
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 March 2025
Sec. Renal Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1526694
Background: Kidney stones are a major public health concern, and their prevalence has increased significantly in recent decades. While urinary albumin-to-creatinine ratio (UACR) is a recognized marker for kidney disease, its relationship with kidney stones, especially within the normal UACR range, remains unclear. The purpose of this study was to investigate the association between UACR levels within the normal range and the risk of developing kidney stones.
Methods: We analyzed data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2009 to 2018, focusing on adults aged 20 years and older with available UACR data. Using weighted multivariable logistic regression and restricted cubic spline (RCS) models, we assessed the relationship between UACR levels and the prevalence of kidney stones, adjusting for relevant covariates. Subgroup analyses were also performed to evaluate the consistency of this association across demographic and health-related factors.
Results: The study found that higher UACR levels within the normal range were significantly associated with an increased likelihood of developing kidney stones. Specifically, individuals in the highest quartile of UACR had a 36% higher odds of kidney stones compared to those in the lowest quartile (OR: 1.36, 95% CI: 1.04-1.77). A non-linear, dose-response relationship was observed between UACR levels and kidney stone risk (P < 0.001), with the association remaining consistent across various demographic subgroups.
Conclusion: Elevated UACR levels, even within the normal range, are strongly associated with a higher risk of kidney stones. This finding highlights the potential of UACR as a valuable biomarker for assessing kidney stone risk in clinical practice.
Kidney stones are a significant public health issue globally, particularly prevalent in industrialized nations (1, 2). Over the past few decades in America, there has been a significant increase in the prevalence of kidney stones, impacting millions and adversely affecting their overall well-being (2, 3). The prevalence of kidney stones in the United States is approximately 9-10% of adults, with a growing incidence across various demographic groups, including men and women of different age groups, ethnicities, and socioeconomic statuses (4). The recurrence rate of kidney stones is notably high, with studies showing that up to 50% of individuals who have had a kidney stone will experience another episode within 10 years (1, 5, 6). This recurrence rate is particularly concerning given the painful nature of kidney stone episodes and the associated risk of chronic kidney disease (CKD) over time. The formation of kidney stones is influenced by a wide range of factors, including diet, genetics, hydration levels, and underlying health conditions (6–8). Increased dietary salt and oxalate intake, insufficient fluid consumption, and sedentary behavior are key contributors to the rising incidence (9–11). As a result, the recurrent nature of kidney stones further emphasizes the need for early identification, effective management, and preventive measures (6, 12).
Elevated levels of albumin in urine, known as albuminuria, are considered a warning sign of early renal impairment (13), and the urinary albumin-to-creatinine ratio (UACR) serves as a tool for monitoring the initial stages of chronic kidney disease (CKD) (14–16). Although a UACR within normal limits is typically considered indicative of healthy kidney function, recent studies have begun to focus on the health implications of minor variations within this range (17, 18). Specifically, increases in UACR within the normal range may reflect subtle, undetected renal impairments, which might be associated with a heightened risk of developing kidney stones (19, 20). Our analysis focuses on these minor fluctuations within the normal UACR range, which may have clinical significance for the early prevention and holistic treatment of kidney stones.
Here, based on data from the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2018, we reveal the benefits of monitoring UACR within normal limits for the prevention and control of kidney stones on a public health scale. It offers crucial insights for the development of focused prevention strategies and the enhancement of clinical practices.
The study utilized information from the NHANES, a continuous program designed to evaluate the health and nutritional well-being of Americans. Around 5,000 individuals from the U.S. are involved each year, contributing information on their demographics, socioeconomic status, eating patterns, and health status. The data is gathered through in-person interviews and thorough physical assessments that include physiological measurements and lab tests. The process of obtaining ethical approval and informed consent was approved by the Institutional Review Board of the National Center for Health Statistics.
In this observational study, data was collected from the NHANES database covering a period from 2009 to 2018. Initially, 49,693 potential participants were assessed based on predefined criteria for inclusion and exclusion. Participants were excluded if they were younger than 20 years old (20,858 individuals), lacked historical kidney stone data (65 individuals), had incomplete UACR data or UACR levels exceeding 30 mg/g (5,130 individuals), or were missing key covariate data (5,900 individuals). After applying these exclusions, a total of 17,740 individuals met the criteria and were included in the final dataset for analysis. For further specifics, please refer to Figure 1.
Urine specimens were carefully prepared and stored at an ideal temperature of -30°C for further examination. The presence of urinary albumin was determined through a solid-phase fluorescence immunoassay technique, and the amount of urinary creatinine was measured using an enzymatic quantification method. The NHANES website provides a complete summary of the laboratory methodology (21). Following the NHANES guidelines, urinary albumin and creatinine levels underwent standardization and calibration utilizing the recognized gold standard approach. The UACR was presented in units of milligrams per gram (mg/g).
The verification of the precision of participants’ self-reported history of kidney stones was achieved through inquiring, “Have you experienced kidney stones in the past?” This approach to confirmation is backed by existing research (22, 23). Consequently, individuals who affirmed the question were classified as having experienced kidney stones in the past.
The research incorporated various factors linked to UACR levels and the risk of kidney stone formation, divided into demographic, lifestyle, and health-related categories. Demographically, the study considered age, gender, ethnicity, marital status, education, and economic hardship. Lifestyle factors included alcohol consumption patterns (classified as never having consumed more than 12 drinks in their lifetime, former drinkers who had more than 12 drinks annually but not in the past year, and current drinkers who had more than 12 drinks in their lifetime and at least once in the past year) (24), smoking habits (determined by a history of smoking over 100 cigarettes), sedentary behavior (daily sitting time, excluding sleep, with categories for less than 5 hours and 5 hours or more), and physical exercise (assessed by the time spent on moderate to vigorous activities for at least 10 consecutive minutes daily, with inactivity defined as less than 10 minutes per day) (25). Sedentary behavior was defined based on a participant’s reported sitting time during activities such as sitting at school, at home, commuting, reading, watching television, or using a computer, excluding time spent sleeping. Health metrics encompassed the Body Mass Index (BMI), serum uric acid, the estimated glomerular filtration rate (eGFR), as well as conditions such as diabetes, hypertension, hyperlipidemia, and cardiovascular disease (CVD). These data were gathered utilizing standardized surveys and medical evaluations. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (26):
Where SCr represents serum creatinine (mg/dL), and the values are adjusted based on the patient’s age, gender, and race (if Black).
Adhering to the NHANES protocol for sampling and weighting helped ensure that the sample was representative, with appropriate modifications to the weights. Specifically, we used the “Full sample 2year MEC exam weight” provided in the NHANES dataset. The statistical methods included analyzing continuous data (mean values and standard deviations) and categorical data (counts and proportions), using weighted linear regression for the former and chi-square tests for the latter.
We utilized a multivariable logistic regression analysis to explore the potential association between UACR and the risk of kidney stones. UACR was evaluated both as a continuous measure and in a categorized manner, divided into quartiles with the lowest quartile serving as the control group (18). For each regression model, we computed the odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs). The crude model was not adjusted for any covariates, whereas Model 1 included adjustments for age, gender, ethnicity, educational attainment, marital status, and socioeconomic status. Model 2 further incorporated adjustments for smoking habits, alcohol intake, BMI, serum uric acid, eGFR, sedentary lifestyle, exercise levels, hypertension, diabetes, hyperlipidemia, and CVD. To delve into the potential dose-response relationship between UACR and the likelihood of kidney stones, we employed restricted cubic spline (RCS) regression analysis. Additionally, subgroup analyses were conducted to verify the consistency of our results across different demographic and health-related variables. To assess the predictive ability of UACR for kidney stone formation, we conducted a receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC) was calculated to evaluate the discriminative ability of UACR for distinguishing between individuals with and without kidney stones. All statistical computations were performed using R software, version 4.3.2, with statistical significance defined as P<0.05.
In this analysis, data from 17,740 participants spanning five NHANES cycles from 2009 to 2018 were examined. The study categorized participants into four groups based on their UACR. Table 1 outlines the demographic characteristics of the cohort, with a mean age of 46.44 years (± 0.30), 50.75% female participants, and a weighted prevalence of kidney stones of 9.64%. The findings suggest that individuals with elevated UACR values, even within what is considered normal, are more likely to be older, predominantly female, of Mexican American ethnicity, unmarried (including divorced, separated, or widowed), have lower educational levels, lower income ratios, lower BMI, lower serum uric acid level, be smokers, non-drinkers, lead sedentary lifestyles, and have a history of hypertension, diabetes, hyperlipidemia, and CVD. Additionally, there is a significant correlation between higher UACR levels and a higher likelihood of developing kidney stones.
Table 2 reveals a substantial positive link between UACR levels and the prevalence of kidney stones, assessed in both continuous and categorical manners. Initial unadjusted calculations showed that a 1 mg/g increment in UACR was associated with a 3% rise in the probability of kidney stone formation (95% CI: 1.01-1.04, P < 0.0001). This correlation persisted as significant in Model 2, which was comprehensively adjusted, yielding an odds ratio (OR) of 1.01 (95% CI: 1.00-1.03, P = 0.04). Within the fully adjusted quartile analysis, the risk escalated by 35% for the third quartile (95% CI: 1.08-1.70, P = 0.01) and by 36% for the fourth quartile (95% CI: 1.04-1.77, P = 0.02) when contrasted with the lowest quartile. Furthermore, employing a RCS regression analysis, a potential dose-response relationship was identified, characterized by a U-shaped distribution with an inflection point at 12.2 (Figure 2).

Figure 2. Illustration highlighting the relationship between UACR levels and the risk of developing kidney stones. The ORs, depicted by solid lines, have been adjusted to account for various factors including gender, age, ethnicity, educational attainment, marital status, economic hardship, BMI, uric acid, smoking habits, alcohol consumption, physical activity, sedentary behavior, eGFR, presence of hypertension, diabetes, hyperlipidemia, and CVD. Additionally, the 95% CIs, represented by shaded regions, have been considered to provide a comprehensive view of the data.
Figure 3 presents the stratified analysis outcomes, highlighting a noticeable positive correlation between UACR and the prevalence of kidney stones across diverse population segments. Particularly, in individuals aged 20-40 years (OR = 1.03, 95% CI: 1.01-1.05), with lower (OR = 1.02, 95% CI: 1.01-1.04) or higher (OR = 1.02, 95% CI: 1.00-1.05) income poverty ratios, BMI ranging from 25-29.99 (OR = 1.02, 95% CI: 1.01-1.04), smokers (OR = 1.02, 95% CI: 1.01-1.04), current alcohol consumers (OR = 1.02, 95% CI: 1.00-1.03), those engaging in frequent physical activity (OR = 1.02, 95% CI: 1.00-1.04), those with shorter sitting durations (OR = 1.03, 95% CI: 1.01-1.04), and those without a history of hypertension (OR = 1.02, 95% CI: 1.00-1.04), those free from diabetes (OR = 1.02, 95% CI: 1.00-1.03), those without CVD (OR = 1.02, 95% CI: 1.00-1.03) and those diagnosed with hyperlipidemia (OR = 1.02, 95% CI: 1.01-1.04), the correlation was notably stronger, with all P-values below 0.05. Furthermore, significant interactions between UACR and kidney stone risk were noted in different age brackets (P < 0.05), whereas no such interactions were observed in other demographic groups (P > 0.05). Additionally, the estimates consistently revealed the same trend across all subgroups.
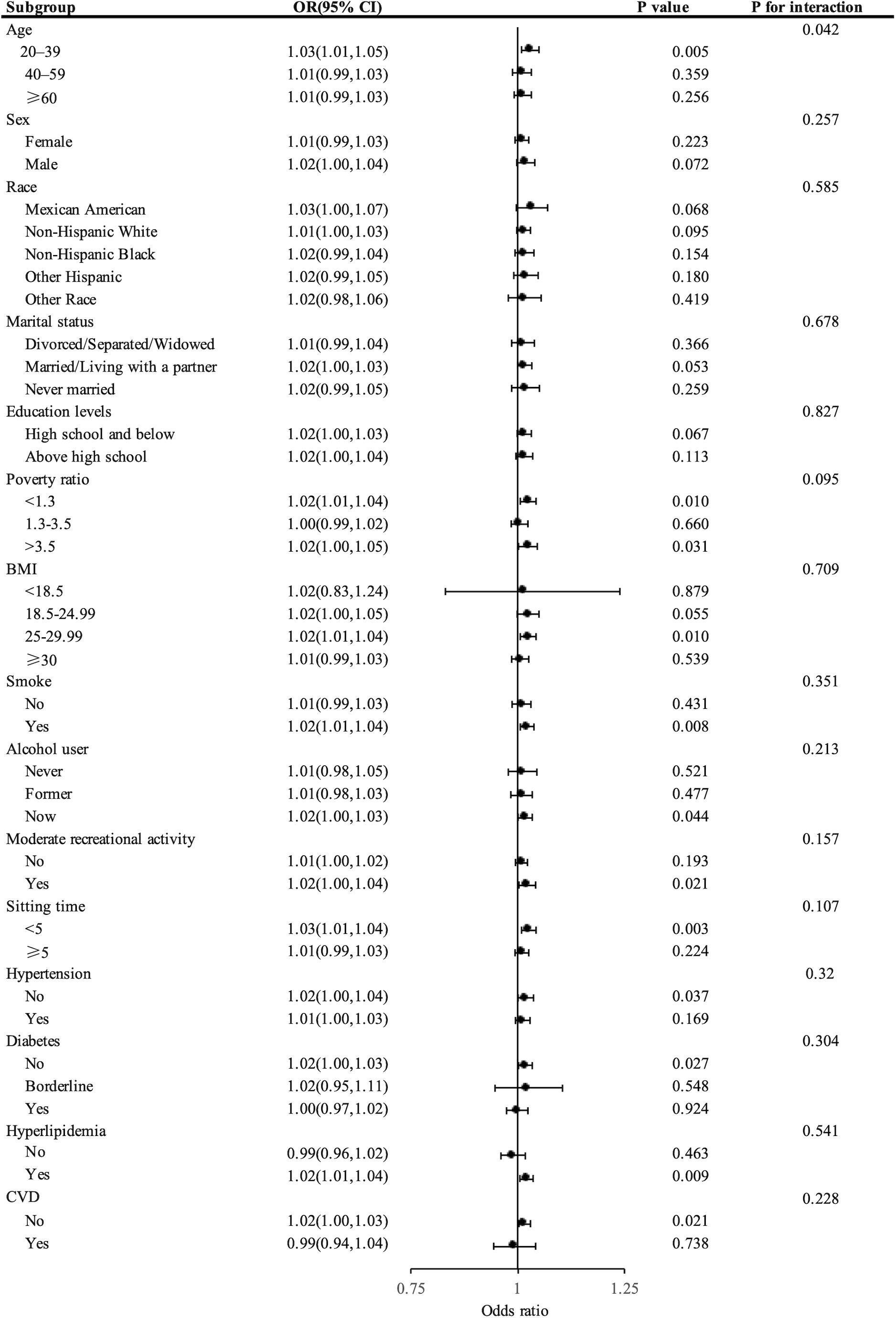
Figure 3. Forest plot showing stratified analysis of the correlation between UACR levels and the risk of developing kidney stones.
To further assess the predictive ability of UACR for kidney stone formation, we conducted a ROC curve analysis. The AUC for the ROC curve was 0.5527, which suggests a moderate ability of UACR to discriminate between individuals with and without kidney stones (Supplementary Figure 1).
The research draws on data from the NHANES to explore the potential link between normal levels of UACR and the likelihood of developing kidney stones. The findings suggest that even a minor increase in UACR within the normal range is associated with a higher risk of kidney stones, especially in individuals in the third and fourth quartiles when compared to those in the lowest quartile. The application of RCS regression models uncovered a non-linear, inverted U-shaped correlation between UACR and the risk of kidney stones, indicating a dose-response relationship. Additionally, this robust positive association was observed across various populations, underscoring the consistency and reliability of our findings.
The formation of kidney stones is a complex phenomenon involving multiple biochemical processes, including supersaturation of solutes in urine, nucleation, growth, aggregation, and eventual deposition on renal tissue (27–29). Minor urinary excretion of albumin may reflect subtle physiological changes in the kidneys that could indirectly promote stone formation by altering the biochemical environment of urine (30). For example, minor tubular damage could lead to protein leakage into the urine, potentially altering urine pH, which influence stone formation (31). Additionally, minor renal function changes may alter the concentration of inhibitory substances like citrate (32), which typically help prevent stone formation. Thus, even normal-range variations in UACR could significantly impact the risk of kidney stones.
Further studies indicate that patients with higher UACR have elevated inflammation scores (33), which suggests that increased UACR is not only a marker of CKD but also a possibly underrecognized risk factor for kidney stone formation (34, 35). Inflammatory processes may increase the permeability of renal tubules and surrounding tissues, facilitating more albumin to enter the urine (36). This increased protein excretion could activate proximal tubular epithelial cells, promoting the expression of, chemokines, and cytokines, further influencing the stone formation process (37). Additionally, the kidneys might indirectly affect stone formation probabilities by regulating urine pH and solute concentrations (38). The increase in UACR may reflect a subtle imbalance in renal function, particularly in handling calcium and phosphate, which could lead to increased urinary concentrations of these minerals and ultimately promote stone formation (28, 32). Moreover, renal function impairment might affect the activation of vitamin D (39), influencing calcium absorption and bone health, which may indirectly increase the risk of kidney stones (40).
Therefore, monitoring UACR could be valuable not only for assessing the risk of CKD but also for its potential utility in predicting kidney stone risk. This understanding underscores the importance of adopting a more comprehensive assessment approach in clinical practice to better prevent and manage kidney diseases such as kidney stones. Our study suggests that even individuals with UACR within the normal range but at higher normal values should undergo regular monitoring and possibly further evaluation and intervention, such as dietary adjustments, increased water intake, and appropriate medication, to reduce the formation of kidney stones. This emphasizes the relevance of UACR as a biomarker in public health and clinical practice.
A significant advantage of this research is the utilization of NHANES, a nationwide, representative dataset that offers crucial insights into the link between UACR levels and the likelihood of kidney stone formation. This large-scale population base offers sufficient statistical power to explore the potential links between UACR and kidney stone risk. Moreover, our use of RCS regression models allows us to explore the non-linear relationships between UACR and kidney stone risk, an aspect not extensively covered in previous studies.
However, the study is constrained by its cross-sectional design, preventing causal inferences. Cross-sectional studies can only capture data at one point in time, making it uncertain whether heightened UACR levels are a cause or a consequence of elevated kidney stone risk. Additionally, the study relies on self-reported history of kidney stones, which may be subject to recall bias. This could potentially lead to misclassification or underreporting of the true prevalence of kidney stones. Furthermore, the study does not capture incident cases or clinically confirmed diagnoses, which may result in underestimation of the true prevalence. The study also relies on a single urine measurement per participant, and variations in UACR levels within the normal range could affect the reliability and generalizability of the results. Although we adjusted for various covariates, there may be additional unmeasured factors such as dietary intake, genetic predispositions, or specific medications that could influence the risk of kidney stones. These unaccounted factors could introduce potential confounding or bias. Future research should adopt a prospective cohort design and perform multiple UACR measurements to more accurately determine the causal relationship between UACR and kidney stones. Additionally, considering the multifactorial nature of stone formation involving various environmental, dietary, and genetic factors, future studies should also consider these potential confounders to fully understand the association between UACR and the risk of kidney stones.
This study reveals a significant connection between elevated UACR levels within the normal range and an augmented risk of kidney stones, highlighting the potential value of monitoring UACR levels in the prevention and control of kidney stones. These findings provide new perspectives and evidence for future research and clinical practice.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving humans were approved by NCHS Research Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because data pertinent to the participants were gathered from the NHANES database, which is publicly available, thus bypassing the requirement for further consent. Consistent with national regulations and institutional policies, obtaining written informed consent from the subjects was not required for this research.
YD: Conceptualization, Investigation, Writing – original draft. CZ: Conceptualization, Investigation, Writing – original draft. DZ: Writing – original draft. YL: Data curation, Methodology, Writing – review & editing. YL: Data curation, Methodology, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Hunan Provincial Natural Science Foundation (grant number 2025JJ81032).
We are deeply thankful to the NHANES databases for granting us access to such invaluable data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1526694/full#supplementary-material
1. Scales CD Jr., Smith AC, Hanley JM, Saigal CS, P Urologic Diseases in America. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
2. Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. (2003) 63:1817–23. doi: 10.1046/j.1523-1755.2003.00917.x
3. Trinchieri A, Coppi F, Montanari E, Nero Del A, Zanetti G, Pisani E. Increase in the prevalence of symptomatic upper urinary tract stones during the last ten years. Eur Urol. (2000) 37:23–5. doi: 10.1159/000020094
4. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C D, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007-2018 data. Eur Urol Focus. (2021) 7:1468–75. doi: 10.1016/j.euf.2020.08.011
5. Hill AJ, Basourakos SP, Lewicki P, Wu X, Arenas-Gallo C, Chuang D, et al. Incidence of kidney stones in the United States: the continuous national health and nutrition examination survey. J Urol. (2022) 207:851–6. doi: 10.1097/JU.0000000000002331
6. Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones. Nat Rev Dis Primers. (2016) 2:16008. doi: 10.1038/nrdp.2016.8
7. Ferraro PM, Bargagli M, Trinchieri A, Gambaro G. Risk of kidney stones: influence of dietary factors, dietary patterns, and vegetarian-vegan diets. Nutrients. (2020) 12(3):779. doi: 10.3390/nu12030779
8. Di X, Xiang L, Jian Z, Xia Z, Luo D. Association between urinary phthalate metabolites and nephrolithiasis in adults: A cross-sectional analysis with NHANES 2007-2018. Chemosphere. (2023) 337:139436. doi: 10.1016/j.chemosphere.2023.139436
9. Siener R. Nutrition and kidney stone disease. Nutrients. (2021) 13(6):1917. doi: 10.3390/nu13061917
10. Littlejohns TJ, Neal NL, Bradbury KE, Heers H, Allen NE, Turney BW. Fluid intake and dietary factors and the risk of incident kidney stones in UK biobank: A population-based prospective cohort study. Eur Urol Focus. (2020) 6:752–61. doi: 10.1016/j.euf.2019.05.002
11. Li Y, Di X, Liu M, Wei J, Li T, Liao B, et al. Association between daily sitting time and kidney stones based on the National Health and Nutrition Examination Survey (NHANES) 2007-2016: a cross-sectional study. Int J Surg. (2024) 110:4624–32. doi: 10.1097/JS9.0000000000001560
12. Peerapen P, Thongboonkerd V. Kidney stone prevention. Adv Nutr. (2023) 14:555–69. doi: 10.1016/j.advnut.2023.03.002
13. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. (2015) 4:57–73. doi: 10.5527/wjn.v4.i1.57
14. Babazono T, Hanai K, Yokoyama Y, Uchiyama K. Association between 1-year changes in urinary albumin-to-creatinine ratio and kidney disease progression in Japanese individuals with diabetes: a historical cohort study. Clin Exp Nephrol. (2023) 27:1001–9. doi: 10.1007/s10157-023-02380-8
15. Sun AJ, Thomas IC, Velaer KN, Ganesan C, Song S, Pao AC, et al. The urine albumin-to-creatinine ratio and kidney function after nephrectomy. J Urol. (2020) 204:231–8. doi: 10.1097/JU.0000000000001005
16. Park JI, Baek H, Kim BR, Jung HH. Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: A population-based study. PloS One. (2017) 12:e0171106. doi: 10.1371/journal.pone.0171106
17. Mahemuti N, Zou J, Liu C, Xiao Z, Liang F, Yang X. Urinary albumin-to-creatinine ratio in normal range, cardiovascular health, and all-cause mortality. JAMA Netw Open. (2023) 6:e2348333. doi: 10.1001/jamanetworkopen.2023.48333
18. Ming L, Wang D, Zhu Y. Association between urinary albumin-to-creatinine ratio within normal range and hypertension among adults in the United States: Data from the NHANES 2009-2018. Clin Cardiol. (2023) 46:622–31. doi: 10.1002/clc.24012
19. Erman A, Rahamimov R, Mashraki T, Levy-Drummer RS, Winkler J, David I, et al. The urine albumin-to-creatinine ratio: assessment of its performance in the renal transplant recipient population. Clin J Am Soc Nephrol. (2011) 6:892–7. doi: 10.2215/CJN.05280610
20. John M, Hussain S, Prayle A, Simms R, Cockcroft JR, Bolton CE. Target renal damage: the microvascular associations of increased aortic stiffness in patients with COPD. Respir Res. (2013) 14:31. doi: 10.1186/1465-9921-14-31
21. (CDC), C.f.D.C.a.P. NHANES serum, plasma, and urine specimens. (2025). Available online at: https://www.cdc.gov/nchs/nhanes/spu-specimens/ (Accessed February 20, 2025).
22. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. (1993) 328:833–8. doi: 10.1056/NEJM199303253281203
23. Di X, Liu S, Xiang L, Jin X. Association between the systemic immune-inflammation index and kidney stone: A cross-sectional study of NHANES 2007-2018. Front Immunol. (2023) 14:1116224. doi: 10.3389/fimmu.2023.1116224
24. Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in U.S. Adults: A prospective cohort study. Ann Intern Med. (2021) 174:167–74. doi: 10.7326/M20-1340
25. Vasquez E, Batsis JA, Germain CM, Shaw BA. Impact of obesity and physical activity on functional outcomes in the elderly: data from NHANES 2005-2010. J Aging Health. (2014) 26:1032–46. doi: 10.1177/0898264314535635
26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
27. Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. (2005) 115:2598–608. doi: 10.1172/JCI26662
28. Alelign T, Petros B. Kidney stone disease: an update on current concepts. Adv Urol. (2018) 2018:3068365. doi: 10.1155/2018/3068365
29. Khan SR, Glenton PA, Backov R, Talham DR. Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int. (2002) 62:2062–72. doi: 10.1046/j.1523-1755.2002.00676.x
30. Koeda Y, Tanaka F, Segawa T, Ohta M, Ohsawa M, Tanno K, et al. Comparison between urine albumin-to-creatinine ratio and urine protein dipstick testing for prevalence and ability to predict the risk for chronic kidney disease in the general population (Iwate-KENCO study): a prospective community-based cohort study. BMC Nephrol. (2016) 17:46. doi: 10.1186/s12882-016-0261-3
31. Imenez Silva PH, Mohebbi N. Kidney metabolism and acid-base control: back to the basics. Pflugers Arch. (2022) 474:919–34. doi: 10.1007/s00424-022-02696-6
32. Patel PM, Kandabarow AM, Druck A, Hart S, Blackwell RH, Kadlec A, et al. Association of impaired renal function with changes in urinary mineral excretion and stone composition. Urology. (2020) 141:45–9. doi: 10.1016/j.urology.2020.03.023
33. Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. (2012) 7:1938–46. doi: 10.2215/CJN.03500412
34. Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. (2020) 173:426–35. doi: 10.7326/M20-0529
35. Okubo A, Nakashima A, Doi S, Doi T, Ueno T, Maeda K, et al. High-normal albuminuria is strongly associated with incident chronic kidney disease in a nondiabetic population with normal range of albuminuria and normal kidney function. Clin Exp Nephrol. (2020) 24:435–43. doi: 10.1007/s10157-019-01842-2
36. Abbate M, Zoja C, Corna D, Capitanio M, Bertani T, Remuzzi G. In progressive nephropathies, overload of tubular cells with filtered proteins translates glomerular permeability dysfunction into cellular signals of interstitial inflammation. J Am Soc Nephrol. (1998) 9:1213–24. doi: 10.1681/ASN.V971213
37. Zoja C, Benigni A, Remuzzi G. Protein overload activates proximal tubular cells to release vasoactive and inflammatory mediators. Exp Nephrol. (1999) 7:420–8. doi: 10.1159/000020640
38. Sohnel O, Grases F. Supersaturation of body fluids, plasma and urine, with respect to biological hydroxyapatite. Urol Res. (2011) 39:429–36. doi: 10.1007/s00240-011-0387-5
39. Ganimusa I, Chew E, Lu EM. Vitamin D deficiency, chronic kidney disease and periodontitis. Medicina (Kaunas). (2024) 60(3):420. doi: 10.3390/medicina60030420
Keywords: urinary albumin-to-creatinine ratio, kidney stones, national health and nutrition examination survey, cross-sectional study, U.S. adults
Citation: Du Y-Z, Zhang C-T, Zeng D-M, Li Y and Liu Y-F (2025) Association between urinary albumin-to-creatinine ratio within normal range and kidney stones in U.S. adults: a cross-sectional observational study. Front. Endocrinol. 16:1526694. doi: 10.3389/fendo.2025.1526694
Received: 12 November 2024; Accepted: 25 February 2025;
Published: 14 March 2025.
Edited by:
Mohamed El-Sherbiny, Almaarefa University, Saudi ArabiaReviewed by:
Dalia Elsherbini, Mansoura University, Mansoura, EgyptCopyright © 2025 Du, Zhang, Zeng, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li, bGl5b25nMDcyNEAxMjYuY29t; Yi-Fu Liu, bHlmMTM3NTU2MDU3NzYyMDIyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.