
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 11 April 2025
Sec. Thyroid Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1512417
Thyroid cancer is a malignant tumor of the endocrine system. Papillary thyroid carcinoma (PTC) is the most common form of thyroid cancer and has a comparatively better prognosis. An autoimmune disease called Hashimoto’s thyroiditis (HT) affects the thyroid and can cause lymphocyte infiltration in the thyroid tissue as well as hypothyroidism, which is characterized by increased levels of a certain antibody. It is currently assumed that there is a connection between PTC and HT. HT may increase the incidence of PTC and improve its prognosis by regulating gene expression, participating in common signaling pathways, and creating a specific immune microenvironment. In this review, we summarized the relationship between HT and PTC as well as the effects of coexisting HT on PTC and the possible mechanisms, thereby providing new perspectives for future research.
Thyroid cancer is the most common tumor in the endocrine system and has the highest five-year relative survival rate of all cancers (1). Malignant thyroid neoplasms can be classified into seven types based on molecular biology and pathological features: follicular thyroid carcinoma, papillary thyroid carcinoma, invasive encapsulated follicular variant papillary thyroid carcinoma, oncocytic thyroid carcinoma, differentiated high-grade thyroid carcinoma, poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma (2). Among them, PTC is the most common type and the prognosis of the different subtypes also varies as well (2).
HT is one of the most common autoimmune diseases and the leading cause of hypothyroidism and shares epidemiological features with thyroid tumors: the incidence is higher in women than in men (3, 4). HT is caused by T cells mistakenly attacking thyroid tissue, resulting in the lymphoplasmacytic infiltration (5). Its diagnosis is based on the symptoms of hypothyroidism and the monitoring of thyroid peroxidase antibody (TPOAb), ultrasound examination can also provide assistance in differential diagnosis (5).
The role of inflammation and the immune system in the development of cancer is a concept that has been widely discussed and accepted in recent years. By providing a range of bioactive molecules, inflammatory cells can contribute to the formation of the tumor microenvironment and thereby supporting cancer progression (6, 7). Chronic inflammation and autoimmune responses often precede the development of certain cancers, indicating a correlation between sustained immune processes and the development of tumors (6, 8).
As a type of chronic immune disease, HT exerts multiple influences on the progression of PTC, including increasing the risk of developing PTC and improving prognosis, reflecting the relationships between them on multiple fronts (9, 10). Thus, by contrasting PTC with and without HT, this review provides new theoretical and experimental insights for clinical treatment while summarizing the effects of HT on PTC and the correlation between them.
Patients suffering from HT have an increased risk of developing thyroid cancer, especially PTC, in contrast to other thyroid cancers. Some retrospective studies have suggested a stronger association between HT and PTC, with this association being even more pronounced in nodal HT (10–12). Because it is an immune-mediated disease of the thyroid, HT does not have a significant impact on the overall cancer incidence in affected individuals, but it can lead to a pro-inflammatory state, and the elevated levels of thyroid-stimulating hormone (TSH) may contribute to the development of PTC by affecting the TSH receptor, which may play a role in the development of cancer (12–14). In contrast to the higher incidence of PTC and HT in women, a clear pattern is emerging within the HT-affected population in which men are more likely to develop PTC (15). The current perspective suggests that the occurrence of thyroid cancer in male patients has a higher degree of invasiveness at the time of diagnosis (16).
The conclusions suggest that HT can be regarded as a risk factor for PTC and plays an important role in both the development and progression of PTC. However, in children, current research is inconclusive as to whether HT increases the risk of developing PTC, indicating the need for further prospective studies (17, 18). It is also recommended to pay more attention to thyroid nodules found during ultrasound examination, especially in people with HT (19).
Because it is a form of differentiated thyroid cancer, the treatment of choice for PTC is surgery. The prognosis is significantly better compared to other malignancies and the 5-year survival rate is more positive (20, 21). In the current consensus, PTC is prone to lymph node metastasis(LNM) (22). Because PTC has a generally good prognosis, there is no discernible relationship between the presence of HT in PTC and overall survival, nor is there a significant effect of LNM on the overall survival rate (23, 24). Therefore, the usefulness of overall survival as an indicator is not very important when we talk about how HT affects the prognosis of PTC.
The metastasis of PTC to lymph nodes is influenced by a variety of factors. As for central lymph node metastasis (CLNM), well-established factors such as male gender, tumor diameter over 1 cm, age, etc. are known, but the influence of HT on CLNM in PTC is still largely under debate (24, 25). Given that the BRAFV600E mutation is known to be a risk factor for LNM and that HT is negatively correlated with both the aggressiveness and metastasis of PTC and the BRAF mutation rate, it is reasonable to conclude that HT lowers the risk of LNM (25–29). Multifocal papillary thyroid carcinoma (MPTC) is also covered by this result (30).
However, some studies suggest that the coexistence of PTC and HT may not have a beneficial effect on LNM and may even act as a separate risk factor for LNM as opposed to a protective factor (14, 30, 31). These results appear to contradict previous conclusions.
The existence of these contradictory results may be attributed to a variety of factors. This contradictory situation may be partially explained by a more refined categorization and discussion of age groups and tumor sizes, as the implications of having HT differ for younger and older populations, and patients with HT are often diagnosed at a younger age (31–33).
Recent studies have shown that among other markers associated with the prognosis of PTC, patients with coexisting HT may have a lower incidence of vascular invasion, a longer recurrence-free survival period, a lower mortality and recurrence rate, and fewer cases of extrathyroidal extension (9, 34–37).
Nevertheless, it should be emphasized that there is still a great of disagreement about how HT affects neural invasion as well as the multifocality and bilaterality of PTC (9, 34, 38). Further investigation is required to substantiate certain conclusions that are not sufficiently supported by the available data.
Given the various factors discussed above, the general conclusion can be drawn that HT is a favorable factor for the prognosis of PTC, meaning that the prognosis is generally favorable for PTC patients who also suffer from HT, despite some controversy and different findings is better from various studies on the influence of HT on certain prognostic indicators of PTC (39–41).
Reviewing the previous results, it is clear that HT may have a dual impact on PTC. It can increase the likelihood of PTC occurring, but when present alongside PTC, it can also improve its prognosis. The underlying reasons for this complex influence appear to be due to various factors ranging from genetic elements to the immune system. BRAF belongs to the RAF kinase family and is involved in the Ras/Raf/MEK/ERK signaling pathway, which is associated with cell proliferation and growth (42). Thyroid cancer frequently has BRAF mutations, and the most common mutation found in PTC is the BRAFVal600Glu substitution (BRAFV600E) (26).
This mutation is not uncommon in PTC in both adults and children and has high specificity (43). Certain morphological features can specifically identify the presence of the BRAFV600E mutation in PTC (44). Moreover, traits linked to BRAF mutations include hypoechogenicity, irregular margins, and micro/macrocalcifications seen on ultrasound (45). Previous studies suggest that the overexpression of NIBAN1 and miRNA-222-3p in PTC may result from the BRAFV600E mutation, which in turn may facilitate PTC metastases (46, 47). It can also reduce the primary cilia to some extent, thereby promoting the invasion of PTC (48). Accordingly, it is thought to be related to the aggressiveness of thyroid cancer and includes features such as vascular invasion, LNM and extrathyroidal extension (26, 49, 50).
The frequency of BRAFV600E mutations is significantly lower in PTC cases associated with HT, and even in cases where BRAF mutations occur, HT can partially neutralize this effect (51–54). This could potentially be the reason why PTC patients with HT have a better prognosis.
BRAFV600E can lead to differential expression of specific long noncoding RNAs (lncRNAs), which are predominantly involved in calcium signaling pathway, MAPK signaling pathway and other signaling pathways (55). Additionally, the expression of certain lncRNAs has differential patterns in PTC and papillary thyroid carcinoma with Hashimoto’s thyroiditis (PTC-HT). For example, the expression of BRAF-activated non-coding RNA (BANCR), which is involved in the proliferation and invasion signaling pathways of PTC, was found to be decreased in PTC, moderately increased in HT, and significantly decreased in PTC-HT (56, 57). This dual effect may, to some extent, account for the consequence of a higher incidence of PTC in patients with HT.
The RET proto-oncogene and its rearranged form, the RET/PTC, are found in both PTC and HT, with a prevalence of approximately 20% in PTC, and HT is significantly associated with it. Initially considered specific for PTC diagnosis, RET/PTC is now identified in some benign disease like HT as detection sensitivity improves (58, 59).This process arises from chromosomal rearrangements that affect the MAPK signaling pathway and lead to its sustained activation (60). The most common rearrangements are RET/PTC-1 and RET/PTC-3 (61). Among them, activation of the most frequently observed RET/PTC1 can lead to the downregulation of immune checkpoints and thus play a role in tumor progression (62). It is important to note that patients with benign nodules who test positive for RET/PTC may experience faster growth rates (63). Whether RET/PTC can be considered an early event in the development of malignant tumors remains to be confirmed, current research on RET/PTC in HT is limited and controversial, warranting further investigation (64, 65). In summary, there is insufficient evidence to conclude that detecting RET/PTC mutations in the context of HT indicates the presence of malignant tumors. Moreover, its prognostic impact in PTC when present alone is limited (66).
In targeted therapy for PTC, experiments using organoid models have demonstrated that combining BRAF inhibitors, such as vemurafenib and dabrafenib, with inhibitors of MEK, RTK, CDK, and other targets produces better results, inhibiting cell proliferation while modulating the immune response (67, 68).
Additionally, although PTC is classified as a kind of cancer and HT is classified as an autoimmune disease, there is significant overlap in the genetic factors associated with both. Among them, ADH1B, ABR, SERPINA1, and LPAR5 have been identified as key genes, with EGR1 serving as a common transcription factor for SERPINA1, ABR and LPAR5 (69). Furthermore, HIF-1α and PD-L1 are important upstream regulatory factors in this process (70).
In terms of gene expression, the changes in PTC-HT are more pronounced than in HT alone, indicating a certain association between HT and the progression of PTC (69, 70). Among them, PD-L1, as a tumor marker associated with the malignancy of PTC, is positive in some PTC tissues, and its expression is even more pronounced in the context of HT (71–73). This might occur because of the various cytokines that HT, being a chronic inflammatory condition, produces and its subsequent effects on the tumor microenvironment (74). The microenvironment formed by HT can inhibit immune responses via the PD-1/PD-L1 pathway, and in tumor immunology, PD-L1 aids in facilitating the process of immune evasion (75). In the absence of PD-L1, T cells show enhanced antitumor activity (76) (Figure 1A).
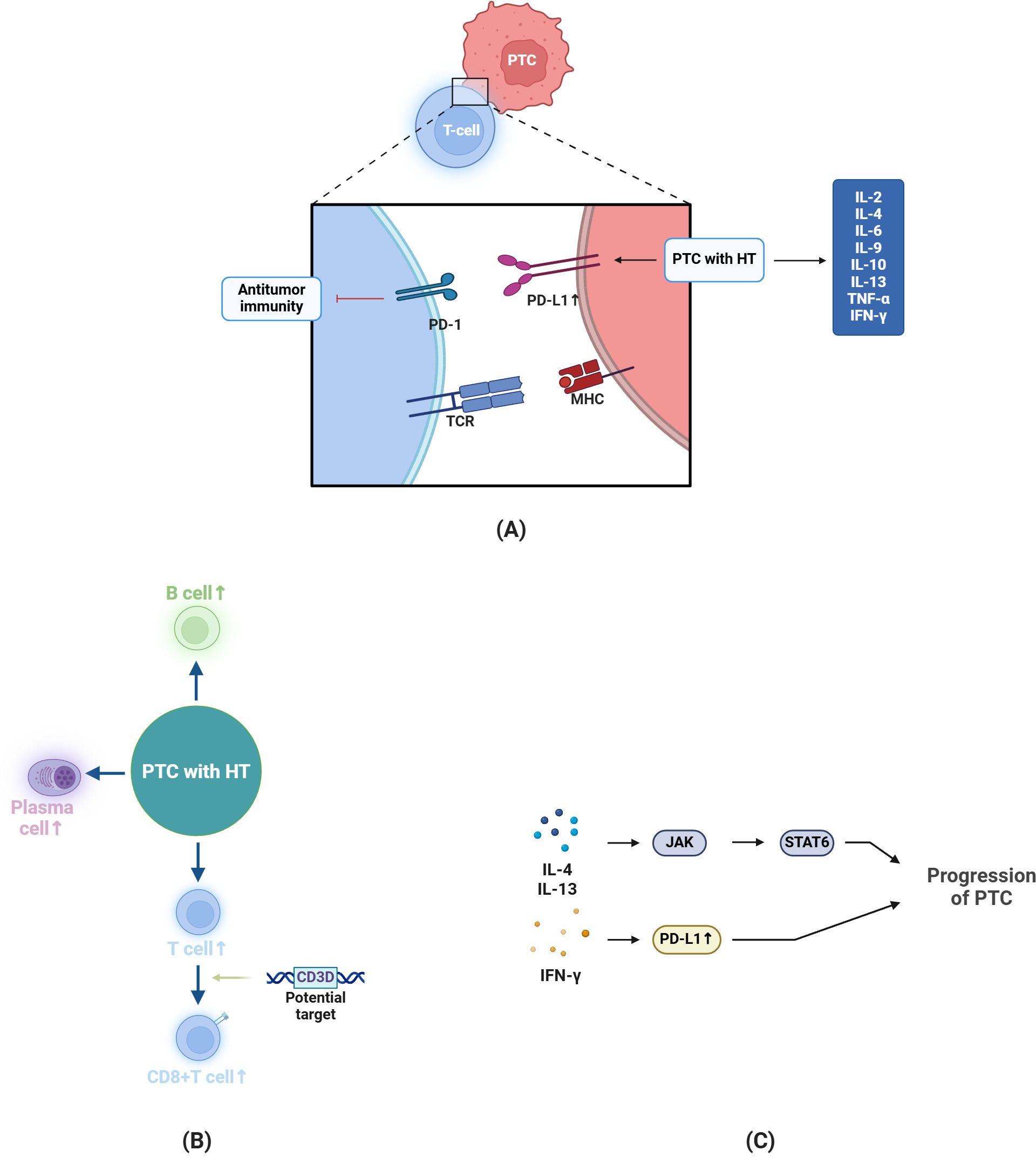
Figure 1. Changes and Potential Mechanisms in PTC-HT. (A) In antitumor immunity, T cells function via TCR binding to MHC molecules. In PTC patients with HT, the expression of PD-L1 is more pronounced, promoting tumor progression. Moreover, compared to PTC without HT, cytokines like IL-2 are upregulated. (B) In the case of PTC-HT, the number of B cells, plasma cells and T cells will increase, among which CD3D is a potential target to enhance the activation and (C) IL-4 and IL-13 can promote tumor progression through JAK-STAT pathway, and IFN-γ can promote PD-L1 expression in PTC cells to induce immune escape. The figure in this review is created by BioRender.com.
Additionally, HT exhibits upregulated lactotransferrin (LTF) and CCL21 expression compared to PTC, while PTC-HT has higher expression of SERPINA1 and DMBT1 (77, 78). LTF, an immune-related factor, is associated with tumor invasion and growth when downregulated, CCL21 is related to the infiltration of lymphocytes into tissues, SERPINA1 is involved in multiple pathways in PTC progression and its high expression can inhibit the roles of KRAS and TNF-α in their signaling pathways via the NF - κB pathway, while highly - expressed DMBT1 can also suppress PTC through immune pathways (77–80). In conclusion, the distinct levels of factor expression between the two conditions provide an explanation for the improved prognosis in PTC-HT compared to PTC alone.
Since it is an autoimmune disease, the role of specific antibodies in the progression of PTC is essential and should not be overlooked. In HT, activation of cellular and humoral immunity to certain autoantigens may have effects on thyroid endocrine function (81). TPOAb and thyroglobulin antibody (TgAb) are both autoantibodies against the thyroid, with TPOAb being a diagnostic hallmark for HT (5). In patients with coexisting HT and PTC, elevated TPOAb levels (>1300 IU/mL) correlate with the multifocality of PTC, and preoperative TPOAb and TgAb levels may, to some extent, predict the risk of recurrence in these patients (82–84).
In patients with HT, TPOAb acts as a protective factor, whereas TgAb tends to be a risk factor that promotes the progression of PTC (85). Studies have indicated that high levels of TgAb IgG4 are a risk factor for the development of PTC, which is related to differences in epitopes (86).
Increased levels of TPOAb and TgAb can lead to elevated TSH expression, which subsequently stimulates the secretion of VEGF, and patients with HT who have higher levels of TSH are more susceptible to developing PTC, because TSH, by acting on the TSH receptor, can to some degree facilitate the progression of PTC (85–88). TSH can reduce the expression of p53 and E-Cadherin, counteract the cellular senescence induced by the BRAFV600E mutation, thereby promoting the progression of the tumor (89). This could partially explain the influence of elevated TSH levels on the increased incidence of PTC. Overall, the different levels of specific antibodies in PTC-HT patients may influence the prognosis of patients with PTC (90).
In terms of metabolism, PTC shares similar metabolic pathways regardless of the presence of HT (91). However, in PTC-HT, there is a significant increase in serum concentrations of glutamate and lysine, while the concentration of alanine decreases in comparison, which correlates negatively with TPOAb levels (91, 92).
The significance of the tumor microenvironment in cancer progression should not be overlooked, as it can confer tumors with signature characteristics at an earlier stage, which is particularly the case in the progression of PTC. Research has confirmed that the presence of HT can alleviate oxidative stress caused by PTC (7).
By promoting angiogenesis, VEGF supplies the tumor with nutrients (93). Compared to patients with PTC alone, those with PTC-HT exhibit lower expression levels of VEGF (94). In thyroid tissue, E-cadherin and its activator TGF-β1 are more highly expressed in patients with PTC complicated by HT, while the expression of N-cadherin and ICAM-1 is reduced (95). The two play different roles: N-cadherin tends to promote tumor cell metastasis, while E-cadherin can normally inhibit tumor invasion and its loss is associated with the epithelial-mesenchymal transition (EMT) (95, 96). Therefore, it can be concluded that HT generally exerts an inhibitory effect on the invasiveness of PTC.
The immune system can monitor and attack tumor cells, which is one of its functions. After cancer cells emerge, the immune system will try to attack and eliminate them. In some tumors, T - cell infiltration predicts a better prognosis. As a type of T cell, cytotoxic T lymphocytes (CTLs) can identify and specifically kill tumor cells after accepting antigens (97). This process relies on MHC I. regulatory T cells (Tregs), whose main function is immune suppression and which are often associated with a poor prognosis in tumor immunity, are also a current direction of immunotherapy by specifically targeting the tumor cell microenvironment (98, 99) (Figure 1A).
In terms of immunity, one of the characteristics of cancer is to evade the immune environment, and specific surface molecules under immune response promote the progression of the tumor (85). HT plays a significant role in the immune evasion mechanisms of PTC, which is also related to its impact on the tumor microenvironment. Patients with HT show a decrease in Tregs, which are involved in immune homeostasis regulation, and an increase in the CD4 to CD8 ratio (4). In addition, the thyroid is infiltrated by lymphocytes, predominantly T cells (4). Compared with other non-autoimmune thyroiditis, the number of CD8+ T cells in HT is significantly increased, which is associated with cytotoxic effects that lead to follicular destruction (100).The majority of B cells are located in the thyroid tissue and there are no significant fluctuations in the amount of circulating B cells (101).
In PTC-HT, as compared to PTC alone, there is an increased presence of CD3+, CD4+, CD8+ cells, B lymphocytes, and plasma cells in the thyroid tissue. As already mentioned, the density of CD4+ cells is higher than that of CD8+ cells (102–104). A higher number of CD8+ T cells is positively correlated with the disease-free survival of PTC patients (105). CD3D is a target in signal transduction that enhances the effector function of CD8+ T cells. Through an increase in CD8+ cell count, HT may have an impact on the activation of STAT6 and the tumor microenvironment (105, 106) (Figures 1B, C).
Compared to PTC alone, patients with HT have increased production of IL-2, IL-4, IL-6, IL-9, IL-10, IL-13, TNF-α, and IFN-γ, and there is also an increase in the expression of MHC I (related to IL-2 and IL-10) (107–110). While IL-6 helps thyroid cancer acquire EMT and stem cell-like properties, increased levels of IL-4 and IL-10 can also encourage the expression of anti-apoptotic proteins (111) (Figures 1A, C).
The different levels of immune cells and cytokines can explain why PTC with HT may have a better prognosis. In PTC with HT, T cells, especially CTLs and Tregs, dominate the tumor microenvironment (112). CD8+ T cell immunity against tumors relies on MHC I, whose absence can lead to immune evasion. HT is associated with increased IL-2 expression, which boosts MHC I expression, creating a significant difference in MHC I levels between PTC and PTC-HT (110). However, there’s insufficient research to draw definitive conclusions about B lymphocytes in this context (113).
In addition, it is noteworthy that HT is also strongly associated with other thyroid malignancies, including medullary thyroid carcinoma(MTC) and thyroid lymphoma(TL), but appears to be weakly associated with undifferentiated thyroid cancer and follicular thyroid carcinoma (114).
Although the relationship between HT and MTC remains controversial, a study has shown that their association is more significant when gender is considered (11, 115). HT may affect serum calcitonin (sCt) levels but doesn’t negate the strong suspicion of MTC when sCt is high (116, 117). Similar to other thyroid diseases, ultrasound can provide diagnostic value for MTC in patients with HT (118).
TL is a very rare disease in clinical practice, and it is usually manifested by rapid enlargement of neck masses and even compression of airway (119, 120). As an autoimmune disease, HT is associated with a higher risk of TL, and they both exhibit clonal bands with sequence similarity (121, 122). In some cases, primary TL has similar characteristics to HT under ultrasound and should be identified (123).
Pathologically, PTC typically exhibits a papillary architecture and a propensity for lymphatic metastasis (22). HT presents with focal lesions, and its immunohistochemical characteristics are similar to those of PTC (124). PTC associated with HT tends to show tumorous fibrosis and anastomosing pseudovascular spaces on pathological examination (125).
In aspects of ultrasound and fine needle aspiration biopsy (FNAB), patients with PTC-HT have a lower proportion of large areas of calcification and psammoma bodies but a higher frequency of dense calcifications compared to PTC without HT (126). However, overall, the coexistence of HT does not significantly impact the outcomes of preoperative ultrasonography and FNAB (127). Furthermore, applying ultrasound findings and serological markers to deep learning may aid in the future diagnosis and detection of HT (128).
In patients with autoimmune thyroid diseases, the use of thyroid ultrasound examination helps in the early detection of PTC (19, 129). In ultrasonography, HT patients who demonstrate patterns of echogenic foci may be at an increased risk for PTC (130). Preoperative ultrasound examinations in PTC patients may be helpful in predicting the occurrence of CLNM (131). However, it is important to recognize that the presence of HT may complicate the interpretation of preoperative ultrasound findings related to CLNs in patients with PTC (132).
For thyroid cancer, surgery is the most common and traditional treatment method (22). When there is evidence of spread, a comprehensive clearance of the thyroid and any compromised tissues is attempted (22). In addition, treatment with radioactive iodine can also be carried out postoperatively in individual cases (22). Drawing from previous conclusions, patients with HT are at an elevated risk for the development of PTC, and thus, total thyroidectomy may be considered for them (133). With HT, thyroid surgery is more likely to encounter adhesions, and patients are at a higher risk of developing transient hypocalcemia postoperatively, which requires attention (134). Patients with PTC who have coexisting HT tend to respond better to radioactive iodine treatment (135). Nonetheless, to guarantee the efficacy of radioactive iodine therapy, it’s critical to modify the recommended dosage (135).
Endoscopic thyroidectomy is a relatively new surgical treatment method that does not increase the incidence of postoperative complications compared to open surgery and is considered a safe and reliable approach (136). The concomitant presence of HT may increase the duration of the procedure but does not have a significant impact on the results (137).
In PTC, common mutations include BRAFV600E and RET/PTC fusion. Given the favorable prognosis of PTC patients after surgery, targeted therapy is considered for those with potential poor prognosis, such as coexistence of RET/PTC and TERT promoter mutations (66). Sunitinib (mainly inhibits RET/PTC1 and RET/PTC3) and Sorafenib (inhibits RAF) in TKI have a certain effect for patients with advanced DTC or RAI-refractory DTC (138).
In recent years, the incidence of thyroid tumors has increased, with papillary thyroid carcinoma being the most common form of thyroid cancer. It has distinct pathological features and indicators and both diagnostic and therapeutic approaches are well establishedclinically. The prognosis of PTC is one of the best compared to other types of cancer, making it one of the most well-researched diseases currently.
Hashimoto’s thyroiditis is an autoimmune disease caused by an abnormal immune system, which is the most common cause of primary hypothyroidism in non-iodine deficiency conditions. Patients may present with hyperthyroidism, hypothyroidism during the disease. The combination of clinical symptoms and the determination of hormones and antibodies in the blood helps to diagnose the disease, in which TPOAb is more specific and histological examination is not necessary.
Currently, numerous studies have demonstrated a link between PTC and HT. This article reviews the potential impacts of HT on PTC and the relationship between them, summarizing the latest research advancements from recent years.
HT exerts a dualistic effect on PTC. On the one hand, patients with HT as one of the risk factors for PTC are more susceptible to developing PTC. This susceptibility is associated with genetic changes, cytokine release, the inflammatory environment induced by HT, and higher TSH levels, all of which contribute significantly to shaping the tumor microenvironment. Additionally, HT and PTC share many common genes and similar metabolic pathways, which may determine their similarities in some signaling pathways, thus predisposing individuals to PTC. On the other hand, compared to patients without HT, those with PTC-HT often have a better prognosis. This is related to the different expression levels of factors such as E-cadherin and VEGF, as well as the presence of specific antibodies. Moreover, because HT is an autoimmune disease, immune cells in the tumor microenvironment of PTC will show significant differences in whether HT is accompanied or not. Because of the existence of these two effects, in the presence of HT, the diagnosis and treatment of PTC needs to be considered more circumstances, and specific diagnosis and treatment plans should be developed according to the different circumstances of individuals.
In the field of diagnosis and treatment, although the presence of HT has some impact on PTC, it does not affect the results of ultrasound and FNAB to such an extent that it would affect the assessment. Given the conclusion that HT is a risk factor for PTC, it is recommended to increase the use of ultrasound examinations in HT patients in clinical practice to detect the presence of PTC early and achieve better treatment outcomes. If PTC is confirmed and the patient also has HT, decisions regarding lymph node dissection should be made with greater caution. In addition, the dosage of radioactive iodine treatment should be adjusted to the specific conditions.
In the current research on HT and thyroid tumors, the association between HT and DTC (especially PTC) has been the most prominent, but other types of tumors (such as MTC with a poor prognosis) should still be concerned, although the probability of occurrence may be lower.
However, TL should be paid more attention to patients with HT. Although the occurrence probability is not high, once it occurs, it may be manifested as pressure on the trachea and esophagus, and its ultrasonic diagnosis and treatment plan are different from that of general thyroid tumors, so more care should be taken.
Unlike PTC, more cases are reported in the analysis discussing the association between HT and other malignancies, which may be related to the small number of cases, and the mechanism is not as fully explored as between PTC and HT. However, based on existing studies and discussions, no matter what kind of association, the role played by the chronic inflammatory characteristics of HT cannot be ignored.
Melanoma also has BRAF mutations, and clinical trials have been conducted to test the effects of BRAF inhibitors. In PTC, the presence of BRAF mutations often means a worse prognosis, and the combination of inhibitors targeting BRAF with multiple other inhibitors may lead to better outcomes.
RET/PTC is seen in a subset of PTC patients and was initially considered a specific feature, but it is now possible to detect this chromatin rearrangement in benign disease. There is still debate about this phenomenon: are there microscopic lesions that have not been detected by histology? It should be noted that even in benign lesions, the presence of RET/PTC may indicate a faster growth rate of nodules. Similarly, the corresponding inhibitors may have greater significance in patients with poor prognosis.
In the future, patients with HT should undergo more frequent follow-up, especially in groups more susceptible to developing PTC, such as individuals with BRAFV600E mutations or RET/PTC. The dual effects of HT on PTC require that treatment plans should consider the presence of HT to select the most effective therapeutic strategies. By focusing on the similarities between HT and PTC in the future, we can better explore the pathogenesis of PTC, thereby enabling more precise treatments.
SY: Writing – original draft. HZ: Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
First of all, I would like to express my deepest gratitude to my supervisors for the support they gave me throughout the writing process of this article. I am also grateful to my fellow students and my institution for their support on this journey. Finally, I would like to thank my family and friends for their constant encouragement during this time.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Christofer Juhlin C, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel concepts in nomenclature and grading. Endocr Relat Cancer. (2023) 30. doi: 10.1530/ERC-22-0293
3. Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. (2023) 401:1878–90. doi: 10.1016/S0140-6736(23)00457-9
4. Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, et al. Hashimotos' thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. (2019) 33:101367. doi: 10.1016/j.beem.2019.101367
5. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment. Pol Arch Intern Med. (2022) 132. doi: 10.20452/pamw.16222
6. Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Paparo SR, et al. Thyroid autoimmune disorders and cancer. Semin Cancer Biol. (2020) 64:135–46. doi: 10.1016/j.semcancer.2019.05.019
7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
8. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
9. Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, et al. Prevalence of hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open. (2021) 4:e2118526. doi: 10.1001/jamanetworkopen.2021.18526
10. Xu J, Ding K, Mu L, Huang J, Ye F, Peng Y, et al. Hashimoto's thyroiditis: A "Double-edged sword" in thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:801925. doi: 10.3389/fendo.2022.801925
11. Resende de Paiva C, Grønhøj C, Feldt-Rasmussen U, von Buchwald C. Association between hashimoto's thyroiditis and thyroid cancer in 64,628 patients. Front Oncol. (2017) 7:53. doi: 10.3389/fonc.2017.00053
12. Boi F, Pani F, Calò PG, Lai ML, Mariotti S. High prevalence of papillary thyroid carcinoma in nodular Hashimoto's thyroiditis at the first diagnosis and during the follow-up. J Endocrinol Invest. (2018) 41:395–402. doi: 10.1007/s40618-017-0757-0
13. Jackson D, Handelsman RS, Farrá JC, Lew JI. Increased incidental thyroid cancer in patients with subclinical chronic lymphocytic thyroiditis. J Surg Res. (2020) 245:115–8. doi: 10.1016/j.jss.2019.07.025
14. Harmantepe AT, Ozdemir K, Bayhan Z, Kocer B. The underestimated impact of hashimoto thyroiditis on thyroid papillary carcinoma. Updates Surg. (2024) 76:1085–9. doi: 10.1007/s13304-024-01854-y
15. Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol. (2014) 140:1021–6. doi: 10.1007/s00432-014-1629-z
16. Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. (2010) 6:1771–9. doi: 10.2217/fon.10.127
17. Lee YJ, Cho YJ, Heo YJ, Chung E-J, Choi YH, Kim J-I, et al. Thyroid nodules in childhood-onset Hashimoto's thyroiditis: Frequency, risk factors, follow-up course and genetic alterations of thyroid cancer. Clin Endocrinol (Oxf). (2021) 95:638–48. doi: 10.1111/cen.14490
18. Sur ML, Gaga R, Lazăr C, Lazea C, Aldea C, Sur D. Papillary thyroid carcinoma in children with Hashimoto's thyroiditis - a review of the literature between 2000 and 2020. J Pediatr Endocrinol Metab. (2020) 33:1511–7. doi: 10.1515/jpem-2020-0383
19. Jie Y, Ruan J, Luo M, Liu R. Ultrasonographic, clinical, and pathological features of papillary thyroid carcinoma in children and adolescents with or without Hashimoto's thyroiditis. Front Oncol. (2023) 13:1198468. doi: 10.3389/fonc.2023.1198468
20. Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet. (2023) 401:1531–44. doi: 10.1016/S0140-6736(23)00020-X
21. Boucai L, Zafereo M, Cabanillas ME. Thyroid cancer: A review. JAMA. (2024) 331:425–35. doi: 10.1001/jama.2023.26348
22. Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. (2014) 65:125–37. doi: 10.1146/annurev-med-061512-105739
23. Liang J, Zeng W, Fang F, Yu T, Zhao Y, Fan X, et al. Clinical analysis of Hashimoto thyroiditis coexistent with papillary thyroid cancer in 1392 patients. Acta Otorhinolaryngol Ital. (2017) 37:393–400. doi: 10.14639/0392-100X-1709
24. Lau J, Lee J, Mahipal M, Yang SP, Tan WB, Yuan NK, et al. Hashimoto's thyroiditis on outcomes in papillary thyroid cancer revisited: experience from South East Asia. Ann R Coll Surg Engl. (2022) 104:465–71. doi: 10.1308/rcsann.2021.0224
25. Ma H, Li L, Li K, Wang T, Zhang Y, Zhang C, et al. Hashimoto's thyroiditis, nodular goiter or follicular adenoma combined with papillary thyroid carcinoma play protective role in patients. Neoplasma. (2018) 65:436–40. doi: 10.4149/neo_2018_170428N317
26. Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. (2022) 34:9–18. doi: 10.1097/CCO.0000000000000797
27. Wang Y, Zheng J, Hu X, Chang Q, Qiao Y, Yao X, et al. A retrospective study of papillary thyroid carcinoma: Hashimoto's thyroiditis as a protective biomarker for lymph node metastasis. Eur J Surg Oncol. (2023) 49:560–7. doi: 10.1016/j.ejso.2022.11.014
28. Song WJ, Um IC, Kwon SR, Lee JH, Lim HW, Jeong YU, et al. Predictive factors of lymph node metastasis in papillary thyroid cancer. PloS One. (2023) 18:e0294594. doi: 10.1371/journal.pone.0294594
29. Battistella E, Pomba L, Costantini A, Scapinello A, Toniato A. Hashimoto's thyroiditis and papillary cancer thyroid coexistence exerts a protective effect: a single centre experience. Indian J Surg Oncol. (2022) 13:164–8. doi: 10.1007/s13193-022-01515-9
30. Zhu F, Shen YB, Li FQ, Fang Y, Hu L, Wu YJ. The effects of hashimoto thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma: A retrospective chinese cohort study. Med (Baltimore). (2016) 95:e2674. doi: 10.1097/MD.0000000000002674
31. Wang L, Chen J, Yuan X, Wang J, Sun L, Jiang J, et al. Lymph node metastasis of papillary thyroid carcinoma in the context of Hashimoto's thyroiditis. BMC Endocr Disord. (2022) 22:12. doi: 10.1186/s12902-021-00923-2
32. Ma B, Chen X, Zhao Z, Yin X, Ji Q, Zhou Y, et al. Coexisting CLT in PTC is an independent predictor of tumor aggressiveness for patients aged under 55: a retrospective analysis of 635 patients. BMC Endocr Disord. (2022) 22:55. doi: 10.1186/s12902-022-00945-4
33. Yan C, He X, Chen Z, Wang Y. Central compartment lymph nodes have distinct metastatic patterns in different age groups. Front Endocrinol (Lausanne). (2022) 13:807431. doi: 10.3389/fendo.2022.807431
34. Tang Q, Pan W, Peng L. Association between Hashimoto thyroiditis and clinical outcomes of papillary thyroid carcinoma: A meta-analysis. PloS One. (2022) 17:e0269995. doi: 10.1371/journal.pone.0269995
35. Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, et al. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol. (2015) 20:463–73. doi: 10.1007/s10147-014-0754-7
36. Ryu YJ, Yoon JH. Chronic lymphocytic thyroiditis protects against recurrence in patients with cN0 papillary thyroid cancer. Surg Oncol. (2020) 34:67–73. doi: 10.1016/j.suronc.2020.03.008
37. Marotta V, Sciammarella C, Chiofalo MG, Gambardella C, Bellevicine C, Grasso M, et al. Hashimoto's thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. (2017) 24:485–93. doi: 10.1530/ERC-17-0085
38. Osborne D, Choudhary R, Vyas A, Kampa P, Abbas LF, Chigurupati HD, et al. Hashimoto's thyroiditis effects on papillary thyroid carcinoma outcomes: A systematic review. Cureus. (2022) 14:e28054. doi: 10.7759/cureus.28054
39. Moon S, Chung HS, Yu JM, Yoo HJ, Park JH, Kim DS, et al. Associations between hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: A meta-analysis of observational studies. Endocrinol Metab (Seoul). (2018) 33:473–84. doi: 10.3803/EnM.2018.33.4.473
40. Song E, Jeon MJ, Park S, Kim M, Oh H-S, Song DE, et al. Influence of coexistent Hashimoto's thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (Oxf). (2018) 88:123–8. doi: 10.1111/cen.13475
41. Cao J, Sun Y, Liu Y, Xu Y, Li X, Zhang W, et al. The impact of Hashimoto's thyroiditis on the clinical outcome of papillary thyroid cancer after radioactive iodine therapy: a propensity score matching study. Endocrine. (2024) 87(1):178–87. doi: 10.1007/s12020-024-03973-3
42. Karoulia Z, Gavathiotis E, Poulikakos PI. New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. (2017) 17:676–91. doi: 10.1038/nrc.2017.79
43. Zhou B, Lu X, Hei H, Zhang S, Li Y, Fang J, et al. Single BRAFV600E mutation is not associated with aggressive biological behavior in adolescent and pediatric papillary thyroid carcinoma. Cancer Cytopathol. (2023) 131:716–23. doi: 10.1002/cncy.22746
44. Turchini J, Sioson L, Clarkson A, Sheen A, Delbridge L, Glover A, et al. The presence of typical "BRAFV600E-like" Atypia in papillary thyroid carcinoma is highly specific for the presence of the BRAFV600E mutation. Endocr Pathol. (2023) 34:112–8. doi: 10.1007/s12022-022-09747-9
45. Khadra H, Deniwar A, Mohsin K, Monlezun D, Kandil E. Can suspicious ultrasound features predict BRAFV600E status in papillary thyroid cancer? Eur Thyroid J. (2018) 7:205–10. doi: 10.1159/000489851
46. Gao Y, Xiang D, Li W, Zheng X, Wang L, Li Z, et al. BRAFV600E Mutation-Responsive miRNA-222-3p Promotes Metastasis of Papillary Thyroid Cancer Cells via Snail-Induced EMT. Front Endocrinol (Lausanne). (2022) 13:843334. doi: 10.3389/fendo.2022.843334
47. Diana P, Ribeiro Carneiro TN, Cerutti JM, Kuroshu RM, Carvalheira GMG. Transcriptomic analysis reveals that NIBAN1 overexpression is associated with BRAFV600E mutation and increases the aggressiveness of thyroid cancer. Genes Dis. (2024) 11:101094. doi: 10.1016/j.gendis.2023.101094
48. Ma C-X, Ma X-N, Liu J-J, Guan C-H, Li Y-D, Zhao N, et al. The BRAFV600E mutation maintains the aggressiveness of papillary thyroid cancers requiring downregulation of primary cilia. Mol Cell Endocrinol. (2024) 581:112113. doi: 10.1016/j.mce.2023.112113
49. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. (2011) 7:569–80. doi: 10.1038/nrendo.2011.142
50. Ye Z, Xia X, Xu P, Liu W, Wang S, Fan Y, et al. The prognostic implication of the BRAF V600E mutation in papillary thyroid cancer in a chinese population. Int J Endocrinol. (2022) 2022:6562149. doi: 10.1155/2022/6562149
51. Kim WW, Ha TK, Bae SK. Clinical implications of the BRAF mutation in papillary thyroid carcinoma and chronic lymphocytic thyroiditis. J Otolaryngol Head Neck Surg. (2018) 47:4. doi: 10.1186/s40463-017-0247-6
52. Xie M, Xu ZX, Dai M, Zhu YY. Correlation between the clinicopathological features of papillary thyroid carcinoma complicated with hashimoto's thyroiditis, BRAF V600E gene mutation, and RET gene rearrangement. J Coll Physicians Surg Pak. (2024) 34:445–50. doi: 10.29271/jcpsp.2024.04.445
53. Issa PP, Omar M, Buti Y, Issa CP, Chabot B, Carnabatu CJ, et al. Hashimoto's thyroiditis minimizes lymph node metastasis in BRAF mutant papillary thyroid carcinomas. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10082051
54. Janicki L, Patel A, Jendrzejewski J, Hellmann A. Prevalence and Impact of BRAF mutation in patients with concomitant papillary thyroid carcinoma and Hashimoto's thyroiditis: a systematic review with meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1273498. doi: 10.3389/fendo.2023.1273498
55. Goedert L, Plaça JR, Fuziwara CS, MaChado MCR, Plaça DR, Almeida PP, et al. Identification of long noncoding RNAs deregulated in papillary thyroid cancer and correlated with BRAFV600E mutation by bioinformatics integrative analysis. Sci Rep. (2017) 7:1662. doi: 10.1038/s41598-017-01957-0
56. Zhang Y, Lu K-N, Ding J-W, Peng Y, Pan G, Teng L-S, et al. Identification of long noncoding RNAs associated with the clinicopathological features of papillary thyroid carcinoma complicated with hashimoto's thyroiditis. Front Oncol. (2022) 12:766016. doi: 10.3389/fonc.2022.766016
57. Zhang J, Yao L, Guo Y. Interaction of BANCR in the relationship between Hashimoto's thyroiditis and papillary thyroid carcinoma expression patterns and possible molecular mechanisms. J Gene Med. (2024) 26:e3663. doi: 10.1002/jgm.3663
58. Marotta V, Guerra A, Sapio MR, Vitale M. RET/PTC rearrangement in benign and Malignant thyroid diseases: a clinical standpoint. Eur J Endocrinol. (2011) 165:499–507. doi: 10.1530/EJE-11-0499
59. Wirtschafter A, Schmidt R, Rosen D, Kundu N, Santoro M, Fusco A, et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope. (1997) 107(1):95–100. doi: 10.1097/00005537-199701000-00019
60. Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int J Med Sci. (2019) 16:450–60. doi: 10.7150/ijms.29935
61. Ashwini BR, Nirmala C, Natarajan M, Biligi DS. A study to evaluate association of nuclear grooving in benign thyroid lesions with RET/PTC1 and RET/PTC3 gene translocation. Thyroid Res. (2023) 16:21. doi: 10.1186/s13044-023-00161-9
62. Denning K, Smyth P, Cahill S, Li J, Flavin R, Aherne S, et al. ret/PTC-1 expression alters the immunoprofile of thyroid follicular cells. Mol Cancer. (2008) 7:44. doi: 10.1186/1476-4598-7-44
63. Sapio MR, Guerra A, Marotta V, Campanile E, Formisano R, Deandrea M, et al. High growth rate of benign thyroid nodules bearing RET/PTC rearrangements. J Clin Endocrinol Metab. (2011) 96:E916–E9. doi: 10.1210/jc.2010-1599
64. Sheils OM, O'Eary JJ, Uhlmann V, Lättich K, Sweeney EC. ret/PTC-1 activation in hashimoto thyroiditis. Int J Surg Pathol. (2000) 8:185–9. doi: 10.1177/106689690000800305
65. Cyniak-Magierska A, Wojciechowska-Durczyńska K, Krawczyk-Rusiecka K, Zygmunt A, Lewiński A. Assessment of RET/PTC1 and RET/PTC3 rearrangements in fine-needle aspiration biopsy specimens collected from patients with Hashimoto's thyroiditis. Thyroid Res. (2011) 4:5. doi: 10.1186/1756-6614-4-5
66. Zhang W, Lin S, Wang Z, Zhang W, Xing M. Coexisting RET/PTC and TERT promoter mutation predict poor prognosis but effective RET and MEK targeting in thyroid cancer. J Clin Endocrinol Metab. (2024) 109:3166–75. doi: 10.1210/clinem/dgae327
67. Chen D, Su X, Zhu L, Jia H, Han B, Chen H, et al. Papillary thyroid cancer organoids harboring BRAFV600E mutation reveal potentially beneficial effects of BRAF inhibitor-based combination therapies. J Transl Med. (2023) 21:9. doi: 10.1186/s12967-022-03848-z
68. Mohanty A, Afkhami M, Reyes A, Pharaon R, Yin H, Li H, et al. Exploring markers of immunoresponsiveness in papillary thyroid carcinoma and future treatment strategies. J Immunother Cancer. (2024) 12. doi: 10.1136/jitc-2023-008505
69. Liu T-T, Yin D-T, Wang N, Li N, Dong G, Peng M-F. Identifying and analyzing the key genes shared by papillary thyroid carcinoma and Hashimoto's thyroiditis using bioinformatics methods. Front Endocrinol (Lausanne). (2023) 14:1140094. doi: 10.3389/fendo.2023.1140094
70. Zhang L, Zhou L, Feng Q, Li Q, Ge M. Mutation of hashimoto's thyroiditis and papillary thyroid carcinoma related genes and the screening of candidate genes. Front Oncol. (2021) 11:813802. doi: 10.3389/fonc.2021.813802
71. Dell'Aquila M, Granitto A, Martini M, Capodimonti S, Cocomazzi A, Musarra T, et al. PD-L1 and thyroid cytology: A possible diagnostic and prognostic marker. Cancer Cytopathol. (2020) 128:177–89. doi: 10.1002/cncy.22224
72. Shi R-L, Qu N, Luo T-X, Xiang J, Liao T, Sun G-H, et al. Programmed death-ligand 1 expression in papillary thyroid cancer and its correlation with clinicopathologic factors and recurrence. Thyroid. (2017) 27:537–45. doi: 10.1089/thy.2016.0228
73. Lubin D, Baraban E, Lisby A, Jalali-Farahani S, Zhang P, Livolsi V. Papillary thyroid carcinoma emerging from hashimoto thyroiditis demonstrates increased PD-L1 expression, which persists with metastasis. Endocr Pathol. (2018) 29:317–23. doi: 10.1007/s12022-018-9540-9
74. Santana VB, Krüger VM, Abrahão MCY, Cantú PLM, Brackmann RL, Pandolfi GM, et al. Chronic lymphocytic thyroiditis with oncocytic metaplasia influences PD-L1 expression in papillary thyroid carcinoma. Head Neck Pathol. (2024) 18:14. doi: 10.1007/s12105-024-01618-5
75. D'Andréa G, Lassalle S, Guevara N, Mograbi B, Hofman P. From biomarkers to therapeutic targets: the promise of PD-L1 in thyroid autoimmunity and cancer. Theranostics. (2021) 11:1310–25. doi: 10.7150/thno.50333
76. Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. (2017) 8:14572. doi: 10.1038/ncomms14572
77. Liu C, Pan Y, Li Q, Zhang Y. Bioinformatics analysis identified shared differentially expressed genes as potential biomarkers for Hashimoto's thyroiditis-related papillary thyroid cancer. Int J Med Sci. (2021) 18:3478–87. doi: 10.7150/ijms.63402
78. Zhang Y, Xie X, Zhou H, Li B, Ding L, Cai Z, et al. Identification of SERPINA1 promoting better prognosis in papillary thyroid carcinoma along with Hashimoto's thyroiditis through WGCNA analysis. Front Endocrinol (Lausanne). (2023) 14:1131078. doi: 10.3389/fendo.2023.1131078
79. Gan X-X, Li Y-Y, Li S-J, Mo S-S, Feng J-H, Shen F, et al. Significance of DMBT1 in papillary thyroid carcinoma concurrent with hashimoto's thyroiditis. Front Oncol. (2021) 11:680873. doi: 10.3389/fonc.2021.680873
80. Du X, Chen W. Bioinformatic analysis of serpina1 expression in papillary thyroid carcinoma and its potential association with Hashimoto's thyroiditis. Discovery Oncol. (2025) 16:356. doi: 10.1007/s12672-025-02079-0
81. Vargas-Uricoechea H. Molecular mechanisms in autoimmune thyroid disease. Cells. (2023) 12. doi: 10.3390/cells12060918
82. Dong S, Xia Q, Wu Y-J. High TPOAb levels (>1300 IU/mL) indicate multifocal PTC in hashimoto's thyroiditis patients and support total thyroidectomy. Otolaryngol Head Neck Surg. (2015) 153:20–6. doi: 10.1177/0194599815581831
83. Song E, Oh H-S, Jeon MJ, Chung KW, Hong SJ, Ryu JS, et al. The value of preoperative antithyroidperoxidase antibody as a novel predictor of recurrence in papillary thyroid carcinoma. Int J Cancer. (2019) 144:1414–20. doi: 10.1002/ijc.31944
84. Xu S, Huang H, Qian J, Wang X, Xu Z, Liu S, et al. Prognostic value of the preoperative and early trends in postoperative serum thyroglobulin antibody levels among patients with papillary thyroid carcinoma and concomitant Hashimoto's thyroiditis. Endocrine. (2023) 80:392–8. doi: 10.1007/s12020-022-03283-6
85. Ehlers M, Schott M. Hashimoto's thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends Endocrinol Metab. (2014) 25:656–64. doi: 10.1016/j.tem.2014.09.001
86. Yu Y, Zhang J, Lu G, Li T, Zhang Y, Yu N, et al. Clinical relationship between igG4-positive hashimoto's thyroiditis and papillary thyroid carcinoma. J Clin Endocrinol Metab. (2016) 101:1516–24. doi: 10.1210/jc.2015-3783
87. Ma Y, He J, Shen N, Guo R. Expression of NIS, VEGF-A and thyroid autoantibody in papillary thyroid carcinoma with or without hashimoto's disease. ORL J Otorhinolaryngol Relat Spec. (2019) 81:281–6. doi: 10.1159/000501620
88. Lee IS, Hsieh A-T, Lee T-W, Lee T-I, Chien Y-M. The association of thyrotropin and autoimmune thyroid disease in developing papillary thyroid cancer. Int J Endocrinol. (2017) 2017:5940367. doi: 10.1155/2017/5940367
89. Zou M, Baitei EY, Al-Rijjal RA, Parhar RS, Al-Mohanna FA, Kimura S, et al. TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene. (2016) 35:1909–18. doi: 10.1038/onc.2015.253
90. Wen X, Wang B, Jin Q, Zhang W, Qiu M. Thyroid antibody status is associated with central lymph node metastases in papillary thyroid carcinoma patients with hashimoto's thyroiditis. Ann Surg Oncol. (2019) 26:1751–8. doi: 10.1245/s10434-019-07256-4
91. Sun D, Zhang Y, Wang D, Zhao X, Han R, Li N, et al. Experimental study on changes in metabolic mechanism of papillary thyroid carcinoma complicated with Hashimoto's thyroiditis. Heliyon. (2023) 9:e20661. doi: 10.1016/j.heliyon.2023.e20661
92. Hellmann A, Turyn J, Zwara A, Korczynska J, Taciak A, Mika A. Alterations in the amino acid profile in patients with papillary thyroid carcinoma with and without Hashimoto's thyroiditis. Front Endocrinol (Lausanne). (2023) 14:1199291. doi: 10.3389/fendo.2023.1199291
93. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. (2019) 176:1248–64. doi: 10.1016/j.cell.2019.01.021
94. Lopes NMD, Lens HHM, da Silva Brito WA, Bianchi JK, Marinello PC, Cecchini R, et al. Role of papillary thyroid carcinoma patients with Hashimoto thyroiditis: evaluation of oxidative stress and inflammatory markers. Clin Transl Oncol. (2022) 24:2366–78. doi: 10.1007/s12094-022-02891-y
95. Kim SJ, Lee S-E, Kim YI, Nam-Goong IS, Jung HW, Kim ES. Papillary thyroid cancer with Hashimoto's thyroiditis attenuates the tumour aggressiveness through the up-regulation of E-cadherin and TGF-β expression. Clin Exp Med. (2023) 23:833–40. doi: 10.1007/s10238-022-00857-6
96. Sisto M, Ribatti D, Lisi S. Cadherin signaling in cancer and autoimmune diseases. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms222413358
97. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
98. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. (2003) 9:606–12.
99. Obradovic A, Ager C, Turunen M, Nirschl T, Khosravi-Maharlooei M, Iuga A, et al. Systematic elucidation and pharmacological targeting of tumor-infiltrating regulatory T cell master regulators. Cancer Cell. (2023) 41:933–49.e11. doi: 10.1016/j.ccell.2023.04.003
100. Ben-Skowronek I, Szewczyk L, Ciechanek R, Korobowicz E. Interactions of lymphocytes, thyrocytes and fibroblasts in Hashimoto's thyroiditis: an immunohistochemical and ultrastructural study. Horm Res Paediatr. (2011) 76:335–42. doi: 10.1159/000331857
101. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. (2015) 14:174–80. doi: 10.1016/j.autrev.2014.10.016
102. Sulaieva O, Selezniov O, Shapochka D, Belemets N, Nechay O, Chereshneva Y, et al. Hashimoto's thyroiditis attenuates progression of papillary thyroid carcinoma: deciphering immunological links. Heliyon. (2020) 6:e03077. doi: 10.1016/j.heliyon.2019.e03077
103. Pan J, Ye F, Yu C, Zhu Q, Li J, Zhang Y, et al. Papillary thyroid carcinoma landscape and its immunological link with hashimoto thyroiditis at single-cell resolution. Front Cell Dev Biol. (2021) 9:758339. doi: 10.3389/fcell.2021.758339
104. Cui L, Zhang C, Ding H, Feng D, Huang H, Lu Z, et al. Clonal distribution and intratumor heterogeneity of the TCR repertoire in papillary thyroid cancer with or without coexistent hashimoto's thyroiditis. Front Immunol. (2022) 13:821601. doi: 10.3389/fimmu.2022.821601
105. Li Y, Zang Y, Fan T, Li Z, Li A, Lv W, et al. Transcriptomic signatures associated with autoimmune thyroiditis in papillary thyroid carcinoma and cancer immunotherapy-induced thyroid dysfunction. Comput Struct Biotechnol J. (2022) 20:2391–401. doi: 10.1016/j.csbj.2022.05.019
106. Sulaieva O, Chernenko O, Selesnov O, Nechay O, Maievskyi O, Falalyeyeva T, et al. Mechanisms of the impact of hashimoto thyroiditis on papillary thyroid carcinoma progression: relationship with the tumor immune microenvironment. Endocrinol Metab (Seoul). (2020) 35:443–55. doi: 10.3803/EnM.2020.35.2.443
107. Zivancevic-Simonovic S, Mihaljevic O, Majstorovic I, Popovic S, Markovic S, Milosevic-Djordjevic O, et al. Cytokine production in patients with papillary thyroid cancer and associated autoimmune Hashimoto thyroiditis. Cancer Immunol Immunother. (2015) 64:1011–9. doi: 10.1007/s00262-015-1705-5
108. Lu Z-W, Hu J-Q, Liu W-L, Wen D, Wei W-J, Wang Y-L, et al. IL-10 restores MHC class I expression and interferes with immunity in papillary thyroid cancer with hashimoto thyroiditis. Endocrinology. (2020) 161(10):1–11. doi: 10.1210/endocr/bqaa062
109. Yang SW, Kang S-H, Kim KR, Choi IH, Chang HS, Oh YL, et al. Do helper T cell subtypes in lymphocytic thyroiditis play a role in the antitumor effect? J Pathol Transl Med. (2016) 50:377–84. doi: 10.4132/jptm.2016.07.25
110. Hu J-Q, Lei B-W, Wen D, Ma B, Zhang T-T, Lu Z-W, et al. IL-2 enhanced MHC class I expression in papillary thyroid cancer with Hashimoto's thyroiditis overcomes immune escape in vitro. J Cancer. (2020) 11:4250–60. doi: 10.7150/jca.38330
111. Galdiero MR, Varricchi G, Marone G. The immune network in thyroid cancer. Oncoimmunology. (2016) 5:e1168556. doi: 10.1080/2162402X.2016.1168556
112. Banerjee S, Nahar U, Dahiya D, Mukherjee S, Dey P, Gupta R, et al. Role of cytotoxic T cells and PD-1 immune checkpoint pathway in papillary thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:931647. doi: 10.3389/fendo.2022.931647
113. Pani F, Caria P, Yasuda Y, Makoto M, Mariotti S, Leenhardt L, et al. The immune landscape of papillary thyroid cancer in the context of autoimmune thyroiditis. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14174287
114. Abbasgholizadeh P, Naseri A, Nasiri E, Sadra V. Is Hashimoto thyroiditis associated with increasing risk of thyroid Malignancies? A systematic review and meta-analysis. Thyroid Res. (2021) 14:26. doi: 10.1186/s13044-021-00117-x
115. Zayed AA, Ali MKM, Jaber OI, Suleiman MDJ, Ashhab AA, Al Shweiat WM, et al. Is Hashimoto's thyroiditis a risk factor for medullary thyroid carcinoma? Our experience and a literature review. Endocrine. (2015) 48:629–36. doi: 10.1007/s12020-014-0363-2
116. Rosario PW, Calsolari MR. Influence of chronic autoimmune thyroiditis and papillary thyroid cancer on serum calcitonin levels. Thyroid. (2013) 23:671–4. doi: 10.1089/thy.2012.0564
117. Mousa U, Gursoy A, Ozdemir H, Moray G. Medullary thyroid carcinoma in a patient with Hashimoto's thyroiditis diagnosed by calcitonin washout from a thyroid nodule. Diagn Cytopathol. (2013) 41:644–6. doi: 10.1002/dc.21850
118. Kim HY, Park NH. Concurrent medullary carcinoma and hashimoto's thyroiditis: A case report with an emphasis on US features. J Korean Soc Radiol. (2023) 84:1146–51. doi: 10.3348/jksr.2022.0109
119. Sharma A, Jasim S, Reading CC, Ristow KM, Villasboas Bisneto JC, Habermann TM, et al. Clinical presentation and diagnostic challenges of thyroid lymphoma: A cohort study. Thyroid. (2016) 26:1061–7. doi: 10.1089/thy.2016.0095
120. Otsuka Y, Yasuda M, Tokumasu K, Hasegawa K, Otsuka F. Hashimoto's thyroiditis and primary thyroid lymphoma. QJM. (2020) 113:691–2. doi: 10.1093/qjmed/hcaa002
121. Moshynska OV, Saxena A. Clonal relationship between Hashimoto thyroiditis and thyroid lymphoma. J Clin Pathol. (2008) 61:438–44. doi: 10.1136/jcp.2007.051243
122. Ghafouri AM, Alzaidi S, Al-Kaabi BB, Awadh MA, Bakhsh D, Alharbi A. Thyroid B-cell lymphoma in the background of hashimoto's thyroiditis: A case report and literature review. Cureus. (2024) 16:e57359. doi: 10.7759/cureus.57359
123. Li P, Zhang H. Ultrasonography in the diagnosis and monitoring of therapy for primary thyroid lymphoma. Ultrasound Q. (2019) 35:246–52. doi: 10.1097/RUQ.0000000000000414
124. Chui MH, Cassol CA, Asa SL, Mete O. Follicular epithelial dysplasia of the thyroid: morphological and immunohistochemical characterization of a putative preneoplastic lesion to papillary thyroid carcinoma in chronic lymphocytic thyroiditis. Virchows Arch. (2013) 462:557–63. doi: 10.1007/s00428-013-1397-1
125. Di Pasquale M, Rothstein JL, Palazzo JP. Pathologic features of Hashimoto's-associated papillary thyroid carcinomas. Hum Pathol. (2001) 32:24–30. doi: 10.1053/hupa.2001.21138
126. Ohmori N, Miyakawa M, Ohmori K, Takano K. Ultrasonographic findings of papillary thyroid carcinoma with Hashimoto's thyroiditis. Intern Med. (2007) 46:547–50. doi: 10.2169/internalmedicine.46.1901
127. Baser H, Ozdemir D, Cuhaci N, Aydin C, Ersoy R, Kilicarslan A, et al. Hashimoto's thyroiditis does not affect ultrasonographical, cytological, and histopathological features in patients with papillary thyroid carcinoma. Endocr Pathol. (2015) 26:356–64. doi: 10.1007/s12022-015-9401-8
128. Zhang Q, Zhang S, Pan Y, Sun L, Li J, Qiao Y, et al. Deep learning to diagnose Hashimoto's thyroiditis from sonographic images. Nat Commun. (2022) 13:3759. doi: 10.1038/s41467-022-31449-3
129. Januś D, Kujdowicz M, Wójcik M, Taczanowska-Niemczuk A, Kiszka-Wiłkojć A, Górecki W, et al. Ultrasound evolution of parenchymal changes in the thyroid gland with autoimmune thyroiditis in children prior to the development of papillary thyroid carcinoma - a follow-up study. Front Endocrinol (Lausanne). (2023) 14:1172823. doi: 10.3389/fendo.2023.1172823
130. Wang G, Nie F, Wang Y, Wang P, Wang L, Fan X, et al. Value of echogenic foci in diagnosing papillary thyroid carcinoma and predicting aggressive biological behavior. J Ultrasound Med. (2022) 41:1237–45. doi: 10.1002/jum.15815
131. Chen S, Niu C, Peng Q, Tang K. Sonographic characteristics of papillary thyroid carcinoma with coexistent hashimoto's thyroiditis in the preoperative prediction of central lymph node metastasis. Front Endocrinol (Lausanne). (2021) 12:556851. doi: 10.3389/fendo.2021.556851
132. Tan H-L, Nyarko A, Duan S-L, Zhao Y-X, Chen P, He Q, et al. Comprehensive analysis of the effect of Hashimoto's thyroiditis on the diagnostic efficacy of preoperative ultrasonography on cervical lymph node lesions in papillary thyroid cancer. Front Endocrinol (Lausanne). (2022) 13:987906. doi: 10.3389/fendo.2022.987906
133. Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of Malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto's thyroiditis. Eur Surg Res. (2010) 45:333–7. doi: 10.1159/000320954
134. Gan X, Feng J, Deng X, Shen F, Lu J, Liu Q, et al. The significance of Hashimoto's thyroiditis for postoperative complications of thyroid surgery: a systematic review and meta-analysis. Ann R Coll Surg Engl. (2021) 103:223–30. doi: 10.1308/rcsann.2020.7013
135. Kwon H, Choi JY, Moon JH, Park HJ, Lee WW, Lee KE. Effect of Hashimoto thyroiditis on low-dose radioactive-iodine remnant ablation. Head Neck. (2016) 38 Suppl 1:E730–E5. doi: 10.1002/hed.24080
136. Li X, Ding W, Zhang H. Surgical outcomes of endoscopic thyroidectomy approaches for thyroid cancer: a systematic review and network meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1256209. doi: 10.3389/fendo.2023.1256209
137. Wang MF, Xia H, Cai J. The impact of coexisting Hashimoto's thyroiditis on the feasibility of endoscopic thyroidectomy in papillary thyroid carcinoma. Heliyon. (2024) 10:e26793. doi: 10.1016/j.heliyon.2024.e26793
Keywords: cancer, papillary thyroid carcinoma, Hashimoto’s thyroiditis, autoimmune disease, thyroid
Citation: Yao S and Zhang H (2025) Papillary thyroid carcinoma with Hashimoto’s thyroiditis: impact and correlation. Front. Endocrinol. 16:1512417. doi: 10.3389/fendo.2025.1512417
Received: 16 October 2024; Accepted: 27 March 2025;
Published: 11 April 2025.
Edited by:
Giusy Elia, University of Pisa, ItalyReviewed by:
Oana Stanoiu-Pinzariu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaCopyright © 2025 Yao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Zhang, emhhbmdoOTlAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.