
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 27 February 2025
Sec. Adrenal Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1533295
This article is part of the Research TopicEnhancing Adrenal Tumor Diagnostics: Biomarkers and Molecular MechanismsView all 11 articles
A correction has been applied to this article in:
Corrigendum:
 Yushi Peng1
Yushi Peng1 Fangansheng Chen1
Fangansheng Chen1 Rui Yao1
Rui Yao1 Junping Lan2
Junping Lan2 Yinuo Fu3
Yinuo Fu3 Kaifeng Ye3
Kaifeng Ye3 Zhiqiang Wang1
Zhiqiang Wang1 Qianxiu Zhao4
Qianxiu Zhao4 Xiaowei Ji1
Xiaowei Ji1 Kang Xia4
Kang Xia4 Guoqing Zhu5
Guoqing Zhu5 Kewen Zheng6
Kewen Zheng6 Xuemei Gu4*†
Xuemei Gu4*† Kun Tang1,7,8*†
Kun Tang1,7,8*†Objective: The study aimed to investigate the diagnostic value of 18F-AlF-NOTA-Pentixafor PET/CT in subtyping primary aldosteronism (PA).
Methods: This study enrolled 88 patients with PA or nonfunctional adenoma (NFA) for 18F-Pentixafor PET/CT scan. Of these, 20 patients underwent adrenal venous sampling (AVS), and 65 underwent adrenalectomy and postoperative follow-up.
Results: In 88 patients, 76 were diagnosed with unilateral PA (UPA), 4 were diagnosed with bilateral PA (BPA), and 8 were diagnosed with NFA, resulting in a total of 95 lesions. To identify UPA, visual analysis received a specificity of 94.12% and a sensitivity of 89.74%. The optimal cutoff values for SUVmax at 5.45, the lesion-to-normal adrenal ratio (LAR) at 1.43, and lesion-to-liver ratio (LLR) all yielded a specificity of 100% and a sensitivity of 79.49%, 83.33%, and 80.77%, respectively. In 15 adrenal lesions with similar uptake to contralateral and adjacent normal adrenal tissue (defined as warm lesions), 7 were confirmed as UPA, 4 were confirmed as BPA, and 4 were confirmed as NFA. Furthermore, among the 20 patients who underwent AVS, the concordance rate of AVS and PET/CT visual analysis for PA subtyping was 65.00%.
Conclusions: The CXCR4-targeted 18F-AlF-NOTA-pentixafor PET/CT is a valuable noninvasive tool for diagnosing UPA, demonstrating high sensitivity and specificity. More attention should be paid to warm adrenal lesions for their high diagnostic ambiguity probability.
Primary aldosteronism (PA), resulting from excessive aldosterone secretion by the adrenal cortex, is a clinical syndrome characterized by hypertension with or without hypokalemia (1). Studies have demonstrated that PA is a common syndrome that may be a primary contributor to hypertension pathogenesis, and the prevalence of PA reaches over 20% in individuals with resistant hypertension (2, 3). Compared to primary hypertension, PA is more likely to lead to serious complications, such as aortic coarctation, severe arrhythmias, stroke, and renal failure (4). Currently, the most effective treatment for PA is unilateral adrenalectomy, and patients who undergo this procedure have considerably greater biochemical and clinical remission rates than those who receive medications, with both immediate and long-term benefits (5). PA cases can be subtyped as unilateral primary aldosteronism (UPA) and bilateral primary aldosteronism (BPA). UPA includes cases of unilateral autonomous excessive aldosterone secretion without contralateral secretion, as well as cases of bilateral autonomous asymmetric excessive aldosterone secretion whose dominant side has a clear advantage in secretory activity compared to the contralateral gland. BPA refers to cases of bilateral autonomous symmetrical excessive aldosterone secretion (6–8). The first-line treatment for UPA is surgery, and medication therapy is the recommended treatment for BPA. Thus, PA subtyping is essential for the optimal treatment management of PA patients.
At the present stage, the subtyping diagnosis of PA is mainly based on adrenal imaging and adrenal venous cannulation (AVS) to determine the location and functionality of the lesion (9). Computed tomography (CT) is the first choice for adrenal imaging. However, small adenomas or nodules with a diameter of less than 1 cm are often missed. It is hard to determine functional laterality of PA and differentiate between functional and non-functional adrenal nodule, with an overall accuracy of 60% to 70% in the diagnosis of PA subtyping (10). AVS is considered to be the gold standard for PA subtyping, which can clarify the existence of unilateral dominant secretion. However, since AVS is invasive, costly, and technically challenging, and carries risks of intubation failure and postoperative complications, it is difficult to conduct this procedure on a large scale in hospitals of all levels.
Radionuclide functional imaging is considered a promising novel noninvasive method for PA subtyping and has been investigated in prior research. As the most extensively utilized radiotracer for functional imaging, 18F-FDG tracks glucose uptake and metabolism, predominantly in tumor imaging, but exhibits no significant uptake in aldosterone-secreting adenomas (11, 12). Metomidate is an inhibitor of aldosterone synthase (CYP11B2). Research suggests that 11C-metomidate (11C-MET) PET/CT imaging exhibits promising diagnostic efficacy for aldosterone-producing adenomas (APA), with sensitivities ranging from 55% to 76% and specificities from 44% to 87% (13–15). Despite these findings, the clinical application of 11C-MET and its fluorine-18 analogue is constrained by the requirement for pre-treatment with dexamethasone. This underscores the critical need for alternative diagnostic methodologies to enhance PA subtyping.
C-X-C chemokine receptor 4 (CXCR4), a typical G protein-coupled receptor that stimulates cell migration and activation upon activation, is highly expressed on APA cell membranes and is significantly correlated with aldosterone synthase (CYP11B2) expression (16, 17). Numerous recent studies have demonstrated the clinical value of CXCR4-targeted 68Ga-Pentixafor PET/CT in the subtyping diagnosis of PA. Nevertheless, the inherent limitations of 68Ga, such as its short half-life and the low scalability of 68Ge/68Ga generators, have hindered its clinical application. These limitations can be addressed through the usage of 18F-labeled alternatives (18). Currently, there are few reports, both domestically and internationally, on the synthesis of CXCR4-targeted 18F-Pentixafor PET/CT. Moreover, to the best of our knowledge, no research has been published on the application of 18F-Pentixafor for diagnosing PA.
Therefore, the aim of this study was to develop a robust synthesis method for the novel molecular probe 18F-AlF-NOTA-Pentixafor, evaluate its biodistribution, and, most importantly, investigate its clinical application potential.
The 18F- isotope was produced by proton irradiation of an 18O-H2O target in the cyclotron and passed through a QMA column to capture the 18F-. The 18F- was then eluted with 0.5 mL of saline into the reaction tube, where a mixed solution containing 10 μL of aqueous AlCl3 (1 μg/mL), 200 μL of pH=4 acetate buffer, and 0.6 mL of precursor solution (200 μg in 0.6 mL acetonitrile) was added sequentially. The reaction mixture was heated to 105 ℃ for 15 min, and upon cooling to room temperature, it was transferred to an HLB column for purification. The mixture was rinsed with 20 mL of water for injection, eluted with 2 mL of 50% ethanol, and the final solution was filtered through a 0.22 μm sterile filter membrane. The final product was a colorless, transparent liquid with an ethanol content not exceeding 10% and a pH ranging from 5 to 8. The synthetic chemical formula is provided in Figure 1.
Patients diagnosed with PA or NFA, aged 18-80 years, at the First Affiliated Hospital of Wenzhou Medical University between May 25th, 2023, and April 30th, 2024 were recruited for the study (Figure 2). All participants provided written informed consent, and the study protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Approval Number: KY2024-R245).
The diagnosis of PA was based on the clinical guidelines from the subcommittee of the Endocrine Society (1). All patients underwent PA screening using the plasma aldosterone–renin ratio (ARR) and received adrenal CT scans (slice thickness: 1.25 mm). Patients who tested positive in the screening (ARR > 30 pg·mL−1/pg·mL−1)) proceeded to confirmatory testing with a captopril challenge test (CCT) and seated saline infusion test (SSIT). All included patients underwent a 1-mg overnight dexamethasone suppression test (ODST) to exclude cortisol co-secretion in PA patients or mild autonomous cortisol secretion in NFA subjects. Patients who had unilateral or bilateral adrenal nodule(s) with clear borders identified on CT but presenting negative CCT and SSIT results or negative screening test results, along with normal serum potassium levels, were included as NFA. P The PA group was further subtyped into UPA and BPA. PA meeting any of the following criteria were classified as UPA: 1) unilateral lesion identified in AVS; 2) adrenal cortical adenoma confirmed by postoperative pathology, and complete or partial biochemical and clinical remission after surgery based on the Primary Aldosteronism Surgery Outcome criteria (19) (follow-up time: 1-6 months). PA meeting any of the following criteria were classified as BPA: 1) bilateral lesions identified in AVS; 2) adrenal cortical hyperplasia confirmed by postoperative pathology, and partial or no biochemical and clinical remission after surgery based on the Primary Aldosteronism Surgery Outcome criteria (follow-up time: 1-6 months) (20). Subtype diagnosis was determined based on postoperative pathological and follow-up results when two criteria were contradictory. All enrolled patients underwent 18F-AlF-NOTA-Pentixafor PET/CT imaging.
Patients maintained a normal diet without any special preparation. 18F-AlF-NOTA-Pentixafor was administered intravenously at a dose of 0.1 mCi/kg. Approximately 60 minutes after the 18F-AlF-NOTA-Pentixafor injection, a local PET/CT scan of the upper abdomen was performed. Twenty-one patients diagnosed with or suspected of PA, who underwent 18F-AlF-NOTA-Pentixafor PET/CT scan between July 27th and Oct. 19th in 2023 at the first affiliated hospital of Wenzhou Medical University got additional PET/CT scan at 20 minutes post-injection (p.i.). Studies were performed on two dedicated PET/CT scanners (Gemini TF 64, Philips Medical Systems, Netherlands, and uMI Panorama 35S, equipped with third-generation TOF 3D acquisition technology) with the following parameters: 120 kV, 80 mA, pitch of 0.829, tube rotation time of 0.5 seconds per rotation, and reconstruction thickness and interval of 5.0 mm.
Two nuclear medicine physicians certified by the committee, who were blinded to the patient’s clinical information, evaluated the PET/CT data. Any inconsistencies in their evaluation were resolved through negotiation, and the final decision was reached through consensus. Lesions were considered positive (also referred to as hot lesions) on PET/CT based on visual analysis if the adrenal nodule(s) showed higher uptake than the normal ipsilateral and contralateral adrenal glands. Adrenal lesions with similar uptake to the contralateral and adjacent normal adrenal tissue on visual assessment were classified as warm lesions. Adrenal lesions with lower uptake than the contralateral and adjacent normal adrenal tissue on visual assessment were classified as cold lesions. Warm and cold lesions were collectively referred to as negative lesions. The main lesion was identified as the adrenal nodule with the highest 18F-AlF-NOTA-Pentixafor uptake and clear borders in patients with multifocal nodules. Lesions identified on CT, or those with no abnormalities on CT but suspected of increased tracer uptake on PET, were designated as regions of interest, and the maximal standardized uptake value (SUVmax) was measured in these regions. The liver SUVmax was defined as the average SUVmax of five round spheres, each with a diameter of 2 cm, selected from the liver. Specific uptake value ratios, such as the lesion-to-liver ratio (LLR) and the lesion-to-normal adrenal ratio (LAR), were calculated. Additionally, in 18 patients who underwent AVS, SUVmax values within each adrenal gland were recorded. The side with the higher SUVmax in both adrenal glands was considered the dominant side. The lateralization index (LI) based on SUVmax was calculated as (SUVmax of dominant side)/(SUVmax of non-dominant side).
Patients diagnosed with PA underwent AVS within 3 months of completing 18F-pentixafor PET/CT to determine the lateralization of aldosterone hypersecretion. The decision on whether to perform AVS was made by endocrinologists with over 20 years of experience based on the clinical indicators, PET/CT results, and the patient’s inclination. AVS without adrenocorticotropic hormone stimulation was performed in the morning between 8:00 AM and 12:00 PM. Successful catheterization is considered if the adrenal cortical cortisol/peripheral venous cortisol ratio (ie, selectivity index) is 2 or greater.
A diagnosis of UPA was made if LI based on AVS was 4 or greater or 2 to 4 in combination with contralateral suppression or CT showing a typical adenoma on the dominant side, while those with LI based on AVS of less than 2 or 2 to 4 without meeting the previously described criteria were diagnosed as BPA (21, 22).
The pathological results of all surgical patients were independently assessed by a pathologist who was blinded to the clinical and imaging findings.
At least one follow-up was conducted by endocrinologists within 1 to 6 months after surgery (routine follow-up was scheduled at 1, 3, and 6 months post-surgery). For patients with PA, the Postoperative Aldosterone Outcome Score (PASO) system was used to evaluate their outcomes, categorizing patients into three groups: (1) Cure: blood pressure is normal without the use of antihypertensive medication; normal serum potassium and ARR, or ARR remains elevated but aldosterone levels can be suppressed in confirmatory testing; (2) Improvement: blood pressure has decreased to normal or has remained relatively unchanged with a reduction in antihypertensive medication use; normal serum potassium levels, elevated ARR, but plasma aldosterone levels have decreased by 50% compared to pre-surgery levels, or confirmatory test results have improved compared to baseline; (3) No improvement: antihypertensive medication dosage remains the same or has increased, and blood pressure has not improved or has worsened; low serum potassium and/or elevated ARR and/or aldosterone levels are not suppressed in confirmatory testing (19).
IBM SPSS Statistics 25.0 was used for statistical analysis. The Kolmogorov-Smirnov (K-S) test was employed to assess normality initially. Normally distributed data were reported as mean ± SD (χ ± S), and non-normally distributed data were reported as median (P25, P75). Nonparametric tests were used for between-group comparisons. Categorical data were presented as frequencies (percentages), and between-group comparisons were conducted using χ² or Fisher’s exact test when appropriate. Correlation analysis was performed using Pearson or Spearman correlation analysis. The diagnostic value of SUVmax, LLR, LAR, and LI of 18F-AlF-NOTA-pentixafor PET/CT was evaluated using receiver operating characteristic (ROC) curve analysis. Data visualization was conducted using GraphPad Prism 9.3 software and Origin 2022. This study employed a 2-tailed test, and a P value less than 0.05 was considered statistically significant for all tests.
18F-AlF-NOTA-pentixafor was synthesized with radiochemical yields of (37 ± 8.5) %. Radiochemical purities were > 99.5% as confirmed by radio-TLC.
The biodistribution of 18F-AlF-NOTA-pentixafor in 21 patients diagnosed with or suspected of PA at 20 minutes and 60 minutes post-injection (p.i.) is shown in Figure 3. Among these patients, six patients were later diagnosed with UPA, and one patient was diagnosed with BPA. All the organs in the upper abdomen (including adrenal gland lesions) exhibited higher mean SUVmean at 20 minutes p.i. than it at 60 minutes p.i., except for gallbladder. Specifically, the spleen exhibited the highest uptake at 20 minutes p.i., while the gallbladder exhibited the highest uptake at 60 minutes p.i. The adrenal gland lesion exhibited the highest SUVmean in the upper abdomen. In 6 UPA patients, LAR and LLR at 60 minutes p.i. (2.57[95%CI, 0.71-4.43] and 5.00[95%CI, 1.96-8.04]) was higher than LAR and LLR at 20 minutes p.i. (2.28[95%CI, 0.80-3.77] and 4.28[95%CI, 1.64-6.92]) but with no statistical differences (P=0.766 and 0.657, respectively) (Figure 4).
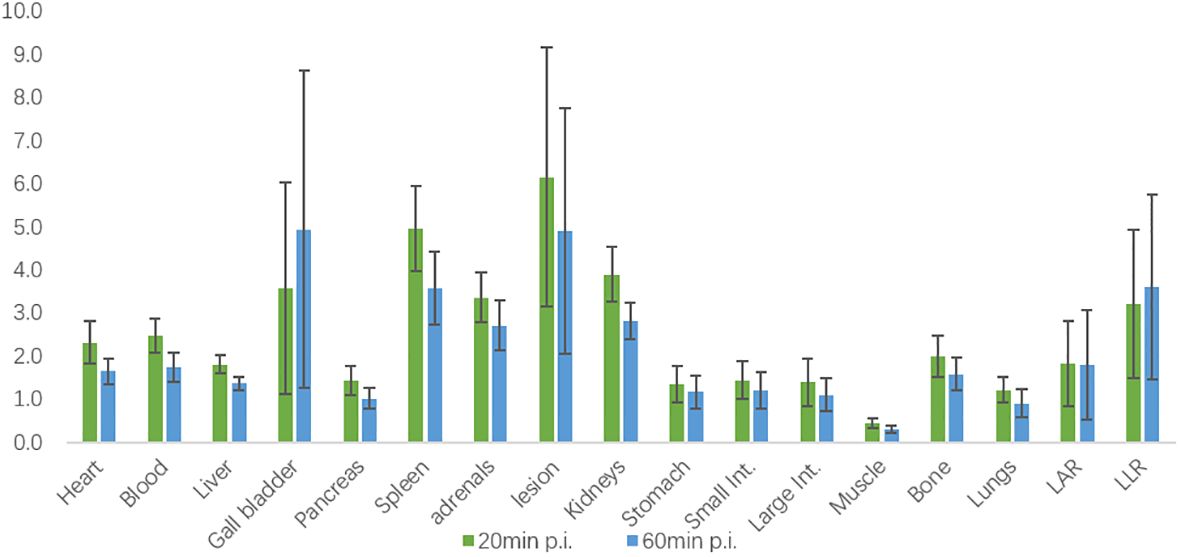
Figure 3. Biodistribution of 18F-AlF-NOTA-pentixafor in 21 patients diagnosed or suspected of PA at 20 minutes and 60 minutes post-injection (p.i.) measured by SUVmean.

Figure 4. Biodistribution of 18F-AlF-NOTA-pentixafor in 6 UPA patients at 20 minutes and 60 minutes post-injection (p.i.) measured by SUVmean.
Overall, there were 45 men and 43 women in our cohort (mean age, 53.52 ± 10.28 years). Among the 88 included patients, 20 underwent AVS and 65 underwent adrenalectomy. Based on clinical evaluation, pathology, and follow-up data, 76 cases were diagnosed as UPA, 4 cases as BPA, and 8 cases as NFA (UPA lesions were classified as surgically eligible lesions, BPA and NFA lesions were classified as surgically ineligible lesions). No significant demographic differences (including age, gender, and BMI) were observed among the three groups. Compared with the UPA group, the BPA group exhibited statistically lower SUVmax, while the NFA group demonstrated statistically higher serum potassium and plasma renin concentration (PRC), and lower plasma aldosterone concentration (PAC), ARR, and SUVmax (Table 1, Figure 5). No significant statistical differences were observed in semi-quantitative parameters, including SUVmax, LAR, and LLR, between the BPA group and the NFA group (as shown in Table 1).
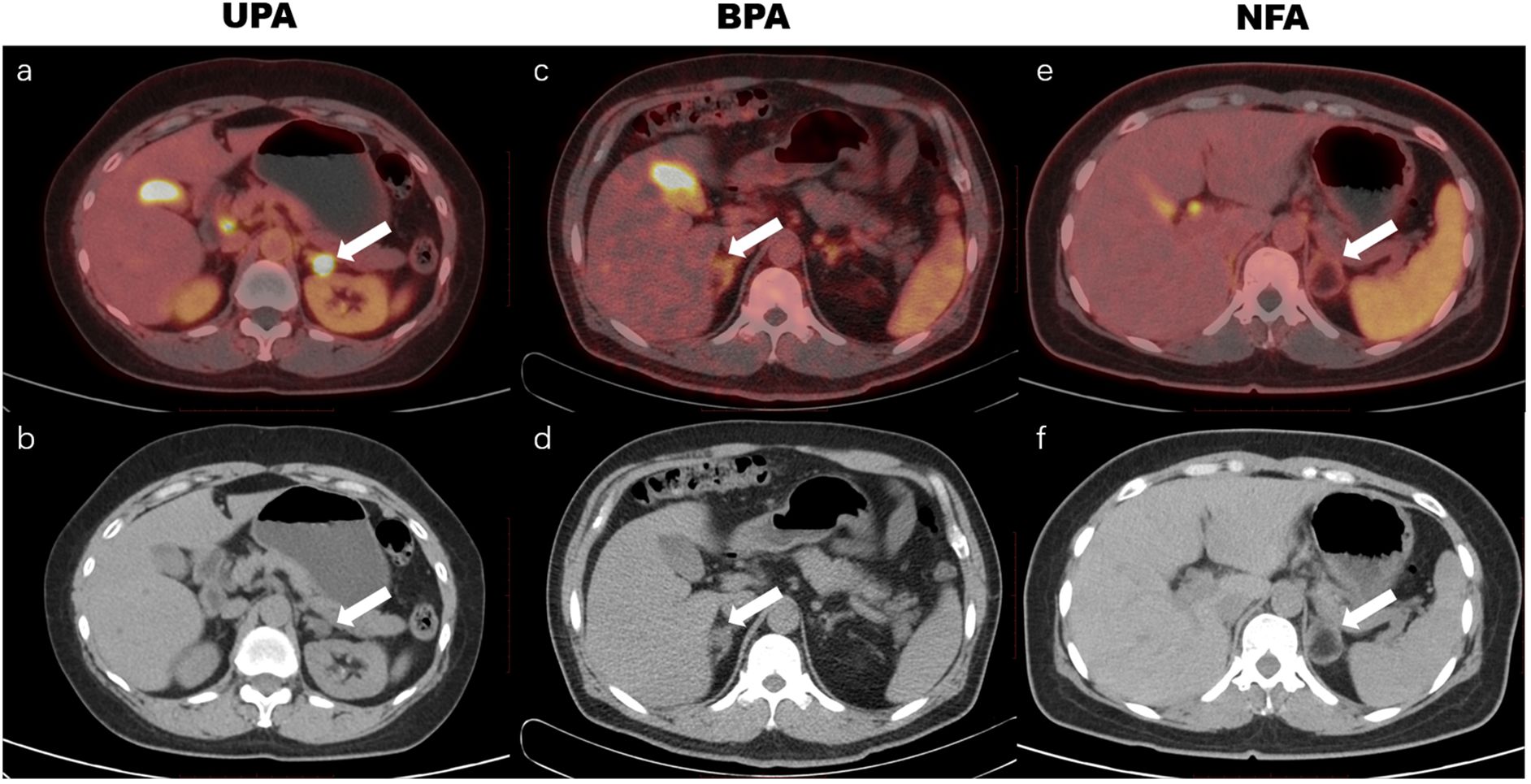
Figure 5. 18F-AlF-NOTA-pentixafor PET/CT imaging of patients with unilateral primary aldosteronism (UPA), bilateral primary aldosteronism (BPA), and nonfunctional adenoma (NFA). The UPA patient was a 61-year-old woman with hypertension for 10 years and hypokalemia for 2 years, with maximum blood pressure of 163/105 mm Hg, and minimum blood potassium of 2.69 mmol/L. A positive uptake (SUVmax of 31.5, white arrow in a, b) was observed in the left adrenal nodule, and postoperative pathology revealed a left adrenal cortical adenoma. The BPA patient was a 74-year-old man with hypertension for 13 years and hypokalemia, maximum blood pressure of 160/99 mm Hg, and minimum blood potassium of 3.05 mmol/L. 18F-AlF-NOTA-pentixafor PET/CT showed a warm lesion in the right adrenal (white arrow in c, d) with an SUVmax of 4.0. The lateralization index (LI) based on adrenal venous sampling (AVS) was 1.3, which indicated bilateral lesions. The NFA patient was a 46-year-old woman with an incidental adrenal nodule and aldosterone/renin ratio of 138.5, without hypertension or hypokalemia. The 18F-AlF-NOTA-pentixafor PET/CT exhibited a cold lesion in the left adrenal (white arrow in e, f) with an SUVmax of 2.9, and postoperative pathology revealed a left adrenal medullary lipoma.
Besides, we conducted a correlation analysis among SUVmax and clinical characteristics. The results showed that SUVmax was negatively correlated with blood potassium concentration (r = − 0.444; P < 0.001) and age (r = − 0.334; P = 0.001), and positively correlated with PAC (r = 0.287; P = 0.007) and ARR (r = 0.265; P = 0.013) (partly shown in Figure 6). There was no correlation of SUVmax with sex, BMI, duration of hypertension, systolic blood pressure, diastolic blood pressure, or lesion diameter. In PA patients, a significant correlation was observed between SUVmax and lesion diameter (r = 0.351; P = 0.001).
In 88 patients, 95 lesions exhibited abnormalities in PET or CT scans (comprising 78 cases of surgically eligible lesions and 17 cases of surgically ineligible lesions). A total of 78 UPA and 4 BPA lesions were confirmed by AVS and/or postoperative pathology, while the remaining 13 NFA lesions were confirmed through clinical evaluation and/or postoperative follow-up evidence. Among the 95 lesions, 71/78 surgically eligible lesions demonstrated positive findings (sensitivity 89.74%), and 16/17 surgically ineligible lesions showed negative findings (specificity 94.12%) in visual analysis of 18F-AlF-NOTA-pentixafor PET/CT. The median SUVmax, LAR, and LLR of the surgically eligible lesions were 9.85(5.70,16.90), 2.85(1.66,4.52), and 5.90(3.59,9.75), respectively, significantly higher than those of the surgically ineligible lesions of 3.40(2.90,3.90), 0.92(0.83,1.11), 2.06(1.71,2.38).
Figure 7 showed LAR based on 18F-pentixafor SUVmax had a higher AUROC (0.961 [95%CI, 0.899-1.023]) than SUVmax of the lesion (0.931 [95% CI, 0.841-1.021]), visual analysis (0.919 [95%CI, 0.830-1.009]), and LLR based on SUVmax (0.901 [95% CI, 0.785-1.016]) to diagnose surgically eligible lesions. Thereinto, the AUROC of LAR was statistically higher than that of LLR (P=0.008). To achieve the maximized Youden index, the optimal cutoff LAR, SUVmax, and LLR was 1.43, 5.45, and 3.20, respectively, with a sensitivity of 83.33%, 79.49%, and 80.77%. No patients with surgically ineligible lesions would be misdiagnosed as surgically eligible lesions using these cutoffs with specificity of 100% in all (shown in Table 2).
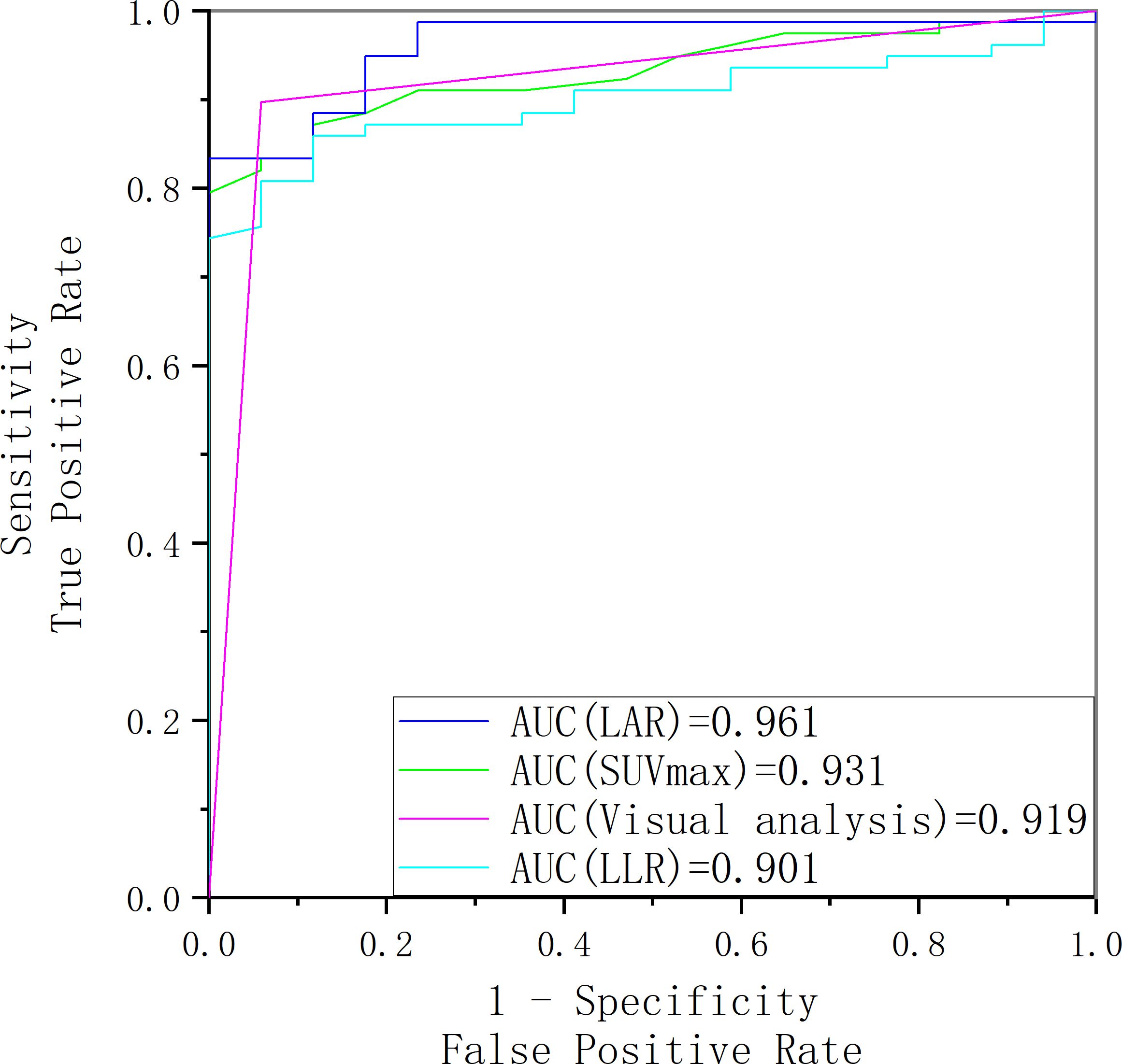
Figure 7. The receiver operating characteristic curve of SUVmax, LAR, LLR and visual analysis for identifying surgically eligible lesions and surgically ineligible lesions.
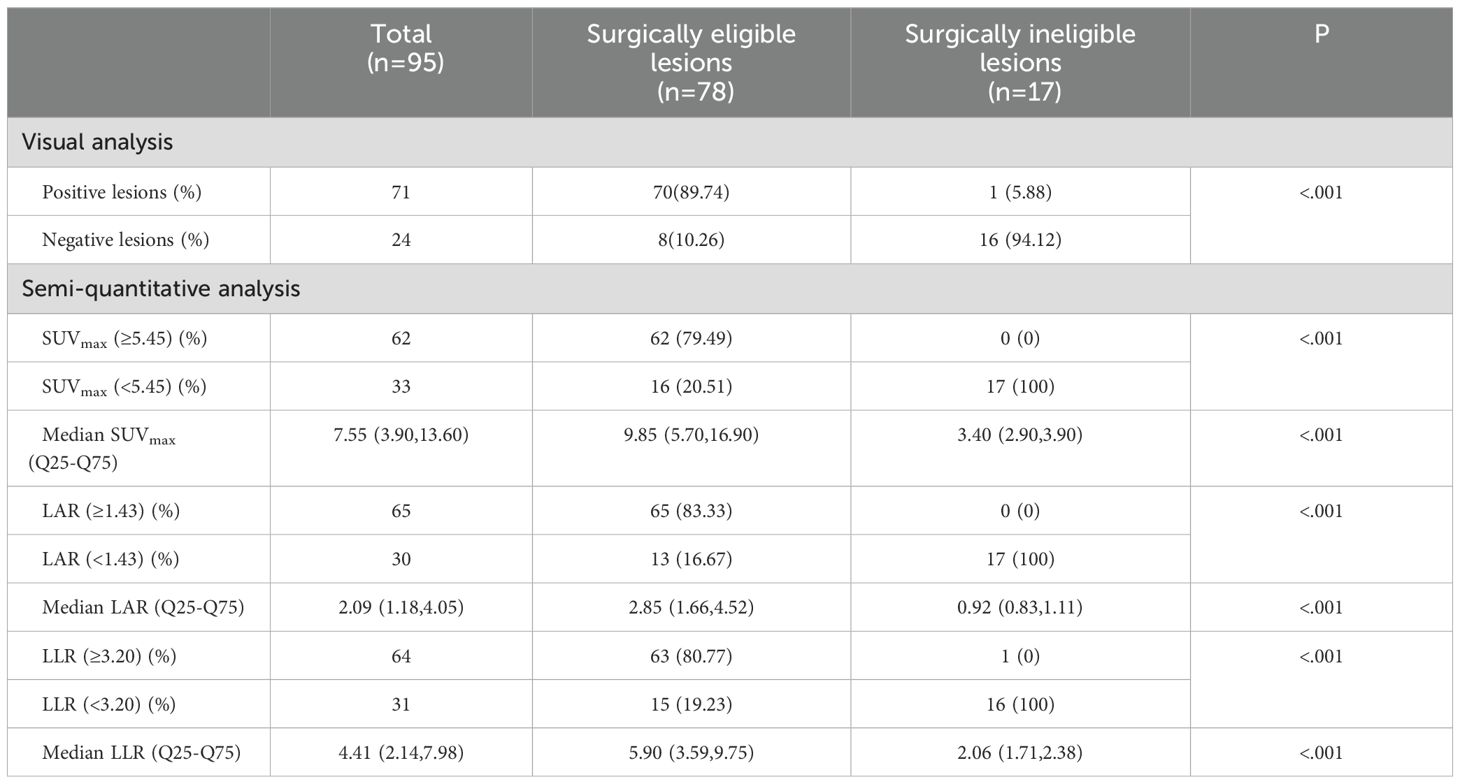
Table 2. Visual and semi-quantitative analysis of 18F-AlF-NOTA-pentixafor PET/CT in surgically eligible lesions and surgically ineligible lesions.
To further evaluate the diagnostic value of 18F-AlF-NOTA-pentixafor PET/CT for lesions with different diameters, we divided 95 lesions into two groups: lesions with diameter < 1 cm and ≥ 1 cm. Among 95 lesions, 13 lesions examined with PET/CT were < 1 cm (10 nodules, 1 nodular thickening, 1 diffuse thickening, and 1 positive lesion without morphological abnormalities), while 82 were ≥ 1 cm in diameter (76 nodules and 6 nodular thickening). The diagnostic performance of 18F-AlF-NOTA-pentixafor PET/CT in identifying UPA was further analyzed in these 2 groups of patients and no significant demographical or biochemical differences were observed between the groups (Supplementary Table 1). The small lesion group (< 1 cm in diameter) has higher optimal cutoffs of metabolic parameters (SUVmax=5.75, LAR=1.43, LLR=3.65) than the big lesion group (≥ 1 cm in diameter) (SUVmax=5.15, LAR=1.47, LLR=3.20). Compared to the small lesion group, the large lesion group demonstrated higher sensitivity, specificity, and accuracy either in visual analysis or semi-quantitative parameters (Table 3). For lesions < 1 cm in diameter, LAR of 1.43 identified 9 UPA out of 11 lesions with an accuracy of 84.62% (sensitivity of 81.82%, specificity of 100%) (Table 3). For lesions ≥ 1 cm in diameter, visual analysis identified 61 UPA out of 67 lesions, with an accuracy of 92.68% (sensitivity of 91.04%, specificity of 100%) for identifying UPA.
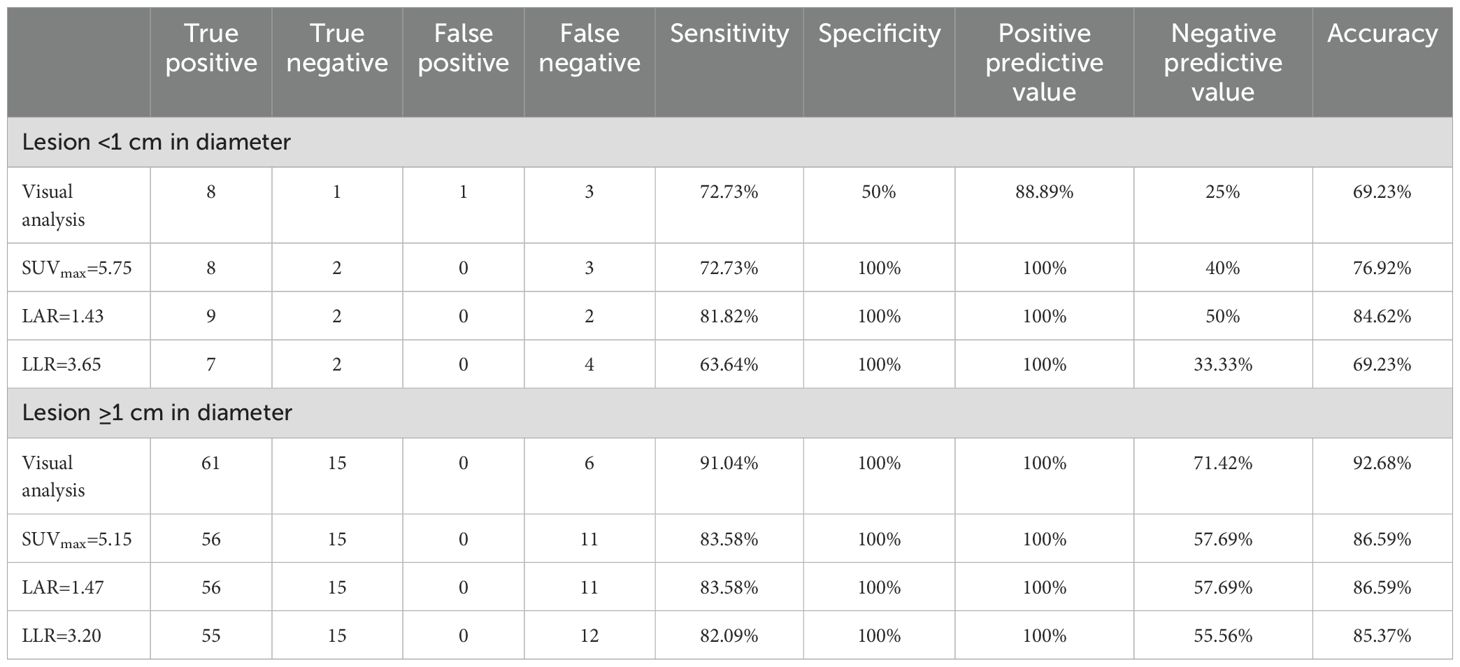
Table 3. Diagnostic efficacy of 18F-AlF-NOTA-pentixafor PET/CT for lesions < 1 cm and ≥ 1 cm in diameter based on visual and semiquantitative analysis.
Since 7 out of 76 UPA patients showed negative findings in visual analysis, we decided to analyze the misdiagnosed reason further. All 7 missing UPA were warm lesions, with patients’ clinical and biochemical characteristics shown in Table 4.
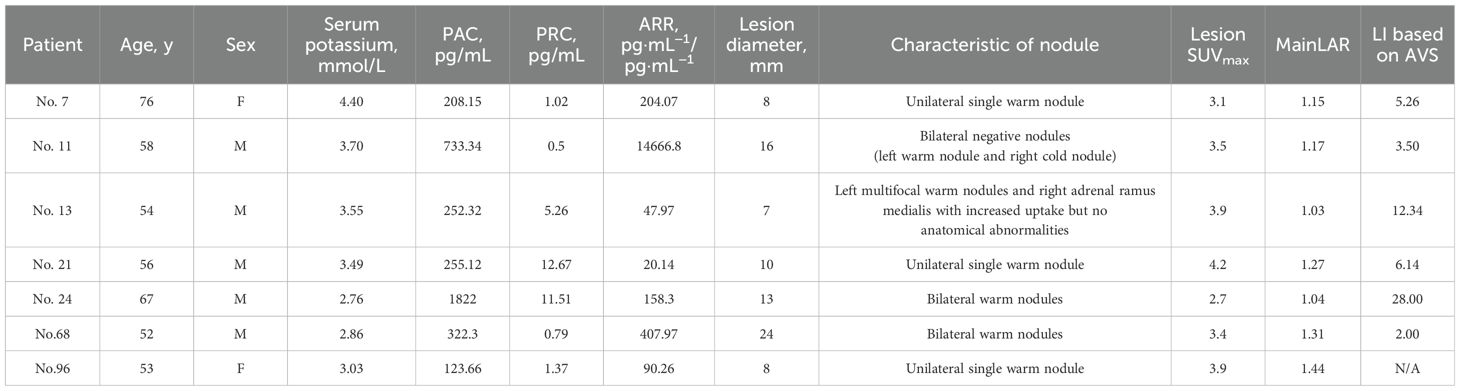
Table 4. Clinical and biochemical characteristics of patients misdiagnosed by 18F-AlF-NOTA-Pentixafor PET/CT visual analysis.
All 69 positive main lesions (hot lesions) out of 88 patients were proved UPA while all 4 cold main lesions were proved NFA in clinical. In 15 warm lesions, 7 lesions were proved UPA, 4 lesions were proved BPA, and 4 were proved NFA. There was a high correlation between the final diagnosis and the degree of the main lesion uptake (χ2 = 48.502; P < 0.001) (Table 5).
Considering that the ultimate subtyping diagnosis needs to specify the hypersecretion side of the adrenal gland rather than simply distinguishing between unilateral or bilateral cases, we further conduct an analysis of the concordance rate of AVS and PET/CT visual diagnosis in 20 patients who underwent AVS (Table 6). Of 11 UPA patients identified by PET/CT visual analysis, 10 were confirmed as having ipsilateral dominant secretion by AVS. The single patient with non-matching laterality between AVS and PET/CT showed multifocal warm nodules on the dominant secretion side of AVS, while the contralateral adrenal ramus medialis showed increased uptake but no anatomical abnormalities. Additionally, 3 of 9 patients with BPA identified by PET/CT were confirmed as having BPA by AVS. Hence, the total concordance rate of AVS and PET/CT visual diagnosis was 65.00% (13/20). The clinical characteristics of 7 patients with a disagreement between PET/CT visual diagnosis and AVS were shown in Supplementary Table 2. The ROC analysis in the 20 patients showed that the area under the curve (AUC) for SUVmax and LI based on SUVmax reached 0.667 and 0.765, respectively, with the optimal cutoff values of SUVmax=5.15 and LI=1.12 (Figure 8). However, employing SUVmax=5.15 and LI based on SUVmax=1.12 for subtyping diagnosis did not result in an improvement in the total concordance rates (65.00% (13/20); 55.00% (11/20)) when compared to visual analysis.
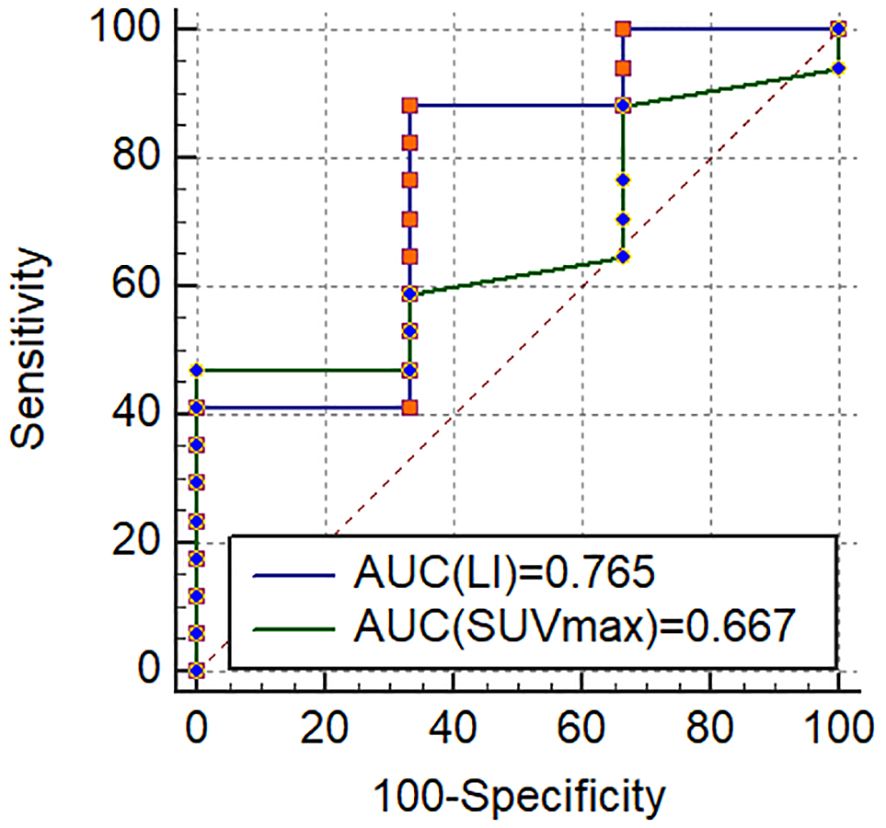
Figure 8. The receiver operating characteristic curves for diagnosis of UPA in 20 patients who underwent AVS.
68Ga-Pentixafor is the predominant CXCR4 imaging radiotracer, but it faces practical, regulatory, and economic barriers associated with 68Ge/68Ga-generators (23, 24). These concerns could potentially be resolved through the introduction of a fluorine-18-labeled alternative with high production yield, enabling centralized production and distribution to remote PET centers. In our study, we successfully synthesized 18F-AlF-NOTA-pentixafor and for the first time applied 18F-pentixafor to clinical practice. The results indicated 18F-pentixafor PET/CT, as a non-invasive examination, could play an important role in the subtyping diagnosis of PA.
Our study demonstrates that 18F-pentixafor uptake in adrenal gland lesions of patients diagnosed or suspected of PA is higher than in any other organ in the upper abdomen, with excellent adrenal gland lesion/background ratios (LAR and LLR) for 18F-AlF-NOTA-pentixafor. As we did not synthesize 68Ga-pentixafor in our hospital, we roughly compared our biodistribution data of 18F-AlF-NOTA-pentixafor with that of 68Ga-NOTA-pentixafor in other studies and found similar biodistribution and high variability of the gall bladder in both radiotracers (25). Previous research by Hu et all (26) has suggested SUVmax of early 68Ga-pentixafor PET imaging may be better than SUVmax of delayed imaging. Yet in our study, LAR and LLR at 60 minutes p.i. were relatively higher than those at 20 minutes p.i. in 6 UPA patients with no statistical differences, which might be attributable to the small data set. Therefore, further validation with larger sample sizes is needed to determine the optimal time for performing 18F-pentixafor PET/CT scan.
Several previous studies have underscored the significance of targeted CXCR4 PET/CT in subtyping the diagnosis of primary aldosteronism (PA); however, their focus was solely on 68Ga-pentixafor. It is essential to explore the clinical applicability of the promising targeted CXCR4 radiotracer, 18F-pentixafor. In our study, SUVmaxs of the BPA group and NFA group were statistically lower than those of the UPA group. Furthermore, 18F-AlF-NOTA-pentixafor proved highly accurate in distinguishing surgically eligible lesions from those in the surgically ineligible lesions. The results indicated that 89.74% of surgically eligible lesions showed positive uptake in PET/CT, while 94.12% of surgically ineligible lesions (including BPA and NFA lesions) exhibited similar or lower contralateral and adjacent normal adrenal tissue uptake in PET/CT. In comparison to visual analysis, the optimal cutoff values of the semi-quantitative parameters including SUVmax (5.45), LAR (1.43), and LLR (3.20) show lower sensitivity (79.49, 83.33, 80.77) and higher specificity (100% in all cases). These results were consistent with the study of Zheng et al, which included 66 APA, 33 IHA, and 21 NFA patients to get 68Ga-pentixafor PET/CT scan (20). It is worth mentioning that, although Xiangya Hospital classified PA patients as APA and IHA in this study, these are substantially equivalent to UPA and BPA. However, the optimal cutoff SUVmax (5.45) of 18F-pentixafor in our study is relatively lower than that of 68Ga-pentixafor (7.65), which might be explained by the differences between two nuclide-labeled radiotracers (18, 25, 27). Additionally, among semi-quantitative parameters, LAR emerged as the most effective indicator for 18F-pentixafor in our study, while LLR was identified as the best indicator for 68Ga-pentixafor in the study by Zheng et al. This correspondence may be substantiated by the comparison of tumor-to-organ ratios between 18F-AlF-NOTA-pentixafor and 68Ga-pentixafor as described by Poschenrieder et al (25).
The small size of nodules was considered to limit the subtype diagnosis of adrenal CT, and a few previous PET/CT studies (17, 20, 28) dispute the diagnostic disadvantage associated with nodules < 1 cm in diameter. Our study found that in lesions < 1 cm in diameter, the sensitivity of PET/CT visual analysis was as high as 72.73%. Additionally, our study indicated a positive correlation between SUVmax and lesion diameter in PA patients (r=0.351, P=0.001), a finding also reported in prior studies (20, 28). Given that previous studies have reported a negative correlation between adenoma size and CYP11B2 expression (29) and a positive correlation between the expression level of CXCR4 and CYP11B2, the expression density of CXCR4 in micro-APA should theoretically be higher than that in macro-APA. We deem the uncertainty in the relationship between SUVmax and nodule diameter may be attributed to the restricted spatial resolution of PET/CT imaging, which was also mentioned by prior research (30).
Our study showed that the positive predictive value (PPV) of the main positive lesions to diagnose UPA and the main cold lesions to diagnose NFA was 100%. However, in 15 warm lesions, 7 were proved UPA, 4 were proved BPA, and 4 were proved NFA. In previous studies, adrenal lesions with a similar uptake to contralateral and adjacent normal adrenal tissue were diagnosed as non-functional nodules by PET/CT (20, 28), which caused 5 missed cases out of 12 with a false negative rate of 41.66 in our study. This finding revealed that warm lesions were more likely to cause diagnostic ambiguity and be misdiagnosed. Therefore, PET/CT diagnosis in such lesions should be more careful, and multi-dimensional analyses (including semi-quantitative parameter analysis and nodule characteristic analysis) are necessary. In 20 patients who underwent AVS, the concordance rate of AVS and PET/CT visual diagnosis was 65.00%. Our study suggested that the adrenal gland with higher 18F-pentixafor uptake but no anatomical abnormalities might be misdiagnosed as dominant secretion side by PET/CT visual diagnosis. Therefore, it was unreliable to rely solely on radiotracer uptake for determining laterality, and anatomical findings should be taken into consideration as well.
There are several limitations to this study. First, it was a single-center study with a small sample size, especially in the AVS patients. Second, since there are only 4 BPA patients confirmed by AVS in our study, the results may be biased due to the uneven distribution of the sample size. More confirmed BPA samples are required. Third, the sample size of lesions < 1 cm in diameter was small. Fourth, resected adrenal lesions were not routinely sent for CYP11B2 staining in our hospital and the pathological diagnosis of adrenal lesions was based solely on morphology with hematoxylin-eosin (HE) staining.
In summary, this study demonstrates that CXCR4-targeted 18F-AlF-NOTA-pentixafor PET/CT is a valuable noninvasive tool to diagnose UPA with high sensitivity and specificity and to guide further treatment options. Adrenal lesions with a similar uptake to contralateral and adjacent normal adrenal tissue are more likely to cause diagnostic ambiguity and misdiagnosis, requiring more attention from nuclear medicine physicians.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee in Clinical Research (ECCR) of the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YP: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Formal analysis, Software, Visualization, Writing – original draft. FC: Data curation, Visualization, Writing – review & editing. RY: Validation, Visualization, Writing – review & editing. JL: Data curation, Writing – review & editing. YF: Data curation, Writing – review & editing. KY: Data curation, Writing – review & editing. ZW: Data curation, Methodology, Writing – review & editing. XJ: Data curation, Writing – review & editing. GZ: Data curation, Methodology, Writing – review & editing. KZ: Data curation, Methodology, Writing – review & editing. XG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – review & editing. KT: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. QZ: Data curation, Validation, Writing – review & editing. KX: Data curation, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the patients for their participation and the research nurses, secretaries, and technicians at the participating hospitals for their skillful work with subject recruitment, PET/CT scan and data recording, and all authors’ contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1533295/full#supplementary-material
1. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
2. Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertension. (2020) 38:1841–8. doi: 10.1097/hjh.0000000000002441
3. Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: A cross-sectional study. Ann Internal Med. (2020) 173:10–20. doi: 10.7326/m20-0065
4. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. (2018) 39:1057–88. doi: 10.1210/er.2018-00139
5. Wachtel H, Fraker DL. Therapeutic outcomes with surgical and medical management of primary aldosteronism. Curr Cardiol Rep. (2021) 23:89. doi: 10.1007/s11886-021-01516-0
6. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. (2021) 9:876–92. doi: 10.1016/S2213-8587(21)00210-2
7. Zuo R, Liu S, Xu L, Pang H. Key to the treatment of primary aldosteronism in secondary hypertension: subtype diagnosis. Curr Hypertens Rep. (2023) 25:471–80. doi: 10.1007/s11906-023-01269-x
8. Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. (2021) 106:42–54. doi: 10.1210/clinem/dgaa484
9. Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. (2009) 151:329–37. doi: 10.7326/0003-4819-151-5-200909010-00007
10. Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). (2017) 87:665–72. doi: 10.1111/cen.13442
11. Patel D, Gara SK, Ellis RJ, Boufraqech M, Nilubol N, Millo C, et al. Fdg pet/ct scan and functional adrenal tumors: A pilot study for lateralization. World J Surg. (2016) 40:683–9. doi: 10.1007/s00268-015-3242-y
12. Akkuş G, Güney IB, Ok F, Evran M, Izol V, Erdoğan Ş, et al. Diagnostic efficacy of 18F-fdg pet/ct in patients with adrenal incidentaloma. Endocrine Connections. (2019) 8:838–45. doi: 10.1530/ec-19-0204
13. Abe T, Naruse M, Young WF Jr., Kobashi N, Doi Y, Izawa A, et al. A novel cyp11b2-specific imaging agent for detection of unilateral subtypes of primary aldosteronism. J Clin Endocrinol Metab. (2016) 101:1008–15. doi: 10.1210/jc.2015-3431
14. Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (Pet)-ct for lateralizing aldosterone secretion by conn’s adenomas. J Clin Endocrinol Metab. (2012) 97:100–9. doi: 10.1210/jc.2011-1537
15. Soinio M, Luukkonen AK, Seppänen M, Kemppainen J, Seppänen J, Pienimäki JP, et al. Functional imaging with 11c-metomidate pet for subtype diagnosis in primary aldosteronism. Eur J Endocrinol. (2020) 183:539–50. doi: 10.1530/eje-20-0532
16. Walenkamp AME, Lapa C, Herrmann K, Wester HJ. Cxcr4 ligands: the next big hit? J Nucl Med. (2017) 58:77s–82s. doi: 10.2967/jnumed.116.186874
17. Heinze B, Fuss CT, Mulatero P, Beuschlein F, Reincke M, Mustafa M, et al. Targeting cxcr4 (Cxc chemokine receptor type 4) for molecular imaging of aldosterone-producing adenoma. Hypertension (Dallas Tex: 1979). (2018) 71:317–25. doi: 10.1161/hypertensionaha.117.09975
18. Kwon D, Lozada J, Zhang Z, Zeisler J, Poon R, Zhang C, et al. High-contrast cxcr4-targeted (18)F-pet imaging using a potent and selective antagonist. Mol Pharm. (2021) 18:187–97. doi: 10.1021/acs.molpharmaceut.0c00785
19. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. (2017) 5:689–99. doi: 10.1016/s2213-8587(17)30135-3
20. Zheng Y, Long T, Peng N, Zhen M, Ye Q, Zhang Z, et al. The value of targeting cxcr4 with 68ga-pentixafor pet/ct for subtyping primary aldosteronism. J Clin Endocrinol Metab. (2023) 109:171–82. doi: 10.1210/clinem/dgad421
21. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. (2014) 63:151–60. doi: 10.1161/hypertensionaha.113.02097
22. Monticone S, Viola A, Rossato D, Veglio F, Reincke M, Gomez-Sanchez C, et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol. (2015) 3:296–303. doi: 10.1016/s2213-8587(14)70069-5
23. Decristoforo C, Pickett RD, Verbruggen A. Feasibility and availability of 68Ga-labelled peptides. Eur J Nucl Med Mol Imaging. (2012) 39 Suppl 1:S31–40. doi: 10.1007/s00259-011-1988-5
24. Velikyan I. 68ga-based radiopharmaceuticals: production and application relationship. Mol (Basel Switzerland). (2015) 20:12913–43. doi: 10.3390/molecules200712913
25. Poschenrieder A, Osl T, Schottelius M, Hoffmann F, Wirtz M, Schwaiger M, et al. First (18)F-labeled pentixafor-based imaging agent for pet imaging of cxcr4 expression in vivo. Tomogr (Ann Arbor Mich). (2016) 2:85–93. doi: 10.18383/j.tom.2016.00130
26. Hu J, Xu T, Shen H, Song Y, Yang J, Zhang A, et al. Accuracy of gallium-68 pentixafor positron emission tomography-computed tomography for subtyping diagnosis of primary aldosteronism. JAMA Netw Open. (2023) 6:e2255609. doi: 10.1001/jamanetworkopen.2022.55609
27. Conti M, Eriksson L. Physics of pure and non-pure positron emitters for pet: A review and a discussion. EJNMMI Phys. (2016) 3:8. doi: 10.1186/s40658-016-0144-5
28. Gao Y, Ding J, Cui Y, Li T, Sun H, Zhao D, et al. Functional nodules in primary aldosteronism: identification of cxcr4 expression with (68)Ga-pentixafor pet/ct. Eur Radiol. (2023) 33:996–1003. doi: 10.1007/s00330-022-09058-x
29. Ono Y, Nakamura Y, Maekawa T, Felizola SJ, Morimoto R, Iwakura Y, et al. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension (Dallas Tex: 1979). (2014) 64:438–44. doi: 10.1161/hypertensionaha.113.02944
Keywords: primary aldosteronism, CXCR4, 18 F-pentixafor, PET/CT, subtyping, treatment
Citation: Peng Y, Chen F, Yao R, Lan J, Fu Y, Ye K, Wang Z, Zhao Q, Ji X, Xia K, Zhu G, Zheng K, Gu X and Tang K (2025) The value of targeted CXCR4 18F-AlF-NOTA-pentixafor PET/CT for subtyping primary aldosteronism. Front. Endocrinol. 16:1533295. doi: 10.3389/fendo.2025.1533295
Received: 23 November 2024; Accepted: 07 February 2025;
Published: 27 February 2025.
Edited by:
Henrik Falhammar, Karolinska Institutet (KI), SwedenReviewed by:
Piotr Kmieć, Medical University of Gdansk, PolandCopyright © 2025 Peng, Chen, Yao, Lan, Fu, Ye, Wang, Zhao, Ji, Xia, Zhu, Zheng, Gu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemei Gu, Z3V4bUB3bXUuZWR1LmNu; Kun Tang, a3VudGFuZzAwN0AxNjMuY29t
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.