
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 03 March 2025
Sec. Clinical Diabetes
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1528801
 S. K. Wangnoo1
S. K. Wangnoo1 Sanjay Kumar Bhadada2
Sanjay Kumar Bhadada2 Faraz Farishta3
Faraz Farishta3 Girithara Gopalakrishnan Jayaram Naidu4
Girithara Gopalakrishnan Jayaram Naidu4 Indira Pattnaik5
Indira Pattnaik5 K. N. Manohar6
K. N. Manohar6 K. P. Singh7
K. P. Singh7 Sandeep Kumar Gupta8
Sandeep Kumar Gupta8 H. S. Bharath9
H. S. Bharath9 Sujoy Ghosh10*
Sujoy Ghosh10*Objective: To determine the post-marketing safety profile of a once-daily fixed-dose combination (FDC) of dapagliflozin (10 mg) and saxagliptin (5 mg) given orally for 24 weeks or until discontinuation, in Indian patients with type 2 diabetes mellitus (T2DM) who are on stable dose of metformin.
Design: Prospective, single-arm, multicenter study
Setting: Adult patients with T2DM enrolled from April 2021 to March 2023 across 9 study sites in India
Outcome measures: The primary objective was to determine the adverse event (AE) profile of the FDC. Additionally, we assessed changes in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), systolic blood pressure, and body weight at 24 weeks, compared to baseline.
Results: Of the 196 patients (median age [range]: 53 [20 to 78] years) analyzed, 61.2% were males with mean ± standard deviation [SD] duration of T2DM of 7.1 ± 5.7 years. Overall, 111 (56.6%) presented with ≥1 comorbidity; the most frequent being hypertension (57; 29.1%). At 24 weeks, a total of 22 patients (11.2%) experienced 40 AEs; the majority of them had mild AEs. The most frequent AEs included urinary tract infection (5; 2.6%), pyrexia (5; 2.6%), nasopharyngitis (3; 1.5%), and balanoposthitis (3; 1.5%). The AEs of special interest reported were genital tract infection (3; 1.5%) and hypoglycemia (1; 0.5%). No serious AEs were reported. None of the AEs required treatment discontinuation. Three (1.5%) patients had AEs leading to temporary interruption of the study drug. No deaths were reported in this study. The mean absolute change in HbA1c (1.2% ± 1.1%), FPG (24.4 ± 62.9 mg/dL), and weight (2.1 ± 4.0 kg) from baseline to 24 weeks was statistically significant (p < 0.0001).
Conclusion: Our study demonstrated the safety and efficacy of once-daily FDC of dapagliflozin and saxagliptin when added to metformin in Indian patients with T2DM.
The incidence of diabetes has consistently shown an upward trend, particularly in Southeast Asian nations, with India ranking second to China as per the disease burden (1–3). According to the International Diabetic Federation Diabetic Atlas (2021), about 74.2 million people in India have diabetes mellitus, majorly type 2 diabetes mellitus (T2DM), and this number is expected to reach 125 million by 2045 (3). Despite therapeutic interventions, more than two-thirds of patients have poor glycemic control (glycated hemoglobin [HbA1c] ≥7%) (4, 5).
The Asian-Indian phenotype of T2DM presents a unique cardiometabolic risk profile, necessitating a multifaceted therapeutic approach for optimal glycemic control and prevention of cardiovascular complications (6). As a result, the rational combination of different therapeutic options is crucial in prioritizing patient-centric management. The American Diabetes Association recommends initial combination therapy for patients with HbA1c levels 1.5% to 2.0% above the target (7). Sodium-glucose cotransporter-2 inhibitors (SGLT2i) and dipeptidyl peptidase 4 inhibitors (DPP4i) are preferred options due to their superior efficacy in glycemic control along with reduction in cardiovascular risk and an acceptable safety profile, particularly in the context of Indian diabetic setting (8).
Numerous studies have demonstrated that the combination of dapagliflozin (an SGLT2i) and saxagliptin (a DPP4i) with metformin resulted in better glycemic control along with the reduction in body weight and blood pressure compared to individual agents, with an acceptable safety profile (9–13). Available evidence demonstrated comparable safety and tolerability of dapagliflozin and saxagliptin combination to individual agents added to metformin (13, 14). Additionally, the incidence of urinary and genital tract infections was lower with the combination regimen, compared to the sequential add-on regimen (13).
Recent trends in prescription pattern practices indicate an increased preference for fixed-dose combinations (FDCs) owing to their simplified dosing schedule and reduced pill burden, contributing to enhanced treatment compliance (15). A once-daily FDC of dapagliflozin and saxagliptin was approved for the management of T2DM by the European Medicines Agency in 2016 (16) and the Food and Drug Administration in 2017 (17). It received marketing approval from the Drug Controller General of India in 2019 (18). This FDC is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2DM who have inadequate glycemic control with metformin and/or sulfonylurea and either of the individual components of the combination or who already received treatment with dapagliflozin and saxagliptin (16). Considering limited data on the safety of this FDC in the Indian population, the present study was conducted in response to the mandate from the Heath Authority of India to determine the post-marketing safety profile of QTERN® (FDC of dapagliflozin 10 mg and saxagliptin 5 mg) among Indian patients with T2DM.
This was a prospective, single-arm, multicenter study conducted at 9 study sites across India between April 2021 and March 2023. The study protocol (NCT04445714) was approved by the Independent Ethics Committees/Institutional Review Boards of all the participating centers. The study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation, good clinical practices, good pharmacoepidemiology practices (GPP), and the applicable legislations. Written informed consent was obtained from all the patients before enrollment. The reporting has been done following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort studies (19).
The study included patients aged ≥18 years with T2DM (HbA1c ranging between >7% and ≤10%) on a stable dose of anti-diabetic medications for ≥3 months before enrollment. Patients with type 1 diabetes mellitus, prior treatment with an SGLT2i, glucagon-like peptide-1 agonist, or DPP4i, severe hepatic impairment, moderate to severe renal impairment, cardiovascular disease within 3 months prior to enrollment, and severe uncontrolled hypertension were excluded. Detailed inclusion and exclusion criteria are presented in Supplementary Table S1.
Enrolled patients were administered once-daily FDC of dapagliflozin 10 mg and saxagliptin 5 mg orally at the same time of a day for 24 weeks or until discontinuation of the study drug due to either adverse events (AEs) or major and/or frequent hypoglycemic events, or other reasons such as consent withdrawal by the patient, whichever occurred earlier. All the patients were on stable dose of metformin (ranging between 1000 and 2000 mg) at enrollment. Patients were allowed to receive additional glucose-lowering drugs as per investigator’s discretion. Concomitant use of weight-loss medications, antiviral drugs, and systemic glucocorticoids was prohibited.
The primary objective was to determine the safety of dapagliflozin and saxagliptin FDC at 24 weeks by evaluating the incidence of AEs, serious AEs, AEs leading to discontinuation, AEs of special interest (AESIs) including major hypoglycemic events, urinary and genital tract infections, volume depletion, diabetic ketoacidosis, fractures, renal events, and hospitalization for heart failure (HHF). The secondary objective included efficacy parameters at 24 weeks, assessed by the changes in HbA1c, fasting plasma glucose (FPG), systolic blood pressure (SBP), and body weight at 24 weeks compared to baseline. At baseline, data were collected for demographics, medical history, HbA1c, FPG, vital signs, physical examination, electrocardiogram (ECG), and laboratory parameters and entered into electronic case report forms. Additionally, data regarding concomitant glucose-lowering medications were also recorded at baseline. Subsequently, patients underwent follow-up assessments at Weeks 8, 16, and 24 (Supplementary Figure S1).
A sample size of 200 patients was deemed to be adequate to estimate AEs associated with the FDC at the incidence rate of 8%, with a margin of error of 4% and overall incidence rate of AEs at 50%, with a margin of error of 7%. The safety population included all enrolled patients who had signed the informed consent form and received at least one dose of the FDC. All reported AEs were included in the analysis.
Statistical analyses were performed using SAS (version 9.4 or higher) software (SAS Institute Inc USA). Data were summarized using descriptive statistics. Continuous variables were summarized using the number of observations, mean, standard deviation (SD), median, and range as appropriate. Categorical values were summarized using the number of observations and percentages. The mean change in HbA1c from baseline to 24 weeks was analyzed using a paired t-test/Wilcoxon signed-rank test at a 5% level of significance. The last treatment observation was carried forward to impute the missing data at 24 weeks. A p-value < 0.05 was considered statistically significant.
Of the 204 patients screened, 196 patients were enrolled in the study; 173 (88.3%) patients completed the study, and 23 (11.7%) patients discontinued the study. The patient disposition along with reasons for discontinuation is presented in Figure 1. The median age of patients was 53 years (range: 20 to 78 years); the majority were males (120; 61.2%) with a mean ± SD body weight of 74.2 ± 12.6 kg and mean ± SD body mass index of 28.0 ± 4.4 kg/m2. The mean ± SD duration of T2DM was 7.1 ± 5.7 years and the mean ± SD baseline HbA1c was 8.6% ± 0.8%. Overall, 111 patients (56.6%) presented with ≥1 comorbidity. The most frequent comorbidities were hypertension (57; 29.1%) and dyslipidemia (36; 18.4%) (Table 1). Commonly prescribed glucose-lowering medications in addition to background metformin included glimepiride, glibenclamide, and gliclazide.
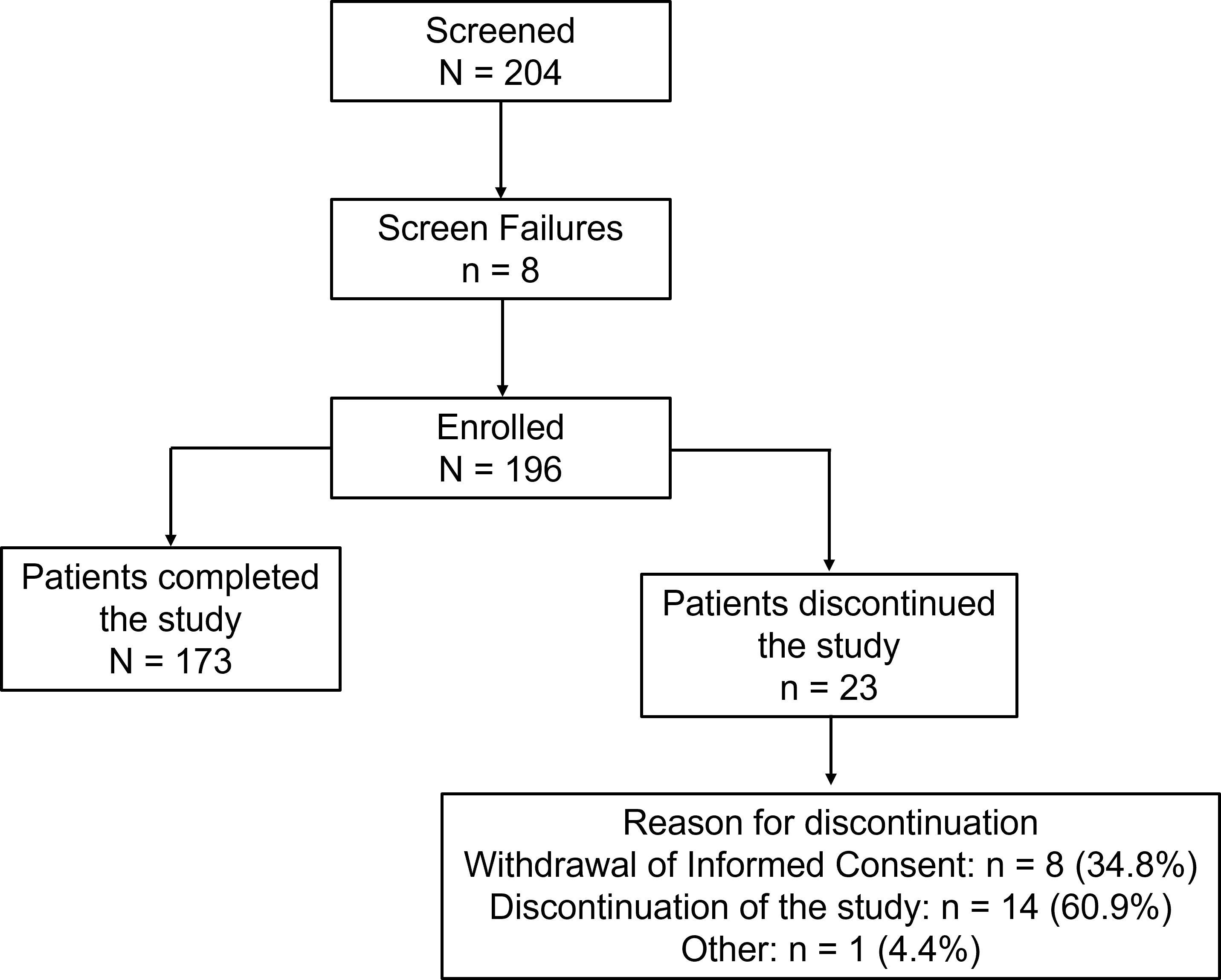
Figure 1. Patient disposition of the study. Screened patients are those who signed the informed consent. Percentages for the ‘reasons for discontinuation’ are based on the number of patients who discontinued the study.
Of the 196 patients, data on safety were available for 142 patients (72.4%), with data missing for 54 (27.6%) patients. A total of 22 patients (11.2%) experienced 40 AEs. All AEs were treatment-emergent, and 13 (6.6%) patients had AEs that were not related to the study drug. Eighteen patients (9.2%) required drug treatment for AEs and 3 (1.5%) patients had AEs leading to temporary interruption of the study drug. Most patients (n = 20; 10.2%) had AEs that were mild in severity and recovered without sequelae and 4 (2.0%) patients were recovering at the end of follow-up. No SAEs, AEs leading to treatment discontinuation, or death were reported during the study (Figure 2). The most frequent AEs reported by the patients included urinary tract infection (n = 5; 2.6%), pyrexia (n = 5; 2.6%), nasopharyngitis (n = 3; 1.5%), and balanoposthitis (n = 3; 1.5%) (Table 2). The AESIs reported were genital tract infection (n = 3; 1.5%) and hypoglycemia (n = 1; 0.5%). The hypoglycemic event was mild and was self-treated by the patient without any treatment intervention or assistance. No new cases of HHF were reported.
No clinically significant changes were observed in hematology, clinical chemistry, urinalysis, physical examination, vital signs, or ECG during the study visits, until Week 24 (Supplementary Tables S2, S3).
The mean ± SD change in HbA1c from baseline to 24 weeks was -1.2% ± 1.1% (95% confidence interval [CI], -1.34 to -1.03; p < 0.0001); the mean ± SD HbA1c was 7.4% ± 1.0% at Week 24 (Figure 3A). The mean ± SD FPG at baseline was 161.8 ± 52.3 mg/dL, which significantly decreased to 136.7 ± 42.0 mmol/L following 24 weeks of treatment (p < 0.0001) (Figure 3B). Additionally, there was a significant reduction in the mean body weight at 24 weeks, compared to baseline (72.2 ± 11.5 kg versus 74.2 ± 12.6 kg, respectively); the mean ± SD change in body weight was -2.1 ± 4.0 kg (95% CI, -2.66 to -1.51; p < 0.0001). The mean ± SD change in SBP from baseline to Week 24 was -0.2 ± 12.9 mmHg (95% CI, -2.34 to 1.91; p = 0.8416) (Figure 3C).
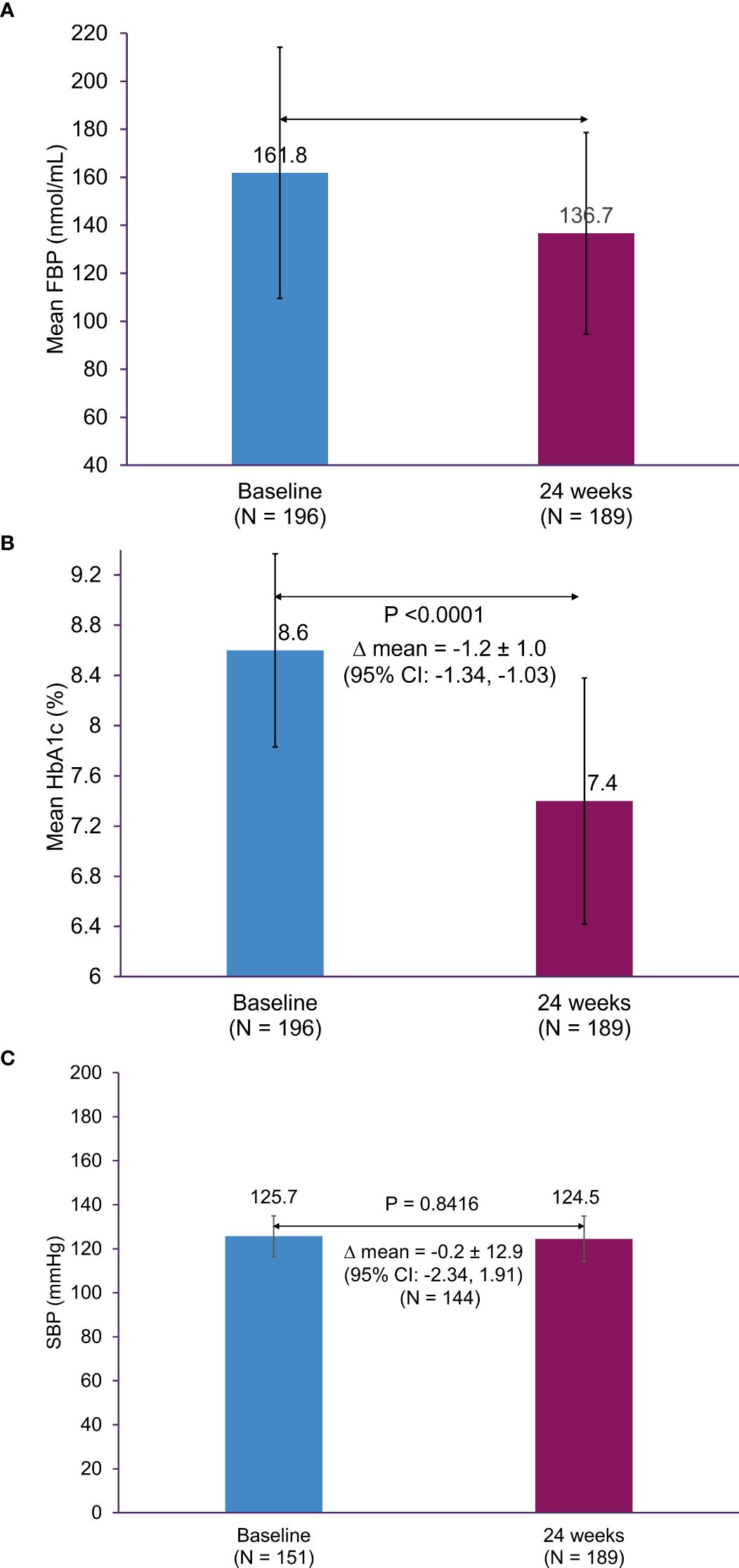
Figure 3. Change in HbA1c, FPG, and SBP from baseline to 24 weeks. (A) HbA1c, (B) FPG, and (C) SBP. CI, confidence interval; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; SBP, systolic blood pressure; SD, standard deviation.
Overall, the mean ± SD compliance with the study drug combination was 98.7% ± 2.9% at 8 weeks which remained consistent at 16 weeks (97.6% ± 4.6%) and 24 weeks (98.1% ± 5.9%). Overall, a median of 100% drug compliance was reported during the study.
The FDC of dapagliflozin and saxagliptin has the potential to improve patient outcomes among Indian patients with T2DM, who are often burdened with a high prevalence of comorbidities, thus necessitating treatment with multiple medications. Previous research has indicated that transitioning from multiple drugs to an FDC regimen led to significantly higher treatment adherence (20–23). These studies suggest that FDCs present a rational approach to T2DM management, providing enhanced treatment compliance due to reduced dosing complexity and decreased pill burden. This approach is particularly beneficial for India where several logistical and socio-economic factors may hinder access to healthcare and medication adherence. Several studies on the safety and efficacy of the FDC of dapagliflozin and saxagliptin have been conducted in regions outside of India (14, 24), but Indian patients have a distinct cardiometabolic profile. Therefore, it is essential to evaluate the safety profile of the FDC in Indian patients. A recent Indian consensus study on the use of the FDC of dapagliflozin and saxagliptin emphasizes the urgent need for its use in the Indian population (25). However, before its widespread use, it is crucial to assess its safety profile in this specific population. The present study was conducted as a part of local regulatory requirements to provide post-marketing safety data for the FDC of dapagliflozin and saxagliptin in Indian patients with T2DM. Our results indicated that the FDC was safe and well tolerated with no new safety concerns. Additionally, a significant reduction in HbA1c levels, FPG, and body weight was observed at 24 weeks.
With regards to the safety profile of the FDC of dapagliflozin and saxagliptin, there were no new unexpected safety concerns. Additionally, no new cases of HHF were reported in this study. The majority of AEs were mild in severity and were not related to the study drugs. The Phase 3 trials have reported urinary tract infections in 3.1% to 5.2% of patients, genital infections in 1.6% to 5.0%, nasopharyngitis in 3.7%, and influenza in 1.0% to 5.0%, with FDC of dapagliflozin and saxagliptin, over the 24-week treatment (11–13, 26). However, in the current study, the overall incidence of urinary tract infections was 2.6% and nasopharyngitis was 1.5%. Also, the overall incidence of nausea (1%), vomiting (1%), headache (0.5%), and cardiac disorders (0.5%) is lower than that reported in the previous studies (headache: 3.5% to 5.6% and diarrhea: 1.9% to 2.2%) (12, 13). Considering the small sample size in our study, larger studies with long-term follow-up period are needed.
A meta-analysis of 8 randomized clinical trials demonstrated that patients treated with the combination of dapagliflozin and saxagliptin experienced a higher frequency of hypoglycemia compared to either agent as an add-on to metformin. On the contrary, genital infections were less frequently reported with the combination regimen, compared to dapagliflozin alone added to metformin (14). However, in our study, AESIs were reported in 4 patients, with 1 mild hypoglycemic event (0.5%) which resolved with dietary intervention, and genital infections in 3 (1.5%) patients. These observations are consistent with a post-hoc pooled safety analysis, which revealed that the combination of dapagliflozin and saxagliptin was safe and well tolerated, whether administered concurrently or sequentially with metformin therapy, over a 24-week treatment duration (13). Moreover, there were no clinically significant abnormalities with regard to vital signs, hematology, clinical chemistry, physical examination, and ECG in our study. Overall, these findings demonstrate an acceptable safety profile of the FDC of dapagliflozin and saxagliptin as an add-on to metformin among Indian patients with T2DM.
Our study also assessed short-term glycemic control with the FDC over 24 weeks. Previous investigations have documented the mean change from baseline in HbA1c ranging from -0.5% to -1.7% at 24 weeks and from -1.2% to -1.4% over the duration of 52 weeks following treatment with the combination of dapagliflozin and saxagliptin in patients with T2DM (9–12, 27–29). Consistent with these findings, our study reported a mean change in HbA1c of -1.2% at 24 weeks. Along similar lines, prior investigations also reported a reduction in FPG levels from baseline with this combination, ranging from -9 to -38 mg/dL at 24 weeks (9–12) and -26 to -35.8 mg/dL at 52 weeks (10, 28). Our study also reports a significant mean change in FPG of -24.4 mg/dL from baseline to Week 24 (p < 0.0001), aligning with the previous observations (9–12). Further, findings from a meta-analysis indicated a significantly greater reduction in HbA1c and FPG levels from baseline with the combination of dapagliflozin and saxagliptin as add-on to metformin, compared to individual agents (14).
Weight management is a critical treatment goal for individuals with T2DM, considering the comorbidity burden in Indian patients. Accordingly, treatment guidelines advocate for the incorporation of glucose-lowering regimens that actively support patients in achieving weight reduction goals (30). Our study showed a reduction in the mean body weight (74.2 ± 12.6 kg versus 72.2 ± 11.5 kg) with the mean change from baseline of 2.1 kg following 24 weeks of treatment (p < 0.0001). These results corroborate prior findings with the mean change in body weight from baseline ranging from -1.5 to -2.1 kg at 24 weeks (9, 10, 12, 27, 29) and -2.3 to -3.2 kg at 52 weeks (10, 27, 28), with the combination of dapagliflozin and saxagliptin. These findings highlighted that effective glycemic control is achieved with the use of a combination of dapagliflozin and saxagliptin without a corresponding increase in body weight. However, further comparative studies focusing the real-world effectiveness of dapagliflozin and saxagliptin with other glucose-lowering agents may provide conclusive results.
While, recent meta-analyses have revealed a significant reduction in SBP with the dual combination of dapagliflozin and saxagliptin added to metformin, when compared to either dapagliflozin or saxagliptin added to metformin (14, 31), our study did not find a statistically significant reduction in SBP at 24 weeks.
The use of dapagliflozin and saxagliptin FDC presents a rational approach for achieving optimal glycemic control and better tolerability while minimizing pill burden. Incorporating a once-daily FDC regimen holds promise in enhancing patient compliance, particularly for Indian patients with T2DM, burdened with multiple comorbidities.
Limitations of this study include absence of a control group, which prevented comparison with other oral antidiabetic agents and lack of stratified efficacy assessment for HbA1c or body mass index. The short follow-up time and small sample size also limit the generalizability of our findings. Additionally, due to real-world nature of the study, safety data was missing for >25% of patients alongside. Concomitant medications including glucose-lowering medications were recorded at baseline alone, also the impact of these medications including baseline metformin on the overall safety and efficacy parameters was not analyzed. This study was conducted as a part of regulatory commitment however, with the increased utilization of the FDC, comprehensive reporting of real-world data can be generated providing further evidence on the efficacy and tolerability of the FDC in the Indian population. Conducting long-term studies with larger sample size might yield more information regarding the effectiveness and safety profile of this combination as well as the incidence of rare AEs.
This was the real-world study for assessing the safety of dapagliflozin and saxagliptin FDC in Indian population. Our study demonstrated that the FDC is safe and well tolerated with no new safety signals compared to the available safety data on individual components. With significant improvement in glycemic control and body weight reduction, this FDC may be a suitable treatment option for patients with T2DM inadequately controlled with metformin. However, large-scale, long-term, comparative studies with robust methodology may help uncover further safety limitations and efficacy benefits providing greater insights into the clinical utility of this FDC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by 1. ECR/13/Inst/UP/2013/RR-19- Institutional Ethics Committee M.V Hospital & Research Centre2. ECR/1116/Inst/TG/2018 – 21 - Institutional Ethics Committee Thumbay Hospital New Life3. ECR/213/Inst/TN/2013/RR-19 Institutional Ethics Committee KG Hospital - K. Govindaswamy Naidu Medical Trust 4. ECR/68/inst/OR/2013/RR-19 - Instituitional Ethics Committee Sparsh Hospital5. ECR/28/Inst/PB/2013/RR-19 Institutional Ethics Committee Fortis Hospital 6. ECR/35/Inst/WB/2013/RR-19 IPGME&R Research Oversight Committee7. ECR/5/Inst/DL/2013/RR-19 Instutional Ethics Committee-Clinical StudiesIndraprastha Apollo Hospitals8. ECR/34/Inst/KA/2013/RR-19 Ethics committee of Manipal Hospitals9. ECR/25/Inst/CH/2013/RR-20 Institutional Ethics Committee Post Graduate Institute of Medical Education and Research. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SW: Writing – review & editing. SB: Writing – review & editing. FF: Writing – review & editing. GJ: Writing – review & editing. IP: Writing – review & editing. KM: Writing – review & editing. KS: Writing – review & editing. SG: Writing – review & editing. HB: Writing – review & editing. SG: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project is funded by AstraZeneca Pharma India Limited.
The authors would like to thank Dr Ranjini Sen, Nandhini S, and Akshaya Sivakumar of AstraZeneca Pharma India Ltd for supervising the study. The authors also would like to thank Parul Rishi and Dr. Suvarna Chavan of Fortrea Scientific Pvt Ltd, for providing medical writing assistance in accordance with GPP 2022 guidelines (https://www.ismpp.org/gpp-2022).
Author HB was employed by the company AstraZeneca Pharma India Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1528801/full#supplementary-material
AE, adverse event; AESI, adverse events of special interest; DPP4i, dipeptidyl peptidase 4 inhibitors; ECG, electrocardiogram; FDC, fixed-dose combination; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HHF, hospitalization for heart failure; SBP, systolic blood pressure; SD, standard deviation; SGLT2i, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus.
1. Kumar A, Gangwar R, Ahmad Zargar A, Kumar R, Sharma A. Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Curr Diabetes Rev. (2024) 20:105–14. doi: 10.2174/1573399819666230413094200
2. Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. (2021) 69:2932–8. doi: 10.4103/ijo.IJO_1627_21
3. India diabetes report 2000 — 2045 . Available online at: https://diabetesatlas.org/data/ (Accessed May 20, 2024).
4. Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res Care. (2019) 7:e000654. doi: 10.1136/bmjdrc-2019-000654
5. Rajan Y, Bhabhor H, Kharde A, Kakadiya J, Varsadiya K, Damor A. Poor glycaemic control and it’s risk factors among diabetes patients in an urban area of western India. Natl J Community Med. (2024) 15:47–55. doi: 10.55489/njcm.150120243602
6. Unnikrishnan R, Gupta PK, Mohan V. Diabetes in south asians: phenotype, clinical presentation, and natural history. Curr Diabetes Rep. (2018) 18:30. doi: 10.1007/s11892-018-1002-8
7. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S158–78. doi: 10.2337/dc24-S009
8. Chadha M, Das AK, Deb P, Gangopadhyay KK, Joshi S, Kesavadev J, et al. Expert opinion: optimum clinical approach to combination-use of SGLT2i + DPP4i in the Indian diabetes setting. Diabetes Ther. (2022) 13:1097–114. doi: 10.1007/s13300-022-01219-x
9. Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. (2015) 38:376–83. doi: 10.2337/dc14-1142
10. Handelsman Y, Mathieu C, Prato SD, Johnsson E, Kurlyandskaya R, Iqbal N, et al. Sustained 52-week efficacy and safety of triple therapy with dapagliflozin plus saxagliptin versus dual therapy with sitagliptin added to metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab Wiley. (2019) 21:883. doi: 10.1111/dom.13594
11. Matthaei S, Catrinoiu D, Celiński A, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. (2015) 38:2018–24. doi: 10.2337/dc15-0811
12. Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. (2015) 38:2009–17. doi: 10.2337/dc15-0779
13. Del Prato S, Rosenstock J, Garcia-Sanchez R, Iqbal N, Hansen L, Johnsson E, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: Post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab. (2018) 20:1542–6. doi: 10.1111/dom.13258
14. Zhuang Y, Song J, Ying M, Li M. Efficacy and safety of dapagliflozin plus saxagliptin vs monotherapy as added to metformin in patients with type 2 diabetes. Med (Baltimore). (2020) 99:e21409. doi: 10.1097/MD.0000000000021409
15. Tiwari K, Bisht M, Kant R, Handu SS. Prescribing pattern of anti-diabetic drugs and adherence to the American Diabetes Association’s (ADA) 2021 treatment guidelines among patients of type 2 diabetes mellitus: A cross-sectional study. J Fam Med Prim Care. (2022) 11:6159–64. doi: 10.4103/jfmpc.jfmpc_458_22
16. QTERN (dapagliflozin and saxagliptin) Summary of product characteristics (2021). Available online at: https://www.ema.europa.eu/en/documents/product-information/qtern-epar-product-information_en.pdf (Accessed May 23, 2024).
17. QTERN® (dapagliflozin and saxagliptin) tablets (2017). FDA. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209091s000lbl.pdf (Accessed May 23, 2024).
18. AstraZeneca India receives marketing permission for QTERN®, once-daily anti-diabetes treatment for adults with type 2 diabetes (2019). Available online at: https://www.astrazeneca.in/media/press-releases/2019/astrazeneca-India-receives-marketing-permission-for-qtern.html (Accessed July 5, 2025).
19. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PloS Med. (2007) 4:e297. doi: 10.1371/journal.pmed.0040297
20. Böhm A-K, Schneider U, Aberle J, Stargardt T. Regimen simplification and medication adherence: Fixed-dose versus loose-dose combination therapy for type 2 diabetes. PloS One. (2021) 16:e0250993. doi: 10.1371/journal.pone.0250993
21. Baumgartner A, Drame K, Geutjens S, Airaksinen M. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. (2020) 12:190. doi: 10.3390/pharmaceutics12020190
22. Hutchins V, Zhang B, Fleurence RL, Krishnarajah G, Graham J. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin Taylor Francis;. (2011) 27:1157–68. doi: 10.1185/03007995.2011.570745
23. Paoli CJ, Linder J, Gurjar K, Thakur D, Wyckmans J, Grieve S. Effectiveness of single-tablet combination therapy in improving adherence and persistence and the relation to clinical and economic outcomes. J Health Econ Outcomes Res. (2024) 11:8–22. doi: 10.36469/001c.91396
24. Vilsbøll T, Ekholm E, Johnsson E, Garcia-Sanchez R, Dronamraju N, Jabbour SA, et al. Efficacy and safety of dapagliflozin plus saxagliptin versus insulin glargine over 52 weeks as add-on to metformin with or without sulphonylurea in patients with type 2 diabetes: A randomized, parallel-design, open-label, Phase 3 trial. Diabetes Obes Metab. (2020) 22:957–68. doi: 10.1111/dom.13981
25. Ghosh S, Wangnoo SK, Chittawar S, Damodharan S, Kadam Y, Kalra P, et al. Dapagliflozin-saxagliptin combination - the quest for optimal glycemic control with cardio-renal protection in type 2 diabetes mellitus: an expert consensus in Indian settings. J Endocrinol Metab. (2024) 14:128–48. doi: 10.14740/jem.v14i3.946
26. Rosenstock J, Perl S, Johnsson E, García-Sánchez R, Jacob S. Triple therapy with low-dose dapagliflozin plus saxagliptin versus dual therapy with each monocomponent, all added to metformin, in uncontrolled type 2 diabetes. Diabetes Obes Metab. (2019) 21:2152–62. doi: 10.1111/dom.13795
27. Müller-Wieland D, Kellerer M, Cypryk K, Skripova D, Rohwedder K, Johnsson E, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. (2018) 20:2598–607. doi: 10.1111/dom.13437
28. Frias JP, Gonzalez-Galvez G, Johnsson E, Maaske J, Testa MA, Simonson DC, et al. Efficacy and safety of dual add-on therapy with dapagliflozin plus saxagliptin versus glimepiride in patients with poorly controlled type 2 diabetes on a stable dose of metformin: Results from a 52-week, randomized, active-controlled trial. Diabetes Obes Metab. (2020) 22:1083–93. doi: 10.1111/dom.13997
29. Vilsbøll T, Ekholm E, Johnsson E, Dronamraju N, Jabbour S, Lind M. Dapagliflozin plus saxagliptin add-on therapy compared with insulin in patients with type 2 diabetes poorly controlled by metformin with or without sulfonylurea therapy: A randomized clinical trial. Diabetes Care Am Diabetes Association;. (2019) 42:1464–72. doi: 10.2337/dc18-1988
30. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2023. Diabetes Care. (2023) 46:S140–57. doi: 10.2337/dc23-S009
Keywords: prospective, single-arm, dapagliflozin, saxagliptin, fixed-dose combination, diabetes mellitus type II, India, safety
Citation: Wangnoo SK, Bhadada SK, Farishta F, Jayaram Naidu GG, Pattnaik I, Manohar KN, Singh KP, Gupta SK, Bharath HS and Ghosh S (2025) Safety and efficacy of fixed-dose combination of dapagliflozin and saxagliptin in patients with type 2 diabetes mellitus – a phase 4 study in India. Front. Endocrinol. 16:1528801. doi: 10.3389/fendo.2025.1528801
Received: 15 November 2024; Accepted: 31 January 2025;
Published: 03 March 2025.
Edited by:
Wen-hong Li, University of Texas Southwestern Medical Center, United StatesCopyright © 2025 Wangnoo, Bhadada, Farishta, Jayaram Naidu, Pattnaik, Manohar, Singh, Gupta, Bharath and Ghosh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sujoy Ghosh, ZHJzdWpveWdob3NoMjAwMEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.