
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 March 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1521247
Background: A major problem that affects women of reproductive age globally is sterility. A new statistic called Relative Fat Mass (RFM) provides an accurate representation of the percentage of total body fat in people. This study aims to investigate the relationship between RFM and sterility in fertility-age American women.
Methods: This study employed a cross-sectional design using data collected from NHANES between 2013 and 2018. The association between RFM and sterility was investigated using logistic regression analysis, controlling for a number of variables. The results were more resilient when RFM was transformed into a four-category variable in order to further examine the patterns of the association between different RFM levels and sterility. The dose-response association between RFM and sterility was illustrated using restricted cubic spline (RCS) analysis. Sensitivity and subgroup analyses were also conducted to assess the robustness and consistency of the results.
Results: This study included 3,197 women aged 18–45, consisting of 2,854 non-sterile participants and 343 sterile participants. First, in the fully adjusted model, RFM and the prevalence of sterility had a positive correlation (OR = 1.05, 95% CI = 1.01–1.09). When converting RFM from a continuous to a categorical variable, the prevalence of sterility was significantly greater in the highest quartile than in the lowest quartile (OR = 2.59, 95% CI = 1.40–4.82). Furthermore, RFM and sterility prevalence were found to be positively linearly correlated by RCS analysis, with sterility rates sharply increasing as RFM levels rose. The positive correlation between RFM and the frequency of sterility was shown to be constant throughout various populations, according to subgroup analysis across stratified parameters. Finally, sensitivity analysis further confirmed the reliability and consistency of the study’s findings.
Conclusion: A representative sample of American women of reproductive age showed a positively correlation between RFM and the prevalence of sterility. RFM may help identify women at risk for sterility, and waist circumference management could potentially help lower the risk of sterility.
After a year or more of consistent, unprotected sexual activity, a couple’s failure to conceive is known as sterility (1). Globally, about one in six couples of reproductive age face sterility challenges, affecting millions of families worldwide (2, 3). The National Survey of Family Growth reports that 6% of married women in America who are of reproductive age are sterile and 12% have impaired fertility, meaning they are unable to conceive or carry a pregnancy to term (4). Sterility is often perceived as a misfortune, bringing severe negative impacts on families and even society (5). It is commonly associated with anxiety, depression, sleep and eating disorders (6–8), as well as sexual and marital problems (9, 10). The fundamental processes of sterility remain unclear, despite the fact that it is widely acknowledged as a serious medical problem.
A new worldwide epidemic (11), obesity is defined by the buildup of adipose tissue (12). Body mass index, or BMI, is a recognized indicator of obesity; however, it has limitations, as it cannot distinguish between fat mass and muscle mass (13), nor can it reflect the distribution of fat across the body (14). A new body fat measurement called Relative Fat Mass (RFM) takes into account height, waist circumference, and sex to provide an accurate representation of the proportion of total body fat (15). Compared to BMI, RFM offers higher diagnostic value (16). In evaluating conditions including metabolic syndrome, heart disease, and diabetes type 2, prior research has shown that RFM has greater sensitivity and specificity (17–19). RFM is very useful in predicting and assessing these conditions as a measure of total body fat percentage. Reports indicate that adipose tissue negatively affects female fertility (20). Alterations in the secretion levels of hormones, such as leptin, can influence steroidogenesis and directly impact embryonic development. Additionally, the endometrium is susceptible to these changes, with evidence suggesting that stromal decidualization is impaired in obese women (21). We examined the relationship between RFM levels and the prevalence of sterility in women aged 18–45 years using data from the NHANES. This research may contribute to the development of future prevention or treatment strategies for sterility.
A continuous nationwide survey conducted in two-year cycles, the NHANES was created to systematically assess the nutritional status and overall health of Americans. The trial has been approved by an ethical review board, and each participant provided written informed consent. The data offers researchers valuable public health information for analyzing various health trends and relationships. NHANES data gathered from 2013 to 2018 were used in this investigation. The inclusion criteria for participants were: (1) adults aged 18–45 years; (2) female participants; (3) participants with complete sterility data; and (4) participants with complete RFM data. After applying these criteria, the final study population was selected for further analysis.
RFM is calculated based on waist circumference (WC), height, and sex. Professionally qualified medical technicians at the Mobile Examination Center (MEC) gathered the measurement data. RFM is calculated as follows: RFM = 64 − (20 × height/WC) + (12 × sex). Since this study focuses solely on female participants, the sex coefficient is set to 1 (22).
Self-reported answers to the reproductive health questionnaire (variable name: RHQ074) were used to make the sterility diagnosis. Researchers surveyed participants with questions such as “Have you tried to become pregnant for a year?” If the answer was “Yes,” it indicated sterility. The reliability of this measure has been validated in previous studies.
Several confounders, including demographic traits, lifestyle choices, and medical problems, were included in this study in order to thoroughly examine the connection between RFM and sterility. Age, menarche age, race, PIR (Poverty Income Ratio), and educational attainment were among the demographic factors. Three PIR categories—<1, 1 to <3, and ≥3—were distinguished. Total household income was divided by the poverty threshold to calculate PIR. Drinking alcohol and smoking were lifestyle factors. During one’s lifetime, smoking was defined as consuming more than 100 cigarettes. Previously consuming more than 12 alcoholic beverages was considered alcohol consumption. Diabetes, high blood pressure, high cholesterol, and pelvic infections were defined as health condition variables based on self-reports or physician diagnoses.
This study used NHANES data gathered from 2013 to 2018 to do a cross-sectional analysis. This study employed the WTMEC2YR weight for weighted analysis following the sample weighting guidelines provided by NHANES. After screening for eligible participants, descriptive analyses were performed based on sterility status. Means (standard deviations) were used to represent continuous variables, whereas percentages were used to represent categorical data. The logistic regression analysis was used to calculate the 95% CI and OR for the association between RFM and sterility. In order to investigate patterns across various RFM ranges, RFM was transformed into a four-category variable to increase the results’ robustness. An investigation of the dose-response association between RFM and sterility was conducted using a limited cubic spline approach. The study’s dependability was further strengthened by subgroup analyses based on lifestyle and health characteristics, which looked at the possible connection between RFM and sterility. All data analyses, carried out with R software (version 4.2.4), were considered statistically significant if P < 0.05.
Data from 29,400 people in all were taken out of the NHANES database. Following the use of the screening procedure (Figure 1), 3,197 participants—2,854 of whom were not sterile and 343 of whom were—were included in the final analysis. Sterility status-stratified baseline features are shown in Table 1. The sterile group’s participants were more likely to have smoked in the past and were usually older than the non-sterile group. They also exhibited higher prevalence rates of diabetes, hypertension, hyperlipidemia, and pelvic infections. Notably, sterile participants had higher RFM levels, suggesting a potential association between RFM and sterility.
To examine the connection between RFM and the prevalence of sterility, a logistic regression analysis was conducted. The results are shown in Table 2. RFM and sterility prevalence were shown to be positively correlated in Model 1 (OR = 1.07, 95% CI = 1.04–1.10). The results remained stable after stepwise adjustments for different covariates. The fully adjusted model showed that for every unit increase in RFM, the prevalence of sterility increased by 5% (OR = 1.05, 95% CI = 1.01–1.09). RFM was converted from a continuous to a categorical variable, and the highest RFM quartile was significantly associated with a greater prevalence of sterility (OR = 2.59, 95% CI = 1.40–4.82). RFM and the prevalence of sterility had a positive linear connection, according to Figure 2’s RCS analysis, with sterility rates rising dramatically as RFM levels increased. According to these findings, there is a significant positive link between RFM and sterility.
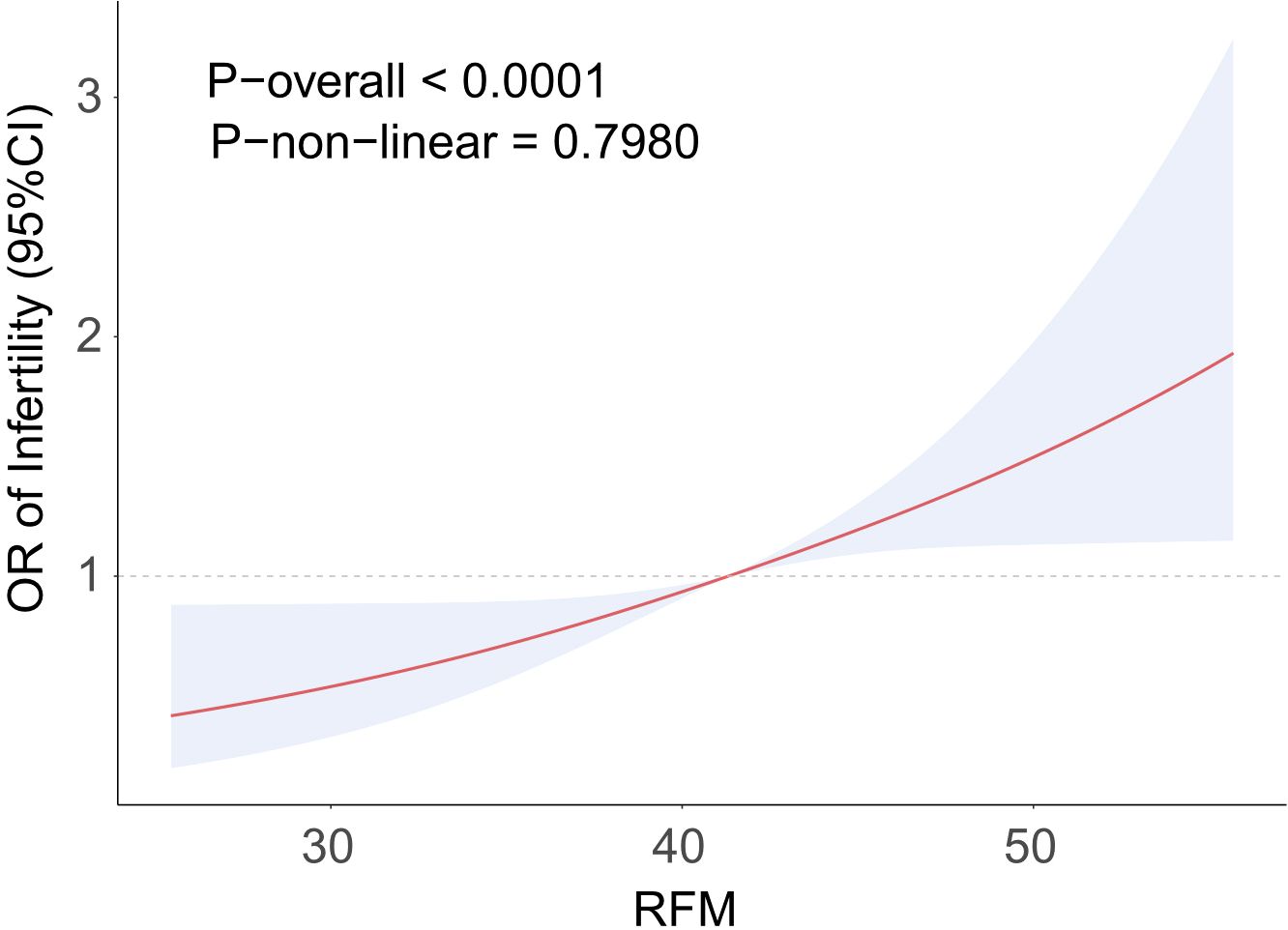
Figure 2. RCS curve fits the Association of RFM with STERILITY. Adjusted for age, Race, Educational level, PIR, Smoke, Drinking, Activity status, Hypertension, Hypercholesterolemia, Diabetes, Pelvic infection, Menarche age, Was pregnant.
The potential correlation between RFM and sterility was examined by using Model 3 to perform subgroup analyses with stratified factors, such as smoking, alcohol use, diabetes, hypertension, hyperlipidemia, and pelvic infections (Table 3). The results showed that the positive association between RFM and sterility prevalence was consistent across all categories. Importantly, there was an interaction effect between alcohol consumption and the prevalence of sterility and RFM, indicating that there is a stronger positive correlation between RFM and sterility prevalence among alcohol users.
Women whose menarche age was under 10 years old were excluded in a sensitivity analysis to increase the study’s robustness and reliability. After exclusion, 2,871 participants remained, including 2,579 non-sterile participants and 292 sterile participants. RFM and the prevalence of sterility were positively correlated across all model changes, as Table 4 illustrates. This trend remained stable even when RFM was transformed into a categorical variable. These results provide more evidence of the study’s consistency and dependability.
This cross-sectional study sought to determine if RFM was associated with the prevalence of sterility in 3,197 people. The findings showed that sterility rates increased dramatically as RFM levels increased, indicating a strong link between RFM and sterility frequency. RFM and the prevalence of sterility also showed a positive linear connection, according to RCS analysis. The reliability and robustness of our results were further confirmed by sensitivity and subgroup analysis. The prevalence of sterility may be predicted by RFM, and controlling obesity as defined by RFM may help reduce the risk of sterility.
This is the first research that we are aware of that looks into the relationship between RFM and sterility. Compared with the traditional obesity indicator BMI, RFM is a novel obesity index that reflects both total body fat percentage and trunk fat percentage and has been shown to provide higher accuracy (16, 23). The findings of this study indicate a positive association between RFM and sterility prevalence. These results align with previous research suggesting a link between weight gain and increased sterility risk. An increased risk of sterility was linked to a greater weight-adjusted waist index, a measure of central obesity, in a cross-sectional analysis of 3,526 women in the United States of reproductive age (OR = 1.42, 95% CI: 1.22–1.65) (24). The body shape index (ABSI) and sterility risk were positively correlated in another study that included 433 women with an sterile diagnosis (OR = 1.56, 95% CI: 1.21–2.00) (25). Similarly, a case-control study of 116,678 women across 14 U.S. states reported that sterility risk increased with a higher BMI at age 18 (26). This study reached similar conclusions, highlighting the positive impact of maintaining a healthy weight on natural conception rates (27, 28). Additionally, we found that RFM and sterility were positively correlated, with those with higher WWI having a higher chance of sterility. These findings suggest that obesity may increase the risk of sterility through various mechanisms, such as hormonal imbalances caused by excessive adipose tissue and the release of pro-inflammatory factors contributing to sterility.
Although there are a number of possible pathways linked to the onset of sterility, the fundamental mechanisms that connect RFM to sterility are still unclear. Sterility and RFM may be positively correlated through a number of routes. First, excessive fat accumulation can lead to excessive aromatization of androgens in peripheral fat, resulting in elevated estrone levels. This can disrupt the hypothalamic-pituitary-gonadal axis during the ovarian cycle, ultimately affecting menstrual cycles and ovulation (29, 30). Second, high levels of adipose tissue release a variety of hormones and cytokines (31), including inflammatory factors and leptin (32, 33), which can impair oocyte vitality and quality (20), negatively impacting female fertility. The pro-inflammatory factors produced and released by excessive adipose tissue can accumulate in multiple tissues, causing a detrimental effect known as lipotoxicity (34). In obese women, lipotoxicity may exacerbate systemic inflammation and insulin resistance, which are considered potential mechanisms for obesity-induced damage to oocyte organelles (35). Additionally, leptin can interfere with follicle maturation and oocyte quality by activating the MAPK pathway and reducing cAMP-regulated steroid production in human granulosa cells (36).
Our study has a number of advantages. First off, this study is the first to investigate the relationship between RFM and sterility risk in women of reproductive age in the United States. With a large sample size, we were able to obtain more precise and reliable results. Our results show that RFM and the prevalence of sterility are positively correlated, and that this correlation is steady and constant rather than random. Second, we adjusted for confounding variables by considering demographics and illnesses associated with chronic illness. The need for more targeted sterility prevention strategies was highlighted when the relationship between RFM and sterility in different categories was ultimately examined utilizing stratified subgroup and sensitivity analysis. Nevertheless, there are a number of limitations to our study. Initially, the cross-sectional design restricts the capacity to establish causality about the association between RFM and sterility. Second, the questionnaire-based definition of infertility used in this study has been widely validated in previous large-scale epidemiological studies. However, we acknowledge its potential limitations. Future research should incorporate stricter diagnostic criteria and larger-scale studies to further validate our findings.
A representative sample of American women of reproductive age showed a positively correlation between RFM and the prevalence of sterility. RFM may help identify women at risk for sterility, and waist circumference management could potentially help lower the risk of sterility.
Publicly available datasets were analyzed in this study. This data can be found here: National Health and Nutrition Examination : Survey https://www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by the research ethics review board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MS: Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YL: Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing. XY: Conceptualization, Methodology, Validation, Writing – review & editing. XM: Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are appreciative that the National Center for Medical Research at the Institute of Prevention and Control of Disorders has made the National Health and Nutritional Evaluation Survey available to all citizens of the country.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, Body Mass Index; CI, Confidence Interval; NHANES, National Health and Nutrition Examination Survey; OR, Odds Ratio; PIR, Poverty Income Ratio; RCS, Restricted Cubic Spline; RFM, Relative Fat Mass; WC, Waist Circumference.
1. Szamatowicz M, Szamatowicz J. Proven and unproven methods for diagnosis and treatment of infertility. Adv Med Sci. (2020) 65:93–6. doi: 10.1016/j.advms.2019.12.008
2. Gerrits T, Van Rooij F, Esho T, Ndegwa W, Goossens J, Bilajbegovic A, et al. Infertility in the Global South: Raising awareness and generating insights for policy and practice. Facts views Vision ObGyn. (2017) 9:39–44.
3. Greil AL, Johnson KM, McQuillan J, Lacy N. Are prior pregnancy outcomes relevant for models of fertility-specific distress or infertility helpseeking? Hum Fertil (Camb). (2011) 14(3):160–6. doi: 10.3109/14647273.2011.587229
4. Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the National Survey of Family Growth. Natl Health Stat Rep. (2013) 1-18:1.
5. Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. (2008) 14:605–21. doi: 10.1093/humupd/dmn042
6. Szkodziak F, Krzyżanowski J, Szkodziak P. Psychological aspects of infertility. A systematic review. J Int Med Res. (2020) 48:300060520932403. doi: 10.1177/0300060520932403
7. Volgsten H, Skoog Svanberg A, Ekselius L, Lundkvist O, Sundström Poromaa I. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum Reprod (Oxford England). (2008) 23:2056–63. doi: 10.1093/humrep/den154
8. Volgsten H, Skoog Svanberg A, Ekselius L, Lundkvist O, Sundström Poromaa I. Risk factors for psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Fertility sterility. (2010) 93:1088–96. doi: 10.1016/j.fertnstert.2008.11.008
9. Agostini F, Monti F, De Pascalis L, Paterlini M, La Sala GB, Blickstein I. Psychosocial support for infertile couples during assisted reproductive technology treatment. Fertility sterility. (2011) 95:707–10. doi: 10.1016/j.fertnstert.2010.06.011
10. Iris A, Aydogan Kirmizi D, Taner CE. Effects of infertility and infertility duration on female sexual functions. Arch gynecology obstetrics. (2013) 287:809–12. doi: 10.1007/s00404-012-2633-7
11. Karam JG, El-Sayegh S, Nessim F, Farag A, McFarlane SI. Medical management of obesity: an update. Minerva endocrinologica. (2007) 32:185–207.
12. Scaglione R, Argano C, Di Chiara T, Licata G. Obesity and cardiovascular risk: the new public health problem of worldwide proportions. Expert Rev Cardiovasc Ther. (2004) 2:203–12. doi: 10.1586/14779072.2.2.203
13. Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. (2008) 32 Suppl 3:S56–9. doi: 10.1038/ijo.2008.87
14. Merchant RA, Seetharaman S, Au L, Wong MWK, Wong BLL, Tan LF, et al. Relationship of fat mass index and fat free mass index with body mass index and association with function, cognition and sarcopenia in pre-frail older adults. Front Endocrinol. (2021) 12:765415. doi: 10.3389/fendo.2021.765415
15. Kobo O, Leiba R, Avizohar O, Karban A. Relative fat mass (RFM) as abdominal obesity criterion for metabolic syndrome. Eur J Internal Med. (2019) 63:e9–e11. doi: 10.1016/j.ejim.2019.03.002
16. Woolcott OO, Bergman RN. Relative fat mass (RFM) as a new estimator of whole-body fat percentage ─ A cross-sectional study in American adult individuals. Sci Rep. (2018) 8:10980. doi: 10.1038/s41598-018-29362-1
17. Kobo O, Leiba R, Avizohar O, Karban A. Relative fat mass is a better predictor of dyslipidemia and metabolic syndrome than body mass index. Cardiovasc Endocrinol Metab. (2019) 8:77–81. doi: 10.1097/XCE.0000000000000176
18. Efe S, Karagoz A, Dogan C, Bayram Z, Kalkan S, Altıntas MS, et al. Relative Fat Mass Index can be solution for obesity paradox in coronary artery disease severity prediction calculated by SYNTAX Score. Postgraduate Med J. (2021) 97:434–41. doi: 10.1136/postgradmedj-2020-138926
19. Suthahar N, Wang K, Zwartkruis VW, Bakker SJL, Inzucchi SE, Meems LMG, et al. Associations of relative fat mass, a new index of adiposity, with type-2 diabetes in the general population. Eur J Internal Med. (2023) 109:73–8. doi: 10.1016/j.ejim.2022.12.024
20. Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol endocrinology: RB&E. (2018) 16:22. doi: 10.1186/s12958-018-0336-z
21. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertility sterility. (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017
22. Cichosz SL, Rasmussen NH, Vestergaard P, Hejlesen O. Is predicted body-composition and relative fat mass an alternative to body-mass index and waist circumference for disease risk estimation? Diabetes Metab syndrome. (2022) 16:102590. doi: 10.1016/j.dsx.2022.102590
23. Wang J, Guan J, Huang L, Li X, Huang B, Feng J, et al. Sex differences in the associations between relative fat mass and all-cause and cardiovascular mortality: A population-based prospective cohort study. Nutrition metabolism Cardiovasc diseases: NMCD. (2024) 34:738–54. doi: 10.1016/j.numecd.2023.10.034
24. Wen Z, Li X. Association between weight-adjusted-waist index and female infertility: a population-based study. Front Endocrinol. (2023) 14:1175394. doi: 10.3389/fendo.2023.1175394
25. Yang Q, Wuliu J, Zeng L, Huang J, Tang G, Zhang J, et al. Association between a body shape index and female infertility: a cross-sectional study. BMC women’s Health. (2024) 24:486. doi: 10.1186/s12905-024-03335-1
26. Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J obstetrics gynecology. (1994) 171:171–7. doi: 10.1016/0002-9378(94)90465-0
27. Hoek A, Wang Z, van Oers AM, Groen H, Cantineau AEP. Effects of preconception weight loss after lifestyle intervention on fertility outcomes and pregnancy complications. Fertility sterility. (2022) 118:456–62. doi: 10.1016/j.fertnstert.2022.07.020
28. Pavli P, Triantafyllidou O, Kapantais E, Vlahos NF, Valsamakis G. Infertility improvement after medical weight loss in women and men: A review of the literature. Int J Mol Sci. (2024) 25(3):1909. doi: 10.3390/ijms25031909
29. Ennab F, Atiomo W. Obesity and female infertility. Best Pract Res Clin obstetrics Gynaecology. (2023) 89:102336. doi: 10.1016/j.bpobgyn.2023.102336
30. Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. (2007) 22(2):414–20. doi: 10.1093/humrep/del400
31. Richard AJ, White U, Elks CM, Stephens JM. Adipose tissue: physiology to metabolic dysfunction. Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, Herder WW de, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. South Dartmouth (MA: Endotext, MDText.com, Inc. Copyright © 2000-2024, MDText.com, Inc. (2000).
32. Engin AB. Adipocyte-macrophage cross-talk in obesity. Adv Exp Med Biol. (2017) 960:327–43. doi: 10.1007/978-3-319-48382-5
33. Villanueva-Carmona T, Cedó L, Madeira A, Ceperuelo-Mallafré V, Rodríguez-Peña MM, Núñez-Roa C, et al. SUCNR1 signaling in adipocytes controls energy metabolism by modulating circadian clock and leptin expression. Cell Metab. (2023) 35:601–619.e10. doi: 10.1016/j.cmet.2023.03.004
34. Sørensen TI, Virtue S, Vidal-Puig A. Obesity as a clinical and public health problem: is there a need for a new definition based on lipotoxicity effects? Biochim Biophys Acta. (2010) 1801(3):400–4. doi: 10.1016/j.bbalip.2009.12.011
35. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome–an allostatic perspective. Biochim Biophys Acta. (2010) 1801:338–49. doi: 10.1016/j.bbalip.2009.12.006
Keywords: relative fat mass, sterility, waist circumference, cross-sectional study, NHANES
Citation: Sun M, Lu Y, Yang X and Mao X (2025) Association between relative fat mass and sterility in women of reproductive age in the United States: results from the 2013–2018 NHANES. Front. Endocrinol. 16:1521247. doi: 10.3389/fendo.2025.1521247
Received: 16 November 2024; Accepted: 17 February 2025;
Published: 03 March 2025.
Edited by:
Gedis Grudzinskas, Independent Researcher, London, United KingdomReviewed by:
Sarbjeet Makkar, Washington University in St. Louis, United StatesCopyright © 2025 Sun, Lu, Yang and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaogang Mao, dGptdW14Z0AxNjMuY29t; Xi Yang, eWFuZ3gwMzE2QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.