- 1Department of Endocrinology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Endocrinology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Objective: To systemically assess the relationship between serum osteocalcin levels and the progression of diabetic kidney disease (DKD) in the Chinese population.
Methods: The PubMed, Web of Science, CNKI, Wanfang Database, VIP and Chinese Medical Journal full-text Database were searched. Two investigators independently reviewed the literature and extracted data based on predetermined inclusion and exclusion criteria. The Newcastle-Ottawa scale was used to assess the quality of the literature. The statistical analysis was performed using Stata16 software.
Results: A total of 20 case-control studies encompassed 4 565 cases, consisting of 643 healthy controls (CN), 1 649 individuals with simple diabetes mellitus (DM), 1 305 with microalbuminuria (MI), and 968 with macroalbuminuria (MA). The meta-analysis results indicated that the serum osteocalcin levels in MI group were significantly lower than those in CN group and DM group [SMD = -1.15, 95% CI (-1.46, -0.85), P < 0.01; and SMD = -0.53, 95% CI (-0.69, -0.37), P < 0.01, respectively], and lower in the MA group compared to the CN group [SMD = -1.28, 95% CI (-1.79, -0.76), P < 0.01]. In the MA group, the serum osteocalcin levels were considerably lower compared to those in DM group and MI group [SMD = -0.93, 95% CI (-1.28, -0.58), P < 0.01; and SMD = -0.41, 95% CI (-0.65, -0.17), P < 0.01, respectively].
Conclusion: The serum osteocalcin levels are typically reduced and show a negative correlation with the severity of proteinuria in Chinese patients with DKD. This indicates a decline in bone formation at early-stage in DKD patients, which worsens as the disease progresses.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/,identifier CRD42024580324.
1 Introduction
Diabetic kidney disease (DKD) is a primary microvascular complication affecting 20 - 40% of patients with diabetes. Nowadays, it represents the leading cause of End-stage renal disease (ESRD) worldwide. The incidence of ESRD due to DKD continues to rise annually (1). Consistent and elevated urinary albumin excretion is a key characteristic of DKD and plays a crucial role in the early detection and evaluation of the risk of DKD advancement (2). The kidney plays an important role in the regulatory system for bone and mineral metabolism. Patients with diabetic kidney disease exhibit an increased risk of osteoporosis attributable to decreased renal 25-hydroxyvitamin D-1α hydroxylase activity, enhanced urinary excretion of vitamin D-binding protein (VDBP) resulted from increased permeability of the glomerular filtration membrane, and imbalance in calcium and phosphorus metabolism in serum and bone (3). Research has indicated that an increased risk of brittle bone fractures in DKD patients is associated with reduced bone mass and impaired bone quality (4).
Osteocalcin (OC) is a non-collagenous protein consisting of 46 to 50 amino acids. Osteoblasts generate it, primarily depositing it in the bone matrix, with a minor portion entering the bloodstream. Serum osteocalcin is a marker of bone formation, can reflect the activity of osteoblasts, and is also a specific and sensitive indicator of bone transformation and bone mineralization in the body (5). Recent studies have shown a notable inverse relationship between osteocalcin and urinary albumin - to - creatinine ratio (UACR) in diabetic patients, indicating that low osteocalcin levels are strongly linked to the onset and progression of DKD (6). And the recent prospective cohort study also confirmed that low osteocalcin levels are associated with an increased risk of DKD (7). Therefore, this study took Chinese patients with DKD as the research objects and conducted a systematic review and meta-analysis of the results of published case-control studies, aiming to explore the relationship between serum osteocalcin levels and DKD progression and provide new ideas and evidence-based medical evidence for the clinical prevention and treatment of bone metabolism abnormalities in patients with DKD.
2 Data and methods
This meta-analysis was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This study has been registered on PROSPERO (CRD42024580324).
2.1 Literature screening criteria
Inclusion criteria: (1) publicly published case-control studies; (2) According to Mogensen standard (8) and American Diabetes Association (ADA) standard (9), DKD is divided into three stages according to urinary albumin excretion rate (UAER) or UACR: simple diabetes mellitus (UAER < 30 mg/24 h, or UACR < 30 mg/g); microalbuminuria (30 ≤ UAER < 300 mg/24 h, or 30 ≤ UACR < 300 mg/g); macroalbuminuria (UAER ≥ 300 mg/24 h, or UACR ≥ 300 mg/g); (3) all cases are Chinese population; (4) no cases involve the use of medicines that impact bone metabolism or have any other conditions influencing bone metabolism; (5) the literature that was published multiple times within the same study is chosen based on having a large sample size.
Exclusion criteria: (1) repeated publication of literature; (2) unclear diagnostic criteria of the case; (3) patients have chronic kidney disease other than DKD or other diseases affecting bone metabolism; (4) patients need dialysis treatment; (5) patients use drugs that affect bone metabolism; (6) research data are incomplete or unable to extract valid data; (7) review, animal experiments or non-case-control studies.
2.2 Document retrieval strategy
PubMed, Web of Science, China Knowledge Network (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP), and Chinese Medical Journal full-text Database were searched to collect the case-control studies related to serum osteocalcin and DKD from the establishment of the database to September 2024. The subject words combined with free words are used for retrieval. The search words include Chinese and English forms of osteocalcin, N-terminal osteocalcin, bone γ-carboxyglutamate protein, Diabetic Kidney Disease, Diabetic Nephropathy, and others.
2.3 Data extraction
All literature data were independently extracted by two researchers and then merged and proofread. Begin by removing duplicate literature. Next, a preliminary screening based on the inclusion and exclusion criteria will be conducted through titles and abstracts. Then, the full text of the selected literature will be reviewed to confirm if the study meets the criteria for inclusion in the analysis. If discrepancies arise during this time, the corresponding author will help make decisions. Data is collected by creating an Excel table that includes the first author, publication year, sample size, grouping standards, serum osteocalcin levels, and other relevant details.
2.4 Document quality evaluation
The study utilized the Newcastle-Ottawa scale (NOS) to assess bias risk. The scale consists of eight questions across three aspects: population selection, inter-group comparability, and measurement of exposure. It concretely consists of 4 entries on selection (adequacy of the case definition; representativeness of the cases; selection of Controls; definition of Controls), 1 entry on comparability (comparability of cases and controls on the basis of the design or analysis), and 3 entries on exposure (ascertainment of exposure; same method of ascertainment for cases and controls; non-response rate); all entries are worth a maximum of 1 point except for the one on comparability, which is worth a maximum of 2 points, with a maximum total score of 9 points. The higher total score show better quality of the study.
2.5 Statistical methods
Stata16 software was used for Meta-analysis. The study utilized standard mean difference (SMD) as the impact index for the continuous variables, with each effect being assigned a point estimate and a 95% confidence interval (CI). A difference was considered statistically significant if P ≤ 0.05. The heterogeneity between the results was included by Q test and I2 quantitative analysis. If P ≥ 0.1 and I2 ≤ 50%, the research results show no statistical difference in heterogeneity, and the fixed effect model is used for Meta analysis. If not, there are statistical differences in heterogeneity, and the random effect model is used for Meta analysis. Publication bias was assessed using a funnel plot, while sensitivity analysis was conducted to ensure the stability and reproducibility of the research findings. Potential publication bias was examined by Begg and Egger tests, with statistical significance set at P < 0.05.
3 Result
3.1 Results of literature retrieval
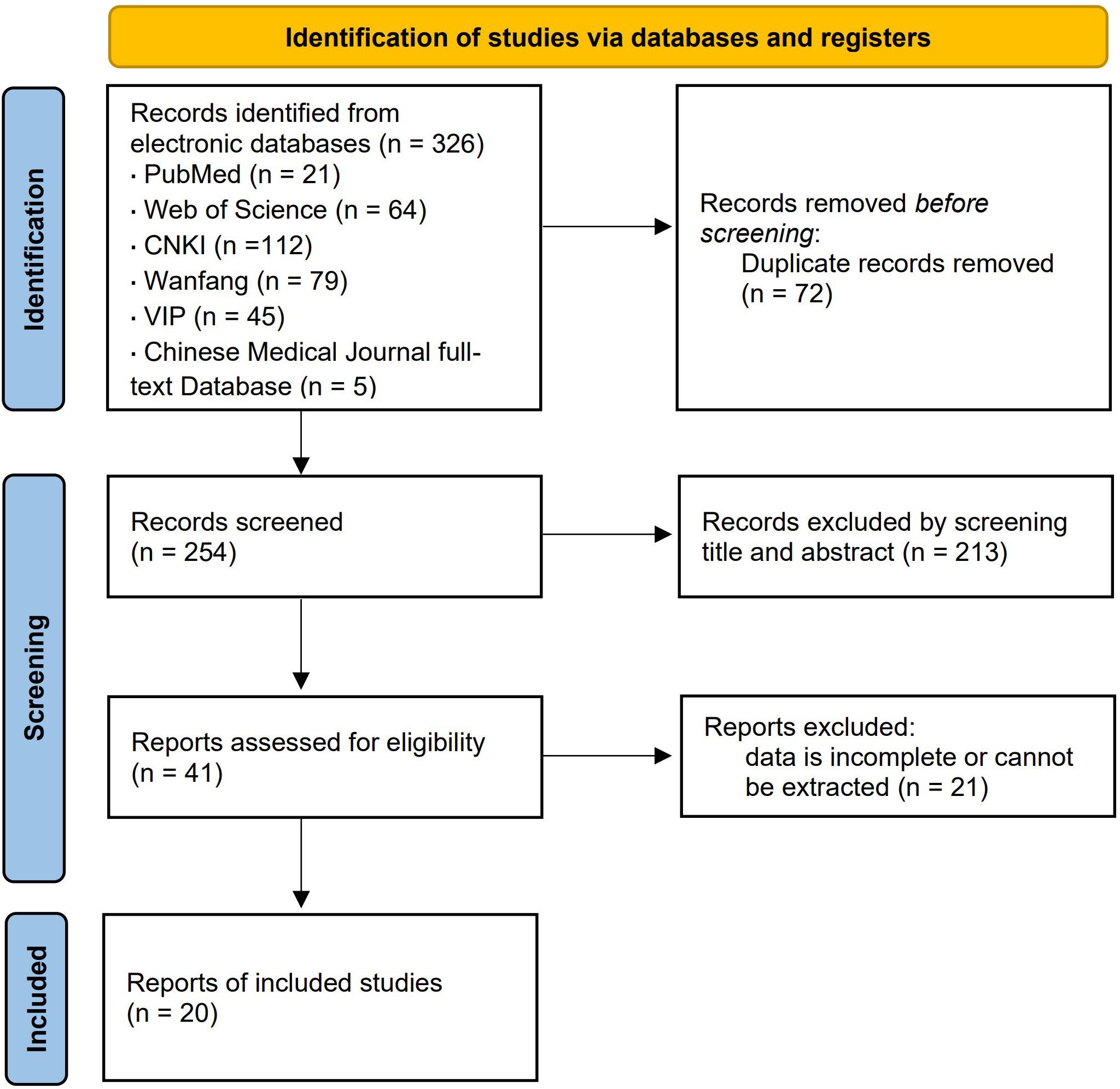
A total of 326 related literature was found, and 254 articles were acquired following re-selection and primary screening. After reviewing the entire text, 20 case-control studies were analyzed, with a total sample size of 4 565 cases, distributed among healthy control (CN) group (n = 643), simple diabetes mellitus (DM) group (n = 1 649), microalbuminuria (MI) group (n = 1 305), and macroalbuminuria (MA) group (n = 968). The literature screening process and results are shown in Figure 1.
3.2 Basic characteristics and quality evaluation of included literature
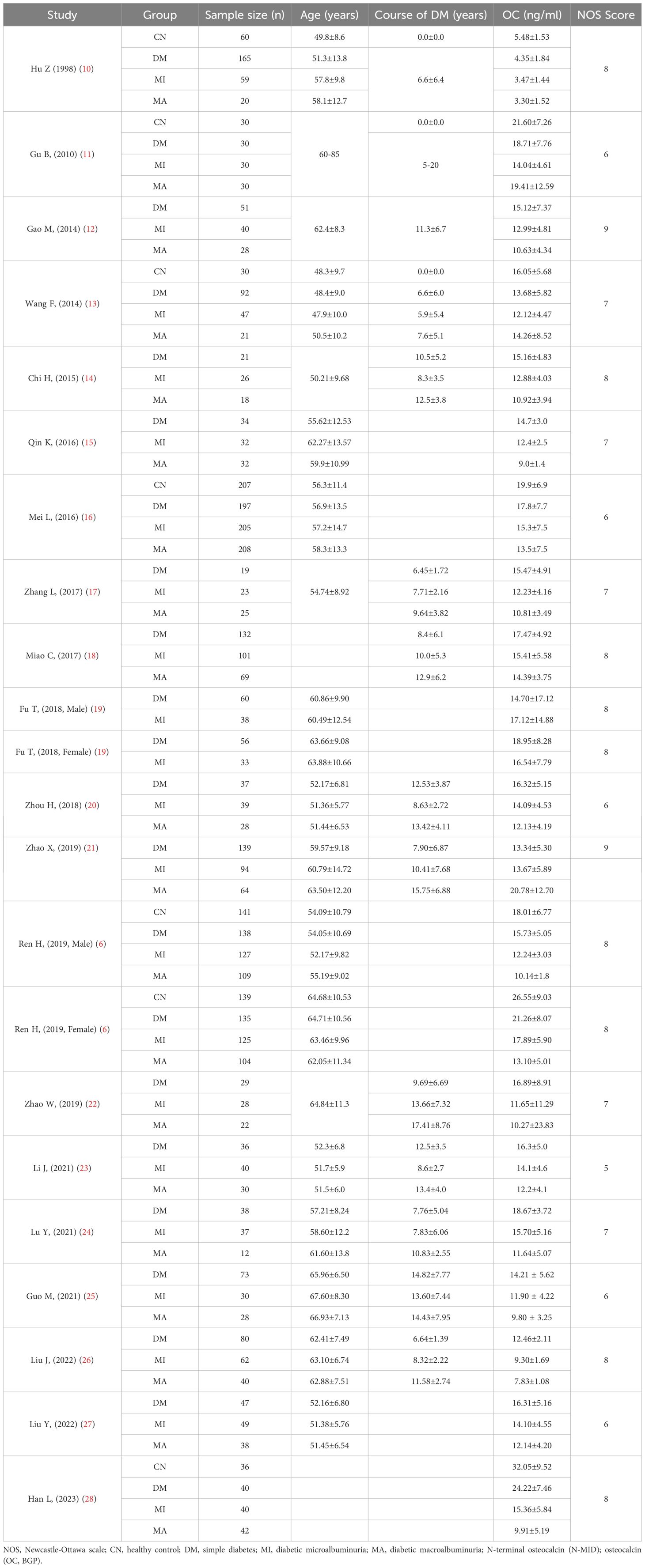
A total of 20 studies were included, all of which were case-control studies, all of which were Chinese population, involving a total of 4565 samples. The Newcastle-Ottawa Scale is used to evaluate the quality of 20 included studies. Following a quality assessment, each piece of literature received a score ranging from 5 to 9, indicating a medium to high level of quality. The basic characteristics of the included study and the NOS score of the literature are shown in Table 1.
3.3 Results of meta-analysis
3.3.1 Comparison of serum osteocalcin between MI group and CN group
6 studies (6, 10, 11, 13, 16, 28) were compared between the MI group (n = 633) and the CN group (n = 643). Heterogeneity analysis showed Q = 34.05, I2 = 82.4%, P < 0.01, and the random effect model was applied. The meta-analysis results indicated a significant decrease in serum osteocalcin levels in the MI group compared to the CN group [SMD = -1.15, 95% CI (-1.46, -0.85), P < 0.01]. As shown in Figure 2.
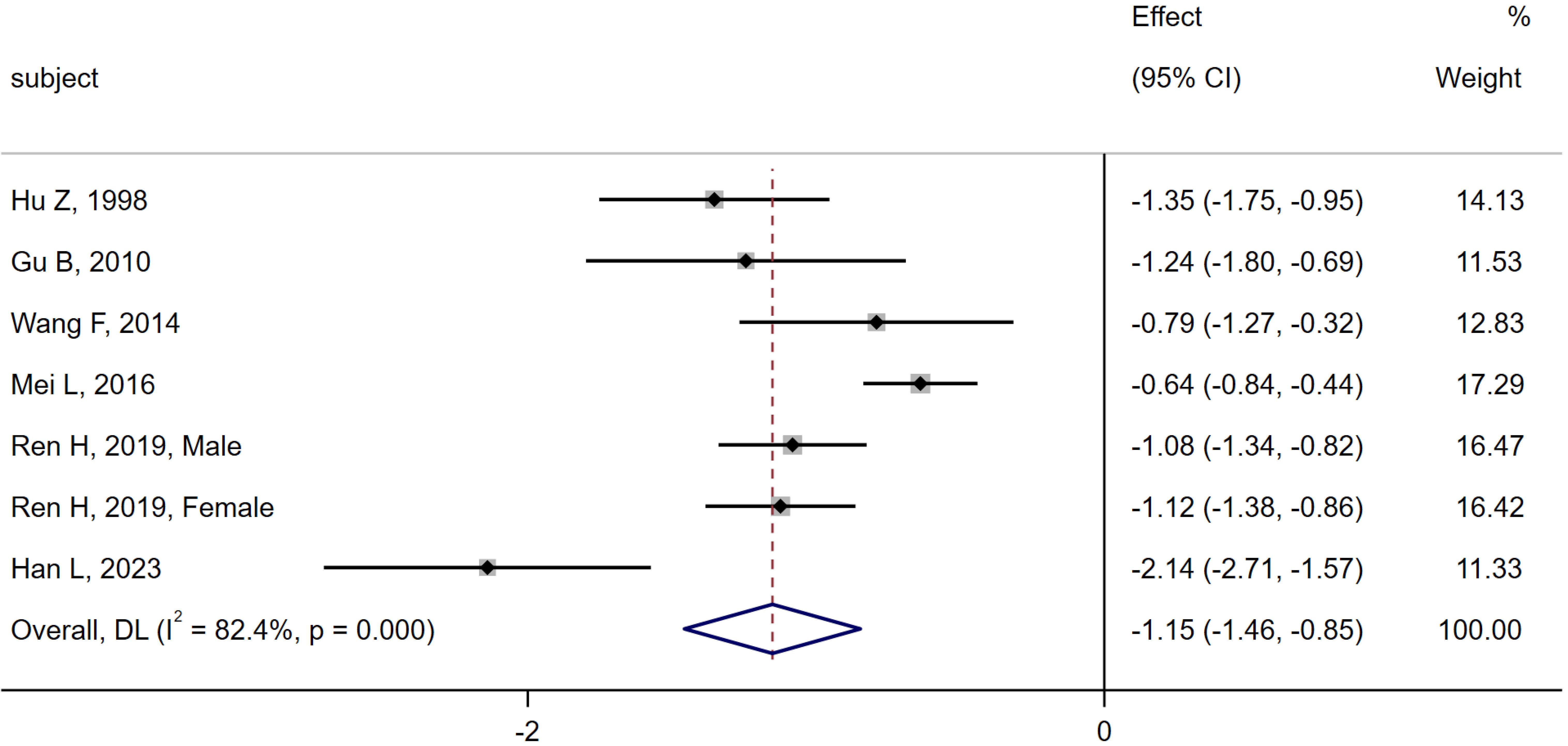
Figure 2. Meta-analysis forest plot comparing serum osteocalcin levels between MI group and CN group.
3.3.2 Comparison of serum osteocalcin between MI group and DM group
20 studies (6, 10–28) were compared between the MI group (n = 1 305) and the DM group (n = 1 649). Heterogeneity analysis indicated Q = 88.21, I2 = 76.2%, P < 0.01, and the random effect model was used for analysis. The meta-analysis results showed that the serum osteocalcin level in the MI group was significantly lower than that in the DM group [SMD = -0.53, 95% CI (-0.69, -0.37), P < 0.01]. As shown in Figure 3.
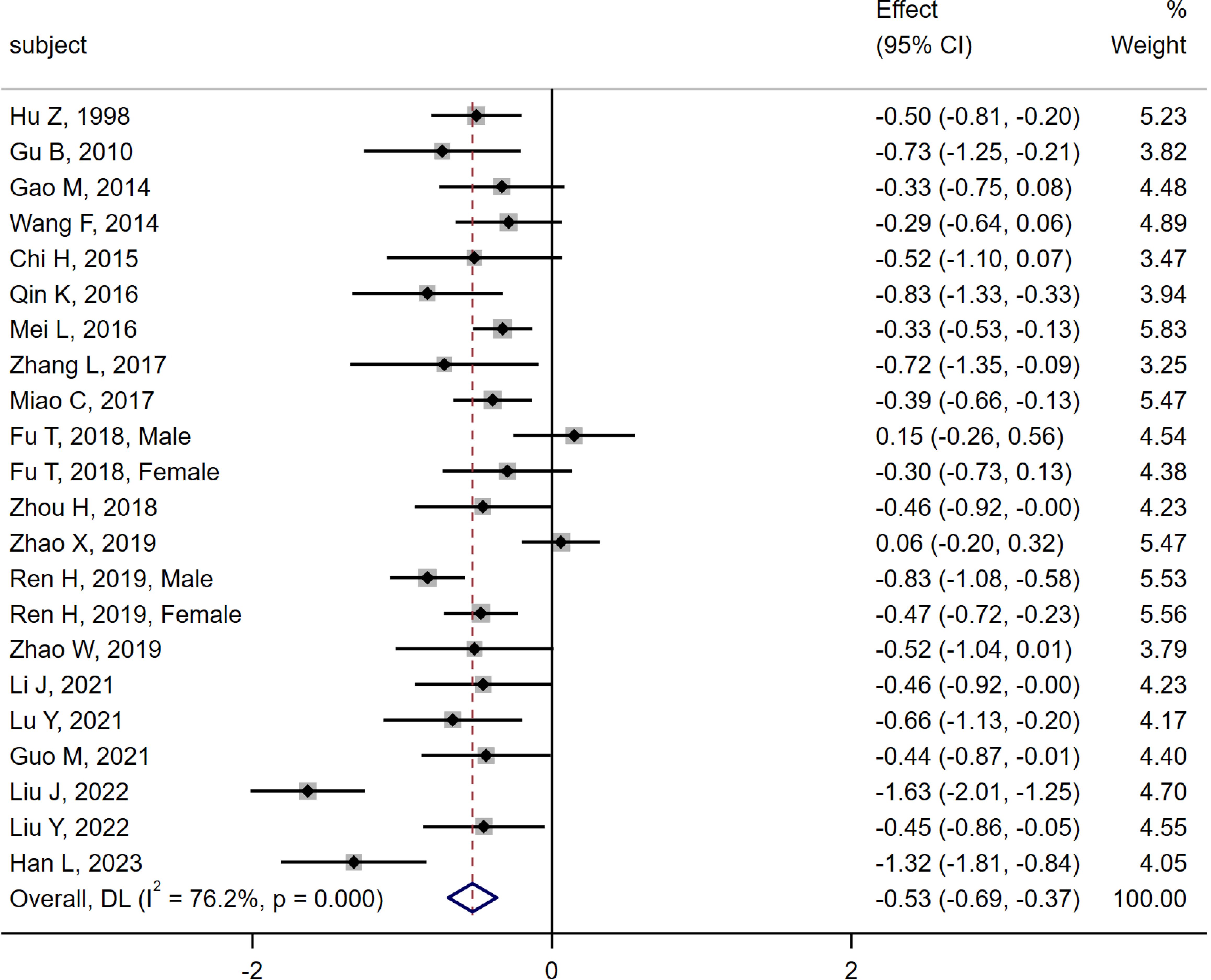
Figure 3. Meta-analysis forest plot comparing serum osteocalcin levels between MI group and DM group.
3.3.3 Comparison of serum osteocalcin between MA group and CN Group
6 studies (6, 10, 11, 13, 16, 28) were compared between the MA group (n = 534) and the CN group (n = 643). Heterogeneity analysis indicated Q=82.08, I2 = 92.7%, P < 0.01, thus the random effect model was used. The meta-analysis showed a significant decrease in serum osteocalcin levels in the MA group compared to the CN group [SMD = -1.28, 95% CI (-1.79, -0.76), P < 0.01]. As shown in Figure 4.
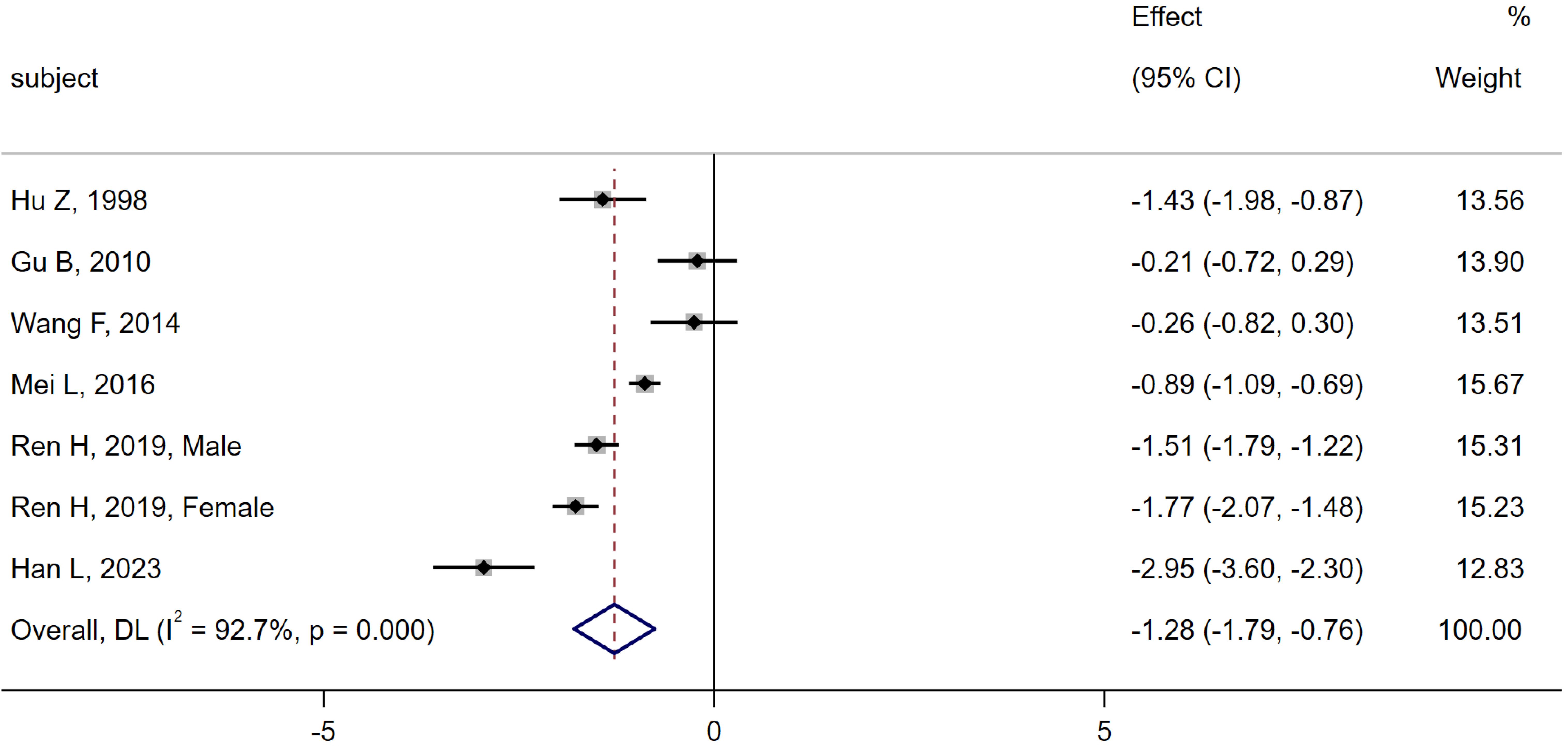
Figure 4. Meta-analysis forest plot comparing serum osteocalcin levels between MA group and CN group.
3.3.4 Comparison of serum osteocalcin between MA group and DM group
19 studies (6, 10–18, 20–28) were compared between the MA group (n = 968) and the DM group (n = 1 533). Heterogeneity analysis indicated Q = 278.45, I2 = 93.2%, P < 0.01, so the random effect model was used. The results of the meta-analysis showed that the level of serum osteocalcin in the MA group was significantly lower than that in the DM group [SMD = -0.93, 95% CI (-1.28, -0.58), P < 0.01]. As shown in Figure 5.
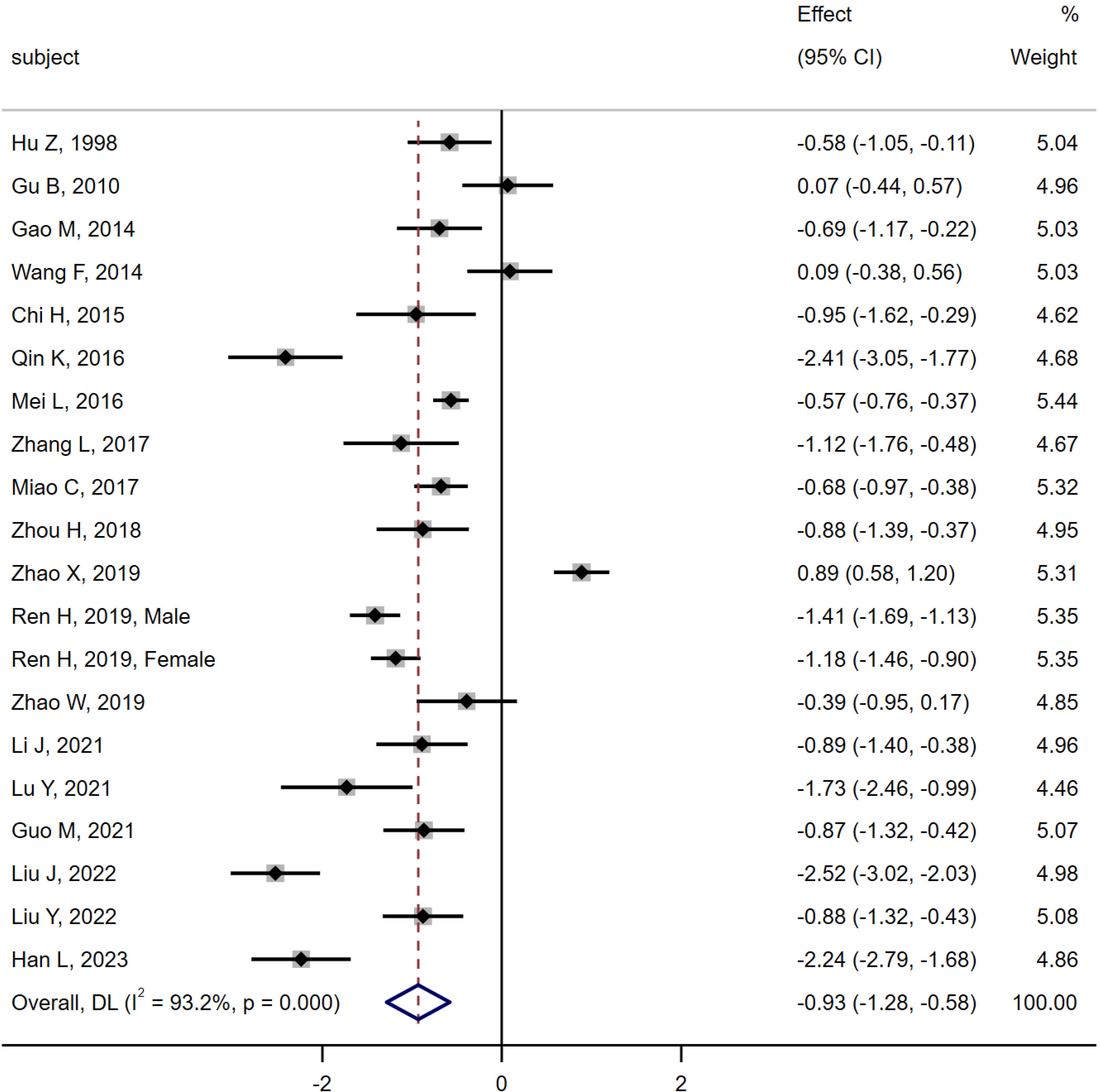
Figure 5. Meta-analysis forest plot comparing serum osteocalcin levels between MA group and DM group.
3.3.5 Comparison of serum osteocalcin between MA group and MI group
19 studies (6, 10–18, 20–28) were compared between the MA group (n = 968) and the DM group (n = 1 234). The results of heterogeneity analysis were as follows Q =133.04, I2 = 85.7%, P < 0.01, using random effect model. The results of the meta-analysis showed that the level of serum osteocalcin in the MA group was significantly lower than that in the MI group [SMD = -0.41, 95% CI (-0.65, -0.17), P < 0.01]. As shown in Figure 6.
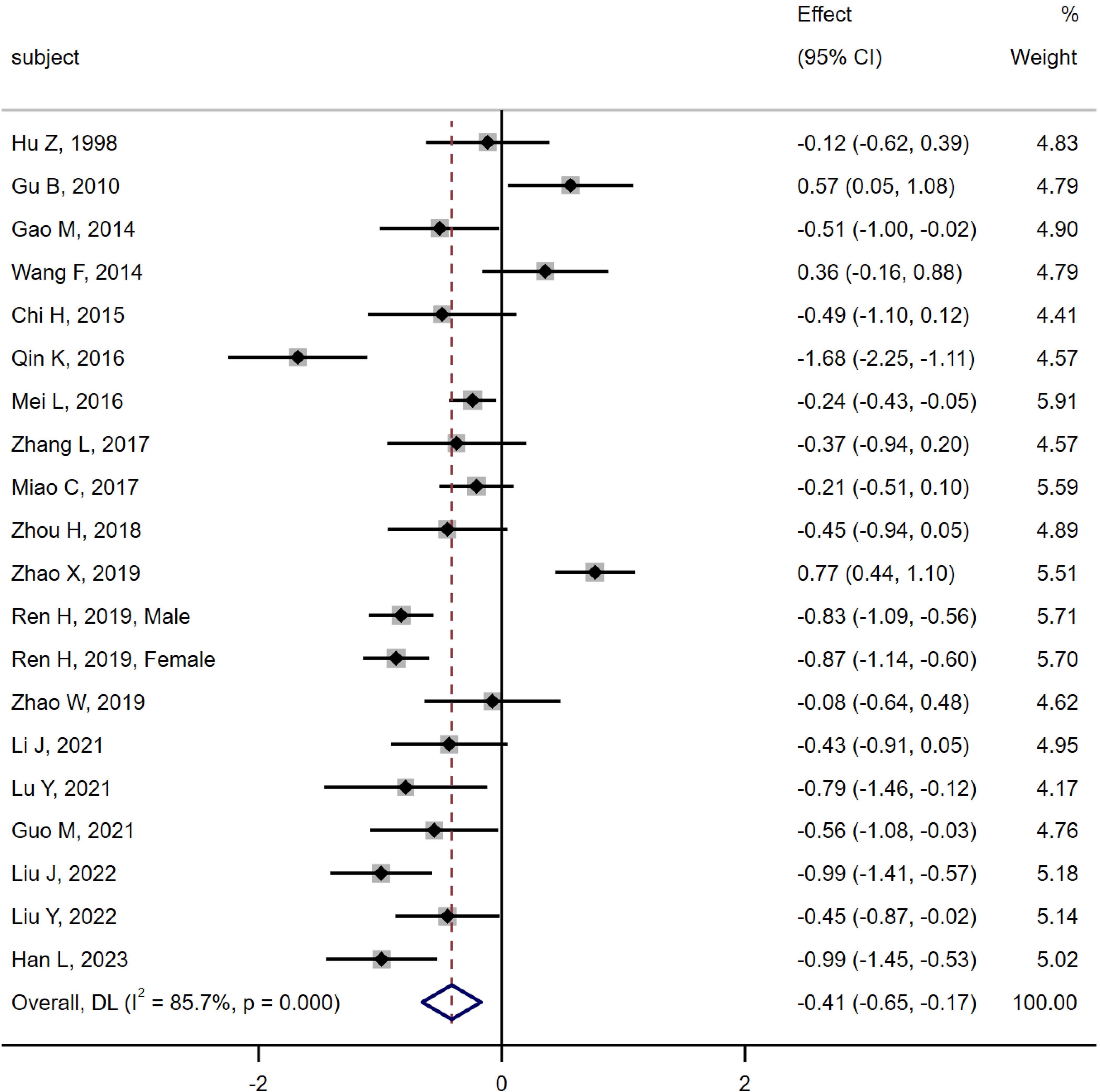
Figure 6. Meta-analysis forest plot comparing serum osteocalcin levels between MA group and MI group.
3.4 Sensitivity analysis
The sensitivity of each group included in the study was analyzed by excluding a single research one by one. The study results showed that the combined SMD value and the 95% CI were not significantly changed before and after exclusion in all study groups, with no statistically significant difference appearing. The preceding meta-analysis is robust and the findings are dependable (Figure 7).

Figure 7. Sensitivity analysis. (A) Sensitivity analysis of MI group and CN group; (B) Sensitivity analysis of MI group and DM group; (C) Sensitivity analysis of MA group and CN group; (D) Sensitivity analysis of MA group and DM group; (E) Sensitivity analysis of MA group and MI group.
3.5 Publication bias
Publication bias was assessed by drawing funnel plots, which showed some graphical asymmetry (Figure 8), indicating publication bias. The reasons may be related to the poor quality of some literature, the complexity of the sample population, and the existence of unpublished negative results. The Begg test and Egger test results showed no statistical difference in the potential publication bias analysis of the combined effect size of the included groups. The details are as follows: MI group vs CN group: Begg’s test, P = 0.133, Egger’s test, P = 0.081; MI group vs DM group: Begg’s test, P=0.063, Egger’s test, P=0.236; MA group vs CN group: Begg’s test, P=0.368, Egger’s test, P=0.745; MA group vs DM group: Begg’s test, P=0.256, Egger’s test, P=0.251; MA group vs MI group: Begg’s test, P=0.974, Egger’s test, P=0.823.
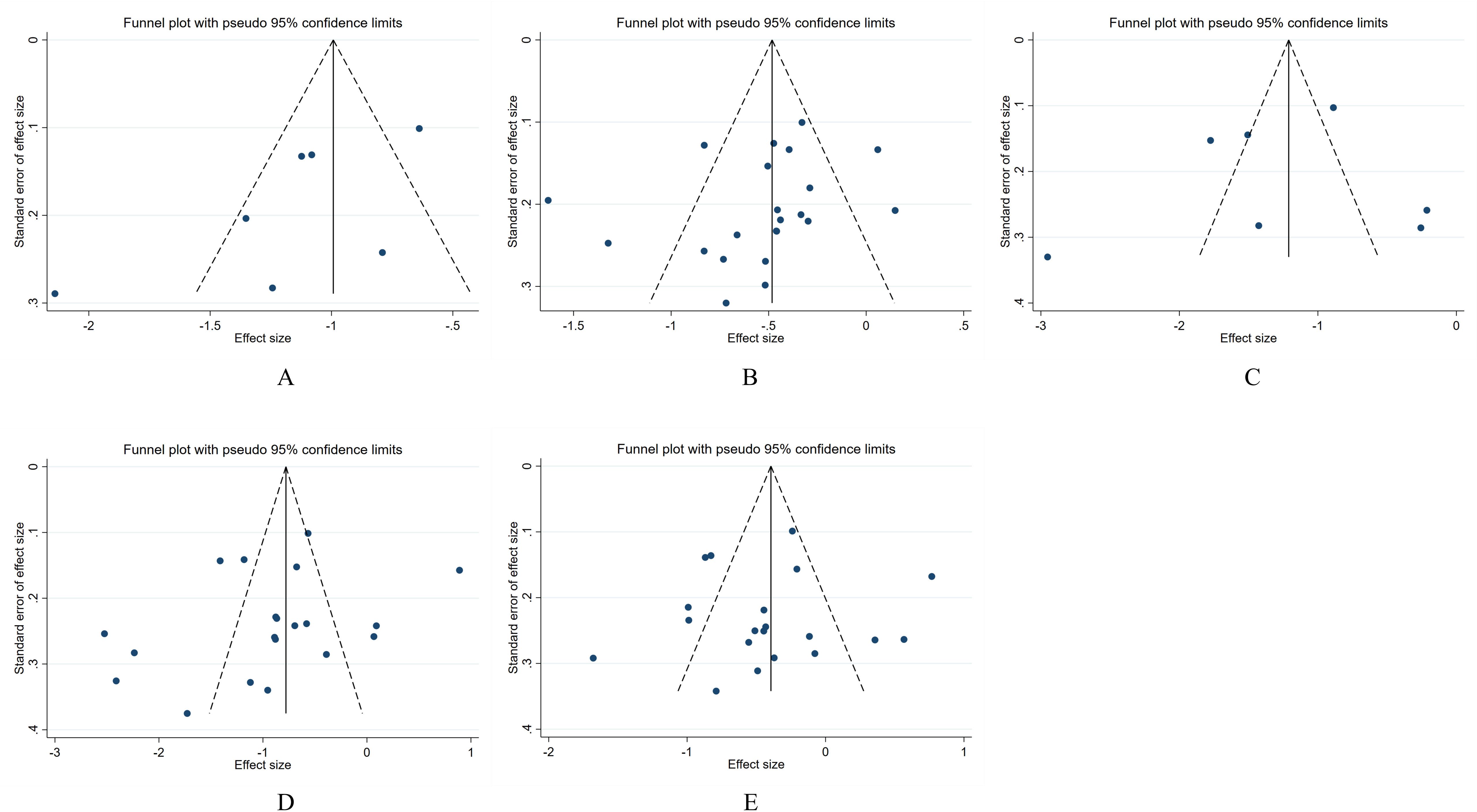
Figure 8. Publication bias of the relationship between serum osteocalcin levels and the progression of DKD in the Chinese population. (A) Analysis of publication bias between MI group and CN group; (B) Analysis of publication bias between MI group and DM group; (C) Analysis of publication bias between MA group and CN group; (D) Analysis of publication bias between MA group and DM group; (E) Analysis of publication bias between MA group and MI group.
4 Discussion
The prevalence of osteoporosis in China is increasing quickly due to the aging population. The survey found that osteoporosis affects 19.2% of individuals over 50 years old in China and 32.0% of those over 65 years old, with women accounting for 51.6% of cases (29). And studies have shown that patients with diabetes and/or chronic kidney disease have a higher risk of osteoporosis and brittle fractures than the general population (30, 31). DKD is not only a common microvascular complication of diabetes, but also the main cause of chronic kidney disease and ESRD (2). Therefore, the DKD population should pay more attention to the screening and prevention of osteoporosis to avoid fractures.
Osteoporosis has no noticeable symptoms in the early stages, but abnormal bone metabolism occurs. Many patients are not detected and diagnosed until fractures occur, which often places a heavy burden on individuals, families, and society. Dual energy X-ray absorptiometry (DXA) is currently the preferred method for diagnosing osteoporosis in clinical settings. However, it has limits in early diagnosis, predication of fracture risk and evaluation of therapeutic effect. On the contrary, although bone metabolism indicators are not suitable for diagnosing osteoporosis, they can reflect early bone metabolism abnormalities due to their high sensitivity and specificity (32), thus are functional in differential diagnosis, risk prediction, bone turnover type judgment, and treatment evaluation of osteoporosis (33), and are widely used in clinical practice.
Osteocalcin, encoded by the bone γ-carboxyglutamate protein gene, is the only non-collagenous protein secreted by osteoblasts during osteogenesis, and the expression of its biological activity is vitamin K-dependent (34). Serum osteocalcin is relatively stable and can directly reflect the status of bone formation, so it has been used as a clinical indicator of bone formation markers (33). Interestingly, new studies have found that osteocalcin, although secreted by osteoblasts during bone formation, is released into the circulation during bone resorption, and is therefore also recognized as a bone turnover marker (35).
This meta-analysis indicates that the serum osteocalcin levels in Chinese patients with DKD are significantly lower than in healthy population and patients with simple DM. With the increase of the diabetes course and the severity of proteinuria in DKD patients, the serum osteocalcin level exhibits a gradual decline, indicating that patients with DKD are characterized by a decrease in osteoblast activity at the early stage and low conversion type of bone turnover.
The mechanism of reduced bone formation in patients with DKD has not been fully elucidated and may be related to the following factors. First, renal damage: In patients with DKD, there is impaired renal filtration and reabsorption functions leading to increased loss of calcium, phosphorus, and various vitamins from the kidney. This results in decreased raw materials for bone formation, reduced function and activity of osteoblasts, and an imbalance between bone formation and resorption, ultimately causing osteoporosis (36). In addition, DKD patients can indirectly stimulate parathyroid hormone secretion, causing secondary hyperparathyroidism, which leads to increased osteoclast activity and decreased bone mineralization (37), further exacerbating the bone conversion imbalance. Second, 25-hydroxyvitamin D deficiency: The decrease of serum 25-hydroxyvitamin D level was common in patients with DKD, and the degree of decrease was positively correlated with the progress of DKD (38). 25-hydroxyvitamin D deficiency can promote the dysfunction of calcium and phosphorus reabsorption in renal tubules, inhibit osteocalcin synthesis by osteoblasts, decrease bone mineralization, and increase the risk of osteoporosis (36). Third, hyperglycemia: In DKD patients, insulin deficiency or resistance leads to poor control of blood glucose and disorder of glucose and lipid metabolism, which can lead to the accumulation of pathological products such as advanced glycation end products (AGEs) (39) and proinflammatory cytokines (ICs) (40), thus reducing bone formation and osteoblast activity and increasing bone resorption and osteoclast activity. And other studies have shown that chronic hyperglycemia can reduce osteocalcin gene transcription and osteoblast differentiation at the gene transcription level (41, 42). In addition, long-term hyperglycemia will also cause microvascular lesions in the body. when bone microvascular lesions occur, the bone blood supply is insufficient and bone metabolism is out of balance, resulting in the destruction of bone microstructure and the decrease of bone formation and bone mineralization level (43). Fourth, insulin resistance: Insulin at a biological dose can enhance osteoblast proliferation, differentiation, and activity while reducing osteoclast activity, but in cases of insulin resistance, there is a disruption in bone energy metabolism leading to increased bone matrix consumption, decreased bone formation, reduced bone turnover, and increased bone fragility (44). Fifth, diabetic gastrointestinal dysfunction: Patients with DKD have been experiencing diabetes for an extended period, leading to complications such as gastrointestinal dysfunction. Due to reduced gastrointestinal absorption function and dietary restrictions imposed by diabetes, the intake of calcium, phosphorus, and vitamins may not suffice to support normal bone formation, resulting in decreased bone formation (45). Sixth, antidiabetic therapy: Cipriani and his colleagues reviewed the published studies and found that long-term use of antidiabetic drugs such as insulin and thiazolidinediones can have a negative impact on bone health (46), which is one of the risk factors for fracture and osteoporosis. Seventh, senium: Human serum osteocalcin levels peak in young individuals and subsequently drop with aging and declining exercise ability (47, 48). Correspondingly, the proliferation and differentiation of osteoblasts decreased during senescence, and the physiological function reduced, resulting in a decrease in the level of bone formation. Furthermore, the outdoor activities of the elderly decreased, the synthesis of 25-hydroxyvitamin D decreased, and the effect of bone stress was weakened, which further promoted the reduction of bone formation. In summary, DKD is a chronic microvascular complication of diabetes, and most of its patients are elderly and have a long course of disease. The decrease in bone formation in patients with DKD may be the result of various pathological conditions caused by prolonged diabetes and aging (Figure 9).
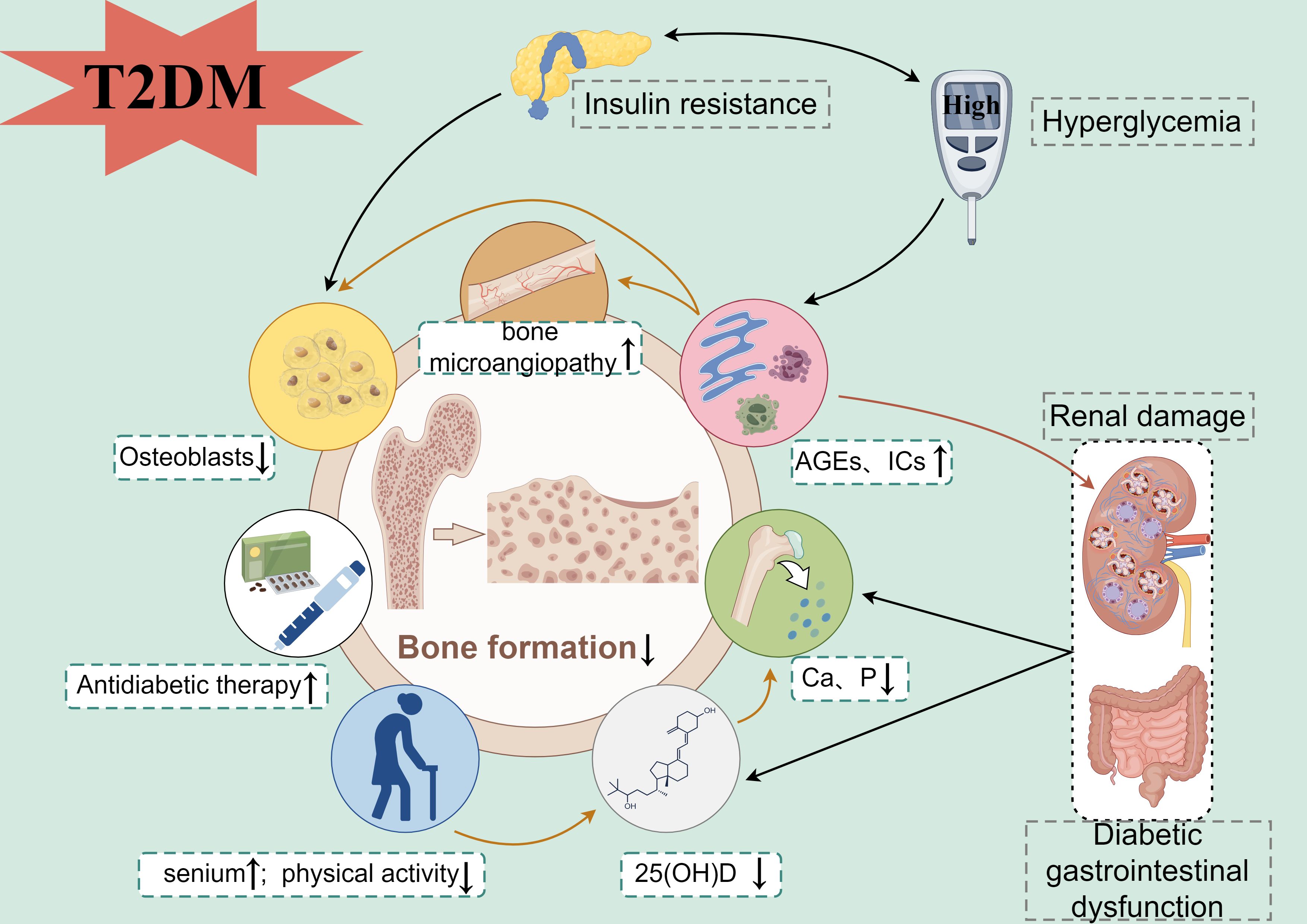
Figure 9. Mechanism map of bone formation reduction in patients with DKD (By Figdraw). T2DM, type 2 diabetes mellitus; AGEs, advanced glycation end products; ICs, pro-inflammatory cytokines; Ca, Calcium; P, Phosphorus; 25(OH)D, 25-hydroxyvitamin D.
Interestingly, the four included studies (11, 13, 19, 21) showed that patients in the MA group had higher osteocalcin levels than those in the MI or DM group, inconsistent with the results obtained from the meta-analysis. The reasons may be related to the changed lifestyle and the decline in the different degrees of renal function in the macroalbuminuria population. Patients with macroalbuminuria have more obvious clinical symptoms and abnormal test indicators, which may force them to pay more attention to lifestyle and physical activity, affecting the original bone turnover. Furthermore, patients with macroalbuminuria or renal failure have significantly increased renal function impairment, the role of renal tubules gradually changes from compensatory increase to decrease, and secondary hyperparathyroidism results in pathological enhancement of both osteoblast and osteoclast activities (49). At this time, the transformation of osteoclast to osteoblast is accelerated, serum osteocalcin levels also increase, and bone turnover changes from a low turnover type in early DKD to a high one. It shows that bone metabolism in DKD patients may have inconsistent characteristics at different stages of renal damage.
The limitations of this study are as follows. First, some included studies have small sample sizes, particularly for MA groups, which may reduce the reliability of the meta-analysis. Second, the methodological quality of this study is relatively low. Despite rigorous screening of included studies according to uniform criteria, there is still biggish heterogeneity in the meta-analysis, which may be related to differences in study design, sample population selection, and measurement methods of OC among included studies. Third, this study only included the Chinese population, and the lack of diversity in population selection may limit the universality of the results to other ethnic groups with different genetic backgrounds and regions. Forth, due to the complexity of the sample population, the study could not exclude confounding factors such as diet, physical activity, and other comorbidities. Finally, the funnel plots show that this study has publication bias, which may affect the accurate judgment of the association between osteocalcin and DKD. Therefore, to verify this study, more clinical studies with rigorous design, authentic records, and large sample sizes are needed. Recommending that future research on serum osteocalcin levels and the progression of DKD should focus more on improving methodological quality, clearly recording the characteristics of the study population, unifying serum osteocalcin measurement methods, and expanding the sample size and the representativeness of the study population, to provide better evidence-based medical evidence for subsequent research and clinical practice.
5 Conclusion
This research indicates that the decrease in serum osteocalcin is prevalent among Chinese patients with DKD. This reduction is strongly related to the severity of proteinuria, indicating a decline in bone formation at the early stages of DKD that worsens as the disease progresses. However, due to the limitations of the quantity and quality of the included studies, the above conclusions need to be verified by more high-quality, large-sample clinical studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
XH: Validation, Investigation, Writing – review & editing, Writing – original draft, Data curation. XW: Writing – review & editing, Supervision, Data curation. CC: Writing – review & editing, Supervision, Data curation. JG: Writing – review & editing, Investigation. XQ: Writing – review & editing, Investigation. JY: Writing – review & editing, Validation, Methodology. LH: Writing – review & editing, Validation, Supervision, Methodology. SX: Writing – review & editing, Validation, Supervision, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Chinese Medicine Science and Technology Development Plan Project of Jiangsu Province (ZD202319), Science and Technology Development Project from Jiangsu Provincial Health Commission (K2023022), and Special Project for Promoting Directors of Department from Jiangsu Province Hospital of Chinese Medicine (Y2022ZR08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng HT, Xu X, Lim PS, Hung KY. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000-2015. Diabetes Care. (2021) 44:89–97. doi: 10.2337/dc20-1913
2. Expert Group of Chinese Society of Nephrology. Chinese guidelines for diagnosis and treatment of diabetic kidney disease. Chin J Nephrol. (2021) 37:255–304. doi: 10.3760/cma.j.cn441217-20201125-00041
3. Tasnim N, Dutta P, Nayeem J, Masud P, Ferdousi A, Ghosh AS, et al. Osteoporosis, an inevitable circumstance of chronic kidney disease: A systematic review. Cureus. (2021) 13:e18488. doi: 10.7759/cureus.18488
4. Hauge SC, Frost M, Hansen D. Understanding bone disease in patients with diabetic kidney disease: a narrative review. Curr Osteoporos Rep. (2020) 18:727–36. doi: 10.1007/s11914-020-00630-2
5. Komori T. Functions of osteocalcin in bone, pancreas, testis, and muscle. Int J Mol Sci. (2020) 21:7513. doi: 10.3390/ijms21207513
6. Ren H, Ma X, Shao Y, Han J, Yang M, Wang Q. Correlation between serum miR-154-5p and osteocalcin in males and postmenopausal females of type 2 diabetes with different urinary albumin creatinine ratios. Front Endocrinol. (2019) 10:542. doi: 10.3389/fendo.2019.00542
7. Ye X, Yu R, Jiang F, Hou X, Wei L, Bao Y, et al. Osteocalcin and risks of incident diabetes and diabetic kidney disease: A 4.6-year prospective cohort study. Diabetes Care. (2022) 45:830–6. doi: 10.2337/dc21-2113
8. American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes—2024. Diabetes Care. (2024) 47:S20–42. doi: 10.2337/dc24-S002
9. Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. (1983) 32:64–78. doi: 10.2337/diab.32.2.s64
10. Hu Z, Zhang H, Mao T. Changes of serum osteocalcin in patients with diabetic nephropathy. Chin J Osteoporos. (1998) 4:43–45+80.
11. Gu B, Lu JY, Yi Y, Wang HQ, Dong BY. Bone metabolism in different phase of elder type 2 diabetic nephropathy patients. Chin J Postgrad Med. (2010) 33:1–4. doi: 10.3760/cma.j.issn.1673-4904.2010.28.001
12. Gao M, Wang DF, Lin YC, Yang HJ, Lv XN, Fei N, et al. Clinical study of bone mineral density and bone metabolism markers in patients with diabetic nephropathy. Chin J Osteoporos. (2014) 20:166–70. doi: 10.3969/j.issn.1006-7108.2014.02.012
13. Wang F, Qin GJ, Li ZZ, Zhang YH, Wang XJ, Shao MW. Effect of chronic kidney disease of male patients aged 25 to 65 years with diabetes mellitus on bone metabolism. Chin J Diabetes. (2014) 22:811–3. doi: 10.3969/j.issn.1006-6187.2014.09.012
14. Chi HY, Zhou YP, Wang YD. The serum levels of N-MID, PINP, and β-CTX in patients with different stage of diabetic nephropathy. Chin J Osteoporos. (2015) 21:855–7. doi: 10.3969/j.issn.1006-7108.2015.07.020
15. Qin K. Changes in bone metabolism markers and bone mineral density in male patients with type 2 diabetic nephropathy. Shanxi Med University. (2016).
16. Mei LP, Sun XD, Ruan Y. Research on the changes of biochemical markers of bone turnover and bone density in male patients with different stages of diabetic nephropathy. Chin J Health Lab Tec. (2016) 26:74–7.
17. Zhang LM, Li J, Li HY, Gao Y, Bi CY, Tian Y. Differences and relationships of bone mineral density and serum N-terminal osteocalcin, type I collagen amino-terminal propeptide in patients with diabetic nephropathy at different stages. Chin J Gerontol. (2017) 37:4522–4. doi: 10.3969/j.issn.1005-9202.2017.18.040
18. Miao CX, Feng ZP. A correlation analysis of urinary albumin excretion rate and biochemical markers of bone metabolism in patients with type 2 diabetes mellitus. Chin J Osteoporos. (2017) 23:1596–9. doi: 10.3969/j.issn.1006-7108.2017.12.012
19. Fu T. The relevant study of urinary microalbumin and bone mineral density and bone metabolism markers in patients with type 2 diabetes mellitus. Kunming Med University. (2019).
20. Zhou H, Deng XH. The change and clinical significance of bone mineral density and bone metabolism in patients with different stage of type 2 diabetic nephropathy. MMJC. (2018) 20:27–30. doi: 10.3969/j.issn.1672-9463.2018.07.008
21. Zhao X, Zhang XM, Yuan N, Yu XF, Ji LN. Associations of bone mineral density and bone metabolism indices with urine albumin to creatinine ratio in chinese patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. (2019) 127:50–5. doi: 10.1055/a-0762-0341
22. Zhao WJ, Li HF, Li M, Tian LL, Xie HJ, Ma L. Investigation of the correlation between bone turnover index and bone mass in early elderly individuals with type 2 diabetes mellitus. J Qiqihar Med University. (2019) 40:2125–7. doi: 10.3969/j.issn.1002-1256.2019.17.005
23. Li JY, Li G. Analysis of correlation between type 2 diabetic nephropathy and bone mineral density in middle-aged and elderly males. China Prac Med. (2021) 16:36–9. doi: 10.14163/j.cnki.11-5547/r.2021.21.013
24. Lu YY, Hu YS, Wang Y, Chen L, Xu Y. The relationship of bone conversion markers and urinary albumin creatinine ratio in type 2 diabetes. Chin J Clin Healthc. (2021) 24:495–8. doi: 10.3969/J.issn.1672-6790.2021.04.013
25. Guo MY, Wang SJ. Changes of bone metabolism and related factors in elderly female patients with type 2 diabetic nephropathy. Henan Med Res. (2021) 30:997–1000. doi: 10.3969/j.issn.1004-437X.2021.06.010
26. Liu J, Liu Y, Pan D. Correlation between urinary albumin excretion rate and serum P1NP, osteocalcin and 25(OH)D3 levels in patients with type 2 diabetes mellitus. Hainan Med J. (2022) 33:21–4. doi: 10.3969/j.issn.1003-6350.2022.01.006
27. Liu YH. Changes and clinical significance of bone mineral density and bone metabolism markers in patients with type 2 diabetic nephropathy in different stages. Sys Med. (2022) 7:21–4. doi: 10.19368/j.cnki.2096-1782.2022.02.021
28. Han L, Wang S, Ma J, Song N, Wang Z, Yao M. Changes of Serum Bone Metabolism Indexes and Ultrasonic Bone Mineral Density in Patients with Diabetic Nephropathy at Different Stages and their effects on Diabetic Renal Microvascular Complications. Pak J Med Sci. (2023) 39:656–61. doi: 10.12669/pjms.39.3.6650
29. Chinese Society of Osteoporosis and Bone Mineral Research. Guidelines for the diagnosis and treatment of primary osteoporosis (2022). Chin Gen Pract. (2023) 26:1671–91. doi: 10.12114/j.issn.1007-9572.2023.0121
30. Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone–osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. (2012) 8:297–305. doi: 10.1038/nrendo.2011.233
31. Khairallah P, Nickolas TL. Updates in CKD-associated osteoporosis. Curr Osteoporos Rep. (2018) 16:712–23. doi: 10.1007/s11914-018-0491-3
32. Brown JP, Don-Wauchope A, Douville P, Albert C, Vasikaran SD. Current use of bone turnover markers in the management of osteoporosis. Clin Biochem. (2022) 109-110:1–10. doi: 10.1016/j.clinbiochem.2022.09.002
33. Bone Metabolism Expert Group of〚Chinese Journal of Osteoporosis〛. Expert consensus on clinical application of biochemical indicators of bone metabolism (2023 Revision). Chin J Osteoporos. (2023) 29:469–76. doi: 10.3969/j.issn.1006-7108.2023.04.001
34. Al-Suhaimi EA, Al-Jafary MA. Endocrine roles of vitamin K-dependent- osteocalcin in the relation between bone metabolism and metabolic disorders. Rev Endocr Metab Disord. (2020) 21:117–25. doi: 10.1007/s11154-019-09517-9
35. Chi PJ, Hung SY, Hsiao FT, Liou HH, Tsai JP. Serum osteocalcin concentration as an independent biomarker of osteoporosis in patients with chronic kidney disease. Clin Nephrol. (2022) 98:1–9. doi: 10.5414/CN110705
36. Hsu CY, Chen LR, Chen KH. Osteoporosis in patients with chronic kidney diseases: A systemic review. Int J Mol Sci. (2020) 21:6846. doi: 10.3390/ijms21186846
37. Coyne DW, Andress DL, Amdahl MJ, Ritz E, de Zeeuw D. Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant. (2013) 28:2260–8. doi: 10.1093/ndt/gft227
38. Du Y, Jiang HW, Li CQ, Xie Y. Correlation between 25-hydroxyvitamin D and the risk of type 2 diabetic nephropathy in Chinese population: a meta-analysis. Hainan Med J. (2021) 32:119–27. doi: 10.3969/j.issn.1003-6350.2021.01.033
39. Gao L, Liu C, Hu P, Wang N, Bao X, Wang B, et al. The role of advanced glycation end products in fracture risk assessment in postmenopausal type 2 diabetic patients. Front Endocrinol (Lausanne). (2022) 13:1013397. doi: 10.3389/fendo.2022.1013397
40. Serban AI, Stanca L, Geicu OI, Dinischiotu A. AGEs-induced IL-6 synthesis precedes RAGE up-regulation in HEK 293 cells: an alternative inflammatory mechanism? Int J Mol Sci. (2015) 16:20100–17. doi: 10.3390/ijms160920100
41. Bilotta FL, Arcidiacono B, Messineo S, Greco M, Chiefari E, Britti D, et al. Insulin and osteocalcin: further evidence for a mutual cross-talk. Endocrine. (2018) 59:622–32. doi: 10.1007/s12020-017-1396-0
42. Wu M, Ai W, Chen L, Zhao S, Liu E. Bradykinin receptors and EphB2/EphrinB2 pathway in response to high glucose-induced osteoblast dysfunction and hyperglycemia-induced bone deterioration in mice. Int J Mol Med. (2016) 37:565–74. doi: 10.3892/ijmm.2016.2457
43. Mauricio D, Gratacòs M, Franch-Nadal J. Diabetic microvascular disease in non-classical beds: the hidden impact beyond the retina, the kidney, and the peripheral nerves. Cardiovasc Diabetol. (2023) 22:314. doi: 10.1186/s12933-023-02056-3
44. Conte C, Epstein S, Napoli N. Insulin resistance and bone: a biological partnership. Acta Diabetol. (2018) 55:305–14. doi: 10.1007/s00592-018-1101-7
45. Sobh MM, Abdalbary M, Elnagar S, Nagy E, Elshabrawy N, Abdelsalam M, et al. Secondary osteoporosis and metabolic bone diseases. J Clin Med. (2022) 11:2382. doi: 10.3390/jcm11092382
46. Cipriani C, Lauriero G, Tripepi G, Ferrari S, Bover J, Ravera M, et al. Effect of antidiabetic drugs on bone health in patients with normal renal function and in chronic kidney disease (CKD): insight into clinical challenges in the treatment of type 2 diabetes. J Clin Med. (2023) 12:7260. doi: 10.3390/jcm12237260
47. Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. (2016) 23:1078–92. doi: 10.1016/j.cmet.2016.05.004
48. Berger JM, Karsenty G. Osteocalcin and the physiology of danger. FEBS Lett. (2022) 596:665–80. doi: 10.1002/1873-3468.14259
Keywords: osteocalcin, diabetic kidney disease, bone metabolism, case-control study, meta-analysis
Citation: Hu X, Wang X, Cai C, Guo J, Qian X, Yu J, Huang L and Xie S (2024) Serum osteocalcin levels are inversely associated with UACR in Chinese DKD patients: a meta-analysis of 20 clinical studies. Front. Endocrinol. 15:1514713. doi: 10.3389/fendo.2024.1514713
Received: 21 October 2024; Accepted: 14 November 2024;
Published: 02 December 2024.
Edited by:
Yun Shen, Pennington Biomedical Research Center, United StatesReviewed by:
Jiawei Meng, Trine University, United StatesHuaqing Zheng, The University of Utah, United States
Copyright © 2024 Hu, Wang, Cai, Guo, Qian, Yu, Huang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaofeng Xie, eGllc2YzNTYyQHNpbmEuY29t; Liji Huang, amlsaTg4QHNpbmEuY29t
 Xiaolan Hu
Xiaolan Hu Xiyu Wang1
Xiyu Wang1 Liji Huang
Liji Huang