
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 06 March 2025
Sec. Neuroendocrine Science
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1536247
 Iulia Petria1,2
Iulia Petria1,2 Rita Indirli1,2*
Rita Indirli1,2* Beatrice Mantovani1
Beatrice Mantovani1 Valeria Lanzi1,2
Valeria Lanzi1,2 Giovanna Mantovani1,2
Giovanna Mantovani1,2 Emanuele Ferrante1
Emanuele Ferrante1Background: Transient hyponatremia due to syndrome of inappropriate antidiuresis (SIAD) is a frequent (20-50%) complication of traumatic brain injury (TBI), but it rarely persists or recurs. There are only few published reports of patients suffering from non-transient hyponatremia due to chronic SIAD after TBI. We report two more cases with this condition.
Case 1: A 36-year-old woman suffering from major depression and treated with olanzapine reported severe TBI after a severe fall. Following head injury, she developed severe hyponatremia, which was managed with fluid restriction and salt supplementation. Upon hospital discharge, 7 months after trauma, mild hyponatremia was still reported (Na 134 mmol/L), which dropped to severe hyponatremia in a week despite continuation of treatment, and spontaneously returned to normal. Two months later, the patient presented one more episode of moderate hyponatremia without clear triggering events. Pituitary hormones were normal and urinary sodium and urinary and plasma osmolality supported the diagnosis of SIAD. Therefore, tolvaptan 7.5 mg daily was started, with sustained normalization of sodium levels. When olanzapine was stopped, discontinuation of tolvaptan was attempted. However, serum sodium dropped again and tolvaptan had to be resumed, with natremia remaining within normal range at follow-up. Consistently, olanzapine-related hyponatremia could be ruled out and post-traumatic SIAD confirmed.
Case 2: A 37-year-old man experienced TBI with diffuse axonal injury falling during a mountain trip. Over the following year, he presented two episodes of tonic-clonic seizures accompanied by the biochemical finding of moderate-severe hyponatremia. Hyponatremia resolved following hypertonic (3% NaCl) saline infusion, and valproate treatment was started after the second episode. In the following outpatient visits, a progressive decrease of serum sodium from 141 mmol/L to 132 mmol/L was observed, with other tests consistent with SIAD. Therefore, considering the high risk of recurrent seizures as well as the concomitant treatment with valproate, tolvaptan 7.5 mg every other day was started and normal sodium levels have been maintained since then.
Conclusions: We report two cases of recurrent SIAD following TBI, with multiple hyponatremic episodes after initial presentation. This highlights the importance of long-term follow-up of electrolyte abnormalities after head injury.
Neuroendocrine disturbances secondary to traumatic brain injury (TBI) have been reported since the early 1900s (1). Various degrees of anterior hypopituitarism are described (2), as well as posterior pituitary dysfunctions (3). Given that TBI is a growing public health issue (4), potentially associated endocrine dysfunctions deserve particular attention.
In the chronic phase after TBI (at least 3 months after trauma), the prevalence of hypopituitarism ranges between 15 and 50% in different studies, with the most common hormone deficiencies being, in decreasing order of prevalence: growth hormone (GH), adrenocorticotropic hormone (ACTH), gonadotropins (follicle stimulating hormone, FSH, and luteinizing hormone, LH), and thyroid stimulating hormone (TSH) (1). One of the few prospective studies available showed that any degree of hypopituitarism was present in 33% of victims of TBI at 3 months, with this percentage decreasing to 12% at 12 months (5).
In the early phase following TBI, neurohypophysis dysfunctions are common, with consequent imbalance in plasma sodium levels: in particular, hyponatremia affects 20–50% of patients and is mainly caused by syndrome of inappropriate antidiuresis (SIAD) (3). Hypernatremia due to vasopressin deficiency (central diabetes insipidus) is likewise described in the acute phase of TBI, with a reported incidence of 3–26% (6).
In general, hyponatremia is the most common disorder of body fluid and electrolyte balance. It varies in severity, as assessed biochemically by serum sodium concentration (“mild”, between 130 and 135 mmol/L, “moderate”, between 125 and 129 mmol/L, and “severe”, below 125 mmol/L), and clinically by the presence of symptoms [“moderately severe”, accompanied by nausea without vomiting, confusion, or headache, and “severe”, with vomiting, cardiorespiratory distress, somnolence, seizures, or a Glasgow Come Scale (GCS) score ≤ 8] (7). Even when asymptomatic, natremia below 135 mmol/L is associated with increased morbidity and mortality (8). While acute hyponatremia (defined as hyponatremia that is documented to exist <48 hours) is mainly responsible for neurological manifestations, chronic hyponatremia is associated with a greater risk of falls, osteoporosis, fractures, gait instability, and cognitive decline, as well as prolonged hospitalization (9). As regards TBI, the neurological manifestations of hyponatremia and acute brain injury are quite similar, therefore differentiation may be difficult. Moreover, correlation between hyponatremia occurrence and trauma severity is conflicting. Trauma severity can be assessed both clinically, e.g., with presenting GCS score, and by imaging, e.g., according to computed tomography (CT) abnormalities. On the one hand, it appears that mild and moderate head injuries are complicated by hyponatremia more frequently than severe head traumas; on the other hand, CT grading of neurological damage seems to better correlate with severity and occurrence of hyponatremia (10).
Hyponatremia due to SIAD following TBI is generally mild and transient. Born et al. (11) suggested a distinction between early syndrome and late syndrome: the former is rarer, with an onset of SIAD between the second and fourth day following TBI, less severe neurological dysfunction, and mostly associated with skull base fractures; the latter is more common, with an onset of SIAD between the seventh and the nineteenth day after TBI with a peak of incidence on the ninth day, more extensive neurologic dysfunctions, and moderate-severe degrees of hyponatremia. While the early syndrome is primarily related to encephalic trauma lesions, the late syndrome is presumably multifactorial, also connected to intensive care procedures (11). Lohani and Devkota (10) describe a clustering of cases at the end of the first week and at the beginning of the second week, with a mean duration of 1.78 (1-3) days.
There are only few published reports of patients suffering from persistent or recurrent hyponatremia due to chronic SIAD after TBI. We report two additional cases with this condition, highlighting the need for follow-up in these patients.
A 36-year-old woman was referred to our Centre for chronic hyponatremia. She was affected with hypothyroidism secondary to total thyroidectomy for toxic multinodular goiter, allergic asthma and major depression. Eight months earlier, she had a major trauma following a fall from a first-floor window. She reported severe TBI with multiple cerebellar, frontobasal, and temporal lacerated-contused foci, diffused subarachnoid hemorrhage, multiple fractures of skull and facial massif, fracture of L1 vertebra and ribs and pulmonary and liver contusion. The patient underwent multiple neurosurgical interventions (osteodural decompression, cranioplasty, and vertebral stabilization). During her seven-month hospital stay, she developed hyponatremia, which was managed with fluid restriction and oral salt supplementation. Therapy at discharge comprised oral salt supplementation (NaCl 1 g three times daily), levothyroxine 100 µg daily, pregabalin 25 mg twice daily, olanzapine 1.25 mg twice daily, and paracetamol 500 mg daily, which guaranteed adequate pain control. Plasmatic sodium level on discharge was still compatible with mild hyponatremia (Na 134 mmol/L), dropped to severe hyponatremia in a week (Na 123–121–123 mmol/L) despite continuation of treatment, and spontaneously returned to normal (Na 133–134–135 mmol/L). Plasma potassium level remained within limits. Follow-up computed tomography (CT) scan showed no intracranial hemorrhages, a very thin left fronto-temporal subdural hygroma, and a frontal malacic gliotic focus.
When the patient came to our attention one month after discharge, she presented normal serum sodium (Na 142 mmol/L). On physical examination, blood pressure was 110/60 mmHg with a heart rate of 60 beats per minute. Reported fluid intake was of 1000 mL daily, while urinary output was about 1250 mL per day. The assessment of pituitary hormones documented normal basal and stimulated anterior pituitary function (Table 1). Kidney function, full blood count, and blood glucose were also within normal ranges. Normal natremia was confirmed (Na 140 mmol/L), while spot urine analysis revealed urinary sodium excretion (U-Na) of 192.1 mmol/L, urinary potassium excretion (U-K) of 106.2 mmol/L, and urinary specific gravity of 1025. Therefore, transient SIAD secondary to TBI was hypothesized. Given the stability of normal sodium plasma levels, withdrawal of salt supplementation was attempted.

Table 1. Patients’ exams at initial diagnosis of SIAD (syndrome of inappropriate antidiuresis) in the two cases presented.
However, one month after treatment discontinuation, the patient developed moderate hyponatremia again (Na 127 mmol/L), with low plasma chloride (95.8 mmol/L, n.v. 96-108), normal plasma potassium (K 4.5 mmol/L), and optimal levothyroxine replacement therapy (TSH 2 mIU/L). She did not report any symptom of hyponatremia, and remained clinically euvolemic. Low plasma sodium levels were subsequently confirmed (Na 129–126.6 mmol/L), and urinary sodium and urinary (U-Osm) and plasma osmolality (P-Osm) supported the diagnosis of moderate euvolemic hypotonic hyponatremia (P-Osm 262 mOsm/Kg, U-Osm 625 mOsm/Kg, U-Na 93.2 mmol/L, U-K 38.5 mmol/L). Treatment with tolvaptan 15 mg daily was started. One week afterwards, natremia was normalized (Na 138 mmol/L). The patient remained euvolemic and reported an increase in thirst and urinary output (2500 mL daily). Tolvaptan dosage was reduced to 7.5 mg daily and then to 7.5 mg every other day, and sodium levels remained normal at follow-up.
Ten months afterwards, on neurological indication, treatment with pregabalin and olanzapine was withdrawn, and after 45 days natremia still remained within range (Na 141 mmol/L). Therefore, an attempt was made to stop tolvaptan, however one month later mild hyponatremia recurred, with plasma sodium dropping to 134 mmol/L. This excluded drug-induced hyponatremia and confirmed post-TBI SIAD as the most likely diagnosis. Tolvaptan 7.5 mg every other day was reintroduced, and natremia at one-month follow-up was 141 mmol/L (Figure 1).

Figure 1. Case 1: trend of serum sodium levels over time, with associated treatments. (A) Severe hyponatremia following TBI (natremia value unavailable), managed with fluid restriction and oral salt supplementation; (B) mild hyponatremia at hospital discharge, dropping to severe hyponatremia, despite continuation of fluid restriction and oral salt supplementation; (C) Spontaneous return to normal natremia, so treatment withdrawal was attempted; (D) Recurrence of moderate hyponatremia; tolvaptan was started; (E) mild hyponatremia after tolvaptan withdrawal; eventual reintroduction of tolvaptan and subsequent maintenance of normal natremia.
A 35-year-old man came to our attention for a previous episode of severe hyponatremia, documented when he presented to the emergency department with tonic-clonic seizures (Na 124 mmol/L, Cl 86 mmol/L, U-Na 28 mmol/L). Over the preceding 16 hours he reported nausea, however he maintained regular food and fluid intake of about 2.5 L daily as usual, and denied any vomiting. He was not on any medications when he was admitted to hospital. His medical history comprised a severe concussive cranioencephalic trauma after a fall during a mountain hike the previous year.
Following seizures, he was hospitalized to complete the diagnostic work-up. Brain magnetic resonance revealed post-traumatic diffused axonal injury, with cortical and subcortical hemosiderin residues. Pituitary imaging was normal, without evidence of signal alteration, except for a minimal thinning of the terminal portion of the stalk, with inhomogeneous enhancement; the neurohypophysis was recognizable. Hyponatremia promptly responded to infusion of hypertonic (3% NaCl) saline solution, and the patient was prescribed fluid restriction of 1200 mL daily. Exams during hospital stay were consistent with SIAD (Table 1). He was discharged with the prescription of fluid restriction, neurological follow-up, and no specific medications.
On our first visit, one month later, he displayed normal natremia (Na 143 mmol/L). On physical examination, he appeared euvolemic, and blood pressure was 110/70 mmHg. The previous symptomatic episode of hyponatremia was then deemed transient and two alternative differential diagnoses were considered: either SIAD following TBI, or hyponatremia secondary to the release of antidiuretic hormone during nausea and subsequent acute dilutional hyponatremia. Given the normalization of plasma sodium levels, an attempt was made to discontinue fluid restriction, and the patient suffered no further overt hyponatremic episodes over the subsequent months. Indeed, the following sodium levels were recorded: 135 mmol/L (4 months after the first episode), 137 mmol/L (at 5 months), 135 mmol/L (at 6 months), 142 mmol/L (at 10 months).
However, the following year he presented a second episode of seizures, not preceded by other symptoms, including nausea, vomiting, or diarrhea. No clear triggers for hyponatremia development could be identified. Electrocardiogram was normal. Moderate hyponatremia (Na 126 mmol/L) was observed on this occasion, therefore hypertonic (3% NaCl) saline infusion was administered, with gradual increase of serum sodium (from 128 to 133.1 mmol/L). Chronic treatment with valproic acid 300 mg twice daily was started on neurological indication. This new episode supported the diagnosis of post-TBI SIAD, with seizures being likely facilitated by hyponatremia in a patient with a reduced epileptogenic threshold. Given that valproate is a known possible pharmacological cause of hyponatremia (8), the patient was prescribed frequent control of plasma sodium levels. In the following visits, a progressive reduction of natremia from 141 mmol/L to 132 mmol/L was observed. In particular, the patient reported asthenia, agitation, and dizziness during the days preceding the occurrence of moderate hyponatremia, but he denied any nausea, vomiting, diarrhea, or excessive fluid intake. Therefore, considering the high risk of recurrent seizures, as well as the concomitant treatment with valproate, tolvaptan 15 mg daily was initiated. While on therapy, plasma sodium rose to 145 mmol/L and patient reported excessive thirst and increased fluid intake (from 2000 mL to 3000 mL daily). For these reasons, tolvaptan was gradually down-titrated to 7.5 mg every other day, and normal sodium levels have been maintained. Antiepileptic treatment with valproate was replaced with lacosamide 100 mg twice daily, while natremia remained normal and the patient reported no further seizures (Figure 2).
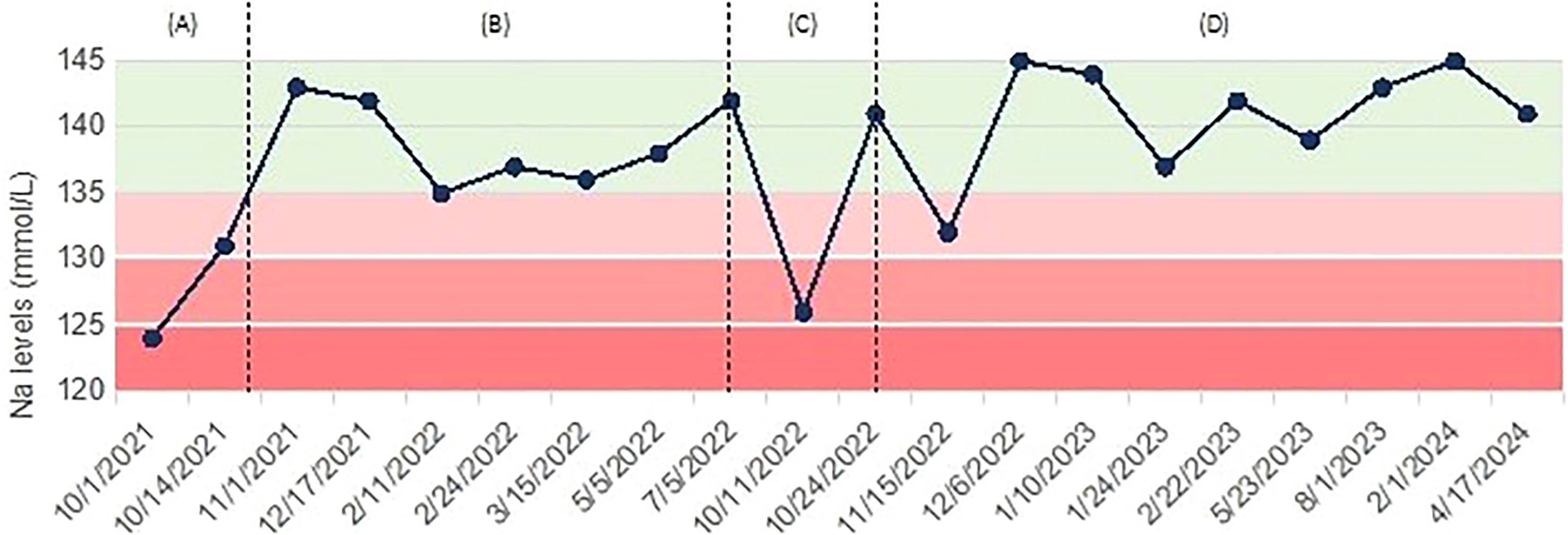
Figure 2. Case 2: trend of serum sodium levels over time, with associated treatments. (A) Severe hyponatremia presenting with tonic-clonic seizure, managed with hypertonic (3% NaCl) saline; (B) maintenance of normal natremia, no treatment; (C) recurrence of moderate hyponatremia, treated with hypertonic (3% NaCl) saline; (D) persistence of mild hyponatremia and start of tolvaptan.
We describe two cases of relapsing hyponatremia secondary to SIAD following TBI. Potentially confounding factors have been addressed in both cases (pharmacologic-induced hyponatremia due to olanzapine in the first one, and epilepsy treatment in the second one).
SIAD is a heterogeneous condition. In general, the most frequent etiologies include malignancies (e.g., small-cell lung carcinoma), disorders of the central nervous system (e.g., vascular insults), medications (e.g., proton-pump inhibitors, antidepressants) (12), pulmonary disorders (e.g., pneumonia), transient conditions (e.g., nausea, pain), and genetic causes (e.g., activating mutations in the vasopressin 2 receptor) (9). Mechanisms of onset of SIAD after TBI are not fully elucidated. It has been hypothesized that damage to the pituitary stalk or to the posterior pituitary, as well as a disorganized activity of the hypothalamic osmoreceptors and of the brainstem cardiovascular centers, may lead to inappropriate non-osmotic release of antidiuretic hormone (13, 14). TBI-associated pain and stress also concur to stimulate antidiuretic hormone secretion (7). While this is generally a temporary effect (10), as in most cases reported hyponatremia resolves after a brief period of fluid restriction, our cases support the possibility that the damage may persist, and hyponatremia may relapse. The second case described herein highlights that the inappropriate release of antidiuretic hormone with subsequent hyponatremia development can be erratic and recur even after a prolonged interval, complicating the clinical management of these patients.
Actually, hyponatremia after TBI rarely persists or recurs, in the absence of secondary causes: Agha et al. (15) describe a series of 102 TBI patients, with chronic SIAD occurring only in two cases (one with concomitant obstructive hydrocephalus, and the other with treatment with selective serotonin reuptake inhibitor citalopram).
The other reports available of non-transient, i.e., withstanding or recurrent hyponatremia following TBI are summarized in Table 2 (16–20). Of these, two reported a relapsing trend (19) (20), and only one was managed with chronic tolvaptan treatment (20). Overall, there is little data available in literature about long-term follow-up of electrolyte balance in patients suffering from TBI; our case reports contribute to this field. As a general indication, we suggest monitoring electrolytes monthly during the first six months after TBI, and subsequently every 3-6 months according to clinical progress.
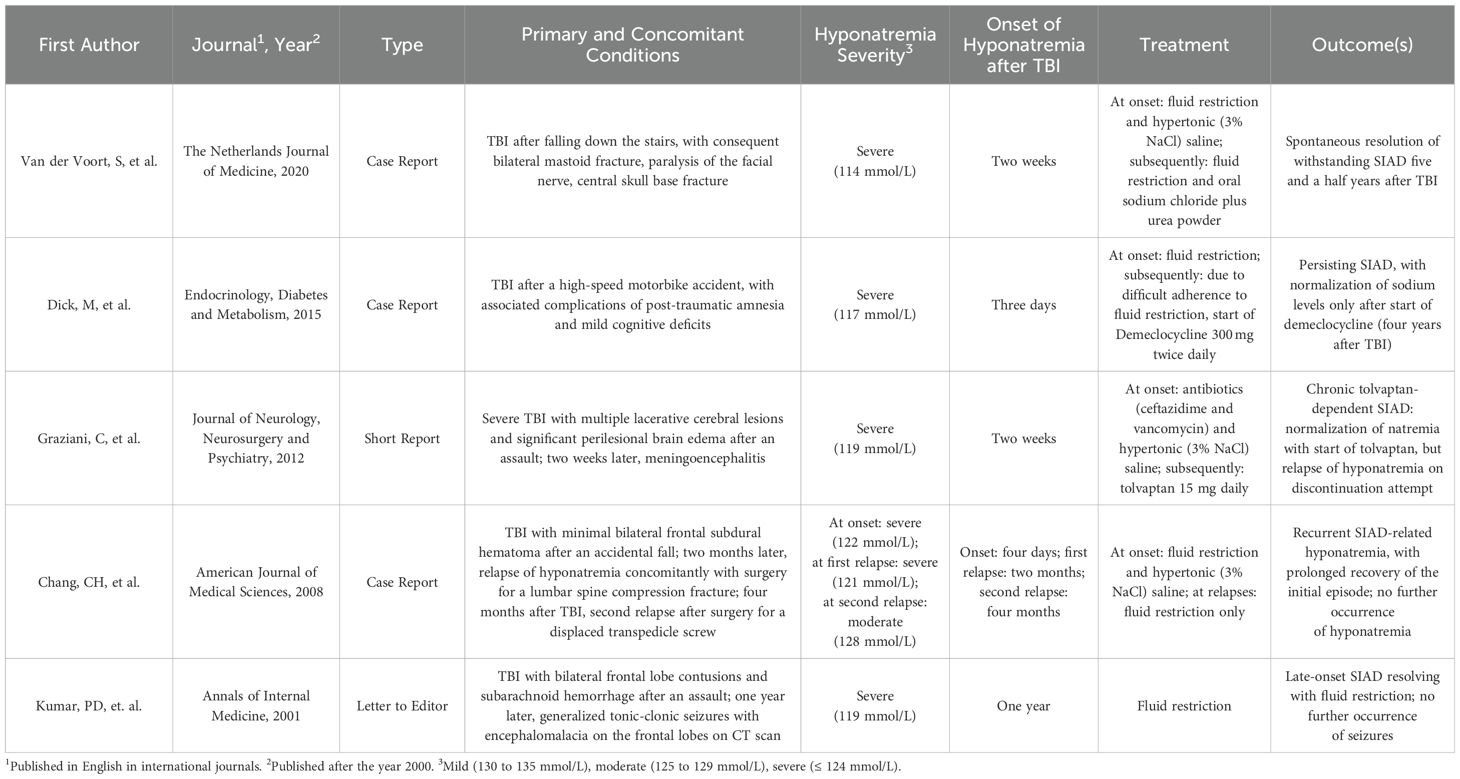
Table 2. Summary of case reports of non-transient (i.e. persistent or relapsing) hyponatremia after traumatic brain injury (TBI), with treatemnt and outcomes.
Antipsychotic use may be associated with reporting of hyponatremia; however, it is difficult to estimate the real incidence of this adverse drug reaction based on existing literature data (21). Periodic monitoring of natremia should be maintained, especially after start of therapy, and when additional risk factors for hyponatremia co-exist. Moreover, some antipsychotics, including olanzapine, have also been reported to have a beneficial effect on polydipsia and hyponatremia in schizophrenic patients (22). In the first case we report on, natremia remained normal after olanzapine discontinuation but dropped with subsequent tolvaptan withdrawal attempt, thus supporting post-traumatic SIAD as the most likely diagnosis.
Antiepileptic drugs are also known to be associated with hyponatremia: in particular, carbamazepine use was the most important risk factor for the development of hyponatremia in a large cohort of adult epileptic patients, and add-on valproate treatment increased the risk (23). A recent review of case reports concluded that valproate-associated SIAD is a rare phenomenon, still caution is warranted for the occurrence of hyponatremia (24).
Management of hyponatremia depends on the underlying cause, severity (as indicated by the presence of neurological symptoms), and speed of onset (7). Acute symptomatic hyponatremia is generally corrected with hypertonic saline (NaCl 3%). Chronic hyponatremia management needs a controlled and limited correction, in order to avoid the neurological complications of osmotic demyelination (17). According to European clinical practice guidelines for hyponatremia (7), fluid restriction is recommended as first-line treatment in chronic hyponatremia without severe or moderately severe symptoms, while the following can be considered equal second-line treatments for SIAD: increasing solute intake with 0.25–0.50 g/kg per day of urea or a combination of low-dose loop diuretics and oral salt supplementation. Both cases we describe were eventually treated with tolvaptan, an inhibitor of the receptor 2 of the antidiuretic hormone, which decreases the expression of aquaporin on renal collecting duct cells, thereby promoting free water clearance (20). Response in terms of rise in natremia is generally rapid, therefore caution to use the lowest effective dose of the drug should be used, in order to avoid overcorrection and reduce the risk of osmotic demyelination (25). For patients necessitating long-term treatment with tolvaptan, periodic attempts of discontinuation, e.g., every 1 or 2 years, can be considered, with tight monitoring of sodium levels.
The two cases reported here emphasize that hyponatremia secondary to SIAD may be a persistent or recurrent complication after TBI. Symptoms of neuroendocrine dysfunctions following TBI might be subtle, therefore clinical suspicion needs to remain high, and monitoring of plasma sodium levels should be maintained even longer than the acute phase. Maintaining an appropriate treatment for SIAD could be considered, in order to prevent recurrence of hyponatremia and avoid further risk.
“My experience with hyponatremia began after a serious accident I suffered almost three years ago, when I fell from the window of my first-floor home while I was closing a shutter. I reported a severe head injury, a burst vertebral fracture, and several rib injuries. I was hospitalized for about a month, undergoing a decompressive craniotomy and a reduction of the vertebral fracture. Subsequently, I followed a rehabilitation program of approximately seven months.
The referral to the Policlinico Hospital in Milan was suggested by a doctor at the rehabilitation clinic, for an evaluation of pituitary function in the context of chronic hyponatremia, which worsened and had been managed with intravenous saline solutions and salt tablets up to that point.
Following the initial visit, the salt tablets were discontinued to monitor sodium levels. Subsequently, given the declining condition, I was prescribed the drug tolvaptan, a treatment that has continued effectively to date and has allowed me to keep an important issue under control. I am currently in follow-up for the continuation of therapy and periodic check-ups.”.
“Sodium, for common people, is only famous because of water brand advertisements.
My approach with hyponatremia happened 3 years ago, following my first epileptic seizure, probably due to a thickening in the pituitary stalk after a severe traumatic brain injury due to a fall while hiking in the mountains.
Before that moment, I always considered myself “a heavy drinker” of water, however afterwards I had to get used to the fact that I would only be so on the days I take my medicine (tolvaptan), with subsequent very frequent urination (up to twenty times within four hours initially), even at night.
After two years, I think I have reached a stable situation, I drink and urinate regularly; I take in less fluids than before, still I live my days without rushing to the toilet, as used to happen before.
I am not sure whether it is subjective, but lately, on the days I take tolvaptan, I feel like still water does not quench my thirst as before; I prefer drinking sparkling water and even fizzy drinks.”
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
IP: Writing – original draft. RI: Writing – review & editing. BM: Writing – review & editing. VL: Writing – review & editing. GM: Writing – review & editing. EF: Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Ricerca Corrente funds from the Italian Ministry of Health to Fondazione IRCSS Ca’ Granda Policlinico.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tanriverdi F, Schneider HJ, Aimaretti G, Masel BE, Casanueva FF, Kelestimur F. Pituitary dysfunction after traumatic brain injury: A clinical and pathophysiological approach. Endocr Rev. (2015) 36:305–42. doi: 10.1210/er.2014-1065
2. Tanriverdi F, Senyurek H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. High Risk of Hypopituitarism after Traumatic Brain Injury: A Prospective Investigation of Anterior Pituitary Function in the Acute Phase and 12 Months after Trauma. J Clin Endocrinol Metab. (2006) 91:2105–11. doi: 10.1210/jc.2005-2476
3. Tudor RM, Thompson CJ. Posterior pituitary dysfunction following traumatic brain injury: review. Pituitary. (2019) 22:296–304. doi: 10.1007/s11102-018-0917-z
4. Najem D, Rennie K, Ribecco-Lutkiewicz M, Ly D, Haukenfrers J, Liu Q, et al. Traumatic brain injury: classification, models, and markers. Biochem Cell Biol. (2018) 96:391–406. doi: 10.1139/bcb-2016-0160
5. Aimaretti G, Ambrosio MR, Di Somma C, Gasperi M, Cannavò S, Scaroni C, et al. Residual pituitary function after brain injury-induced hypopituitarism: A prospective 12-month study. J Clin Endocrinol Metab. (2005) 90:6085–92. doi: 10.1210/jc.2005-0504
6. Hannon MJ, Crowley RK, Behan LA, O’Sullivan EP, O’Brien MMC, Sherlock M, et al. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. J Clin Endocrinol Metab. (2013) 98:3229–37. doi: 10.1210/jc.2013-1555
7. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemiaThe guidelines were peer reviewed by the owner societies and by external referees prior to publication. Eur J Endocrinol. (2014) 170:G1–47. doi: 10.1530/EJE-13-1020
8. Janicic N, Verbalis JG. Evaluation and management of hypo-osmolality in hospitalized patients. Endocrinol Metab Clin North Am. (2003) 32:459–81. doi: 10.1016/S0889-8529(03)00004-5
9. Martin-Grace J, Tomkins M, O’Reilly MW, Thompson CJ, Sherlock M. Approach to the patient: hyponatremia and the syndrome of inappropriate antidiuresis (SIAD). J Clin Endocrinol Metab. (2022) 107:2362–76. doi: 10.1210/clinem/dgac245
10. Lohani S, Devkota UP. Hyponatremia in patients with traumatic brain injury: etiology, incidence, and severity correlation. World Neurosurg. (2011) 76:355–60. doi: 10.1016/j.wneu.2011.03.042
11. Born JD, Hans P, Smitz S, Legros JJ, Kay S. Syndrome of inappropriate secretion of antidiuretic hormone after severe head injury. Surg Neurol. (1985) 23:383–7. doi: 10.1016/0090-3019(85)90212-5
12. De Picker L, Van Den Eede F, Dumont G, Moorkens G, Sabbe BGC. Antidepressants and the risk of hyponatremia: A class-by-class review of literature. Psychosomatics. (2014) 55:536–47. doi: 10.1016/j.psym.2014.01.010
13. Kirkman MA, Albert AF, Ibrahim A, Doberenz D. Hyponatremia and brain injury: historical and contemporary perspectives. Neurocrit Care. (2013) 18:406–16. doi: 10.1007/s12028-012-9805-y
14. Wong LL, Verbalis JG. Systemic diseases associated with disorders of water homeostasis. Endocrinol Metab Clin North Am. (2002) 31:121–40. doi: 10.1016/S0889-8529(01)00007-X
15. Agha A, Thornton E, O’Kelly P, Tormey W, Phillips J, Thompson CJ. Posterior pituitary dysfunction after traumatic brain injury. J Clin Endocrinol Metab. (2004) 89:5987–92. doi: 10.1210/jc.2004-1058
16. van der Voort S, de Graaf J, de Blok K, Sekkat M. Persevering syndrome of inappropriate antidiuretic hormone secretion after traumatic brain injury. Neth J Med. (2020) 78:290–3.
17. Dick M, Catford SR, Kumareswaran K, Hamblin PS, Topliss DJ. Persistent syndrome of inappropriate antidiuretic hormone secretion following traumatic brain injury. Endocrinol Diabetes Metab Case Rep. (2015) 2015:150070. doi: 10.1530/EDM-15-0070
18. Kumar PD, Nartsupha C, Koletsky RJ. Delayed syndrome of inappropriate antidiuretic hormone secretion 1 year after a head injury. Ann Intern Med. (2001) 135:932–3. doi: 10.7326/0003-4819-135-10-200111200-00027
19. Chang CH, Liao JJ, Chuang CH, Lee CT. Recurrent hyponatremia after traumatic brain injury. Am J Med Sci. (2008) 335:390–3. doi: 10.1097/MAJ.0b013e318149e6f1
20. Graziani G, Cucchiari D, Aroldi A, Angelini C, Gaetani P, Selmi C. Syndrome of inappropriate secretion of antidiuretic hormone in traumatic brain injury: when tolvaptan becomes a life saving drug. J Neurol Neurosurg Psychiatry. (2012) 83:510–2. doi: 10.1136/jnnp-2011-300576
21. Mannesse CK, van Puijenbroek EP, Jansen PAF, van Marum RJ, Souverein PC, Egberts TCG. Hyponatraemia as an adverse drug reaction of antipsychotic drugs. Drug Saf. (2010) 33:569–78. doi: 10.2165/11532560-000000000-00000
22. Littrell KH, Johnson CG, Peabody CD. Effects of olanzapine on polydipsia and intermittent hyponatremia. J Clin Psychiatry. (1997) 58:5856. doi: 10.4088/JCP.v58n1206c
23. Yamamoto Y, Takahashi Y, Imai K, Ohta A, Kagawa Y, Inoue Y. Prevalence and risk factors for hyponatremia in adult epilepsy patients: Large-scale cross-sectional cohort study. Seizure. (2019) 73:26–30. doi: 10.1016/j.seizure.2019.10.013
24. Heflin K, ElSayed M. Valproate-induced severe symptomatic hyponatremia in a patient with schizoaffective disorder: a case study and literature review. Eur Psychiatry. (2023) 66:S556–7. doi: 10.1192/j.eurpsy.2023.1174
25. Onuigbo MAC, Agbasi N. Severe symptomatic acute hyponatremia in traumatic brain injury responded very rapidly to a single 15 mg dose of oral tolvaptan; a Mayo Clinic Health System hospital experience – need for caution with tolvaptan in younger patients with preserved renal function. J Ren Inj Prev. (2017) 6:26–9. doi: 10.15171/jrip.2017.05
Keywords: hyponatremia, syndrome of inappropriate antidiuresis (SIAD), traumatic brain injury (TBI), neurohypophysis, tolvaptan, case report
Citation: Petria I, Indirli R, Mantovani B, Lanzi V, Mantovani G and Ferrante E (2025) Recurrent hyponatremia due to syndrome of inappropriate antidiuresis after traumatic brain injury: two case reports. Front. Endocrinol. 16:1536247. doi: 10.3389/fendo.2025.1536247
Received: 28 November 2024; Accepted: 17 February 2025;
Published: 06 March 2025.
Edited by:
Amnon Schlegel, The University of Utah, United StatesReviewed by:
Ploutarchos Tzoulis, University College London, United KingdomCopyright © 2025 Petria, Indirli, Mantovani, Lanzi, Mantovani and Ferrante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Indirli, cml0YS5pbmRpcmxpQHVuaW1pLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.