
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 14 March 2025
Sec. Clinical Diabetes
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1462210
Background: Diabetic retinopathy (DR) is becoming a more widespread public concern worldwide, leading to visual impairments. It has become the leading cause of blindness among working-age adults globally, despite established treatments that can reduce the risk by 60%.
Objective: This study aimed to determine the incidence of diabetic retinopathy and its predictors among adult patients with diabetes in public hospitals in Central and Southern Ethiopia.
Methods: A hospital-based follow-up study was conducted in selected public hospitals in Central and Southern Ethiopia. A total of 376 participants of newly diagnosed adult diabetes were enrolled from 2015-2023 and the follow-up the date was from date of enrolment to the development of events. The data were collected by reviewing their records and entered in Epi-data version 4.6.0.2 and exported to STATA version 14 for analysis. Descriptive statistics of the variables were obtained. The Weibull model with gamma frailty distribution was fitted. Bivariable and multivariable analyses were done, and variables with a p-value less than 0.05 and a corresponding 95% confidence interval in the final model were used. The model of adequacy was checked.
Results: 376 adult diabetic patient records were reviewed with the mean baseline age (± standard deviation) of 34.8±10 years. The univariate frailty was statistically significant (Theta=0.236 (0.131, 0.496)). A total of 376 adult patients with diabetes were followed for 682.894 person-years. Overall, an incidence rate of 14.06/100 person-years. Proteinuria (AHR = 2.21: 95% CI: 1.45, 3.57), cardiovascular disease (AHR = 2.23: 95% CI: 1.34, 4.03), and type II DM (AHR = 2.87: 95% CI: 1.30, 6.13) were identified as significant predictors of diabetic retinopathy.
Conclusion: Overall incidence rate of diabetic retinopathy was high. The most effective way to protect our vision from diabetic retinopathy is to manage diabetes effectively and offer support to high-risk individuals with diabetes. Therefore, healthcare professionals and relevant health authorities should target on addressing these factors in their initiatives to prevent diabetic retinopathy in diabetic patients.
Diabetes is a serious, chronic condition where the body either cannot produce sufficient insulin, produces no insulin at all, or is unable to effectively use the insulin it produces (1). According to the International Diabetes Federation (IDF) 2021, the global prevalence of diabetes among individuals aged 20 to 79 years is 537 million, representing 9.3% of the population in this age group, with 79.4% of cases occurring in low- and middle-income countries (LMICs) (2). This number is expected to rise to 643 million by 2030 and further increase to 782 million by 2045. Additionally, it is estimated that over 6.7 million people aged 20–79 died from diabetes-related causes in 2021 (2, 3).
In low- and middle-income countries are experiencing a large share of the rapid increase in the prevalence of this disease (4). Over the next 20 years, sub-Saharan African (SSA) nations are predicted to see the fastest global increases in the number of patients with diabetes (5). According to estimates, 77% of global DM epidemic burden in the twenty-first century will fall on developing nations like Ethiopia (6). This also plays a part in Africa’s higher early-life morbidity and mortality. In 2017, it was estimated 5.2% of Ethiopians aged 20 to 79 had diabetes, with 2.6 million cases of the disease reported nationwide (2, 7). Patients with diabetes, especially T2DM, are at increased risk of both microvascular and macrovascular disease (8–11). Nearly all patients with T1DM and over 60% of patients with T2DM will develop retinopathy within the first two decades of diagnosis, which is one of the complications of DM (10).
Diabetic retinopathy (DR) is increasingly recognized as a global public health concern and the most common cause of acquired blindness in adults (12). Despite available treatments that can lower the risk by 60% (13, 14), it remains the leading cause of blindness and vision loss among working-age adults worldwide. DR accounts for approximately 2.6% of global blindness (15) and 4.8% of visual impairment (16). In 2020, around 103.12 million people were affected by DR globally, and this number is projected to rise to 160.5 million by 2045, with low- and middle-income countries facing a disproportionately higher burden (17). Studies in Africa revealed that DR has been found with pooled prevalence of 19-29% (18–23). In Ethiopia had a 19.48% of DR (21, 24). Many risk factors have been linked to diabetic DR such as age (24, 25), the length of DM (24, 25), poor medication adherence (26), poor glycemic control (26, 27), obesity (28), and hypertension, which speed up the development of DR in diabetic patients (25, 26, 29, 30). According to the study, hyperglycemia only accounts for 11% of the overall risk of DR; the remaining 89% may be attributable to other possible risk factors (31). The majority of epidemiological studies on DR conducted in Ethiopia and other eastern African countries have so far only estimated using a single facility. Therefore, the objective of this study was to determine the incidence and its predictors of DR among patients with diabetes in public hospitals in Central and Southern Ethiopia.
A hospital-based follow-up study design was conducted from June 1/2023 to July 30/2023 among adult patients with diabetes in five randomly selected public hospitals in the central and southern regions of Ethiopia. In both regions, there are 19 zones and three special woredas (districts) in combination with an estimated total population of more than 16.9 million. During the year of this study, there were 52 public hospitals (compressive specialized, referral, general, and primary) in the region (32, 33). The randomly selected hospitals provide general and specialty health care services along with teaching and research activities. It also provides comprehensive diabetes-related services. The diabetic clinic is a former hospital clinic where care and follow-ups are given to patients with all types of diabetes.
The source population was all adult patients with diabetes who were followed up at chronic disease follow-up units in selected public hospitals in the central and southern regions of Ethiopia from the year January, 2015 - January, 2023. But, those all selected newly diagnosed adult patients with diabetes were the study population. All adult patients with diabetes aged ≥ 18 years who were diagnosed between January, 2015, and January, 2023, were included in the study. However, those whose date of initiation was not recorded, who had gestational DM, or who had DM or retinopathy at the same time of diagnosis were excluded.
STATA version 14 used to determine sample size by using the Schoenfeld formula (34) based on the power approach by considering predictors significantly associated with the incidence of adverse drug reactions (ADRs) from previous studies with the hazard ratios for three predictors significantly associated with diabetic retinopathy in a study conducted at the Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar (35), were calculated (Table 1). By considering under the following assumptions: Cox proportional hazard model, 95% confidence level, 80% power, 10% withdrawal probability.
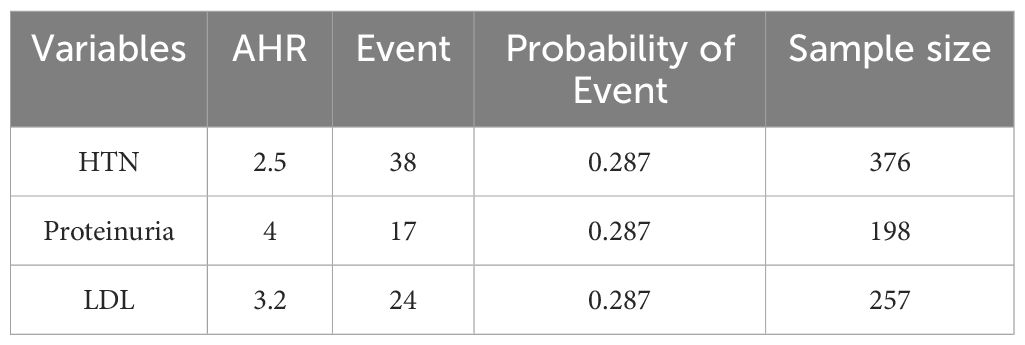
Table 1. Minimum sample size calculated for predictors significantly associated with adult patients with diabetes in public hospitals in the central and southern regions of Ethiopia.
The Schoenfeld formula [Schoenfeld, 1983 (24)] was used for manual calculation:
Where: n = Total sample size, HR is the hazard ratio of selected covariates, P1 is the proportion of subjects in the exposure group, p2= probability of a harm occurring as result of exposure to hazard (1-p1), E = number of events and P (E) is the probability of an event from a previous study.
Therefore, the final sample size for this study was 376. Patients who met the inclusion criteria were included in the study. The sampling procedure followed the same approach used in a similar study (36, 37). Initially, five public hospitals were selected by using simple random sampling method. Then, a sampling frame was created in Excel spread sheets using Health Management and Information Systems (HMIS) card numbers/patient’s medical registration number from each randomly selected hospital’s diabetic registration book for those who fulfilled the inclusion criteria were selected in randomly selected hospitals. After that, the calculated sample size was proportionally distributed to each randomly selected hospital. To select study subjects from each of the randomly selected hospitals, a simple random sampling (SRS) technique was used to select 376 records using a computer-generated random number table. Finally, the selected patient’s medical registration numbers were helped to dig out data from the electronic sheet, follow up patient’s folder, and diabetic registration in the chronic follow-up clinic.
DR was defined by both direct and indirect ophthalmoscopy assessments performed by physicians confirmed by fundus photography. DR was defined as a microvascular complication of diabetes that was evaluated by clinical examination or indirect ophthalmoscopy by ophthalmologists and classified as present (yes) or absent (no) from the charts based on ophthalmologists’ decisions (38).
The time to diabetic retinopathy was defined as the duration, measured in months, between the diagnosis of diabetes mellitus and the development of diabetic retinopathy (38). Anevent of interest in our study was adult patients with diabetes those who experienced diabetic retinopathy during the follow-up period (38).
The clinical and epidemiological data for this study were gathered from various sources, including diabetes mellitus (DM) intake forms, DM registration logbooks, electronic databases, patient cards, and monthly follow-up charts. Patient intake forms, clinical records, and laboratory results for biomarkers were reviewed using a structured and pretested questionnaire. This questionnaire was developed based on the follow-up charts used in the hospital and various reviewed literature (36–40) published in English. Health Management and Information Systems (HMIS) card numbers or patients' medical registration numbers were used to locate individual patient records or their data in the electronic database. Baseline data were collected from the date patients began regular follow-up treatment and were tracked until the study's conclusion, the occurrence of an event, or censoring during the study period. The data collection was carried out by health workers from the chronic disease follow-up unit, who were selected based on their experience and educational qualifications.
To maintain data quality, a well-designed and pretested data extraction checklist was utilized. Data collectors received training and detailed explanations regarding the study's objectives and the data collection process. They were also familiarized with the checklist, and strict supervision was provided throughout the data collection phase.
The collected data were entered into Epi-data version 4.6.0.2 and then exported to STATA version 14 for further analysis. Appropriate data management techniques were employed to ensure that the data were suitable for analysis. Descriptive statistics were conducted to describe the study population. Patients were counted as a censor, if lost to follow-up, if transferred to another health facility before developing retinopathy, who died, or if not develop DR at the end of follow-up. The survival time was calculated in months using the time between the date of diagnosis of diabetes mellitus and the date of the event (DR) or the date of censoring. The outcome of each patient was categorized into censor or event (diabetic retinopathy). The incidence rate with respect to person-time at risk was calculated.
Different survival analysis models were fitted and compared to select best-fitted model. The conventional Cox proportional hazard model may not fit the data well all the time, and may leads to incorrect inferences when all levels of relevant covariates have not been observed or included. The good fitted model was selected for multivariable analysis, by comparing parametric and semi-parametric model. Even though individuals are similar based on the observed variables some individuals are frailer than others, since there are random variables that varies over the population frailty model was carried out to examine predictors of DR. To examine random variables that vary over the population univariate frailty model was carried out.
An associated variable was tested at a 95% CI and summarized by using an adjusted hazard ratio (AHR) and any variable with a P-value less than 0.05 was taken as statistically significant. The adequacy of the model was checked by the Nelson–Aalen cumulative hazard function against the Cox–Snell residual technique (41). The results were presented in a table, figure, and graph.
Ethical approval and a letter of cooperation were obtained from the Institutional Review Board of Wachemo University, College of Medicine and Health Sciences with reference number: wcu/8456/2023 and the hospital were informed about the study objectives through a written letter. Informed consent was waived by the Institutional Review Committee of Wachemo University. Confidentiality was maintained at all levels of the study. The data were stored on a secured password protection system. All procedures were conducted based on the regulations, guidelines, and principles of the Helsinki Declaration.
Patients and the public were not involved in the design of the study, the conduct of the study or the dissemination of the findings.
A total of 376 adult diabetic patient records were reviewed with the mean baseline age (± standard deviation) of 34.8 ± 10 years. Of the total study participants, half of 191 (50.6%) were females, and about 199 (52.9%) were married. About one-third 210 (55.9%) of patients had a college education or above, and about 90 (23.9%) of patients were Government employed. Of the total study participants, greater than one-half, 102 (27.1%), were follow-up in Wolaita Sodo compressive specialized hospital and about 281 (74.7%) were from urban residents (Table 2).
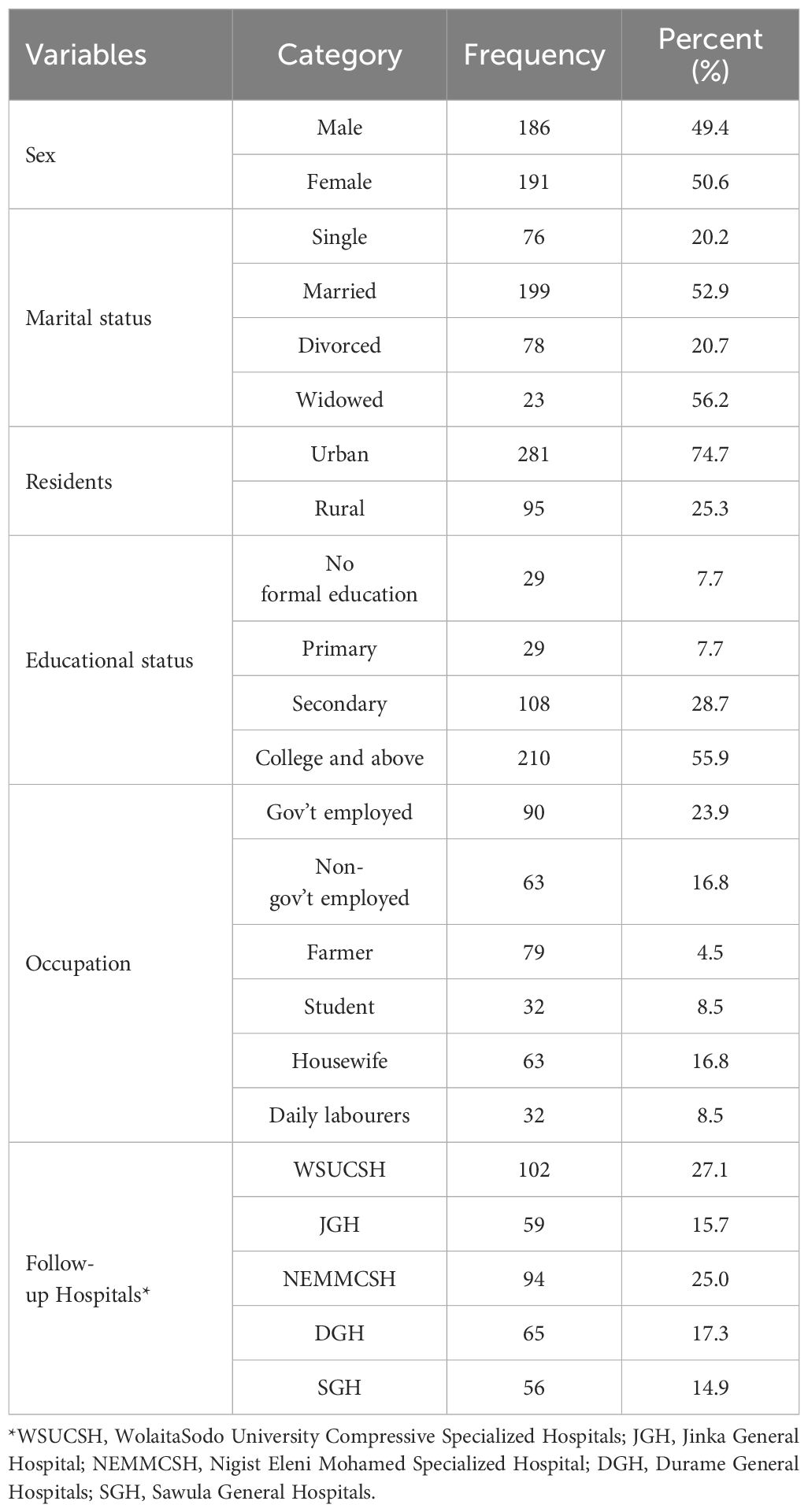
Table 2. Socio-demographic characteristics of the respondents with patients with diabetes at follow-up at public hospitals in the central and southern regions of Ethiopia.
The median duration of DM treatment of patients was 20.2 months, with IQRs of 18.4 and 29.3 months. Three-fourths (n=282, 75.2%) of the patients were receiving insulin treatment, and approximately 73% of patients had good adherence to treatment. In this study, while 17.9% of patients had a history of smoking tobacco products, nearly 12.5% of patients had a history of alcoholic drinks. Among the study subjects, one-fourth (96, 25.5%) of patients had developed retinopathy (Table 3).
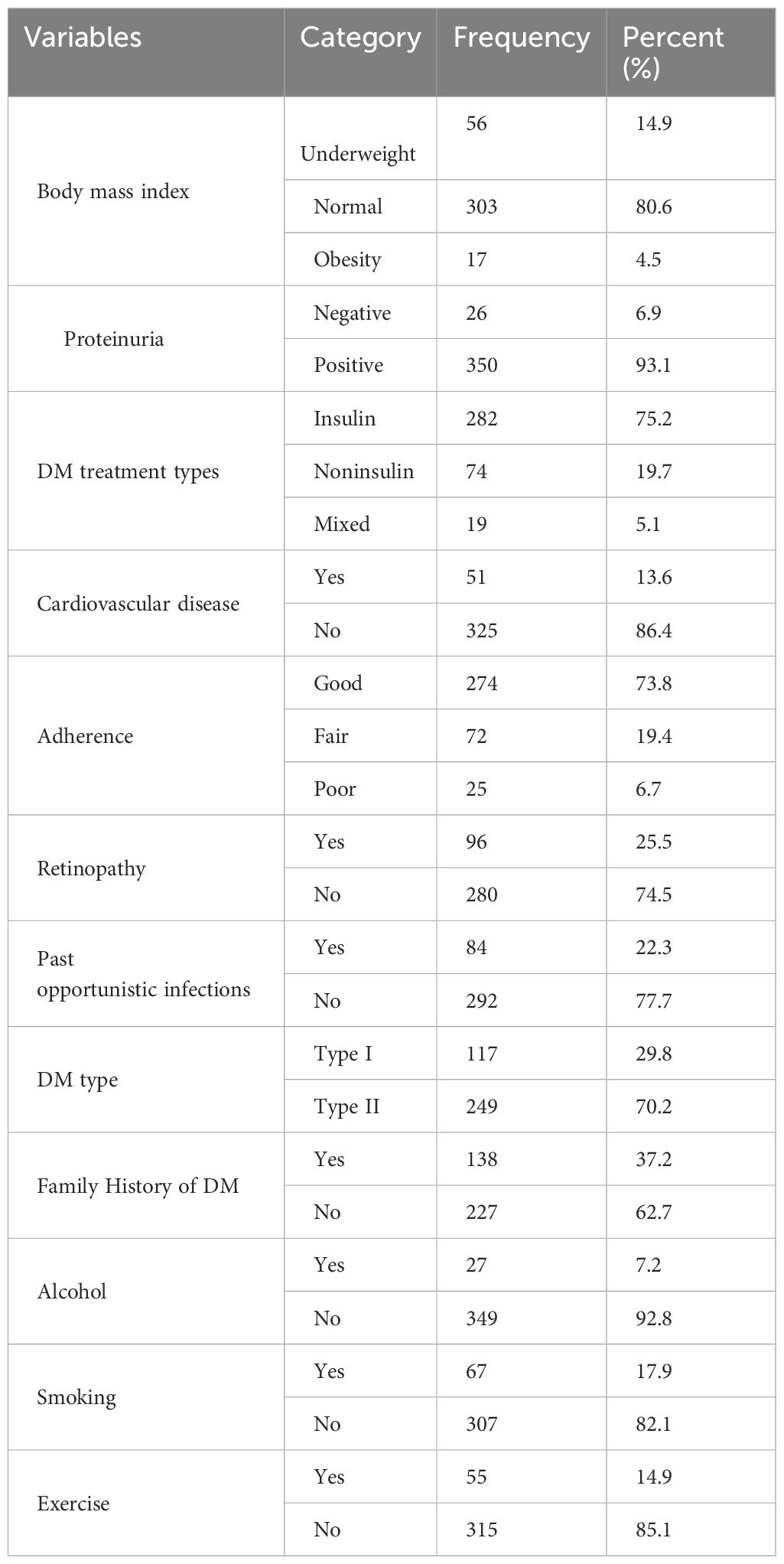
Table 3. Baseline clinical and behavioral characteristics of patients with diabetes on follow-up in public hospitals in the central and southern regions of Ethiopia.
The patients were followed for a minimum of 2.8 months and a maximum of 59.3 months, with a median follow-up time of 19.3 months and an IQR of 16.6to 26.9. Out of 376 study participants who were followed during the investigation,231 (61.43%) were alive and continued their treatment in the health facilities, 11 (2.92%) were lost follow up, 29(5.05%) were transferred to other facility, 9 (2.39%) were died, and 96 (26.5%) developed retinopathy. The incidence rate was 14.06/100 person-years (PY) (approximately fourteen cases per 100 PY of observation), with a 95% CI of (9.82-17.61).
However looking at the Kaplan–Meier survival for CVD, adherence, body mass index is not enough to be certain of proportionality even though few variable fulfil criteria and overall KM go down (Figures 1, 2).
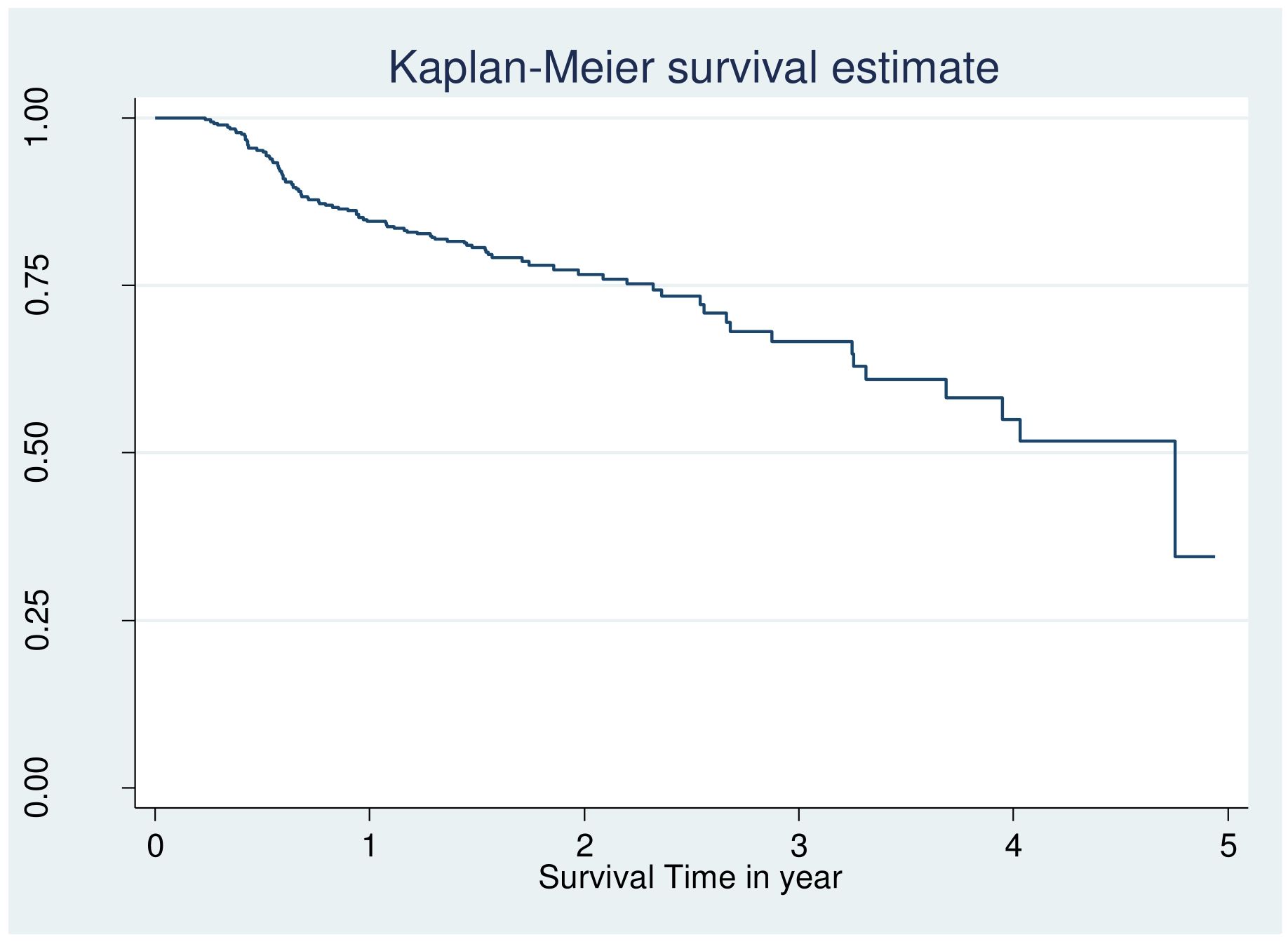
Figure 1. Overall Kaplan Meier failure estimate of retinopathy among patients with DM at public hospital in central and southern, Ethiopia, 2023.
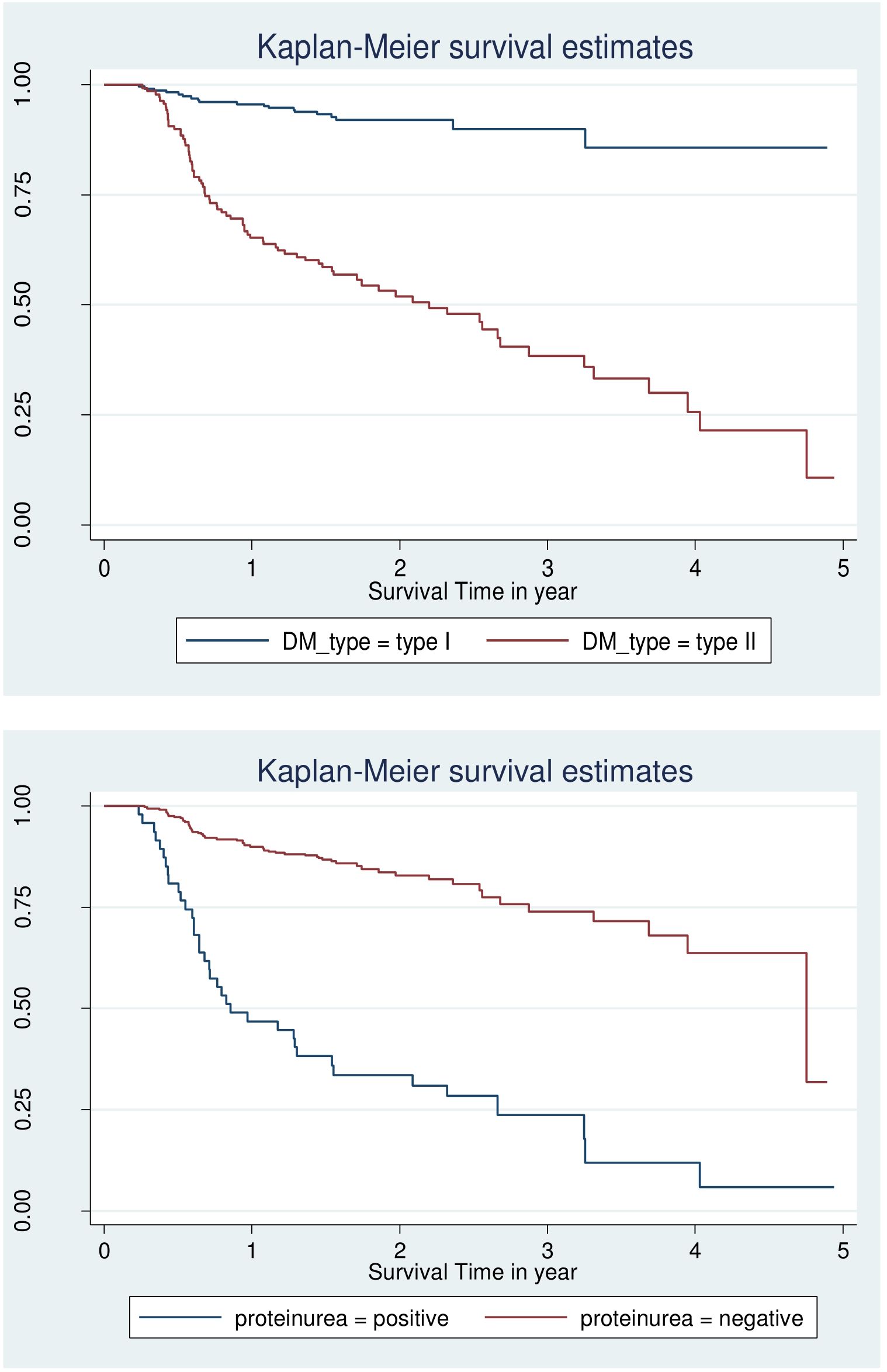
Figure 2. Log-log survival plot by DM type and protein uria for patients with DM at selected public hospital in central and southern, Ethiopia, 2023.
Different techniques of survival analysis were tried to find a model that best fits the data. First, the proportional hazard model was fitted; then, the Weibull, exponential, log-logistic, Gompertz and log-normal parametric survival models were fitted. Finally, these parametric models with the gamma and inverse Gaussian univariate frailty models were fitted. Out of which the one that gave the best fit was selected based on AIC and BIC. Schoenfeld residual test for the Global test was insignificant indicating the proportional hazard assumption holds (Table 4).
Based on the p-value of the Bivariable Cox proportional hazard regression analysis, twelve variables with p-value ≤ 0.20 were identified as potential candidate variables for the multivariable Cox proportional hazard regression model. These were sex, educational status, BMI, CVD, protienuria, adherence, family history of DM, DM type, past opportunistic infections, smoking, and alcohol. In multivariable cox proportional hazard regression analysis, CVD, DM type, and protienuria showed statistically significant associations with the incidence of Diabetic retinopathy (Table 5).
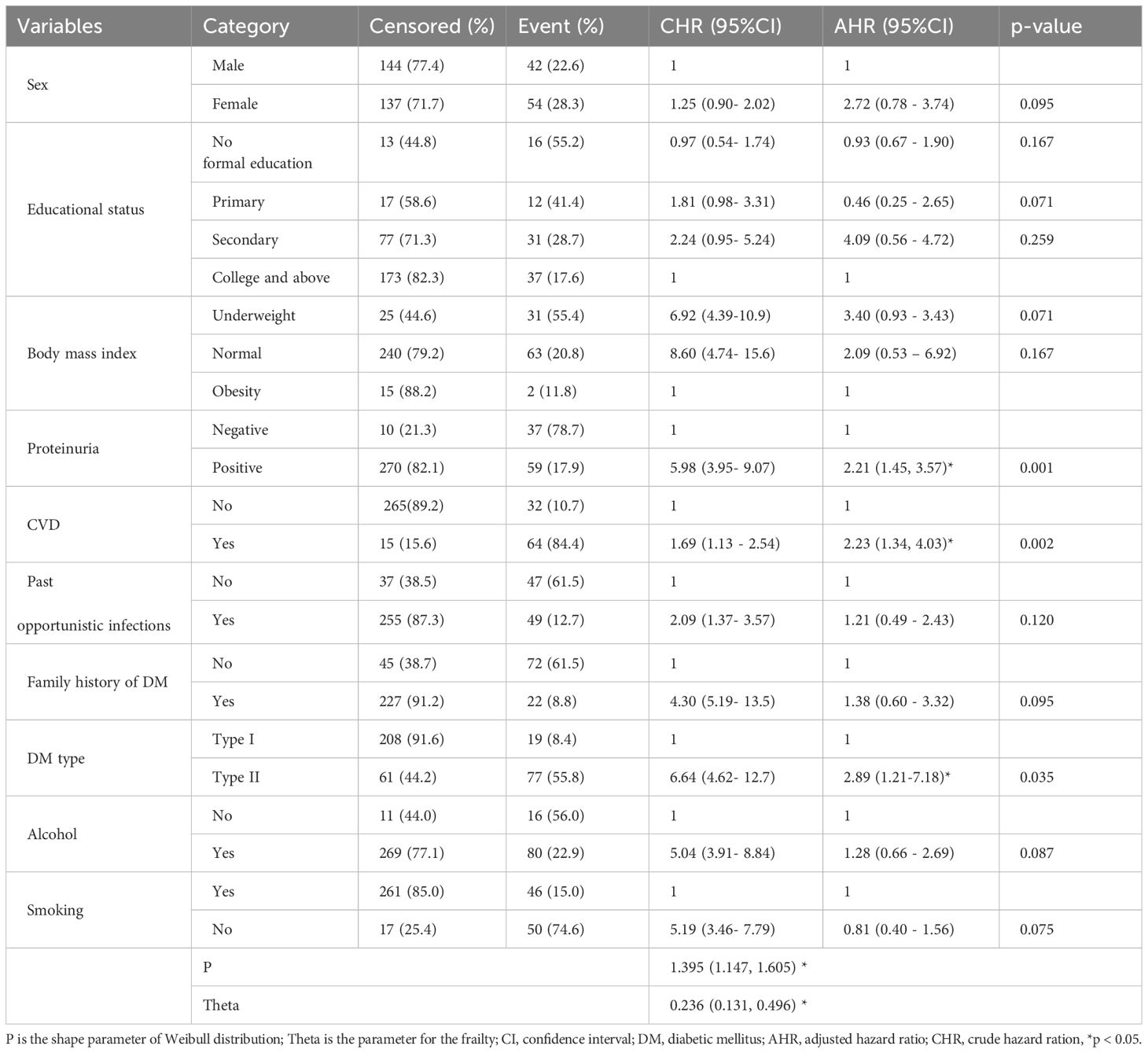
Table 5. Bivariate and multivariable cox proportional hazard regression analysis results for predictors affecting diabetic retinopathy in selected public hospitals of Central and South region of Ethiopia.
The Cox-Snell residual plot is approximately linear through the origin with a slope of 1 which indicates that the fitted Cox model is adequate (Figure 3).
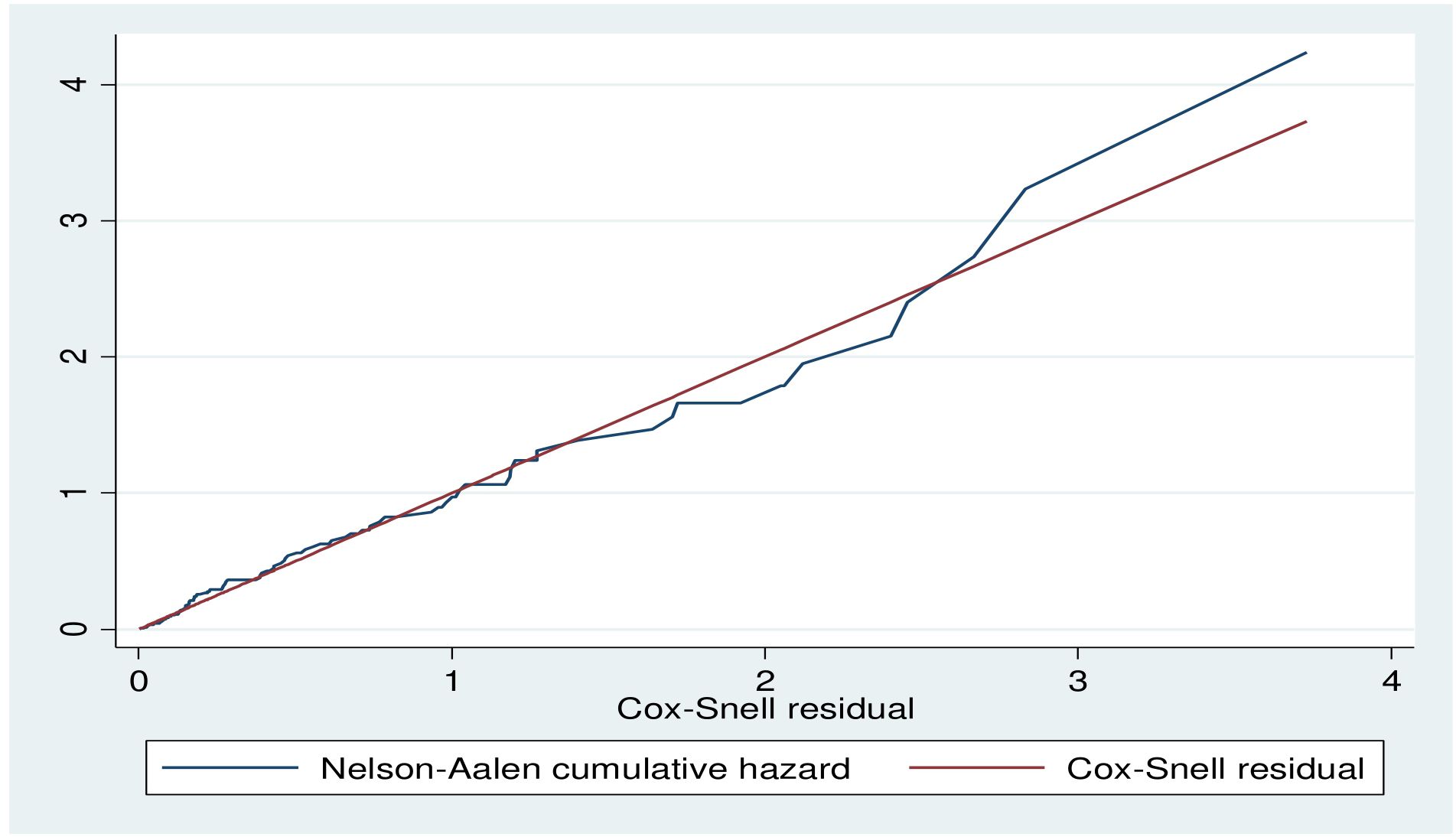
Figure 3. Cox-Snell residual plot of Diabetic retinopathy among DM patient public hospital in central and southern, Ethiopia, 2023.
This study sought to ascertain the incidence of diabetic retinopathy and its risk factors among patients with diabetes in the central and southern regions of Ethiopia. According to this study, the cumulative density of diabetic retinopathy was 14.06/100 person-years (PY) with a 95% CI of (9.82-17.61). This finding was comparable with those of studies conducted in Addis Ababa, Ethiopia (32), in ArbamichGeneral Hospital, Ethiopia (40), in Jimma Medical Center, Ethiopia (39), in Japan (42), in Spain (43) and in United Kingdom (44). This might be due to the use of a matching service delivery approach at the diabetic clinic in the facilities.
The current study revealed that the incidence was higher than that of studies conducted in China (45), Spain (46), the United States (47), and Australia (48). This discrepancy may be the result of variation in the study participants’, demographics, early diagnosis, and follow-up care for diabetic patients. Additionally, the incidence of DR may be reduced in in China, Spain, the United States, and Australia by having strict monitoring of complications and high-quality healthcare systems (47).
The results of this study, however, were lower than those of studies carried out in Bangladesh (49), England (50), Kenya (51),or South Africa, where a retrospective follow-up analysis revealed a cumulative incidence of DR 47/1000 people over the course of a 7 years follow-up study (52).This difference might be due to the study period and study population used in the respective studies, as the follow-up years and screening programs could all contribute factors.
This study identified proteinuria as a risk factor for DR. The hazard of DR was 2.21 times greater than among patients with positive proteinuria than those with negative proteinuria. This finding is in line with those of retrospective cohort studies conducted in the Felege Hiwot Comprehensive Specialized Hospital, Ethiopia (35) and Iran (53). On the other hand, a study revealed that, there was no difference between the presence and absence of DR in terms of the albumin excretion rate (AER) (35). This may be connected to retinal pathologic features linked to inflammatory processes in kidney infection. These processes include the thickening of the retinal basement membrane, which causes DR, circulatory irregularities, and decreased vascular responsiveness (54). Furthermore, this can be a result of the different study designs and study focuses.
CVD has been identified as another risk factor for the incidence of DR among adult diabetic patients. The hazard of DR among patients with diabetes with CVD comorbidities was 2.23 times greater than that among patients with diabetes with no CVD. This finding is consistent with those of studies conducted in China (45), Japan (42), Taiwan (55), Dessie, Ethiopia (56), Southwest Ethiopia (39), in Northwest Ethiopia (35), and ArbamichGeneral Hospital, Ethiopia (40). This finding is also analogous to those of studies conducted in Hong Kong (57) and Denmark (58). This could be the result of chronic hyperglycemia in the as of hypertension activating the renin-angiotensin system, which led to an increase in the level of Angiotensin II (AII) in vitreous fluid in patients with diabetics macular edema and DR. Drastic reduction in blood flow is ultimately caused by enhanced vascular permeability and neovascularization (59). Also, this significant link between the outcome variable and hypertension might be due to the repeated clinical coexistence of hypertension and DM (57). Furthermore, hypertension itself might cause complications of DM, such as DR, through changes in the morphology of the vessel at the retina, such as hemorrhages, hard exudates, and others (60).
In this study, an expected hazard ratio of DR was 2.89 (95%CI 1.21–7.18) higher in patients with T2DM than patients with T1DM while keeping other covariates keeping constants. These findings are consistent with a study performed at Ayder Referral Hospital in Ethiopia (61) and in Addis Ababa, Ethiopia (32), which revealed that T2DM were more likely than T1DM patients to experience microvascular problems earlier in life. This difference may be attributed to the fact that aging is more prevalent among Type 2 Diabetes Mellitus (T2DM) patients compared to Type 1 Diabetes Mellitus (T1DM) patients. Furthermore, the onset of T2DM tends to decrease with age, and microvascular diabetic complications can develop over similar durations in both types (62). In contrast to our findings, studies conducted in Scotland (43) and Spain (63) reported that T1DM patients were more likely to develop complications than T2DM patients. These discrepancies could be due to differences in the study populations. For instance, the Spanish study included all participants with T1DM and T2DM who were 2 years or older at the start of the study (not at the time of diagnosis), whereas our study only included patients who were 18 years or older at diagnosis. This may have excluded a significant portion of T1DM patients, potentially influencing the results.
Even though the current study had some strengthen such there are some drawbacks of the current study such as; as small sample used and a result of the study focusing on hospital patients, it does not accurately represent the incidence of DR in the general diabetic population as undiagnosed subjects may have been excluded so that more randomized and stratified sampling method and large sample size across different healthcare facilities with extending the follow-up period with prospective study design further required. Because the study was retrospective, we missed some important diabetes complications that had a significant association with DR, such as lipid profile, self-monitoring of blood glucose practice, hemoglobin level, presence/absence of other diabetic complications, and residents, because we obtained the data through chart review.
The overall incidence of retinopathy among patients with diabetes was 14.06/100 person-years observations. For this study, the predicted median follow-up time was 57 months. CVD, proteinuria, and diabetes type were identified as predictors of DR. To reduce diabetic retinopathy the best strategy to protect our eyesight from diabetic retinopathy is to keep our diabetes under control and provide high-risk individuals with diabetes. Health professionals and relevant authorities should focus on diabetic patients who also have CVD and proteinuria as part of their efforts to reduce the risk of diabetic retinopathy. Regular monitoring, evaluation, and documentation of these factors are essential. Healthcare providers in diabetes follow-up clinics should work to enhance patients' self-care practices and overall quality of life to decrease the incidence of diabetic retinopathy. Additionally, they should ensure that all diabetic patients receive the WHO-recommended eye evaluation at least twice a year. While follow-up frequency may vary depending on blood glucose control, it is advisable for patients to undergo a general assessment during each follow-up, particularly focusing on ocular health.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval and a letter of cooperation were obtained from the Institutional Review Board of Wachemo University, College of Medicine and Health Sciences, and the hospital was informed about the study objectives through a written letter. Informed consent was waived by the Institutional Review Committee of Wachemo University. Confidentiality was maintained at all levels of the study. The data were stored on a secured password protection system. All procedures were conducted based on the regulations, guidelines and principles of the Helsinki Declaration, patients, and public involvement. Patients and the public were not involved in the design of the study, the conduct of the study or the dissemination of the findings. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of study was retrospective cohort study and used secondary data (chart review) rather than no direct contact with participants.
TY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BY: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. AA: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MJ: Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
Our heartfelt gratitude goes to Wachemo University, College of Health and Medical Sciences for support with all necessary services. Additionally, we appreciate the support from hospital administrations and data collectors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, Body Mass Index; DM, Diabetes Mellitus; DR, Diabetic Retinopathy; HMIS, Health Management and Information Systems; IQR, Interquartile Ranges; MRN, Medical Registration Number; PM, Person month; T2DM, Type two Diabetes Mellitus.
1. Davis J, Fischl AH, Beck J, Browning L, Carter A, Condon JE, et al. 2022 National standards for diabetes self-Management education and support. Diabetes Care. (2022) 45:484–94. doi: 10.2337/dc21-2396
2. International Diabetes Federation. IDF diabetes atlas (2021). Available online at: https://diabetesatlas.org/atlas/tenth-edition/https://diabetesatlas.org/atlas/tenth-edition/27 (Accessed July 22, 2023).
3. Tesfaye B, Alebel A, Gebrie A, Zegeye A, Tesema Leshargie C, Ferede A, et al. Diabetes mellitus and its association with hypertension in Ethiopia: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2019) 156:107838. doi: 10.1016/j.diabres.2019.107838
6. Nandeshwar S, Jamra V, Pal D. Indian diabetes risk score for screening of undiagnosed diabetic subjects of Bhopal city. Natl J Community Med. (2020) 1:176–7.
7. C. D. Control, Prevention. National Diabetes Statistics Report Vol. 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services (2017).
8. Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. (2000) 342:905–12. doi: 10.1056/NEJM200003303421301
9. Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clinics North America. (2014) 43:103–22. doi: 10.1016/j.ecl.2013.09.005
10. Hu G, Jousilahti P, Tuomilehto J. Joint effects of history of hypertension at baseline and type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur Heart J. (2007) 28:3059–66. doi: 10.1093/eurheartj/ehm501
11. Grossman E, Messerli FH, Goldbourt U. High blood pressure and diabetes mellitus: are all antihypertensive drugs created equal? Arch Intern Med. (2000) 160:2447–52.
12. Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. (2007) 14:179–83. doi: 10.1080/09286580701396720
14. Sowers JR. Recommendations for special populations: diabetes mellitus and the metabolic syndrome. Am J Hypertens. (2003) 16:41s–5s. doi: 10.1016/j.amjhyper.2003.07.009
15. Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. (2013) 1:e339–49. doi: 10.1016/S2214-109X(13)70113-X
16. Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. (2004) 82:844–51.
17. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
18. Mathenge W, Bastawrous A, Peto T, Leung I, Yorston D, Foster A. Prevalence and correlates of diabetic retinopathy in a population-based survey of older people in Nakuru, Kenya. Ophthalmic Epidemiol. (2014) 21:169–77. doi: 10.3109/09286586.2014.903982
19. Kyari F, Tafida A, Sivasubramaniam S, Murthy GV, Peto T, Gilbert CE. Prevalence and risk factors for diabetes and diabetic retinopathy: results from the Nigeria national blindness and visual impairment survey. BMC Public Health. (2014) 14:1299. doi: 10.1186/1471-2458-14-1299
20. AlSawahli H, Mpyet CD, Ezzelarab G, Hassanin I, Shalaby M, Safa O, et al. Population-based cross-sectional prevalence survey of diabetes and diabetic retinopathy in Sohag-Egypt, 2019. BMJ Open. (2021) 11:e047757. doi: 10.1136/bmjopen-2020-047757
21. Fite RO, Lake EA, Hanfore LK. Diabetic retinopathy in Ethiopia: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. (2019) 13:1885–91. doi: 10.1016/j.dsx.2019.04.016
22. Abebe N, Kebede T, Addise D. Review article diabetes in Ethiopia 2000–2016–prevalence and related acute and chronic complications; a systematic review. Afr J Diabetes Med. (2017) 25:7–12.
23. Lebeta KR, Argaw Z, Birhane BW. Prevalence of diabetic complications and its associated factors among diabetes mellitus patients attending diabetes mellitus clinics; institution based cross sectional study. Am J Health Res. (2017) 5:38. doi: 10.11648/j.ajhr.20170502.13
24. Ejigu T, Tsegaw A. Prevalence of diabetic retinopathy and risk factors among diabetic patients at university of Gondar tertiary eye care and training center, North-West Ethiopia. Middle East Afr J Ophthalmol. (2021) 28:71. doi: 10.4103/meajo.meajo_24_21
25. Chisha Y, Terefe W, Assefa H. Incidence and factors associated with diabetic retinopathy among diabetic patients at arbaminch general hospital, gamo gofa Zone (longitudinal follow up data analysis). J Diabetol. (2017) 8. doi: 10.4103/jod.jod_6_17
26. Garoma D, Merga H, Hiko D. Determinants of diabetic retinopathy in Southwest Ethiopia: a facility-based case-control study. BMC Public Health. (2020) 20:503. doi: 10.1186/s12889-020-08652-2
27. Mersha GA. Prevalence of diabetic retinopathy and associated risk factors among adult diabetes attending at Debre Tabor General Hospital, Northwest Ethiopia. J Diabetes Metab. (2021) 12:7.
28. Dirani M, Xie J, Fenwick E, Rees G, Wong TY, Lamoureux EL. Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci. (2011) 52:4416–21. doi: 10.1167/iovs.11-720825
29. Tilahun M, Gobena T, Dereje D, Welde M, Yideg G. Prevalence of diabetic retinopathy and its associated factors among diabetic patients at Debre Markos Referral Hospital, Northwest Ethiopia, 2019: hospital-based cross-sectional study. Diabetes Metab Syndr Obes Targets Ther. (2020) 13:2179–87. doi: 10.2147/DMSO.S260694
30. Knowler WC, Bennett PH, Ballintine EJ. Increased incidence of retinopathy in diabetics with elevated blood pressure. A six-year follow-up study in Pima Indians. N Engl J Med. (1980) 302:645–50. doi: 10.1056/NEJM198003203021201
31. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes. (2008) 57:995–1001. doi: 10.2337/db07-1618
32. Ethiopia Regions. (2023). Worldstatesmen.org. Available online at: http://www.worldstatesmen.org/Ethiopia_Regions.html (Accessed March 26, 2023).
33. Central Ethiopia, southern Ethiopia regional states established. Available online at: https://www.ena.et/web/eng/w/eng_3222547:~:text=The%20Central%20Ethiopia%20region%20constitutes,Chief%20Administrator%20of20the%20region (Accessed July 26, 2023).
34. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. (1983), 499–503. doi: 10.2307/2531021
35. Takele MB, Boneya DJ, Alemu HA, Tsegaye TB, Birhanu MY, Alemu S, et al. Retinopathy among adult diabetics and its predictors in northwest Ethiopia. J Diabetes Res. (2022) 2022:1362144. doi: 10.1155/2022/1362144
36. Magliah SF, Bardisi W, Al Attah M, Khorsheed MM. The prevalence and risk factors of diabetic retinopathy in selected primary care centers during the 3−year screening intervals. J Family Med Primary Care. (2018) 7.
37. Tilahun E, Workina A, Habtamu A, Tufa H, Abebe F, Fikadu A, et al. Survival, incidence, and predictors of diabeticneuropathy among type 2 diabetic patients in hospitals of Addis Ababa. Front Clin Diabetes Healthc. (2024) 5:1386426. doi: 10.3389/fcdhc.2024.1386426
38. Azeze TK, Sisay MM, Zeleke EG. Incidence of diabetes retinopathy and determinants of time to diabetes retinopathy among diabetes patients at Tikur Anbessa Hospital, Ethiopia: a retrospective follow up study. BMC Res notes. (2018) 11:1–6. doi: 10.1186/s13104-018-3660-7
39. Debele GR, Kanfe SG, Weldesenbet AB, Ayana GM, Jifar WW, Raru TB. Incidence of diabetic retinopathy and its predictors among newly diagnosed type 1 and type 2 diabetic patients: A retrospective follow-up study at tertiary health-care setting of Ethiopia. Diabetes Metab Syndr Obes. (2021) 14:1305–13. doi: 10.2147/DMSO.S300373
40. Chisha Y, Terefe W, Assefa H, Lakew S. Prevalence and factors associated with diabetic retinopathy among diabetic patients at Arbaminch General Hospital, Ethiopia: cross sectional study. PloS One. (2017) 12:e0171987. doi: 10.1371/journal.pone.0171987
41. Hosmer Dw LS, May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. 2nd Edition. Canada: John Wiley & Sons, Inc (2008).
42. Kawasaki R, Tanaka S, Tanaka S, Yamamoto T, Sone H, Ohashi Y, et al. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia. (2011) 54:2288–94. doi: 10.1007/s00125-011-2199-0
43. Romero-Aroca P, Navarro-Gil R, Valls-Mateu A, SagarraAlamo R, Moreno-Ribas A, Soler N. Differences in incidence of diabetic retinopathy between type 1 and 2 diabetes mellitus: A nine-year follow-up study. Br J Ophthalmol. (2017) 101:1346–51. doi: 10.1136/bjophthalmol-2016-310063
44. Thomas RL, Dunstan F, Luzio SD, Chowdury SR, Hale SL, North RV, et al. Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: Retrospective analysis. BMJ. (2012) 344:e874. doi: 10.1136/bmj.e874
45. Liu L, Wu J, Yue S, Geng J, Lian J, Teng W, et al. Incidence density and risk factors of diabetic retinopathy within type 2 diabetes: a five-year cohort study in China (Report 1). Int J Environ Res Public Health. (2015) 12:7899–909. doi: 10.3390/ijerph120707899
46. Salinero-Fort MA, San-Andres-Rebollo FJ, de-Burgos-lunar C, Arrieta-Blanco FJ, Gomez-Campelo P, Group M. Four-year incidence of diabetic retinopathy in a Spanish cohort: the MADIABETES study. PloS One. (2013) 8:e76417. doi: 10.1371/journal.pone.0076417
47. Wang SY, Andrews CA, Herman WH, Gardner TW, Stein JD. Incidence and risk factors for developing diabetic retinopathy among youths with type 1 or type 2 diabetes throughout the United States. Ophthalmology. (2017) 124:424–430. doi: 10.1016/j.ophtha.2016.10.031
48. Cikamatana L, Mitchell P, RochtChina E, Foran S, Wang JJ. Five-year incidence and progression of diabetic retinopathy in a defined older population: the Blue Mountains Eye Study. Eye. (2007) 21:465–71. doi: 10.1038/sj.eye.6702771
49. Ahmed KR, Karim MN, Bhowmik B, Habib SH, Bukht MS, et al. Incidence of diabetic retinopathy in Bangladesh: A 15-year follow-up study. J Diabetes. (2012) 4:386–91. doi: 10.1111/j.1753-0407.2012.00208.x
50. Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care. (2012) 35:592–6. doi: 10.2337/dc11-0943
51. Bastawrous A, Mathenge W, Wing K, Bastawrous M, Rono H, Weiss HA, et al. The incidence of diabetes mellitus and diabetic retinopathy in a population-based cohort study of people age 50 years and over in Nakuru, Kenya. BMC Endocr Disord. (2017) 17:19. doi: 10.1186/s12902-017-0170-x
52. Thomas RL, Distiller L, Luzio S, Melville V, Chowdhury SR, Kramer B, et al. Incidence and progression of diabetic retinopathy within a private diabetes mellitus clinic in South Africa. J Endocrinology Metab Diabetes South Afr. (2015) 20:127–33. doi: 10.1080/16089677.2015.1090159
53. Janghorbani M, Amini M, Ghanbari H, Safaiee H. Incidence of and risk factors for diabetic retinopathy in Isfahan, Iran. Ophthalmic Epidemiol. (2003) 10:81–95. doi: 10.1076/opep.10.2.81.13893
54. Grunwald JE, Alexander J, Ying G-S, Maguire M, Daniel E, Xie D, et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch Ophthalmol. (2012) 130:1136–44. doi: 10.1001/archophthalmol.2012.1800
55. Tung T-H, Chen S-J, Liu J-H, Lee F-L, Li A-F, Shyong MP, et al. A community-based follow-up study on diabetic retinopathy among type 2 diabetics in Kinmen. Eur J Epidemiol. (2005) 20:317–323. doi: 10.1007/s10654-004-6651-z
56. Seid MA, Akalu Y, Gela YY, Belsti Y, Diress M, Fekadu SA, et al. Microvascular complications and its predictors among type 2 diabetes mellitus patients at Dessie town hospitals, Ethiopia. Diabetol Metab Syndrome. (2021) 13:86. doi: 10.1186/s13098-021-00704-w
57. Wat N, Wong RLM, Wong IYH. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med J. (2016). doi: 10.12809/hkmj164869
58. Broe R, Rasmussen ML, Frydkjaer-Olsen U, Olsen BS, Mortensen HB, Peto T, et al. The 16-year incidence, progression and regression of diabetic retinopathy in a young population-based Danish cohort with type 1 diabetes mellitus: the Danish cohort of pediatric diabetes 1987 (DCPD1987). Acta Diabetol. (2014) 51:413–20. doi: 10.1007/s00592-013-0527-1
59. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. (1998) 317:703–13.
60. Tomić M, Ljubić S, Kaštelan S, Gverović Antunica A, Jazbec A, Poljičanin T. Inflammation, haemostatic disturbance, and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators Inflamm. (2013) 2013:1–10. doi: 10.1155/2013/818671
61. Berihun L, Muluneh EK. Correlates of time to microvascular complications among diabetes mellitus patients using parametric and non-parametric approaches: a case study of Ayder referral hospital Ethiopia. (2017) 10:65–80. doi: 10.4314/ejst.v10i1.5
62. Koopman RJ, Mainous AG, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Family Med. (2005) 3:60–3. doi: 10.1370/afm.214
Keywords: diabetic retinopathy, predictors, incidence, diabetes mellitus, Ethiopia
Citation: Yakob T, Abraham A, Yakob B and Jaldo MM (2025) Incidence of diabetic retinopathy and its predictors among adult patients with diabetes in Ethiopia: a frailty model. Front. Endocrinol. 16:1462210. doi: 10.3389/fendo.2025.1462210
Received: 09 July 2024; Accepted: 27 January 2025;
Published: 14 March 2025.
Edited by:
Ramkumar Kunka Mohanram, SRM Institute of Science and Technology, IndiaReviewed by:
Weihua Yang, Southern Medical University, ChinaCopyright © 2025 Yakob, Abraham, Yakob and Jaldo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tagese Yakob, eWl0YWdlc3VqQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.