
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 21 March 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1520362
Objective: There has been substantial research conducted recently on the effect of myo-inositol (MI) on human reproduction. However, it still remains ambiguous about the therapeutic efficacy of MI in infertile women undergoing in vitro fertilization embryo transfer (IVF-ET). This systematic review and meta-analysis was carried out to investigate the efficacy of MI on IVF outcomes.
Methods: Literatures were searched in the PubMed, Web of Science, Cochrane Library, ScienceDirect and Wanfang databases. The methodological quality was assessed using the Cochrane Risk of Bias tool. Data were pooled using a random- or fixed-effects model according to study heterogeneity. The results are expressed as odds ratio (OR) or mean difference (MD) with 95% confidence intervals (CIs). Heterogeneity was measured by the I2 statistic. The protocol was prospectively registered with PROSPERO (CRD42024582149).
Results: Eleven eligible studies with 981 participants reported the IVF outcomes of the MI group versus the control group. The synthesis results showed that the metaphase II (MII) oocyte rate was higher in the MI group than in the control group (OR 1.55, 95% CI 1.04-2.31, P=0.03). For polycystic ovary syndrome (PCOS) women, as well as non-obese PCOS women, a statistically significant improvement in MII oocyte rate were assumed after taking MI (OR 1.97, 95% CI 1.20-3.25, P<0.01; OR 1.92, 95% CI 1.09-3.37, P=0.02) while there is no statistically significant advancement showed in the poor ovary responder (POR) women(OR 0.97, 95% CI 0.35-2.68, P=0.95). The fertilization rate was higher in the MI group than in the control group (OR 1.62, 95% CI 1.21-2.16, P<0.01), for PCOS, non-obese PCOS and POR women (OR 1.59, 95% CI 1.16-2.18, P<0.01; OR 1.87, 95% CI 1.52-2.31, P<0.01; OR 2.42, 95% CI 1.48-3.95, P<0.01).
Conclusions: Our results suggest that MI supplementation improves the MII oocyte rate and the fertilization rate. More high-grade evidence from prospective randomized studies is warranted.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024582149.
IVF-ET is supposed to help infertile couples to achieve pregnancy (1). With the breakthrough of novel technologies in ovulation induction protocols and laboratory, the successful rate in IVF has progressively risen (2). However, there is still a considerable number of infertile couples who cannot conceive through IVF. Improving the quantity and quality of embryos is a crucial factor contributing to the success of IVF-assisted reproduction (3–5). Great efforts have been made to discover adjuvant therapy or supplementation attempting to get ideal IVF outcomes (6, 7).
As a supplementation in IVF, inositol has attracted increasing attention. It is a compound that occurs naturally in many foods and is an essential component of the vitamin B group (8). Studies have shown a correlation between the level of inositol in follicular fluid and the quality of oocytes (9). It was also confirmed that women who achieved pregnancy through in vitro fertilization and embryo transfer had higher levels of inositol in their follicular fluid than non-pregnant women (10). MI, one of the nine different forms of inositol, can be converted into inositolphosphoglycan within the human body, which acts as a secondary messenger involved in insulin signal transduction, primarily regulating the activation of glucose transporters and glucose utilization (11, 12). Therefore, its function as an insulin sensitizer is used to treat PCOS of which insulin resistance is one of the typical symptoms (13–16). Although researches conducted to investigate the effectiveness of inositol on PCOS women attending intracytoplasmic sperm injection (ICSI) programs indicated no significant improvement on the quality of oocyte or embryo or pregnancy rates, the combination use of d-chiro-inositol (DCI), another form of inositol, left the conclusion undetermined, because DCI was reported to act differently with MI may even have a negative effect on oocyte quality (17, 18). Moreover, MI is also utilized in non-PCOS women in IVF, including normal responders and poor responders (19, 20), since it plays a crucial role in cell growth and follicle-stimulating hormone (FSH)signal transduction (21, 22), which is associated with oocyte maturation and embryo development.
At present, the data regarding the therapeutic efficacy of MI in infertile women undergoing IVF is still rather unclear. Thus, this systematic review and meta-analysis aims to systematically review and summarize the evidence examining the impact of MI on IVF outcomes to further optimize clinical treatment strategy.
The growing number of observations have focused on the effect of MI in assisted reproductive technologies since the concentration of MI in the follicular fluid was found directly correlates with the quality of oocytes and embryos in 2002 (9). In this systematic review, we searched for studies published in the last two decades until July 2024 in the following databases: PubMed, Cochrane Library, Web of Science, Science Direct, and WANFANG Database. A combination of MeSH terms and free words were used. The main search terms were ‘myoinositol’ or ‘inositol’ and ‘IVF’ or ‘in vitro fertilization’ or ‘ICSI’. The language was restricted to English and Chinese in the searches. A hand-search of reference lists of the included studies or relevant recent reviews was conducted to identify potential data resources.
Titles and abstracts were screened independently in duplicate by 2 reviewers, and disagreements were resolved by discussion, with a third reviewer to adjudicate if needed. The identified studies were reviewed to be included or excluded by 1 reviewer and verified by the second reviewer. The study selection process for the systematic review is shown in Figure 1.
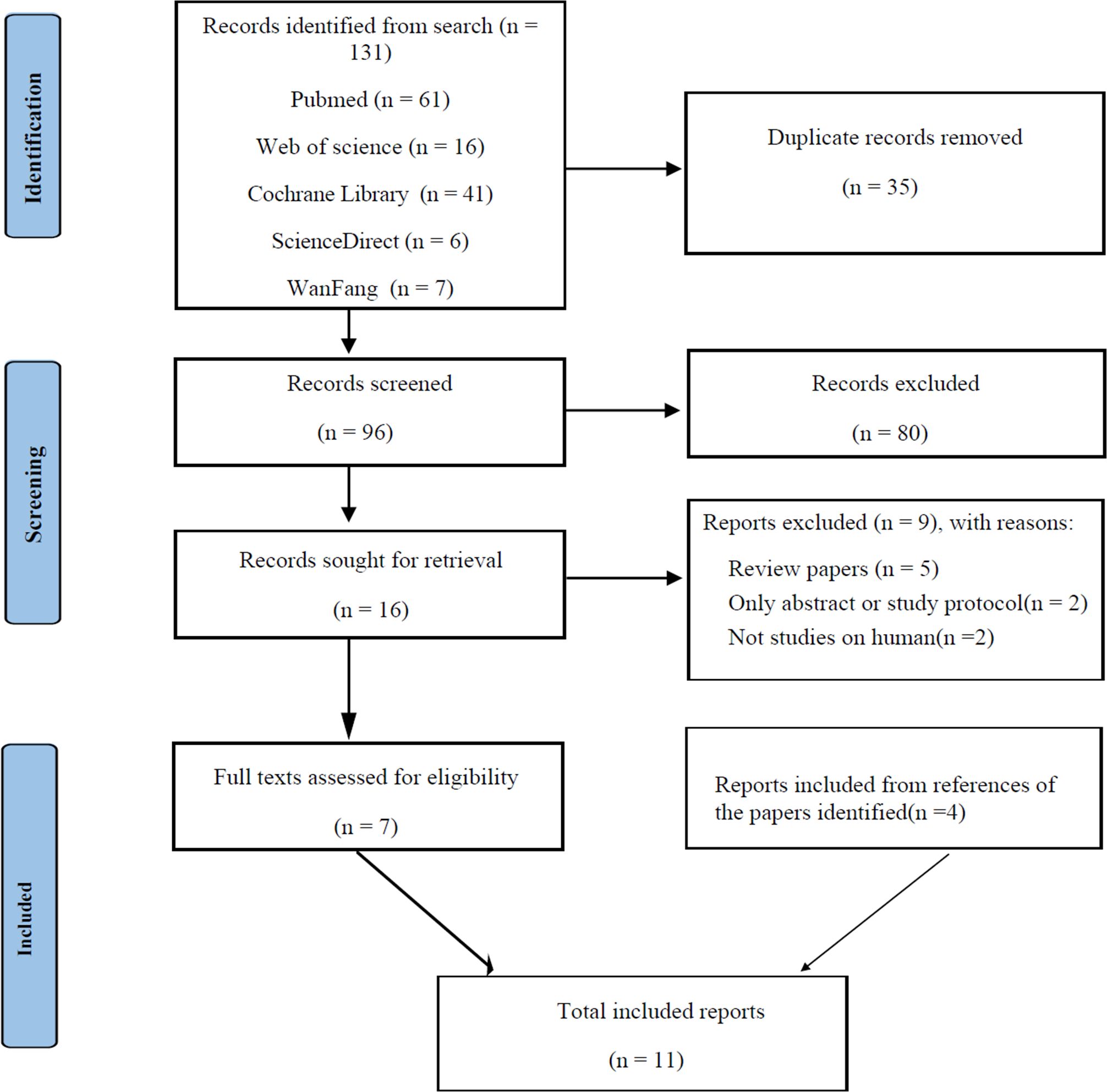
Figure 1. PRISMA diagram for literature search process and total number of studies screened and included at each stage.
We included randomized controlled trials and controlled observational studies that compared IVF outcomes between MI adjuvant therapy and usual care or placebo. The inclusion criteria were as follows: (1) female participants diagnosed with infertility, undergoing IVF or ICSI. (2) with MI adjuvant therapy being the main difference between the intervention group and the control group.
In this meta-analysis, we excluded studies published only as abstracts or repeated publications, as well as studies with co-interventions used, except folic acid, as the effect of folic acid (FA) on outcomes was thought to be minimal.
The methodological quality of all the selected studies was assessed by two reviewers independently. For randomized studies, quality assessment was performed by following Cochrane Handbook for Systematic Reviews of Interventions, information on the randomization method, allocation concealment, blinding, intention-to-treat analysis and follow-up rate was assessed (Supplementary Table S1). The Newcastle-Ottawa Scale (NOS) was used to evaluate the methodological quality of the three non-randomized studies (Supplementary Table S2). Any disagreements were resolved by discussion.
For each study, data obtained from the manuscript included the first author, year of publication, country of origin, study design, participant characteristics and intervention protocol, etc. All the data were extracted by one researcher and verified by a second researcher.
This meta-analysis was performed using Review Manager 5.4.1 (23). Heterogeneity was assessed using the chi-squared and I2 tests, with significant heterogeneity defined as P < 0.05 or I2 > 40%. Random effects models were adopted when P< 0.05 or I2>40%; otherwise, fixed-effects models were used. For continuous data, MD with 95% CI was used to express the effect estimate, while pooled OR with 95% CI was used for categorical data. Subgroup analysis was conducted according to the work-up of the participants (PCOS/POR) if there was a difference among the population for one individual indicator between studies. In addition, a sensitivity analysis was conducted to examine the heterogeneity and differences in outcomes as well as publication bias using funnel plots to assess the bias.
A total of 131 articles were identified by the literature search. First, 35 duplicate articles were removed. Initial screening of the titles and abstracts excluded 80 unsuitable articles, and 7 articles remained after reviewing the full text of the identified articles. In addition, 4 articles were retrieved from the references of the identified articles.
Finally, eleven studies were included in this review and meta-analysis. Among them, seven studies reported the IVF outcomes of the MI group versus the control group in PCOS participants, three studies reported the outcomes in POR participants and the remaining reported the results in non-PCOS participants. The pooled sample size was 981 (478 in the observation group and 503 in control group). The study participants were mainly from Europe, the Middle East, and Eastern Asia. The ages of the participants, as well as the study interventions, are presented in Table 1.
The statistical results of the comparison between the MI group and the control group are presented in Table 2, and the forest plots are shown in Supplementary Figures S1-S5.
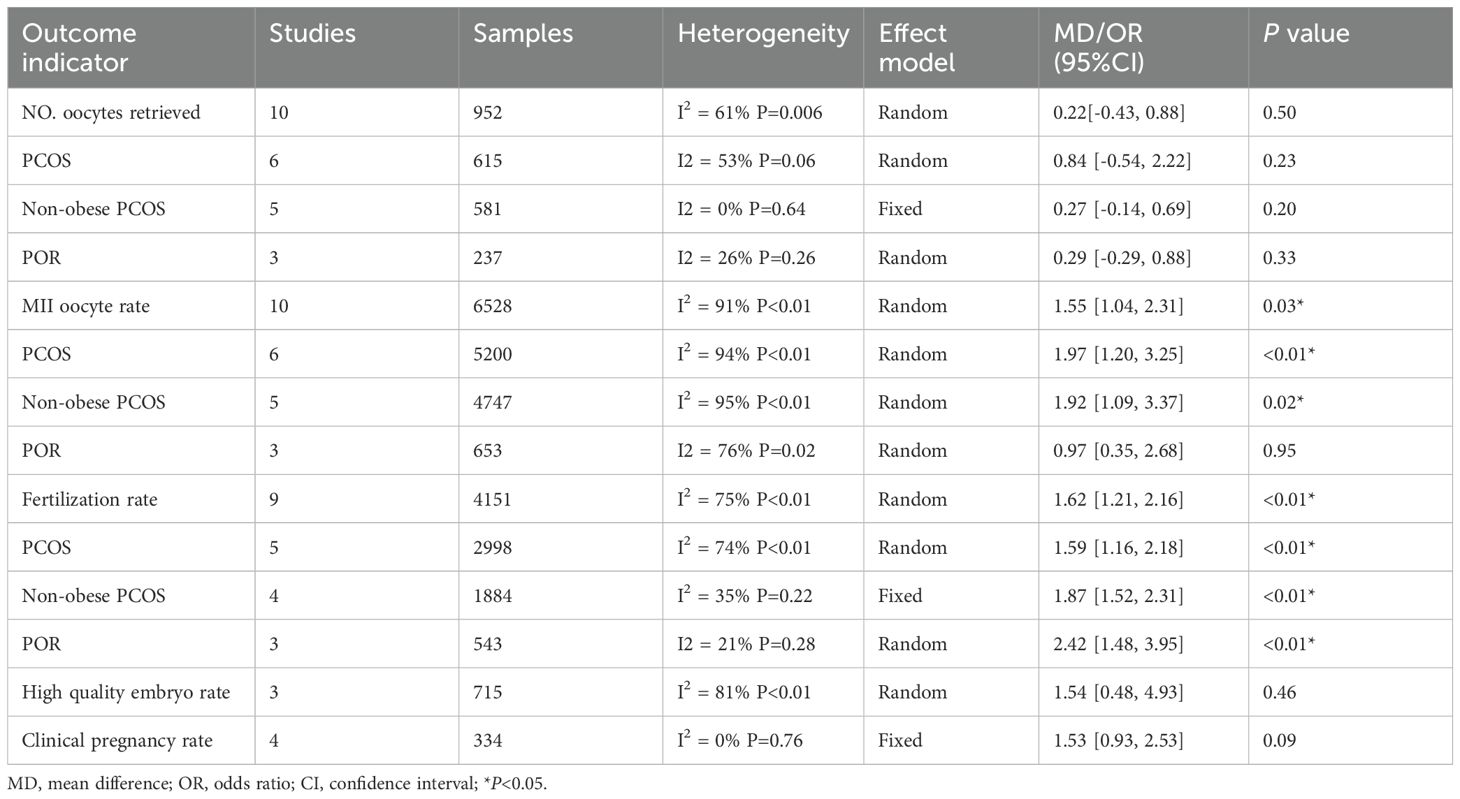
Table 2. Summary of results of meta-analysis of comparison between obsevrvational and control group.
Ten studies (19, 24–32), including reported the Number (No.) Sof oocytes retrieved. Six studies (27–32) involved the PCOS group, three studies (24–26) involved the POR group and the remaining one (19) involved the non-PCOS participants. Pooling of the results from the ten studies did not show a statistically significant difference in No. Oocytes retrieved between the MI group and the control group (MD 0.22, 95% CI -0.43-0.88, P = 0.5; Table 2). Meanwhile, there was no statistically significant improvement in No. oocytes retrieved in the MI group compared with the control group for PCOS participants (MD 0.29, 95% CI -0.29-0.88, P=0.33; Table 2). In addition, 5 studies (27–30, 32) on PCOS reported women enrolled were not obese, the meta-analysis still showed no significant difference in No. oocytes retrieved (MD 0.27, 95% CI -0.14-0.69, P = 0.2; Table 2). The data for POR participants presented no significant improvement, either (MD 0.84, 95% CI -0.54-2.22, P=0.23; Table 2).
Ten studies (19, 24–32) reporting MII oocyte rate showed a statistical significance in the advancement in the MI group (OR 1.55, 95% CI 1.04-2.31, P=0.03; Table 2). Six studies (27–32) involving the PCOS group assumed a statistically significant improvement in MII oocyte rate after taking MI (OR 1.97, 95% CI 1.20-3.25, P<0.01; Table 2), and the significant improvement also showed in the non-obese women (27–30, 32)in this subgroup analysis (OR 1.92, 95% CI 1.09-3.39, P=0.02; Table 2). However, there is no statistically significant advancement showed in the POR subgroup (OR 0.97, 95% CI 0.35-2.68, P=0.95; Table 2) (25–27).
The heterogeneity test for the data synthesis showed that the χ2 value was 1.51, with df =1 and P=0.22, while I2 was 33.9%, suggesting no statistical heterogeneity among the included studies between the subgroups.
Nine studies (19, 24–28, 30, 32, 33) reported fertilization rate. The test for overall effect showed a statistically significant improvement in the fertilization rate in the MI group compared with the control group (OR 1.62, 95% CI 1.21-2.16, P<0.01; Table 2). The five studies, involving the PCOS subgroup (27, 28, 30, 32, 33), also showed a statistically significant improvement in the fertilization rate after taking MI (OR 1.59, 95% CI 1.16-2.18, P<0.01; Table 2). The significant improvement was also observed in the non-obese PCOS women (OR 1.84, 95% CI 1.41-2.40, P<0.01; Table 2). Likewise, the three studies involving the POR subgroup (24–26) showed a statistically significant difference in the fertilization rate between the MI group and the control group (OR 2.42, 95% CI 1.48-3.95, P<0.01; Table 2).
The heterogeneity test for the data synthesis showed that the χ2 value was 2.0, with df=1 and P=0.16, while I2 was 49.9%, suggesting there is statistical heterogeneity among the included studies between the subgroups.
Two studies (19, 30) reported cleavage rate. Lizi F, et al. reported there was 171 out of 202 fertilized oocytes grow to cleavage, and Papaleo E, et al. reported 149/169 after using MI.
Three studies (19, 30, 31) comparing the high-quality embryo rate between the MI group and the control group were included in the data synthesis. The results showed no significant difference between the two groups under a random-effects model (OR 1.54, 95% CI 0.48-4.93, P=0.46; Table 2).
Only one study (27) reported on blastocyst rate, which indicated that there were 89 blastocysts cultivated out of 206 cleavage embryos.
There were three studies (19, 25, 26) reporting on implantation rate, but only Lisi et al. (19) supplied the raw data, 21 out of 112 (18.7%) embryos implanted in the MI supplement group. In the other two studies (25, 26), the implantation rate was 7.94% and 10.8%.
Four studies (19, 24, 29, 30) reported the clinical pregnancy rate. The test for overall effect showed no statistically significant improvement in the clinical pregnancy rate after taking MI (OR1.53, 95% CI 0.93-2.53, P=0.09; Table 2). Furtherly, Pacchiarotti et al. reported 63.3% clinical pregnancy rate, and Papaleo et al. reported 8 out of 30 got pregnancy after using MI when PCOS women undergoing IVF procedure.
Publication bias was assessed using funnel plots. The analysis results for publication and related bias did not suggest evidence of bias (Figure 2).
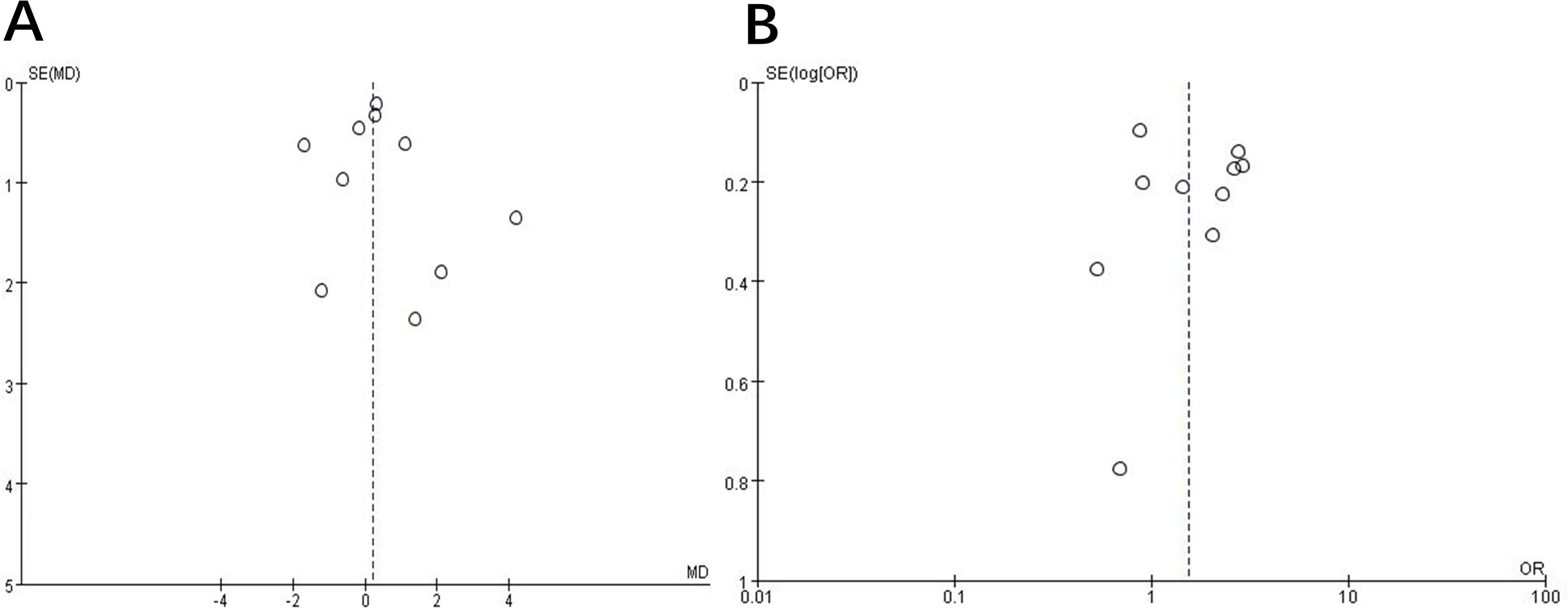
Figure 2. Funnel plot to assess publication and related biases in the systematic review. (A) continuous data; (B) categorical data.
Generally, if the I2 test results exceed 40%, the heterogeneity is considered high. Consequently, a random-effects model is employed for analysis when I2 surpasses 40%; otherwise, a fixed-effects model is utilized. Sensitivity analysis was conducted by sequentially excluding individual studies. Statistically similar results were obtained for each indication except after excluding study of Sene or Tabatabaie while evaluating the sensitivity for the MII oocyte rate in non-obese PCOS women. Overall, the result showed statistical significance (OR 1.92, 95% CI 1.09-3.39, P=0.02), but it showed no significant improvement (OR 1.78, 95% CI 0.91, 3.47, P=0.09; OR 1.74, 95% CI, P=0.10) after deleting these two studies separately despite I2 sustained at 95%, P<0.05. This was likely due to the range of sample sizes. Therefore, data from this systematic analysis should be interpreted with caution until further high-grade evidence emerges.
Increasing evidence has emerged to support the use of inositol in human reproduction, particularly for women with PCOS, but its effectiveness on IVF outcomes remains uncertain. This meta-analysis found that pre-treatment with MI may improve the MII oocyte rate in women undergoing IVF. This improvement remained in the PCOS subgroup as well as the non-obese PCOS women, but not in the POR subgroup. In addition, the positive impact on fertilization rate was consistent among the PCOS, the non-obese PCOS and POR subgroups, although the cleavage rate, high-quality embryo rate, or clinical pregnancy did not improve significantly (34). The results of sensitivity analysis by excluding studies with the most extreme dose variation (MI 2g daily) indicated that the variable dose of MI did not unduly influence the results. The effect of myoinositol supplementation on IVF outcomes in PCOS women with insulin resistance was a predetermined outcome. However, it failed to be analyzed since the studies on PCOS either excluded patients with diabetes or hyperinsulinemia, or did not provide data on insulin resistance.
Studies have indicated a significant positive correlation between MI concentration in follicles and various reproductive factors such as estradiol level in follicular fluid, cleavage rate of fertilized oocytes, embryo stage (± 4 cells), and embryo quality (grade) (9). A higher concentration of inositol in human follicular cells serves as a biological indicator for the improvement of oocyte quality (9, 35). Inositol can exist within cells in a free form or as a binding component of phospholipids or inositol phosphate derivatives (such as inositol triphosphate, etc.) in the plasma membrane, where they are crucial for cell growth, insulin, and FSH activity (36). Inositol serves as a precursor of inositol phosphate, which is a key component of the phosphatidylinositol signal transduction system. This signal transduction system involves the hydrolysis of phosphatidyl-inositol bisphosphate-dependent receptors to generate two important second messengers: trisphosphate (InsP3) and diacylglycerol (DAG) (37). InsP3 diffuses into the cytoplasm and binds to inositol InsP3 receptors on the surface of the endoplasmic reticulum, leading to the release of intracellular calcium oscillations. DAG activates protein kinase C, which modulates various cellular processes such as gamete formation, fertilization, cell proliferation, and development by phosphorylating proteins in diverse cell types (38, 39). Inositol performs various functions at the ovarian level, particularly the role of InsP3 in regulating intracellular calcium concentrations in response to the effects of the luteinizing hormone and FSH (40, 41). The function of this molecule seems to be crucial in the maturation of oocytes (42).
As in our research, MI was reported to contribute to the quality and maturity of oocytes, the cleavage rate, blastocyst expansion, and embryo quality (43). Nevertheless, it has been also demonstrated that in women with PCOS, MI was insufficient to improve the MII, embryo quality or pregnancy rate in women with PCOS (17). In this study, any dose and duration of inositol pretreatment, either MI or DCI, were included. Currently, the data on the effects of DCI on the ovary is inconsistent. The usual ratio of MI/DCI in the follicular fluid is 100:1 but is altered to 0.2:1 in PCOS patients (16). A decrease in MI concentration may lead to excessive DCI due to epimerase overactivation, resulting in depleted MI levels and subsequent decline in embryo quality (11). The dosage, duration of supplementation, and the ratio of two types of inositol are likely influencing factors on its effects which warranted further evaluation by large-sample randomized controlled trial (RCT) studies. Meanwhile, there was evidence showing that increasing the dose of DCI gradually deteriorated oocyte quality and the total amount of r-FSH (18). Therefore, this meta-analysis excluded studies with interventions of DCI supplementation or combination with metformin to ensure the accuracy of the data. Only studies with MI supplementation were included.
MI may enhance oocyte responsiveness to intracellular calcium oscillations during the early stages of fertilization, thereby potentially improving fertilization rate and embryo quality (25). It plays a role in the release of cortical granules, inhibition of polyspermy, meiosis, and subsequent activation of the cell cycle (19). In a mouse model, elevated levels of MI lead to an increase in intracellular calcium oscillations and the end of meiosis (44). The exposure of fully grown mouse germinal vesicle oocytes to MI during in-vitro maturation can enhance meiotic maturation, and the subsequent developmental potential of these oocytes following fertilization. Colazingari G et al. found that MI supplemented embryos displayed a faster cleavage rate and by the end of preimplantation development, the majority of MI supplemented blastocysts was expanded and formed by a higher number of blastomeres (45). Mohammadi F et al. showed that MI could decrease Intracellular reactive oxygen species and increase glutathione and mitochondrial membrane potential levels and consequently prevent oocyte quality reduction and improve fertilization potential in mouse (46). In humans, it has been demonstrated that the incidence of gestational diabetes mellitus was significantly reduced in women supplemented with MI. Moreover the incidence of fetal macrosomia (birth weight > 4000 g), gestational hypertension, preterm delivery, caesarean section, shoulder dystocia, neonatal hypoglycemia, and neonatal transfer to an intensive care unit) did not reveal appreciable differences between the MI and the placebo‐exposed groups (47).
In the present study, the MII oocyte rate did not increase in women with POR, which may be attributed to the multifactorial nature of oocyte quality, encompassing nuclear and mitochondrial genomes, as well as the ovarian and follicle microenvironments influencing cytoplasmic maturation, with inositol being just one contributing factor (48).
Achieving clinical pregnancy not only relies on high-quality embryos but also requires an endometrium with good receptivity, normal immune and coagulation functions as well as consideration for the psychological state of the infertile women. Improving clinical pregnancy rates demands multiple intervention measures rather than relying solely on one method since any abnormality at any stage may result in failed embryo implantation.
Overall, data from this systematic review should be interpreted with caution because of the limitations. Firstly, the robustness of the results depends largely on the quality of the primary studies included in this review. Inclusion of both RCTs and observational studies might introduce methodological heterogeneity in some instances. Meanwhile, adverse events were not reported by the majority of studies. Secondly, substantial disparities in patient selection and the variability in sample sizes decreased the certainty of the evidence overall as women with PCOS and POR accounted for a large proportion of participants in this meta-analysis. Significant statistical heterogeneity (I² >50%) in outcomes, might invalidate fixed-effect models, necessitating cautious interpretation of random-effects results. Consequently, the data derived from this systematic analysis should be interpreted with caution until further high-quality evidence becomes available. Future studies should standardize outcome reporting and prioritize individual participant data meta-analyses to address these gaps.
In conclusion, this meta-analysis demonstrates that MI supplementation improves the MII oocyte rate and the fertilization rate for women undergoing IVF. The uncertainty of the results should be considered with individual preferences when making clinical decisions. Further, clinical research and extensive multicenter randomized controlled trials needed to be conducted to assess its supplementation mode and better understand the working mechanism, establishing a more robust evidence base for clinical practice.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JZ: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. HZ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. WZ: Data curation, Methodology, Writing – review & editing. MJ: Data curation, Methodology, Writing – review & editing. XL: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1520362/full#supplementary-material
IVF-ET, In vitro fertilization and embryo transfer; ICSI, Intracytoplasmic Sperm Injection; POR, Poor ovary response; PCOS, Polycystic ovary syndrome; MI, Myo-inositol; DCI, D-chiro-inositol; OR, Odds ratio; MD, mean difference; CI, Confidence interval; FSH, Follicle-stimulating hormone; No., Number; BMI, Body mass index; RCT, Randomized controlled trial; InsP3, Trisphosphate; DAG, Diacylglycerol.
1. Alpha Scientists In Reproductive Medicine And Eshre Special Interest Group Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. (2011) 22:632–46. doi: 10.1016/j.rbmo.2011.02.001
2. ESHRE Add-ons working group, Lundin K, Bentzen JG, Bozdag G, Ebner T, Harper J, et al. Good practice recommendations on add-ons in reproductive medicine. Hum Reprod. (2023) 38:2062–104. doi: 10.1093/humrep/dead184
3. Rienzi L, Gábor V. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. (2011) 17:34–45. doi: 10.1093/humupd/dmq029
4. Hill GA, Freeman M, Bastias MC, Rogers BJ, Wentz AC. The influence of oocyte maturity and embryo quality on pregnancy rate in a program for in vitro fertilization-embryo transfer. Fertil Steril. (1989) 52:801–6. doi: 10.1016/0028-2243(89)90215-3
5. Paternot G, Debrock S, De ND, D’Hooghe TM, Spiessens C. Semi-automated morphometric analysis of human embryos can reveal correlations between total embryo volume and clinical pregnancy. Hum Reprod. (2013) 28:627–33. doi: 10.1093/humrep/des427
6. ESHRE Working Group on Recurrent Implantation Failure, Cimadomo D, de Los Santos MJ, Griesinger G, Lainas G, Le Clef N, et al. ESHRE good practice recommendations on recurrent implantation failure. Hum Reprod Open. (2023) 3:hoad023. doi: 10.1093/hropen/hoad023
7. Busnelli A, Somigliana E, Cirillo F, Baggiani A, Levi-Setti PE. Efficacy of therapies and interventions for repeated embryo implantation failure: a systematic review and meta-analysis. Sci Rep. (2021) 11:1747. doi: 10.1038/s41598-021-81439-6
8. Kane MT. The effects of water-soluble vitamins on the expansion of rabbit blastocysts in vitro. J Exp Zool. (1988) 245:220–23. doi: 10.1002/jez.1402450211
9. Chiu TT, Rogers MS, Law EL, Briton-Jones CM, Cheung LP, Haines CJ. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte quality. Hum Reprod. (2002) 17:1591–6. doi: 10.1093/humrep/17.6.1591
10. Chiu TTY, Tam PPL. A correlation of the outcome of clinical in vitro fertilization with the inositol content and embryo trophic properties of human serum. J Assist Reprod Genet. (1992) 9:524–30. doi: 10.1007/BF01204248
11. Merviel P, James P, Sarah B, Guillou ML, Véronique K. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod Health. (2021). doi: 10.1186/s12978-021-01073-3
12. Downes CP. The cellular functions of myo-inositol. Biochem SocTrans. (1989) 17:259–68. doi: 10.1042/bst0170259
13. Gerli S, Mignosa M, Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol. (2003) 7:151–9.
14. The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
15. Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. (2011) 27:857–61. doi: 10.3109/09513590.2011.564687
16. Unfer V, Carlomagno G, Rizzo P, Raffone E, Roseff S. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur Rev Med Pharmacol Sci. (2011) 15:452–7.
17. Mendoza N, Pérez L, Simoncini T, Genazzani A. Inositol supplementation in women with polycystic ovary syndrome undergoing intracytoplasmic sperm injection: a systematic review and meta-analysis of randomized controlled trials. Reprod BioMed Online. (2017) 35:529–35. doi: 10.1016/j.rbmo.2017.07.005
18. Isabella R, Raffone E. Does ovary need D-chiro-inositol? J Ovarian Res. (2012) 5:14. doi: 10.1186/1757-2215-5-14
19. Lisi F, Carfagna P, Oliva MM, Rago R, Lisi R, Poverini R, et al. Pretreatment with myo-inositol in non polycystic ovary syndrome patients undergoing multiple follicular stimulation for IVF: a pilot study. Reprod Biol Endocrinol. (2012) 10:52. doi: 10.1186/1477-7827-10-52
20. Facchinetti F, Bizzarri M, Benvenga S, Anna RD', Lanzone A, Soulage C, et al. Results from the International Consensus Conference on Myo-inositol and d-chiro-inositol in Obstetrics and Gynecology: the link between metabolic syndrome and PCOS. Eur J Obstet Gynecol Reprod Biol. (2015) 31:441–6. doi: 10.1016/j.ejogrb.2015.09.024
21. Gilbert P, Pietro C. Phosphoinositides in cell regulation and membrane dynamics. Nature. (2006) 443:651–7. doi: 10.1038/nature05185
22. Joëlle T, Michaël T, Alice P, Nassim A, Perrine M, Corinne B, et al. FSH and its second messenger cAMP stimulate the transcription of human anti-Müllerian hormone in cultured granulosa cells. Mol Endocrinol. (2011) 25:645–55. doi: 10.1210/me.2010-0297
23. Core software for Cochrane Reviews. Available online at: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (Accessed 2 June 2021).
24. Mohammadi S, Eini F, Bazarganipour F, Taghavi SA, Kutenaee MA. The effect of Myo-inositol on fertility rates in poor ovarian responder in women undergoing assisted reproductive technique: a randomized clinical trial. Reprod Biol Endocrinol. (2021) 19:61. doi: 10.1186/s12958-021-00741-0
25. Nazari L, Salehpour S, Hosseini S, Saharkhiz N, Azizi E, Hashemi T, et al. Effect of myo-inositol supplementation on ICSI outcomes among poor ovarian responder patients: A randomized controlled trial. J Gynecol Obstet Hum Reprod. (2020) 49:101698. doi: 10.1016/j.jogoh.2020.101698
26. Caprio F, D’Eufemia MD, Trotta C, Campitiello MR, Ianniello R, Mele D, et al. Myo-inositol therapy for poor-responders during IVF: a prospective controlled observational trial. J Ovarian Res. (2015) 8:37. doi: 10.1186/s13048-015-0167-x
27. Kitaya K, Takaya Y, Nishiyama R, Yamaguchi K, Matsubayashi H, Takeuchi T, et al. Myoinositol supplementation on intracytoplasmic sperm injection outcome in Japanese infertile polycystic ovarian syndrome women with non-obese less-androgenic phenotype: a prospective controlled observational study. Clin Exp Obstetrics Gynecol. (2019) 4:46. doi: 10.12891/ceog4567.2019
28. Tabatabaie M, Amiri S, Golestan Jahromi M, Sene AA, Zandieh Z, Mehdizadeh M, et al. The effect of Myo-Inositol supplement on molecular regulation of folliculogenesis, steroidogenesis, and assisted reproductive technique outcomes in patients with polycystic ovarian syndrome. Mol Biol Rep. (2022) 49:875–84. doi: 10.1007/s11033-021-06833-9
29. Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. (2016) 32:69–73. doi: 10.3109/09513590.2015.1101444
30. Papaleo E, Unfer V, Baillargeon JP, Fusi F, Occhi F, De Santis L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril. (2009) 91:1750–4. doi: 10.1016/j.fertnstert.2008.01.088
31. Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. Effects of Myo-Inositol supplementation on oocyte’s quality in PCOS patients: A double blind trial. Eur Rev Med Pharmacol Sci. (2011) 15:509–14.
32. Akbari Sene A, Tabatabaie A, Nikniaz H, Alizadeh A, Sheibani K, Mortezapour Alisaraie M, et al. The myo-inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: a randomized clinical trial. Arch Gynecol Obstet. (2019) 299:1701–7. doi: 10.1007/s00404-019-05111-1
33. Lesoine B, Regidor P-A. Prospective randomized study on the influence of myoinositol in PCOS women undergoing IVF in the improvement of oocyte quality, fertilization rate, and embryo quality. Int J Endocrinol. (2016), 5:4378507. doi: 10.1155/2016/4378507
34. Beemster P, Groenen P, Steegers-Theunissen R. Involvement of inositol in reproduction. Nutr Rev. (2002) 60. doi: 10.1301/00296640260042748
35. Vitale SG, Rossetti P, Corrado F, Rapisarda AMC, Buscema M. How to achieve high-quality oocytes? The key role of myo-inositol and melatonin. Int J Endocrinol. (2016) 8085:1–9. doi: 10.1155/2016/4987436
36. Fahy M, Kane MT. Incorporation of [3H]inositol into phosphoinositides and inositol phosphates by rabbit blastocysts. Mol Reprod Dev. (1993) 34:391–5. doi: 10.1002/mrd.1080340407
37. Balla T. Inositol-lipid binding motifs: Signal integrators through protein-lipid and protein-protein interactions. J Cell Sci. (2005) 118:2093–104. doi: 10.1242/jcs.02387
38. Berridge MJ. Inositol trisphosphate and calcium signaling. Ann NY Acad Sci. (1993) 361:315–25. doi: 10.1111/j.1749-6632.1995.tb26646.x
39. Goud PT. Inositol 1,4,5-trisphosphate receptor function in human oocytes: calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP(3) are blocked by a specific antibody to the type I receptor. Mol Hum Reprod. (2002) 8:912–18. doi: 10.1093/molehr/8.10.912
40. Zacche MM, Caputo L, Filippis S, Zacchè G, Ferrari A. Efficacy of myo-inositol in the treatment of cutaneous disorders in young women with polycystic ovary syndrome. Gynecol Endocrinol. (2009) 25:508–13. doi: 10.1080/09513590903015544
41. Matsuda M, Tsutsumi K, Kanematsu T, Fukami K, Terada Y, Takenawa T, et al. Involvement of phospholipase C-related inactive protein in the mouse reproductive system through the regulation of gonadotropin levels. Biol Reprod. (2009) 81:681–9. doi: 10.1095/biolreprod.109.076760
42. Lowther KM, Weitzman VN, Maier D, Mehlmann LM. Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biol Reprod. (2009) 81:147–54. doi: 10.1095/biolreprod.108.072538
43. Colazingari S, Treglia M, Najjar R, Bevilacqua A. The combined therapy myo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial. Arch Gynecol Obstet. (2013) 288:1405–11. doi: 10.1007/s00404-013-2855-3
44. Tak T, Chiu Y, Scott M, Rogers C, Briton-Jones C. Effects of myo-inositol on the in-vitro maturation and subsequent development of mouse oocytes. Hum Reprod. (2003) 18:408–16. doi: 10.1093/humrep/deg113
45. Colazingari S, Fiorenza MT, Carlomagno G, Najjar R, Bevilacqua A. Improvement of mouse embryo quality by myo-inositol supplementation of IVF media. J Assist Reprod Genet. (2014) 31:463–9. doi: 10.1007/s10815-014-0188-1
46. Mohammadi F, Ashrafi M, Zandieh Z, Najafi M, Niknafs B. The effect of preincubation time and myo-inositol supplementation on the quality of mouse MII oocytes. J Reprod Infertil. (2020) 21:259–68. doi: 10.18502/jri.v21i4.4330
47. Formoso G, Baldassarre MPA, Ginestra F, Carlucci MA, Bucci I, Consoli A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab Res Rev. (2019) 35:e3154. doi: 10.1002/dmrr.3154
Keywords: myo-inositol, in vitro fertilization embryo transfer, polycystic ovary syndrome, poor ovary responder, outcomes
Citation: Zhang J, Zhang H, Zhou W, Jiang M and Lin X (2025) Effect of myo-inositol supplementation in mixed ovarian response IVF cohort: a systematic review and meta-analysis. Front. Endocrinol. 16:1520362. doi: 10.3389/fendo.2025.1520362
Received: 31 October 2024; Accepted: 26 February 2025;
Published: 21 March 2025.
Edited by:
Giovanna Di Emidio, University of L’Aquila, ItalyReviewed by:
Arturo Bevilacqua, Sapienza University of Rome, ItalyCopyright © 2025 Zhang, Zhang, Zhou, Jiang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanhuan Zhang, MTgyNjg4NjAyMjRAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.