- 1Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, Guangdong, China
- 2The Fourth Clinical Medical College, Guangzhou University of Chinese Medicine, Shenzhen, China
Background: Epidemiological evidence suggests that non-alcoholic fatty liver disease (NAFLD) may increase the risk of atrial fibrillation (AF). However, the findings are inconsistent, and the causality remains to be established.
Methods: We conducted two-step, two-sample Mendelian randomization (MR) analysis to assess the association between genetically predicted NAFLD (i.e. chronically elevated serum alanine aminotransferase levels [cALT], imaging-based and biopsy-confirmed NAFLD) and AF. Subsequently, we further performed Mendelian randomization to investigate the causal relationship between non-alcoholic steatohepatitis (NASH), a subtype of NAFLD, and AF. The inverse variance weighted (IVW) method was used as the primary approach to reveal the potential causation between the exposure and outcome.
Results: There was no significant causal association between NAFLD diagnosed based on cALT, confirmed by imaging, or verified by biopsy, and an increased risk of atrial fibrillation. Furthermore, the results of the IVW method revealed a positive causal effect of NASH on AF (OR=1.113, 95% CI=1.025-1.209, P = 0.011). In the reverse analysis, however, no evidence supported a significant genetic association between AF and NASH (OR=0.974, 95% CI=0.934-1.016, P = 0.214).
Conclusion: A causal relationship existed between NASH and the risk of AF. However, no significant genetic association has been observed between NAFLD and AF risk. This suggests that managing the progression of NAFLD may hold potential value in preventing the onset of AF.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic metabolic liver disease, primarily encompassing simple steatosis and non-alcoholic steatohepatitis (NASH) (1). Simple steatosis is defined as the presence of ≥5% hepatic steatosis (HS) without evidence of hepatocellular injury in the form of hepatocyte ballooning. NASH is defined as the presence of ≥5% HS and inflammation with hepatocyte injury (e.g., ballooning), with or without any fibrosis. NASH represents a more severe pathological manifestation compared to steatosis alone and poses a heightened clinical risk of progressing to liver fibrosis, cirrhosis, and ultimately, hepatocellular carcinoma (2). Meanwhile, a growing body of evidence demonstrates that NAFLD, as a multisystemic disease, affects extrahepatic organs and regulatory mechanisms, thereby elevating the risk of cardiovascular diseases, type 2 diabetes, and other related conditions (3). Recently, the concept of metabolic association with fatty liver disease (MAFLD) has been proposed to better reflect the metabolic basis of the disease, emphasizing its association with obesity, insulin resistance, and cardiovascular risk (4).
As a cardiovascular disease with rapidly rising incidence and prevalence, atrial fibrillation (AF) significantly increases the global disease burden (5). Studies have shown that NAFLD is closely associated with the development of AF (6–8). NAFLD and AF share common risk factors, including obesity, insulin resistance, type 2 diabetes, systemic inflammation (9), and abnormal circadian rhythms (10–12), which can contribute to the development of both diseases. Additionally, the pro-inflammatory and pro-fibrotic states during the progression of NAFLD may contribute to atrial remodeling, thereby increasing the risk of atrial fibrillation (13). However, whether NAFLD is an independent risk factor for AF remains controversial.
Mendelian randomization (MR), a research method used to infer causal relationships between exposures and outcomes from a genetic perspective, is currently being widely applied in medical research (14). To further explore whether NAFLD is an independent risk factor for AF, our study employed the two-sample Mendelian randomization (TSMR) to clarify the potential causal relationships between NAFLD, particularly its advanced phenotype (NASH), and AF.
2 Methods
2.1 Study design
The genetic data pertaining to exposure and outcome variables in this study were derived from summary-level data of genome-wide association studies (GWAS). We performed TSMR analysis with publicly available summary‐level data, which were derived from several large‐scale cohorts (15–17). Declaration of Helsinki statement and informed consent procedure has been described in the original publications of these cohorts. In the first phase, we conducted two-sample Mendelian randomization analysis to investigate the causal relationship between NAFLD and AF. In the second phase, we employed the same two-sample Mendelian randomization approach to examine the causality between NASH (advanced phenotype of NAFLD) and AF. The flowchart of the study design is shown in Figure 1.
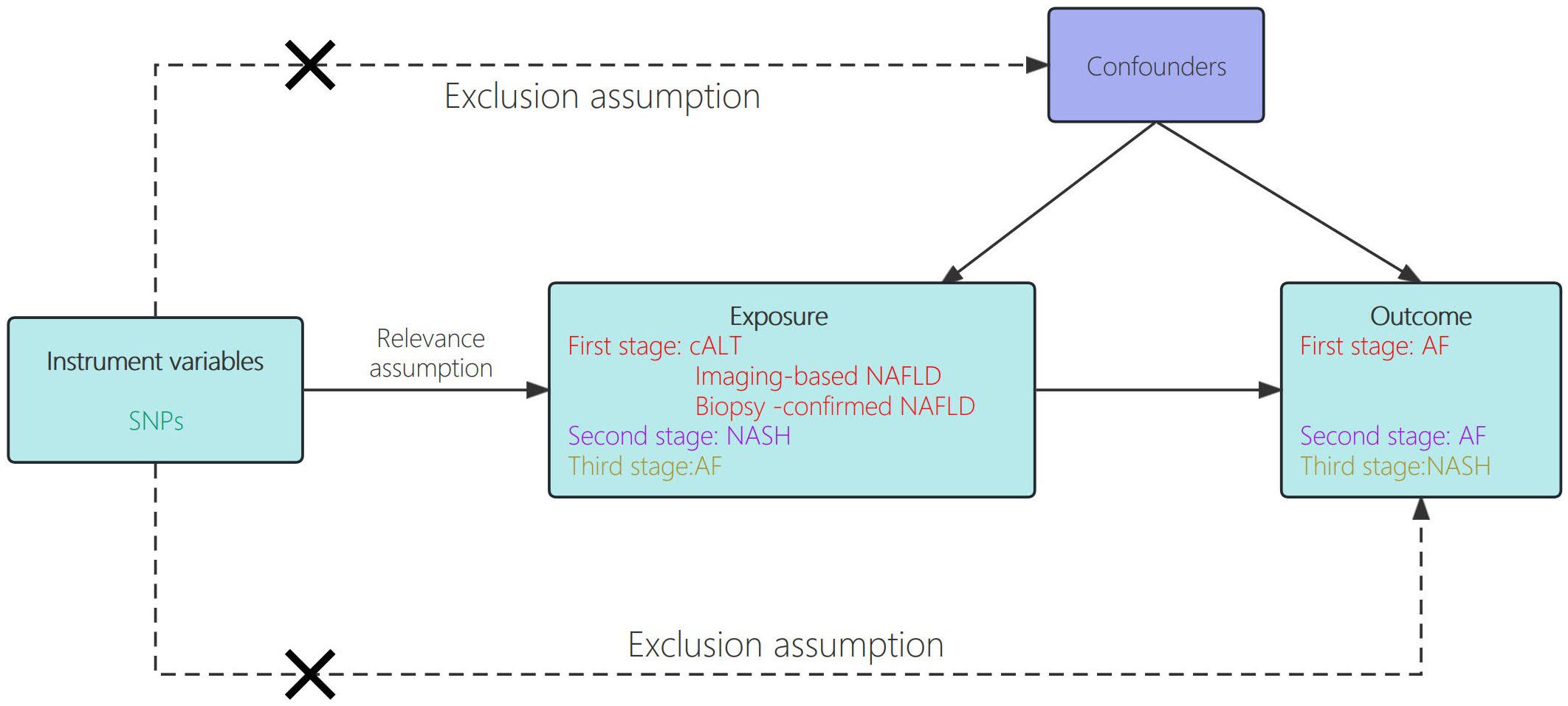
Figure 1. Schematic showing how Mendelian randomization was used to evaluate a causal association between NAFLD/NASH and AF in this study.
2.2 Data sources
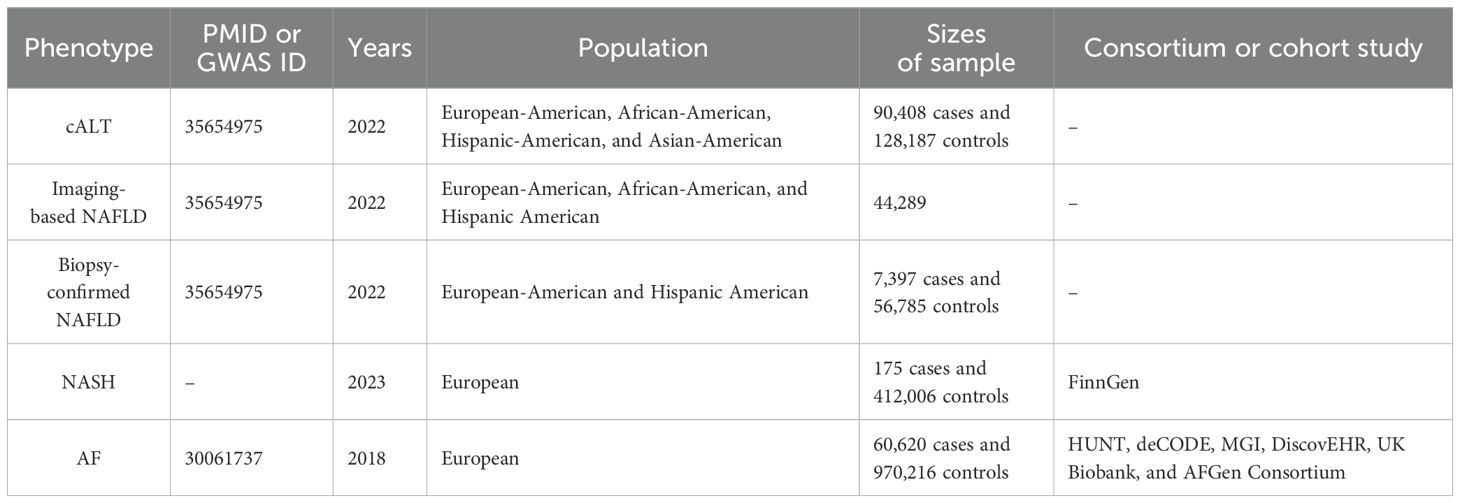
Genetic data relating to NAFLD were derived from a recently published GWAS for cALT, in which NAFLD was defined as an elevated alanine transaminase (ALT) > 40 U/L for men or >30U/L for women during at least two time points at least 6 months apart within a 2‐year period, after exclusion of other liver diseases (15). The study included 90,408 cases of cALT and 128,187 controls from the Million Veteran Program (MVP) database, predominantly composed of European Americans (EA, 75.1%) across four ancestral groups. Overall, 77 independent single-nucleotide polymorphisms (SNPs) were identified with significant associations (P < 5e-8) in the study. Among the SNPs, 22 were replicated in a subsequent imaging-confirmed NAFLD cohort (n=44,289), while 36 were replicated in a biopsy-defined NAFLD cohort comprising 7,397 cases and 56,785 controls. Therefore, NAFLD defined based on cALT, imaging, and biopsy was separately included in the MR analysis.
The SNPs associated with NASH are sourced from the FinnGen R10 dataset within the FinnGen study (16). The study is a large-scale genomics initiative that has analyzed over 500,000 Finnish biobank samples and correlated genetic variation with health data to understand disease mechanisms and predispositions. The project is a collaboration between research organisations and biobanks within Finland and international industry partners. The definition of NASH refers to clinically diagnosed non-alcoholic steatohepatitis (ICD-10 code K73.80). The study included a total of 175 NASH cases and 412,006 control subjects.
Genetic data associated with AF were extracted from the largest GWAS meta-analysis of 60,620 AF cases and 970,216 controls of European ancestry (17). This comprehensive dataset integrated information from notable studies such as the Nord-Trøndelag Health Study (HUNT), deCODE, the Michigan Genomics Initiative (MGl), DiscovEHR, UK Biobank, and the AFGen Consortium. AF cases were defined by clinically diagnosed atrial fibrillation or flutter (ICD-10 code I48 and ICD-9 code 427.3). A detailed description of the GWAS data involved in this study is shown in Table 1.
2.3 Screening for genetic instrumental variables
All instrumental variables included into the ultimate MR analysis are required to fulfill three fundamental assumptions. Firstly, SNPs that are significantly associated with exposure on a genome-wide scale are considered (specifically, p < 5e-8 for cALT, p < 5e-6 for Imaging-based NAFLD, Biopsy-confirmed NAFLD, and NASH, with 1.44e-9 representing 0.05/nSNPs for AF). Secondly, SNPs without linkage disequilibrium (kb = 10000, r² < 0.001) are extracted, and palindromic SNPs with intermediate allele frequencies are excluded. Proxy SNPs were not used in this MR analysis. The results from IVW will serve as the main outcomes for TSMR analysis. Significant associations identified by IVW will undergo further sensitivity analysis, and pleiotropy will be tested using MR-Egger to ensure the effects of horizontal pleiotropy. The strength of the selected genetic instrument was assessed using F statistics, with a mean F‐statistic < 10 regarded as a weak set of instrumental variables.
3 Results
3.1 Causal effects of NAFLD-related traits on AF
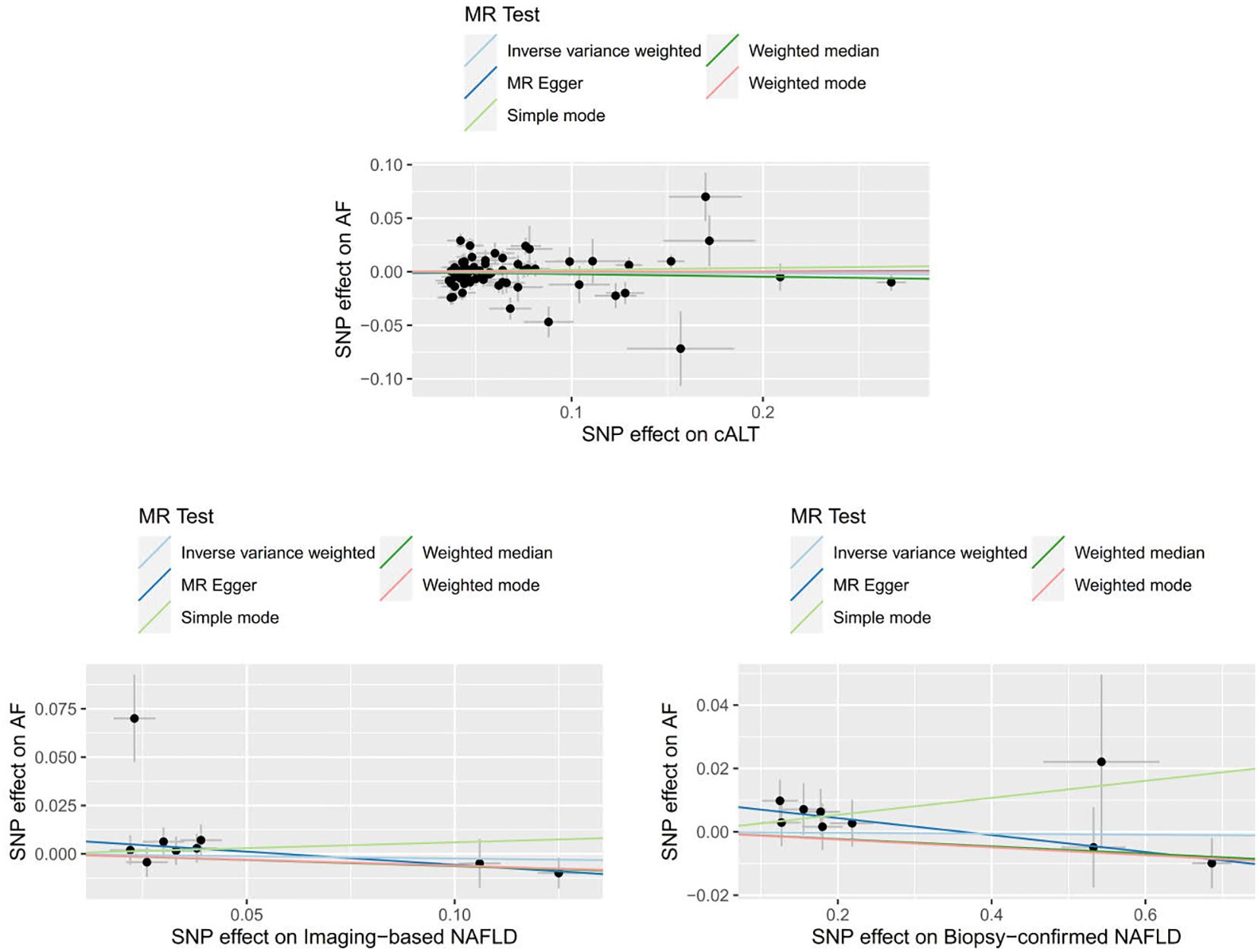
We obtained 67 cALT‐associated SNPs, 9 imaging‐associated SNPs and 9 biopsy‐associated SNPs after removing correlated SNPs (Supplementary Tables 1-3). According to the IVW result, no significant causal relationship between NAFLD-related traits and the risk of AF was found. Furthermore, other MR methods showed consistent results. Figures 2 and 3 present the detailed results of the MR analysis.
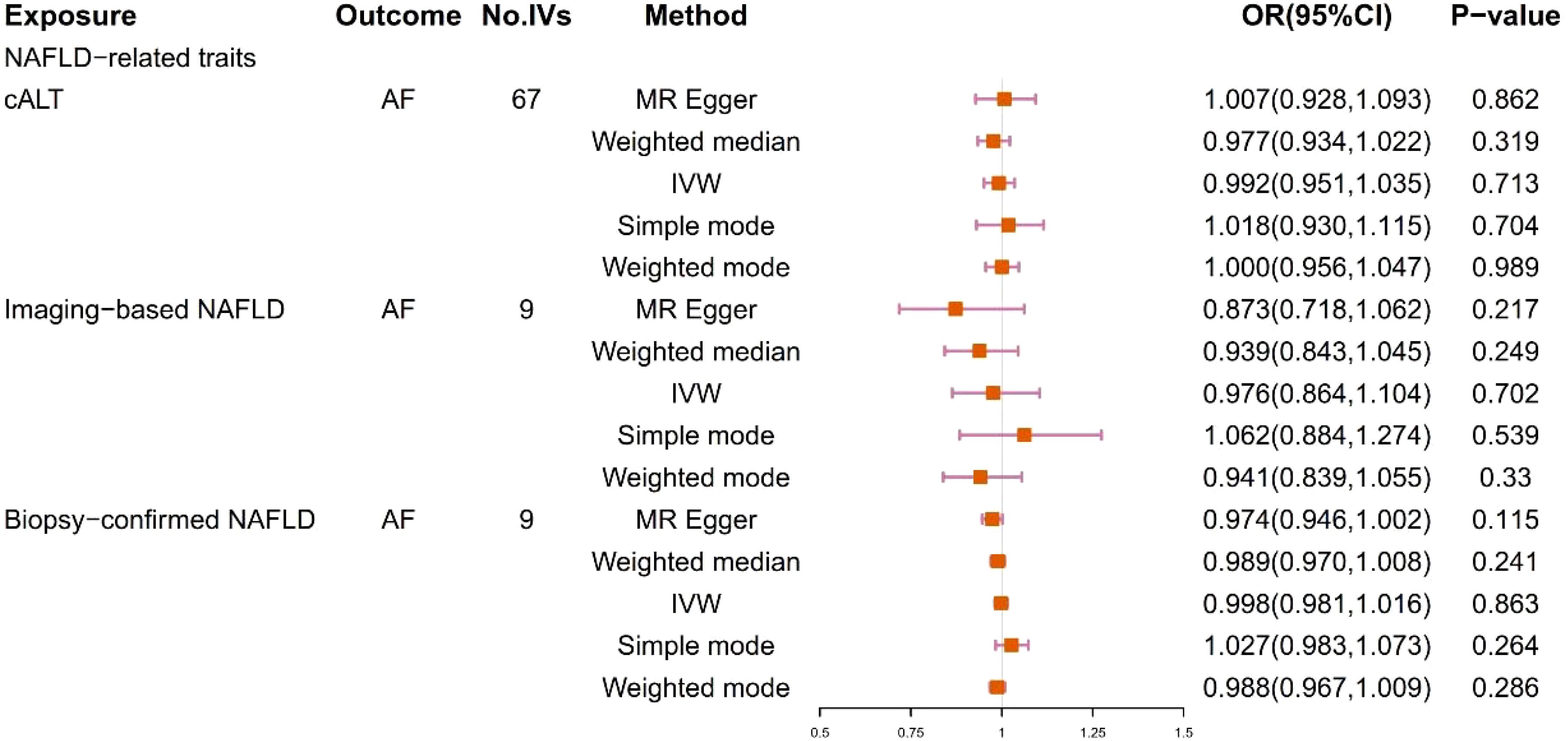
Figure 2. Odds ratios and p-value of MR analysis for the associations between NAFLD and AF. OR, odds ratio; 95% CI, 95% confidence interval.
3.2 Causal effects between NASH and AF
3.2.1 The effect of NASH on AF
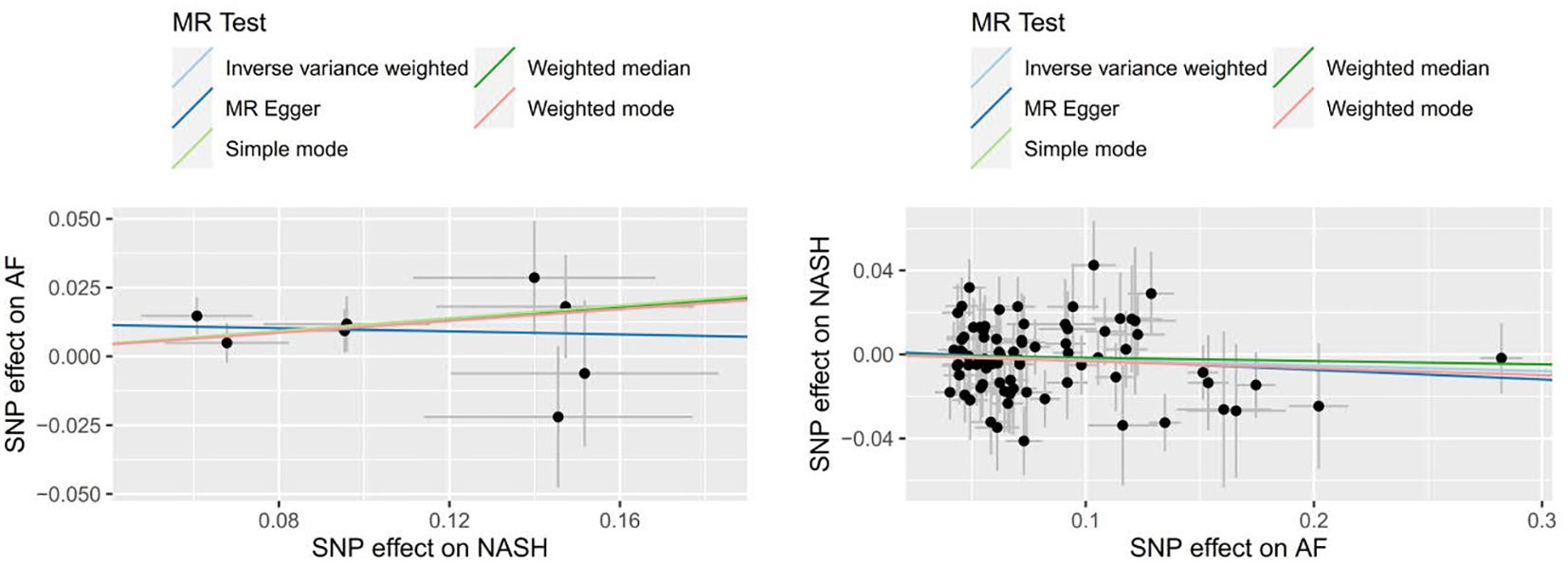
9 SNPs based on clinical diagnosis of NASH were used in MR analysis (Supplementary Table 4). IVW method showed a statistically significant association between genetically predicted NASH and the risk of AF (OR=1.113, 95% CI=1.025-1.209, P = 0.011). The result of Weighted median was consistent with IVW (p=0.045), while the remaining three methods were not statistically significant. Figures 4 and 5 present the detailed results of the MR analysis.
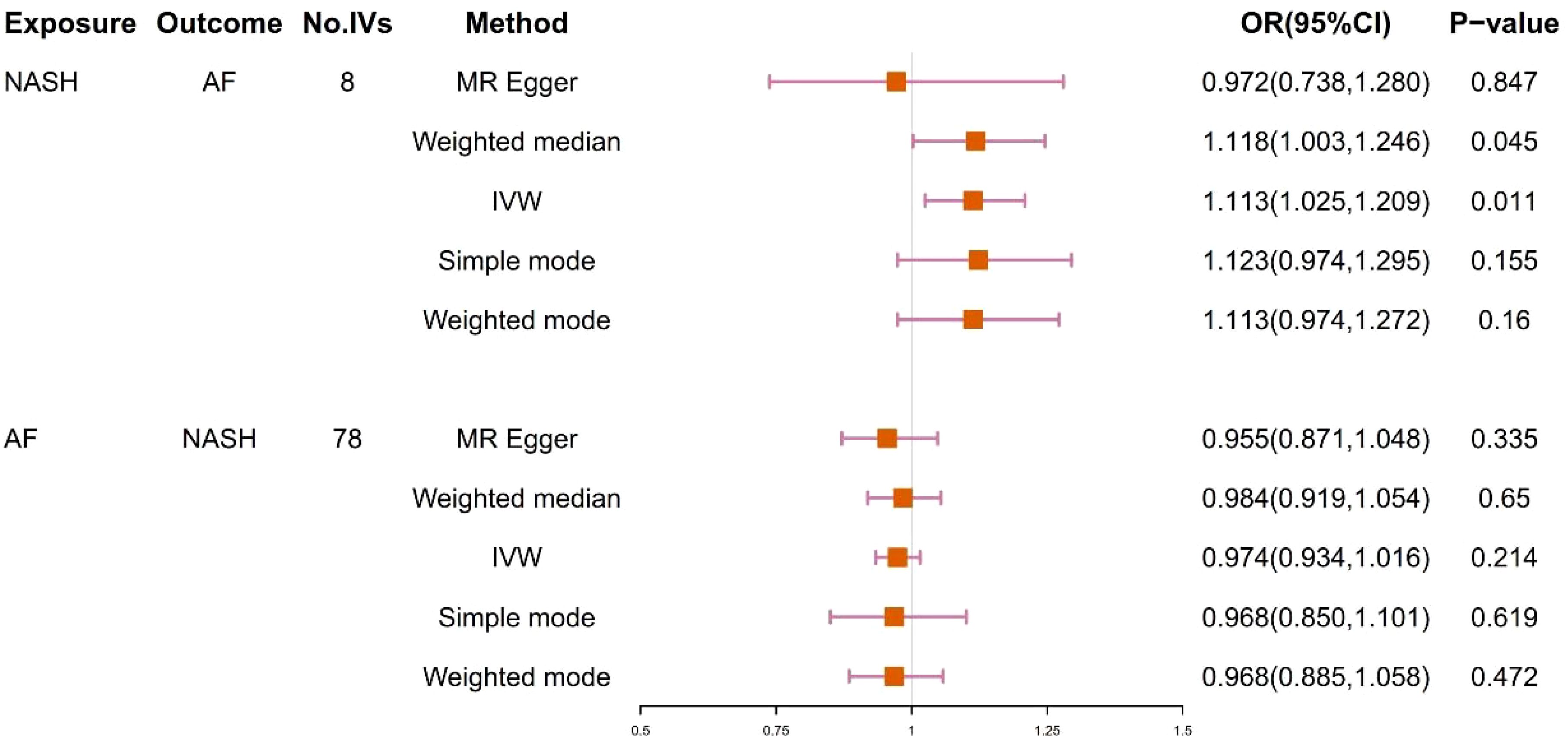
Figure 4. Odds ratios and p-value of MR analysis for the associations between NASH and AF. OR, odds ratio; 95% CI, 95% confidence interval.
Cochran’s Q statistic showed no significant heterogeneity in the estimates of included SNPs (Q=4.883, P=0.674). The intercept of MR-Egger was 0.013 (P = 0.350), which showed no significant horizontal pleiotropy. In addition, the MR-PRESSO Test also showed that there was no horizontal pleiotropy in this study (Global Test P= 0.769), and no potential outliers were found in the result, indicating that the result was robust and reliable (Supplementary Table 6).
3.2.2 The effect of AF on NASH
78 SNPs depending on the clinical diagnosis of AF were used in MR analysis (Supplementary Table 5). There was no significant causal relationship between AF and the risk of NASH according to the result of IVW (OR = 0.974, 95%CI = 0.934–1.016, P = 0.214). In addition, the results of MR Egger, Weighted median, Simple mode and Weighted mode were consistent with IVW. Figures 4 and 5 present the detailed results of the MR analysis.
4 Discussion
Although genetically predicted NAFLD based on cALT diagnosis, imaging and biopsy confirmation was not substantially related to an increased risk of AF, further analysis revealed that NASH, the advanced phenotype of NAFLD, was associated with the risk of developing AF.
4.1 The relationship between NAFLD/NASH and atrial fibrillation
Previous clinical studies have shown that NAFLD is closely related to the occurrence of AF. Käräjämäki et al. (18) reported an elevated incidence of AF in NAFLD patients from the OPERA study, which persisted after adjusting for confounding factors. Roh et al. (7) found an independent link between NAFLD (defined by FLI) and increased AF risk in a healthy Korean cohort. Meta-analyses further confirmed a strong correlation between NAFLD and higher AF risk (6, 19–22). As an advanced phenotype of NAFLD, NASH have risk factors for the development of cardiac abnormalities. Whitsett et al. (23) pointed out that AF is highly prevalent in patients with biopsy-proven Nonalcoholic Steatohepatitis (NASH). However, the Framingham Heart Study showed no association between AF and either computerized tomography or ultrasound-diagnosed hepatic steatosis (24).
Mendelian randomization explored the relationship between NAFLD/NASH and AF from the perspective of genetics. Two previous Mendelian randomization studies have explored the relationship between NAFLD and its subtype NASH and AF (Their exposure data originated from the same GWAS study). Their results showed that there was no significant causal relationship between NAFLD and AF, which is consistent with our Mendelian randomization study of NAFLD and AF. Simultaneously, these two investigations probed the causative association between NASH and AF, and the findings indicated the absence of a causal relationship (25, 26). We conducted a Mendelian randomization analysis based on the newly published FinnGen R10 database to further explore the causal relationship between NASH and AF, and the result revealed that genetically predicted NASH was causally associated with an increased risk of AF(OR=1.113, 95% CI=1.025-1.209, P = 0.011). Subsequently, heterogeneity tests and pleiotropy evaluations suggested the causal relationship was robust and reliable(both P > 0.05). Although the increase in odds ratio may not appear substantial, it still holds important epidemiological and clinical implications. Furthermore, reverse MR analysis suggested that AF was not associated with an increased risk of NASH. The larger sample size and updated genetic information in FinnGen R10 may provide a more robust interpretation of the relationship between NASH and AF.
4.2 Possible mechanisms associated with NASH and atrial fibrillation
The mechanism of AF induced by NAFLD remains unclear, but it is suggested that chronic inflammation, insulin resistance, lipid deposition, and oxidative stress may be involved in the development of AF in NAFLD patients (27). Mild systemic inflammation is a key factor in NAFLD (28). The Pro-inflammatory environment and oxidative stress lead to an increased release of inflammatory factors, ultimately resulting in an elevated risk of AF (29, 30). Furthermore, as NAFLD progresses to the stage of NASH, lipid deposition leads to the occurrence of lipotoxicity, causing further release of inflammatory factors and exacerbating the inflammatory response (31). During the NASH stage, the degree of liver fibrosis progresses significantly compared to the early stage of NAFLD (simple steatosis). Study have shown that the severity of liver fibrosis in NAFLD patients is associated with an increased risk of atrial fibrillation (AF), potentially mediated through mechanisms such as systemic inflammation, metabolic disturbances, and atrial remodeling (32). Furthermore, Hui et al. reported that high TNF-α levels and hypoadiponectinemia are IR-independent features of NASH, and these two factors synergistically exacerbate insulin resistance, oxidative stress, and lipotoxicity, potentially serving as underlying mechanisms linking NASH to AF risk (33).
4.3 The role of NASH in the prevention and treatment of atrial fibrillation
The pathogenesis of atrial fibrillation is still not fully understood, and effective treatment strategies remain limited. Our study provides genetic evidence supporting a causal relationship between NASH and AF risk, which may have implications for clinical practice. When NASH is recognized as a risk factor for atrial fibrillation, patients with NASH, especially those with obesity or metabolic syndrome, should be encouraged to undergo regular screening for atrial fibrillation. In addition, interventions for NASH, such as lifestyle changes (such as weight loss, dietary changes) and anti-inflammatory or anti-fibrotic treatments, may help reduce the risk of atrial fibrillation.
5 Conclusion
In conclusion, this MR study reveals a causal relationship between genetically determined NASH and an elevated risk of AF, while there is no apparent causal relationship between NAFLD and AF. This suggests that controlling the further progression of NAFLD may hold potential value in preventing the occurrence of AF.
6 Limitation
Although our study had enough statistical power to evaluate the causal relationships, the findings should be interpreted with prudence. Furthermore, this study also has some limitations. Firstly, the selected instrumental variables (IVs) capture only a relatively small proportion of the phenotypic genetic variation, potentially leading to bias from weak IVs. Additionally, the standard for the independence test P-value is relatively lenient (P < 5e-6), which could introduce some bias into the results. Secondly, the definition of NAFLD is partially based on cALT levels rather than the presence of NAFLD itself. Thirdly, a possible limitation of the study is the lack of further stratification for the severity of NAFLD and other factors such as gender and age. The absence of subgroup data for NAFLD limits the extension of these stratified analyses. While our study did not stratify by gender due to data limitations, future research should explore gender-specific associations, particularly in postmenopausal women, to better understand the underlying mechanisms. Finally, our focus was primarily on individuals of European descent, which may reduce the generalizability of our study results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
All the summary-level GWAS data used in the analyses are publicly available, and therefore ethical approval was not imperative for this study. Ethical approval for the GWASs can be found in the corresponding GWAS publications cited in the manuscript.
Author contributions
BC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. XS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. JH: Visualization, Writing – review & editing. YG: Conceptualization, Project administration, Software, Supervision, Writing – review & editing. MC: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. YW: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. YY: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. PC: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. XL: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. TL: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. CX: Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. BL: Conceptualization, Project administration, Software, Supervision, Writing – review & editing. QL: Conceptualization, Project administration, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article is supported by the Young Scientists Fund of the National Natural Science Foundation of China (No. 82200349) and Guangdong Basic and Applied Basic Research Foundation of China (No. 2022A1515011647).
Acknowledgments
We want to acknowledge the participants and investigators of the FinnGen study, MVP Consortium, database on atrial fibrillation, and all concerned investigators and consortiums for sharing the GW AS summary statistics on the diseases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1390259/full#supplementary-material
Abbreviations
NAFLD, non-alcoholic fatty liver disease; AF, atrial fibrillation; MR, Mendelian randomization; cALT, chronically elevated serum alanine aminotransferase; NASH, non-alcoholic steatohepatitis; IVW, inverse variance weighted; TSMR, two-sample Mendelian randomization; GWAS, genome-wide association studies; ALT, alanine transaminase; MVP, Million Veteran Program; EA, European Americans; SNPs, single-nucleotide polymorphisms; HUNT, Nord-Trøndelag Health Study; MGl, Michigan Genomics Initiative; FLI, fatty liver Index; IVs, instrumental variables.
References
1. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
2. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
3. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
4. Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014. doi: 10.1053/j.gastro.2019.11.312
5. Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. (2020) 127:4–20. doi: 10.1161/CIRCRESAHA.120.316340
6. Mantovani A, Dauriz M, Sandri D, Bonapace S, Zoppini G, Tilg H, et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int. (2019) 39:758–69. doi: 10.1111/liv.14044
7. Roh JH, Lee JH, Lee H, Yoon YH, Kim M, Kim YG, et al. Association between non-alcoholic fatty liver disease and risk of new-onset atrial fibrillation in healthy adults. Liver Int. (2020) 40:338–46. doi: 10.1111/liv.14236
8. Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. (2020) 40:1594–600. doi: 10.1111/liv.14461
9. Minhas AM, Usman MS, Khan MS, Fatima K, Mangi MA, Illovsky MA. Link between non-alcoholic fatty liver disease and atrial fibrillation: A systematic review and meta-analysis. Cureus. (2017) 9:e1142. doi: 10.7759/cureus.1142
10. Gnocchi D, Custodero C, Sabba C, Mazzocca A. Circadian rhythms: a possible new player in non-alcoholic fatty liver disease pathophysiology. J Mol Med (Berl). (2019) 97:741–59. doi: 10.1007/s00109-019-01780-2
11. Jokl E, Llewellyn J, Simpson K, Adegboye O, Pritchett J, Zeef L, et al. Circadian disruption primes myofibroblasts for accelerated activation as a mechanism underpinning fibrotic progression in non-alcoholic fatty liver disease. Cells. (2023) 12(12):1582. doi: 10.3390/cells12121582
12. Corino VD, Platonov PG, Enger S, Tveit A, Ulimoen SR. Circadian variation of variability and irregularity of heart rate in patients with permanent atrial fibrillation: relation to symptoms and rate control drugs. Am J Physiol Heart Circ Physiol. (2015) 309:H2152–57. doi: 10.1152/ajpheart.00300.2015
13. Packer M. Atrial fibrillation and heart failure with preserved ejection fraction in patients with nonalcoholic fatty liver disease. Am J Med. (2020) 133:170–77. doi: 10.1016/j.amjmed.2019.09.002
14. Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafo MR, et al. Mendelian randomization. Nat Rev Methods Primers. (2022) 2:6. doi: 10.1038/s43586-021-00092-5
15. Vujkovic M, Ramdas S, Lorenz KM, Guo X, Darlay R, Cordell HJ, et al. A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation. Nat Genet. (2022) 54:761–71. doi: 10.1038/s41588-022-01078-z
16. Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
17. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50:1234–39. doi: 10.1038/s41588-018-0171-3
18. Karajamaki AJ, Patsi OP, Savolainen M, Kesaniemi YA, Huikuri H, Ukkola O. Non-alcoholic fatty liver disease as a predictor of atrial fibrillation in middle-aged population (OPERA study). PloS One. (2015) 10:e142937. doi: 10.1371/journal.pone.0142937
19. Zhou BG, Ju SY, Mei YZ, Jiang X, Wang M, Zheng AJ, et al. A systematic review and meta-analysis of cohort studies on the potential association between NAFLD/MAFLD and risk of incident atrial fibrillation. Front Endocrinol (Lausanne). (2023) 14:1160532. doi: 10.3389/fendo.2023.1160532
20. Choi J, Lee SR, Choi EK, Ahn HJ, Kwon S, Park SH, et al. Non-alcoholic fatty liver disease and the risk of incident atrial fibrillation in young adults: A nationwide population-based cohort study. Front Cardiovasc Med. (2022) 9:832023. doi: 10.3389/fcvm.2022.832023
21. Zhou Y, Lai C, Peng C, Chen M, Li B, Wang X, et al. Nonalcoholic fatty liver disease as a predictor of atrial fibrillation: a systematic review and meta-analysis. Postepy Kardiol Interwencyjnej. (2017) 13:250–57. doi: 10.5114/aic.2017.70198
22. Wijarnpreecha K, Boonpheng B, Thongprayoon C, Jaruvongvanich V, Ungprasert P. The association between non-alcoholic fatty liver disease and atrial fibrillation: A meta-analysis. Clin Res Hepatol Gastroenterol. (2017) 41:525–32. doi: 10.1016/j.clinre.2017.08.001
23. Whitsett M, Wilcox J, Yang A, Zhao L, Rinella M, VanWagner LB. Atrial fibrillation is highly prevalent yet undertreated in patients with biopsy-proven nonalcoholic steatohepatitis. Liver Int. (2019) 39:933–40. doi: 10.1111/liv.14018
24. Long MT, Yin X, Larson MG, Ellinor PT, Lubitz SA, McManus DD, et al. Relations of liver fat with prevalent and incident atrial fibrillation in the framingham heart study. J Am Heart Assoc. (2017) 6(5):e005227. doi: 10.1161/JAHA.116.005227
25. Li Z, Zhang B, Li J, Tao Z, Wu Y. Assessing causal relationship between non-alcoholic fatty liver disease and risk of atrial fibrillation. J Hepatol. (2023) 78:e63–65. doi: 10.1016/j.jhep.2022.10.032
26. Chen J, Mei Z, Wang Y, Chen Y, Liu Q. Causal effect of non-alcoholic fatty liver disease on atrial fibrillation. Eur J Intern Med. (2022) 105:114–17. doi: 10.1016/j.ejim.2022.07.007
27. Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. (2018) 15:425–39. doi: 10.1038/s41575-018-0010-0
28. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. (2020) 69:1691–705. doi: 10.1136/gutjnl-2020-320622
29. Haghbin H, Gangwani MK, Ravi S, Perisetti A, Aziz M, Goyal H, et al. Nonalcoholic fatty liver disease and atrial fibrillation: possible pathophysiological links and therapeutic interventions. Ann Gastroenterol. (2020) 33:603–14. doi: 10.20524/aog.2020.0550
30. Wu N, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. (2013) 169:62–72. doi: 10.1016/j.ijcard.2013.08.078
31. MaChado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. (2016) 150:1769–77. doi: 10.1053/j.gastro.2016.02.066
32. Park HE, Lee H, Choi SY, Kim HS, Chung GE. The risk of atrial fibrillation in patients with non-alcoholic fatty liver disease and a high hepatic fibrosis index. Sci Rep. (2020) 10:5023. doi: 10.1038/s41598-020-61750-4
Keywords: atrial fibrillation, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, Mendelian randomization, causal relationship
Citation: Cheng B, Su X, He J, Gu Y, Chen M, Wei Y, Yi Y, Chen P, Lin X, Li T, Xu C, Liu Q and Li B (2025) A Mendelian randomization study reveals a causal association between NASH and the risk of atrial fibrillation. Front. Endocrinol. 16:1390259. doi: 10.3389/fendo.2025.1390259
Received: 23 February 2024; Accepted: 28 February 2025;
Published: 18 March 2025.
Edited by:
Carmine Izzo, University of Salerno, ItalyReviewed by:
Davide Gnocchi, University of Bari Medical School, ItalyDebosree Ghosh, Government General Degree College, Kharagpur II, India
Copyright © 2025 Cheng, Su, He, Gu, Chen, Wei, Yi, Chen, Lin, Li, Xu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Liu, MTM4MDIyNjM5MTZAMTM5LmNvbQ==; Biao Li, bGliaWFvemFja0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Biwei Cheng
Biwei Cheng Xuekang Su
Xuekang Su Jue He2
Jue He2 Mingtai Chen
Mingtai Chen Yumeng Yi
Yumeng Yi Peiying Chen
Peiying Chen