
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 10 March 2025
Sec. Thyroid Endocrinology
Volume 16 - 2025 | https://doi.org/10.3389/fendo.2025.1424248
This article is part of the Research Topic Primary and secondary hyperparathyroidism: from etiology to treatment View all 7 articles
 Shuiping Li1†
Shuiping Li1† Jincheng Qiu1†
Jincheng Qiu1† Xiaoguang Zhang2
Xiaoguang Zhang2 Fuzhen Wang3
Fuzhen Wang3 Xianrong Yang4
Xianrong Yang4 Xiaoyan Chen5
Xiaoyan Chen5 Xiaofang Guo6
Xiaofang Guo6 Zuolin Li1
Zuolin Li1 Min Lin1
Min Lin1 Xiaolian Li1
Xiaolian Li1 Jinghua He1
Jinghua He1 Guorong Lyu7*
Guorong Lyu7* Jiantang Zhang1*
Jiantang Zhang1*Objective: This study compared the efficacy of microwave ablation (MWA) and parathyroidectomy (PTX) in the treatment of secondary hyperparathyroidism (SHPT) and evaluated the improvement of bone metabolic markers (BMMs) and bone mineral density (BMD).
Materials and methods: Eligible patients with SHPT treated between January 2019 and August 2022 were enrolled in the study and were divided into two groups: MWA and PTX. Outcome measures included the treatment success rate, percentage of patients whose intact parathyroid hormone (iPTH) concentration was within the target range, serum calcium (Ca), phosphorus (P), alkaline phosphatase (ALP), osteocalcin (OC), C-terminal cross-linked telopeptide of type I collagen (β-CXT), and BMD. Data on the procedure time, intraoperative blood loss volume, length and cost of hospitalization, incidence of postoperative complications, and recurrence rates were analyzed.
Results: A total of 107 patients with SHPT—48 in the MWA group and 59 in the PTX group— were included in the study. There were no significant differences in baseline data between the two groups (p>0.05). At the final follow-up, both therapies decreased iPTH, Ca, P, ALP, OC, and β-CXT levels and increased BMD (p<0.05). Nonetheless, the decrease in iPTH, ALP, OC, and β-CXT was more pronounced 6 and 12 months after PTX (p<0.05). The percentage of patients whose iPTH level was within the target range was significantly higher in the MWA group (p<0.05). The incidence of severe hypocalcemia was significantly lower in the MWA group (p<0.05).
Conclusion: MWA can improve BMMs and BMD, and is a minimally invasive approach with great potential for treating patients with SHPT who cannot tolerate PTX.
Secondary hyperparathyroidism (SHPT) is a common complication of chronic renal insufficiency. Patients with SHPT generally present with elevated serum intact parathyroid hormone (iPTH) levels and disturbances in serum calcium (Ca) and phosphorus (P) metabolism. Nonetheless, serum calcium levels are usually normal or sometimes low in secondary hyperparathyroidism, distinguishing SHPT from primary and tertiary hyperparathyroidism (1, 2). These dysfunctions lead to increased bone turnover, decreased bone mineral density (BMD), renal osteodystrophy, and vascular calcification, increasing the risk of fractures and cardiovascular mortality (3, 4).
Early-stage SHPT can be treated with oral active vitamin D, intravenous vitamin D analogs, calcimimetics, dialysis (5–7). Parathyroidectomy (PTX) is effective in patients with SHPT who do not respond to drug treatments (8–11). However, some patients with SHPT do not tolerate general anesthesia because of severe organ dysfunction, and a minimally invasive percutaneous approach is required in these cases. The efficacy of thermal ablation and PTX for treating SHPT is similar (12–15). Nonetheless, the former is performed under local anesthesia, with short operation time, high safety, and faster postoperative recovery (16–18). Moreover, thermal ablation is associated with a lower incidence of permanent parathyroid hypothyroidism and hypocalcemia compared with PTX (12–14).
Bone metabolic markers (BMMs), including biochemical markers, bone metabolism regulators, and bone turnover markers (BTMs), can assess bone metabolic status non-invasively (19, 20). BMD reflects the status of bone tissue and predicts the risk of fractures (21). PTX can improve BMMs and BMD in patients with SHPT (22–25). Nonetheless, the effects of thermal ablation on bone metabolism in these patients are limited to iPTH and Ca and P levels, and no systematic study has investigated changes in BMMs and BMD following thermal ablation. This study compared the effects of PTX and microwave ablation (MWA) on BMMs and BMD in patients with SHPT.
Patients with SHPT admitted from two centers between January 2019 and August 2022 were included in the study and were divided into two groups—PTX and MWA—based on patient preferences. All patients received regular dialysis (dialysis modes included hemodialysis and peritoneal dialysis), and the dialysate calcium concentration was 1.25–1.75 mmol/L. After treatment, all patients received oral calcium carbonate 1.8 g/d, calcitriol 2.0 µg/d, and 2 mg/kg/h calcium gluconate intravenous injection, and calcium dosage was adjusted according to serum calcium concentration. All patients underwent parathyroid ultrasound and 99mTc-sestamibi imaging. This retrospective study was approved by the ethical review committees of both institutions ([2021] Ethics Committee Approval for Scientific Research No. 016 and 2021001020).
The inclusion criteria were (a) patients with severe SHPT (SHPT not controlled by dialysis and medications), (b) age 18–85 years, (c) iPTH >600 pg/mL, and (d) ultrasonography revealing one or more parathyroid hyperplasia. The exclusion criteria were (a) patients who had previously undergone single excision or thermal ablation of parathyroid adenomas; (b) patients with obesity (body mass index ≥25 kg/m2), diabetes, or long-term use of glucocorticoids and (c) patients with ectopic parathyroid gland on 99mTc-sestamibi imaging.
Ultrasound-guided MWA of parathyroid nodules was performed at each center by an experienced ultrasound interventionist using an ultrasound scanner (Acuson Sequoia; Siemens Healthineers, Erlangen, Germany) and a 4–10 MHz high-frequency linear probe. Contrast-enhanced ultrasound (CEUS) with SonoVue (Bracco, Milan, Italy) was performed before ablation to determine parathyroid blood supply (Figures 1A, B). The patient was placed in a supine position, and a 22-G puncture needle was used to inject saline around the parathyroid nodules for hydrodissection until the nodules were separated from the surrounding tissue by >5 mm (Figure 1C). A 2.0-mm probe with a 5.0-mm tip (KY-2000; Nanjing Kangyou, Nanjing, China) was inserted in each nodule. Ablation power varied between 25 W and 30 W depending on nodule size. Ablation was performed until the nodules were completely hyperechoic on ultrasonography (Figure 1D). Heart rate and blood pressure were closely monitored during ablation. Ablation was stopped immediately if hoarseness or dysphonia occurred. Hematoma and hypoxia were also monitored, and CEUS was performed following ablation of all target nodules (Figures 1E, F). Additional ablation was performed immediately in lesions with residual contrast enhancement.
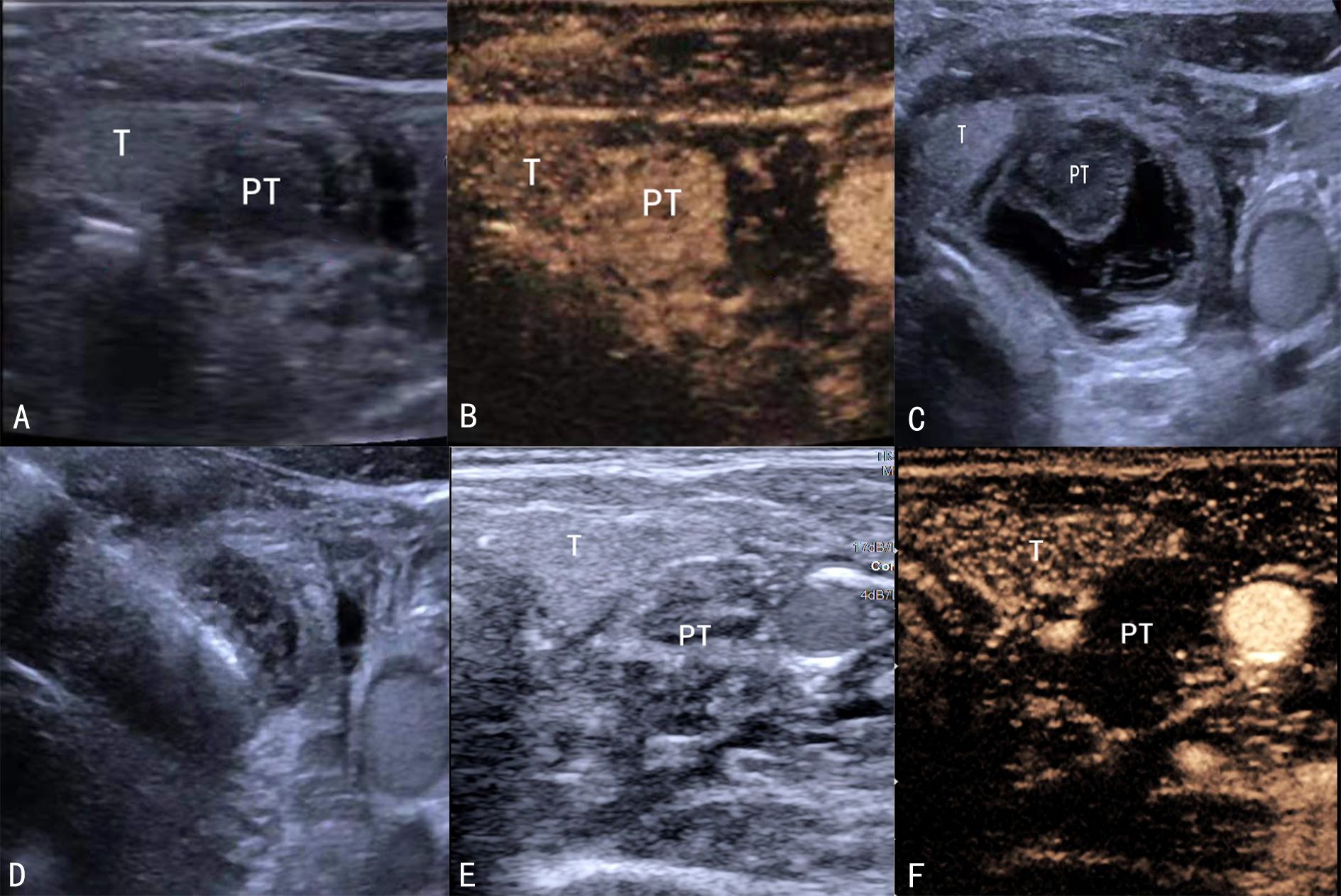
Figure 1. Ultrasound-guided MWA. (A) B-scan before ablation showing a hypoechoic parathyroid nodule. (B) CEUS showing enhanced parathyroid gland nodules. (C) Hydrodissection procedure. (D) The probe is inserted in the parathyroid nodule. (E) B-scan showing the ablation zone. (F) CEUS showing a non-enhancement zone covering the parathyroid nodule. MWA, microwave ablation; CEUS, contrast-enhanced ultrasound.
PTX includes subtotal PTX (sPTX), total PTX combined with autotransplantation (tPTX+AT), and total PTX (tPTX). All our patients underwent tPTX+AT, which was performed by the same experienced surgeon. After general anesthesia, the patient was placed in a supine position. The skin was incised, the subcutaneous tissue and platysma muscle were incised layer by layer, the flap was separated along the inferior platysma fascia, and the white line was cut at the median cervical line. The thyroid was freed, and all parathyroid glands were removed. Approximately 30–60 mg of parathyroid tissue was removed for use. After sufficient hemostasis, the incision was sutured layer by layer. Parathyroid AT was performed to cut three to four fragments of approximately 1 mm3 from the spare parathyroid tissue and was implanted into the muscle layer of the right forearm. The implant sites were marked with silk or metal materials.
iPTH, Ca, P, and alkaline phosphatase (ALP) levels were measured at baseline, 1 and 7 days after treatment, and 1, 3, 6, and 12 months after treatment. Osteocalcin (OC), 25-hydroxy vitamin D [25(OH)D], beta C-terminal cross-linked telopeptide of type I collagen (β-CTX), and BMD of the lumbar spine (LS), femoral neck (FN), and total hip (TH) were measured before treatment and 12 months after treatment. Data on total hospital stay, hospitalization costs, incidence of complications (hypocalcemia, hoarseness, infection/fever, and hematoma), and recurrence rates were collected and analyzed.
The Kidney Disease Improving Global Outcomes (KDIGO) recommends that iPTH concentrations in patients with stage 5 chronic kidney disease (CDK-5) should be 124–558 pg/mL, which is two to nine times the upper limit of normal (26). Treatment success was defined as serum iPTH <300 pg/mL within 7 days after treatment (15). Recurrence was defined as serum iPTH levels >558 pg/mL after successful treatment (15). Hypocalcemia and severe hypocalcemia (SH) are defined as serum Ca concentration <2.0–1.875 mmol/L and <1.875 mmol/L, respectively (normal range 2.0–2.75 mmol/L) (27).
Data analysis was performed using R version 4.3.1 (R Foundation, Vienna, Austria). Normally distributed continuous variables are expressed as means and standard deviations. Non-normally distributed continuous variables are expressed as medians and interquartile ranges. For categorical variables, frequencies and percentages were calculated using cross-tabulation. The groups were compared using the t-test, Wilcoxon rank-sum test, chi-squared test, or Fisher’s exact test. Differences in BMM concentration and BMD between before and 12 months after treatment were analyzed using the paired t-test or Wilcoxon signed-rank test. Between-group differences in serum iPTH, Ca, P, and ALP concentrations at each follow-up were analyzed using generalized estimating equations. All tests were two-sided, and p-values < 0.05 were considered statistically significant.
Baseline demographic and clinical data are shown in Table 1. A total of 107 patients with SHPT (410 nodules)—48 in the MWA group and 59 in the PTX group—were enrolled in the study. The mean age was 51.2 ± 10.1 years (range, 26–75 years). There were no significant differences in sex ratio, age, dialysis history, number of nodules, maximum nodule diameter, and other markers between the two groups (p > 0.05).
In patients treated with MWA, all multiple lesions were completely ablated. The efficacy and safety of MWA and PTX are shown in Table 2. The success rate was marginally lower in the MWA group than in the PTX group, and the recurrence rate was slightly higher in the former; however, these differences were not significant (p > 0.05). The procedure time, intraoperative blood loss, length of hospital stay, and hospitalization costs were significantly lower in the MWA group (p < 0.001). Hypocalcemia was the most common complication after MWA and PTX, with no difference between the groups (p > 0.05). The incidence of SH was significantly higher in the PTX group (p < 0.05). There were no significant between-group differences in the incidence of hoarseness, infection/fever, and hematoma (p > 0.05).
Post-treatment iPTH levels in the two groups are shown in Figure 2. The percentage of patients with iPTH <12 pg/mL was significantly lower in the MWA group at each follow-up (p < 0.05). Nevertheless, the percentage of patients whose iPTH levels were within the target range and iPTH >558 pg/mL was significantly higher in the MWA group at each follow-up (p < 0.05).
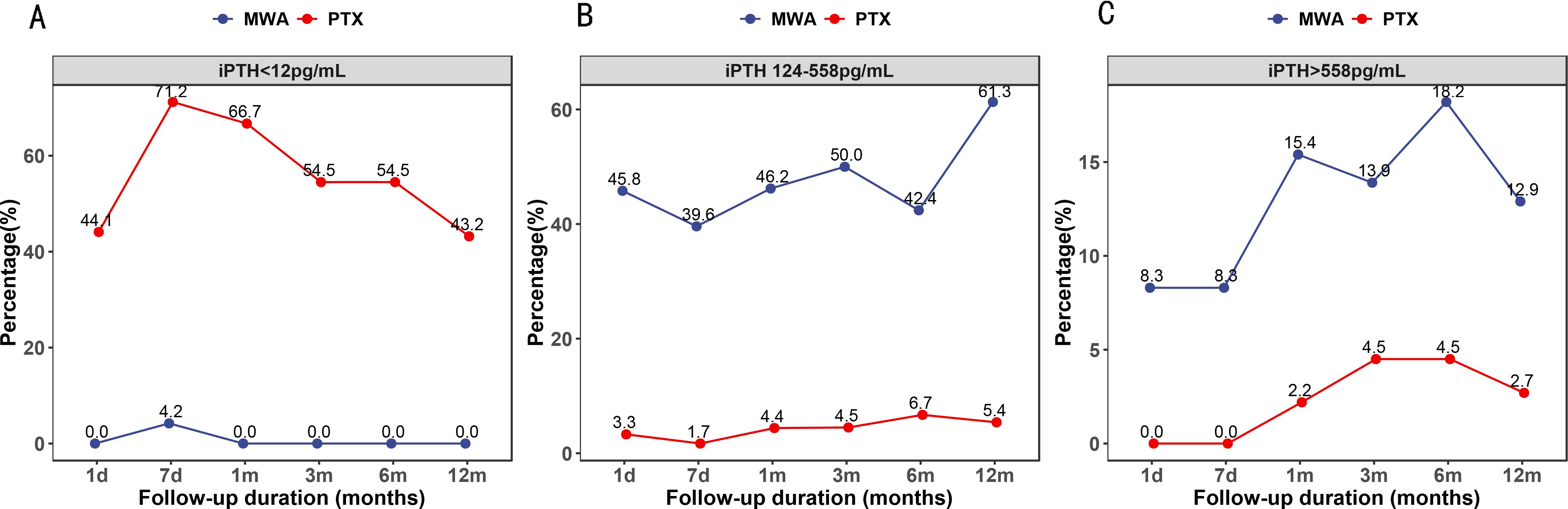
Figure 2. Percentage of patients whose serum intact parathyroid hormone levels were lower than 12 pg/mL (A), within the target range (B), and higher than 558 pg/mL (C).
The effects of treatments on iPTH, Ca, P, and ALP concentrations are shown in Figure 3. Both procedures decreased iPTH levels 1 day postoperatively, and the decrease was more pronounced after PTX (Figure 3A). Both therapies decreased serum Ca up to 1 month postoperatively, and the decrease was more pronounced at 1 and 7 days after MWA (Figure 3B). Similarly, both treatments decreased serum P up to 1 month postoperatively, and concentrations increased gradually within 12 months (Figure 3C). The ALP concentration in the two groups reached a peak at 7 days after treatment, after which it continued to decline. The ALP concentration was lower in the PTX group than in the MWA group at 6 and 12 months after treatment (p < 0.05) (Figure 3D).

Figure 3. (A–D) Mean concentrations of intact parathyroid hormone, calcium, phosphorus, and alkaline phosphatase during the study period. *p < 0.05, **p < 0.01. NS, no statistical difference.
Changes in BTMs and BMD before and 12 months after MWA and PTX are shown in Table 3. There were no significant between-group differences in OC, β-CTX, 25(OH)D, and BMD before treatment (p > 0.05). However, both treatments decreased OC and β-CTX concentrations and increased 25(OH)D levels and BMD in the LS, FN, and TH at 12 months after treatment (p < 0.05), and the 25(OH)D, LS, FN, or TH showed no significant differences between the two groups (p > 0.05). Nonetheless, the effect of PTX on OC and β-CTX levels was more pronounced.
The results showed that MWA and PTX decreased serum iPTH, Ca, P, ALP, OC, and β-CTX and increased serum 25(OH)D levels and BMD in the LS, FN, and TH in patients with SHPT. Both therapies corrected bone metabolism disorders and improved bone density.
The KDIGO recommends that iPTH levels in patients with CDK-5 should not be over-suppressed (26). Sustained low iPTH concentrations may be related to reduced bone formation and an increased incidence of dynamic bone disease, bone pain, and fractures; thus, maintaining ideal iPTH levels is essential for bone turnover (28, 29). Although MWA and PTX reduced iPTH levels, the effect of PTX was more pronounced. Moreover, the percentage of patients whose iPTH concentration was within the target range was higher in the MWA group, and the percentage of patients with iPTH <12 pg/mL was lower, consistent with previous studies (12, 14). This difference may be related to two factors. First, PTX is performed under direct vision; thus, removing parathyroid tissue is easier, whereas thermal ablation can only ablate the hyperplasia of parathyroid tissue identified on ultrasound. Second, although post-ablation CEUS can reveal the active portion of nodules, there may still be residual marginal lesions that cannot be detected by ultrasound.
ALP and OC are markers of osteoblast activity and bone formation (30, 31) while CTX is a marker of osteoclast activity and bone resorption (22, 32). These markers combined reflect bone metabolism status. We found that preoperative ALP, OC, and β-CTX levels were significantly increased in our patients, indicating active bone formation and resorption. Consistent with this finding, Woitge et al. showed that OC and CTX were elevated in patients with renal insufficiency (33). Although MWA and PTX reduced ALP, OC, and β-CTX, the effect of PTX was more pronounced, indicating that both therapies can stimulate bone formation and resorption and maintain bone homeostasis. Among these indicators, ALP showed a brief and significant increase within 7 days after treatment, after which it continued to decline. Similarly, other studies found that PTX caused a short-term increase in ALP in patients with SHPT (22, 23). Moreover, Yajima et al. observed that a rapid decline in iPTH can inhibit bone resorption and cause a significant transient increase in bone formation, as observed using bone biopsy 1 week after PTX (34). This result may explain the transient elevation in ALP levels within 7 days after MWA and PTX.
High bone turnover in SHPT can decrease BMD and is the leading cause of metabolic bone diseases in patients with renal insufficiency (35). Hyperparathyroidism is usually represented as the bone density is lower in the cortical than the trabecular bone (36). We found that baseline BMD was low in most of our patients, with lower BMD in the LS and FN compared to that in TH, and PTX increased BMD, especially in the LS and FN, consistent with the literature (24, 25, 37), suggesting that sites with lower BMD may benefit more from treatment in these patients.
MWA also increased BMD, although the degree of increase was slightly lower than that of PTX. Similarly, Wu et al. found that MWA increased BMD in the LS and FN within 2 years after surgery in patients with primary hyperparathyroidism (pHPT) (38). While the mechanism underlying this increase in BMD remains unclear, it may be related to improvements in biochemical characteristics after PTX (39, 40). Therefore, MWA is also a therapy that can promote bone recovery. However, Nomura et al. found that BMD in patients with pHPT increased significantly and continuously within 6 years after PTX (41). Therefore, we predicted that BMD may also continue to increase over a long period after surgery in patients with SHPT, although longer follow-up is needed to verify the difference between MWA and PTX in improving BMD.
Our data showed that hypocalcemia was the most common complication following PTX and MWA, with no significant differences in incidence rate between the two groups, although PTX was associated with a higher incidence of SH. A possible explanation is that iPTH decreases more rapidly and strongly after PTX than after MWA, leading to a faster transfer of large amounts of serum Ca to previously decalcified bones, resulting in a significant decrease in serum Ca concentrations. Wei et al. found that the number of removed parathyroid glands was an independent risk factor for postoperative SH in patients with SHPT (27). MWA can ablate parathyroid tissue that can be shown on ultrasound, but there may still be a small number of residual lesions. Although the PTX group in this study underwent AT, the iPTH concentration was still significantly lower than that of MWA, which may be related to the non-survival of transplanted parathyroid tissue. A meta-analysis found that the incidence of hypocalcemia was similar between tPTX+AT and sPTX; nonetheless, the former was associated with an increased incidence of severe symptomatic hypocalcemia (42). The incidence of other complications, such as hoarseness, infection/fever, and hematoma, was similar between the two groups, demonstrating that MWA is safe for treating SHPT.
This study has limitations. First, the design was retrospective and had a limited sample size. Second, the follow-up time was short; thus, we were unable to investigate the long-term efficacy and recurrence rates. To address these limitations, future studies with increased sample sizes and longer follow-up times are needed to explore the long-term efficacy of these procedures. In addition, BTM and BMD data were only available before and 12 months after treatment in this study, and the bone density changes in the forearm are not mentioned, although cortical bone loss occurs more usually in SPTH and should be perfected in future studies.
In conclusion, MWA and PTX are effective and safe methods for treating SHPT, and both improve bone metabolism markers and BMD. MWA is a minimally invasive percutaneous approach with great potential for treating patients with SHPT who cannot tolerate PTX. Based on the findings of this study, it is necessary to consider the individual differences of patients in the treatment of SPTH and select the most appropriate option.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Jiantang Zhang, NDUwMjUzMzQ4QHFxLmNvbQ==.
The studies involving humans were approved by Longyan First Affiliated Hospital of Fujian Medical University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SL: Investigation, Methodology, Writing – review & editing, Conceptualization, Formal analysis, Project administration, Supervision, Validation, Visualization. JQ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. XZ: Investigation, Writing – review & editing, Formal analysis, Resources. FW: Formal analysis, Investigation, Resources, Writing – review & editing. XY: Formal analysis, Investigation, Resources, Writing – review & editing. XC: Investigation, Writing – review & editing, Data curation, Software. XG: Investigation, Writing – review & editing. ZL: Investigation, Writing – review & editing, Funding acquisition. ML: Funding acquisition, Investigation, Writing – review & editing. XL: Investigation, Writing – review & editing, Software. JH: Investigation, Writing – review & editing, Data curation. GL: Investigation, Writing – review & editing, Conceptualization, Project administration, Supervision, Validation, Visualization. JZ: Investigation, Writing – review & editing, Methodology, Data curation, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Natural Science Foundation of Fujian Province (2021J011441).
The authors thank Editage for the English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. (2015) 10:98–109. doi: 10.2215/CJN.12941213
2. Jamal SA, Miller PD. Secondary and tertiary hyperparathyroidism. J Clin Densitom. (2013) 16:64–8. doi: 10.1016/j.jocd.2012.11.012
3. Goldsmith D, Kothawala P, Chalian A, Bernal M, Robbins S, Covic A. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of fracture and need for parathyroidectomy in CKD. Am J Kidney Dis. (2009) 53:1002–13. doi: 10.1053/j.ajkd.2009.02.010
4. Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. (2011) 26:1948–55. doi: 10.1093/ndt/gfq219
5. Komaba H, Moriwaki K, Goto S, Yamada S, Taniguchi M, Kakuta T, et al. Cost-effectiveness of cinacalcet hydrochloride for hemodialysis patients with severe secondary hyperparathyroidism in Japan. Am J Kidney Dis. (2012) 60:262–71. doi: 10.1053/j.ajkd.2011.12.034
6. Sezer S, Tutal E, Bal Z, Uyar ME, Bal U, Cakir U, et al. Differential influence of vitamin D analogs on left ventricular mass index in maintenance hemodialysis patients. Int J Artif Organs. (2014) 37:118–25. doi: 10.5301/ijao.5000289
7. Zhang Q, Li M, You L, Li H, Ni L, Gu Y, et al. Effects and safety of calcimimetics in end stage renal disease patients with secondary hyperparathyroidism: a meta-analysis. PloS One. (2012) 7:e48070. doi: 10.1371/journal.pone.0048070
8. Jia X, Wang R, Zhang C, Cui M, Xu D. Long-term outcomes of total parathyroidectomy with or without autoimplantation for hyperparathyroidism in chronic kidney disease: A meta-analysis. Ther Apher Dial. (2015) 19:477–85. doi: 10.1111/1744-9987.12310
9. Gao XF, Li JD, Guo L, Guo SS, Zhang R, Gou YL, et al. Effect of hybrid blood purification treatment on secondary hyperparathyroidism for maintenance hemodialysis patients. Blood Purif. (2018) 46:19–26. doi: 10.1159/000486844
10. Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol. (2018) 13:952–61. doi: 10.2215/CJN.10390917
11. Chen L, Wang K, Yu S, Lai L, Zhang X, Yuan J, et al. Long-term mortality after parathyroidectomy among chronic kidney disease patients with secondary hyperparathyroidism: a systematic review and meta-analysis. Ren Fail. (2016) 38:1050–8. doi: 10.1080/0886022X.2016.1184924
12. Jiang B, Wang X, Yao Z, Wu H, Xiao L, Gong H, et al. Microwave ablation vs. parathyroidectomy for secondary hyperparathyroidism in maintenance hemodialysis patients. Hemodial Int. (2019) 23:247–53. doi: 10.1111/hdi.12740
13. Zhao J, Qian L, Teng C, Yu M, Liu F, Liu Y, et al. A short-term non-randomized controlled study of ultrasound-guided microwave ablation and parathyroidectomy for secondary hyperparathyroidism. Int J Hyperthermia. (2021) 38:1558–65. doi: 10.1080/02656736.2021.1904153
14. Diao Z, Qian L, Teng C, Zhang N, Liang J, Kong L, et al. Microwave ablation versus parathyroidectomy for severe secondary hyperparathyroidism in patients on hemodialysis: a retrospective multicenter study. Int J Hyperthermia. (2021) 38:213–9. doi: 10.1080/02656736.2021.1885754
15. Ren M, Zheng D, Wu J, Liu Y, Peng C, Shen W, et al. Efficacy and safety of radiofrequency ablation versus parathyroidectomy for secondary hyperparathyroidism in dialysis patients: a single-center retrospective study. Sci Rep. (2022) 12:10289. doi: 10.1038/s41598-022-14623-x
16. Zhuo L, Peng LL, Zhang YM, Xu ZH, Zou GM, Wang X, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-A pilot study. Radiology. (2017) 282:576–84. doi: 10.1148/radiol.2016151875
17. Diao Z, Liu X, Qian L, Liu J, Liu S, Liu W. Efficacy and its predictor in microwave ablation for severe secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. (2016) 32:614–22. doi: 10.1080/02656736.2016.1194485
18. Zhao J, Qian L, Zu Y, Wei Y, Hu X. Efficacy of ablation therapy for secondary hyperparathyroidism by ultrasound guided percutaneous thermoablation. Ultrasound Med Biol. (2016) 42:1058–65. doi: 10.1016/j.ultrasmedbio.2015.08.021
19. Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. (2016) 20:846–52. doi: 10.4103/2230-8210.192914
20. Wu CH, Chang YF, Chen CH, Lewiecki EM, Wüster C, Reid I, et al. Consensus statement on the use of bone turnover markers for short-term monitoring of osteoporosis treatment in the Asia-Pacific region. J Clin Densitom. (2021) 24:3–13. doi: 10.1016/j.jocd.2019.03.004
21. Black DM, Bauer DC, Vittinghoff E, Lui LY, Grauer A, Marin F, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. (2020) 8:672–82. doi: 10.1016/S2213-858730159-5
22. Ge Y, Yang G, Wang N, Zha X, Yu X, Mao H, et al. Bone metabolism markers and hungry bone syndrome after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Int Urol Nephrol. (2019) 51:1443–9. doi: 10.1007/s11255-019-02217-y
23. Ma L, Zhao S, Li Z. Effects of parathyroidectomy on bone metabolism in haemodialysis patients with secondary hyperparathyroidism. Scand J Clin Lab Investig. (2017) 77:527–34. doi: 10.1080/00365513.2017.1354256
24. Okada M, Tominaga Y, Tomosugi T, Hiramitsu T, Ichimori T, Sato T. Predictors of bone mineral density improvement after parathyroidectomy for secondary hyperparathyroidism: A retrospective single-center analysis. World J Surg. (2021) 45:2777–84. doi: 10.1007/s00268-021-06186-1
25. Fang L, Wu J, Luo J, Wen P, Xiong M, Cao J, et al. Changes in bone mineral density after total parathyroidectomy without autotransplantation in the end-stage renal disease patients with secondary hyperparathyroidism. BMC Nephrol. (2018) 19:142. doi: 10.1186/s12882-018-0934-1
26. Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. (2017) 92:26–36. doi: 10.1016/j.kint.2017.04.006
27. Wei Y, Peng LL, Zhao ZL, Li Y, Yu MA. Risk factors of severe hypocalcemia after US-guided percutaneous microwave ablation of the parathyroid gland in patients with secondary hyperparathyroidism. J Bone Miner Res. (2020) 35:691–7. doi: 10.1002/jbmr.3934
28. Ott SM, Malluche HH, Jorgetti V, Elder GJ. Importance of bone turnover for therapeutic decisions in patients with CKD-MBD. Kidney Int. (2021) 100:502–5. doi: 10.1016/j.kint.2021.05.024
29. Ginsberg C, Ix JH. Diagnosis and management of osteoporosis in advanced kidney disease: a review. Am J Kidney Dis. (2022) 79:427–36. doi: 10.1053/j.ajkd.2021.06.031
30. Naylor K, Eastell R. Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol. (2012) 8:379–89. doi: 10.1038/nrrheum.2012.86
31. Karsenty G. Update on the biology of osteocalcin. Endocr Pract. (2017) 23:1270–4. doi: 10.4158/EP171966.RA
32. Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. (2017) 63:464–74. doi: 10.1373/clinchem.2016.259085
33. Woitge HW, Pecherstorfer M, Li Y, Keck AV, Horn E, Ziegler R, et al. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res. (1999) 14:792–801. doi: 10.1359/jbmr.1999.14.5.792
34. Yajima A, Tanaka K, Tominaga Y, Ogawa Y, Tanizawa T, Inou T, et al. Early changes of bone histology and circulating markers of bone turnover after parathyroidectomy in hemodialysis patients with severe hyperparathyroidism. Clin Nephrol. (2001) 56:27–34. doi: 10.1046/j.1464-410x.2001.02308.x
35. Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. (1999) 56:1084–93. doi: 10.1046/j.1523-1755.1999.00617.x
36. Abdelhadi M, Nordenström J. Bone mineral recovery after parathyroidectomy in patients with primary and renal hyperparathyroidism. J Clin Endocrinol Metab. (1998) 83:3845–51. doi: 10.1210/jcem.83.11.5249
37. Lu KC, Ma WY, Yu JC, Wu CC, Chu P. Bone turnover markers predict changes in bone mineral density after parathyroidectomy in patients with renal hyperparathyroidism. Clin Endocrinol (Oxf). (2012) 76:634–42. doi: 10.1111/j.1365-2265.2011.04265.x
38. Wu W, Zhou Q, Xu S, An S, Shen F, Li H, et al. Two-year changes of biochemical profiles and bone mineral density after percutaneous ultrasound-guided microwave ablation for primary hyperparathyroidism. Endocrine. (2021) 71:476–83. doi: 10.1007/s12020-020-02511-1
39. Lee D, Walker MD, Chen HY, Chabot JA, Lee JA, Kuo JH. Bone mineral density changes after parathyroidectomy are dependent on biochemical profile. Surgery. (2019) 165:107–13. doi: 10.1016/j.surg.2018.04.065
40. Miguel GA, Carranza FH, Rodríguez JCR, Ramos MA, Pablos DL, Herrero EF, et al. Trabecular bone score, bone mineral density and bone markers in patients with primary hyperparathyroidism 2 years after parathyroidectomy. Horm Metab Res. (2019) 51:186–90. doi: 10.1055/a-0850-8679
41. Nomura R, Sugimoto T, Tsukamoto T, Yamauchi M, Sowa H, Chen Q, et al. Marked and sustained increase in bone mineral density after parathyroidectomy in patients with primary hyperparathyroidism; a six-year longitudinal study with or without parathyroidectomy in a Japanese population. Clin Endocrinol (Oxf). (2004) 60:335–42. doi: 10.1111/j.1365-2265.2004.01984.x
Keywords: microwave ablation, parathyroidectomy, secondary hyperparathyroidism, bone metabolic markers, bone mineral density
Citation: Li S, Qiu J, Zhang X, Wang F, Yang X, Chen X, Guo X, Li Z, Lin M, Li X, He J, Lyu G and Zhang J (2025) Comparison of microwave ablation and parathyroidectomy for treating severe secondary hyperparathyroidism. Front. Endocrinol. 16:1424248. doi: 10.3389/fendo.2025.1424248
Received: 09 July 2024; Accepted: 24 February 2025;
Published: 10 March 2025.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Shuangxin Liu, Guangdong Provincial People’s Hospital, ChinaCopyright © 2025 Li, Qiu, Zhang, Wang, Yang, Chen, Guo, Li, Lin, Li, He, Lyu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiantang Zhang, NDUwMjUzMzQ4QHFxLmNvbQ==; Guorong Lyu, bGdyX2ZldXNAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.