
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 05 March 2025
Sec. Renal Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1544223
Background: Cirrhosis is a leading cause of global disease burden, with high mortality, particularly in critically ill patients. The blood urea nitrogen to creatinine ratio (BCR) is a straightforward biochemical indicator of renal excretory function and is linked to negative outcomes across different conditions. However, the relationship between BCR and mortality in critically ill patients with cirrhosis is unclear, The purpose of this study is to explore this question.
Methods: A retrospective cohort study was performed utilizing the MIMIC-IV database. We divided BCR into quartiles and evaluated 180-day and 365-day mortality as the primary outcomes. Kaplan-Meier survival analysis and multivariate Cox regression modeling were used to assess the link between BCR and mortality. Linear relationships were further determined using restricted cubic spline (RCS) curves, and finally, subgroup analyses were also performed.
Results: In our study of 2,816 critically ill cirrhotic patients, elevated BCR was significantly linked to higher mortality at both 180 and 365 days. The top BCR quartile showed a 45% higher risk of 180-day mortality (HR=1.45, 95% CI: 1.21-1.73) and a 38% higher risk of 365-day mortality (HR=1.38, 95% CI: 1.17-1.63) relative to the bottom quartile. RCS analysis demonstrated a notable linear correlation between BCR and mortality risk. Subgroup analyses indicated a stronger association between BCR and mortality among older patients.
Conclusion: In critically ill cirrhotic patients, elevated BCR values are strongly linked to increased mortality risk. Our research highlights BCR’s potential as a prognostic marker for cirrhosis, especially in elderly patients.
Cirrhosis denotes the irreversible alterations in liver structure and function resulting from chronic damage, typically manifesting as hepatocyte necrosis, fibrosis, and nodule formation (1). Liver disease is responsible for 2 million deaths globally each year, accounting for 4 percent of all deaths, which are mainly attributable to complications of cirrhosis and hepatocellular carcinoma, cirrhosis is ranked as the 11th leading cause of death (2, 3). Cirrhosis-related mortality declined from 20.0/100,000 person-years in 1980 to 15.8/100,000 person-years in 2010 (4). Mortality has declined markedly in East Asia, North Africa/Middle East and high-income Asia, and the Pacific, but has also increased in many other parts of the world, including South Asia, Central Asia, and Eastern Europe (5). In addition, cirrhosis generates a significant number of disability-adjusted life-years (DALYs) and is the 15th leading cause of DALYs globally (3). Cirrhosis mortality is affected by factors like alcohol consumption, viral hepatitis (e.g., hepatitis B and C), and non-alcoholic fatty liver disease (6). Cirrhosis progresses from the asymptomatic phase (compensated cirrhosis) to the symptomatic phase (decompensated cirrhosis). Decompensated cirrhosis refers to a state where the liver function is no longer able to meet the body’s demands, with significant manifestations of liver failure, such as ascites, hepatic encephalopathy, or variceal bleeding (1). Patients with critically ill cirrhosis are usually admitted to the Intensive Care Unit (ICU) due to severe complications of cirrhosis (7). These patients frequently encounter complications such as multi-organ failure, infections, and bleeding, which exacerbate their condition and heighten the risk of mortality (8). Nearly 40 percent of patients with cirrhosis develop infections during admission or hospitalization. The most common types of infections included spontaneous bacterial peritonitis (22.5-25%), urinary tract infections (21.4-28.5%), respiratory tract infections (9.9-16.4%), skin and soft tissue infections (8.5-12.2%), and secondary bacterial peritonitis (4%), and the presence of any infection in patients with cirrhosis was associated with a 4-fold increase in mortality (3). Recent studies suggest that the prognosis of cirrhotic patients is closely associated with clinical parameters such as the Child-Pugh score, model for end-stage liver disease (MELD) score, liver function status, comorbidities, and infection severity (5, 9). Subsequent studies have built on this foundation by further refining the score to include additional biomarkers with independent predictive value, including the United Kingdom Model for End-Stage Liver Disease (UKELD) incorporating serum sodium, the MELD-Plus score incorporates albumin, total cholesterol, age, and length of hospital stay (10, 11). The MELD-EEG adds an electroencephalogram (EEG), which responds to the presence of hepatic encephalopathy, further increasing predictive accuracy (12). In addition, different strata have been created. For different strata, such as patients with acute or chronic liver failure (ACLF), the European Foundation for the Study of Chronic Liver Failure (CLIF) developed the CLIF Organ Failure (CLIF-OF) score (13, 14). In addition to the above composite scores, an independent index of heart rate variability has also been shown to be associated with cirrhosis and independently predicted mortality in cirrhotic patients (15). In conclusion, the identification of more biomarkers with independent predictive value is important for the prognostic management of cirrhosis.
The blood urea nitrogen to creatinine ratio (BCR) is a simple biochemical indicator of the kidney’s ability to excrete urea and creatinine (16). Recent studies have shown a strong link between high BCR levels and negative clinical outcomes in various patient groups, such as those with acute kidney injury, acute decompensated heart failure, chronic heart failure, and acute myocardial infarction (17). In the context of chronic heart failure, an increased BCR has been linked to heightened mortality (18). Moreover, the potential of the BCR as a prognostic marker for stroke is underlined by a large sample study showing a significant 19% increase in stroke risk in participants in the lowest quintile compared with those in the third quintile of the BCR (19). Acute kidney injury (AKI) is a prevalent comorbidity among cirrhotic patients, impacting up to 50% of hospitalized cirrhotic patients and 58% of ICU patients (20). Renal insufficiency occurs in about 20% of patients with cirrhosis, and fluctuations in creatinine in these patients are closely related to hepatic and renal impairment, and an elevated BCR may imply reduced hepatic metabolic function and impaired renal perfusion (21). Previous studies have shown that BCR is associated with short-term mortality in patients with cirrhosis and is a better predictor of mortality than MELD in patients with cirrhosis, but there is currently a lack of clarity regarding the association between different levels of BCR and mortality in patients with severe cirrhosis (22, 23).
We propose that a high BCR correlates with negative inpatient clinical outcomes in critically ill liver cirrhosis patients. This study assesses the link between BCR and overall mortality in critically ill liver cirrhosis patients using data from the Medical Information Mart for Intensive Care (MIMIC) database data, exploring BCR’s potential as a prognostic biomarker.
Our retrospective cohort study utilized the publicly accessible MIMIC-IV (version 2.2) database, comprising de-identified health data of patients admitted to the intensive care unit at Beth Israel Deaconess Medical Center from 2008 to 2019 (24). Lin Li, an author of the paper, was granted access to the database (Record No: 66829958). The MIMIC-IV database usage received approval from the review boards of both the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Informed consent was not required due to the anonymization of patient health information in the database (25). Our study adhered to the ethical principles of the Declaration of Helsinki.
The diagnosis of cirrhosis relies on the International Classification of Diseases, Ninth Edition (ICD-9), including 5715, 5712, 5716, and Tenth Edition (ICD-10) codes, including k7469, k7460, k7031, k7030, and k743. The study initially enrolled 5,871 cirrhosis patients, excluding those with ICU stays under 24 hours, under 18 years of age, or lacking blood urea nitrogen or creatinine data. A total of 2,816 patients participated in this study (Figure 1).
Follow-up began at the time of ICU admission and continued until one year after the patient was discharged from the hospital or at the end of death. Patients who were alive as of the end of the study period were defined as right censored.
Baseline characteristics related to cirrhosis, such as demographics, vital signs, lab results, comorbidities, and disease severity scores, were extracted using SQL scripts from the GitHub repository (https://github.com/MIT-LCP/mimic-iv) (26). The study considers demographic factors such as age and gender; Vital sign variables including weight, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), respiratory rate, transcutaneous arterial oxygen saturation (SpO2), and temperature; Laboratory results including white blood cell (WBC), red blood cell (RBC), platelets, hemoglobin, RDW, albumin, sodium, potassium, calcium, chloride, glucose, anion gap, international normalized ratio (INR), prothrombin time (PT) and partial thromboplastin time (PTT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST); Comorbidities include atrial fibrillation, respiratory failure, acute kidney injury (AKI), hypertension, diabetes mellitus (DM), heart failure, myocardial infarction, malignant tumors, and liver transplantation; and disease severity scores such as sequential organ failure assessment score (SOFA), acute physiology score III (APS III), systemic inflammatory response syndrome score (SIRS), simplified acute physiology score II (SAPS II), and oxford acute severity of illness score (OASIS). All laboratory variables were derived from the first measurements of the patients at the time of admission.
MissForest interpolates better than established interpolation methods for data with missing values in the range of 10-30% (27). We chose to exclude variables with more than 20% missing data based on existing studies, while variables with less than 20% missing data were estimated using the Random Forest method in the R software missForest package (27, 28).
The main exposure variable in this analysis was BCR, treated as a continuous variable and divided into four categories according to its quartiles: Q1 ≤ 14, 14<Q2 ≤ 20, 20<Q3 ≤ 27, and Q4>27 (16). Patient mortality at 180 and 365 days was the primary outcome of this study.
Participant baseline characteristics are categorized by BCR quartiles. The Kolmogorov-Smirnov test was utilized to evaluate the normality of the variables. Normally distributed continuous variables are expressed as mean ± standard error, whereas skewed continuous variables are expressed as median with interquartile range (IQR). Categorical variables are expressed as frequencies (%). Continuous variables between groups were compared using one-way ANOVA or the Mann-Whitney U test, based on distribution normality. Categorical data comparisons utilized chi-square or Fisher’s exact tests, depending on suitability.
We employed Kaplan-Meier (KM) analysis and multivariate Cox regression modeling to evaluate the relationship between BCR and mortality in cirrhosis patients. To ensure the validity of the Cox regression model, we tested the proportional hazards assumption, the results are presented in Supplementary Figure 1. We developed three models: Model 1 is unadjusted; Model 2 is adjusted for age and body weight; and Model 3 was further adjusted for variables that differed in the baseline datasheet including WBC, RDW, hemoglobin, potassium, chloride, glucose, anion gap, INR, AST, SOFA, APS III, SIRS, heart rate, AKI, diabetes, sepsis and liver transplantation. To explore the potential linear relationship between BCR and mortality in cirrhosis patients, we applied restricted cubic spline (RCS) regression, adjusting for variables in Model 3. Subgroup analyses evaluated the consistency between BCR and mortality among patients with cirrhosis. These analyses were categorized by age, sex, presence of AKI, hypertension, diabetes, heart failure, and myocardial infarction. Interaction effects between subgroups were further statistically assessed.
All analyses were performed using R software (version 4.2.2), and a two-sided P value of less than 0.05 was considered statistically significant.
The study categorized severe hepatic cirrhosis patients into four groups (Q1 to Q4) according to BCR quartiles and examined the baseline characteristic differences among these groups (Table 1). The results showed a significant difference in the age distribution of patients with increasing BCR, with the proportion of patients aged ≥60 years increasing and then decreasing, and being highest in group Q3 (P<0.05). Mean body weight showed a trend of increasing and then decreasing from Q1 to Q4 and was highest in group Q3 (P<0.05). In terms of laboratory parameters, leukocytes, potassium, chloride, and glucose increased with increasing BCR, while anion gap and AST showed an opposite trend (P<0.05). In addition, erythrocytes and hemoglobin first increased and then decreased with increasing BCR levels, while PTT and INR showed opposite trends. In terms of disease severity scores, SOFA, APSIII, SIRS, and OASIS first decreased and then increased with increasing BCR. Regarding vital signs, the heart rate initially decreased before increasing with the rise in BCR. In terms of complications, respiratory failure, AKI, and DM changed significantly with increasing BCR (all P values <0.05). Regarding patient prognostic indicators, patients with the highest BCR levels had the highest all-cause mortality at 180 and 365 days.
Figure 2 presents the KM survival analysis evaluating the association between BCR and mortality in critically ill cirrhosis patients. Figure 2A illustrates the KM survival curve for 180-day mortality. The results showed that as the BCR quartile increased, the 180-day mortality of the patients significantly increased. Patients in the highest BCR quartile exhibited an increased mortality risk compared to those in the lowest quartile. This trend was further confirmed in the KM survival curve for 365-day mortality in Figure 2B. By Log-rank test, we found significant differences in the survival curves between different BCR quartiles (P < 0.05).
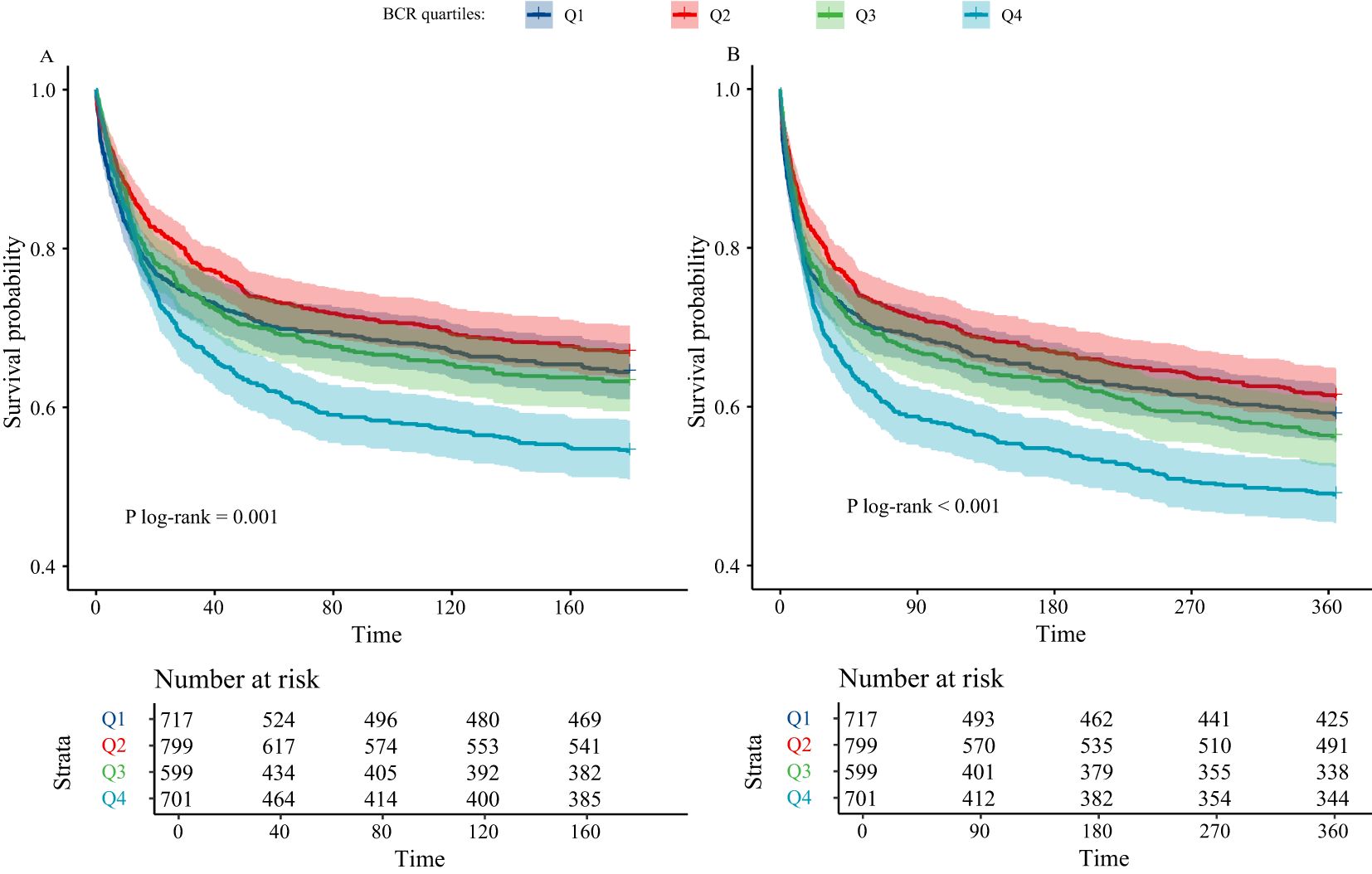
Figure 2. Kaplan-Meier analysis of critically ill cirrhotic patients according to BCR quartiles (A, 180-day mortality; B, 365-day mortality; BCR, blood urea nitrogen to creatinine ratio; BCR quartiles: Q1 ≤ 14, 14<Q2 ≤ 20, 20<Q3 ≤ 27, and Q4>27).
Our study investigated the association between BCR and all-cause mortality in critically ill cirrhosis patients using a multivariate Cox regression model (Table 2). The results showed that the BCR ratio was significantly associated with both 180-day and 365-day mortality. In Model 1, without corrections, For every 1-unit rise in BCR, there was a corresponding 1% increase in the risk of death at both 180 and 365 days (P<0.001). Even after adjusting for additional confounders in Model 3, the association between BCR and mortality persisted significantly (P<0.001).

Table 2. The association between BCR and all-cause mortality in critically ill patients with cirrhosis.
For BCR quartiles, the highest quartile (Q4) showed a significant increase in both 180-day and 365-day mortality compared with the lowest quartile (Q1). Model 3 demonstrated a heightened risk of mortality, with a 45% increase for 180-day mortality (HR=1.45, 95% CI: 1.21-1.73) and a 38% increase for 365-day mortality (HR=1.38, 95% CI: 1.17-1.63). In addition, the association between different BCR quartiles and mortality was also differential (P for trend <0.001).
Figure 3 presents an RCS analysis examining the association between BCR and mortality risk at 180 days (Figure 3A) and 365 days (Figure 3B) in critically ill cirrhosis patients. The RCS curves indicate a significant linear correlation between BCR and 180-day mortality risk (nonlinear P = 0.364), with increased BCR values associated with a higher risk of death. The 180-day risk of death in cirrhotic patients increased with higher BCR values. Similarly, a similar linear association was found in the 365-day mortality study (nonlinear P = 0.423).
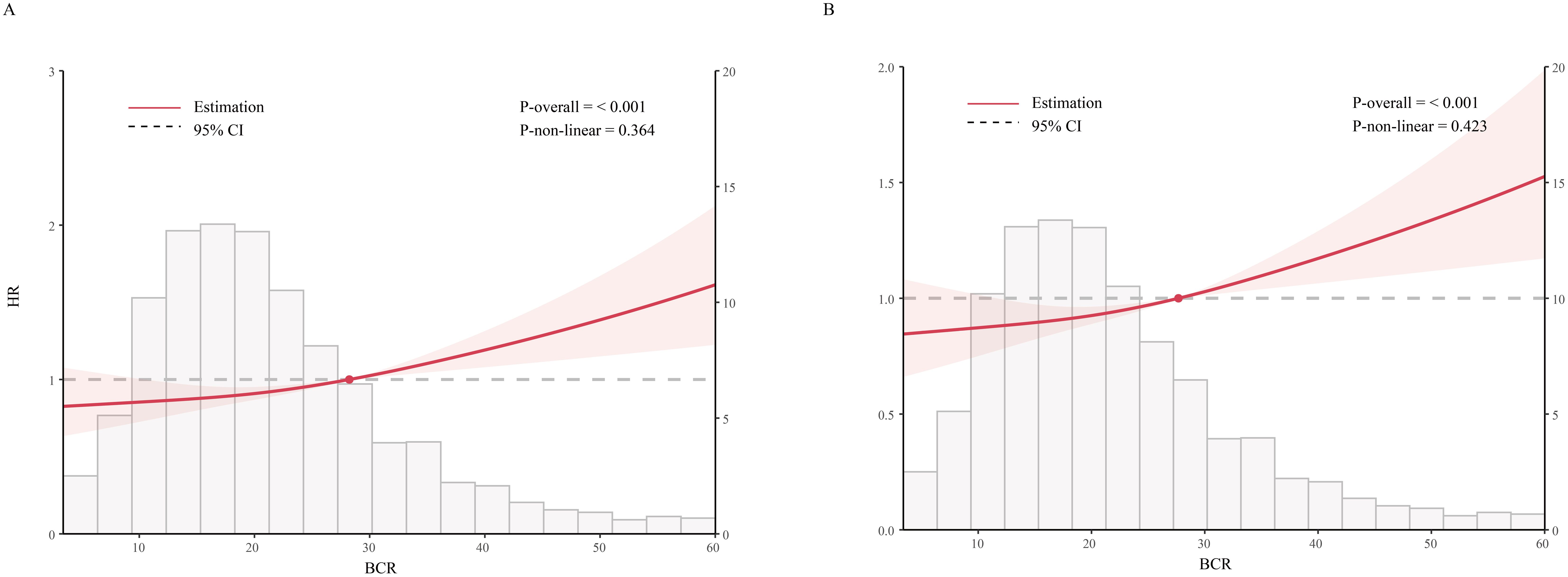
Figure 3. Restricted cubic spline analysis of mortality risk with BCR in critically ill patients with cirrhosis (A, 180-day mortality; B, 365-day mortality; BCR, blood urea nitrogen to creatinine ratio).
We employed a forest plot to illustrate the relationship between BCR and mortality at 180 and 365 days in critically ill cirrhosis patients with varying clinical characteristics (Figure 4). Specifically, positive associations between BCR and all-cause mortality were shown in patients of different ages, gender, AKI, hypertension, DM, heart failure, and myocardial infarction. The interaction test results indicated a notable variance in the relationship between BCR and mortality among critically ill cirrhosis patients across different age subgroups. The positive correlations between BCR and mortality at 180 and 365 days were more pronounced in patients over 60 compared to those under 60. In other subgroups, no significant interactions were found.
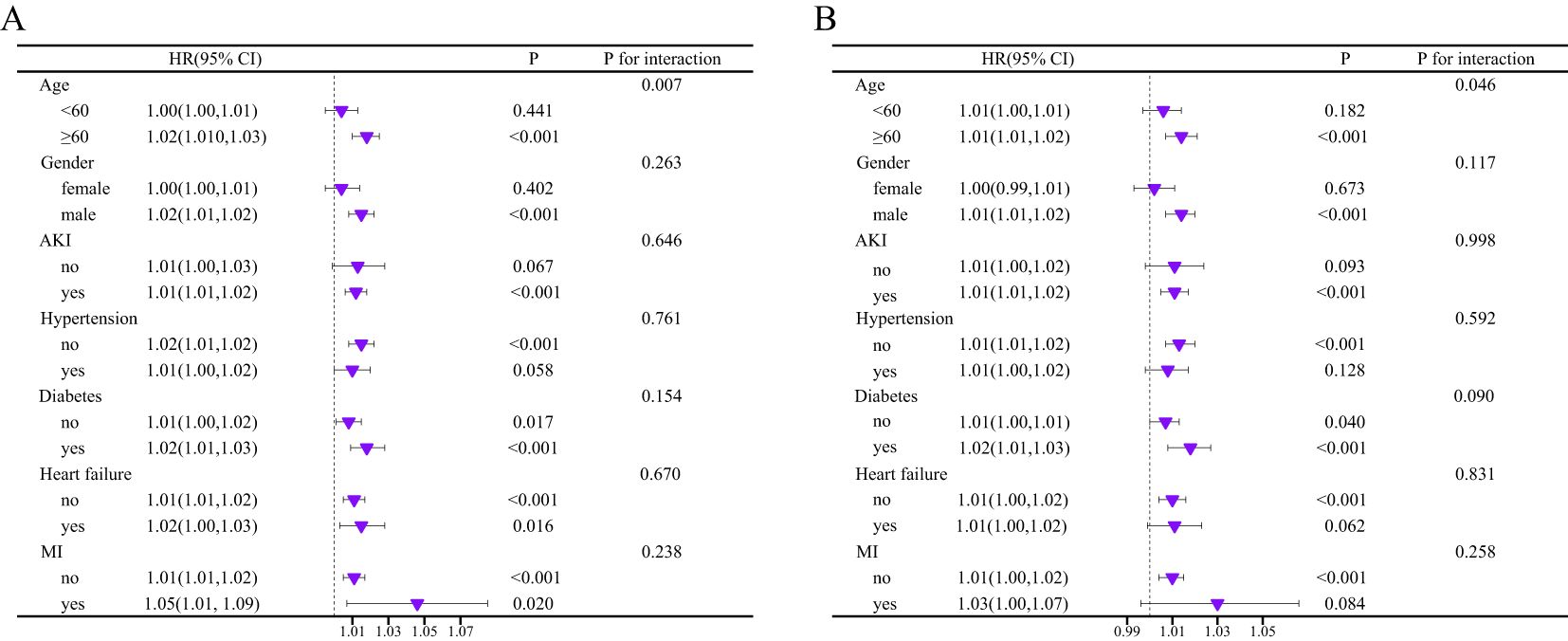
Figure 4. Subgroup analysis of the association between BCR and mortality risk of critically ill cirrhosis (A, 180-day mortality; B, 365-day mortality).
This study explored the association between BCR and mortality in critically ill cirrhosis patients. Our analysis of MIMIC-IV data indicates a strong link between high BCR and higher mortality risk in patients. KM survival analysis and multivariate Cox regression models consistently showed that elevated BCR quartiles significantly increased mortality at both 180 and 365 days. RCS analysis demonstrated a significant linear correlation between increased BCR values and elevated mortality risk. Subgroup analyses and interaction effects revealed a stronger association between BCR and mortality in older patients.
BCR, as a simple biochemical marker, has gained increasing recognition for its prognostic significance in various clinical settings. Previous research has demonstrated a significant association with negative clinical outcomes, such as acute kidney injury, heart failure, cerebral infarction, and ischemic stroke (29–33). A prospective cohort study of over 50,000 participants, with an average follow-up of 7.9 years, found that elevated BCR levels were linked to a higher stroke risk (19). Additionally, BCR is linked to a heightened risk of insulin resistance (34). The uniform association between BCR and mortality across various studies underscores the significance of this marker in forecasting patient outcomes. In patients with chronic heart failure, Paolo et al. found that a higher BCR was associated with adverse outcomes in chronic heart failure patients, independent of estimated glomerular filtration rate (eGFR) and N-terminal pro-brain natriuretic peptide (NT-proBNP), and may be associated with pathophysiological mechanisms such as neurohormonal activation and changes in renal blood flow (18). Our study supports the role of BCR as a prognostic indicator in cirrhosis patients, aligning with previous findings.
The relationship between BCR and mortality in cirrhotic patients involves complex mechanisms. BCR reflects the kidney’s ability to excrete urea and creatinine, and elevated levels may indicate impaired renal function, a known predictor of poor outcomes in cirrhosis (17, 35). In addition, extreme impairment of renal function in patients with cirrhosis leads to hepatorenal syndrome, which is characterized by a decrease in renal blood flow and glomerular filtration rate, which also leads to an elevated BCR, which likewise increases the poor prognosis of patients with cirrhosis (36).
The subgroup analysis identified a notable interaction between age and BCR as predictors of mortality. Older patients with higher BCR levels exhibited a more pronounced increase in mortality risk. This interaction might result from age-related renal function decline, which is associated with higher BCR values. Elevated BCR may indicate a poorer prognosis in older patients with cirrhosis (37). Additionally, older patients often have a higher burden of comorbidities that, in conjunction with elevated BCR, contribute to a higher risk of mortality (38). This finding emphasizes the importance of considering age as a modifier when evaluating the prognostic value of BCR in cirrhotic patients.
Our study highlights the potential of blood urea nitrogen to creatinine ratio (BCR) as a prognostic marker for patients with cirrhosis. The BCR has several advantages over existing prognostic scores such as the Child-Pugh score and the MELD score. It is a direct biochemical indicator of both renal and hepatic function. In addition, the BCR avoids the subjectivity of certain markers (e.g., ascites and encephalopathy) in the Child-Pugh score and the laboratory heterogeneity of creatinine and INR measurements in the MELD score, as well as gender bias (39–41). Our findings suggest that the BCR can be a valuable adjunct to existing prognostic tools, especially in critically ill patients where an accurate prognosis is essential for clinical decision-making.
While our study provides valuable insights into predicting mortality in critically ill cirrhotic patients, it has limitations. Firstly, our analysis is retrospective, which limits the ability to establish causality and may introduce biases inherent in observational studies. Secondly, the single-center origin of our patient cohort may restrict the applicability of our findings to diverse populations and healthcare environments. Thirdly, despite adjusting for many confounders, some unincluded variables such as laboratory tests, genetic factors, lifestyle, and specific treatments may still affect the relationship between BCR and mortality, and thus the scope of attention in the interpretation of the conclusions. Fourth, due to missing data from retrospective studies, we were unable to obtain all the metrics for either the MELD score or the ACLF score, so they were not included in the analyses, and we will take these two scores into account in future prospective studies. Our study adds to the increasing evidence highlighting the significance of BCR in managing critically ill cirrhotic patients, despite certain limitations.
In cirrhotic patients admitted to the ICU, a higher BCR is associated with increased short- and long-term mortality. Therefore, the measurement of BCR may be helpful in the prognostic management of cirrhotic patients in the ICU. Additional prospective studies are required to confirm our results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YY: Writing – original draft. LL: Writing – original draft. YC: Writing – original draft. YL: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are grateful to all of the participants for their valuable contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1544223/full#supplementary-material
1. Gines P, Krag A, Abraldes JG, Sola E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
2. Fang K, Yang Q, Lin Y, Zheng L, Wang HL, Wu J. Global cirrhosis prevalence trends and attributable risk factors-an ecological study using data from 1990-2019. Liver Int. (2022) 42:2791–9. doi: 10.1111/liv.v42.12
3. Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
4. Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. (2014) 12:145. doi: 10.1186/s12916-014-0145-y
5. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. (2020) 18:2650–66. doi: 10.1016/j.cgh.2019.07.060
6. Orntoft NW, Sandahl TD, Jepsen P, Vilstrup H. Short-term and long-term causes of death in patients with alcoholic hepatitis in Denmark. Clin Gastroenterol Hepatol. (2014) 12:1739–44.e1. doi: 10.1016/j.cgh.2014.04.020
7. Cho J, Choi SM, Yu SJ, Park YS, Lee CH, Lee SM, et al. Bleeding complications in critically ill patients with liver cirrhosis. Korean J Intern Med. (2016) 31:288–95. doi: 10.3904/kjim.2014.152
8. Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. (2011) 9:897–901. doi: 10.1016/j.cgh.2011.07.007
9. Peng Y, Qi X, Guo X. Child-Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: A systematic review and meta-analysis of observational studies. Med (Baltimore). (2016) 95:e2877. doi: 10.1097/MD.0000000000002877
10. Neuberger J, Gimson A, Davies M, Akyol M, O’Grady J, Burroughs A, et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. (2008) 57:252–7. doi: 10.1136/gut.2007.131730
11. Kartoun U, Corey KE, Simon TG, Zheng H, Aggarwal R, Ng K, et al. The MELD-Plus: A generalizable prediction risk score in cirrhosis. PLoS One. (2017) 12:e0186301. doi: 10.1371/journal.pone.0186301
12. Montagnese S, De Rui M, Schiff S, Ceranto E, Valenti P, Angeli P, et al. Prognostic benefit of the addition of a quantitative index of hepatic encephalopathy to the MELD score: the MELD-EEG. Liver Int. (2015) 35:58–64. doi: 10.1111/liv.2015.35.issue-1
13. Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. (2014) 61:1038–47. doi: 10.1016/j.jhep.2014.06.012
14. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. (2013) 144:1426–37, 1437.e1-9. doi: 10.1053/j.gastro.2013.02.042
15. Bhogal AS, De Rui M, Pavanello D, El-Azizi I, Rowshan S, Amodio P, et al. Which heart rate variability index is an independent predictor of mortality in cirrhosis? Dig Liver Dis. (2019) 51:695–702. doi: 10.1016/j.dld.2018.09.011
16. Han D, Zhang L, Zheng S, Xu F, Li C, Yang R, et al. Prognostic value of blood urea nitrogen/creatinine ratio for septic shock: an analysis of the MIMIC-III clinical database. BioMed Res Int. (2021) 2021:5595042. doi: 10.1155/2021/5595042
17. Brookes EM, Power DA. Elevated serum urea-to-creatinine ratio is associated with adverse inpatient clinical outcomes in non-end stage chronic kidney disease. Sci Rep. (2022) 12:20827. doi: 10.1038/s41598-022-25254-7
18. Tolomeo P, Butt JH, Kondo T, Campo G, Desai AS, Jhund PS, et al. Independent prognostic importance of blood urea nitrogen to creatinine ratio in heart failure. Eur J Heart Fail. (2024) 26:245–56. doi: 10.1002/ejhf.v26.2
19. Peng R, Liu K, Li W, Yuan Y, Niu R, Zhou L, et al. Blood urea nitrogen, blood urea nitrogen to creatinine ratio and incident stroke: The Dongfeng-Tongji cohort. Atherosclerosis. (2021) 333:1–8. doi: 10.1016/j.atherosclerosis.2021.08.011
20. Lin B, Xiao W, Huang P, Lin X, Lin Y, Lin J, et al. Association between serum magnesium concentrations and the risk of developing acute kidney injury in patients with cirrhosis: a retrospective cohort study based on the MIMIC-IV database. Ren Fail. (2024) 46:2368088. doi: 10.1080/0886022X.2024.2368088
21. Urrunaga NH, Mindikoglu AL, Rockey DC. Renal dysfunction in cirrhosis. Curr Opin Gastroenterol. (2015) 31:215–23. doi: 10.1097/MOG.0000000000000168
22. Chen YW, Wu CJ, Chang CW, Lee SY, Sun FJ, Chen HH. Renal function in patients with liver cirrhosis. Nephron Clin Pract. (2011) 118:c195–203. doi: 10.1159/000321384
23. Abendaño-Rivera DF, Sánchez-Sánchez CY, Cazarin-Chávez K, Diego-Salazar PM, Santana-Vargas D, Tijera MFH-DL, et al. BUN/creatinine ratio associated with mortality in patients with cirrhosis and acute kidney injury. Ann Hepatol. (2024) 29:101439. doi: 10.1016/j.aohep.2024.101439
24. Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. (2000) 101:E215–20. doi: 10.1161/01.CIR.101.23.e215
25. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
26. Oyelade T, Forrest E, Moore KP, O’Brien A, Mani AR. Parenclitic network mapping identifies response to targeted albumin therapy in patients hospitalized with decompensated cirrhosis. Clin Transl Gastroenterol. (2023) 14:e00587. doi: 10.14309/ctg.0000000000000587
27. Stekhoven DJ, Buhlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. (2012) 28:112–8. doi: 10.1093/bioinformatics/btr597
28. Liu L, Zhu Z, Yu K, Zhang W, Pu J, Lv Y, et al. Association between stress hyperglycemia ratio and all-cause mortality in critically ill patients with atrial fibrillation: insights from a MIMIC-IV study. Front Endocrinol (Lausanne). (2024) 15:1412159. doi: 10.3389/fendo.2024.1412159
29. Uchino S, Bellomo R, Goldsmith D. The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury. Clin Kidney J. (2012) 5:187–91. doi: 10.1093/ckj/sfs013
30. Wang Y, Xu X, Shi S, Gao X, Li Y, Wu H, et al. Blood urea nitrogen to creatinine ratio and long-term survival in patients with chronic heart failure. Eur J Med Res. (2023) 28:343. doi: 10.1186/s40001-023-01066-x
31. Jiang WF, Deng ML. Prognostic impact of blood urea nitrogen/creatinine ratio changes in patients with acute ischemic stroke. Clin Neurol Neurosurg. (2022) 215:107204. doi: 10.1016/j.clineuro.2022.107204
32. Huang S, Guo N, Duan X, Zhou Q, Zhang Z, Luo L, et al. Association between the blood urea nitrogen to creatinine ratio and in−hospital mortality among patients with acute myocardial infarction: A retrospective cohort study. Exp Ther Med. (2023) 25:36. doi: 10.3892/etm.2022.11735
33. Chen T, Li AP, Gong Q, Zhou L, Zhao YX, Zhou ZW, et al. The association of blood urea nitrogen to creatinine ratio and the prognosis of critically ill patients with cerebral infarction: A cohort study. Mediators Inflammation. (2022) 2022:2151840. doi: 10.1155/2022/2151840
34. Lee J, Hwang IC, Ahn HY. Association between blood urea nitrogen-to-creatinine ratio and insulin sensitivity. Diabetes Metab. (2024) 50:101521. doi: 10.1016/j.diabet.2024.101521
35. Rajakumar A, Appuswamy E, Kaliamoorthy I, Rela M. Renal dysfunction in cirrhosis: critical care management. Indian J Crit Care Med. (2021) 25:207–14. doi: 10.5005/jp-journals-10071-23721
36. Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. (2020) 370:m2687. doi: 10.1136/bmj.m2687
37. Guppy M, Thomas ET, Glasziou P, Clark J, Jones M, O’Hara DV, et al. Rate of decline in kidney function with age: a systematic review. BMJ Open. (2024) 14:e089783. doi: 10.1136/bmjopen-2024-089783
38. Xiang Z, Wang H, Li H. Comorbidity risk and distribution characteristics of chronic diseases in the elderly population in China. BMC Public Health. (2024) 24:360. doi: 10.1186/s12889-024-17855-w
39. Ruf A, Dirchwolf M, Freeman RB. From Child-Pugh to MELD score and beyond: Taking a walk down memory lane. Ann Hepatol. (2022) 27:100535. doi: 10.1016/j.aohep.2021.100535
40. Schneider MD, Sarrazin C. Management of HCV-associated liver cirrhosis. Visc Med. (2016) 32:96–104. doi: 10.1159/000445330
Keywords: cirrhosis, mortality, blood urea nitrogen to creatinine ratio (BCR), outcome prediction, severely ill patients
Citation: Yi Y, Li L, Chen Y and Luo Y (2025) Interaction between age and blood urea nitrogen to creatinine ratio on mortality in patients with severe cirrhosis: a retrospective cohort study from the MIMIC database. Front. Endocrinol. 16:1544223. doi: 10.3389/fendo.2025.1544223
Received: 12 December 2024; Accepted: 14 February 2025;
Published: 05 March 2025.
Edited by:
Alireza Mani, University College London, United KingdomReviewed by:
Yen Yi Tan, University College London, United KingdomCopyright © 2025 Yi, Li, Chen and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yawen Luo, MTc3MTgwNjQyMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.