
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 26 March 2025
Sec. Adrenal Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1525844
This article is part of the Research Topic Circadian Rhythm in Adrenal Endocrinology View all 4 articles
 Tomohiro Otani1†
Tomohiro Otani1† Takahito Miyake1†
Takahito Miyake1† Takumi Ota1†
Takumi Ota1† Daisuke Yarimizu1
Daisuke Yarimizu1 Yuuki Nakagawa1
Yuuki Nakagawa1 Iori Murai1
Iori Murai1 Hitoshi Okamura1,2
Hitoshi Okamura1,2 Emi Hasegawa1
Emi Hasegawa1 Masao Doi1*
Masao Doi1*The mammalian circadian timing system is organized in a hierarchy, with the master clock residing in the suprachiasmatic nucleus (SCN) of the hypothalamus and subsidiary peripheral clocks in peripheral tissues. Because of the diversity of peripheral tissues and cell-types in the body, the existence of autonomous clock and identification of its potential entrainment signals need to be empirically defined on a cell type-by-cell type basis. In this study, we characterized the basic circadian clock properties of the adrenal zona glomerulosa cells, or ZG cells. Using isolated adrenal explants from Per2Luc mice, dissociated ZG cells from Per2-dluc rats, and a related human adrenocortical cell line H295R, we showed that ZG cells possess genetically-encoded, self-sustained and cell-autonomous circadian clock. As to the potential entrainment signals, angiotensin II (Ang II) caused phase-dependent phase-shifts of adrenal ZG cells in cultured slices. Ang II treatment also drove initiation (or reset) of circadian clock gene expression in H295R cells with associated immediate up-regulation of PER1 and E4BP4 mRNA expression. We found that the type I Ang II receptor blocker CV11974, one of the most widely used clinical drugs for hypertensive diseases, caused attenuation of the phase resetting of H295R cells. Our in vitro data provide a basis to understand and argue for the adrenal gland ZG cells as a component of autonomous and entrainable peripheral clocks.
Most aspects of physiology and behavior display circadian rhythms driven by an endogenous clock (1, 2). In mammals, a master pacemaker in the suprachiasmatic nucleus (SCN) regulates downstream oscillators in peripheral tissues (3–5). In all clock cell types, regardless of central vs. peripheral, the basic clock system is composed of three elementary elements: an oscillator that oscillates even under constant conditions; an input that enables the oscillator to synchronize with environmental cycles or internal time cues; and an output that transmits the oscillator’s signals into rhythmic gene expression and physiology, as referred to as the Eskinogram model (6). Within the oscillator, opposing effects of transcriptional activators and repressors constitute interlocked feedback loops (7–9). Briefly, the transcriptional activators CLOCK and BMAL1 drive transcription of Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2) via binding to E-box elements present in their promoters. Once the repressor proteins PER and CRY reach a critical concentration, they form complexes and repress their own expression by inhibiting the CLOCK/BMAL1 complexes. In addition to E-box elements, the promoter regions of Per genes contain D-boxes, through which the PAR bZip family of transcriptional activators (DBP, TEF and HLF) and the related repressor E4BP4 exert their opposing effects on the expression of target genes (10–15). The retinoic acid receptor-related orphan receptor binding elements (RRE) also provide a shared site for RORs (activator) and REV-ERBs (repressor), thereby forming a loop, in which REV-ERBα/β proteins indirectly regulate expression of Per genes by suppressing the transcription of Bmal1 and E4bp4 through the RRE on their promoters (15–18). In principle, phase entrainment or resetting of the clock must impact the activities or levels of the molecular components of these loops, and the induction of Per1 gene expression is believed to be a critical step in this process (19–23). Other parallel mechanisms that contribute to entrainment might exist. It would be expected that these unknown input pathways would also impinge on the loops described above.
The phase of the molecular clocks in peripheral tissues are influenced by various external and/or endogenous signals. Numerous endogenous neural, humoral, and metabolic factors, including glucocorticoids, insulin, glucose, retinoic acid, and Ang II have been reported to affect circadian gene expression in cultured cell lines (24–28). External time signals such as feeding and physical activity also affect peripheral tissue clocks (29, 30). It is also reported that several medically prescribed drugs (medical external inputs) drastically change clock gene expression (31–34). Because of the diversity of peripheral tissues and cell-types in the body, the existence of autonomous clock and identification of its potential entrainment signal(s), either external (e.g., light, food intake) or internal (hormonal, neuronal, body temperature), need to be empirically defined on a cell type-by-cell type basis.
In the present study, we focused our attention to evaluating the basic circadian clock properties of adrenal ZG cells. The zona glomerulosa, termed ZG, is a specific component of the adrenal gland cortex. The ZG cells are located in the outermost zone of the cortex and responsible for synthesizing and secreting the steroid hormone aldosterone (35). As a critical element of the renin–angiotensin–aldosterone system (RAAS), these cells express the aldosterone-synthesizing enzyme (AS, or Cyp11b2) under the control of Ang II and play a role in blood pressure homeostasis (36). We previously found that mice lacking the core circadian clock components Cry1 and Cry2 exhibit hyperplasia of ZG cells and resultantly show salt-sensitive hypertension phenotype (37). However, it is still not known (remains unexplored) whether ZG cells possess endogenous circadian oscillators, and if so, what their phase (time) entrainment signals are. This current situation contrasts to the extensive research on the circadian aspect of the adrenal gland in the hypothalamic-pituitary-adrenal (HPA) axis (38–41). There are several adrenal-cortex in situ hybridization data in the literature, which suggest for the circadian gene expression in the region of ZG layer (38, 42); however, direct evidence to show the rhythmicity of ZG cells is missing. Within this paradigm, in the present study, we have demonstrated the presence of autonomous clock in ZG cells and tested their potential entrainment capacity to Ang II and Ang II receptor antagonist (CV11974) using different cell culture systems. Isolated adrenal explants of Per2Luc mice, dissociated ZG cells from Per2-dluc rats, and a related human adrenocortical cell line H295R, were used in this study.
Per2-dluc transgenic rats (43), Per2Luc knock-in mice (44), and ClockΔ19/Δ19 mice (45) were bred and genotyped as described previously (46–48). Animals were housed under a regular 12-h light/12-h dark (LD) cycle, maintained at 22 ± 2°C, with free access to food and water. All animal experiments were conducted in compliance with the Ethical Regulations of Kyoto University and performed under protocols approved by the Animal Care and Experimentation Committee of Kyoto University.
Adrenals from Per2Luc mice (male, 8–12 weeks old) were harvested between ZT5–7 and were sliced into a section of 0.3 mm thickness (49) and separately cultured on a Millicell membrane (PICMORG50, Millipore) with serum-free minimum essential medium (MEM) containing 20 mM HEPES, 36 mM glucose, 100 units/ml penicillin, 100 μg/ml streptomycin with 1% ITS+ Premix (BD Biosciences), and 1 mM D-luciferin (Promega), in 35-mm dish. The bioluminescence from the cultured adrenal slice was measured with a highly sensitive cryogenic CCD camera (600 series, Spectral Instruments) coupled to a microscope (Axiovert 135TV, Carl Zeiss) at 35°C. Successive images were acquired every 20 min with an exposure time of 20 min. Background noise was removed from the images by applying a median filter (Metamorph Software, Molecular devices) to the whole stack of pictures, before measuring luminescence intensity from the area of interest. Luminescence intensity data were filtered by a low-pass filter with a cutoff threshold of 72 cycles/day to eliminate stochastic ultradian oscillations. Where indicated, Ang II (Peptide institute) was applied to culture medium at the final concentration of 100 nM. Phase shifts were calculated as the time-interval between the second and third peaks minus the time-interval between the first and second peaks.
The rat glomerulosa cells were obtained from adrenal glands of male rats weighing 200–250 g (7–8 weeks old) and isolated according to the method previously established by Payet et al. (50, 51). In brief, Per2-dluc rats were sacrificed at ZT5–7 and adrenal glands were immediately removed and dissected. The capsules were separated from fasciculata-reticularis by manual compression and incubated for 20 min at 37°C in oxygenized MEM (4 capsules/ml) containing 2 mg/ml collagenase and 125 units/ml deoxyribonuclease. After incubation, cells were disrupted mechanically, filtered through Cell Strainer (100 μm, BD Biosciences), and centrifuged for 10 min at 300g. The cell pellet was resuspended in OPTI-MEM medium supplemented with 2% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (52–54). The cells were then plated in 35-mm dishes (4 capsules/dish) and cultured with repeated medium changes at 24-h intervals for 3 days before experiments.
H295R cells (ATCC, CRL-2128) were cultured in DMEM/F12 supplemented with 2.5% Nu serum (BD Biosciences) and 1% ITS premix (BD Biosciences) as described previously (47). Cells were transfected with the following reporter plasmids using Lipofectamine LTX (Thermo Fisher Scientific): (i) pGL4.25 PER1 CRE×3-WT-Luc2CP, in which a tandem repeat of the sequence corresponding to the human PER1 CRE with flanking sequences (positions –1950 to –1921; 5′-TTC TTC CGC TTT GAC GTC ACT GCT GTC TCC-3′) was cloned into [luc2CP/minP] (Promega), (ii) pGL4.25 PRE1 CRE×3-Mut-Luc2CP, which is the same as (i) except that CRE sequences were mutated to 5′-TCACATAA-3′, (iii) pGL4.25 E4BP4 NBRE-like×3-WT-Luc2CP, in which a tandem repeat of the sequence corresponding to the human E4BP4 NBRE-like (5′-TGACCTTG-3′) with flanking sequences (positions +4452 to +4481) was cloned into pGL4.25, (iv) pGL4.25 E4BP4 NBRE×3-WT-Luc2CP, in which a tandem repeat of the human E4BP4 NBRE (5′-TGACCTTT-3′) with flanking sequences (positions +4845 to +4874) was cloned into pGL4.25, and (v) pGL4.25 E4BP4 NBRE×3-Mut-Luc2CP, which is the same as (iv) except that the human E4BP4 NBRE sequences were mutated to 5′-TGA ATTCT-3′. Bioluminescence recording was performed a day after transfection.
A luciferase reporter (Luc2P) driven by a mouse or human Per2 promoter sequence (positions –1670 to +53 for mouse; –1840 to +108 for human) was inserted between the inverted terminal repeat (ITR) sequences in pAAV-MCS2 plasmid (Addgene, Plasmid #46954) to obtain pAAV-mPer2-Luc2P or pAAV-hPER2-Luc2P. Recombinant AAV-DJ vectors were produced with a triple-transfection helper-free method as described (55). The day after AAV transduction, cells were split and equally plated into 35-mm dishes containing medium supplemented with 1 mM D-luciferin.
Bioluminescence recording was performed using a dish-type luminometer AB-2550 Kronos Dio (ATTO) under 5% CO2 atmosphere at 37°C. Luminescence was measured at 20-min intervals, with an exception of 30-min intervals in pharmacological experiments (due to drug application during intervals between measurements). To evaluate dose dependency, CV11974 was administrated at a concentration of 0.001, 0.01, 0.1, and 1 μM. Where indicated, cells were treated with Ang II (100 nM), CV11974 (1 μM) or PD123319 (1 μM, Abcam). For enhancer activity reporter assay, values were normalized to the luminescence intensity that was recorded 30 min before Ang II stimulation. For single cell level recording, successive images were acquired with a highly sensitive cryogenic CCD camera (600 series, Spectral Instruments) with a 20 min exposure time for each picture (3 pictures/h). A median filter was applied to all images to eliminate cosmic-ray-induced background noise. Cell viability test and morphological inspection verify that the cells treated with CV or PD (each at 1 μM) for 24 h remained viable and morphologically unaffected (Supplementary Figure S1). Trypan blue extraction assay was used for viability test (56). The half-life of CV and PD in H295R culture is not known (57).
Cells were lysed in Laemmli buffer and immunoblotting was performed according to our standard method (58) with antibodies against NGFIB (Santa Cruz, sc-5569, 1:500 dilution), E4BP4 (Santa Cruz, sc-9549, 1:1000), and β-actin (Sigma Aldrich, A5441, 1:1000). The data were normalized to the expression levels of β-actin. For qRT-PCR, cells were harvested in TRIzol reagent (Thermo Fisher). Total cell RNA was extracted using RNeasy micro kit (Qiagen) and converted to complementary DNA with SuperScript VILO cDNA synthesis kit (Thermo Fisher). Quantitative real-time PCR was achieved using the Platinum SYBR Green qPCR SuperMix-UDG with ROX (Thermo Fisher) with StepOnePlus system (Applied Biosystems). The data were normalized to a non-oscillatory housekeeping gene, RPLP0 mRNA levels. The primer sets used in this study included: PER1, Fw: 5′-GCA TCT CAG CGG AGC TCA CA-3′, Rv: 5′-GAG GCT GTA GGC AAT GGA ACT G-3′, PER2, Fw: 5′-GTG CAG CTC CAC CCT AGT GA-3′, Rv: 5′-GAT TTT CCT GCT CCA TGG GTT GAT G-3′, CRY1, Fw: 5′-AGC AAA CTC ACC TGT TGA AGC AAG G-3′, Rv: 5′-GCT GCA ACA GTA TTC CTC CTG AAT G-3′, BMAL1, Fw: 5′-AGT CTG TCT TCA AGA TCC TCA ACT AC-3′, Rv: 5′-CTG GAA GTC CAG TTT TTG CAT CTA TG-3′, DBP, Fw: 5′-GAA CCC GAC CCA GCT GAT CT-3′, Rv: 5′-CTT GGC TGC CTC GTT GTT CTT GT-3′, E4BP4, Fw: 5′-CCC GAG AGC AGG AAC ACG AT-3′, Rv: 5′-ACC CTA TCT ATG TGT GTA GGA GAA C-3′, and RPLP0, Fw: 5′-ATG CAG CAG ATC CGC ATG T-3′, Rv: 5′-TTG CGC ATC ATG GTG TTC TT-3′. For temperature entrainment, cells were subjected to 24-h temperature cycles with a 12-h warm phase (37°C) and a 12-h cold phase (33°C) for three days, and then released into constant 37°C conditions.
Isolated rat glomerulosa cells were seeded on poly-D-lysine-coated coverslips and cultured for 12 h. Cells were fixed with acetone for 10 min at –20°C, immediately rehydrated in 0.1 M phosphate buffered saline (PBS) for 5 min, and incubated with mouse anti-CYP11B2 antibody (Millipore, MAB6021, 1:100) in PBS containing 0.3% Triton X-100 (PBX) for 5 days at 4°C. After washing with PBX, the samples were incubated with Alexa594-conjugated donkey anti-mouse IgG (Thermo Fisher, 1:5000) for 12 h at 4°C. Nuclei were visualized by staining with 4′,6′-diamino-2-phenylindole (DAPI).
Radioisotopic in situ hybridization was performed with following gene-specific probes: for Agtr1a, nucleotides 1387–2138 (GenBank, NM_177322); for Agtr1b, nucleotides 1298–2015 (NM_175086); for Cyp11b2, nucleotides 1744−2456 (S85260). The corresponding cDNA fragment was cloned and used as a template for the generation of riboprobes. The riboprobes were radiolabeled with [33P]UTP (PerkinElmer), using a standard protocol for the cRNA synthesis. In situ hybridization was performed as described (37). Briefly, paraformaldehyde fixed tissues were frozen and sectioned at a thickness of 30 μm. Then, the free-floating tissue sections were transferred through 4 × SSC, proteinase K (1 μg/ml, 0.1 M Tris-HCl [pH 8.0]; 50 mM EDTA) for 15 min at 37°C, 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min, and 4 × SSC for 10 min. The sections were then incubated in the Denhardt’s hybridization buffer containing 55% formamide, 10% dextran sulfate, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 0.6 M NaCl, 0.2% N-laurylsarcosine, 500 μg/ml tRNA, 0.25% SDS, 10 mM dithiothreitol (DTT) and radiolabeled riboprobes for 16 h at 60°C. Following a high-stringency posthybridization wash, the sections were treated with RNase A. Air-dried sections were exposed to X-ray films (Kodak Biomax).
Cosinor analysis and Rayleigh’s uniformity test were performed using Python 3.8. Western blot band intensities were quantified using ImageJ software. All statistical analyses were performed using GraphPad Prism 8, using the statistical tests for each figure as indicated in the legend.
To test expression of the core clock gene in mouse adrenal, we used isolated adrenal explants from Per2Luc knock-in mice, which facilitate the analysis of circadian expression of Per2 in tissues and cells (44). Bioluminescence imaging revealed abundant PER2::LUC luminescence located mostly in the outer layer of the adrenal cortex, where ZG cells are located (Figure 1A); These observations are similar to those reviewed by other researchers (59). We found that ZG cells in Per2Luc explants exhibit persistent luminescence oscillations for more than a week in culture, with a period length of approximately 24 hours (23.78 ± 0.27 h, mean ± SEM, n = 5 biologically independent slices) (Figure 1B). The PER2::LUC luminescence rhythms were abolished in adrenals from Per2Luc; ClockΔ19/Δ19 mice (Figure 1A), indicating that these rhythms are generated by the endogenous circadian clock mechanism.
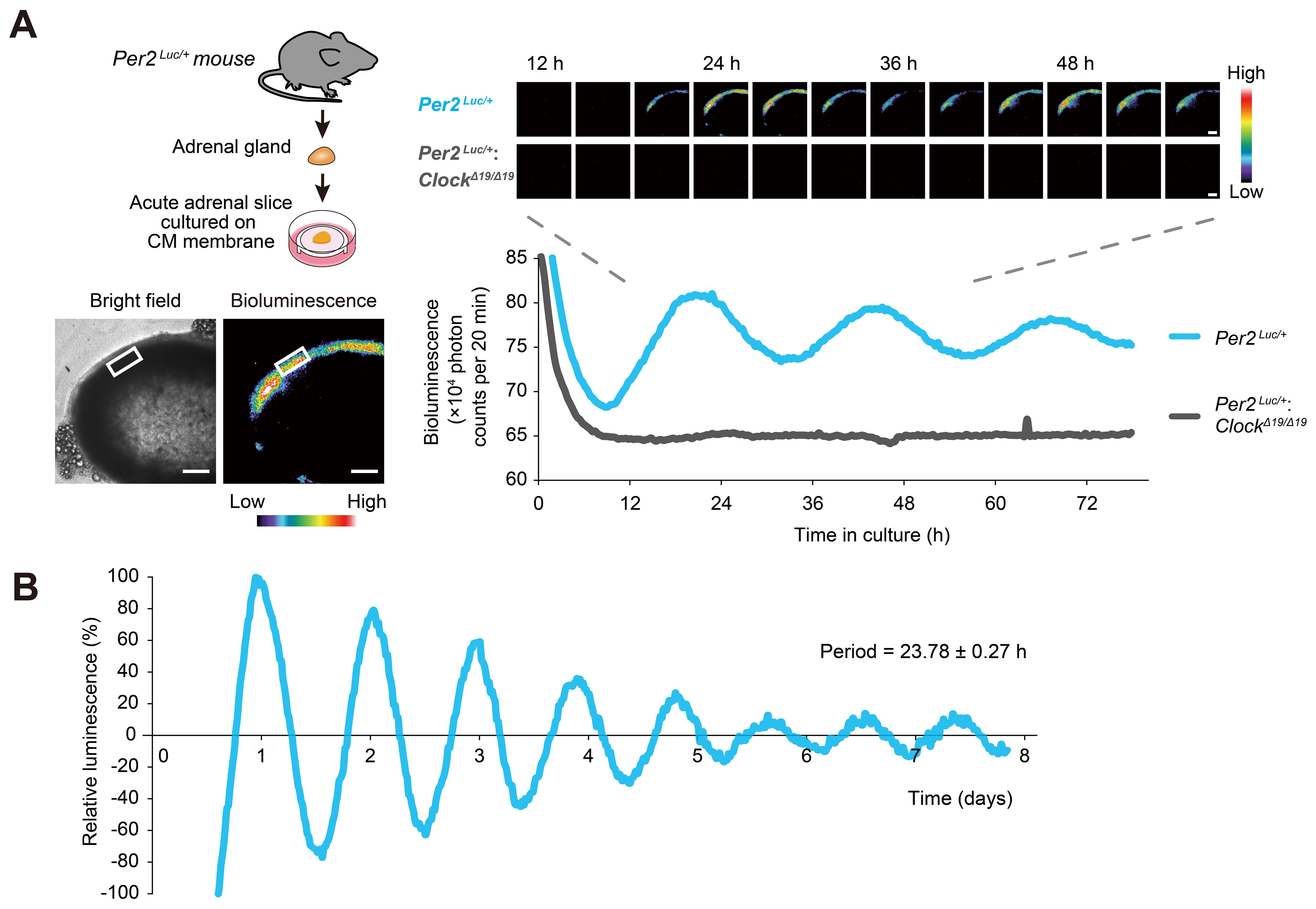
Figure 1. Circadian oscillations exhibited by adrenal ZG cells. (A) Time-lapse images of circadian PER2::LUC bioluminescence obtained from Per2Luc/+ and Per2Luc/+: ClockΔ19/Δ19 mouse adrenal slices. Intensity was traced from a region of the adrenal cortex outer layer containing ZG cells (white boxes) over 80 h. Bioluminescence intensity is represented in pseudo-color scale. Scale bars, 200 μm. (B) Representative long-term bioluminescence recording of the ZG in Per2Luc/+ adrenal from five independent experiments. Data were detrended by 24-h moving average. The maximum bioluminescence was set to 100%.
Next, we tested dissociated cell culture of ZG cells (Figure 2A). For this particular purpose, we used Per2-dluc rat (43), since isolation of primary ZG cells has been established in rats (50, 51). Immunocytochemistry confirmed that approximately 90 percent of the cells isolated were immunolabelled for the ZG cell-marker Cyp11b2, an enzyme responsible for aldosterone production, verifying enrichment of ZG cells as reported (54) (Figure 2A). The freshly isolated rat ZG cells were maintained with repeated medium changes at 24-h intervals for 3 consecutive days (for establishment of primary cell culture) and subsequently maintained under constant culture conditions without medium change for an additional 96 h for luminescence tracing. Under these conditions, sustained circadian bioluminescence rhythms were observed (Figure 2A), indicating that cultured ZG cells can act as a circadian oscillator.
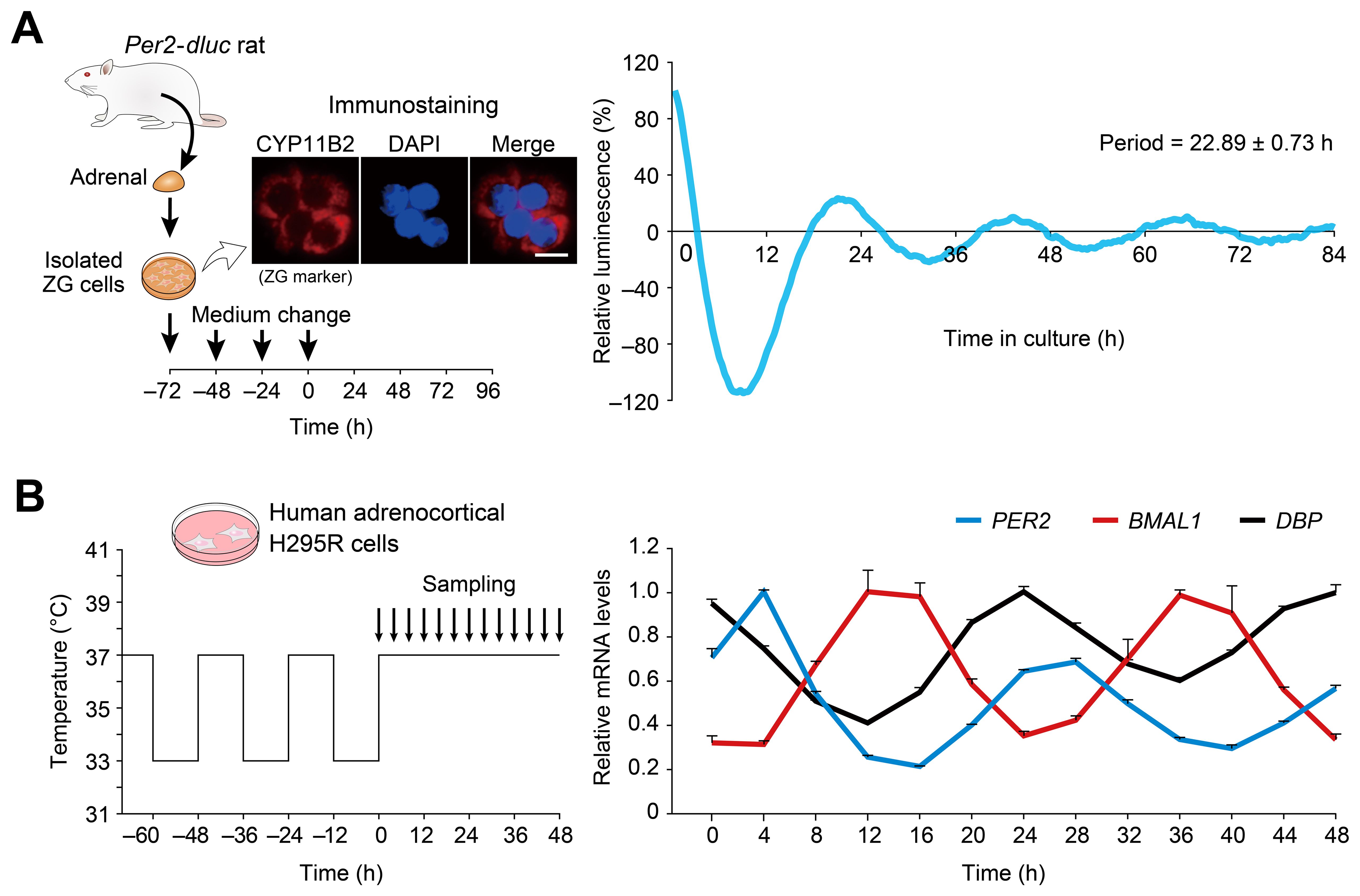
Figure 2. Circadian oscillations displayed by dispersed cell culture of ZG cells and human H295R adrenocortical cells. (A) Representative Per2-dluc bioluminescence recording of dissociated rat primary ZG cells. ZG cells were entrained by 24-h interval medium changes and then released into constant conditions with no medium change. The data were detrended by 24-h moving average and plotted from the last medium change. Immunocytochemistry for CYP11B2 confirmed the isolation of ZG cells from rat adrenal glands. Scale bar, 10 μm. Periods were determined from three measurements. (B) Circadian oscillation of clock genes in human adrenocortical H295R cells. Cells pre-synchronized to 37°C/33°C temperature cycles were harvested under constant 37°C temperature conditions. The data were normalized to RPLP0. The peak mRNA values of each gene were set to 1. n = 3 biological replicates per timepoint. Values are means ± SEM.
As an extension of the above investigation, we also asked whether the human adrenocortical cell line H295R exhibits circadian oscillation (Figure 2B). H295R cells have been widely utilized as a model system for studying ZG (60), including aldosterone biosynthesis (61, 62). However, it is currently not known whether this human model cell line shares similar clock gene expression.
Temperature is known to entrain (or synchronize) cellular clocks of many tissues in vitro (63–65). We cultured H295R cells for 3 days with a temperature cycle (12 h at 37°C, 12 h at 33°C) and placed them in constant temperature conditions (37°C). Cells were harvested every 4 h over a 48-h period to study circadian variations. Quantitative RT-PCR analysis (Figure 2B) revealed that the expression levels of PER2 and BMAL1—representatives of two major feedback loops within the clockwork—showed high-amplitude circadian oscillations. PER2 exhibited its circadian peak expression 8 h earlier than BMAL1, and DBP mRNA expression fluctuated in the opposite phase of BMAL1—a phasic relationship consistent with the current molecular model of the circadian clock (7–9). Our data indicate that mouse and rat ZG cells and human H295R cells all retain a functional clockwork.
Because Ang II is a potent physiological regulator of ZG function (35), we hypothesized that Ang II may modulate circadian rhythms in ZG. In situ hybridization using radioisotope-labeled probes for the Ang II type 1 receptor (AT1R) subtypes Agtr1a and Agtr1b confirmed expression of AT1Rs in the mouse ZG (Figure 3A), further prompting us to test Ang II’s effect on ZG clock. When Ang II was administered to cultured adrenal slices at different phases of luminescence, it caused phase-dependent phase-shifts (delay versus advance) (Figure 3B): administration at the luminescence peak (12 h after the trough) significantly delayed the phase, whereas administration 6 h after the peak advanced the phase significantly (P = 0.001 for phase delay, P = 0.045 for phase advance, unpaired two-sided Student’s t test, Figure 3C). These results demonstrate that mouse ZG contains an Ang II-regulatable circadian clock.

Figure 3. Ang II elicits phase-dependent phase shifts of the adrenal ZG clock. (A) Autoradio-graphs showing expression of Agtr1a, Agtr1b, and Cyp11b2 in the mouse adrenal section. (B) Phase shifts of PER2::LUC rhythm after Ang II treatment in adrenal slices. Arrows indicate the time of Ang II or vehicle administration. Luminescence of ZG was traced. The peak and trough values were adjusted to 100 and 0, respectively. (C) Quantification of the magnitude of phase shifts shown in (B). Phase delays and advances are plotted as negative and positive values, respectively. n = 3–4 slices per condition. Values are means ± SEM. *P < 0.05, **P < 0.01, unpaired two-sided Student’s t test.
The human adrenocortical H295R cells were also treated with Ang II to test for the resetting effect of Ang II stimulation (Figure 4). To do this, H295R cells were seeded onto 24-well plates and cultured for 2 days to confluency; then, after Ang II treatment, cells were harvested at 0, 2, and every 4 hour over a 68-h period (n = 3 replicates; total 57 wells, Figure 4A). Quantitative RT-PCR analysis revealed rhythmical expression of PER1, PER2, BMAL1, CRY1, DBP, and E4BP4 that continued over 3 circadian cycles after Ang II treatment (cosinor analysis, available in Supplementary Table S1). There was an acute and transient upregulation of PER1 mRNA expression immediately after Ang II stimulation. In addition, a similarly prominent (~ 6 fold) upregulation of mRNA expression was also identified for E4BP4, while no appreciable elevation was observed following vehicle administration (see Supplementary Figure S2). These observations, taken together, indicate that Ang II stimulation has the ability to reset or (re)initiate circadian clock gene expression in H295R cells.
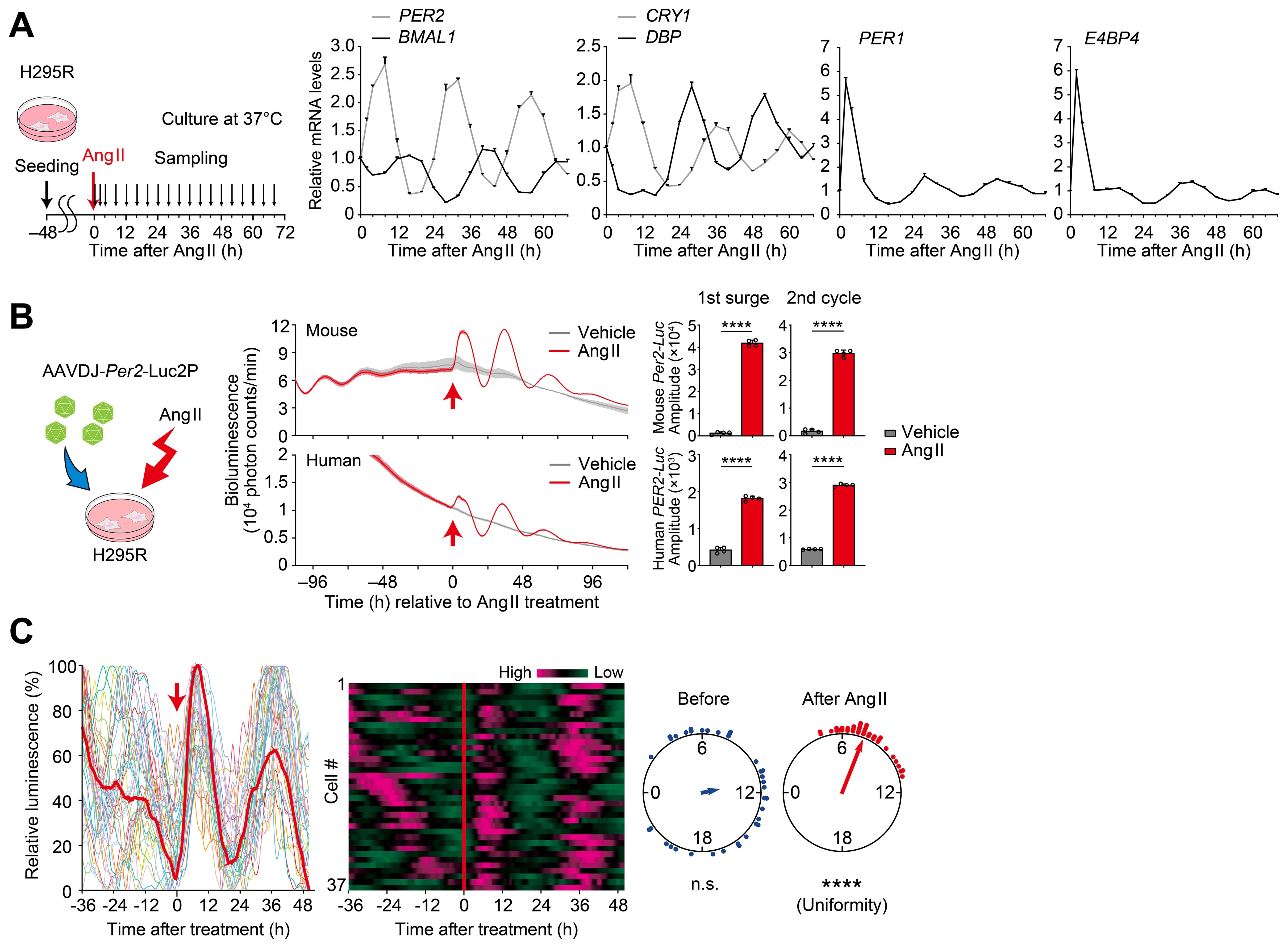
Figure 4. Ang II resets circadian rhythms in H295R cells. (A) Circadian expression profiles of representative core clock genes and clock-controlled genes in H295R cells. Cells were treated with Ang II at Time 0 and were harvested at 0, 2, and every 4 hour over a 68-h period. Values are means ± SEM from n = 3 biological replicates per timepoint. Results of cosinor analysis of the clock gene expression profiles are available in Supplementary Table S1. (B) A cartoon for viral infection to H295R cells and luminescence traces of cells harboring a luciferase reporter under the control of human or mouse Per2 promoter. Arrows indicate the time of Ang II or vehicle administration. Bar graphs illustrate the amplitude of the first surge and second cycle of luminescence following Ang II administration. Data are the means ± SD from n = 4 biologically independent experiments. (C) Single-cell bioluminescence tracing in H295R cells expressing mouse Per2-Luc reporter. Heat maps show individual cellular luminescence, where magenta corresponds to peak bioluminescence and green to trough. Rayleigh plot shows phase distribution of acrophase of individual cells before and after Ang II treatment. n = 37 cells. Statistics in (B), unpaired two-sided Student’s t test; in (C), Rayleigh’s uniformity test. ****P < 0.0001.
In order to facilitate real-time tracing of circadian clock gene expression, we next employed a luciferase reporter system. We generated AAV encoding a luciferase under the PER2 promoter from either human or mouse origin and transduced it to H295R cells. Then, luminescence was traced both before and after Ang II stimulation, in culture (Figure 4B). Luminescence rhythmicity gradually dampened before Ang II treatment. Following Ang II administration, a significant and high-amplitude surge was observed, along with sustained luminescence oscillations in subsequent cycles (P < 0.0001, for the first surge and second circadian cycle, vs vehicle control, Figure 4B). Single-cell-level analysis of circadian rhythms was further performed by CCD video recording of H295R cells before and after Ang II treatment (Figure 4C). We observed that upon Ang II treatment, bioluminescence rhythms of individual cells were immediately resynchronized. This was evident as the vertical alignment of peak bioluminescence across all traced cells in the heatmap of cellular rhythms after Ang II treatment. These data illustrate the phase-resetting of individual cells [see also ref. (66)].
CV11974 (CV) is a receptor antagonist specific to AT1R. We found that CV treatment dose-dependently suppressed the rhythm resetting of H295R cells (Figure 5A). In this experiment, a Per2-Luc reporter was also virally introduced into H295R cells. As the CV dose increased (0, 1, 10, 100, and 1000 nM), the Per2-Luc expression induced by Ang II decreased accordingly. This resulted in a similar dose-dependent attenuation of the Per2-Luc rhythm amplitude in the following circadian cycles (Figure 5A). Moreover, CV shortened the duration of the initial surge of the Per2-Luc expression, which in turn influenced the phase of the Per2-Luc rhythms in the subsequent circadian cycles (Figure 5B; compare phases across different CV doses). We observed that the Ang II type 2 receptor inhibitor PD did not affect either the amplitude or phase of the Per2-Luc rhythm in the same H295R cells (Supplementary Figure S3). Without Ang II, CV treatment had no effect on the Per2-Luc expression (Supplementary Figure S4), consistent with a conjecture that AT1R is not basally activated in the culture conditions that we used for H295R cells (67–69).
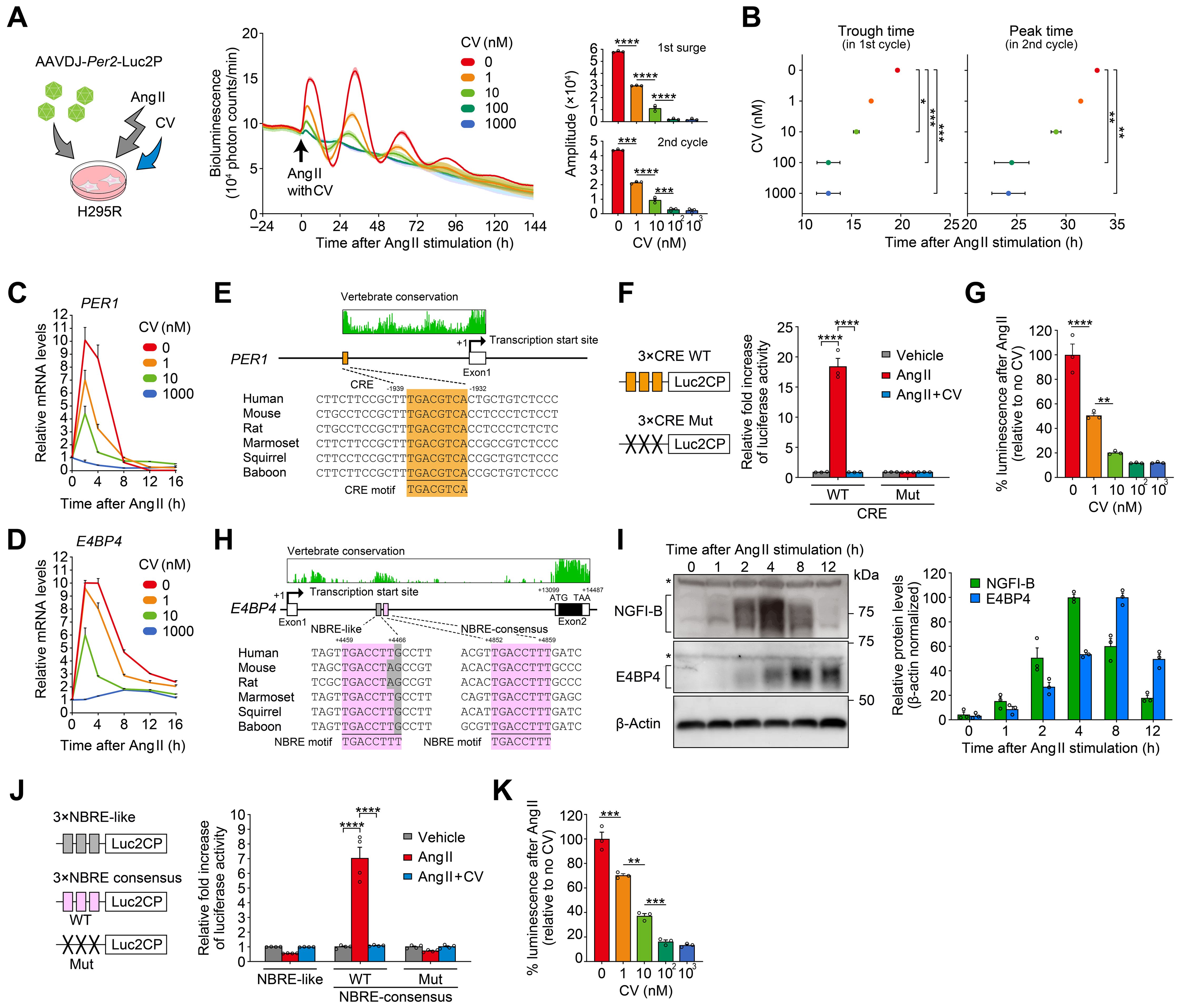
Figure 5. Ang II-induced clock resetting through a mechanism involving upregulation of PER1 and E4BP4. (A, B) Effects of increasing concentrations of CV on Ang II-induced circadian luminescence in H295R cells. Cells were transduced with a luciferase reporter under the control of mouse Per2 promoter. Arrows indicate the time of Ang II/CV treatment. Bar graphs in (A) illustrate the amplitude of the first surge and second cycle of luminescence following Ang II administration with different doses of CV. Plots in (B) show the first trough and second peak phase of the luminescence rhythm following Ang II treatment. n = 3 biological replicates for each CV concentration. Traces in (A) are expressed as means ± SD. (C, D) Effects of CV on Ang II-induced PER1 and E4BP4 mRNA expression in H295R cells. n = 3 biological replicates per datapoint. (E) Sequence alignment of CRE located in the promoter of PER1. The sequences of CRE are compared among mammalian species along with the consensus CRE motif (5′-TGACGTCA-3′). Genomic positions, relative to the transcription start site (+1), are indicated along with the conservation scores obtained from the UCSC Genome Browser (https://genome.ucsc.edu/). (F) Relative reporter activities of CRE×3-Luc2CP and its mutant (Mut, TCACATAA). Cells received vehicle, Ang II, or Ang II plus CV (1 µM). n = 3 biological replicates. (G) Dose-dependent effects of CV on CRE reporter activity after Ang II stimulation. n = 3 biological replicates for each CV concentration. (H) Sequence alignment of two candidate NRBEs, both located in the intron 1 of E4BP4. The sequences of NBRE-like (left) and NBRE-consensus (right) are aligned among species with the consensus NGFI-B binding motif (5′-TGACCTTT-3′ or 5′-AAAGGTCA-3′). E4BP4 consists of two exons. (I) Immunoblots showing the protein expression profiles of NGFI-B and E4BP4 after Ang II stimulation. Bar graph shows protein quantification data (n = 3 biological replicates). β-Actin serves as a loading control. Asterisks indicate nonspecific bands. Uncropped blots are available in Supplementary Figure S5. (J) Relative reporter activities of NBRE-like×3-Luc2CP, NBRE-consensus×3-Luc2CP, and its mutant (Mut, TGAATTCT). n = 4 biological replicates. (K) Dose-dependent effect of CV on NBRE-consensus reporter activity after Ang II stimulation. n = 3 biological replicates for each CV concentration. Statistics in (A, B, G, K) were one-way ANOVA followed by Tukey’s multiple comparisons test; in (F) and (J), two-way ANOVA followed by Sidak’s multiple comparison test. Values are means ± SEM; *P < 0.05, **P < 0.01, ***P< 0.001, ****P < 0.0001.
Furthermore, we observed that the magnitude of the initial induction of endogenous PER1 and E4BP4 mRNA expression in Ang II-treated H295R cells was also decreased by CV treatment in a dose-dependent manner (Figures 5C, D). Considering that CV is one of the most commonly used clinical drugs for hypertension treatment, its dose-dependent effects on clock gene expression imply a previously unstudied potential action of the drug [for known actions of CV, see (70)].
It has been reported that PER1 is acutely responsive to various stimuli through the cAMP-response element (CRE) located in its promoter and that Ang II causes upregulation of CRE- mediated gene transcription in H295R cells (71–76). Consistently, the isolated human PER1 CRE sequence (5′-TGACGTCA-3′) significantly increased reporter transcription upon Ang II stimulation, and CV treatment dose-dependently reduced this activation (see Figures 5E−G for PER1). However, CRE has not previously been implicated in the regulation of E4BP4 (77).
Previous studies investigating Ang II-downstream signaling in H295R cells (61, 78–80) revealed two independent, DNA-binding transcription factor families — ATF/CREB family and NGFI-B family — that mediate transient transcription of a different set of genes upon Ang II stimulation. Because CRE sequence (ATF/CREB-binding motif) is not present in the human E4BP4 locus, we rather propose that the NGFI-B-responsive element (NBRE, 5′-TGA CCTTT-3′) may play a role in the Ang II/CV-dependent mRNA induction of E4BP4 (Figures 5H−K). E4BP4 consists of two exons. Sequence conservation analysis revealed that the intron 1 contains a highly conserved segment (+4283 to +5181, relative to the transcription start site) harboring two putative NBRE sequences at +4459 to +4466 (NBRE-like) and +4852 to +4859 (NBRE-consensus), but no CRE sites (see Figure 5H). Western blots (Figure 5I) confirmed acute accumulation of NGFI-B, noticeable as early as 2 h after Ang II treatment, consistent with previous reports (61, 68). E4BP4 protein levels peaked around 8 h after Ang II treatment (Figure 5I). Subsequently, we assessed the potential cis-element function using reporter vectors containing tandem repeats of the putative cis-element with flanking regions (NBRE-consensus×3-luc and NBRE-like×3-luc) (Figure 5J). In H295R cells, Ang II significantly increased the reporter activity of NBRE-consensus, and CV effectively blocked this effect. Contrastingly, neither Ang II nor CV had any effect on the NBRE-like sequence, indicating the sequence specificity of Ang II-induced transactivation in H295R cells. We confirmed that mutation of the NBRE-consensus sequence abolished its Ang II-dependent enhancer activity (Figure 5J, Mut). Finally, we applied CV at the same various doses as used in Figure 5A. We found that the upregulation of NBRE-consensus reporter activity was dose-dependently reduced by CV, in a manner similar to the changes observed in endogenous E4BP4 mRNA expression in H295R cells (Figures 5D, K). We thus propose that the NBRE sequence in intron 1 may be involved, at least partly, in the Ang II-regulated transcription of E4BP4 in H295R cells.
There still remain several cell types whose circadian clock functionality has yet to be tested. Here, we showed that the circadian clock resides in cells in adrenal ZG and its related human H295R cell line. Our data indicate that they are genetically-encoded, self-sustained and cell-autonomous circadian clock, and responsive to external Ang II stimulation. Consistent with the reaction to Ang II, ZG cells were found to express Ang II receptor type I subtypes, Agtr1a and Agtr1b. Furthermore, we observed that the type I receptor specific blocker CV modulates the level of clock gene expression in Ang II-stimulated H295R cells. Given that CV is widely used for hypertension treatment, its dose-dependent effect on clock gene expression may warrant consideration as a potential additional action of this drug (discussed below).
In the present study, we provide evidence that Ang II-regulatable cell-autonomous circadian oscillators reside in the adrenal gland ZG cells. Time-lapse bioluminescence microscopy of isolated adrenal glands from Per2Luc reporter mice revealed abundant and robustly cycling Per2::Luc luminescence in the adrenal ZG cells, which was completely abolished in Clock mutant mice (Figure 1A). These data thus demonstrate that the oscillations in ZG cells are driven by the endogenous, genetically encoded circadian clock. Self-sustainable oscillations are also presented by dissociated rat ZG cells and human H295R cells (Figure 2). Furthermore, we found that circadian rhythms in ZG can be modulated by external Ang II treatment in culture; Ang II treatment produced phase-dependent phase-shifts (Figure 3). Using H295R cells, we also showed phase resetting that acutely occurs in response to Ang II stimulation. Our single-cell data suggest that the immediate phase-resetting of individual cellular rhythms results in the emergence of overt rhythms at the cell population level [cf. ref. (66)] (Figure 4). Altogether, our results provided evidence to show the presence of Ang II-responsive, cell-autonomous circadian clocks in adrenal ZG and its related H295R cells.
The Ang II type 1 receptors have been localized in vascular smooth muscles, adrenal gland, kidney, heart, brain, platelets, and placenta (81). Previous in vitro studies with cultured cells indicate that Ang II signaling through AT1R has the ability to phase reset the clock gene expression in cardiomyocytes and vascular smooth muscle cells (28, 82). However, with regard to in vivo relevance, no previous studies demonstrate the effect of endogenous Ang II on circadian clock function in peripheral tissues. Particularly, it is still unclear whether Ang II functions as an endogenous entrainment signal for peripheral tissues such as the ZG cells we identified in this study. In this regard, it is noteworthy that a modest diurnal rhythm of plasma Ang II concentration was reported for humans and rats (83–87) while plasma angiotensinogen levels do not exhibit significant circadian oscillation (88). Thus, in vivo role of Ang II as a systemic entrainer remains to be studied in our future research. Clinically, Ang II itself is not used for therapeutic purposes. By contrast, CV is one of the most widely prescribed drugs for clinical treatment of hypertension and related cardiovascular diseases such as heart failure, myocardial infarction and diabetic nephropathy (89–93). Thus, exogenously given (or taken) CV may rather warrant attention from a practice point of view. It is not known or considered if CV affects the ZG circadian clock, in addition to its authorized actions, in clinical use. However, given our observations of its dose-dependent effect on clock gene expression, CV introduction with once-daily dosing (at a fixed timepoint or a more temporally random scheme) may have an additional influence on the clock phase in ZG; CV may enhance the rhythmicity of clock gene expression at certain regimes, while at other intervals, it could potentially diminish that rhythmicity. Although purely hypothetical, these implications may be of use in considering the repertoire of actions associated with this drug.
E4bp4 (also known as Nfil3) has been recognized as a key regulator in the field of immune system and neurobiology, as well as in the circadian clock biology. In CD4+ T cells, E4bp4 is upregulated by chronic antigen stimulation and mediates upregulation of production of IL-10 and IL-13, which helps prevent excessive immune responses (94). In natural killer (NK) cells, E4bp4 activation enhances the cytotoxic activity, contributing to cancer suppression (95, 96). In epilepsy, reduced expression of E4bp4 in neurons causes an increased incidence of seizure (97). E4bp4 activation in microglia restrains microglial cell activation via ERK1/2 signaling and alleviates delirium-associated cognitive decline (98). In terms of circadian biology, on the other hand, the direct functional role of E4bp4 is still not fully understood (99–101); knockout studies show that E4bp4 is dispensable for maintaining circadian rhythms of mouse locomotor activity (99, 101), while knockdown of E4bp4 lengthened the circadian period in cultured rat-1 cells (100), indicating that E4bp4 is not an essential requirement of the central clock function. Nevertheless, it is still possible that E4bp4 may contribute to peripheral clock time-keeping and/or time-resetting system of certain tissue/cell types. Yoshitane et al. demonstrated that in in vitro cultured mouse embryonic fibroblast cells, acidic stress (pH 7.0 → pH 6.6) caused acute upregulation of E4bp4 mRNA-protein expression and showed that genetic deficiency of E4bp4 resulted in abrogated phase-resetting due to acid stress in the cells (101), indicating the potential of E4bp4 as a phase-modulator for peripheral cells. In our study, we observed that E4BP4 mRNA-protein expression is strongly and acutely upregulated by Ang II to the levels almost comparable to those of acute induction of PER1 in H295R cells (Figure 4A). PER1 induction has long been considered to be involved in phase resetting of cells (19–23); however, remaining phase-resetting capacities of PER1 knockout cells (U2OS cells) to several resetting stimuli in culture (serum shock and forskolin) (102) and the fact that grafting Per1 knockout embryonic fibroblasts into wild-type mice results in synchronization with the host’s rhythm in vivo (103) support the idea that Per1 induction is not the sole control point for resetting the clock and rather suggest that the responsible mechanism relies on a broader network of changes beyond Per1 induction (101, 104). In this light, exploration of the extent to which E4BP4 contributes to Ang II-induced clock resetting will be a subject of our future study (12, 105); additional investigations using PER1 and E4BP4 double deficient cells and KO mice will be required to assess the mechanism of Ang II-induced molecular clock resetting.
Collectively, in the present study, we have elucidated the presence of Ang II-responsive molecular clock in rodent adrenal ZG cells and adrenocortical H295R cells. Because of the diversity of peripheral tissues and cell-types, the existence of autonomous clock and identification of its potential entrainment signal(s), either external (e.g., light, food intake) or internal (hormonal, neuronal, body temperature), need to be empirically defined on a cell type-by-cell type basis. Our studies showing the presence of autonomous clock in ZG cells and its potential entrainment capacity to Ang II and its receptor antagonist CV (a potential exogenous stimulus) may provide a basis to understand in vitro circadian properties of ZG cells as a possible component of various peripheral clocks.
There are a number of limitations in our study. Firstly, we did not test all entrainment methods for each culture condition. Temperature entrainment was tested for H295R cells because it is a common method for cell culture entrainment (26, 65, 106–108) and regarded as a physiologically relevant entrainment cue in the body (109, 110). In the rat primary ZG cell culture, however, temperature cycles (changes) negatively affect cell viability upon establishment of primary culture; it requires repeated medium exchange during the initial development (at 37°C). We leveraged these conditions, instead of applying temperature cycles, for entrainment. In our study, we focused on the effect of Ang II and did not test for the effects of other hormones such as mineralocorticoid and glucocorticoid on the ZG circadian clock. Given the proximity between ZG and the zona fasciculata (ZF), where glucocorticoids are produced, a question remains as to the interaction between ZG clock and ZF clock. Secondly, only male rodents were used in the present study, while H295R cells are derived from a female patient with adrenocortical carcinoma (111). Considering known sex-dependent differences in adrenal functions, including stress responsivity (112), tissue renewal (113) and AT1R expression (114–116), further in vivo studies will be required to investigate sex-derived differences in clock function in the ZG, including the rhythmicity of AT1R availability. Thirdly and lastly, Ang II-responsive element(s) responsible for E4BP4 induction remains unclear. We found a functional NBRE site in the intron 1 of E4BP4. However, the extent to which this element contributes to Ang II-responsiveness of E4BP4 in vivo (in H295R cells) needs to be verified by cells harboring specific mutation on this site. We do not exclude the possibility that other cis-regulatory elements that we could not identify in this study may also contribute to the up-regulation of E4BP4 in response to Ang II as well as to other stimuli.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by The Animal Care and Experimentation Committee of Kyoto University. The study was conducted in accordance with the local legislation and institutional requirements.
ToO: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. TM: Data curation, Formal analysis, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing, Methodology. TaO: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. DY: Investigation, Writing – review & editing. YN: Investigation, Writing – review & editing. IM: Investigation, Writing – review & editing. HO: Methodology, Writing – review & editing. EH: Data curation, Formal analysis, Writing – review & editing. MD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22H04987, 22K09771 and 24H02306 to MD; 24K02178 to TM), the Basis for Supporting Innovative Drug Discovery and Life Science Research program of the Japan Agency for Medical Research and Development (JP21am0101092), SRF, Astellas Foundation for Research on Metabolic Disorders (to MD) as well as the Takeda Science Foundation and the Research Foundation for Pharmaceutical Sciences (to TM). ToO is supported by a Japan Science and Technology Agency SPRING fellowship.
The authors thank Y. Takahashi, F. Yamazaki, R. Komatsu and M. Matsuo for their technical assistance. We also thank all members of the Doi Laboratory for their technical assistance and guidance on this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1525844/full#supplementary-material
Supplementary Table 1 | Characteristics of circadian mRNA expression rhythms in H295R cells, related to Figure 4A. MESOR (midline-estimating statistic of rhythm), a rhythm-adjusted 24-hour mean; amplitude, half of total predictable change in rhythm, defined by rhythmic function fitted to data; acrophase, peak time of a fitted cosine curve; period, the time span of a complete rhythmic cycle estimated from the fitted cosine function; R-factor, goodness-of-fit parameter for curve fits.
1. Panda S. Circadian physiology of metabolism. Science. (2016) 354:1008–15. doi: 10.1126/science.aah4967
2. Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. (2016) 354:994–9. doi: 10.1126/science.aah4965
3. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. (2012) 35:445–62. doi: 10.1146/annurev-neuro-060909-153128
4. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. (2010) 72:517–49. doi: 10.1146/annurev-physiol-021909-135821
5. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. (2003) 4:649–61. doi: 10.1038/nrn1177
6. Evans JA, Schwartz WJ. On the origin and evolution of the dual oscillator model underlying the photoperiodic clockwork in the suprachiasmatic nucleus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. (2024) 210:503–11. doi: 10.1007/s00359-023-01659-1
7. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. (2017) 18:164–79. doi: 10.1038/nrg.2016.150
8. Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. (2020) 21:67–84. doi: 10.1038/s41580-019-0179-2
9. Rosbash M, Bradley S, Kadener S, Li Y, Luo W, Menet JS, et al. Transcriptional feedback and definition of the circadian pacemaker in Drosophila and animals. Cold Spring Harb Symp Quant Biol. (2007) 72:75–83. doi: 10.1101/sqb.2007.72.062
10. Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H. Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol. (2000) 20:4773–81. doi: 10.1128/MCB.20.13.4773-4781.2000
11. Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. (2001) 15:995–1006. doi: 10.1101/gad.873501
12. Doi M, Okano T, Yujnovsky I, Sassone-Corsi P, Fukada Y. Negative control of circadian clock regulator E4BP4 by casein kinase Iepsilon-mediated phosphorylation. Curr Biol. (2004) 14:975–80. doi: 10.1016/j.cub.2004.05.043
13. Ohno T, Onishi Y, Ishida N. A novel E4BP4 element drives circadian expression of mPeriod2. Nucleic Acids Res. (2007) 35:648–55. doi: 10.1093/nar/gkl868
14. Akashi M, Ichise T, Mamine T, Takumi T. Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock. Mol Biol Cell. (2006) 17:555–65. doi: 10.1091/mbc.e05-05-0396
15. Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. (2005) 37:187–92. doi: 10.1038/ng1504
16. Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. (2002) 110:251–60. doi: 10.1016/S0092-8674(02)00825-5
17. Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. (2005) 12:441–8. doi: 10.1038/nsmb925
18. Takeda Y, Jothi R, Birault V, Jetten AM. RORgamma directly regulates the circadian expression of clock genes and downstream targets in vivo. Nucleic Acids Res. (2012) 40:8519–35. doi: 10.1093/nar/gks630
19. Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, and, to light. Cell. (1997) 91:1055–64. doi: 10.1016/S0092-8674(00)80495-X
20. Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the transcript. Cell. (1997) 91:1043–53. doi: 10.1016/S0092-8674(00)80494-8
21. Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, et al. Inhibition of light- or glutamate-induced expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. (1999) 19:1115–21. doi: 10.1523/JNEUROSCI.19-03-01115.1999
22. Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem. (2003) 278:718–23. doi: 10.1074/jbc.M209241200
23. Schwartz WJ, Tavakoli-Nezhad M, Lambert CM, Weaver DR, de la Iglesia HO. Distinct patterns of Period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc Natl Acad Sci U S A. (2011) 108:17219–24. doi: 10.1073/pnas.1107848108
24. Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. (2002) 277:44244–51. doi: 10.1074/jbc.M206233200
25. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. (2000) 289:2344–7. doi: 10.1126/science.289.5488.2344
26. Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, et al. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell. (2019) 177:896–909.e20. doi: 10.1016/j.cell.2019.02.017
27. McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. (2001) 105:877–89. doi: 10.1016/S0092-8674(01)00401-9
28. Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, et al. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. (2001) 104:1746–8. doi: 10.1161/hc4001.098048
29. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. (2000) 14:2950–61. doi: 10.1101/gad.183500
30. Gabriel BM, Zierath JR. Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol. (2019) 15:197–206. doi: 10.1038/s41574-018-0150-x
31. Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nat Med. (2001) 7:356–60. doi: 10.1038/85507
32. Terazono H, Hamdan A, Matsunaga N, Hayasaka N, Kaji H, Egawa T, et al. Modulatory effects of 5-fluorouracil on the rhythmic expression of circadian clock genes: a possible mechanism of chemotherapy-induced circadian rhythm disturbances. Biochem Pharmacol. (2008) 75:1616–22. doi: 10.1016/j.bcp.2008.01.011
33. Tampakakis E, Gangrade H, Glavaris S, Htet M, Murphy S, Lin BL, et al. Heart neurons use clock genes to control myocyte proliferation. Sci Adv. (2021) 7:eabh4181. doi: 10.1126/sciadv.abh4181
34. Cunningham PS, Kitchen GB, Jackson C, Papachristos S, Springthorpe T, van Dellen D, et al. ClinCirc identifies alterations of the circadian peripheral oscillator in critical care patients. J Clin Invest. (2023) 133:e162775. doi: 10.1172/JCI162775
35. Seccia TM, Caroccia B, Gomez-Sanchez EP, Gomez-Sanchez CE, Rossi GP. The biology of normal zona glomerulosa and aldosterone-producing adenoma: pathological implications. Endocr Rev. (2018) 39:1029–56. doi: 10.1210/er.2018-00060
36. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. (2012) 350:151–62. doi: 10.1016/j.mce.2011.07.034
37. Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. (2010) 16:67–74. doi: 10.1038/nm.2061
38. Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. (2006) 4:163–73. doi: 10.1016/j.cmet.2006.07.002
39. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. (2008) 105:20970–5. doi: 10.1073/pnas.0806962106
40. Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol. (2011) 32:451–65. doi: 10.1016/j.yfrne.2011.07.003
41. Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. (2017) 38:3–45. doi: 10.1210/er.2015-1080
42. Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am J Physiol Regul Integr Comp Physiol. (2003) 285:R561–9. doi: 10.1152/ajpregu.00783.2002
43. He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. The disruption of circadian clockwork in differentiating cells from rat reproductive tissues as identified by in vitro real-time monitoring system. J Endocrinol. (2007) 193:413–20. doi: 10.1677/JOE-07-0044
44. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. (2004) 101:5339–46. doi: 10.1073/pnas.0308709101
45. Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. (1994) 264:719–25. doi: 10.1126/science.8171325
46. Yamaguchi Y, Okada K, Mizuno T, Ota T, Yamada H, Doi M, et al. Real-time recording of circadian per1 and per2 expression in the suprachiasmatic nucleus of freely moving rats. J Biol Rhythms. (2016) 31:108–11. doi: 10.1177/0748730415621412
47. Sasaki L, Hamada Y, Yarimizu D, Suzuki T, Nakamura H, Shimada A, et al. Intracrine activity involving NAD-dependent circadian steroidogenic activity governs age-associated meibomian gland dysfunction. Nat Aging. (2022) 2:105–14. doi: 10.1038/s43587-021-00167-8
48. Doi M, Ishida A, Miyake A, Sato M, Komatsu R, Yamazaki F, et al. Circadian regulation of intracellular G-protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat Commun. (2011) 2:327. doi: 10.1038/ncomms1316
49. Doi M, Shimatani H, Atobe Y, Murai I, Hayashi H, Takahashi Y, et al. Non-coding cis-element of Period2 is essential for maintaining organismal circadian behaviour and body temperature rhythmicity. Nat Commun. (2019) 10:2563. doi: 10.1038/s41467-019-10532-2
50. Payet N, Lehoux JG. Aldosterone and corticosterone stimulation by ACTH in isolated rat adrenal glomerulosa cells: interaction with vasopressin. J Physiol (Paris). (1982) 78:317–21.
51. Gallo-Payet N, Payet MD. Excitation-secretion coupling: involvement of potassium channels in ACTH-stimulated rat adrenocortical cells. J Endocrinol. (1989) 120:409–21. doi: 10.1677/joe.0.1200409
52. Mazzocchi G, Rossi GP, Malendowicz LK, Champion HC, Nussdorfer GG. Endothelin-1[1-31], acting as an ETA-receptor selective agonist, stimulates proliferation of cultured rat zona glomerulosa cells. FEBS Lett. (2000) 487:194–8. doi: 10.1016/S0014-5793(00)02352-8
53. Semplicini A, Ceolotto G, Baritono E, Malendowicz LK, Andreis PG, Sartori M, et al. Adrenomedullin stimulates DNA synthesis of rat adrenal zona glomerulosa cells through activation of the mitogen-activated protein kinase-dependent cascade. J Hypertens. (2001) 19:599–602. doi: 10.1097/00004872-200103001-00012
54. Nogueira EF, Vargas CA, Otis M, Gallo-Payet N, Bollag WB, Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol. (2007) 39:365–74. doi: 10.1677/JME-07-0094
55. Miyake T, Tanaka K, Inoue Y, Nagai Y, Nishimura R, Seta T, et al. Size-reduced DREADD derivatives for AAV-assisted multimodal chemogenetic control of neuronal activity and behavior. Cell Rep Methods. (2024) 4:100881. doi: 10.1016/j.crmeth.2024.100881
56. Tainaka M, Doi M, Inoue Y, Murai I, Okamura H. Circadian PER2 protein oscillations do not persist in cycloheximide-treated mouse embryonic fibroblasts in culture. Chronobiol Int. (2018) 35:132–6. doi: 10.1080/07420528.2017.1316731
57. Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. (2000) 355:637–45. doi: 10.1016/S0140-6736(99)10365-9
58. Fujita Y, Miyake T, Shao X, Aoki Y, Hasegawa E, Doi M. Omeprazole Induces CYP3A4 mRNA Expression but Not CYP3A4 Protein Expression in HepaRG Cells. Biol Pharm Bull. (2024) 47:1218–23. doi: 10.1248/bpb.b24-00161
59. Leliavski A, Dumbell R, Ott V, Oster H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms. (2015) 30:20–34. doi: 10.1177/0748730414553971
60. Wang T, Rainey WE. Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol. (2012) 351:58–65. doi: 10.1016/j.mce.2011.08.041
61. Ota T, Doi M, Yamazaki F, Yarimizu D, Okada K, Murai I, et al. Angiotensin II triggers expression of the adrenal gland zona glomerulosa-specific 3beta-hydroxysteroid dehydrogenase isoenzyme through de novo protein synthesis of the orphan nuclear receptors NGFIB and NURR1. Mol Cell Biol. (2014) 34:3880–94. doi: 10.1128/MCB.00852-14
62. Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, et al. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology. (1993) 133:1555–61. doi: 10.1210/endo.133.4.8404594
63. Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. (2002) 12:1574–83. doi: 10.1016/S0960-9822(02)01145-4
64. Miyake T, Inoue Y, Shao X, Seta T, Aoki Y, Nguyen Pham KT, et al. Minimal upstream open reading frame of Per2 mediates phase fitness of the circadian clock to day/night physiological body temperature rhythm. Cell Rep. (2023) 42:112157. doi: 10.1016/j.celrep.2023.112157
65. Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. (2010) 330:379–85. doi: 10.1126/science.1195262
66. Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. (2004) 119:693–705. doi: 10.1016/j.cell.2004.11.015
67. Suzuki J, Otsuka F, Inagaki K, Takeda M, Ogura T, Makino H. Novel action of activin and bone morphogenetic protein in regulating aldosterone production by human adrenocortical cells. Endocrinology. (2004) 145:639–49. doi: 10.1210/en.2003-0968
68. Yarimizu D, Doi M, Ota T, Okamura H. Stimulus-selective induction of the orphan nuclear receptor NGFIB underlies different influences of angiotensin II and potassium on the human adrenal gland zona glomerulosa-specific 3beta-HSD isoform gene expression in adrenocortical H295R cells. Endocr J. (2015) 62:765–76. doi: 10.1507/endocrj.EJ15-0211
69. Strajhar P, Tonoli D, Jeanneret F, Imhof RM, Malagnino V, Patt M, et al. Steroid profiling in H295R cells to identify chemicals potentially disrupting the production of adrenal steroids. Toxicology. (2017) 381:51–63. doi: 10.1016/j.tox.2017.02.010
70. Gleiter CH, Jagle C, Gresser U, Morike K. Candesartan. Cardiovasc Drug Rev. (2004) 22:263–84. doi: 10.1111/j.1527-3466.2004.tb00146.x
71. Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci U S A. (2002) 99:7728–33. doi: 10.1073/pnas.102075599
72. Gau D, Lemberger T, von Gall C, Kretz O, Le Minh N, Gass P, et al. Phosphorylation of CREB Ser142 regulates light-induced phase shifts of the circadian clock. Neuron. (2002) 34:245–53. doi: 10.1016/S0896-6273(02)00656-6
73. Doi M, Cho S, Yujnovsky I, Hirayama J, Cermakian N, Cato AC, et al. Light-inducible and clock-controlled expression of MAP kinase phosphatase 1 in mouse central pacemaker neurons. J Biol Rhythms. (2007) 22:127–39. doi: 10.1177/0748730406298332
74. Wang XL, Bassett M, Zhang Y, Yin S, Clyne C, White PC, et al. Transcriptional regulation of human 11beta-hydroxylase (hCYP11B1). Endocrinology. (2000) 141:3587–94. doi: 10.1210/endo.141.10.7689
75. Nogueira EF, Rainey WE. Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology. (2010) 151:1060–70. doi: 10.1210/en.2009-0977
76. Meier RK, Clark BJ. Angiotensin II-dependent transcriptional activation of human steroidogenic acute regulatory protein gene by a 25-kDa cAMP-responsive element modulator protein isoform and Yin Yang 1. Endocrinology. (2012) 153:1256–68. doi: 10.1210/en.2011-1744
77. Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. (2004) 119:1041–54. doi: 10.1016/j.cell.2004.10.032
78. Gu J, Wen Y, Mison A, Nadler JL. 12-lipoxygenase pathway increases aldosterone production, 3’,5’-cyclic adenosine monophosphate response element-binding protein phosphorylation, and p38 mitogen-activated protein kinase activation in H295R human adrenocortical cells. Endocrinology. (2003) 144:534–43. doi: 10.1210/en.2002-220580
79. Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. (2004) 18:279–90. doi: 10.1210/me.2003-0005
80. Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. (2004) 84:489–539. doi: 10.1152/physrev.00030.2003
81. Burnier M. Angiotensin II type 1 receptor blockers. Circulation. (2001) 103:904–12. doi: 10.1161/01.CIR.103.6.904
82. Herichova I, Soltesova D, Szantoova K, Mravec B, Neupauerova D, Vesela A, et al. Effect of angiotensin II on rhythmic per2 expression in the suprachiasmatic nucleus and heart and daily rhythm of activity in Wistar rats. Regul Pept. (2013) 186:49–56. doi: 10.1016/j.regpep.2013.06.016
83. Kala R, Fyhrquist F, Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest. (1973) 31:363–5. doi: 10.3109/00365517309084318
84. Richards AM, Nicholls MG, Espiner EA, Ikram H, Cullens M, Hinton D. Diurnal patterns of blood pressure, heart rate and vasoactive hormones in normal man. Clin Exp Hypertens A. (1986) 8:153–66. doi: 10.3109/10641968609074769
85. Rittig S, Matthiesen TB, Pedersen EB, Djurhuus JC. Circadian variation of angiotensin II and aldosterone in nocturnal enuresis: relationship to arterial blood pressure and urine output. J Urol. (2006) 176:774–80. doi: 10.1016/S0022-5347(06)00594-5
86. Naito Y, Tsujino T, Matsumoto M, Okuda S, Sakoda T, Ohyanagi M, et al. The mechanism of distinct diurnal variations of renin-angiotensin system in aorta and heart of spontaneously hypertensive rats. Clin Exp Hypertens. (2009) 31:625–38. doi: 10.3109/10641960903406993
87. Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res. (2017) 40:413–22. doi: 10.1038/hr.2016.166
88. Nishijima Y, Kobori H, Kaifu K, Mizushige T, Hara T, Nishiyama A, et al. Circadian rhythm of plasma and urinary angiotensinogen in healthy volunteers and in patients with chronic kidney disease. J Renin Angiotensin Aldosterone Syst. (2014) 15:505–8. doi: 10.1177/1470320314557584
89. Carey RM, Moran AE, Whelton PK. Treatment of hypertension: A review. JAMA. (2022) 328:1849–61. doi: 10.1001/jama.2022.19590
90. Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. (2003) 362:759–66. doi: 10.1016/S0140-6736(03)14282-1
91. Demers C, McMurray JJ, Swedberg K, Pfeffer MA, Granger CB, Olofsson B, et al. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. JAMA. (2005) 294:1794–8. doi: 10.1001/jama.294.14.1794
92. Rossing K, Christensen PK, Hansen BV, Carstensen B, Parving HH. Optimal dose of candesartan for renoprotection in type 2 diabetic patients with nephropathy: a double-blind randomized cross-over study. Diabetes Care. (2003) 26:150–5. doi: 10.2337/diacare.26.1.150
93. Husain A, Azim MS, Mitra M, Bhasin PS. A review on candesartan: pharmacological and pharmaceutical profile. J Appl Pharm Sci. (2011) 1:12–7.
94. Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. (2011) 12:450–9. doi: 10.1038/ni.2020
95. Yang M, Li D, Chang Z, Yang Z, Tian Z, Dong Z. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J Exp Med. (2015) 212:253–65. doi: 10.1084/jem.20141703
96. Tang PM, Zhou S, Meng XM, Wang QM, Li CJ, Lian GY, et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat Commun. (2017) 8:14677. doi: 10.1038/ncomms14677
97. Zhang T, Yu F, Xu H, Chen M, Chen X, Guo L, et al. Dysregulation of REV-ERBalpha impairs GABAergic function and promotes epileptic seizures in preclinical models. Nat Commun. (2021) 12:1216. doi: 10.1038/s41467-021-21477-w
98. Chen M, Zhang L, Shao M, Du J, Xiao Y, Zhang F, et al. E4BP4 coordinates circadian control of cognition in delirium. Adv Sci (Weinh). (2022) 9:e2200559. doi: 10.1002/advs.202200559
99. Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PloS Biol. (2009) 7:e1000181. doi: 10.1371/journal.pbio.1000181
100. Yamajuku D, Shibata Y, Kitazawa M, Katakura T, Urata H, Kojima T, et al. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. (2011) 585:2217–22. doi: 10.1016/j.febslet.2011.05.038
101. Yoshitane H, Asano Y, Sagami A, Sakai S, Suzuki Y, Okamura H, et al. Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun Biol. (2019) 2:300. doi: 10.1038/s42003-019-0522-3
102. Park J, Lee K, Kim H, Shin H, Lee C. Endogenous circadian reporters reveal functional differences of PERIOD paralogs and the significance of PERIOD: CK1 stable interaction. Proc Natl Acad Sci U S A. (2023) 120:e2212255120. doi: 10.1073/pnas.2212255120
103. Pando MP, Morse D, Cermakian N, Sassone-Corsi P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell. (2002) 110:107–17. doi: 10.1016/S0092-8674(02)00803-6
104. Kon N, Hirota T, Kawamoto T, Kato Y, Tsubota T, Fukada Y. Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat Cell Biol. (2008) 10:1463–9. doi: 10.1038/ncb1806
105. Doi M, Nakajima Y, Okano T, Fukada Y. Light-induced phase-delay of the chicken pineal circadian clock is associated with the induction of cE4bp4, a potential transcriptional repressor of cPer2 gene. Proc Natl Acad Sci U S A. (2001) 98:8089–94. doi: 10.1073/pnas.141090998
106. Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med. (2017) 9:eaal2774. doi: 10.1126/scitranslmed.aal2774
107. Droin C, Paquet ER, Naef F. Low-dimensional dynamics of two coupled biological oscillators. Nat Phys. (2019) 15:1086–94. doi: 10.1038/s41567-019-0598-1
108. Tu HQ, Li S, Xu YL, Zhang YC, Li PY, Liang LY, et al. Rhythmic cilia changes support SCN neuron coherence in circadian clock. Science. (2023) 380:972–9. doi: 10.1126/science.abm1962
109. Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science. (2021) 371:eabd0951. doi: 10.1126/science.abd0951
110. Miyake T, Inoue Y, Maekawa Y, Doi M. Circadian clock and body temperature. Adv Exp Med Biol. (2024) 1461:177–88. doi: 10.1007/978-981-97-4584-5_12
111. Gazdar AF, Oie HK, Shackleton CH, Chen TR, Triche TJ, Myers CE, et al. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. (1990) 50:5488–96.
112. Martinez GJ, Appleton M, Kipp ZA, Loria AS, Min B, Hinds TD Jr. Glucocorticoids, their uses, sexual dimorphisms, and diseases: new concepts, mechanisms, and discoveries. Physiol Rev. (2024) 104:473–532. doi: 10.1152/physrev.00021.2023
113. Lyraki R, Schedl A. Adrenal cortex renewal in health and disease. Nat Rev Endocrinol. (2021) 17:421–34. doi: 10.1038/s41574-021-00491-4
114. Gao X, Yamazaki Y, Tezuka Y, Omata K, Ono Y, Morimoto R, et al. Gender differences in human adrenal cortex and its disorders. Mol Cell Endocrinol. (2021) 526:111177. doi: 10.1016/j.mce.2021.111177
115. Owonikoko TK, Fabucci ME, Brown PR, Nisar N, Hilton J, Mathews WB, et al. In vivo investigation of estrogen regulation of adrenal and renal angiotensin (AT1) receptor expression by PET. J Nucl Med. (2004) 45:94–100.
Keywords: chronobiology, circadian rhythm, zona glomerulosa, angiotensin II, candesartan, chronotherapy
Citation: Otani T, Miyake T, Ota T, Yarimizu D, Nakagawa Y, Murai I, Okamura H, Hasegawa E and Doi M (2025) Identification of angiotensin II-responsive circadian clock gene expression in adrenal zona glomerulosa cells and human adrenocortical H295R cells. Front. Endocrinol. 16:1525844. doi: 10.3389/fendo.2025.1525844
Received: 10 November 2024; Accepted: 06 March 2025;
Published: 26 March 2025.
Edited by:
Birgit Harbeck, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Alessandra Porcu, University of South Carolina, United StatesCopyright © 2025 Otani, Miyake, Ota, Yarimizu, Nakagawa, Murai, Okamura, Hasegawa and Doi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masao Doi, ZG9pbWFzYW9AcGhhcm0ua3lvdG8tdS5hYy5qcA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.