
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 11 April 2025
Sec. Systems Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1533516
Ethylene oxide (EO) is a crucial organic compound commonly utilized in industrial and medical products. Food and Drug Administration (FDA)-approved EO sterilization sterilizes about 50% of sterile medical devices in the U.S. Animal and human studies have suggested that EO exposure may result in severe health problem. However, studies evaluating the relationship between EO exposure and sex hormones in human populations are still lacking. Therefore, further investigation into EO’s effects on humans is essential. This cross-sectional study within the U.S. National Health and Nutrition Examination Survey (NHANES),2013–2016 examined the relationship between EO-hemoglobin adducts (HbEO) and sex hormones. HbEO was found to be inversely associated with estradiol (E2) and positively associated with the ratio of total testosterone (TT) to E2 and sex hormone-binding globulin (SHBG) levels in adult males. Such associations HbEO and E2 and SHBG were non-linear in male adults. However, no significant associations were found between HbEO and sex steroids across various age groups of females and all male age groups except for adults. Thus, our study provides evidence that EO may potentially serve as an endocrine disruptor in the environment, affecting the levels of sex hormones in adult males.
Ethylene oxide (EO) is a crucial organic compound extensively employed in industrial and medical applications. It is extensively utilized as a disinfectant and sterilizing agent in numerous industrial processes to ensure product quality and safety (1). Additionally, it is employed in the manufacturing of various chemical products including ethylene glycol, emulsifiers, and surfactants (2). Despite its widespread applications, EO can cause potential hazards to human health. It exists in a gaseous form at room temperature, easily entering the respiratory system and bind to DNA and proteins. The blood biomarker N-(2-hydroxyethyl) valine, known as the hemoglobin adduct of EO (HbEO), is a valuable indicator of ethylene oxide exposure (3). In-vitro studies demonstrated that EO exposure exhibited genotoxic and mutagenic effects (4, 5). In population-based studies, it has been observed that long-term contact with EO may cause severe health issues such as cancer, cardiovascular diseases, diabetes, neurological impairments, respiratory diseases, as well as adverse reproductive outcomes (6–10). EO gas presents a significant health risk to residents, patients, and workers living in proximity to the currently hundreds of active EO-emitting facilities across the U.S. These individuals may be exposed to medical devices sterilized with EO within hospital environments, or directly as employees within EO-emitting manufacturing plants (11, 12). Although EO has previously been considered a “potentially hazardous air pollutant” in 2016, the U.S. Environmental Protection Agency (EPA) reclassified EO as a known human carcinogen based on new data, which indicate that the toxicity of EO is 30 times higher than previously estimated (5, 13). Given that there are a significant number of facilities emitting EO located in densely populated areas worldwide, understanding the comprehensive health risks associated with EO exposure is an urgent public health priority.
Sex hormones serve a crucial function in triggering and sustaining human reproductive well-being (14). Important human sex hormones include TT, E2, and SHBG play prominent roles (14).The levels of various sex hormones within the body vary at different stages of life. Studies on animals have demonstrated that exposure to EO by inhalation led to elongated estrous cycles in female mice (15), while male mice experienced testicular atrophy in the presence of EO exposure (16). These evidences implied detrimental impacts of EO on the reproductive system in mice. However, currently, studies in human populations evaluating the correlation between exposure to EO and sex steroid hormone are still lacking. Hence, the objective of this research is to investigate the connection between exposure to EO and sex hormones across various demographic segments of the human population.
NHANES survey measures the health and nutrition of American adults, adolescents and children in the most comprehensive way. This survey combines information from interviews and physical examinations, which information from interviews and physical examinations is combined. NHANES is conducted under the National Center for Health Statistics (NCHS), an agency that falls under the jurisdiction of the Centers for Disease Control and Prevention (17).Data collection and laboratory analysis methods for the NHANES are described elsewhere. Questions on demographics, socioeconomics, dietary habits, and health are included in the interviews. Participants gave written consent, and the study’s methods and materials were ethics board-approved. This study used data from the 2013–2016 NHANES to determine blood HbEO, serum TT, E2, and SHBG levels for the United States population. Referring to previous NHANES literature, we deleted participants with missing covariates (18, 19). A total of 20146 participants were enrolled at first, after the exclusion of individuals who were pregnant (n=135), missed data on blood EO (n=15189) and unavailable data on sex hormones (n=601), 4221 participants were included in our final analysis. Figure 1 illustrates the selection process for participants.
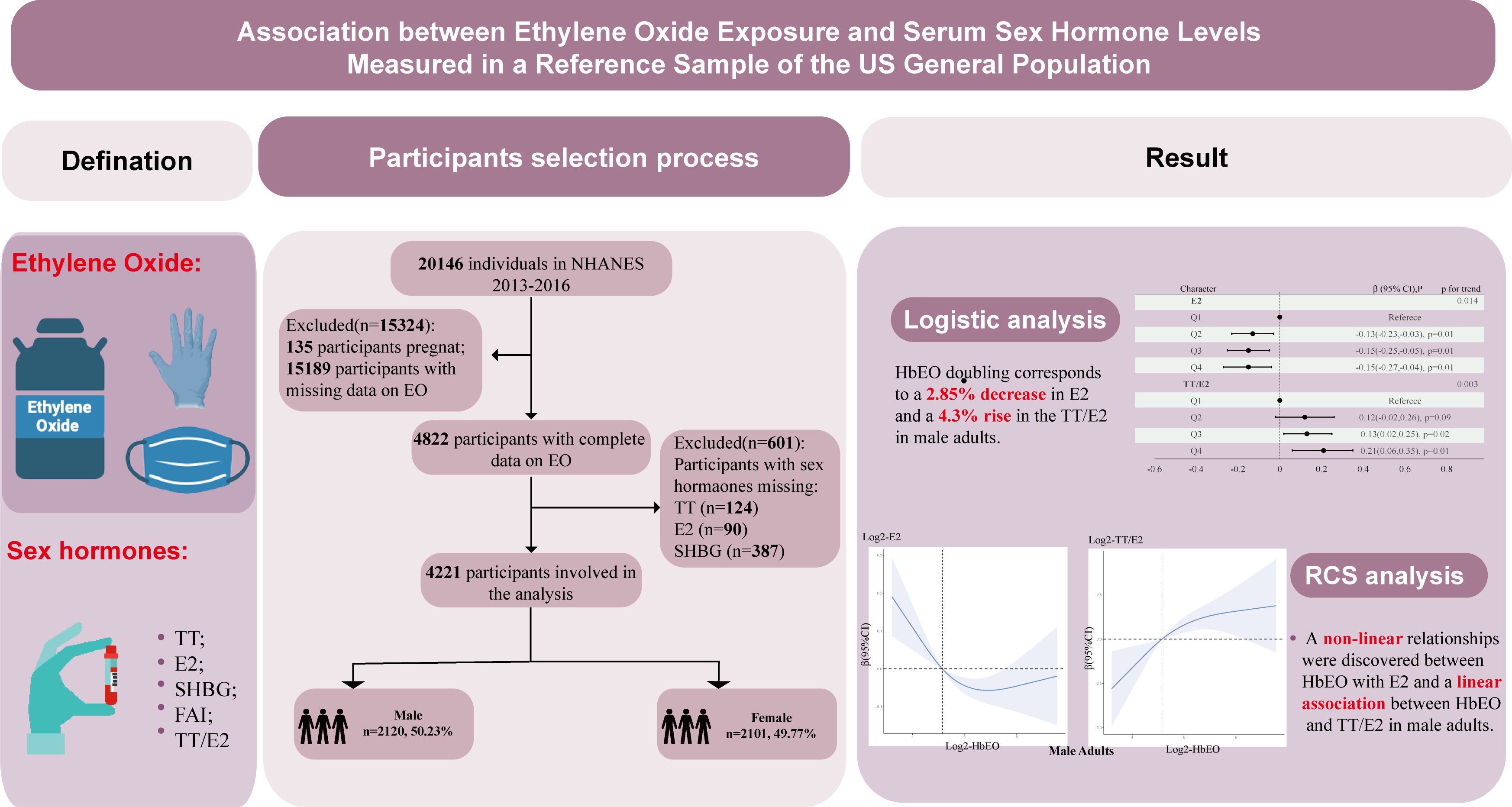
Figure 1. Graphical abstract and flowchart of studied participants selection. TT, total testosterone; E2, total estradiol; SHBG, sex hormone binding globulin; FAI, free androgen index, was calculated as total testosterone (ng/dL)/SHBG (nmol/L), TT/E2 was calculated as TT (ng/dL)/E2 (pm/ml); HbEO, hemoglobin adducts of ethylene oxide.
HbEO, with a longer in vivo half-life than EO, is utilized as an indicator of EO exposure. The measurement of HbEO levels was performed according to the guidelines outlined in the NHANES Laboratory/Medical Technologist Procedures Manual (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/ETHOX_H.htm). Initially, samples of erythrocytes were gathered, preserved at −30°C, and then sent to the National Center for Environmental Health for assessment. Subsequently, The HbEO quantity was measured utilizing high-performance liquid chromatograph-tandem mass spectrometry (HPLC-MS/MS) and the modified Edman reaction. Lastly, as a result, the levels of HbEO were expressed as picomoles adducts per gram of hemoglobin. Additionally, a comprehensive quality control program, incorporating both external and internal surveillance, was established to oversee and assess the accuracy and reliability of the analytical testing process.
A serum sample was prepared, preserved at -20°C, and shipped to the National Center for Environmental Health to be tested. To measure TT and E2, isotope dilution-liquid chromatograph-tandem mass spectrometry (ID-LC-MS/MS) was applied. The levels of SHBG were measured using a chemiluminescent assay. Detailed technical information about these methods are available in other sources (20, 21). The lower limit of detection (LLOD) was 0.75 ng/mL for TT, 2.994 pg/mL for E2, and 0.800 nmol/L for SHBG. For estimating the concentration of circulating free testosterone, the free androgen index (FAI) was used to estimate the level of circulating free testosterone by dividing TT by SHBG (22). The TT to E2 ratio (TT/E2) was utilized as an indirect measure of aromatase activity (23).
Potential confounding factors were also collected as covariates, including age, race/ethnicity, education level, BMI, poverty income ratio (PIR), cotinine and the time of sample collection. Race/ethnicity was classified into Hispanics, non-Hispanic Black, non-Hispanic White, and other races. Education level was classified as less than high school, more than high school, high school, or general educational development (24). BMI was computed by dividing weight (in kilograms) by the square of height (in meters). According to the International Obesity Task Force (IOTF), children were classified as overweight (BMI ≥ IOTF-25), obesity (BMI ≥ IOTF-30) and normal (BMI< IOTF-25) (25). In the adult group, BMI category was classified as < 25 kg/m2 and ≥ 25 kg/m2 (9). The PIR, which signifies the family’s collective socioeconomic standing, was computed by dividing the family income according to the poverty guidelines pertinent to the participant’s household size, coupled with the year and state data (26). Serum cotinine levels were measured and group into 2 categories: <LOD (below the LOD, 0.015 ng/mL) and ≥LOD to take the environmental tobacco exposure into account (27). Moreover, to account for the variations in hormone levels, a six-month time span (November 1 to April 30, May 1 to October 31) incorporated as covariates in the statistical analysis. Physical activity was calculated based on a detailed physical activity survey described previously (28). Total energy intake was calculated from three-day dietary-recall food composition tables (29).Whether the participant has taken prescription medicine is assessed by NHANES interviewers through the following question: “In the past 30 days, have you used or taken any medication that requires a prescription? Please do not include any prescription vitamins or minerals that you may have already mentioned.” according to the NHANES analysis guideline (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/RXQ_RX_H.htm#RXDCOUNT).
Potential confounding factors were also collected as covariates, including age, race/ethnicity, education level, BMI, poverty income ratio (PIR), cotinine and the time of sample collection. Race/ethnicity was classified into Hispanics, non-Hispanic Black, non-Hispanic White, and other races. Education level was classified as less than high school, more than high school, high school, or general educational development (24). BMI was computed by dividing weight (in kilograms) by the square of height (in meters). According to the International Obesity Task Force (IOTF), children were classified as overweight (BMI ≥ IOTF-25), obesity (BMI ≥ IOTF-30) and normal (BMI< IOTF-25) (25). In the adult group, BMI category was classified as < 25 kg/m2 and ≥ 25 kg/m2 (9). The PIR, which signifies the family’s collective socioeconomic standing, was computed by dividing the family income according to the poverty guidelines pertinent to the participant’s household size, coupled with the year and state data (26). Serum cotinine levels were measured and group into 2 categories: <LOD (below the LOD, 0.015 ng/mL) and ≥LOD to take the environmental tobacco exposure into account (27). Moreover, to account for the variations in hormone levels, a six-month time span (November 1 to April 30, May 1 to October 31) incorporated as covariates in the statistical analysis. Physical activity was calculated based on a detailed physical activity survey described previously (28). Total energy intake was calculated from three-day dietary-recall food composition tables (29). Whether the participant has taken prescription medicine is assessed by NHANES interviewers through the following question: “In the past 30 days, have you used or taken any medication that requires a prescription? Please do not include any prescription vitamins or minerals that you may have already mentioned.” According to the NHANES analysis guideline (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/RXQ_RX_H.htm#RXDCOUNT). Urine specimens were obtained during field examination visits and cryopreserved at −20°C prior to analytical processing. Chemical selection criteria required ≥85% detection frequency across samples, resulting in the inclusion of: two phenolic compounds (bisphenol A [BPA] and bisphenol S [BPS]), three phthalate derivatives (monobenzyl phthalate [MBzP], monoisobutyl phthalate [MiBP], and monocarboxyoctyl phthalate [MCOP]). BPA and BPS were quantified via automated online solid-phase extraction coupled with isotope-dilution high-performance liquid chromatography-tandem mass spectrometry (SPE-HPLC-MS/MS). MBzP, MCOP, and MiBP analysis employed HPLC separation with electrospray ionization tandem mass spectrometric detection (HPLC-ESI-MS/MS). Methodological specifications and quality assurance protocols adhere to NHANES laboratory standards (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/EPHPP_H.htm#URD14DLC), (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/PHTHTE_H.htm), polychlorinated biphenyls (PCBs) were measured in serum by high-resolution gas chromatography/isotope-dilution high-resolution mass spectrometry (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/PCBPOL_H.htm). Based on prior literature, we identified three polychlorinated biphenyls (PCBs) with potential endocrine-disrupting effects on sex hormones (30).
Appropriate weighting, in accordance with the recommendations of the NCHS, were employed for each analytical process. Weighted mean value (± standard deviation [SD]) were calculated employing NHANES primary sampling units and strata, with the results being subjected to a weighted t-test for statistical analysis. Frequencies (proportions) for categorical variables were analyzed using the weighted chi-square test. The serum HbEO and sex hormone indicator distributions exhibited a right-skewed pattern, prompting log2 transformation to normalize them for descriptive and regression analyses. The log2-transformed HbEO values were then analyzed as continuous and categorical (divided into quartiles) variables (31). Weighted quartiles for log2-transformed HbEO were calculated within specific sex-age and sex-puberty subgroups, as shown in Supplementary Table S1.
A weighted multiple linear regression was used to calculate β (Standardized coefficients) values and corresponding 95% confidence intervals (CIs) to examine the connections between individual HbEO levels and sex steroid hormone indicators. Restricted cubic spline (RCS) analysis was applied to further examine linear and non-linear relationships between HbEO and sex hormones after adjusting for various potential covariates (32, 33). The Akaike information criterion (AIC) was used to choose the most suitable knots that had the smallest AIC (34).
Considering the marked variations in sex hormone levels between genders and at different stages of development, this analysis was conducted based on age (children: ≤11 years, adolescents: 12-19 years, adults: >19 years) for males and females (35). Grouping 6-19 years old participants as “children” or “adolescents” based solely on their ages may include both pre-pubertal and pubertal individuals in the same groups. Such grouping could result in subgroups with exceptionally high or low sex hormone levels, which might bias the regression analysis relationship between HbEO and sex steroid hormones. Moreover, the impact of log2-HbEO on sex hormones may be influenced by the pubertal stage. To tackle this concern, we further subcategorized individuals into pubertal and prepubertal subgroups based on their serum sex hormone levels and menarcheal status. Individuals were considered to have entered puberty (i.e., classified as pubertal) if their TT levels were equal to or greater than 50 ng/dL (in males) or E2 levels were 20 pg/mL or higher (in females), or if they had experienced the onset of menstruation (in females) (36–38). Those not meeting these criteria were classified as pre-pubertal (i.e., classified as prepubertal). Data regarding girls’ menarche status was collected through inquiries such as: “Have menstrual periods commenced?” (found in the medical questionnaire) and “What age was the first menstrual period?” (included in the reproductive health questionnaire). Female whose answer was “Yes” or provided their age at first menstrual period was recorded as having started menstrual cycles. Furthermore, this analysis performed the multiple linear regression analyses again, stratified by pubertal status in both males and females.
Subgroup analyses with multiplicative interaction terms were performed to show whether the relationship between individual HbEO levels and sex steroid hormones varied by race, age (<45 years or ≥45 years), BMI (<24 or ≥24 kg/m2), energy intake (<2,400 or ≥ 2,400 kcal), physical activity (<200 or ≥ 200 METs-hour/week) and taken prescription medicine (Yes or No) (39).
To evaluate the robustness of our results, 4 sensitivity analyses were conducted to examine the relationship between individual HbEO levels and sex steroid hormones: 1) Multiple imputation for missing data was conducted using the ‘mice’ package (Multivariate Imputation by Chained Equations) in R (40); 2) Energy intake, physical activity, and prescription medication was adjusted additionally; 3) exposure of bisphenol A, phthalates and polychlorinated biphenyls was adjusted additionally; 4) excluded participants aged ≥65 years in the adults. All statistical analyses were performed using R software version 4.1.2, and a two-tailed P value with a significance level of 0.05 was used for all statistical tests to determine significance.
Table 1 summarizes the demographic features of the enrolled participants categorized by sex and age. The study sample comprised a total of 4221 individuals, including 2089 males and 2101 females. The individuals had a mean age of 41.70 years. The detection rates for TT, E2, SHBG, and HbEO in the entire sample were 99.81%, 85.93%, 100%, and 97.49%, respectively. The values below the LLOD were replaced by the lower limit of detection divided by the square root of 2 (LLOD/sqrt (2)) according to the NHANES analysis guideline (https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/ETHOX_H.htm#LBXEOA) (Supplementary Table S2). However, the detection rate for E2 in male children was less than 50%. As a result, the analyses for E2 and TT/E2 were omitted for this particular subgroup. Comparatively, adult participants exhibited greater ethylene oxide exposure levels than adolescents, while adolescents showed higher exposure levels than children, across both genders (Supplementary Table S3).
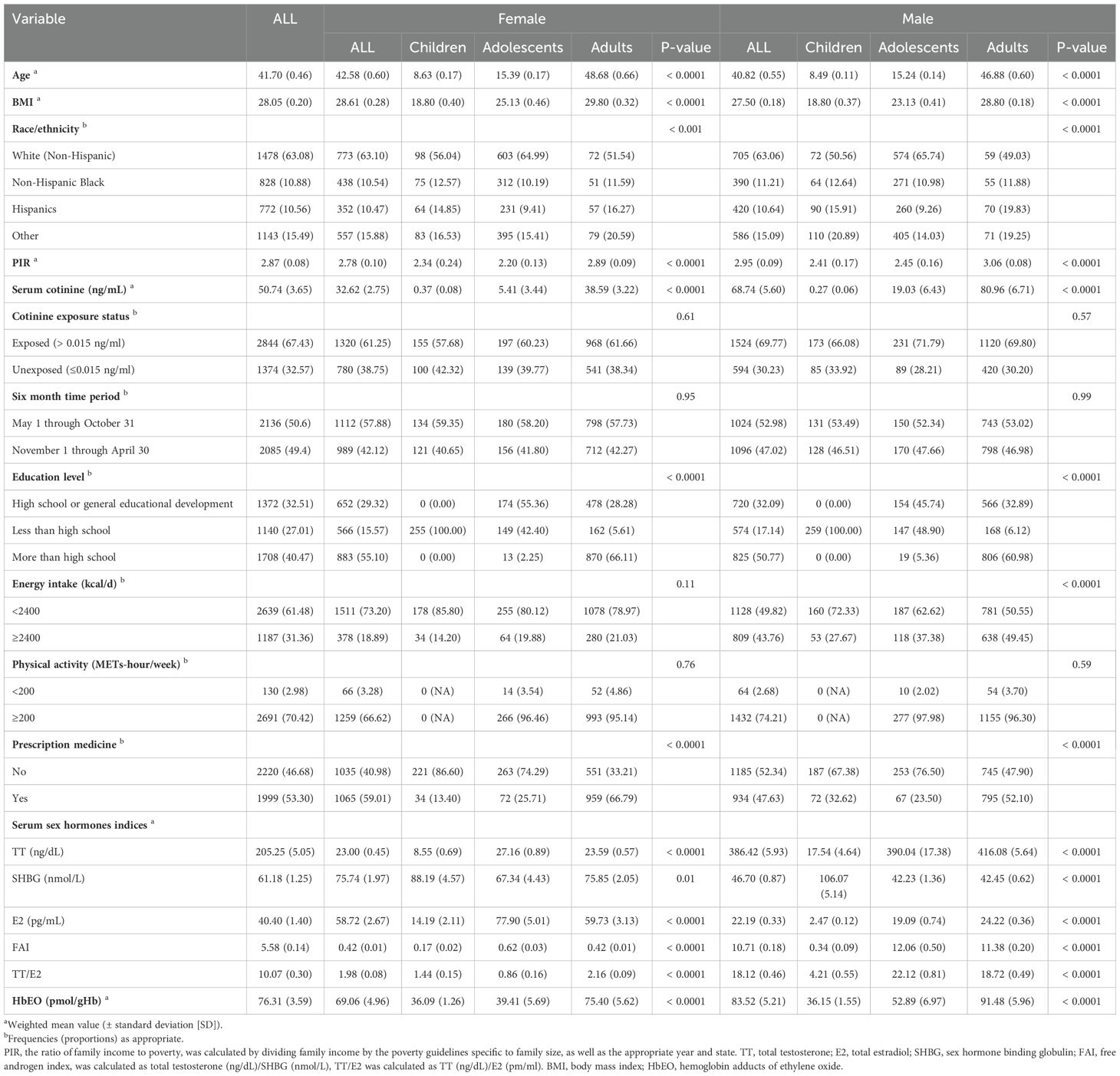
Table 1. Weighted sample characteristics of children (6-11 years old), adolescents (12–19 years old) and adults (≥ 20 years old) with serum sex hormones and HbEO in NHANES 2013–2014.
Given the crucial involvement of sex hormones in the initiation of puberty, the statuses of pre-puberty and puberty were also examined (Supplementary Table S4). The proportion of E2 detections in prepubertal males and females was below 50%. Consequently, assessments of E2 and TT/E2 were not conducted for these demographic subsets. EO exposure was significantly higher in the puberty than that of pre-puberty in males. While there were no significances between pre-puberty and puberty in females.
Figure 2 and Supplementary Table S4 present the relationships between HbEO levels, categorized as continuous and quartiles, and sex hormones across various stages of development. Among males, no substantial correlations were found between HbEO and sex steroid hormones among children and adolescents (Supplementary Table S4). However, in male adults, HbEO doubling corresponds to a 2.85% decrease in E2, a 2.10% increase in SHBG, and a 4.3% rise in the TT/E2 (for E2: p= 0.04, OR = -0.03, 95% CI: -0.06 to 0.00; for SHBG: p= 0.03, OR = 0.03, 95% CI: 0.00 to 0.06; for TT/E2: p< 0.001, OR = 0.06, 95% CI: 0.03 to 0.09). Moreover, consistent associations of serum E2 and TT/E2 levels with HbEO were also observed across quartiles of EO exposure. Serum E2 levels were decreased along with the increased exposure of EO (p for trend=0.014). Similarly, TT/E2 levels were increased accompanied by the increased exposure of EO (p for trend=0.003) (Figure 2). No correlations were identified between EO exposure and sex hormones in female subjects across various categories.
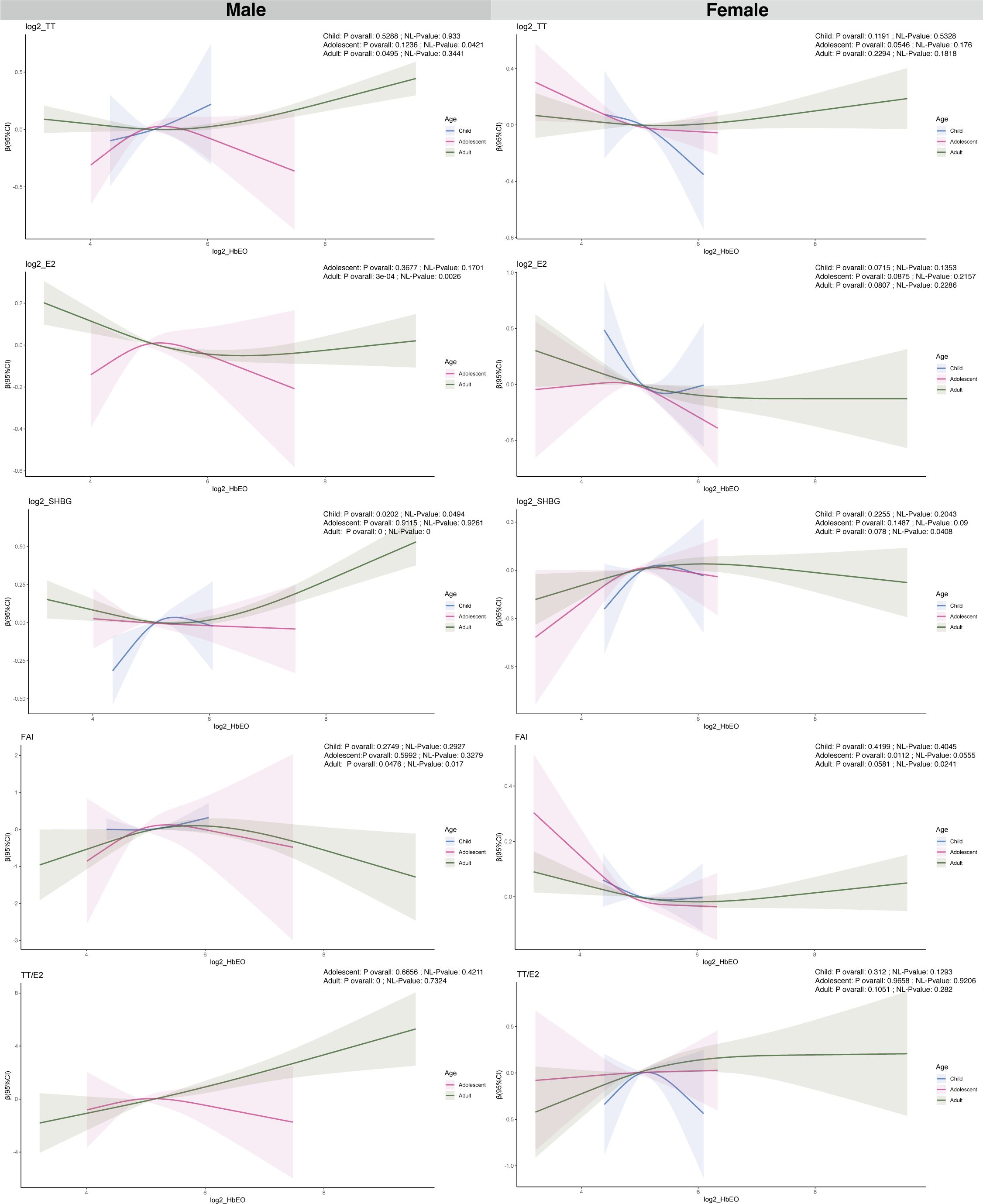
Figure 2. Analysis of the association between log2-HbEO and sex hormones by sex-age groups in participants in NHANES 2013–2016 using the RCS. TT, total testosterone; E2, total estradiol; SHBG, sex hormone binding globulin; FAI, free androgen index, was calculated as total testosterone (ng/dL)/SHBG (nmol/L), TT/E2 was calculated as TT (ng/dL)/E2 (pm/ml). HbEO, hemoglobin adducts of ethylene oxide.
The relationship between the continuous and quartile distributions of HbEO and sex steroid hormones in prepubertal and pubertal status were presented in Supplementary Table S5. There were no significant correlations found between HbEO and sex steroid hormones in prepuberty and puberty, irrespective of gender. When evaluated by quartiles of EO exposure, HbEO (quartile 4 versus 1) showed a positive correlation with SHBG in pre-pubertal males (P = 0.03, OR = 0.21, 95% CI: 0.02, 0.4). In contrast, HbEO (quartile 3 versus 1) was negatively correlation with SHBG in pubertal males (P = 0.03, OR = -0.22, 95% CI: -0.42, -0.03).
A restricted cubic spline (RCS) analysis was utilized for further investigating the linear and nonlinear relationships between HbEO with sex hormones after adjusting for various confounding factors, as depicted in Figure 3 and Supplementary Figure S1. In male children, A nonlinear association was found between HbEO and SHBG (NL-P value = 0.049). A non-linear relationship was found between HbEO and TT in male adolescents (NL-P value = 0.042). In male adult, non-linear relationships were discovered between HbEO with E2, SHBG and FAI (E2: NL-P value = 0.0026; SHBG: NL-P value =0; FAI: NL-P value =0.017). Furthermore, nonlinear associations were found between FAI, SHBG, and HbEO in female adult participants. (NL-P value = 0.041; FAI: NL-P value = 0.024) (Figure 3).

Figure 3. Associations of continuous and quartiles of log2-HbEO with sex hormones in >19-year old adults. (A) TT. (B) E2. (C) SHBG. (D) FAI. (E) TT/E2. TT, total testosterone; E2, total estradiol; SHBG, sex hormone binding globulin; FAI, free androgen index, was calculated as total testosterone (ng/dL)/SHBG (nmol/L), TT/E2 was calculated as TT (ng/dL)/E2 (pm/ml); HbEO, hemoglobin adducts of ethylene oxide.
What’s more, our RCS analysis uncovered a nonlinear relationship between HbEO and SHBG in the prepubertal group among males (NL-P value = 0.0264). While, HbEO has a non-linear association with FAI in female pubertal individuals (NL-P value = 0.0476) (Supplementary Figure S1).
Subgroup analyses were conducted among adult males categorized by age, race, BMI, energy intake, physical activity, and prescription medication to further investigate the consistency of the relationship between the log2-transformed HbEO levels and sex hormones across different groups. These results confirmed a robust association between the log2-transformed HbEO levels and TT, E2, FAI, TT/E2 across different subgroups (P for interaction > 0.05) (Figures 4A, B, D, E). We found that the effect of HbEO on SHBG was significantly dependent on energy intake (interaction P < 0.001). In adult males with an energy intake ≥ 2400 kcal, HbEO was positively associated with SHBG (Figure 4C).
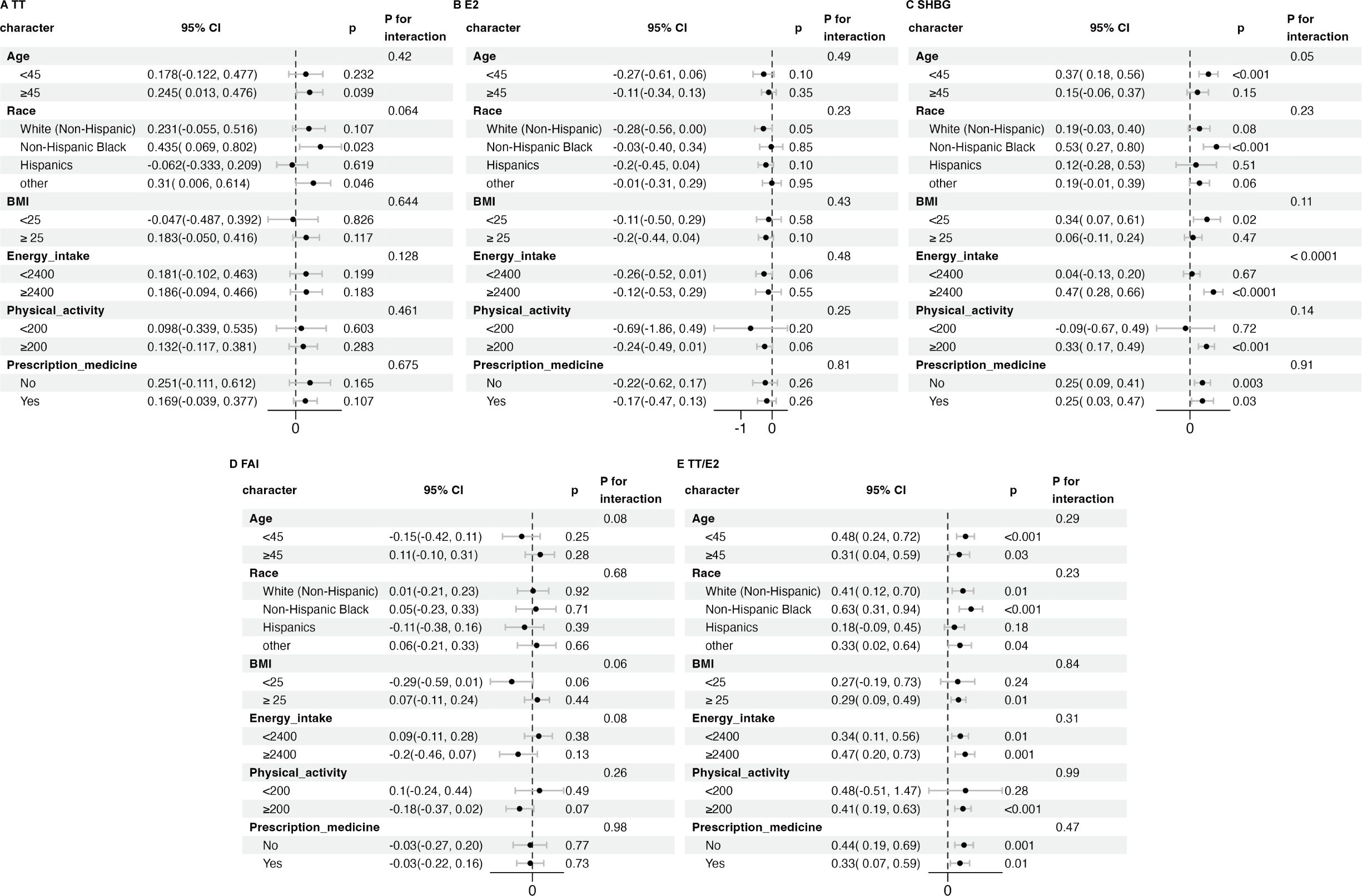
Figure 4. Subgroup analysis of the association between log2-HbEO and sex hormones in subgroups of >19-year old male adults. (A) TT. (B) E2. (C) SHBG. (D) FAI. (E) TT/E2. Each subgroup adjusted for all factors except the stratification factor itself. TT, total testosterone; E2, total estradiol; SHBG, sex hormone binding globulin; FAI, free androgen index, was calculated as total testosterone (ng/dL)/SHBG (nmol/L), TT/E2 was calculated as TT (ng/dL)/E2 (pm/ml). BMI, body mass index; HbEO, hemoglobin adducts of ethylene oxide.
To validate the robustness of our findings, we conducted 4 sensitivity analyses. In all sensitivity analyses, the negative association of HbEO with E2 and TT/E2 remained significant, demonstrating the robustness of our results (Supplementary Tables S6–S12).
This investigation represents the first to explore the connection between EO and sex steroids across various age brackets within a participant population. The findings demonstrated that HbEO was significantly inversely associated with E2 and positively associated with TT/E2 and SHBG levels in adult males. Such associations between HbEO with E2 and SHBG were non-linear in male adults. However, no significant correlation was detected between HbEO and sex steroids in different age female groups. Thus, our study provides evidence that EO may potentially serve as an endocrine disruptor in the environment, affecting the levels of sex hormones in adult males.
EO is a gaseous compound at room temperature and is primarily encountered through inhalation (41). Members of the public may encounter EO through a range of routes, such as breathing polluted air, inhaling tobacco smoke, exposure to vehicle exhaust, or encountering fumes from commercial products in domestic settings (1, 42). a key industrial chemical derived from ethylene, is extensively employed in the manufacturing of diverse compounds that are prevalent in industrial, medical, and consumer goods (4). Nevertheless, in 2016, Ethylene oxide is classified as a carcinogen for humans by both the International Agency for Research on Cancer (IARC) and the United States Environmental Protection Agency (USEPA), signifying that ethylene oxide is harmful to human health’ sake (43). EO sterilization, FDA, is responsible for sterilizing approximately half of all sterile medical devices within the U.S. Workers, especially those in the medical equipment sterilization sector, have been documented to face occupational exposure to EO at elevated levels (2, 44). EO sterilization has also gained great attention in the worldwide effort to combat the COVID-19 pandemic. The rise in requests for personal protective equipment (PPE), including items like face masks, gloves, and protective suits, might have led to a higher exposure to EO. It is noteworthy that single-use sterile medical devices have stringent standard regarding EO residue. Specifically, the allowable residual limit for EO limit content in face masks is established at <10 μg/g. These regulatory standards underscore the importance of conducting interventions limiting the exposure of EO in daily life. HbEO serve as a more stable and sensitive blood biomarker for assessing EO exposure. Prior research has established a direct link between human HbEO levels and exposure to airborne EO (45, 46). In this investigation, we utilized a widely recognized HPLC-MS/MS that integrates a modified Edelman reaction to concurrently measure HbEO level (47). Occupational exposure limits for EO are set by organizations such as the Occupational Safety and Health Administration (OSHA) in the United States. The current OSHA permissible exposure limit (PEL) for EO is 1 ppm (parts per million) as an 8-hour time-weighted average. OSHA regulations require employers to implement controls to reduce EO exposure below the PEL (48). The U.S. Food and Drug Administration (FDA) regulates the use of EO in the sterilization of medical devices and sets limits on the residual amounts of EO left on these devices (49). The variability in EO regulations across different countries and states can lead to inconsistencies in protection levels. There can be challenges in enforcing regulations due to the pervasive use of EO and the difficulty of monitoring and controlling emissions. Workers in industries that use EO, such as healthcare workers and those in the sterilization and chemical manufacturing industries, are at highest risk. Communities located near facilities that emit EO may be at increased risk of exposure. Ensuring compliance with exposure limits requires effective monitoring and enforcement, which can be resource-intensive. Public awareness about the risks of EO exposure is crucial for informed decision-making and for the public to advocate for stronger protections. As more is learned about the health effects of EO, exposure limits may need to be revised and additional controls implemented to protect public health. There may be a need for enhanced research into the health effects of EO and improved methods for monitoring and controlling its emissions.
Only a few animal experiments have explored the relationship between HbEO and sex steroid. Previous studies have found that chronic inhalation of EO leads to a dose-dependent decrease in testes weight and testes’ DNA content in adult male rats (50). Some studies have also found damages to reproductive cells and impaired sperm production in male rats after EO exposure (15, 51). In human studies, Takumi Kagawa found a positive correlation between HbEO and TT, TT/E2. Moderate EO exposure was associated with a decrease in E2 levels (52). Another study by Cao et al., which only explored the relationship between HbEO and TT, found that HbEO was positively correlated with TT, with the association being stronger in males than in females (53). In contrast, our study considered the distinct sex hormone levels across different sexes, age groups, and pubertal status, providing a more comprehensive exploration of the relationship between EO exposure and five sex hormones by sex-age and sex-puberty groups. Consistently, we observed that HbEO was significantly inversely associated with E2 and positively associated with TT/E2 and SHBG levels in adult males. Previous studies have pointed out that sperm production can be influenced by E2 (54, 55). Consequently, this analysis suggested EO exposure may affect sperm production in male adults consistent with previous animal experiments. For TT, our study did not find results consistent with the two aforementioned studies. This discrepancy is likely attributed to the fact that biologically available TT and E2 levels depend on SHBG concentrations. Therefore, in our regression models assessing the relationship between HbEO and TT or E2, we strictly controlled for SHBG. Our findings differ from previous studies, suggesting that the observed increase in TT levels among adult males exposed to HbEO may be mediated through SHBG. Further robust evidence is required to confirm these mechanisms. Additionally, our findings are consistent with results from animal studies, where plasma testosterone concentrations did not significantly change after EO exposure in rats (16). Notably, a prolonged estrus cycle and an increased percentage of the diestrus stage in the presence of EO exposure were observed in female mice (15, 56). Nonetheless, our research did not uncover any substantial correlation between EO exposure and sex hormones in female subjects. Previous papers have also shown different effects of other pollutants on male and female sex hormones (27, 57, 58).Such differences may occur due to the differences in hormonal regulatory networks exist significantly between males and females. In females, the high baseline levels of estrogen may help stabilize hormone dynamics by modulating the secretion of gonadotropic hormones such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby mitigating the disruptive effects of EO (59). Additionally, estrogens may exert protective effects by activating their receptors (Estrogen Receptor (ER) α and ERβ), preserving the integrity of the endocrine system and reducing the oxidative stress or genotoxicity induced by EO exposure. Furthermore, EO may undergo distinct metabolic pathways in males and females. For example, variations in enzymatic activity due to gender differences could result in faster detoxification of EO in female systems. Moreover, lifestyle and environmental factors may play a role; women are more likely to use hormonal medications, such as oral contraceptives, which might modulate hormone levels and overshadow the effects of EO exposure. The dynamic fluctuations in hormone levels during specific life stages, such as premenopause and postmenopause, might also contribute to the difficulty in detecting EO’s effects through statistical analysis. Due to the limited research on the biological mechanisms of EO’s impact on sex hormones in experimental animals, it is challenging to elucidate the mechanistic approach behind the observed differences in EO’s effects on sex hormones between male and female populations. Such complexities underscore the need for further research to comprehensively understand the biological mechanisms underlying EO’s influence on sex hormones in both genders.
The biological processes through which EO influences endocrine hormone levels remain unclear. Endocrine disruptors can indirectly affect serum sex hormone levels through various mechanisms, such as regulating luteinizing hormone and follicle-stimulating hormone signaling, liver excretion processes, and the biofeedback processes of related hormones (60–64). Air pollutants, may primarily act on cholesterol metabolism pathways and hepatic function, thereby influencing sex hormone synthesis (65, 66). Specifically, long-term exposure to EO induces oxidative stress in the body, leading to increased hepatic lipid peroxidation and decreased glutathione reductase activity, which in turn affects lipid metabolism (67). Abnormal lipid metabolism can interfere with sex hormone synthesis. Additionally, EO exposure can trigger inflammation (68, 69). When inflammation occurs, pro-inflammatory cytokines typically activate the hypothalamic-pituitary-adrenal (HPA) axis. This activation helps prevent excessive inflammatory reactions by leveraging the anti-inflammatory effects of glucocorticoids. In this process, the HPA axis may also influence reproductive, growth, and thyroid functions, ultimately altering sex hormone levels. Conversely, abnormal sex hormone levels can modify inflammatory and immune responses (70). Future studies should explore the biological mechanisms involved in the relationship between EO and sex hormones in greater depth.
This study possesses multiple strengths. To the best of our understanding, this investigation represents the earliest utilization of the NHANES dataset to examine the correlation between the degree of EO exposure and sex hormones across a demographically comprehensive sample of the American population by sex-age or sex-puberty status. The database was meticulously filtered through stringent protocols, ensuring standard quality control measures. Large-scale databases, such as NHANES, have been instrumental to researchers and humanity. With their access to diverse populations and comprehensive datasets, these databases facilitate the identification of additional risk factors and disease biomarkers (71–76). Additionally, the levels of TT and E2 were assayed using ID-LC-MS/MS, offering enhanced precision compared to the immunoassay technique (21). Nevertheless, our study is subject to several constraints. Firstly, the lack of precise puberty status data within our study population hinders the thorough adjustment for the effects of puberty’s influence on this finding. In addition, this study carried out analyses using a derived “puberty status” classification, determined by the steroid hormone levels and menarcheal status. These criteria are not reliable for distinguishing pubertal from prepubertal status, as age and steroid hormone levels are not the most dependable indicators of pubertal progression (77). Consequently, unmeasured confounding variables associated with the unquantified puberty status persist. Secondly, as with other epidemiological analysis, our findings may be influenced by unmeasured confounding variables, such as job status and exposure to other chemical and physical pollutants. Thirdly, the absence of data on gonadotropins and key enzymes in steroidogenesis prevented us from investigating the fundamental biological mechanisms involved. Fourthly, The NHANES is a cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC) to estimate the health and nutritional status of the non-institutionalized population in the US, it is does not represent the situation in other countries or regions. Additionally, the NHANES data based on self-reported information such as BMI from individuals, potentially leading to recall bias. In the meanwhile, the measurement error of the exposure may have led our results biased towards the null.
HbEO was significantly inversely associated with E2 and positively associated with TT/E2 and SHBG levels in adult males. Such associations between HbEO with E2 and SHBG were non-linear in male adults. However, no significant correlation was detected between HbEO and sex steroids in different age female groups. Given the cross-sectional study design and the restricted sample size, additional research is required to validate and replicate these results. Furthermore, an in-depth exploration of the specific mechanisms by which EO affects sex hormones in male adults is essential. In addition, it is crucial for relevant U.S. agencies to strengthen adherence to EO exposure limits and regulatory frameworks, and to further refine corresponding research and rule-making for populations with prolonged exposure to EO.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Study protocols for NHANES were approved by the NCHS ethnics review board (Protocol #2011–17, https://www.cdc.gov/nchs/nhanes/about/erb.html?CDC_AAref_Val=https://www.cdc.gov/nchs/nhanes/irba98.htm). All the participants signed the informed consent before participating in the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
WW: Conceptualization, Methodology, Writing – original draft. JW: Resources, Writing – review & editing. ZH: Investigation, Writing – review & editing. QY: Software, Writing – review & editing. KQ: Visualization, Writing – review & editing. YuZ: Methodology, Writing – review & editing. HZ: Software, Validation, Writing – review & editing. YiZ: Visualization, Writing – review & editing. JH: Data curation, Writing – review & editing. JL: Conceptualization, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No. 82270915), Special Fund for Science and Technology Innovation Teams of Shanxi Province (No. 202304051001021), Shanxi Funding for High Level Overseas Returns (2022), Shanxi Province Science and Technology Cooperation and Exchanges Project. (Grant Number: 202204041101024).
The authors thank the American Centers for Disease Control and Prevention for administering the survey and making it accessible online without charge, as well as all the participants for contributing their data. Special thanks to Jing Zhang (Shanghai Tongren Hospital) for his contributions to the NHANES database. His exceptional efforts, the nhanesR package, and his webpage have facilitated our exploration of the NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1533516/full#supplementary-material
1. Kirman CR, Li AA, Sheehan PJ, Bus JS, Lewis RC, Hays SM. Ethylene oxide review: characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J Toxicol Environ Health B Crit Rev. (2021) 24:1–29. doi: 10.1080/10937404.2020.1852988
2. Shintani H. Ethylene oxide gas sterilization of medical devices. Biocontrol Sci. (2017) 22:1–16. doi: 10.4265/bio.22.1
3. Ghosh M, Godderis L. Genotoxicity of ethylene oxide: A review of micronucleus assay results in human population. Mutat Res Rev Mutat Res. (2016) 770:84–91. doi: 10.1016/j.mrrev.2016.05.002
4. Mendes GC, Brandao TR, Silva CL. Ethylene oxide sterilization of medical devices: a review. Am J Infect Control. (2007) 35:574–81. doi: 10.1016/j.ajic.2006.10.014
5. United States Environmental Protection Agency (U.S. EPA). Evaluation of the inhalation carcinogenicity of ethylene oxide (Final report). Washington, DC: US Environmental Protection Agency (2016). Available at: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NCEA&count=10000&dirEntryId=329730&searchall=&showcriteria=2&simplesearch=0&timstype (Accessed December 16, 2023).
6. Jones RR, Fisher JA, Medgyesi DN, Buller ID, Liao LM, Gierach G, et al. Ethylene oxide emissions and incident breast cancer and non-Hodgkin lymphoma in a US cohort. J Natl Cancer Inst. (2023) 115:405–12. doi: 10.1093/jnci/djad004
7. Zeng G, Zhang Q, Wang X, Wu K-H. Association between blood ethylene oxide levels and the risk of cardiovascular diseases in the general population. Environ Sci pollut Res Int. (2021) 28:64921–8. doi: 10.1007/s11356-021-15572-0
8. Guo J, Wan Z, Cui G, Pan A, Liu G. Association of exposure to ethylene oxide with risk of diabetes mellitus: results from NHANES 2013-2016. Environ Sci pollut Res Int. (2021) 28:68551–9. doi: 10.1007/s11356-021-15444-7
9. Huang Q, Li S, Wan J, Nan W, He B. Association between ethylene oxide exposure and prevalence of COPD: Evidence from NHANES 2013-2016. Sci Total Environ. (2023) 885:163871. doi: 10.1016/j.scitotenv.2023.163871
10. Gresie-Brusin DF, Kielkowski D, Baker A, Channa K, Rees D. Occupational exposure to ethylene oxide during pregnancy and association with adverse reproductive outcomes. Int Arch Occup Environ Health. (2007) 80:559–65. doi: 10.1007/s00420-006-0163-y
11. United States Environmental Protection Agency. (U.S. EPA). Actions to protect workers and communities from ethylene oxide (EtO) risk (2023). Available online at: https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/actions-protect-workers-and-communities-ethylene-oxide-eto (Accessed April 13, 2023).
12. Wood A, Howarth M. How federal and state regulatory systems perpetuate environmental injustice in the United States: industrial ethylene oxide emissions as a case study. Environ Justice. (2023) 16:297–308. doi: 10.1089/env.2021.0120
13. O'Kelley L, Swanson B, Bishop-Royse JC. Integrative literature review: Ethylene oxide exposure signs and symptoms. Public Health Nurs. (2023) 40(5):790–809. doi: 10.1111/phn.13216
14. Wang C, Xu Y. Mechanisms for sex differences in energy homeostasis. J Mol Endocrinol. (2019) 62:R129–R43. doi: 10.1530/JME-18-0165
15. Mori K, Kaido M, Fujishiro K, Inoue N, Hori H. Effects of inhalation of ethylene oxide on female rats. J UOEH. (1989) 11:173–9. doi: 10.7888/juoeh.11.173
16. Mori K, Kaido M, Fujishiro K, Inoue N. Testicular toxicity and alterations of glutathione metabolism resulting from chronic inhalation of ethylene oxide in rats. Toxicol Appl Pharmacol. (1989) 101:299–309. doi: 10.1016/0041-008X(89)90278-0
17. CDC National Center for Health Statistics. National health and nutrition examination survey: analytic guidelines, 2011–2014 and 2015–2016. Natl Cent Heal Stat (2018) p. 1–40.
18. You Y, Li J, Zhang Y, Li X, Li X, Ma X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: Insights from population-based and mice studies. CNS Neurosci Ther. (2024) 30:e14783. doi: 10.1111/cns.14783
19. You Y, Chen Y, Liu R, Zhang Y, Wang M, Yang Z, et al. Inverted U-shaped relationship between sleep duration and phenotypic age in US adults: a population-based study. Sci Rep. (2024) 14:6247. doi: 10.1038/s41598-024-56316-7
20. NCHS. Laboratory procedure manual for serum sex hormone binding globulin. National Center for Environmental Health. (2018) Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Sex_Hormone_Binding_Globulin.pdf (Accessed December 10, 2023).
21. NCHS. Laboratory procedure manual for serum total estradiol and total testosterone. National Center for Environmental Health. (2018). Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Total_Estradiol_and_Total_Testosterone.pdf (Accessed December 10, 2023).
22. Wilke TJ, Utley DJ. Total testosterone, free-androgen index, calculated free testosterone, and free testosterone by analog RIA compared in hirsute women and in otherwise-normal women with altered binding of sex-hormone-binding globulin. Clin Chem. (1987) 33:1372–5. doi: 10.1093/clinchem/33.8.1372
23. Perel E, Wilkins D, Killinger DW. The conversion of androstenedione to estrone, estradiol, and testosterone in breast tissue. J Steroid Biochem. (1980) 13:89–94. doi: 10.1016/0022-4731(80)90117-X
24. You Y, Mo L, Tong J, Chen X, You Y. The role of education attainment on 24-hour movement behavior in emerging adults: evidence from a population-based study. Front Public Health. (2024) 12:1197150. doi: 10.3389/fpubh.2024.1197150
25. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. (2000) 320:1240–3. doi: 10.1136/bmj.320.7244.1240
26. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital and Health Statistics Series 2. Data Eval Methods Res. (2013) 161:1–5.
27. Bai R, Huang Y, Wang F, Guo J. Associations of fluoride exposure with sex steroid hormones among U.S. children and adolescents, NHANES 2013-2016. Environ pollut. (2020) 260:114003. doi: 10.1016/j.envpol.2020.114003
28. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. (2000) 32:S498–504. doi: 10.1097/00005768-200009001-00009
29. Wu W, Zhang Z, Qi Y, Zhang H, Zhao Y, Li J. Association between dietary inflammation index and hypertension in participants with different degrees of liver steatosis. Ann Med. (2023) 55:2195203. doi: 10.1080/07853890.2023.2195203
30. Lambertino A, Persky V, Freels S, Anderson H, Unterman T, Awadalla S, et al. Associations of PCBS, dioxins and furans with follicle-stimulating hormone and luteinizing hormone in postmenopausal women: National Health and Nutrition Examination Survey 1999-2002. Chemosphere. (2021) 262:128309. doi: 10.1016/j.chemosphere.2020.128309
31. Zhu X, Kong X, Chen M, Shi S, Cheang I, Zhu Q, et al. Blood ethylene oxide, systemic inflammation, and serum lipid profiles: Results from NHANES 2013-2016. Chemosphere. (2022) 299:134336. doi: 10.1016/j.chemosphere.2022.134336
32. You Y. Accelerometer-measured physical activity and sedentary behavior are associated with C-reactive protein in US adults who get insufficient sleep: A threshold and isotemporal substitution effect analysis. J Sports Sci. (2024) 42:527–36. doi: 10.1080/02640414.2024.2348906
33. You Y, Ablitip A, Chen Y, Ding H, Chen K, Cui Y, et al. Saturation effects of the relationship between physical exercise and systemic immune inflammation index in the short-sleep population: a cross-sectional study. BMC Public Health. (2024) 24:1920. doi: 10.1186/s12889-024-19432-7
34. Li W, Wu H, Xu X, Zhang Y. Environmental exposure to perchlorate, nitrate, and thiocyanate in relation to chronic kidney disease in the general US population, NHANES 2005-2016. Chin Med J (Engl). (2023) 136:1573–82. doi: 10.1097/CM9.0000000000002586
35. Luo K, Liu J, Wang Y, Aimuzi R, Luo F, Ao J, et al. Associations between organophosphate esters and sex hormones among 6-19-year old children and adolescents in NHANES 2013-2014. Environ Int. (2020) 136:105461. doi: 10.1016/j.envint.2020.105461
36. Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. (1997) 82:2849–55. doi: 10.1210/jc.82.9.2849
37. Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, et al. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol. (2011) 45:8160–6. doi: 10.1021/es1038694
38. Rapkin AJ, Tsao JC, Turk N, Anderson M, Zeltzer LK. Relationships among self-rated tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol. (2006) 19:181–7. doi: 10.1016/j.jpag.2006.02.004
39. Qi SF, Cao YJ, Wang HJ, Zhang B, Yan J, Tian QB. Associations of carbohydrate intake with new-onset hypertension subtypes: results from the China health and nutrition survey (2000-2011). Front Nutr. (2021) 8:728774. doi: 10.3389/fnut.2021.728774
40. Shakya PR, Melaku YA, Page A, Gill TK. Association between dietary patterns and adult depression symptoms based on principal component analysis, reduced-rank regression and partial least-squares. Clin Nutr. (2020) 39:2811–23. doi: 10.1016/j.clnu.2019.12.011
41. Thier R, Bolt HM. Carcinogenicity and genotoxicity of ethylene oxide: new aspects and recent advances. Crit Rev Toxicol. (2000) 30:595–608. doi: 10.1080/10408440008951121
42. Jain RB. Associations between observed concentrations of ethylene oxide in whole blood and smoking, exposure to environmental tobacco smoke, and cancers including breast cancer: data for US children, adolescents, and adults. Environ Sci pollut Res Int. (2020) 27:20912–9. doi: 10.1007/s11356-020-08564-z
43. Vincent MJ, Kozal JS, Thompson WJ, Maier A, Dotson GS, Best EA, et al. Ethylene oxide: cancer evidence integration and dose-response implications. Dose Response. (2019) 17:1559325819888317. doi: 10.1177/1559325819888317
44. Tates AD, Grummt T, Tornqvist M, Farmer PB, van Dam FJ, van Mossel H, et al. Biological and chemical monitoring of occupational exposure to ethylene oxide. Mutat Res. (1991) 250:483–97. doi: 10.1016/0027-5107(91)90205-3
45. Boogaard PJ, Rocchi PS, van Sittert NJ. Biomonitoring of exposure to ethylene oxide and propylene oxide by determination of hemoglobin adducts: correlations between airborne exposure and adduct levels. Int Arch Occup Environ Health. (1999) 72:142–50. doi: 10.1007/s004200050353
46. Kirman CR, Hays SM. Derivation of endogenous equivalent values to support risk assessment and risk management decisions for an endogenous carcinogen: Ethylene oxide. Regul Toxicol Pharmacol. (2017) 91:165–72. doi: 10.1016/j.yrtph.2017.10.032
47. Yang M, Frame T, Tse C, Vesper HW. High-throughput, simultaneous quantitation of hemoglobin adducts of acrylamide, glycidamide, and ethylene oxide using UHPLC-MS/MS. J Chromatogr B Analyt Technol BioMed Life Sci. (2018) 1086:197–205. doi: 10.1016/j.jchromb.2018.03.048
48. U.S. Occupational Safety and Health Administration (OSHA). Ethylene oxide. Safety and health topics (2021). Available online at: https://www.osha.gov/SLTC/ethyleneoxide (Accessed November 12, 2023).
49. U.S. Food and Drug Administration (FDA). Guideline for the sterilization of medical devices containing natural rubber latex (2018). Available online at: https://www.fda.gov/media/119358/download (Accessed December 19, 2023).
50. Mori K, Inoue N, Fujishiro K, Hori H, Tanaka I. Effects of chronic inhalation of ethylene oxide on the rat testes. J UOEH. (1988) 10:427–31. doi: 10.7888/juoeh.10.427
51. Mori K, Kaido M. Evaluation of testicular damage by flow cytometry: testicular atrophy caused by ethylene oxide. J UOEH. (1989) 11:37–42. doi: 10.7888/juoeh.11.37
52. Kagawa T. Association between ethylene oxide exposure and sex steroid hormone levels in male adults in the United States. Hum Ecol Risk Assessment: Int J. (2024) 30:164–79. doi: 10.1080/10807039.2024.2303471
53. Cao S, Meng L, Bai H, Yang W, Hu X, Li X. The association between ethylene oxide and testosterone in the United States population: a cross-sectional study based on the National Health and Nutrition Examination Survey (NHANES) 2013-2016. Endocrine. (2024) 86:850–9. doi: 10.1007/s12020-024-03979-x
54. Allan CM, Couse JF, Simanainen U, Spaliviero J, Jimenez M, Rodriguez K, et al. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-alpha mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology. (2010) 151:2800–10 doi: 10.1210/en.2009-1477
55. Ebling FJ, Brooks AN, Cronin AS, Ford H, Kerr JB. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology. (2000) 141:2861–9. doi: 10.1210/endo.141.8.7596
56. Mori K, Kaido M, Fujishiro K, Inoue N, Koide O, Hori H, et al. Dose dependent effects of inhaled ethylene oxide on spermatogenesis in rats. Br J Ind Med. (1991) 48:270–4. doi: 10.1136/oem.48.4.270
57. Zhu YD, Han X, Wang XQ, Ge TX, Liu H, Fan L, et al. Effect of the phthalates exposure on sex steroid hormones in the US population. Ecotoxicol Environ Saf. (2022) 231:113203. doi: 10.1016/j.ecoenv.2022.113203
58. Hu G, Long C, Hu L, Zhang Y, Hong S, Zhang Q, et al. Blood chromium exposure, immune inflammation and genetic damage: Exploring associations and mediation effects in chromate exposed population. J Hazard Mater. (2022) 425:127769. doi: 10.1016/j.jhazmat.2021.127769
59. Genazzani AR, Lucchesi A, Stomati M, Catarsi S, Genazzani AD, Criscuolo M, et al. Effects of sex steroid hormones on the neuroendocrine system. Eur J Contracept Reprod Health Care. (1997) 2:63–9. doi: 10.1080/13625189709049935
60. Shobana N, Kumar MK, Navin AK, Akbarsha MA, Aruldhas MM. Prenatal exposure to excess chromium attenuates transcription factors regulating expression of androgen and follicle stimulating hormone receptors in Sertoli cells of prepuberal rats. Chem Biol Interact. (2020) 328:109188. doi: 10.1016/j.cbi.2020.109188
61. Wirbisky SE, Freeman JL. Atrazine exposure and reproductive dysfunction through the hypothalamus-pituitary-gonadal (HPG) axis. Toxics. (2015) 3:414–50. doi: 10.3390/toxics3040414
62. Schiffer L, Barnard L, Baranowski ES, Gilligan LC, Taylor AE, Arlt W, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J Steroid Biochem Mol Biol. (2019) 194:105439. doi: 10.1016/j.jsbmb.2019.105439
63. Tian M, Wu S, Wang YX, Liu L, Zhang J, Shen H, et al. Associations of environmental phthalate exposure with male steroid hormone synthesis and metabolism: An integrated epidemiology and toxicology study. J Hazard Mater. (2022) 436:129213. doi: 10.1016/j.jhazmat.2022.129213
64. Wierman ME. Sex steroid effects at target tissues: mechanisms of action. Adv Physiol Educ. (2007) 31:26–33. doi: 10.1152/advan.00086.2006
65. Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. (2010) 365:1557–69. doi: 10.1098/rstb.2009.0258
66. Ho SM, Cheong A, Adgent MA, Veevers J, Suen AA, Tam NNC, et al. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol. (2017) 68:85–104. doi: 10.1016/j.reprotox.2016.07.011
67. Katoh T, Higashi K, Inoue N, Tanaka I. Effects of chronic inhalation of ethylene oxide on lipid peroxidation and glutathione redox cycle in rat liver. Res Commun Chem Pathol Pharmacol. (1988) 61:281–4.
68. Rusyn I, Asakura S, Li Y, Kosyk O, Koc H, Nakamura J, et al. Effects of ethylene oxide and ethylene inhalation on DNA adducts, apurinic/apyrimidinic sites and expression of base excision DNA repair genes in rat brain, spleen, and liver. DNA Repair (Amst). (2005) 4:1099–110. doi: 10.1016/j.dnarep.2005.05.009
69. Lynch DW, Lewis TR, Moorman WJ, Burg JR, Groth DH, Khan A, et al. Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol Appl Pharmacol. (1984) 76:69–84. doi: 10.1016/0041-008X(84)90030-9
70. Wei C, Zhang W, Chen J, He Q, Cao L, Zhang P, et al. Systematic analysis between inflammation-related index and sex hormones in American adults: cross-sectional research based NHANES 2013-2016. Front Immunol. (2023) 14:1175764. doi: 10.3389/fimmu.2023.1175764
71. Wang Y, Wang J, Liu J, Zhu H. Immune-related diagnostic markers for benign prostatic hyperplasia and their potential as drug targets. Front Immunol. (2024) 15:1516362. doi: 10.3389/fimmu.2024.1516362
72. Wang JF, Wang JS, Liu Y, Ji B, Ding BC, Wang YX, et al. Knockdown of integrin beta1 inhibits proliferation and promotes apoptosis in bladder cancer cells. Biofactors. (2025) 51:e2150. doi: 10.1002/biof.2150
73. Wang Y, Zhu H, Wang X. Prognosis and immune infiltration analysis of endoplasmic reticulum stress-related genes in bladder urothelial carcinoma. Front Genet. (2022) 13:965100. doi: 10.3389/fgene.2022.965100
74. Wang Y, Zhu H, Xu H, Qiu Y, Zhu Y, Wang X. Senescence-related gene c-Myc affects bladder cancer cell senescence by interacting with HSP90B1 to regulate cisplatin sensitivity. Aging (Albany NY). (2023) 15:7408–23. doi: 10.18632/aging.204863
75. Wang Y, Wang J, He J, Ji B, Pang Z, Wang J, et al. Comprehensive analysis of PRPF19 immune infiltrates, DNA methylation, senescence-associated secretory phenotype and ceRNA network in bladder cancer. Front Immunol. (2023) 14:1289198. doi: 10.3389/fimmu.2023.1289198
76. Wang Y, Li C, He J, Zhao Q, Zhou Y, Sun H, et al. Multi-omics analysis and experimental validation of the value of monocyte-associated features in prostate cancer prognosis and immunotherapy. Front Immunol. (2024) 15:1426474. doi: 10.3389/fimmu.2024.1426474
Keywords: ethylene oxide, endocrine disruptor, sex hormones, total testosterone, estradiol
Citation: Wu W, Wu J, Hou Z, Yan Q, Qin K, Zhao Y, Zhang H, Zhu Y, He J and Li J (2025) Association between ethylene oxide exposure and serum sex hormone levels measured in a reference sample of the US general population. Front. Endocrinol. 16:1533516. doi: 10.3389/fendo.2025.1533516
Received: 24 November 2024; Accepted: 24 March 2025;
Published: 11 April 2025.
Edited by:
Christopher Gerner, University of Vienna, AustriaReviewed by:
Chen Yingjun, Southern Medical University, ChinaCopyright © 2025 Wu, Wu, Hou, Yan, Qin, Zhao, Zhang, Zhu, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Li, amlubGk4MDdAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.