
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 January 2025
Sec. Cardiovascular Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1447053
This article is part of the Research Topic Screening Remnant Lipid Markers in Cardiometabolic Diseases View all 9 articles
Background: Currently, the clinical evidence regarding the prognostic significance of the TyG index in acute myocardial infarction (AMI) patients remains unclear. Our research analyzed the correlation between the TyG index and the risk of mortality in patients with AMI, in order to evaluate the influence of the TyG index on the prognosis of this population.
Methods: 1205 ICU patients with AMI were analyzed in this retrospective cohort analysis, and the necessary data were obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. The study conducted Kaplan-Meier analysis to compare all-cause mortality rates across four groups of patients. The study included logistic regression and Cox regression analysis to examine the correlation among the TyG index and the risk of in-hospital, 28-day, and 90-day mortality.
Results: In our study, 176 (14.61%) patients experienced in-hospital deaths, 198 (16.43%) patients died within 28 days of follow-up, and 189 (23.98%) patients died within 90 days of follow-up. Logistic regression and Cox proportional hazard analyses revealed that the TyG index was an independent predictor of in-hospital, 28-day, and 90-day mortality (OR: 1.406, 95% CI 1.141-1.731, p = 0.001; HR: 1.364, 95% CI 1.118-1.665, p = 0.002; HR: 1.221, 95% CI 1.024-1.445, p = 0.026, respectively). The restricted cubic spline regression model showed that the risk of in-hospital, 28-day, and 90-day mortality increased linearly with increasing TyG index.
Conclusions: The TyG index was significantly associated with an increased risk of mortality in AMI patients. Our findings suggested that the TyG index may be instrumental in identifying patients at high risk for adverse outcomes following AMI.
Despite significant advancements in the diagnosis and management of acute myocardial infarction (AMI), it is still the major cause of death on a global scale (1, 2). Although patients with AMI get treatment involving coronary revascularization, dual antiplatelet therapy, and aggressive lipid-lowering, some individuals, especially those with type 2 diabetic mellitus (T2DM), remain a significant likelihood of experiencing recurrent cardiovascular events (3). Moreover, AMI also imposes a substantial economic burden, particularly in nations with lower and moderate incomes (4). Coronary artery disease (CAD) significantly contributes to illness and death in diabetes (DM) patients, with diabetes-related cardiovascular disease expenditure estimated at $37.3 billion annually (5).
Insulin resistance (IR) is a defining characteristic of metabolic syndrome (MetS) (6), it is a major pathogenic mechanism of DM and a contributing factor to the progression of macrovascular complications in patients, and many studies have demonstrated the role of IR and disorders of glucolipid metabolism in the progression of CAD (7, 8). Methods for the assessment of IR include hypoglycaemic-hyperinsulinaemic clamp test, homeostatic model assessment of insulin resistance (HOMA-IR) insulin resistance index, but these methods are costly (9). In addition, researchers have found that TyG is measured by measuring fasting blood glucose (FBG) and triglycerides, correlates with the above methods in clinical practice and can be utilized as a specific and reliable predictor of IR (10).
IR predisposes individuals to metabolic disorders, including hyperglycemia and dyslipidemia, which are strongly associated with a poor prognosis in CAD. Some studies shown that TyG levels are valuable for prognostic prediction in CAD patients (11–13). Ryo et al. (14) found the higher the index, the worse the prognosis. As the TyG index increases, the danger about cardiovascular mortality or rehospitalization also increases (15). Nevertheless, there is currently insufficient clinical evidence to determine the validity of the TyG index in AMI patients. Our study explored the association between the TyG index and the prognosis of AMI patients admitted to the intensive care unit (ICU), to provide more meaningful information for clinical practice.
Our study is a retrospective observational analysis that uses data from the publicly accessible Medical Information Mart for Intensive Care IV (MIMIC-IV) database (https://mimic.mit.edu). The MIMIC-IV researches stem from an organization between Beth Israel Deaconess Medical Centre (BIDMC) and the Massachusetts Institute of Technology (MIT). Data collected by Beth Israel Deaconess Medical Centre can be identified and transformed by researchers who, of course, have completed human research training and signed data use agreements. Meanwhile, the Beth Israel Deaconess Medical Centre Institutional Review Board has approved the waiver of informed consent and the sharing of research resources (16). In compliance with the applicable requirements, the author acquired a Collaborative Institutional Training Initiative (CITI) license and the requisite authorizations to make use of the MIMIC-IV database.
Our study encompassed a total of 15,274 individuals who suffered from AMI and were hospitalized to the intensive care unit. All participants were 18 years of age or older in the research. Figure 1 displays the flow chart. We exclusively analyzed the initial admission for patients with multiple admissions. In order ensure the precision and accuracy of the data, individuals who did not have AMI data available within 48 hours of being admitted to the ICU, had inadequate data on their glucose and triglyceride (TG) levels, or lacked follow-up data were excluded in our study. Ultimately, we established a final cohort of 1,205 patients, these patients were divided into four groups (Q1, Q2, Q3, Q4) based on the TyG index quartiles, and group Q1 was used as the reference group.
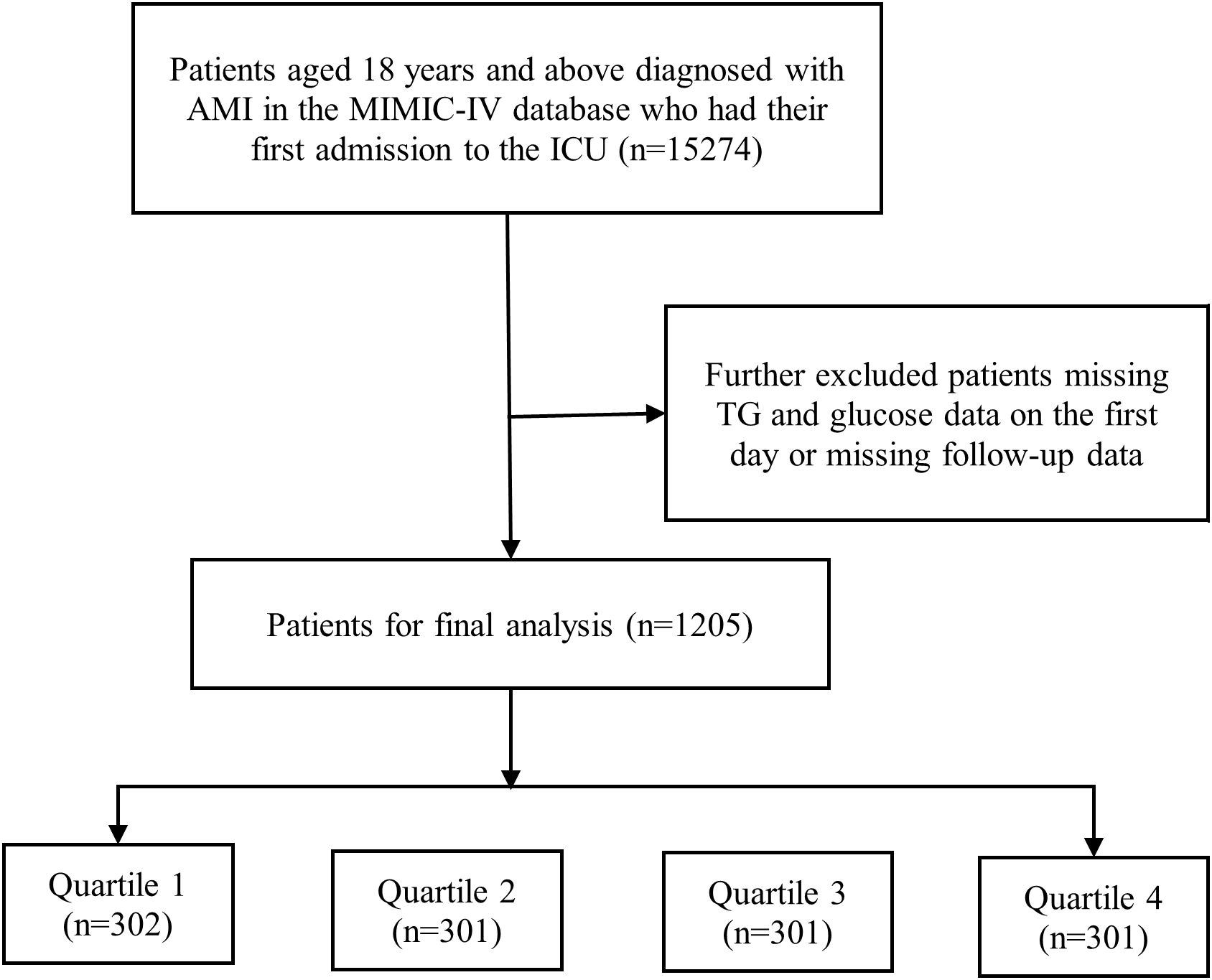
Figure 1. Flow chart of the study participants. AMI, acute myocardial infarction; MIMIC, Medical Information Mart for Intensive Care; TG, triglyceride.
The baseline features of patients were extracted by collecting data using Structured Query Language (SQL) with PostgreSQL (version 16.2). The data included patient demographics (age, gender, body mass index (BMI)), Laboratory tests (white blood cell (WBC), platelet (PLT), red blood cell (RBC), total cholesterol (TC), TG, glucose, triglyceride glucose (TyG), partial thromboplastin time (PTT), prothrombin time (PT), international normalized ratio (INR), serum creatinine (Scr)), comorbidities (anemia, atrial fibrillation (AF), CAD, DM, heart failure, hyperglycemia, hypertension, transient ischemic attack (TIA), peripheral vascular disease (PVD), cerebrovascular disease, dementia, chronic pulmonary disease, renal disease), medication (statin, aspirin, clopidogrel), continuous renal replacement therapy (CRRT) and ventilation. The study’s follow-up period begins on the day of admission and ended upon the relevant endpoints occur.
To reduce potential bias, we excluded variables with missing values greater than 20%. Meanwhile, we carefully used multiple interpolation techniques to fill in the missing values for variables with less than 20% missing values, adopting a robust approach that maintains data consistency and reliability.
In our study, the primary outcome was in-hospital mortality, and secondary outcomes focused on 28-day and 90-day mortality.
We used equation “ln [TG (mg/dl) × FBG (mg/dl)/2]” to calculate the TyG index.
For continuous variables, we used t-tests or analyses of variance (ANOVA) for statistical analyses and reported them as mean ± standard deviation. For categorical variables, analyses were performed using chi-square tests or corrected chi-square tests and expressed as numbers (proportions).
Logistic regression and Cox regression analyses were used to investigate the correlation between the TyG index and the risk of hospitalization, 28-day and 90-day mortality. In Model 1, we only included the TyG index without any additional adjustments. In Model 2, we modified the model to include gender, age, and BMI. Model 3 was adjusted for age, gender, BMI, RBC, WBC, PT, PTT, anemia, AF, CAD, heart failure, statin use, aspirin use, clopidogrel use, ventilation, TG, TC, and INR. This adjustment considered feature selection results and clinical experience.
We employed Kaplan-Meier survival analysis to assess the occurrence of outcome events in separate stratified groups, categorized by the TyG index. We next examined any disparities detected using the log-rank test. In addition, we analyzed potential non-linear relationships between the TyG index and in-hospital mortality, 28-day mortality and 90-day mortality using 3-node multivariate restricted cubic spline (RCS) regression. Subgroup analyses were performed, taking into account gender, age, BMI, heart failure, atrial fibrillation, DM, and hypertension. Subsequently, the p-value for interaction was assessed.
For our statistical analyses, we used SPSS (version 25.0, IBM), STATA (version 17.0), and R (version 4.1.3, Austria) to perform statistical analyses. Statistical significance was defined as a two-sided P value of less than 0.05.
In our retrospective analysis, we examined 1,205 patients diagnosed with AMI using strict criteria for inclusion and exclusion. Their average age was 66.95 ± 13.90 years and 791 (65.64%) were female. Additionally, our study revealed 176 (14.61%) in-hospital deaths, 198 (16.43%) deaths within 28 days of follow-up, and 189 (23.98%) deaths within 90 days of follow-up.
In Table 1, we analyzed the baseline characteristics of AMI patients by the TyG index quartiles. Our findings indicate that patients who have higher TyG indices typically exhibit characteristics such as younger age, greater BMI, and raised levels of WBC, TC, TG, and glucose compared to patients with lower TyG index. In individuals with an elevated TyG index, the risk of developing DM is significantly higher. Furthermore, our analysis showed patients with a higher TyG index were more likely to take aspirin and clopidogrel but less likely to receive statin. Moreover, patients with relatively high TyG had increased mortality relative to those with lower TyG.
Through using multivariate logistics and COX regression analysis, it was determined that the TyG index is independently associated with an increased risk of in-hospital mortality (OR 1.406; 95% CI 1.141-1.731; P = 0.001), 28-day mortality (HR 1.364; 95% CI 1.118-1.665; P = 0.002), and 90-day mortality (HR 1.221; 95% CI 1.024-1.455; P = 0.026). The validity of these findings was additionally verified in both adjusted models 2 and 3. The OR for in-hospital mortality in the highest quartile of the TyG index was 3.091, with a 95% CI of 1.631-5.867. The HR for 28-day mortality was 3.359, with a 95% CI of 1.811-6.230. The HR for 90-day mortality was 2.431, with a 95% CI of 1.409-4.194. These results were obtained using the lowest quartile as a reference (Table 2).
Kaplan-Meier survival analyses were used to assess the 28-day and 90-day mortality across TyG quartile groups. We observed statistical differences in the 28- and 90-day mortality rates in the quartile groups (log-rank p = 0.0060 and p = 0.0488, respectively) (Figure 2).
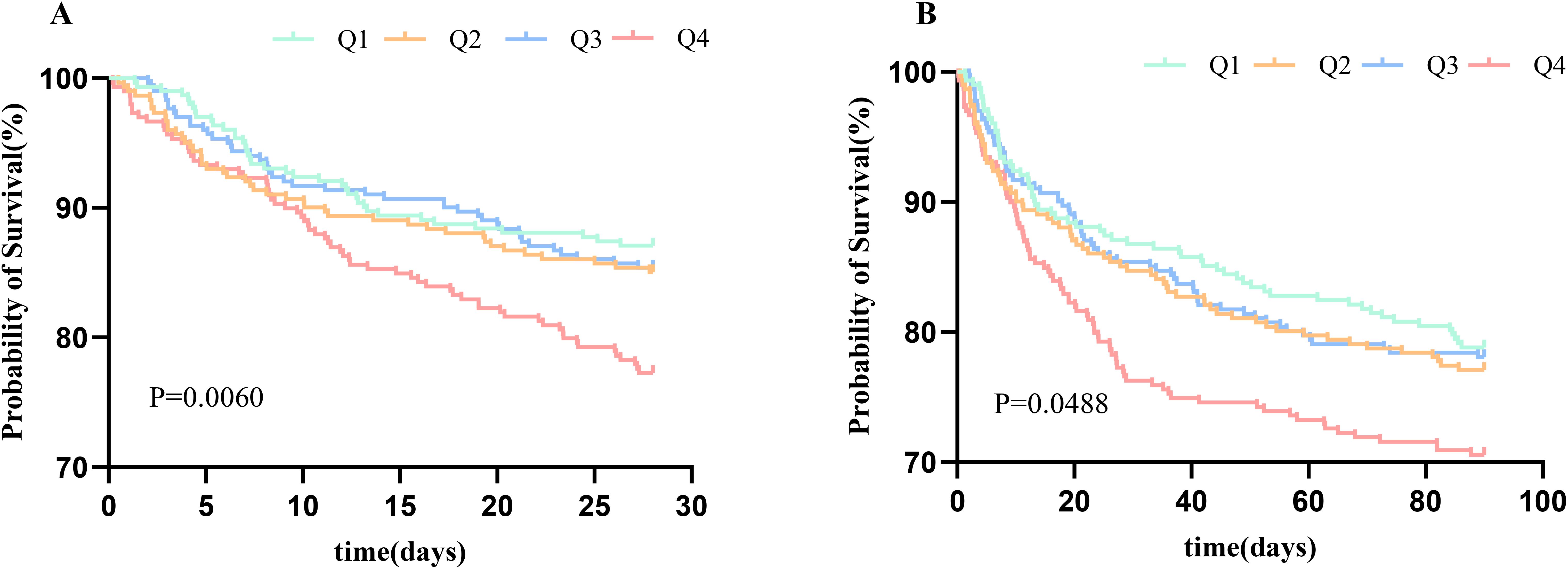
Figure 2. Kaplan–Meier survival analysis curve for 28-day mortality (A) and 90-day mortality (B). TyG, triglyceride glucose index.
Figure 3 displays the RCS analysis results following the adjustment for age, gender, and BMI. We found that an elevated TyG index was linearly associated with higher in-hospital, 28-day, and 90-day mortality risk (P for nonlinear = 0.3256, 0.8293, and 0.4883, respectively).
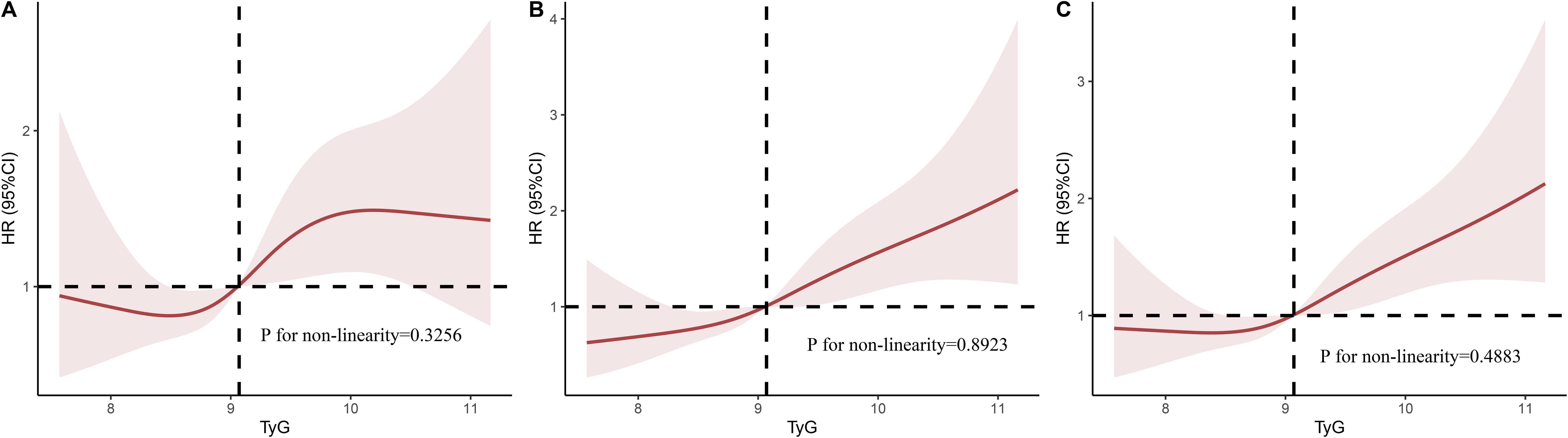
Figure 3. Multivariable RCS regression showed the nonlinear association between the TyG index and in-hospital mortality (A), 28-day mortality (B), and 90-day mortality (C) after adjusted by Age, Gender, BMI. TyG, triglyceride glucose; RCS, restricted cubic spline; OR, odds ratio; HR, hazard ratio.
We conducted stratified analyses based on gender, age, BMI, heart failure, atrial fibrillation, DM, and hypertension to confirm the link between TyG index and mortality. In Figure 4, our study demonstrated no significant interactions between baseline TyG index and stratification variables across subgroups (all p values for interaction > 0.05).
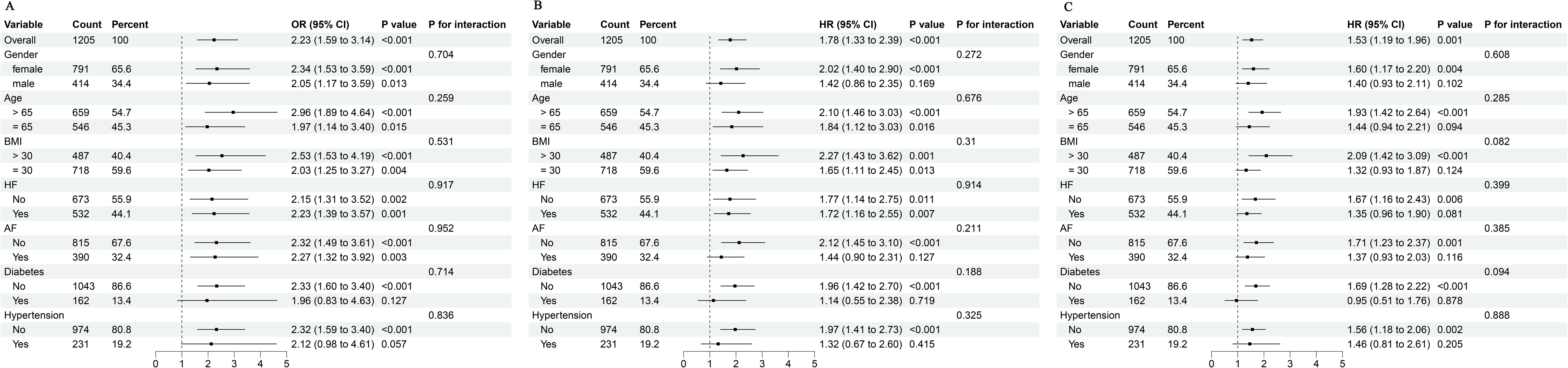
Figure 4. Subgroup analyses for the association of TyG index with in-hospital mortality (A), 28-day mortality (B), and 90-day mortality (C). BMI, body mass index; HF, heart failure; AF, atrial fibrillation; TyG, triglyceride glucose; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
We studied the correlation between the TyG index and mortality in AMI patients. And the results of our research showed an important association between the TyG index and mortality in AMI patients. Particularly, this correlation persists even when adjusted for potential factors that might affect the results. Furthermore, the Kaplan-Meier analyses demonstrated that individuals with elevated TyG indices experienced worse clinical outcomes.
IR is strongly related to several disorders involving the metabolism of glycolipids, including hyperlipidemia, dyslipidemia and hypertension, mitral annular calcification, and cardiovascular prognosis (11, 12, 17). Prior researches demonstrated hypertension is the separate factor which may forecast platelet-dependent thrombosis. It worsens platelet-dependent thrombosis, raises the levels of adhesion molecules and leukocyte occlusion in capillaries, diminishes vasodilation, and hampers the availability of nitric oxide, which is crucial in preventing the formation of microthrombi after AMI (18, 19). Dysregulated insulin signaling impairs available nitric oxide, leading to vascular sclerosis (12). Additionally, IR has a significant impact on CVD through two distinct mechanisms: (1) atheromatous plaque formation. (2) ventricular hypertrophy and diastolic abnormalities (20). Both of these effects contribute to heart failure, impacting the prognosis of patients with CVD. Furthermore, Eddy et al. (21) found that IR may be the primary factor contributing to CAD. In addition, it has been demonstrated that IR is connected to damage in the arterial wall, including reduced vasodilatory function, heightened stiffness of arteries, and elevated coronary artery calcification, which have been identified as contributors to the development of future cardiovascular disease (22). Therefore, alleviation of IR or hyperinsulinemia is expected to be effective in reducing the likelihood of unfavorable outcomes in individuals with AMI. Of course, we emphasize that studies at the cellular and animal levels, as well as clinical trials which include large sample sizes and long-term follow-up are still needed to support this view. Further knowledge of the pathogenesis of IR and how it can be prevented or cured will have a profound impact on AMI.
The TyG index, compared to triglycerides, plasma atherogenicity index, and TG to HDL cholesterol ratio, is more effective in risk detection for cardiovascular events (23). Jin et al. suggest the TyG index might have superior predictive significance compared to the hemoglobin glycation index (HGI) in patients with stable CAD and DM (11). More importantly, the TyG index identifies IR reliably, not only due to the cost-effectiveness of serum TG and glucose level testing but also because it is fast and efficient (24).
Furthermore, the TyG index enables the timely identification of individuals with a heightened susceptibility toDM. After analyzing 2330 patients, Alessandra et al. revealed TyG index was positively correlated with the incidence in symptomatic CAD (25). A study conducted on 438 NSTE-ACS patients demonstrated a strong correlation between the TyG index was associated with the SYNTAX score and was an independent predictor for evaluating CAD severity (26). Hu et al. (27) discovered that patients with an rised TyG index exhibited a markedly increased susceptibility to cardiovascular events, regardless of whether they had DM or not. In CAD patients that received percutaneous coronary intervention (PCI), the TyG index can be a more reliable indicator than FBG or glycated hemoglobin. Additionally, Zhao et al. (28) suggested that the TyG index could predict a poor prognosis in AMI patients who have well-managed levels of LDL-C after PCI. Liu et al. (29) discovered that increased levels of the TyG index were suggestive of worsening IR and were non-linearly linked to all-cause and cardiovascular mortality. Within a study that included 2,830 participants, Zhao et al. (30) discovered a notable correlation between a higher TyG index and arterial stiffness and microvascular damage. In addition, a study of 888 participants with DM but without any prior cardiovascular disease (CVD) revealed that a higher TyG index was linked to an increased likelihood of CAD (31). However, there is an insufficient amount of current information about the correlation between the TyG index and AMI individuals. Our finding that TyG index is an independent factor for all-cause death in AMI patients adds to the existing body of research on the relationship between the TyG index and negative outcomes. Therefore, we appeal to clinicians to pay attention to patients’ glycemic management in IR indicators.
Our study demonstrates that the higher TyG index, the greater risk of negative outcomes in AMI patients, showing that the TyG index can be a beneficial indicator for assessing risk and guiding the clinical care of these patients. Liao et al. observed that the probability of death during hospitalization increased by 1.19 times for each 1-unit rise in the TyG index (32). This is in accordance with our findings. Our study found that when adjusted for confounders, each 1-unit rise in the TyG index was associated with a 1.019-fold rise in the probability of death within 28 days of follow-up. Therefore, clinical practitioners ought to actively manage risk factors associated with cardiovascular disease, including control of lipids and fasting blood glucose. Adopting systematic surveillance and intervention in individuals with high TyG index can significantly decrease the occurrence of negative outcomes in AMI patients.
Nevertheless, our study has limits. Firstly, our study was retrospective and observational, so the findings may indicate correlation more than causation. Therefore, future studies with multicenter prospective studies should be conducted. Secondly, due to the limitations of the MIMIC database, our study could not compare the TyG index with other IR measurement techniques and there was no way to confirm that all blood glucose and lipids were fasting results. Finally, the data was collected only from the United States, so our findings may not be fully applicable to ICU in other countries.
Our findings provide further clinical evidence that the TyG index is valuable in the early identification of AMI patients with poor prognosis, thus helping to guide the clinical management of such patients. We also emphasize the importance of incorporating the TyG index into the daily routine of healthcare workers. Of course, further prospective studies are still needed to confirm our findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board at the BIDMC. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
XS: Investigation, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. YZ: Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Software. JC: Data curation, Investigation, Resources, Writing – review & editing, Writing – original draft. XZ: Investigation, Methodology, Writing – review & editing, Writing – original draft. HL: Investigation, Supervision, Writing – review & editing, Writing – original draft. HS: Resources, Supervision, Writing – review & editing, Writing – original draft, Visualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) for providing data and technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, et al. Management of acute coronary syndrome in the older adult population: A scientific statement from the american heart association. Circulation. (2023) 147. doi: 10.1161/CIR.0000000000001112
2. Bergmark BA, Mathenge N, Merlini PA, Lawrence-Wright MB, Giugliano RP. Acute coronary syndromes. Lancet. (2022) 399:1347–58. doi: 10.1016/S0140-6736(21)02391-6
3. Bjarnason TA, Hafthorsson SO, Kristinsdottir LB, Oskarsdottir ES, Johnsen A, Andersen K. The prognostic effect of known and newly detected type 2 diabetes in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. (2020) 9:608–15. doi: 10.1177/2048872619849925
4. Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. (2015) 386:2145–91. doi: 10.1016/S0140-6736(15)61340-X
5. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes—2023. Diabetes Care. (2023) 46(4):S158–90. doi: 10.2337/dc23-S010
6. Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. (2002) 25(7):1177–84. doi: 10.2337/diacare.25.7.1177
7. Zhang Y, Liu C, Xu Y, Wang Y, Dai F, Hu H, et al. The management correlation between metabolic index, cardiovascular health, and diabetes combined with cardiovascular disease. Front Endocrinol. (2023) 13:1036146. doi: 10.3389/fendo.2022.1036146
8. Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated tyG index predicts progression of coronary artery calcification. Diabetes Care. (2019) 42(8):1569–73. doi: 10.2337/dc18-1920
9. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. (2008) 294(1):E15–26. doi: 10.1152/ajpendo.00645.2007
10. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
11. Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu CG, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med. (2018) 50(7):576–86. doi: 10.1080/07853890.2018.1523549
12. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
13. Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. (2020) 19(1):80. doi: 10.1186/s12933-020-01054-z
14. Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. (2023) 22(1):170. doi: 10.1186/s12933-023-01906-4
15. Hao Q, Yuanyuan Z, Lijuan C. The prognostic value of the triglyceride glucose index in patients with acute myocardial infarction. J Cardiovasc Pharmacol Ther. (2023) 28:10742484231181846. doi: 10.1177/10742484231181846
16. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10(1):1. doi: 10.1038/s41597-022-01899-x
17. Aydınyılmaz F, Özbeyaz NB, Guliyev İ, Algül E, Şahan HF, Kalkan K. Effect of atherogenic index of plasma on pre-percutaneous coronary intervention thrombolysis in myocardial infarction flow in patients with ST elevation myocardial infarction. Angiology. (2024) 75(9):841–8. doi: 10.1177/00033197231185204
18. Wu Y, Zhou L, Yao M, Zhu Y, Ni J, Cui L, et al. Elevated fasting blood glucose is predictive of the severity and poor outcome in nondiabetic patients with cerebral venous thrombosis. J Neurol Sci. (2020) 417:117017. doi: 10.1016/j.jns.2020.117017
19. Pepe M, Zanna D, Cafaro A, Marchese A, Addabbo F, Navarese EP, et al. Role of plasma glucose level on myocardial perfusion in ST-segment elevation myocardial infarction patients. J Diabetes Complications. (2018) 32(8):764–9. doi: 10.1016/j.jdiacomp.2018.05.015
20. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
21. Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: A mathematical analysis. Diabetes Care. (2009) 32(2):361–6. doi: 10.2337/dc08-0854
22. Adeva-Andany MM, Ameneiros-Rodríguez E, Fernández-Fernández C, Domínguez-Montero A, Funcasta-Calderón R. Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes. (2019) 10(2):63–77. doi: 10.4239/wjd.v10.i2.63
23. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. (2016) 46(2):189–97. doi: 10.1111/eci.2016.46.issue-2
24. Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. (2019) 9(1):7320. doi: 10.1038/s41598-019-43776-5
25. da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. (2019) 18(1):89. doi: 10.1186/s12933-019-0893-2
26. Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. (2019) 2019:6891537. doi: 10.1155/2019/6891537
27. Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. (2020) 19(1):116. doi: 10.1186/s12933-020-01091-8
28. Zhao HW, Wang Y, Wang CF, Meng QK. Association between triglyceride glucose index and adverse clinical outcomes in patients with acute myocardial infarction and LDL-C ≤ 1.8 mmol/L who underwent percutaneous coronary intervention: a prospective cohort study. Front Endocrinol. (2024) 14:1323615. doi: 10.3389/fendo.2023.1323615
29. Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. (2021) 7:628109. doi: 10.3389/fcvm.2020.628109
30. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. (2019) 18(1):95. doi: 10.1186/s12933-019-0898-x
31. Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. (2016) 15(1):155. doi: 10.1186/s12944-016-0324-2
Keywords: triglyceride-glucose index, acute myocardial infarction, insulin resistance, all-cause mortality, MIMIC-IV database
Citation: Su X, Zhou Y, Chang J, Zhao X, Li H and Sang H (2025) Association between triglyceride-glucose index and all-cause mortality in critically ill patients with acute myocardial infarction: analysis of the MIMIC-IV database. Front. Endocrinol. 16:1447053. doi: 10.3389/fendo.2025.1447053
Received: 11 June 2024; Accepted: 03 January 2025;
Published: 22 January 2025.
Edited by:
Zhiyuan Wu, Harvard University, United StatesReviewed by:
Manoj Kumar Mahata, Belle Vue Clinic, IndiaCopyright © 2025 Su, Zhou, Chang, Zhao, Li and Sang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiqiang Sang, MTUyMzc4MzA2MzZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡ These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.