, 2.95/1000pys, 3.27/1000pys, 5.33/1000pys. Table 2 showed that the risk of HF was increased by 1.29-fold (HR, 1.29; 95% CI, 1.15-1.45), 1.40-fold (HR, 1.40; 95% CI, 1.29-1.53) and 1.85-fold (HR, 1.85; 95% CI, 1.65-2.06) in the MetS-CRP+, MetS+CRP- and MetS+CRP+ groups, respectively, compared with the MetS-CRP- group. We further examined the interaction between MetS and inflammation (hs-CRP > 3 mg/L) with HF. Before adjustment for covariates, the multiplicative interaction was not statistically significant (p for interaction = 0.39), and the additive interaction metrics were statistically significant (RERI 0.49, 95% CI, 0.13- 0.85; AP 0.15, 95% CI 0.04 - 0.26; SI 1.29, 95% CI 1.07 - 1.55, P value =0.008). After adjustment for covariates, the additive interaction indicator was no longer significant (multiplicative interaction: p for interaction = 0.85, additive interaction: RERI 0.16, 95% CI, -0.08- 0.39; AP 0.08, 95% CI -0.03 - 0.20; SI 1.22, 95% CI 0.90 - 1.65, P value =0.20).
Association of the MetS and components and hs-CRP with the incidence of HF
After adjustment for covariates, there was a 1.41-fold increased risk of HF in patients with MetS (HR, 1.41; 95% CI, 1.31-1.52) compared with those without MetS. In the MetS components of the HF risk study, elevated BP had the strongest association with HF (HR, 1.47; 95% CI, 1.34-1.61). The risk of HF was increased 1.29-fold (95% CI, 1.18-1.41), 1.20-fold (95% CI, 1.11-1.30), and 1.13-fold (95% CI, 1.05-1.22) for elevated WC, elevated FBG, and elevated TG, respectively. Low HDL-C levels did not show a significant effect. Those with hs-CRP levels >3 mg/L had a 1.30-fold increased risk of HF (HR, 1.30; 95% CI, 1.20-1.41) compared with those with hs-CRP levels ≤ 3 mg/L, as shown in Table 3.
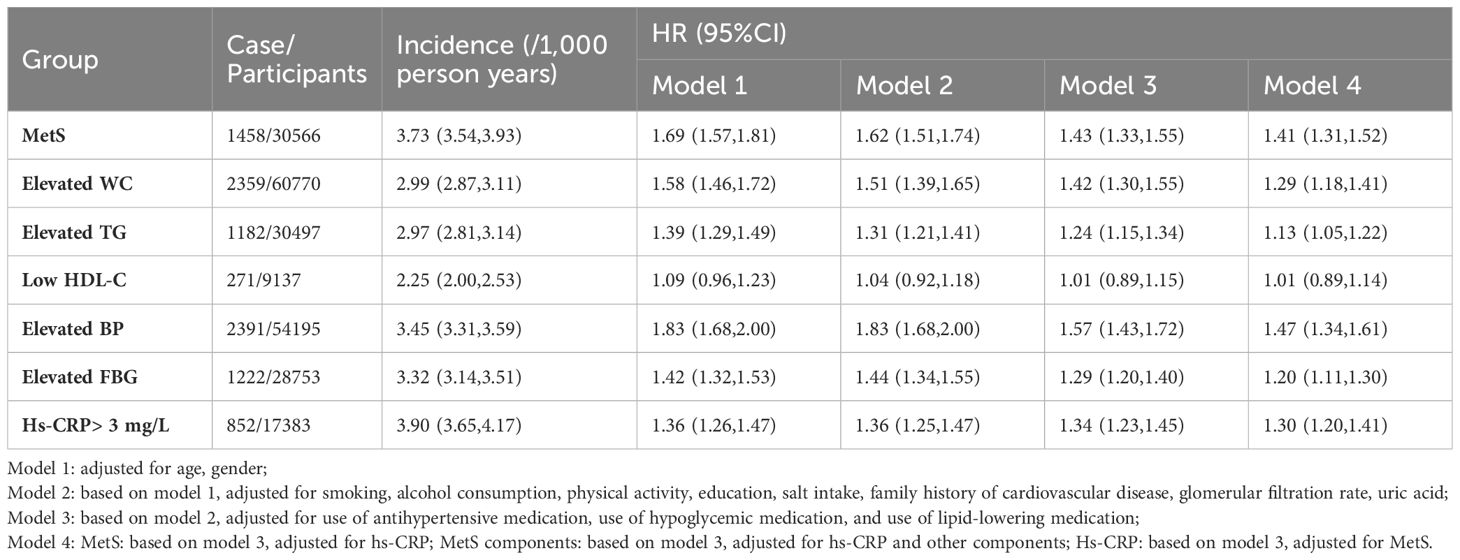
Table 3. Hazard ratios (HRs) for the association of MetS and components or hs-CRP levels with HF Risk.
Age and sex stratified analysis of the association of the combination of MetS and hs-CRP levels with the risk of HF
There was an interaction between groups and age (p for interaction < 0.01). Compared with MetS-CRP-, the risk of HF in the MetS+CRP+ group was increased 2.17-fold (HR, 2.17; 95% CI, 1.83-2.57) in participants with < 60 years and 1.53-fold (HR, 1.53; 95% CI, 1.32-1.78) in participants with ≥ 60 years. Lifestyle and medication information grouped by age were shown in Supplementary Material Supplementary Table S1. There was no interaction between groups and sex (p for interaction >0.05). Compared with MetS-CRP-, the risk of HF in the MetS+CRP+ group was 1.81-fold (HR, 1.81; 95% CI, 1.60-2.04) in males and 1.93-fold (HR, 1.93; 95% CI, 1.47-2.53) in females, respectively. The results were shown in Table 4.
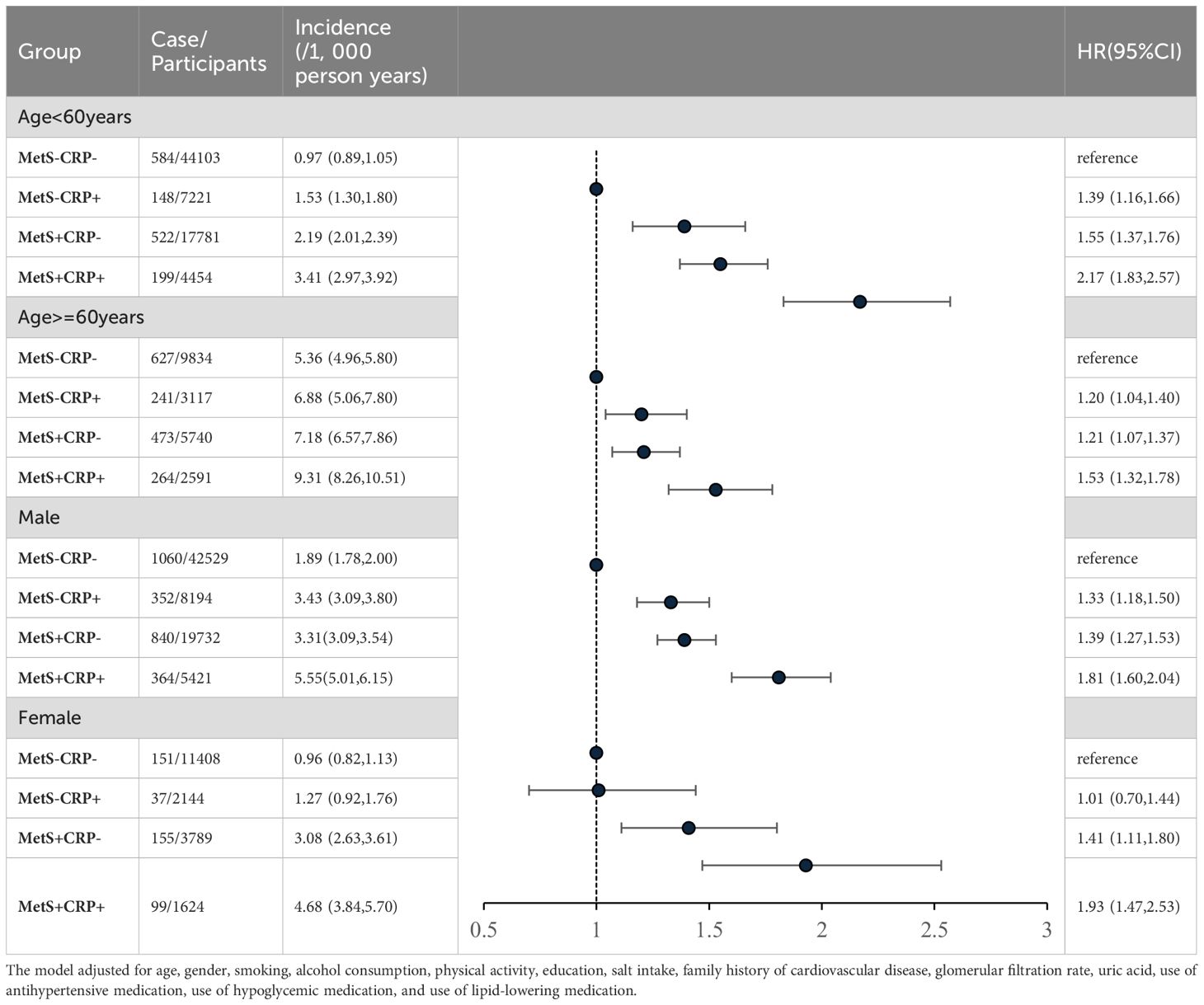
Table 4. Hazard ratios (HRs) for the association of MetS and hs-CRP levels with HF risk by age and gender.
Antihypertensive drugs stratified analysis of the association of the combination of MetS and hs-CRP levels with the risk of HF
There was an interaction between groups and ues of antihypertension medication (p for interaction <0.01). Compared with MetS-CRP-, the risk of HF in the MetS+CRP+ group was increased 1.38-fold (HR, 1.38; 95% CI, 1.12-1.70) in participants with antihypertension medication use and 2.00-fold (HR, 2.00; 95% CI, 1.75-2.27) in participants without antihypertension medication use. We continued to stratify according to the intensity of treatment, divided into monotherapy and combination therapy (at least two types of antihypertensive drugs). After stratification, the HR (HR, 1.27; 95% CI, 1.04-1.56) of the combined treatment of MetS + CRP + group was significantly lower than that of the single drug treatment of MetS + CRP + group (HR, 1.76; 95% CI, 1.26-2.48). The results were shown in Table 5.
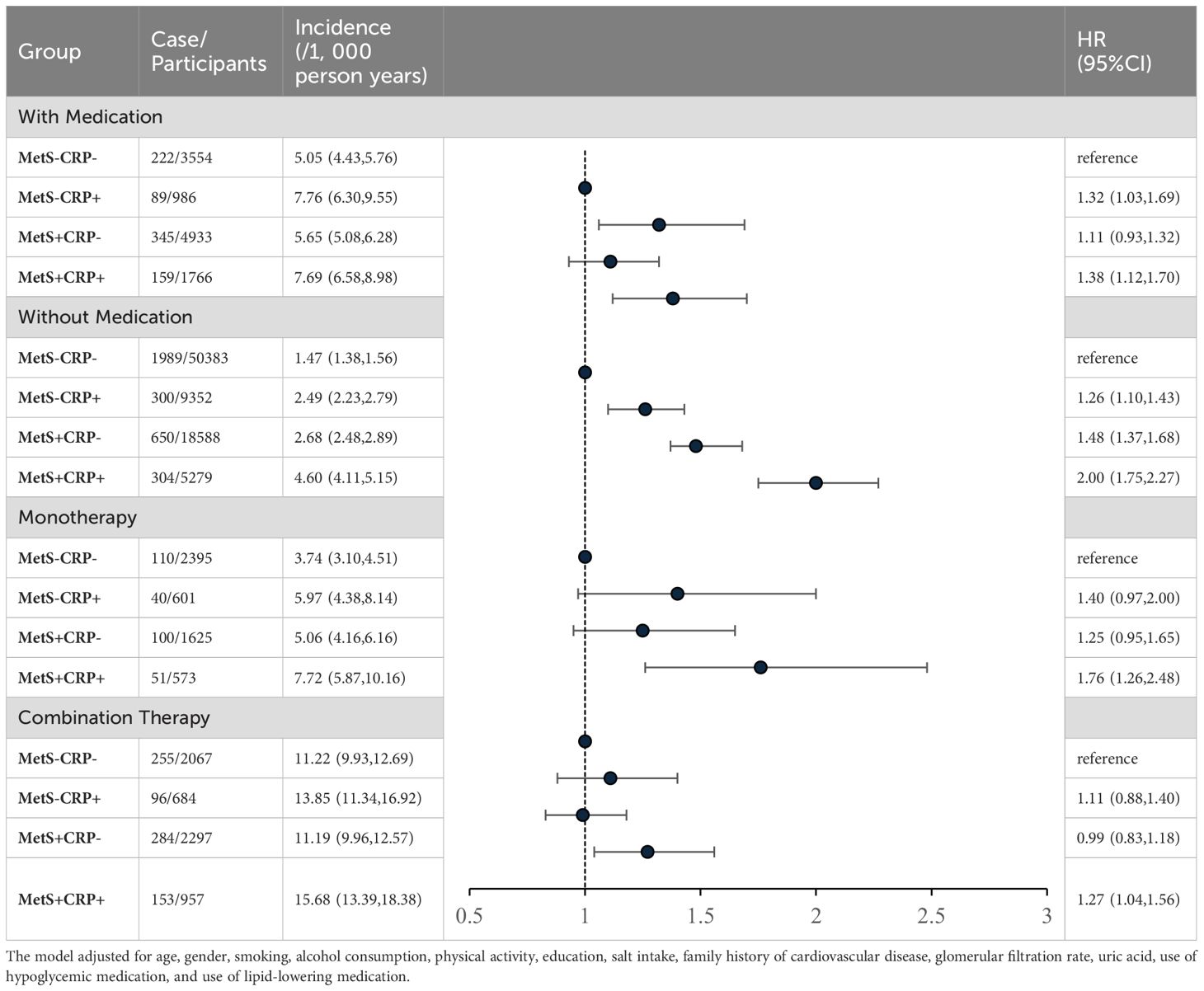
Table 5. Hazard ratios (HRs) for the association of MetS and hs-CRP levels with HF risk by antihypertensive medication.
Sensitivity analysis
We separately excluded participants who had MI (N=403), AF (N=204) before the onset of HF, and the results remained robust after adjustment for covariates. We redefined the MetS population using the IDF definition of MetS and the results remained similar. CRP was grouped at 2 mg/L and the results were consistent with the main analysis. We separately excluded those with hs-CRP >10 mg/L (N=3,682), those with pre-diabetes (N=19,903), those with hypertension (N=42,287), those with diabetes (N=9,059), those with dyslipidemia (N=33,444), those on medication (N=12,878), and those who progressed to MetS (N=33,261), and the results were consistent with the primary outcome. Due to baseline age differences among the four groups, we used matching, and the results were consistent with the main results. The baseline characteristics of the four groups after matching were shown in Supplementary Material: Supplementary Table S2. The results were shown in Table 6.
Discussion
The results showed that the combination of MetS and high hs-CRP increased the risk of HF. Our stratified analysis found that in young people, people with metabolic disorders combined with inflammation had a higher relative risk of HF compared to people with metabolically healthy non-inflammation. This study highlighted the potential importance of MetS and inflammation as part of a strategy to prevent HF, especially when both are present. Especially in young people, early prevention and intervention may have more significant long-term benefits.
Several mechanisms may explain the association between MetS and inflammation with HF. Insulin resistance is the main pathogenesis of MetS (25). Chronic inflammation released pro-inflammatory cytokines such as TNF-α and IL-6, which affect insulin signalling pathways and lead to insulin resistance (26). The coexistence of both exacerbated insulin resistance and impaired two signalling pathways, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) pathways, leading to cardiomyocyte apoptosis and fibrosis (27). Systemic inflammation in patients with MetS also led to activation of the vegetative angiotensin system and the sympathetic nervous system, resulting in volume expansion, increased peripheral resistance, and compensatory myocardial enlargement (7). In addition, the onset of MetS and the binding of CRP to CD32 and CD64 receptors on endothelial cells triggered pro-inflammatory pathways (28). These pathways might increase susceptibility to HF.
We found that the association of MetS with HF was stronger than the association of CRP with HF and provided several possible explanations. First, elevated BP in the MetS component was most strongly associated with HF. According to the 2019 GBD data, the main causes of HF worldwide are ischemic heart disease and hypertensive heart disease. Among them, the proportion of HF caused by hypertensive heart disease was 32.84% (29). Hypertension was a key component of MetS. In China, the awareness, treatment and compliance rates of hypertensive patients were still low compared with those in developed countries (30). Therefore, effective management of hypertension in MetS has become an important direction for improving HF prevention strategies. Second, after we excluded the hypertensive and diabetes populations from the sensitivity analyses, we found that the association between CRP and HF was slightly stronger than that between MetS and HF. This indicated that MetS with combined hypertension and hyperglycemia played a key role in the pathogenesis of HF. The additive effect of MetS combined with hypertension and hyperglycemia on the risk of developing HF was unclear and may be related to activation of insulin resistance and chronic inflammation. Last, the association of CRP with HF with preserved ejection fraction (HFpEF) and with reduced ejection fraction (HFrEF) was controversial. Several studies showed a stronger association between CRP and HFpEF (28, 31). In another study, CRP was independently associated with HFrEF but not with HFpEF (32). However, we were unable to typify due to missing data on ejection fraction. The association between CRP and HF might be underestimated by the different proportions of HF in different types.
After stratified for age, we found that the MetS+CRP+ group had a higher relative risk of HF in those <60 years of age. According to previous studies, the relative risk of cardiovascular disease differed between different age groups for the onset of MetS, hypertension, and diabetes, and this association was particularly strong in the younger population (33–35). Although the incidence and absolute risk of HF were higher in older populations, modifiable clinical risk factors had higher relative and population-attributable risks in younger populations (36). People with early onset of metabolic disease more frequently had concomitant obesity and poor risk factor control (37). Younger people were less likely to take interventions for hypertension, diabetes, etc., and were more likely than older people to have unhealthy lifestyles, which may have increased their relative risk of developing HF. We compared lifestyles and drug use in different age groups and found that the proportion of unhealthy lifestyles such as smoking and drinking was higher in people under 60 years old, and although most of them maintained exercise habits, they may still increased the relative risk of HF due to poor health management. In contrast, people over 60 years old were more inclined to maintain healthy lifestyle habits. Although they had a high prevalence of chronic diseases, they were more active in receiving drug treatment.
Our results found that antihypertensive therapy alleviated the association between participants with metabolic disorders combined with inflammation and the risk of HF, and that combination therapy was more significant than monotherapy. At present, no drugs have been approved for the treatment of MetS itself (38). Our results suggested the importance of targeting antihypertensive treatment to participants with metabolic disorders associated with inflammation. Elevated CRP in patients with MetS might predicted worse subclinical impairment (39, 40), and the included of CRP in the risk assessment of patients with MetS might identified high-risk subgroups who might benefit from intensive antihypertensive therapy.
We did several sensitivity analyses. The MI and AF cause HF (14, 41, 42). We excluded participants with MI and AF that occurred before HF, respectively, and the associations were not significantly changed. It has been suggested that chronic low-grade inflammation be defined as CRP > 2 mg/L (28). Therefore, we repeated the primary analysis and the results were consistent. The definition of MetS included people with pre-diabetes. Studies reported that pre-diabetes increased the risk of HF (43). We excluded this group, and the results remained robust. We observed that the change of HF risk was small after excluding pre-diabetes, and was significantly lower than that of excluding diabetes. Some studies have reported that the more elevated FBG levels, the more likely they are to adversely affect the heart by inducing inflammation, modulating nitric oxide metabolism and increasing oxidative stress (44). These differences may lead to less myocardial damage in prediabetes than in diabetes. In a prospective cohort study of 18,084 patients with cardiovascular disease, a 1 mmol/L increased in FBG was associated with a 1.23-fold increased risk of hospitalisation for HF (45). Individuals without MetS at baseline might progress to MetS during follow-up. Studies showed that the occurrence of MetS, even if only once, might have had long-term adverse effects on cardiovascular health, and this effect was difficult to completely reverse regardless of whether the individual eventually recovered (24). Therefore, we excluded people who had MetS during follow-up, and the results did not change.
It provided a unique perspective on the association of MetS and inflammation with the risk of HF onset. The age range of participants in this study was 18-90 years, which was well represented. Despite these strengths, it was important to acknowledge certain limitations of the study. First, the use of a single biomarker for baseline measurements was a potential limitation. However, the large sample size mitigated this limitation to some extent. In addition, we did not measure other inflammatory markers such as IL-6. We lacked indicators that reflect early organ damage. For example, the Mechanical Energy Efficiency Index (MEEI) was a valuable predictor of HF (46), and elevated CRP, MetS exacerbated myocardial MEEI (39). We lacked data on ejection fraction, and the association of MetS and inflammation with HF needed to be further discussed in the different subtypes of HF.
Conclusion
In the Chinese population, the combination of MetS and high hs-CRP levels increased the risk of HF, especially in young people. Early intervention for metabolic abnormalities combined with inflammation might prevent the development of HF.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Kailuan Hospital (Ethics approval number:200605). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YT: Investigation, Writing – original draft, Writing – review & editing. YXW: Writing – original draft. DZ: Writing – original draft. HS: Writing – original draft. HW: Writing – original draft. PY: Writing – original draft, Writing – review & editing. SW: Writing – review & editing. YW: Writing – original draft. SC: Writing – original draft. YL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0503500); This study was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0504000).
Acknowledgments
The authors thank the investigators who made this cohort study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1544823/full#supplementary-material
Abbreviations
AF, Atrial Fibrillation; BP, Blood Pressure; CI, Confidence Interval; DBP, Diastolic Blood Pressure; EGFR, Estimated Glomerular Filtration Rate; FBG, Fasting Blood Glucose; HDL-C High-density Lipoprotein Cholesterol; HF, Heart Failure; HR, Hazard Ratio; Hs-CRP, High Sensitive C-reactive Protein; LDL-C, Low-density Lipoprotein Cholesterol; Meei, Mechanical Energy Efficiency Index; MetS Metabolic Syndrome; SBP, Systolic Blood Pressure; SUA, Blood Uric Acid; TC, Total Cholesterol; TG, Triglycerides; WC Waist Circumference.
References
1. Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. (2021) 28:1682–90. doi: 10.1093/eurjpc/zwaa147
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Wang H, Chai K, Du M, Wang S, Cai JP, Li Y, et al. Prevalence and incidence of heart failure among urban patients in China: A national population-based analysis. Circulation. Heart failure. (2021) 14:e008406. doi: 10.1161/CIRCHEARTFAILURE.121.008406
3. Wang H, Li Y, Chai K, Long Z, Yang Z, Du M, et al. Mortality in patients admitted to hospital with heart failure in China: a nationwide Cardiovascular Association Database-Heart Failure Centre Registry cohort study. Lancet Global Health. (2024) 12:e611–22. doi: 10.1016/S2214-109X(23)00605-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. (2020) 126:789–806. doi: 10.1161/CIRCRESAHA.119.312321
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Kamimura D, Cain LR, Mentz RJ, White WB, Blaha MJ, DeFilippis AP, et al. Cigarette smoking and incident heart failure: insights from the jackson heart study. Circulation. (2018) 137:2572–82. doi: 10.1161/CIRCULATIONAHA.117.031912
6. He YN, Zhao WH, Zhao LY, Yu DM, Zhang J, Yang XG, et al. Prevalence of metabolic syndrome in Chinese adults in 2010-2012. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. (2017) 38:212–5. doi: 10.3760/cma.j.issn.0254-6450.2017.02.015
7. Gargiulo P, Marsico F, Renga F, Dell’Aversana S, Esposito I, Marciano C, et al. The metabolic syndrome in heart failure: insights to specific mechanisms. Heart failure Rev. (2020) 25:1–7. doi: 10.1007/s10741-019-09838-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Kim TE, Kim H, Sung J, Kim DK, Lee MS, Han SW, et al. The association between metabolic syndrome and heart failure in middle-aged male and female: Korean population-based study of 2 million individuals. Epidemiol Health. (2022) 44:e2022078. doi: 10.4178/epih.e2022078
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. (2003) 107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Liu C, Liu T, Zhang Q, Song M, Zhang Q, Shi J, et al. Temporal relationship between inflammation and metabolic disorders and their impact on cancer risk. J Global Health. (2024) 14:04041. doi: 10.7189/jogh.14.04041
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Varghese M, Song J, Singer K. Age and Sex: Impact on adipose tissue metabolism and inflammation. Mech Ageing Dev. (2021) 199:111563. doi: 10.1016/j.mad.2021.111563
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Liu T, Fan Y, Zhang Q, Wang Y, Yao N, Song M, et al. The combination of metabolic syndrome and inflammation increased the risk of colorectal cancer. Inflammation research: Off J Eur Histamine Res Soc. (2022) 71:899–909. doi: 10.1007/s00011-022-01597-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Song M, Liu T, Liu H, Zhang Q, Zhang Q, Wang Y, et al. Association between metabolic syndrome, C-reactive protein, and the risk of primary liver cancer: a large prospective study. BMC Cancer. (2022) 22:853. doi: 10.1186/s12885-022-09939-w
14. Wang Z, Wang B, Li X, Zhang S, Wu S, Xia Y. Metabolic syndrome, high-sensitivity C-reactive protein levels and the risk of new-onset atrial fibrillation: Results from the Kailuan Study. Nutrition metabolism Cardiovasc diseases: NMCD. (2021) 31:102–9. doi: 10.1016/j.numecd.2020.06.026
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Li Y, Li Y, Gurol ME, Liu Y, Yang P, Shi J, et al. In utero exposure to the Great Chinese Famine and risk of intracerebral hemorrhage in midlife. Neurology. (2020) 94:e1996–2004. doi: 10.1212/WNL.0000000000009407
16. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circulation. Cardiovasc Qual outcomes. (2012) 5:487–93. doi: 10.1161/CIRCOUTCOMES.111.963694
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
17. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). Jama. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
18. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua xin xue guan bing za zhi. (2018) 46:760–89. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004
19. Liu X, Song Q, Wu S, Zhou W, Wang X. Prediabetes and risk for myocardial infarction by hypertension status in a Chinese population: a prospective cohort study. J Hypertens. (2021) 39:77–83. doi: 10.1097/HJH.0000000000002607
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2017) 32:497–507. doi: 10.1093/ndt/gfw425
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine: J Br Diabetic Assoc. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Sung KC, Ryu S, Chang Y, Byrne CD, Kim SH. C-reactive protein and risk of cardiovascular and all-cause mortality in 268 803 East Asians. Eur Heart J. (2014) 35:1809–16. doi: 10.1093/eurheartj/ehu059
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. (2004) 109:1955–9. doi: 10.1161/01.CIR.0000125690.80303.A8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. He D, Zhang X, Chen S, Dai C, Wu Q, Zhou Y, et al. Dynamic changes of metabolic syndrome alter the risks of cardiovascular diseases and all-cause mortality: evidence from a prospective cohort study. Front Cardiovasc Med. (2021) 8:706999. doi: 10.3389/fcvm.2021.706999
25. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. (2022) 23(2):786. doi: 10.3390/ijms23020786
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. (2010) 72:219–46. doi: 10.1146/annurev-physiol-021909-135846
27. Banerjee D, Biggs ML, Mercer L, Mukamal K, Kaplan R, Barzilay J, et al. Insulin resistance and risk of incident heart failure: Cardiovascular Health Study. Circulation. (2013) 6:364–70. doi: 10.1161/CIRCHEARTFAILURE.112.000022
28. Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, et al. C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J Am Coll Cardiol. (2023) 82:414–26. doi: 10.1016/j.jacc.2023.05.035
29. Feng J, Zhang Y, Zhang J. Epidemiology and burden of heart failure in asia. JACC Asia. (2024) 4:249–64. doi: 10.1016/j.jacasi.2024.01.013
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
30. Yin R, Yin L, Li L, Silva-Nash J, Tan J, Pan Z, et al. Hypertension in China: burdens, guidelines and policy responses: a state-of-the-art review. J Hum hypertension. (2022) 36:126–34. doi: 10.1038/s41371-021-00570-z
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. (2010) 55:2129–37. doi: 10.1016/j.jacc.2009.12.045
32. de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. (2018) 3:215–24. doi: 10.1001/jamacardio.2017.4987
33. Huang Z, Wang X, Ding X, Cai Z, Li W, Chen Z, et al. Association of age of metabolic syndrome onset with cardiovascular diseases: the kailuan study. Front Endocrinol. (2022) 13:857985. doi: 10.3389/fendo.2022.857985
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
34. Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. (2020) 75:2921–30. doi: 10.1016/j.jacc.2020.04.038
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Zhao M, Song L, Sun L, Wang M, Wang C, Yao S, et al. Associations of type 2 diabetes onset age with cardiovascular disease and mortality: the kailuan study. Diabetes Care. (2021) 44:1426–32. doi: 10.2337/dc20-2375
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Tromp J, Paniagua SMA, Lau ES, Allen NB, Blaha MJ, Gansevoort RT, et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ (Clinical Res ed.). (2021) 373:n880. doi: 10.1136/bmj.n461
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
37. Steinarsson AO, Rawshani A, Gudbjörnsdottir S, Franzén S, Svensson AM, Sattar N. Short-term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: a longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia. (2018) 61:599–606. doi: 10.1007/s00125-017-4532-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Handelsman Y, Butler J, Bakris GL, DeFronzo RA, Fonarow GC, Green JB, et al. Early intervention and intensive management of patients with diabetes, cardiorenal, and metabolic diseases. J Diabetes its complications. (2023) 37:108389. doi: 10.1016/j.jdiacomp.2022.108389
39. Cefalo CMA, Riccio A, Fiorentino TV, Succurro E, Miceli S, Mannino GC, et al. Metabolic syndrome and C-reactive protein are associated with a reduced myocardial mechano-energetic efficiency. J Clin Endocrinol Metab. (2023) 108:e1264–71. doi: 10.1210/clinem/dgad300
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
40. Chen Y, Ju H, Xie K, Zhao X. Association of inflammatory score with all-cause and cardiovascular mortality in patients with metabolic syndrome: NHANES longitudinal cohort study. Front Immunol. (2024) 15:1410871. doi: 10.3389/fimmu.2024.1410871
41. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nature reviews. Cardiology. (2016) 13:131–47. doi: 10.1038/nrcardio.2015.191
42. Rastogi T, Ho FK, Rossignol P, Merkling T, Butler J, Clark A, et al. Comparing and contrasting risk factors for heart failure in patients with and without history of myocardial infarction: data from HOMAGE and the UK Biobank. Eur J Heart failure. (2022) 24:976–84. doi: 10.1002/ejhf.v24.6
43. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: A meta-analysis. Diabetes Obes Metab. (2021) 23:1746–53. doi: 10.1111/dom.14388
44. Devos P, Chioléro R, Van den Berghe G, Preiser JC. Glucose, insulin and myocardial ischaemia. Curr Opin Clin Nutr Metab Care. (2006) 9:131–9. doi: 10.1097/01.mco.0000214572.97933.d1
45. Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. (2007) 115:1371–5. doi: 10.1161/CIRCULATIONAHA.106.661405
Keywords: metabolic syndrome, C-reactive protein, heart failure, risk factors, cohort study.
Citation: Tian Y, Wang Y, Zhao D, Sun H, Wu H, Yang P, Wu S, Wu Y, Chen S and Li Y (2025) Metabolic syndrome, high-sensitivity C-reactive protein and the risk of heart failure: the Kailuan cohort study. Front. Endocrinol. 16:1544823. doi: 10.3389/fendo.2025.1544823
Received: 13 December 2024; Accepted: 24 March 2025;
Published: 22 April 2025.
Edited by:
Tatsuya Sato, Sapporo Medical University, JapanReviewed by:
Chiara Maria Assunta Cefalo, Sapienza University of Rome, ItalyRanjodh Singh Sandhu, National Institutes of Health (NIH), United States
Copyright © 2025 Tian, Wang, Zhao, Sun, Wu, Yang, Wu, Wu, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li, bGl5dW44MDIyQDE2My5jb20=; Shuohua Chen, Y3NoMDEwNjIwMTFAMTYzLmNvbQ==; Ying Wu, d3l6aGRkQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Yan Tian1†
Yan Tian1† Yanxiu Wang
Yanxiu Wang Shouling Wu
Shouling Wu Shuohua Chen
Shuohua Chen Yun Li
Yun Li