
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 26 February 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1510114
Background: The clinical need for assisted reproduction continued to increase, so did the need for predictive markers of assisted reproductive technology (ART) outcomes. Among all the markers, sperm DNA integrity was paid more and more attention in the assessment of male fertility in recent years, but its clinical value remains still in doubt.
Methods: We conducted a retrospective cohort study. Couples coming to our reproductive center were retrospectively enrolled, and semen and assisted reproductive technology parameters were assessed. Sperm DNA integrity was analyzed using a flow cytometric method. Statistics were analyzed to investigate the relationship of this on semen quality and ART outcomes.
Results: DNA fragmentation index (DFI) was affected by abstinence days and age, and was a directly correlated with sperm quality parameters (p<0.001). Meanwhile high sperm DNA stainability (HDS) showed an unexplainable negative correlation with abstinence days (p<0.05), age (p<0.001) and body mass index (p<0.01).For sperm quality parameters, HDS showed a similar relevance besides abnormal sperm head morphology (p<0.001). Embryo cleavage and implantation rates were significantly negatively related to DFI in fresh intracytoplasmic sperm injection cycles (p<0.01) while HDS showed a positive relationship with high quality embryo rate (p<0.05). For final outcomes, only the live birth rate from fresh intracytoplasmic sperm injection cycles was positively correlated with DFI which is meaningless (p<0.05).
Conclusions: HDS might not be an appropriate marker for male fertility and further studies are needed to identify the efficiency of SDF in clinical practice.
Male factors account for about 40-50% of infertility cases (1). The most common cause of male infertility is sperm abnormalities. Therefore, at present, a large part of laboratory tests for male fertility are focused on sperm quality analysis, such as sperm motility, morphology, viability. However, sperm quality is often not accurately reflected by a single test value (2). Sperm DNA fragmentation (SDF), as a complement to traditional sperm quality analysis, is being paid increasing attention in the clinic. Unlike those of somatic cells, sperm nucleoproteins undergo a histone-to-protamine transition during maturation, which causes a drastic change in the DNA topology, relieving torsional stress. In this progress, chromatin is remodeled by inducing double-strand breaks and their subsequent relegation. SDF increases significantly when this process is aberrant (3). There are various causes of increasing SDF, including apoptosis, defective maturation, oxidative stress, which are affected by many risk factors such as advanced paternal age, diet, life style, chemo-/radio- therapy, obesity, environmental toxicants, infection and testicular trauma (4).
There are many methods for the clinical detection of sperm SDF, including comet assay (5), sperm chromatin dispersion (SCD) (6), terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) (7) and sperm chromatin structure assay (SCSA) (8, 9), the latter two methods being widely used in the clinic. The SCSA test is a high-precision test based on flow cytometry, which needs a large capital expense and a trained technician (10). In contrast to SCSA, the other methods are time-consuming and labor intensive (11). The values obtained by flow cytometry with the commercial SCSA test kit are the DNA fragmentation index (DFI) and high DNA stainability (HDS), which are considered to be indicators of the degree of sperm DNA integrity and sperm chromatin condensation, respectively. With SCSA, sperm DNA breaks can be evaluated indirectly through DNA denaturability. This assay is based on the characteristics of Acridine Orange (AO), which is a cell-permeable dye that shows fluoresces green when bound to native double-stranded DNA and yellow/red when bound to single-stranded DNA (12). Sperm samples were stained and analyzed by flow cytometry, producing a scatter plot, of the ratio of the number of green and red sperm heads. DFI is defined as the percentage of the red spermatozoa, while that of green spermatozoa was defined as HDS (13). Many studies have shown the correlation between traditional semen parameters values and, unexplained recurrent miscarriage, natural conception rates, assisted reproductive technology outcome and DFI (14–17). A few studies have supported the opposite view (18). Contrary to the fact that there is a large amount of data supporting the predicted value of DFI, the clinical definition and significance of HDS are ambiguous (19). In the early years, there were positive views on the predictive value of HDS (20), but recent reports have challenged it (21, 22). Although this parameter was debatable, there were few relevant large-scale statistical studies in recent years. Studies paid more attention on the indication of HDS on sperm quality, and the few studies focusing on assisted reproductive outcomes had a relatively low cycle count.
Here, we assessed the common factors affecting sperm DNA integrity, and how DFI and HDS reflected the sperm quality and ART outcome. It was found that HDS might not be an appropriate indicator of male fertility and assisted reproductive technology outcomes. Our findings provide an experimental basis and guidance for the clinical application of SDF indicators.
This is a single-center, retrospective, observational study. A total of 3970 couples were selected, who had undergone assisted reproductive treatment, including artificial insemination by husband (AIH), in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), in the reproductive center of our hospital from April 2018 to April 2021.
Inclusion criteria: (1) the ovulation induction program was standard or conventional long-term; (2) the age of the female patient was less than 35 years old; (3) both patients had no chromosomal abnormality, and the only factor was the Fallopian tube.
Exclusion criteria: (1) the female patient has reproductive system diseases; (2) the body mass index (BMI) of female patient was abnormal (greater than 24 or less than 18.5); (3) the woman had genetic defects, chronic diseases, etc. (4)the female patient has smoking habit; (5)the male patient has abnormal testicular size. This study was approved by the ethics committee and the patients gave informed consent for participation.
Semen samples were collected by masturbation after 2–7 days of sexual abstinence and were processed for analysis after liquefaction for 60 min at 37°C. Sperm concentration, morphology and motility were assessed according to the WHO laboratory manual for the examination and processing of human semen (5th edition). Spermatozoa on slides were stained with Papanicolaou. 200 cells were morphologically assessed per smear.
DFI was quantified by the SCSA kit (Zhejiang Cellpro Biotech, Ningbo, China). First, the liquefied semen was diluted with 4°C buffer to a sperm concentration of 1×106/mL; secondly, 500 μL of acid solution was added to the diluted sperm suspension and after 30 seconds the dye acridine orange (AO) was added. The flow cytometer was calibrated, and then each sample was measured continuously at least twice in a carousel by the Navios flow cytometer (Beckman Coulter, USA), recording at least 5000 cells in each tube; the gate was set and the cytogram populations were determined according to the previous reference (10). Finally DFI and HDS were calculated with DFI View software for statistical analysis.
For AIH, the husband’s semen sample was obtained by masturbating for 2–7 days of sexual abstinence and optimized by density gradient centrifugation according to the protocol of the optimization kit (Irvine Scientific, Santa ANA, USA) with a final sperm concentration of 20 × 106/mL. Then, the sperm suspension was injected into the female’s uterine cavity.
Conventional gonadotropin-releasing hormone agonists (GnRH-ant, Merck & Co., Ltd., USA), recombinant human follicle stimulating hormone (MerckSerono, USA) were used to induce ovulation. For IVF, the sperm concentration was adjusted to that in the AIH protocol, and added into the culture droplets. Meanwhile the oocytes were added to the prepared fertilization droplets 3-4 h after retrieval and fertilization was assessed at 16-18 h. For ICSI, after oocyte retrieval, the spermatozoa selected from the microscopic field were injected by using an ICSI platform. After fertilization, embryo morphology was scored according to the shape, size, spatial distribution of blastomeres, and cytoplasmic homogeneity.
In order to determine pregnancy, hCG in blood and urine was detected on the 14th day after embryo transfer; positive samples indicated a biochemical pregnancy, and clinical pregnancies were confirmed by B-ultrasound 4-6 weeks after embryo transfer. The outcome parameters of interest were rate of fertilization, embryo cleavage (number of fertilized cleavage embryos/number of fertilized ova)*100%), high-quality embryo (at day 3 of embryo culture, embryos with 6-12 cells and uniform cell size were categorized as good quality embryos), implantation, pregnancy and live births.
SPSS 19.0 (IBM, USA) was used for data analysis. Measured data that were normally distributed were expressed as median (95% confidence interval). T-test was used to compare the differences in DFI and HDS between groups with different smoking habits (at least 3 cigarettes per day). Multivariate linear regression analysis was performed, using abstinence day, BMI and age as the independent variables and DFI, HDS as dependent variables to investigate the impact of these three factors on SDF. Linear regression and correlation analysis was performed, using DFI and HDS as the independent variable and semen parameters as dependent variables, to investigate the predictive value of SDF on sperm quality. The correlation of DFI, HDS and rates of IVF/ICSI fertilization, embryo cleavage, high-quality embryos and implantation were analyzed by the Spearman Rho test. The difference in implantation rates was analyzed by t-Test. The difference in pregnancy rate and live birth rate was analyzed by Chi-squared test; P<0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were constructed for analyzing the sensitivity and specificity of DFI and HDS for predicting seminal quality. The effect was evaluated from the area under the curve (AUC).
11648 ART cycles were examined and the detailed information is shown in Table 1. The influence of factors on SDF revealed that DFI was increased by the increasing of abstinence and age (p < 0.001). HDS was decreased by increasing abstinence (p < 0.05), male age (p < 0.001) and BMI (p < 0.01). Smoking had no significant effect on either of these parameters (Figure 1).
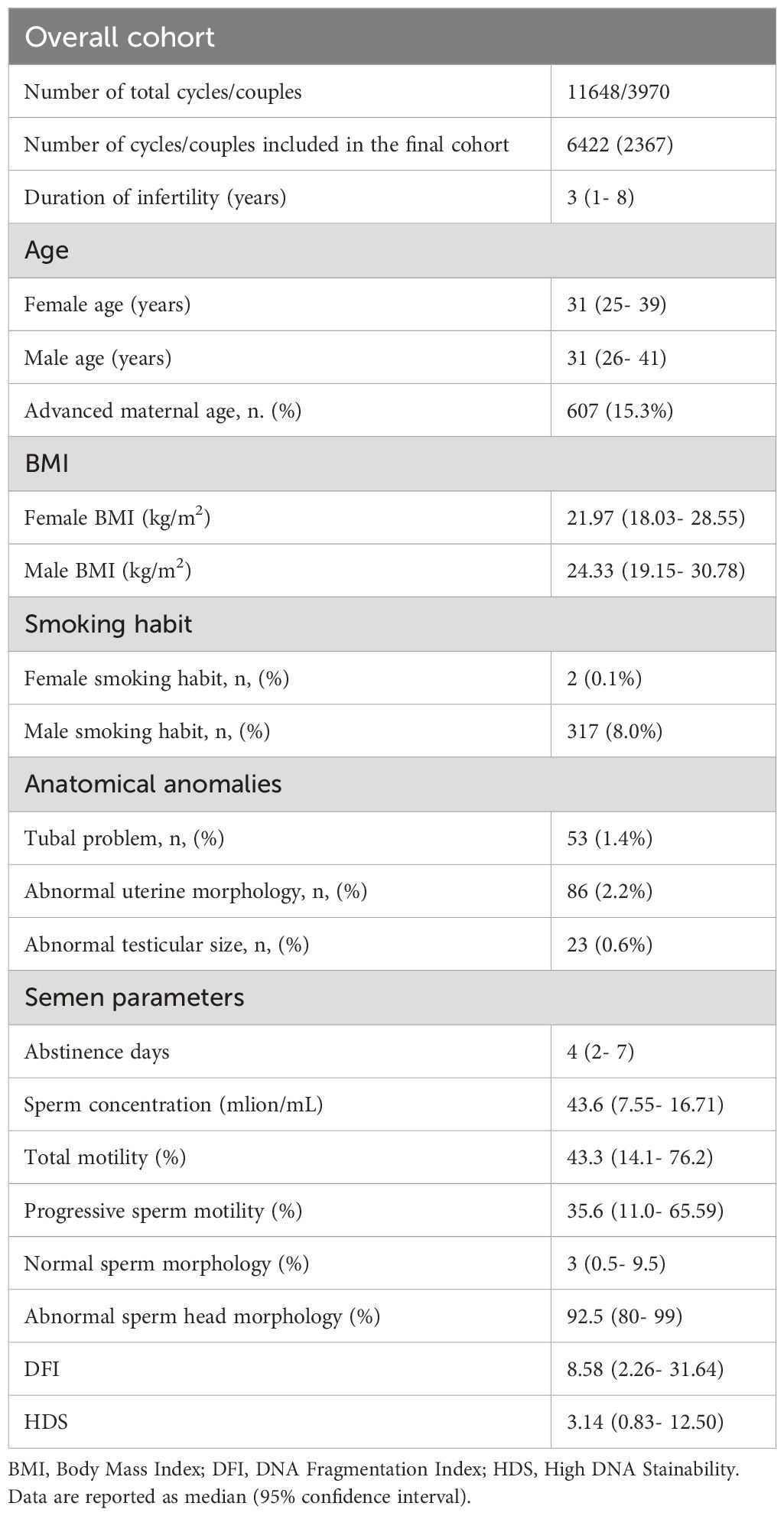
Table 1. Couples characteristics and semen parameters classified according to the World Health Organization manual (5th).
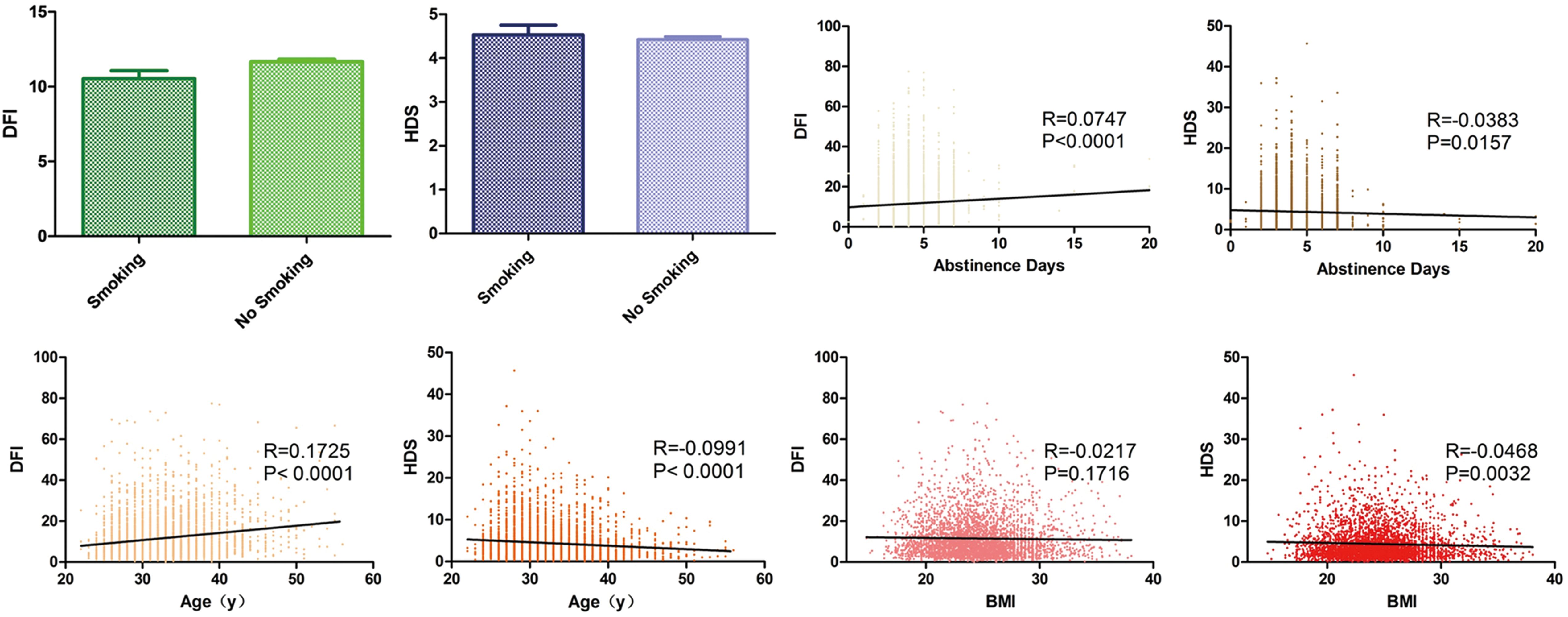
Figure 1. Analysis of SDF impact factors. Column grams representing the effect of smoking on DFI (green) and HDS (blue). Box plots showing correlation between DFI, HDS and abstinence days (yellow), age (orange) and BMI (red). The black line shows the correlation trend. R means correlation coefficient and P means p value.
To explore the predictive value of SDF for sperm quality, linear regression was used for visualizing correlation trends and a significant correlation between DFI and all the sperm quality parameters (p < 0.001) was found. As DFI increased, sperm concentration, motility, progressive motility and normal morphology decreased, whereas the abnormal sperm head percentage increased. HDS showed a similar correlation and trend with sperm concentration, motility, progressive motility and normal morphology (p < 0.001), but no correlation with the abnormal sperm head (Figure 2). From ROC curves of DFI and HDS on seminal quality, the AUC were from 0.6 to 0.8, it is worth noting that the AUC of DFI was larger than that of HDS for total and progressive sperm motility (Figure 3).
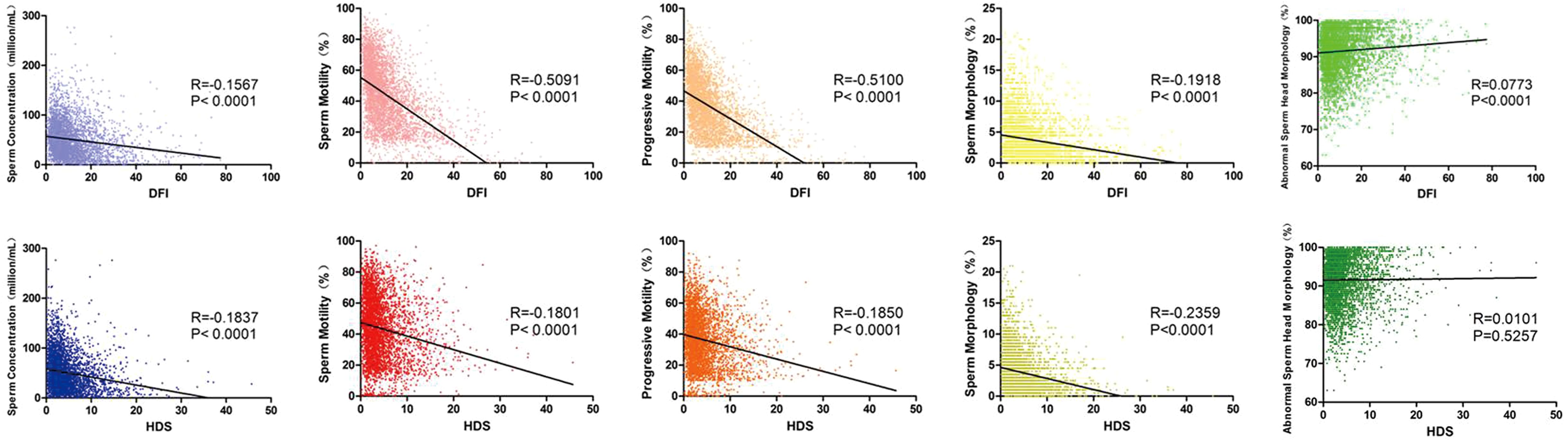
Figure 2. Linear regression and correlation analysis of SDF and sperm quality parameter values. Box plots showing correlation between DFI (upper panel), HDS (lower panel) and sperm concentration (blue), motility (red), progressive motility (orange), sperm morphology (yellow) and abnormal sperm head morphology (green). The black line shows the correlation trend. R means correlation coefficient and P means p value.
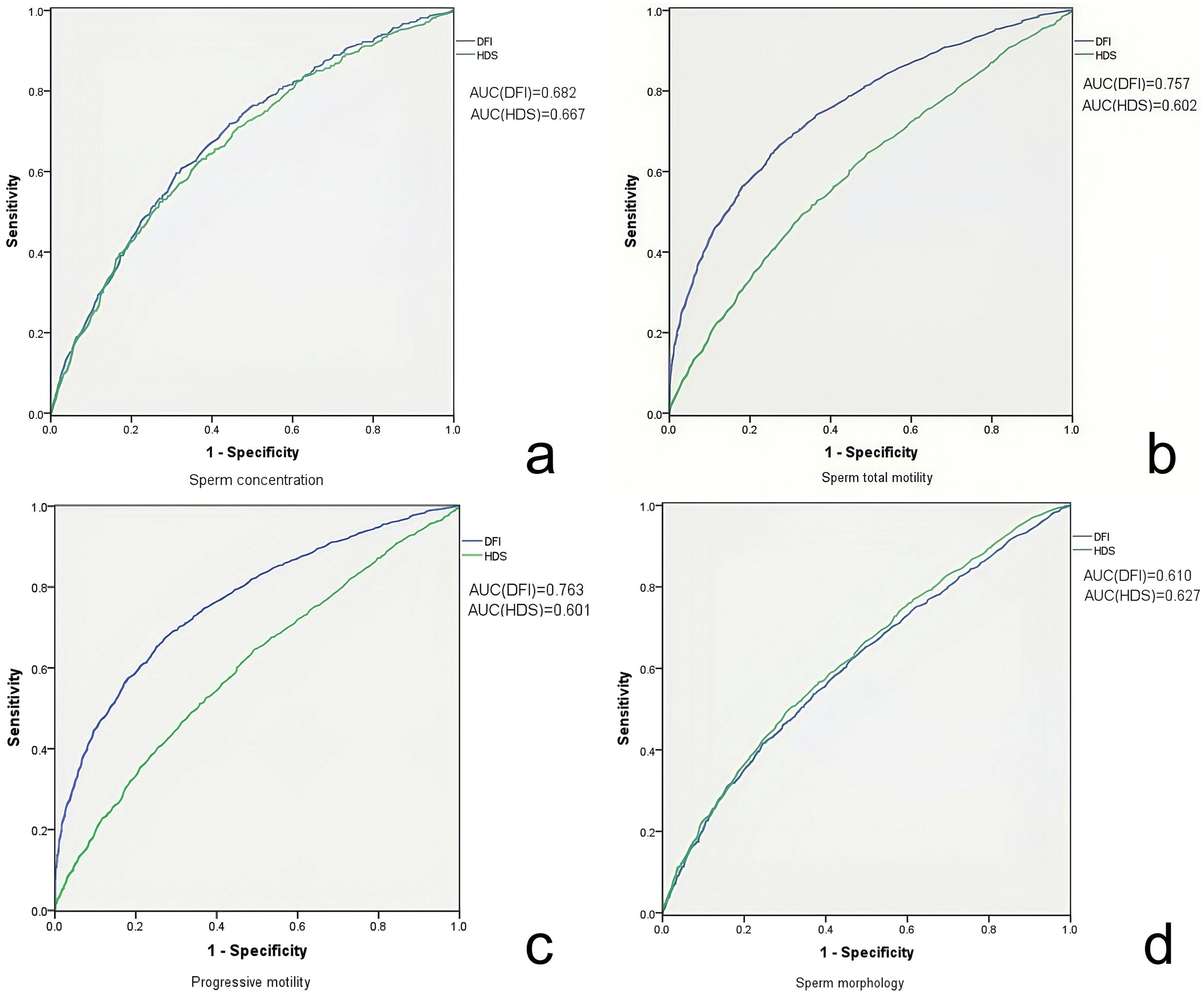
Figure 3. ROC curve analyzing the sensitivity and specificity of DFI and HDS for seminal quality. ROC curve of DFI (blue) and HDS (green) evaluating the sperm concentration (a), total motility (b), progressive motility (c) and morphology (d). The areas under the curve of each ROC curve are indicated respectively in the figure.
For the correlation between SDF and ART outcomes of 6422 cycles, the data are shown in Table 2. We used the Spearman Rho test to investigate the effect of DFI and HDS on ART parameters. In fresh IVF cycles, DFI was not directly related to rate of fertilization, cleavage, high quality embryos or implantation. There was a significant association of HDS with the high quality embryo rate. In ICSI fresh cycles, DFI was found negatively correlated with cleavage rate and implantation rate (p<0.01), while HDS showed no correlation (Table 3).
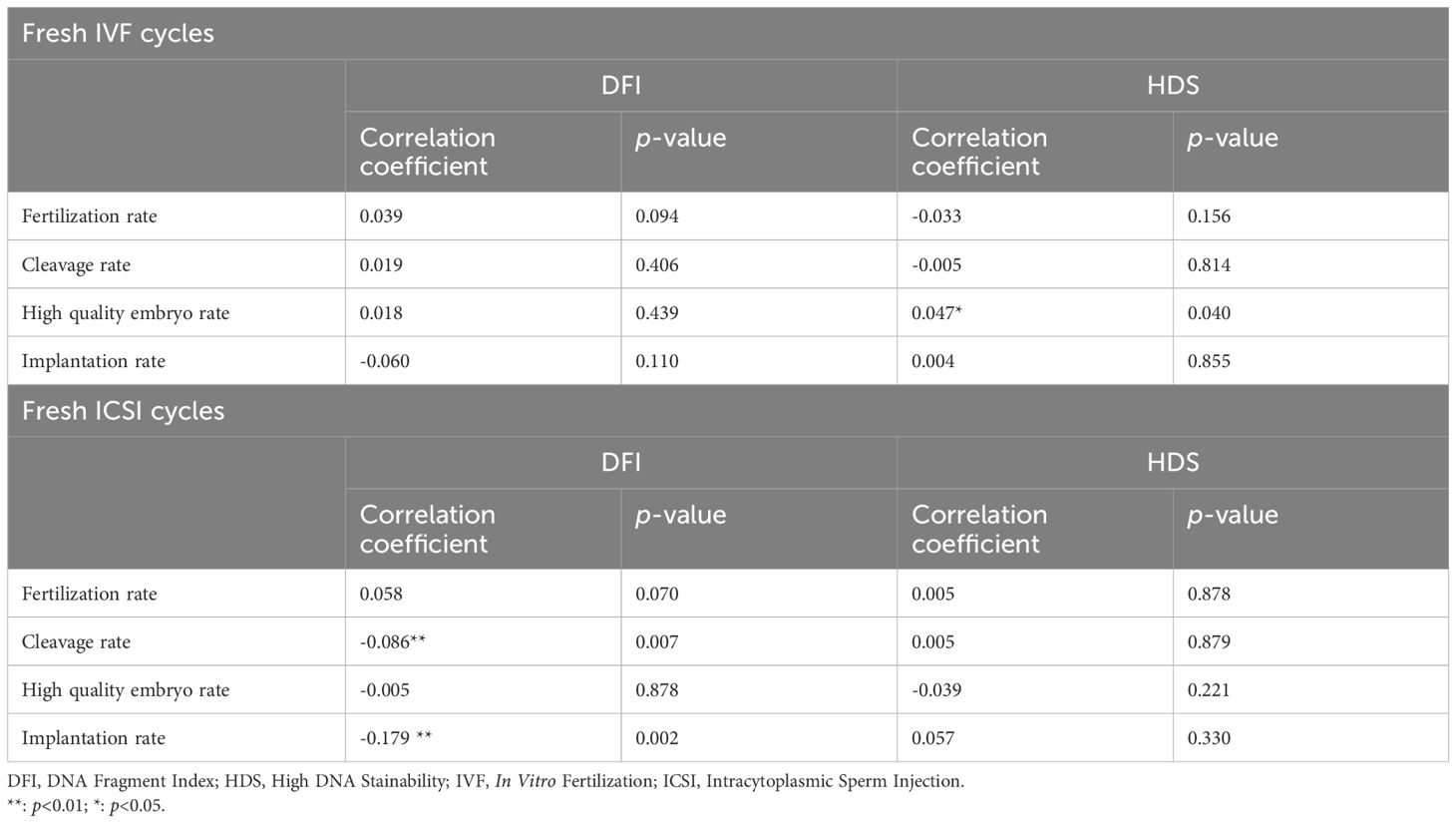
Table 3. Correlation analyses of SDF parameters and ART outcome parameters, sorted by in vitro fertilization (IVF), fresh intracytoplasmic sperm injection (ICSI) cycles.
We divided the pregnancy and live birth data according to the DFI (DFI ≤ 15% for normal, 15%< DFI<30% for subnormal, DFI≥30% for abnormal) and HDS (HDS ≤ 15%, HDS>15% for abnormal). In the fresh ICSI group, the embryo implantation rate of patients with normal DFI was significantly higher than the subnormal DFI group (p<0.05) and abnormal normal (p<0.01). For the correlation between SDF and pregnancy rate, live birth rate, chi-square test was used, but only the live birth rate from fresh ICSI cycles showed a significant difference between those with normal and abnormal DFI (Table 4).
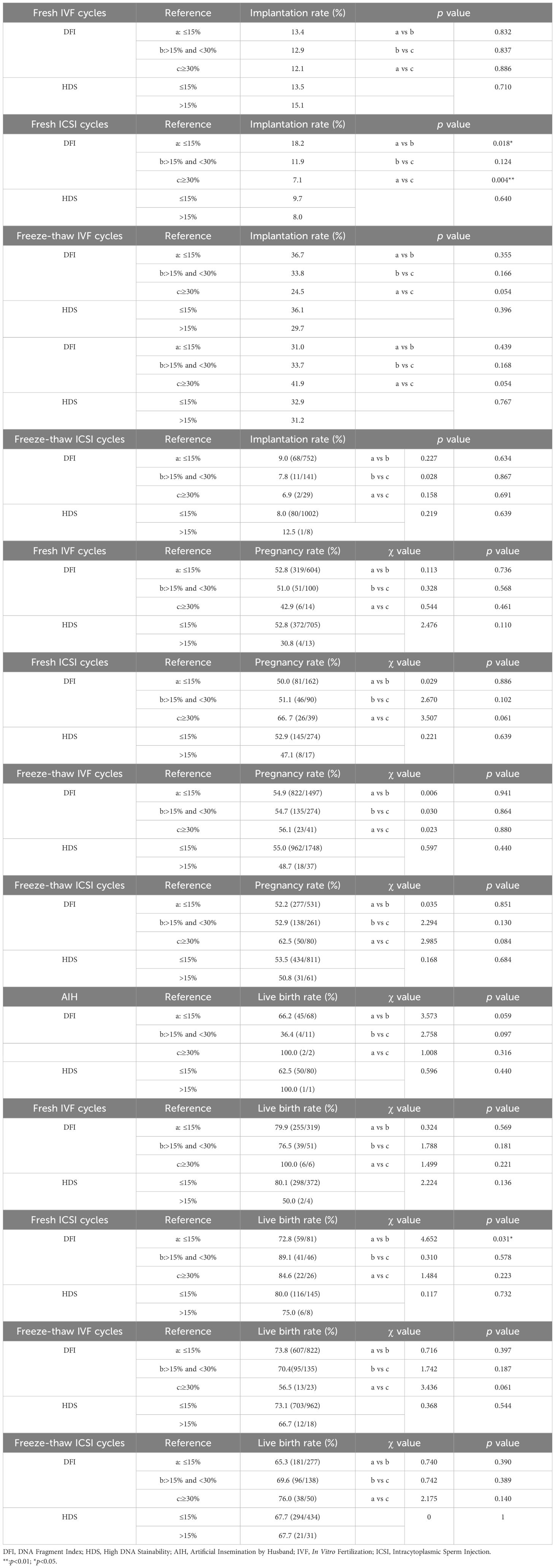
Table 4. T-test of implantation rate and Chi-squared analyses of clinical pregnancy rate, and live birth rate, sorted artificial insemination by husband (AIH), in vitro fertilization (IVF), Intracytoplasmic sperm injection (ICSI) cycles.
As described above, various factors are related to SDF, such as life habit, BMI, male age and abstinence days. In this cohort, the smoking habit had no effect on DFI and HDS results contrary to a previous report (23). But apart from this, DFI was directly related to male age and abstinence days. The older the man was, the more the abstinence days, the higher the DFI. On the other hand, the opposite relationship of age, abstinence days and BMI with HDS shows a lack of clinical predictive value and support of evidence-based medicine for HDS.
DFI had been considered to be a reliable indicator reflecting sperm quality, on the basis of the strong connection with sperm motility (24), morphology (25) and ART outcomes (26). Nevertheless, there are a few reports supporting the opposite view (18). The results observed in our study showed a strong link between DFI and sperm parameter values. The sperm concentration, total and progressive motility, and normal sperm morphology were lower when DFI was higher, which could be explained by the sperm head’s composing mainly sperm nucleus, whose morphology would be affected by the organization of its DNA. DFI was not associated with IVF outcomes, consistent with several other studies, which make the predictive value of DFI an open question (27). The correlation between DFI and cleavage and implantation rates in fresh ICSI cycles indicates the predictive value of DFI on ICSI embryo quality, which has been mentioned in many studies (28). ART outcome of pregnancy and live births showed no relationship with DFI in our study; the only relationship found for DFI in fresh ICSI cycles was inexplicable. As shown in the retrospective cohort study of Deng et al., high DFI does not influence live birth, miscarriage or clinical pregnancy rates (15). There was also no significant difference in the pregnancy rate in intrauterine insemination cycles in another study (29). However, the reports of negative predictive results of DFI remain in the minority. It was confusing that our results showed a negative correlation of DFI with seminal quality but no clear association with reproductive results. We attribute these results to the fact that the DFI obtained here may not fully represent the DNA integrity of the spermatozoon that was ultimately used for ART, and the variability of sperm quality affected the reliability of the analysis. In addition, although we tried to eliminate female factors by inclusion and exclusion criteria, there might still be some shortcomings. Among the various studies on SDF, it is believed by some researchers that sperm DNA fragmentation on the day of fertilization is not associated with ART outcome independently of gamete quality (30). Well designed further studies are still needed.
HDS, as an indicator of the degree of chromatin maturation has been doubted recently. Although HDS values were simultaneously obtained and significantly correlated with the DFI values, before we doubted its clinical value, as HDS was thought to be independent from DFI. Recently, Lu published a short communication pointing out that HDS should not be recommended as a marker for the detection of sperm DNA damage. In his point of view, the establishment of HDS in the detection of sperm DNA damage has no theoretical basis and has also no support from evidence-based medicine (19). However, there were few studies to prove Lu’s point through clinical data statistics. As mentioned in our study, when used to reflect sperm quality, HDS performs well. But the loose association of HDS with normal sperm head morphology cannot be explained. Although several studies had affirmed the predictive value of HDS on ART outcomes, our result showed a poor relationship. Similarly, the positive relationship between HDS and IVF outcomes is confusing, implying the lower the chromatin maturity, the better the ART result. The definition of HDS is questionable; the strong green fluorescence may only represent the integrity of the sperm DNA, and not directly reflect the completion of sperm nucleoprotein replacement. HDS should be ranked by comparing it with other sperm nucleoprotein markers, like transition nuclear proteins 1 (TNP1), pre-protamine 2 (pPRM2), etc. Our statistics raise a question about the clinical use of HDS, and it is hoped that more data studies will emerge to demonstrate whether the clinical value of HDS is overestimated.
In summary, according to our results DFI is still an efficient marker of sperm DNA integrity, while HDS is not so useful. Although whether to choose HDS as a predictor in clinical laboratory is debatable, we must admit that the patients with high HDS had less chance to get their partner pregnant in most reports. Here, we present our data, but cannot refute HDS as an influence factor. We have to face the fact that there were limitations in our research. From the hundreds of men, very few had an HDS >15%, the low number made the statistical analysis results not convincing enough to deny the value of HDS. New markers are still needed to investigate the sperm DNA damage more precisely. Lately, the research team of Huazhong University of Science and Technology proposed a novel parameter, the mean number of sperm DNA breakpoints (MDB), which relies on a novel secondary amplification detection system they developed. The system is based on terminal deoxynucleotidyl transferase and endonuclease IV, which can effectively reflect the number of 3’-OH (equivalent to the number of breakpoints) (31). In Lu’s article, he improved the flow cytometry detection method of DFI through optimizing the conditions of gating (19). We also tried new flow cytometric parameters, such as the heterogeneity of seminal cell size distribution, which has been proved to be a good predictive effect on sperm quality. Nowadays, methods are emerging for assessment of sperm quality. It follows that many indicators have been to rapidly used in clinical testing without good evidence-based medical certification. SDF may reflect sperm quality, but still needs data validation, strict and scientific quality control and improvement of methods and parameters.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by ethics committee of Suzhou Municipal Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LS: Writing – original draft. CZ: Writing – review & editing, Investigation. GW: Investigation, Writing – review & editing. XF: Resources, Writing – review & editing. SY: Funding acquisition, Writing – review & editing. JW: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Suzhou Health Talent Cultivation Project (2024(161)), Biopharmaceutical Industry Innovation (Clinical Trial Capability Improvement)->Medicine-Industrial Collaborative Innovation Research Project (SLJ2022019) and Suzhou Clinical Medical Center funding (Szlcyxzx202106).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. O’Flynn O’Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. (2010) 93:1–12. doi: 10.1016/j.fertnstert.2009.10.045
2. Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. (2014) 102:1502–7. doi: 10.1016/j.fertnstert.2014.10.021
3. Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. (2010) 16:30–6. doi: 10.1093/molehr/gap080
4. Agarwal A, Panner Selvam MK, Baskaran S, Cho CL. Sperm DNA damage and its impact on male reproductive health: a critical review for clinicians, reproductive professionals and researchers. Expert Rev Mol Diagn. (2019) 19:443–57. doi: 10.1080/14737159.2019.1614916
5. Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, Amengual MJ, Prada E, Cortés P, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PloS One. (2012) 7:e44679. doi: 10.1371/journal.pone.0044679
6. Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. (2003) 24:59–66. doi: 10.1002/j.1939-4640.2003.tb02641.x
7. Sharma R, Iovine C, Agarwal A, Henkel R. TUNEL assay-Standardized method for testing sperm DNA fragmentation. Andrologia. (2021) 53:e13738. doi: 10.1111/and.13738
8. Evenson DP, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril. (2020) 114:311–20. doi: 10.1016/j.fertnstert.2020.03.028
9. Vaughan DA, Tirado E, Garcia D, Datta V, Sakkas D. DNA fragmentation of sperm: a radical examination of the contribution of oxidative stress and age in 16 945 semen samples. Hum Reprod. (2020) 35:2188–96. doi: 10.1093/humrep/deaa159
10. Evenson DP. Sperm chromatin structure assay (SCSA®) for fertility assessment. Curr Protoc. (2022) 2:e508. doi: 10.1002/cpz1.v2.8
11. Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil. (2014) 15:2–14.
12. Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. (2002) 23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x
13. Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. (2011) 13:69–75. doi: 10.1038/aja.2010.73
14. Dai Y, Liu J, Yuan E, Li Y, Shi Y, Zhang L. Relationship among traditional semen parameters, sperm DNA fragmentation, and unexplained recurrent miscarriage: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2021) 12:802632. doi: 10.3389/fendo.2021.802632
15. Deng C, Li T, Xie Y, Guo Y, Yang QY, Liang X, et al. Sperm DNA fragmentation index influences assisted reproductive technology outcome: A systematic review and meta-analysis combined with a retrospective cohort study. Andrologia. (2019) 51:e13263. doi: 10.1111/and.2019.51.issue-6
16. Evenson DP, Jost LK, Baer RK, Turner TW, Schrader SM. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol. (1991) 5:115–25. doi: 10.1016/0890-6238(91)90039-I
17. Malić Vončina S, Golob B, Ihan A, Kopitar AN, Kolbezen M, Zorn B. Sperm DNA fragmentation and mitochondrial membrane potential combined are better for predicting natural conception than standard sperm parameters. Fertil Steril. (2016) 105:637–644.e1. doi: 10.1016/j.fertnstert.2015.11.037
18. Green KA, Patounakis G, Dougherty MP, Werner MD, Scott RT Jr, Franasiak JM. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J Assist Reprod Genet. (2020) 37:71–6. doi: 10.1007/s10815-019-01632-5
19. Lu JC. High DNA stainability (HDS) should not be recommended as a marker for the detection of sperm DNA damage. Andrologia. (2022) 54:e14442. doi: 10.1111/and.14442
20. Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. (2004) 81:1289–95. doi: 10.1016/j.fertnstert.2003.09.063
21. Best JC, Kohn T, Patel P, Blachman-Braun R, de Quadros E, Beyhan Z, et al. Elevated sperm DNA fragmentation does not predict recurrent implantation failure. Andrologia. (2021) 53:e14094. doi: 10.1111/and.14094
22. Blachman-Braun R, Best JC, Sandoval V, Lokeshwar SD, Patel P, Kohn T, et al. Sperm DNA fragmentation index and high DNA stainability do not influence pregnancy success after intracytoplasmic sperm injection. F S Rep. (2020) 1:233–8. doi: 10.1016/j.xfre.2020.08.005
23. Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J Androl. (2019) 21:478–85. doi: 10.4103/aja.aja_110_18
24. Campos L, Requejo LC, Miñano C, Orrego JD, Loyaga EC, Cornejo LG. Correlation between sperm DNA fragmentation index and semen parameters in 418 men seen at a fertility center. JBRA Assist Reprod. (2021) 25:349–57. doi: 10.5935/1518-0557.20200079
25. Ferrigno A, Ruvolo G, Capra G, Serra N, Bosco L. Correlation between the DNA fragmentation index (DFI) and sperm morphology of infertile patients. J Assist Reprod Genet. (2021) 38:979–86. doi: 10.1007/s10815-021-02080-w
26. Jiang H, He RB, Wang CL, Zhu J. The relationship of sperm DNA fragmentation index with the outcomes of in-vitro fertilisation-embryo transfer and intracytoplasmic sperm injection. J Obstet Gynaecol. (2011) 31:636–9. doi: 10.3109/01443615.2011.590910
27. Rienzi L, Mazzilli R, Ubaldi FM. Sperm DNA fragmentation to predict embryo development, implantation, and miscarriage: still an open question. Fertil Steril. (2019) 112:466. doi: 10.1016/j.fertnstert.2019.05.016
28. Kim DS, Song SH. Effect of sperm DNA fragmentation on embryo quality in normal responder women in IVF and ICSI. Yonsei Med J. (2020) 61:986–7. doi: 10.3349/ymj.2020.61.11.986
29. Siddhartha N, Reddy NS, Pandurangi M, Muthusamy T, Vembu R, Kasinathan K. The effect of sperm DNA fragmentation index on the outcome of intrauterine insemination and intracytoplasmic sperm injection. J Hum Reprod Sci. (2019) 12:189–98. doi: 10.4103/jhrs.JHRS_22_19
30. Ten J, Guerrero J, Linares Á, Rodríguez-Arnedo A, Morales R, Lledó B, et al. Sperm DNA fragmentation on the day of fertilisation is not associated with assisted reproductive technique outcome independently of gamete quality. Hum Fertil (Camb). (2022) 25:706–715. doi: 10.1080/14647273.2021.1877364
Keywords: infertility, SCSA, DFI, HDS, ART outcomes
Citation: Shen L, Zhang C, Wang G, Fu X, Yang S and Wang J (2025) High sperm DNA stainability might not be an accurate predictive indicator of male fertility and assisted reproductive technology outcomes. Front. Endocrinol. 16:1510114. doi: 10.3389/fendo.2025.1510114
Received: 17 October 2024; Accepted: 11 February 2025;
Published: 26 February 2025.
Edited by:
Kristian Leisegang, University of the Western Cape, South AfricaReviewed by:
Francisco Quereda, Miguel Hernández University of Elche, SpainCopyright © 2025 Shen, Zhang, Wang, Fu, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaxiong Wang, d2p4ODcwNUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.