
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 01 April 2025
Sec. Thyroid Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1539442
Background: Thyroid nodules, often discovered incidentally, typically require long-term monitoring and may contribute to psychological distress. Despite their prevalence, the psychological impact of thyroid nodules remains underexplored.
Methods: This retrospective cohort study used data from the TrinetX platform (2010–2023), encompassing 118 million patients. Patients diagnosed with thyroid nodules were matched to those with thyroid cancer using propensity score matching for age, sex, race, socioeconomic status and comorbidities. The primary outcome was anxiety disorder risk, with secondary outcomes including depression, mood disorder, and insomnia.
Results: After matching, 138,803 pairs were analyzed, with a mean age of 52 years, 70% female, and 66% White. Comorbidities were well-balanced. Patients with thyroid nodules had a significantly higher risk of developing anxiety disorder compared to those with thyroid cancer (HR 1.06; 95% CI: 1.03–1.08). Conversely, thyroid nodule patients had lower risks of depression (HR 0.93; 95% CI: 0.90–0.96), mood disorders (HR 0.95; 95% CI: 0.92–0.98), and insomnia (HR 0.93; 95% CI: 0.89–0.97). Psychotic disorders showed no significant difference (HR 1.03; 95% CI: 0.90–1.17).
Conclusions: This study identifies a significant association between thyroid nodules and increased anxiety risk, while risks for depression, mood disorders, and insomnia were lower compared to thyroid cancer patients. Furthermore, a sensitivity analysis compared thyroid nodule patients to the general population and revealed elevated anxiety risk in patients with nodules, reinforcing that this increased risk is not solely attributable to cancer-related factors. Further research is warranted to confirm these findings and explore mechanisms underlying the psychological impact of thyroid nodules.
Thyroid nodules are a common endocrine condition, with reported prevalence ranging from approximately 10% to 46% in the general population (1, 2). While many nodules are benign, their detection often necessitates long-term surveillance to monitor for potential malignant transformation. There is growing recognition that the need for ongoing monitoring can impose a significant psychological burden on patients (3). In fact, recent studies have demonstrated that patients with thyroid nodules experience greater anxiety and depression compared to individuals without nodules (4), suggesting that the very presence of a thyroid nodule can adversely impact mental health. Patients may face anxiety about the possibility of malignancy, repeated diagnostic procedures, and uncertainty about their health, all of which can contribute to stress.
Moreover, in the context of small thyroid cancers, studies comparing active surveillance with immediate surgery have yielded insights into patient well-being. One long-term study reported that patients who opted for surveillance maintained better overall quality of life and did not show increased anxiety over five years of follow-up compared to those who underwent early surgery (5). However, other research has suggested that living with an untreated thyroid lesion can still provoke considerable worry; for example, patients on active surveillance may experience fear of cancer progression and even decision regret regarding their management choice (6). These findings underscore that the psychological impact of long-term thyroid nodule monitoring is complex and not yet fully understood. In light of the limited and contradictory literature on this topic, further investigation is necessary to clarify the extent and drivers of psychological burden in this patient population.
Accordingly, the present study was undertaken to rigorously evaluate the mental health outcomes of patients undergoing prolonged thyroid nodule surveillance. We specifically sought to address two key research questions: (1) Does prolonged observation of thyroid nodules lead to significant anxiety or other forms of psychological distress in patients? and (2) What patient or nodule-related factors are associated with any such psychological distress during long-term monitoring? We hypothesized that patients under long-term surveillance would exhibit elevated anxiety levels, and that certain characteristics would correlate with greater psychological distress.
This retrospective cohort study utilized the TriNetX database, which comprises data from approximately 118 million patients, spanning from January 1, 2010, to December 31, 2023. TriNetX is a federated, cloud-based network that provides access to electronic medical records from a wide range of healthcare entities, including hospitals, primary care, and specialty treatment providers. The database includes diverse geographic locations, age groups, disease diagnoses, and medications. It aggregates patient data and offers statistical analysis results through a browser-based interface. With a waiver from the Western Institutional Review Board, TriNetX ensures that only aggregated counts and statistical summaries of de-identified data are used, avoiding access to protected health information and primary data collection in retrospective analyses. Additionally, this study received an exemption waiver from the Institutional Review Board (IRB) of Chung Shan Medical University Hospital, identified by the reference number CS2-20121.
To ensure comprehensive follow-up, we required at least two follow-up entries in the database. Eligible cases were those with a recorded diagnosis of thyroid nodules, with the cohort entry (index date) defined as the date of the first recorded diagnosis. Controls were identified as patients with a first-time diagnosis of thyroid cancer, with the index date similarly defined as the first available diagnosis date. Patients with a prior diagnosis of hyperthyroidism or hypothyroidism were excluded from the case. If a patient had both a thyroid nodule and thyroid cancer, they were classified under the control group (thyroid cancer group).
For the control group, patients with other thyroid diseases were not excluded. The severity of thyroid cancer is expected to be the primary driver of psychological outcomes, with the psychological impact of the cancer diagnosis likely dominating that of any coexisting thyroid disorder. Additionally, it is common for patients with thyroid cancer, particularly those undergoing thyroidectomy, to experience subsequent hypo- or hyperthyroidism due to necessary levothyroxine adjustments. This post-thyroidectomy adjustment period often results in temporary or fluctuating thyroid function levels as clinicians optimize dosing. Excluding these cases would reduce the generalizability of our findings, as post-surgical thyroid dysfunction is a frequent aspect of the thyroid cancer care pathway. Including these patients ensures a more representative sample, reflecting the complex, real-world presentation of thyroid cancer patients.
The rationale for excluding patients with hyperthyroidism and hypothyroidism from the thyroid nodule group is based on the well-documented association of these conditions with a higher risk of depression and anxiety (7–12). This approach ensures that psychiatric outcomes are more specifically attributable to the presence of thyroid nodules rather than confounded by other thyroid dysfunctions that independently affect mental health.
We selected thyroid cancer as the comparison group, given that it is the malignant counterpart of benign thyroid nodules and is typically diagnosed with a high degree of accuracy. From a clinical perspective, the risk of psychiatric outcomes associated with benign thyroid nodules is unlikely to match that of thyroid cancer, providing a clear and meaningful distinction for comparative analysis.
Furthermore, to ensure that observed psychiatric outcomes are attributable to exposure in the case and control groups, we excluded patients with any prior psychiatric diagnosis before the index date. This step allows for a more precise evaluation of new psychiatric diagnoses following the identification of thyroid nodules or thyroid cancer.
The primary outcome of this study was the first occurrence of an anxiety disorder, measured starting 90 days after the index date for both the thyroid nodule and thyroid cancer groups, and assessed using hazard ratios (HR). We used the 90-day cutoff to mitigate potential misclassification bias or acute psychological impact immediately following a cancer diagnosis.
Anxiety was chosen as the primary outcome due to its strong alignment with the uncertainty and prolonged surveillance associated with benign thyroid nodules. This ‘wait and watch’ approach often leads to heightened alertness, restlessness, and worry, as patients anticipate potential progression or malignancy despite a benign diagnosis. In addition to anxiety, the study also evaluated the risks of depression, mood disorder and insomnia as secondary outcomes. We selected psychotic disorders as a negative control based on their relatively distinct etiology and lower susceptibility to fluctuations in thyroid-related concerns, thereby helping assess whether the associations observed for anxiety and other disorders were specific to those conditions.
We identified diagnoses of thyroid nodules based on the presence of International Classification of Diseases, 10th Revision (ICD-10) codes E04.1 and E04.2, recorded on or after January 1, 2010. Thyroid cancer diagnoses were identified using ICD-10 code C73. Anxiety disorders were defined using ICD-10 codes F40–F48, while major depressive disorder was defined using ICD-10 code F32, mood disorders using ICD-10 codes F30–F39, and insomnia using ICD-10 code G47.
In addition to these diagnoses, several baseline characteristics and comorbidities were considered, including age, sex, race, socioeconomic status, diabetes, ischemic heart disease, and other chronic pain conditions. Further details on the specific criteria and classification of these comorbidities are provided in Supplementary Table S1.
To address potential data heterogeneity and ensure diagnostic accuracy, we utilized the TriNetX platform, which harmonizes data from diverse healthcare organizations by mapping to standardized terminologies such as ICD-10, Current Procedural Terminology (CPT), and Logical Observation Identifier Name and Codes (LOINC). This process facilitates consistency across the network. Diagnostic codes within TriNetX are derived from electronic health records and are assigned by licensed healthcare providers during patient encounters, reflecting professional clinical judgment. To mitigate misclassification bias, we included only patients with at least two separate diagnostic entries for each condition, thereby reducing the likelihood of errors due to transient or incorrect diagnoses. While these measures enhance data reliability, we acknowledge that inherent limitations in coding practices and data entry may still introduce some degree of bias.
We performed 1:1 propensity score matching (PSM) using the TriNetX platform’s default greedy nearest-neighbor method, without exact matching on specific covariates (13). Baseline characteristics were considered well matched if the standardized mean difference (SMD) was below 0.1 (14). For the analysis of anxiety disorder, PSM was applied to baseline characteristics such as age, sex, race, socio-economic status and comorbidities to ensure comparable cohort pairs. Cohort comparisons were conducted using the log-rank test, and the HR was calculated using a proportional hazards model. The null hypothesis was tested using a chi-square test to assess whether the cohorts were statistically equivalent. All statistical analyses were performed within the TriNetX platform, and statistical significance was defined as a two-sided p-value < 0.05.
Additionally, a subgroup analysis was conducted to assess the risk of anxiety disorders across different follow-up periods, including 1, 2, 3, 5, and 10 years. We also evaluate potential effect modification on the risk of anxiety associated with thyroid nodules, stratifying by age (<30, 30–60, and >60 years), sex (female vs. male), and race (White person, Black person, or Asian). Additionally, we assessed whether the presence of specific comorbidities was associated with an increased risk of anxiety.
Furthermore, we performed a sensitivity analysis comparing thyroid nodule patients with the general population to gain additional insight into the broader psychiatric impact of thyroid nodules. The flow diagram for this sensitivity analysis is presented in Supplementary Figure S1, with baseline characteristics in Supplementary Table S2 and risk estimates in Supplementary Table S3.
The complete flow diagram is shown in Figure 1. This study included a comprehensive cohort of 118 million patients. A total of 935,351 thyroid nodule and 177,349 thyroid cancer cases were initially identified. We excluded patients with hyperthyroidism and hypothyroidism, resulting in 556,157 thyroid nodule cases. Further exclusions were made for patients with a prior history of any psychiatric disease before the index date, leaving 406,539 thyroid nodule cases and 138,933 thyroid cancer controls. Finally, we applied PSM based on age, sex, race, socio-economic status and comorbidities, yielding 138,803 matched pairs for the final analysis.
Table 1 presents the baseline demographic data for this study. Before PSM, baseline characteristics differed markedly between the thyroid nodule and thyroid cancer groups. The average age was 56.5 years for thyroid nodule patients and 51.9 years for thyroid cancer patients. Additionally, White constituted 59.1% of the thyroid nodule group and 66.5% of the thyroid cancer group, with various comorbidities also differing significantly between groups. After PSM, these differences were well-balanced, confirmed by a standardized mean difference (SMD) of less than 0.1. The matched cohort had a mean age of 52 years, 70% female representation, and 66% White. Socioeconomic status and comorbidities were also balanced, with rates of overweight and obesity at 4%, type 2 diabetes at 5%, heart disease at 4.5%, and neoplasms at 14.3%.
Table 2 presents the primary outcomes of this study. Patients with thyroid nodules demonstrated a consistently higher risk of developing anxiety compared to those with thyroid cancer, with an overall HR of 1.06 (95% CI: 1.03–1.08). Conversely, patients with thyroid nodules had a lower risk of depression (HR 0.93; 95% CI: 0.90–0.96), mood disorders (HR 0.95; 95% CI: 0.92–0.98), and insomnia (HR 0.93; 95% CI: 0.89–0.97) than those with thyroid cancer, with similar trends persisting across follow-up intervals (Table 3). Psychotic disorders showed no significant difference between groups (HR 1.03; 95% CI: 0.90–1.17).
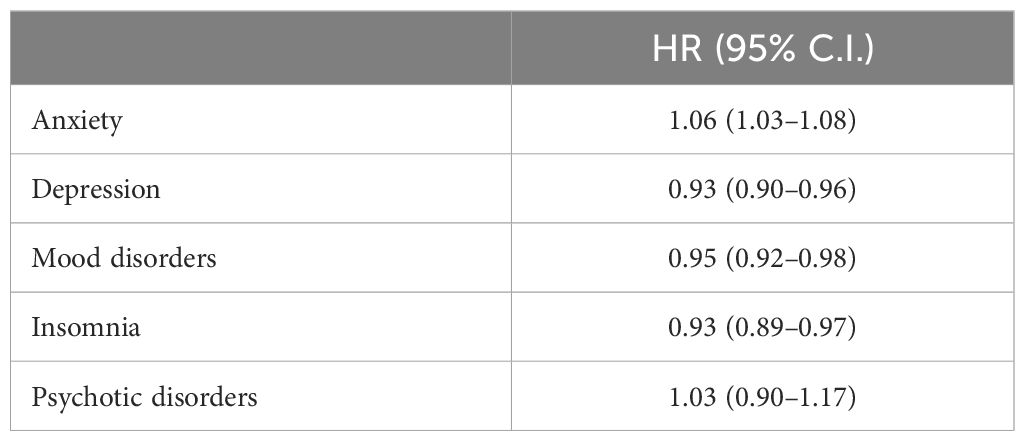
Table 2. Risk of all psychiatric illness exposed to thyroid nodules compared with thyroid cancer (n = 138,308 matched pairs).
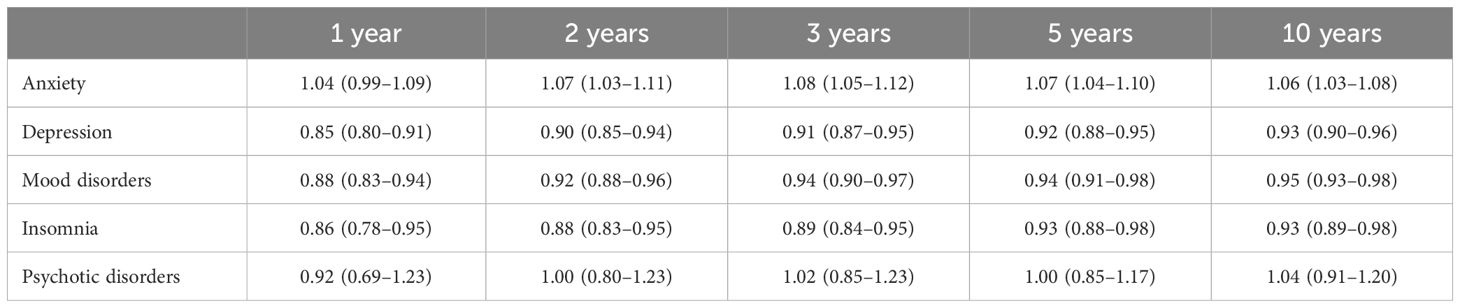
Table 3. Subgroup Analysis of psychiatric disease in patients with thyroid nodules compared to thyroid cancer across different follow-up durations.
Stratified analysis revealed that patients with thyroid nodules had varying psychiatric risks compared to those with thyroid cancer, influenced by age, sex, race, and comorbidities (Table 4). Younger patients (<30 years) with thyroid nodules exhibited notably higher risks of anxiety (HR 1.31; 95% CI: 1.21–1.41) and mood disorders (HR 1.15; 95% CI: 1.04–1.26) than their thyroid cancer counterparts, whereas patients over 60 years showed a reduced risk for anxiety (HR 0.97; 95% CI: 0.93–1.02) and depression (HR 0.89; 95% CI: 0.84–0.94). Females with thyroid nodules demonstrated a higher risk of anxiety (HR 1.07; 95% CI: 1.04–1.10) compared to those with thyroid cancer, while male patients showed no significant differences across psychiatric outcomes. Racial stratification indicated that White patients with thyroid nodules had an increased risk of anxiety (HR 1.09; 95% CI: 1.06–1.12), whereas Black/African American patients exhibited lower risks for depression (HR 0.86; 95% CI: 0.77–0.97) and mood disorders (HR 0.85; 95% CI: 0.76–0.95) relative to the thyroid cancer group.
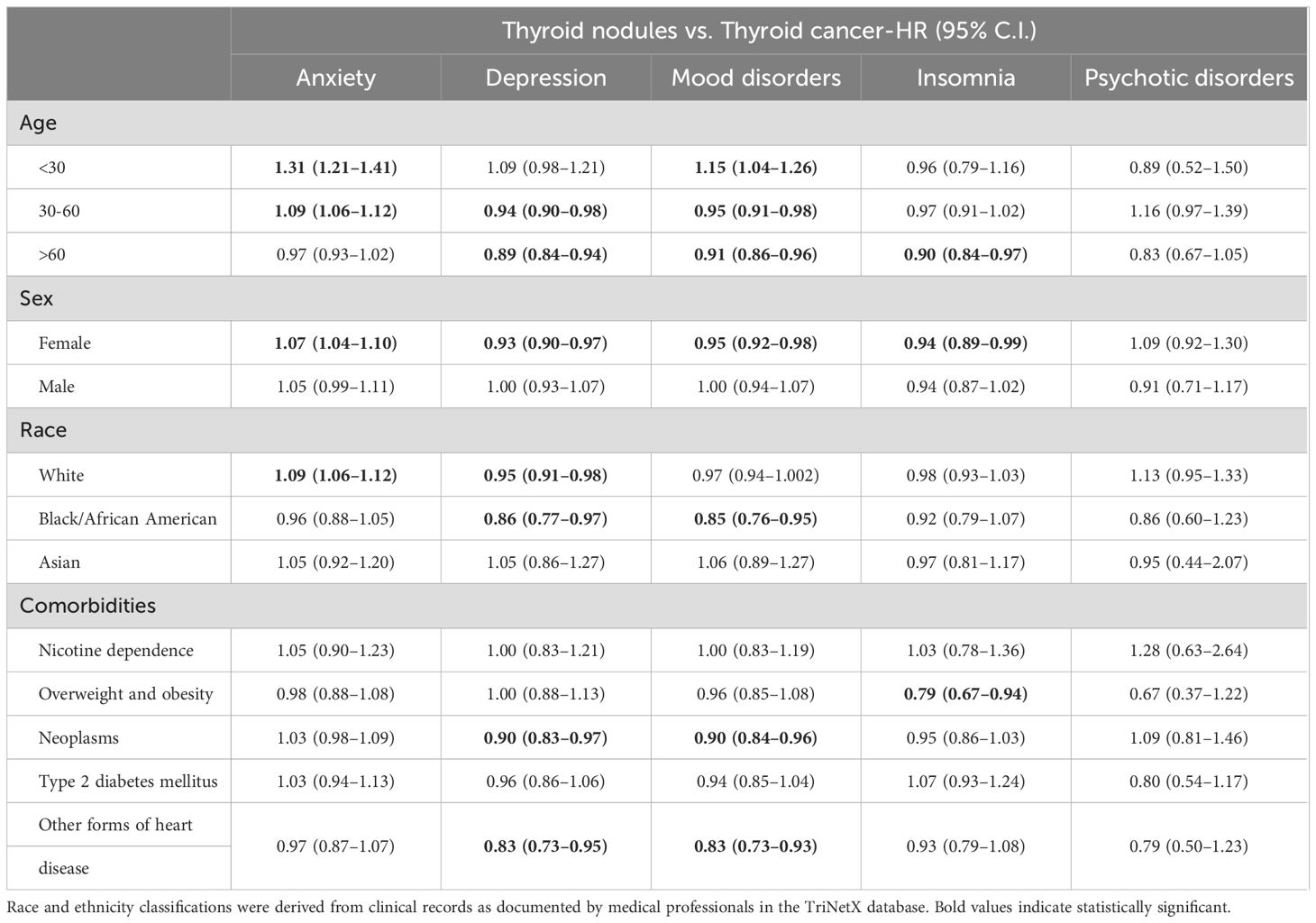
Table 4. Stratification for risk of psychiatric disease exposed to thyroid nodules compared to thyroid cancer.
In the additional sensitivity analysis comparing thyroid nodule patients (n=335,954) to a matched general population without thyroid disease, we observed a significantly higher risk of both anxiety (HR 1.36; 95% CI: 1.34–1.38) and mood disorders (HR 1.16; 95% CI: 1.14–1.18). This finding supports the notion that the increased anxiety risk among thyroid nodule patients exists independently of cancer-specific factors, underscoring the unique psychological burden of nodule surveillance. Detailed data are presented in Supplementary Tables S2, S3.
To the best of our knowledge, this is the largest retrospective study to evaluate the risk of anxiety disorders in patients with thyroid nodules. Despite using thyroid cancer as an extreme control group, our findings reveal that patients with thyroid nodules exhibit a significantly higher risk of developing anxiety disorders compared to those with thyroid cancer, with an overall HR for anxiety of 1.06 (95% CI: 1.03–1.08). This risk remained consistent across various stratifications, including age, sex, and race. Importantly, the study’s reliability is further supported by the lack of an increased risk for psychotic disorders, included as a negative control, which strengthens the validity of our anxiety findings.
Additionally, the year-by-year subgroup analyses confirmed the stability of these results over time, indicating that the association between thyroid nodules and anxiety persists in long-term follow-up. In contrast, patients with thyroid nodules demonstrated a lower risk for other psychiatric outcomes, including depression, mood disorders, and insomnia, relative to thyroid cancer patients. One potential explanation is that a thyroid cancer diagnosis may prompt more frequent healthcare visits and screening for psychiatric comorbidities, thereby increasing the detection of conditions such as depression or insomnia. Alternatively, post-surgical or cancer-related distress could manifest in different psychiatric domains, contributing to higher rates of depression in the thyroid cancer cohort (6).
When compared to the general population in our sensitivity analysis, patients with thyroid nodules had elevated risks of both anxiety (HR 1.36; 95% CI: 1.34–1.38) and mood disorder (HR 1.16; 95% CI: 1.14–1.18). These findings suggest that while thyroid nodules confer a heightened overall risk for psychiatric conditions, the contrasting results observed when comparing nodules to thyroid cancer reflect distinct psychological contexts across different reference groups.
The literature on the relationship between thyroid nodules and psychiatric outcomes is limited, with most studies focusing on thyroid cancer rather than benign thyroid conditions. In a study conducted in China, Li et al. examined distress and sleep disturbance among adults undergoing thyroid nodule screening, diagnosis, and treatment, finding increased psychological distress and sleep issues among those with thyroid nodules (3). Interestingly, Li et al. also explored psychiatric outcomes in thyroid cancer patients, noting a worsening in psychological outcomes. However, our findings contrast with theirs, as we observed that thyroid cancer patients did not exhibit a higher risk of psychiatric illness compared to those with benign thyroid nodules. One potential explanation for this discrepancy may lie in the relatively short follow-up period in the Li et al. study, which might capture only initial psychiatric impacts; in contrast, the extended surveillance often required for thyroid nodules may lead to a sustained, gradual increase in psychiatric risk over time.
Most research on psychological and quality of life impacts in thyroid disorders has centered on thyroid cancer (15–18). For example, studies from Korea assessed quality of life in patients with papillary thyroid microcarcinoma (PTMC), comparing outcomes between active surveillance and surgical intervention (19, 20). Jeon et al. reported that PTMC patients under active surveillance had fewer health-related problems than those who underwent surgery, supporting active surveillance as a viable management option (19). Similarly, Kong et al. found that patients opting for surveillance experienced better psychological and physical health both at baseline and during follow-up, compared to those who underwent immediate surgery (20). While these studies provide valuable insights into managing thyroid cancer, they do not address the unique psychological challenges faced by patients with benign thyroid nodules.
The exact mechanisms underlying the elevated risk of anxiety observed in patients with benign thyroid nodules remain unknown. However, one primary contributing factor may be the inherent uncertainty and prolonged surveillance associated with benign thyroid nodules, often requiring years of regular imaging and follow-up. This ongoing monitoring can lead to anticipatory anxiety, as patients frequently worry about potential nodule progression or malignancy despite the benign diagnosis. In contrast to thyroid cancer, where treatment protocols are typically well-defined, the ‘wait and watch’ approach often recommended for thyroid nodules may impose a sustained psychological burden, heightening patient vigilance and alertness to somatic changes.
Durante et al. demonstrated that among patients with asymptomatic, sonographically or cytologically benign thyroid nodules, most nodules showed no significant size increase over a 5-year follow-up period, and cases of thyroid cancer were rare (21). Current guidelines recommend initial ultrasound follow-up intervals of 6 to 18 months, with less frequent monitoring if the nodule remains stable (22). Additionally, recent updates suggest re-evaluation at 3 to 5 years, with the potential to reclassify or classify nodules as stable, allowing for discontinuation of further follow-up in many cases (23). This structured, extended monitoring can contribute to anticipatory anxiety, emphasizing the need for psychological support alongside routine clinical follow-up.
This study has several notable strengths. It represents the largest retrospective analysis to date on psychiatric risks in patients with benign thyroid nodules, leveraging a large cohort to enhance generalizability. Furthermore, the extended follow-up period, including year-by-year analyses, allowed us to observe trends in psychiatric risk over time, lending greater reliability to our findings. We also utilized a negative control outcome to strengthen causal inference regarding the specificity of the findings.
Nevertheless, the use of EHR data from multiple institutions introduces heterogeneity and potential coding inconsistencies, which may lead to misclassification of both thyroid conditions and psychiatric outcomes. Additionally, although PSM balanced many observable variables, unmeasured confounders (e.g., baseline psychological state, social support systems, and lifestyle factors) could still influence the results.
The findings of this study suggest several important areas for further investigation. First, more rigorous prospective studies or randomized controlled trials are needed to confirm the observed association between thyroid nodules and anxiety, ideally using standardized, validated psychiatric assessment tools. Future research should also investigate thyroid nodule characteristics—such as size, location, duration, or biopsy frequency—to evaluate whether certain subgroups have a heightened psychological risk. Finally, mechanistic studies examining potential hormonal, immunological, or psychosocial pathways are warranted to elucidate how benign thyroid nodules might precipitate or exacerbate psychiatric disorders.
In conclusion, this study demonstrates a significant association between benign thyroid nodules and an elevated risk of anxiety disorder, highlighting the importance of recognizing and addressing psychological distress in these patients. While thyroid nodule patients showed higher anxiety than even those with thyroid cancer, they paradoxically exhibited lower risks of depression, mood disorders, and insomnia relative to the cancer group. Compared to the general population, however, patients with thyroid nodules also displayed increased risks for both anxiety and mood disorders, underscoring the independent psychological burden posed by benign nodule surveillance. These findings underscore the need for clinicians to provide comprehensive education, reassurance regarding the typically benign nature of nodules, and, where appropriate, referrals to mental health professionals or the use of stress management techniques. Further prospective validation is essential to refine these recommendations and tailor interventions effectively.
This population-based study obtained data from the TrinetX platform (accessible at https://trinetx.com/), for which third-party restrictions apply to the availability of this data. The data were used under license for this study with restrictions that do not allow for data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs might be incurred, and a data-sharing agreement would be necessary.
The studies involving humans were approved by Institutional Review Board of Chung Shan Medical University Hospital, identified by the reference number CS2-20121. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
EK: Data curation, Resources, Supervision, Writing – original draft. S-CL: Conceptualization, Investigation, Methodology, Writing – review & editing. C-NH: Investigation, Resources, Supervision, Writing – review & editing. Y-HW: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – review & editing. Y-SY: Formal Analysis, Methodology, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Chung Shan Medical University Hospital (CSH-2022-A-022).
Special thanks for Jing-Yang Huang and all study team for their dedication and support in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1539442/full#supplementary-material
Supplementary Figure 1 | Sensitivity Analysis Comparing Patients with Thyroid Nodules to the General Population.
1. Zhao W, Han C, Shi X, Xiong C, Sun J, Shan Z, et al. Prevalence of Goiter and Thyroid Nodules before and after Implementation of the Universal Salt Iodization Program in Mainland China from 1985 to 2014: A Systematic Review and Meta-Analysis. PloS One. (2014) 9:e109549. doi: 10.1371/journal.pone.0109549
2. Li Y, Jin C, Li J, Tong M, Wang M, Huang J, et al. Prevalence of thyroid nodules in China: A health examination cohort-based study. Front Endocrinol. (2021) 12:676144. doi: 10.3389/fendo.2021.676144
3. Li R, Li G, Wang Y, Bao T, Lei Y, Tian L, et al. Psychological distress and sleep disturbance throughout thyroid nodule screening, diagnosis, and treatment. J Clin Endocrinol Metab. (2021) 106:e4221–30. doi: 10.1210/clinem/dgab224
4. Wang M, Wang S, Yuan G, Gao M, Wang J, Chu Z, et al. Analysis of risk factors for negative emotions in patients with thyroid nodules: A cross-sectional study. Med (Baltimore). (2024) 103:e40548. doi: 10.1097/MD.0000000000040548
5. Kazusaka H, Sugitani I, Toda K, Sen M, Saito M, Nagaoka R, et al. Patient-reported outcomes in patients with low-risk papillary thyroid carcinoma: cross-sectional study to compare active surveillance and immediate surgery. World J Surg. (2023) 47:1190–8. doi: 10.1007/s00268-022-06786-5
6. Sawka AM, Ghai S, Rotstein L, Irish JC, Pasternak JD, Monteiro E, et al. Decision regret following the choice of surgery or active surveillance for small, low-risk papillary thyroid cancer: A prospective cohort study. Thyroid. (2024) 34:626–34. doi: 10.1089/thy.2023.0634
7. Bode H, Ivens B, Bschor T, Schwarzer G, Henssler J, Baethge C. Association of hypothyroidism and clinical depression: A systematic review and meta-analysis. JAMA Psychiatry. (2021) 78:1375. doi: 10.1001/jamapsychiatry.2021.2506
8. Siegmann EM, Müller HHO, Luecke C, Philipsen A, Kornhuber J, Grömer TW. Association of depression and anxiety disorders with autoimmune thyroiditis: A systematic review and meta-analysis. JAMA Psychiatry. (2018) 75:577. doi: 10.1001/jamapsychiatry.2018.0190
9. Ittermann T, Völzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Soc Psychiatry Psychiatr Epidemiol. (2015) 50:1417–25. doi: 10.1007/s00127-015-1043-0
10. Vogel A, Elberling TV, Hørding M, Dock J, Rasmussen AK, Feldt-Rasmussen U, et al. Affective symptoms and cognitive functions in the acute phase of Graves’ thyrotoxicosis. Psychoneuroendocrinology. (2007) 32:36–43. doi: 10.1016/j.psyneuen.2006.09.012
11. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry. (1986) 8:23–8. doi: 10.1016/0163-8343(86)90060-5
12. Engum A, Bjøro T, Mykletun A, Dahl AA. An association between depression, anxiety and thyroid function – a clinical fact or an artefact? Acta Psychiatr Scand. (2002) 106:27–34. doi: 10.1034/j.1600-0447.2002.01250.x
13. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
15. James BC, Aschebrook-Kilfoy B, White MG, Applewhite MK, Kaplan SP, Angelos P, et al. Quality of life in thyroid cancer—assessment of physician perceptions. J Surg Res. (2018) 226:94–9. doi: 10.1016/j.jss.2017.11.069
16. Goldfarb M, Casillas J. Thyroid cancer–specific quality of life and health-related quality of life in young adult thyroid cancer survivors. Thyroid. (2016) 26:923–32. doi: 10.1089/thy.2015.0589
17. Grogan RH, Aschebrook-Kilfoy B, Angelos P. Interventions to improve thyroid cancer survivors’ quality of life. Future Oncol. (2016) 12:1309–11. doi: 10.2217/fon-2016-0052
18. Aschebrook-Kilfoy B, James B, Nagar S, Kaplan S, Seng V, Ahsan H, Angelos P, et al. Risk factors for decreased quality of life in thyroid cancer survivors: initial findings from the north american thyroid cancer survivorship study. Thyroid. (2015) 25:1313–21. doi: 10.1089/thy.2015.0098
19. Jeon MJ, Lee YM, Sung TY, Han M, Shin YW, Kim WG, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: A cross-sectional study. Thyroid. (2019) 29:956–62. doi: 10.1089/thy.2018.0711
20. Kong SH, Ryu J, Kim MJ, Cho SW, Song YS, Yi KH, et al. Longitudinal assessment of quality of life according to treatment options in low-risk papillary thyroid microcarcinoma patients: active surveillance or immediate surgery (Interim analysis of MAeSTro). Thyroid. (2019) 29:1089–96. doi: 10.1089/thy.2018.0624
21. Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, et al. The natural history of benign thyroid nodules. JAMA. (2015) 313:926. doi: 10.1001/jama.2015.0956
22. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
Keywords: thyroid nodule, thyroid cancer, anxiety, depression, mood disorder
Citation: Kornelius E, Lo S-C, Huang C-N, Wang Y-H and Yang Y-S (2025) Anxiety disorders in patients with thyroid nodules vs. thyroid cancer: a retrospective cohort study. Front. Endocrinol. 16:1539442. doi: 10.3389/fendo.2025.1539442
Received: 04 December 2024; Accepted: 13 March 2025;
Published: 01 April 2025.
Edited by:
Xinyu Fang, Nanjing Brain Hospital Affiliated to Nanjing Medical University, ChinaReviewed by:
Dandan Wang, Shanghai Jiao Tong University, ChinaCopyright © 2025 Kornelius, Lo, Huang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Sun Yang, bW9uaWNhMTE5QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.