
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 24 January 2025
Sec. Reproduction
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1472333
This article is part of the Research Topic Male Reproduction and Oxidative Stress, Volume II View all 14 articles
Objective: Abstinence time has been associated with semen quality, but the results remain controversial.
Methods: This study recruited 3052 men undergoing fertility evaluation. Abstinence time (AT) was categorized as short (0-1 day), WHO-recommended (2-7 days) and long (>7 days). Semen parameters including volume, sperm concentration, progressive motility, total motility, total motility sperm count (TMSC), morphology and DNA fragmentation index were assessed for their association with AT.
Results: Short AT was significantly associated with lower semen volume (P< 0.001), sperm concentration (P= 0.01) and TMSC (P< 0.001), while long AT was significantly associated with higher sperm concentration (P= 0.006), reduced progressive motility (P= 0.005) and total motility (P= 0.02), and higher DFI (P< 0.001). Restricted cubic spline models demonstrated a non-linear relationship between AT and the risk of low semen volume (Pnon-linear < 0.001), sperm concentration (Pnon-linear = 0.039) and TMSC (Pnon-linear < 0.001).
Conclusions: Our findings suggest both short and long AT were significantly associated with lower sperm quality, which indicated the importance of maintaining a recommended AT (2-7days) for semen analysis. Additionally, short abstinence periods may be recommended to maintain optimal sperm DNA integrity.
Human spermatozoa are produced in the seminiferous tubules, release into the rete testis, and subsequently enter the epididymis (1). During transit and storage in the epididymis, immature spermatozoa acquire motility and fertilizing potential, ultimately becoming functionally matured spermatozoa. Several factors correlate with semen quality, one of which is ejaculatory abstinence (2). During periods of ejaculatory abstinence, mature sperm mainly accumulate and greatly expose to reactive oxygen species (ROS) in the epididymis (3, 4). Excessive ROS impair semen quality by reducing sperm motility and causing DNA damage (5). Several studies have indicated that semen volume and sperm count increase, while progressive motility decreases, with longer periods of abstinence (6, 7).
The World Health Organization (WHO) recommends an abstinence period of 2-7 days prior to semen collection for standard analysis. This range is considered optimal for obtaining consistent and reliable sperm parameters, such as sperm count, motility, and morphology, without significantly affecting semen quality. In contrast, the European Society of Human Reproduction and Embryology (ESHRE) suggests a shorter abstinence period of 3-4 days. This recommendation is based on the idea that this specific window may better balance sperm concentration and motility while avoiding potential negative effects, such as reduced sperm motility and increased DNA fragmentation index (DFI), which can occur with longer abstinence periods (8). Current evidence regarding the correlation between short or long abstinence periods and semen quality remains unclear. In this large observational study of 3052 men, we conducted logistic analyses to evaluate the relationship between semen parameters and abstinence time categorized as short (0-1 day), recommend (2-7 days) and long (> 7 days).
Between April 2020 and May 2022, a total of 3052 men who undergoing fertility evaluation at the first affiliated hospital of University of Science and Technology of China (USTC) were included in this study. Clinical characteristics were collected, and semen quality were assessed for all participants. Exclusion criteria included azoospermia, testicular cancer, cryptorchidism and genetic defects related to the male reproductive tract. This study was approved by the first affiliated hospital of USTC Ethical Committee (No. 2023-RE-196).
Semen was collected by masturbation and incubated at 37°C for 30 min for liquefaction. The duration of sexual abstinence was recorded following semen collection. Semen parameters, including sperm concentration, progressive motility and total motility, were assessed using a computer-assisted sperm analysis (CASA) system (SAS-II, SAS Medical, Beijing, China). Sperm morphology was analyzed using Diff-Quick staining (Anke Biotechnology, Hefei, China) under a light microscope (CX33, Olympus Corporation, Tokyo, Japan). Leukocytes were determined by peroxidase staining (Anke Biotechnology, Hefei, China). Antisperm antibodies (AsAs) were detected using the mixed antiglobulin reaction (MAR) test (Anke Biotechnology, Hefei, China). Sperm DFI and high DNA stainability (HDS) were measured using the sperm chromatin structure assay (SCSA, Celula, Chengdu, China).
Qualitative variables were presented as frequencies (percentages), and quantitative variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR). Pearson’s chi-square test, Student’s t test and the Mann−Whitney U test were used for comparisons for categorical, parametric and nonparametric data, respectively. Logistic regression was used to examine the association between abstinence time and semen parameters. Restricted cubic spline (RCS) was performed to assess for the dose-response relationships between abstinence time and abnormal semen parameters according to WHO criteria. Covariates initially included age, BMI, smoking, alcohol intake, education, chronic diseases, urogenital infections and varicocele. A P value of less than 0.05 was considered to indicate a statistically significant status. Statistical analyses were performed using GraphPad Prism 9.0 software (San Diego, CA, USA).
Characteristics of the participants categorized by abstinence time (AT) are shown in Table 1. The mean age of the subjects was 30.9 ± 5.0 years, with a mean BMI of 24.7 ± 3.6 kg/m2. Among all participants, 1292 (42.3%) smoked tobacco and 1533 (50.2%) consumed alcohol. Educationally, 575 (18.9%) had completed middle school or lower levels, 538 (17.6%) had completed high school, and 1939 (63.5%) had completed college/university. The incidence rates of chronic diseases, urogenital infections and varicocele were 309 (10.1%), 420 (13.8%) and 318 (10.4%), respectively. No significant differences were observed between abstinence time and age, BMI, smoking, drinking, education, chronic diseases, urogenital infections and varicocele.
Compared to men with recommended AT, those with short AT had significantly lower semen volume (P < 0.001), sperm concentration (P < 0.001) and TMSC (P < 0.001), while men with long AT had significantly higher semen volume (P < 0.001), sperm concentration (P < 0.001), TMSC (P < 0.001) and DFI (P < 0.001) (Table 2). Lower sperm progressive motility and total motility were shown in long AT than those in recommended EA. No significant differences were observed between AT and sperm normal morphology, semen leukocytes, AsAs and HDS. In terms of sperm kinematics, VCL, VSL, VAP, BCF and MAD significantly declined with AT, while ALH, LIN, WOB, and STR significantly increased with AT (Table 3).
As shown in Table 4, logistic regression analysis revealed a significantly positive association between short AT and low semen volume (OR = 3.1, 95% CI = 2.0-4.7, P < 0.001), low sperm concentration (OR = 1.7, 95% CI = 1.1-2.6, P = 0.02) and low TMSC (OR = 2.0, 95% CI = 1.3-3.1, P < 0.001). After adjusting for potential confounders including age, BMI, educational levels, smoking, alcohol consumption, chronic diseases, urogenital infections and varicocele), a high risk of poor semen quality was still observed in men with short AT. Additionally, long AT was significantly negatively associated with low sperm concentration (OR = 0.5, 95% CI = 0.3-0.8, P = 0.007) and positively associated with low sperm progressive motility (OR = 1.5, 95% CI = 1.2-1.9, P = 0.002), low sperm total motility (OR = 1.4, 95% CI = 1.1-1.8, P = 0.007) and high sperm DFI (OR = 2.8, 95% CI = 1.7-4.6, P < 0.001), and those relationship were still observed after adjusting for several confounders. No significant associations were observed for sperm normal morphology (all P > 0.05).
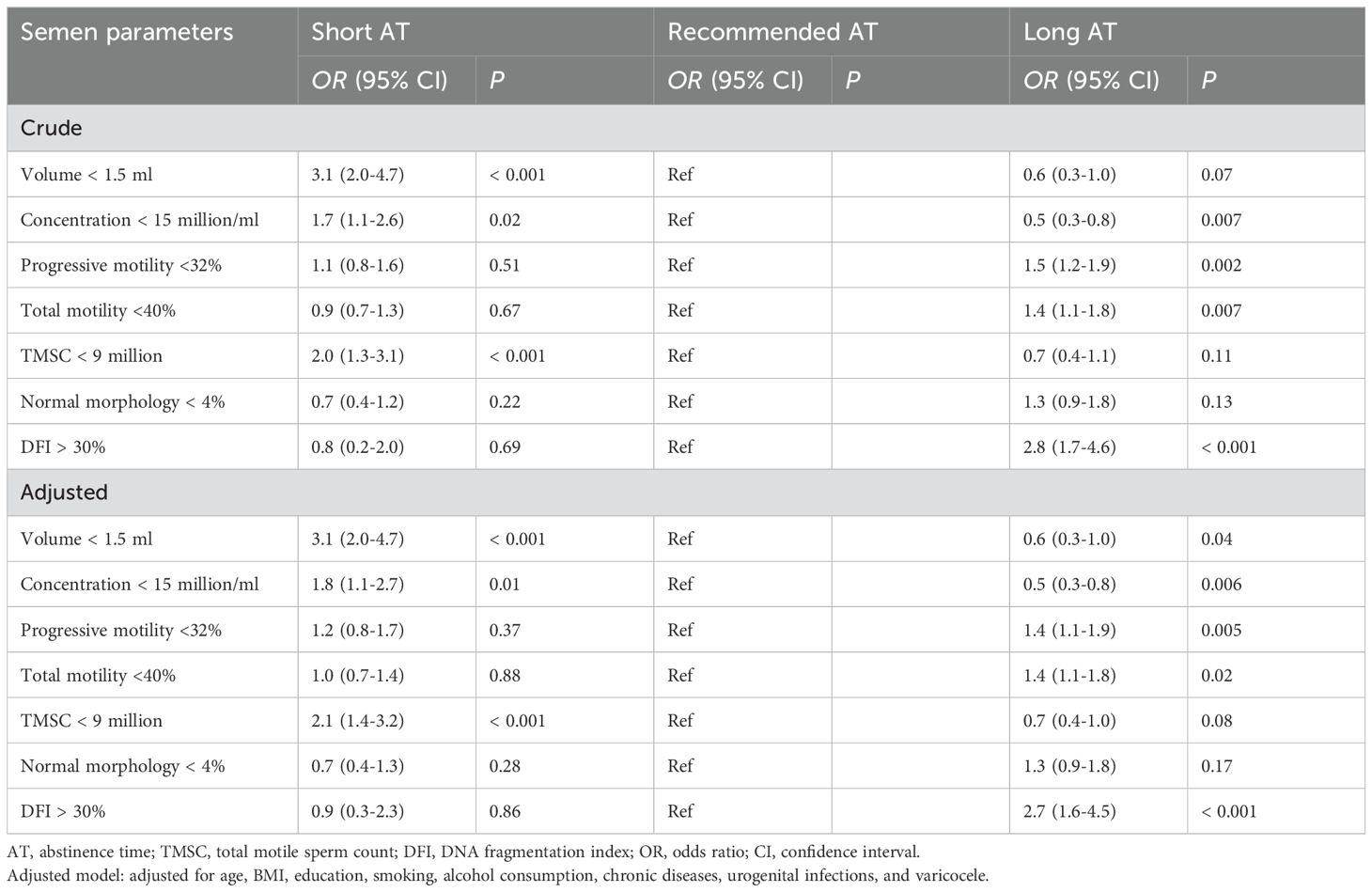
Table 4. Odds ratios (95% confidence intervals) for abnormal semen parameters across abstinence time.
The dose-response relationships between continuous AT and abnormal semen parameters after adjusting for confounders were further explored using RCS (Figure 1). When AT was lower than the median AT in men with recommended AT (4 days), risk of low semen volume (P non-linear < 0.001), sperm concentration (P non-linear = 0.039) and TMSC (P non-linear < 0.001) appeared to decrease with increasing AT, while there was no significant difference for AT longer than 4 days. No significant non-linear relationships were observed between AT and risk of abnormal sperm progressive motility, total motility and sperm DFI.
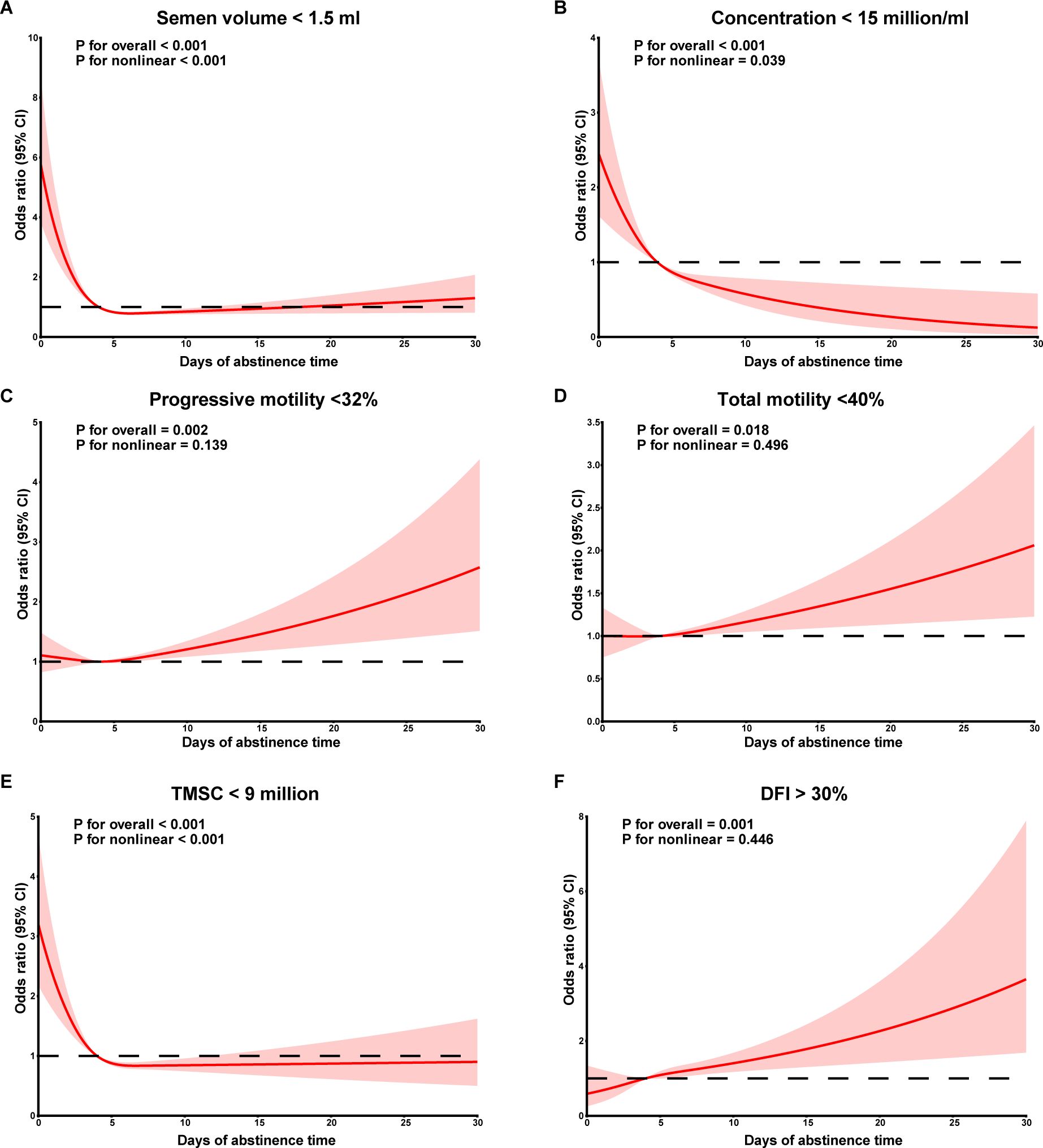
Figure 1. Dose-response curves between abstinence time and semen volume (A), sperm concentration (B), sperm progressive motility (C), sperm total motility (D), TMSC (E), DFI (F). TMSC, total motile sperm count; DFI, DNA fragmentation index.
Semen analysis, including sperm concentration, motility and morphology, has been as the commonly test for evaluating male fertility (9, 10). Several pre-analytical factors, such as time of abstinence and method of semen collection, have been shown to affect semen parameters (11). In this observational study with a relatively large sample size, we assessed the relationship between AT and semen quality in men who undergoing fertility examination. Our results indicate that shorter AT was positively associated with abnormal semen volume, sperm concentration and TMSC, and long AT was positively associated with abnormal sperm progressive motility, total motility and DFI.
The recommended duration of abstinence by the WHO laboratory Manual for the Examination and Processing of Human Semen ranges from 2 to 7 days. The association between AT and semen quality remains controversial (12, 13). Several studies have shown that abstinence duration is significantly linked with semen volume, sperm concentration, motility, normal morphology, and DFI (14–16). However, other studies have not reported significant associations, particularly concerning sperm morphology. Recently, a systematic review suggested that shorter abstinence durations improve semen quality, including lower sperm DFI, higher motility and normal morphology (17). In addition, several guidelines address the beneficial effects of short abstinence duration in reducing sperm DFI (18, 19). However, another review concluded that the effects of short-term abstinence on semen parameters are contradictory and inconclusive (20).
On the other hand, prolonged AT was associated with increased seminal volume, semen leukocytes, sperm concentration, DFI and decreased sperm motility (15). Given that semen volume increases with abstinence duration, the change in sperm count exceeded that in sperm concentration. Recent studies have suggested improvements in sperm count with abstinence periods longer than 5 or 7 days (17, 20), while our study observed TMSC exhibited the most significant changes with increasing AT. As long-term AT appear to reduce sperm motility, TMSC is recommended as a preferred evaluation of semen parameters.
The pathophysiological mechanisms linking abnormal abstinence duration to semen quality remains unclear. Shorter abstinence periods have been reported to affect the duration of sperm retention in the epididymis, which may improve sperm antioxidant capacity and seminal metabolites (21). Prolonged retention of sperm in the epididymis increases susceptibility to damage by ROS (7, 22, 23). ROS at normal physiological levels is essential for the processes of maturation, hyperactivation, capacitation, acrosome reaction, zona pellucida binding and oocyte fusion (24–26). However, an imbalance between ROS and antioxidants leads to oxidative stress, impairing acrosome, mitochondrial, and DNA integrity.
One major strength of our study is its relatively large sample size, which provides high statistical power and precision. The current study also included dose-response relationship between abstinence duration and semen parameters. In addition, we also considered several potential confounding factors, such as age, BMI, drinking, and smoking, that have been previously reported to be associated with semen quality. However, several limitations need to be discussed. First, all semen parameters were obtained from men who undergoing fertility evaluation at a single clinic center and were unable to generalize men with reproductive age. Second, the level of ROS was not assessed in this study, which might affect the relationship between abstinence duration and semen quality. Third, as our study is a retrospective design, potential selection bias is unavoidable. Finally, although we adjusted for several potential confounders, there is still the possibility of residual confounding by unknown factors.
In conclusion, our study showed that both short and long AT were significantly associated with lower sperm quality, which indicated the importance of maintaining a recommended AT for semen analysis. Additionally, short abstinence periods short abstinence periods may be recommended to maintain optimal sperm DNA integrity.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the First Affiliated Hospital of USTC Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
GY: Writing – original draft. QQ: Writing – original draft. XD: Writing – original draft. WZ: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. XZ: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (81901543) and Youth Innovation Talents Project of Colleges and Universities in Guangdong Province (2024KQNCX074).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen GX, Li HY, Lin YH, Huang ZQ, Huang PY, Da LC, et al. The effect of age and abstinence time on semen quality: a retrospective study. Asian J Androl. (2022) 24:73–7. doi: 10.4103/aja202165
2. Cornwall GA, Do HQ, Hewetson A, Muthusubramanian A, Myers C. The epididymal amyloid matrix: structure and putative functions. Andrology. (2019) 7:603–9. doi: 10.1111/andr.12586
3. Potts RJ, Jefferies TM, Notarianni LJ. Antioxidant capacity of the epididymis. Hum Reprod. (1999) 14:2513–6. doi: 10.1093/humrep/14.10.2513
4. Mayorga-Torres BJ, Camargo M, Agarwal A, Du Plessis SS, Cadavid AP, Cardona Maya WD. Influence of ejaculation frequency on seminal parameters. Reprod Biol Endocrinol. (2015) 13:47. doi: 10.1186/s12958-015-0045-9
5. Raei H, Karimi Torshizi MA, Sharafi M, Ahmadi H. Sperm flow cytometric parameters, antioxidant status, and testicular histomorphology in roosters fed diets supplemented with camphor. Poult Sci. (2022) 101:102014. doi: 10.1016/j.psj.2022.102014
6. Ayad BM, Horst GV, Plessis SSD. Revisiting the relationship between the ejaculatory abstinence period and semen characteristics. Int J Fertil Steril. (2018) 11:238–46. doi: 10.22074/ijfs.2018.5192
7. Li Y, Lu T, Wu Z, Wang Z, Yu T, Wang H, et al. Trends in sperm quality by computer-assisted sperm analysis of 49,189 men during 2015-2021 in a fertility center from China. Front Endocrinol (Lausanne). (2023) 14:1194455. doi: 10.3389/fendo.2023.1194455
8. Akhigbe RE, Hamed MA, Dutta S, Sengupta P. Influence of ejaculatory abstinence period on semen quality of 5165 normozoospermic and oligozoospermic Nigerian men: A retrospective study. Health Sci Rep. (2022) 5:e722. doi: 10.1002/hsr2.v5.5
9. Chao HH, Zhang Y, Dong PY, Gurunathan S, Zhang XF. Comprehensive review on the positive and negative effects of various important regulators on male spermatogenesis and fertility. Front Nutr. (2022) 9:1063510. doi: 10.3389/fnut.2022.1063510
10. Deuel JW, Lauria E, Lovey T, Zweifel S, Meier MI, Zust R, et al. Persistence, prevalence, and polymorphism of sequelae after COVID-19 in unvaccinated, young adults of the Swiss Armed Forces: a longitudinal, cohort study (LoCoMo). Lancet Infect Dis. (2022) 22:1694–702. doi: 10.1016/S1473-3099(22)00449-2
11. Bai S, Dou XC, Qi HL, Zhu YS, Zhang YT, Liu YX, et al. Association between semen collection time and semen parameters: an observational study. Asian J Androl. (2023) 25:339–44. doi: 10.4103/aja202268
12. Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. (2016) 94:102–10. doi: 10.1016/j.urology.2016.03.059
13. Du C, Li Y, Yin C, Luo X, Pan X. Association of abstinence time with semen quality and fertility outcomes: a systematic review and dose-response meta-analysis. Andrology. (2024) 12:1224–35. doi: 10.1111/andr.13583
14. Levitas E, Lunenfeld E, Weiss N, Friger M, Har-Vardi I, Koifman A, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. (2005) 83:1680–6. doi: 10.1016/j.fertnstert.2004.12.045
15. Comar VA, Petersen CG, Mauri AL, Mattila M, Vagnini LD, Renzi A, et al. Influence of the abstinence period on human sperm quality: analysis of 2,458 semen samples. JBRA Assist Reprod. (2017) 21:306–12. doi: 10.5935/1518-0557.20170052
16. Xie M, Hammerli S, Leeners B. The association between abstinence period and semen parameters in humans: results in normal samples and different sperm pathology. Life (Basel). (2024) 14:188. doi: 10.3390/life14020188
17. Sokol P, Drakopoulos P, Polyzos NP. The effect of ejaculatory abstinence interval on sperm parameters and clinical outcome of ART. A systematic review of the literature. J Clin Med. (2021) 10:3213. doi: 10.3390/jcm10153213
18. Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: A new guideline for clinicians. World J Mens Health. (2020) 38:412–71. doi: 10.5534/wjmh.200128
19. Agarwal A, Farkouh A, Parekh N, Zini A, Arafa M, Kandil H, et al. Sperm DNA fragmentation: A critical assessment of clinical practice guidelines. World J Mens Health. (2022) 40:30–7. doi: 10.5534/wjmh.210056
20. Hanson BM, Aston KI, Jenkins TG, Carrell DT, Hotaling JM. The impact of ejaculatory abstinence on semen analysis parameters: a systematic review. J Assist Reprod Genet. (2018) 35:213–20. doi: 10.1007/s10815-017-1086-0
21. Lo Giudice A, Asmundo MG, Cimino S, Cocci A, Falcone M, Capece M, et al. Effects of long and short ejaculatory abstinence on sperm parameters: a meta-analysis of randomized-controlled trials. Front Endocrinol (Lausanne). (2024) 15:1373426. doi: 10.3389/fendo.2024.1373426
22. Li Y, Wang S, Li D, Huang Y, Liu H, Zhang X, et al. Short-interval second ejaculation improves sperm quality, blastocyst formation in oligoasthenozoospermic males in ICSI cycles: a time-lapse sibling oocytes study. Front Endocrinol (Lausanne). (2023) 14:1250663. doi: 10.3389/fendo.2023.1250663
23. Atmoko W, Savira M, Shah R, Chung E, Agarwal A. Isolated teratozoospermia: revisiting its relevance in male infertility: a narrative review. Transl Androl Urol. (2024) 13:260–73. doi: 10.21037/tau-23-397
24. Biagi M, Noto D, Corsini M, Baini G, Cerretani D, Cappellucci G, et al. Antioxidant Effect of the Castanea sativa Mill. Leaf Extract on Oxidative Stress Induced upon Human Spermatozoa. Oxid Med Cell Longev. (2019) 2019:8926075. doi: 10.1155/2019/8926075
25. Lazzarino G, Listorti I, Bilotta G, Capozzolo T, Amorini AM, Longo S, et al. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants (Basel). (2019) 8. doi: 10.3390/antiox8040096
Keywords: abstinence time, semen quality, semen parameters, DNA fragmentation index, DFI
Citation: Yao G, Qi Q, Dou X, Zhou W, Bai S and Zhang X (2025) Association of abstinence time with semen quality in men who undergoing fertility evaluation: a cross-sectional study from 3052 participants. Front. Endocrinol. 16:1472333. doi: 10.3389/fendo.2025.1472333
Received: 29 July 2024; Accepted: 09 January 2025;
Published: 24 January 2025.
Edited by:
Kristian Leisegang, University of the Western Cape, South AfricaReviewed by:
Ahmad Mustafa Metwalley, Women’s Health Fertility Clinic, Saudi ArabiaCopyright © 2025 Yao, Qi, Dou, Zhou, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Zhou, enpmZmNjMjAyM0BzaW5hLmNvbQ==; Shun Bai, c2h1bmJhaUB1c3RjLmVkdS5jbg==; Xi Zhang, emhhbmd4aUBnemhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.