- 1Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Sohag University, Sohag, Egypt
- 2Ajyal IVF Center, Ajyal Hospital, Sohag, Egypt
- 3Department of Obstetrics and Gynaecology, University of Alexandria, Bab Sharqi, Alexandria Governorate, Alexandria, Egypt
- 4Alexandria Fertility and IVF Center, Alexandria, Egypt
- 5Basic Medical Sciences Department, College of Medicine, Ajman University, Ajman, United Arab Emirates
- 6Centre of Medical and Bio-Allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
- 7Department of Biomedical Sciences, College of Medicine, Gulf Medical University, Ajman, United Arab Emirates
- 8Department of Obstetrics and Gynaecology, Assiut University, Assiut, Egypt
Oxidative stress (OS) is established as a key factor in the etiology of both male and female infertility, arising from an imbalance between reactive oxygen species (ROS) production and the endogenous antioxidant (AOX) defenses. In men, OS adversely affects sperm function by inducing DNA damage, reducing motility, significantly impairing sperm vitality through plasma membrane peroxidation and loss of membrane integrity, and ultimately compromising overall sperm quality. In women, OS is implicated in various reproductive disorders, including polycystic ovary syndrome, endometriosis, and premature ovarian failure, leading to diminished oocyte quality, disrupted folliculogenesis, and poorer reproductive outcomes. Antioxidant therapy represents a promising intervention to mitigate the harmful effects of ROS on reproductive health in additions to its easy accessibility, safety, and low cost. Despite several findings suggesting improvements in fertility potential with AOX therapy, the data remains inconclusive regarding optimal dosage and combination, duration of treatment, and the specific patient populations most likely to benefit. In this review, we discuss the role of AOXs in the management of infertile couples, focusing on their biological mechanisms, potential adverse effects, therapeutic efficacy, and clinical applications in improving reproductive outcomes in both natural conception and medically assisted reproduction. Additionally, we highlight the current practice patterns and recommendations for AOX supplementation during the course of infertility treatment. Further, we provide an overview on the limitations of the current research on the topic and insights for future studies to establish standardized AOX regimens and to assess their long-term impact on key outcomes such as live birth rates and miscarriage rates.
Introduction
The term oxidative stress (OS) applies when increased amounts of reactive oxygen species (ROS) surpass the antioxidant (AOX) capacity that naturally offers the protective balance (1). While OS plays a major role in the pathogenesis of male infertility (2–4), it is equally important to recognize the critical physiological roles of ROS in normal reproductive functions. ROS are indispensable for processes such as sperm capacitation, hyperactivation, and the acrosome reaction, which are essential for successful fertilization (5, 6). However, an imbalance favoring excessive ROS production can lead to oxidative damage, negatively affecting male fertility potential and reproductive outcomes (7, 8). Also, OS has been linked with many female infertility conditions such as polycystic ovary syndrome (PCOS) (9, 10), endometriosis (11–13), premature ovarian failure (POF) (14), and recurrent miscarriages (RM) (15).
Hence, AOX therapy to enhance fertility by protecting against the harmful effects of ROS is theoretically feasible. AOXs are not regulated as pharmaceutical drugs, thus allowing their utilization without prescription. Indeed, AOXs are extensively consumed due to their easy availability through different retail outlets (16). Also, the relatively low cost and good safety profile of AOX products are appealing to both healthcare providers and infertile couples. However, a significant diversity exists among practitioners in terms of prescribing AOXs for the purpose of infertility treatment (17).
In this review, we comprehensively discussed the types, mechanisms of action and safety profile of AOXs that are commonly prescribed in infertility clinics. We also highlight the attitudes and practice patterns of clinicians towards AOX prescription during the course of infertility treatment, and compare them with current recommendations by professional societies. Additionally, we discuss the impact of AOX therapy on fertility outcomes in men and women under natural and assisted reproduction conditions. Furthermore, we highlight limitations of the current research on utility of AOXs for treatment of infertile couples and the need for well-designed studies that can bridge the gap between research and practice in this regard.
Types, natural sources and mechanism of action of antioxidants
An AOX is a natural substance that provides protection to the living cell against oxidative damage and deleterious effects of ROS (18). Under normal circumstances, there is a fine balance that enables AOXs to neutralize excess ROS, and maintain a small amount of ROS necessary for normal cell functions. Thus, when ROS production increases to harmful levels, AOXs play a key role in neutralizing them. In the living cell, the AOX defense system is generally classified into enzymatic and non-enzymatic elements.
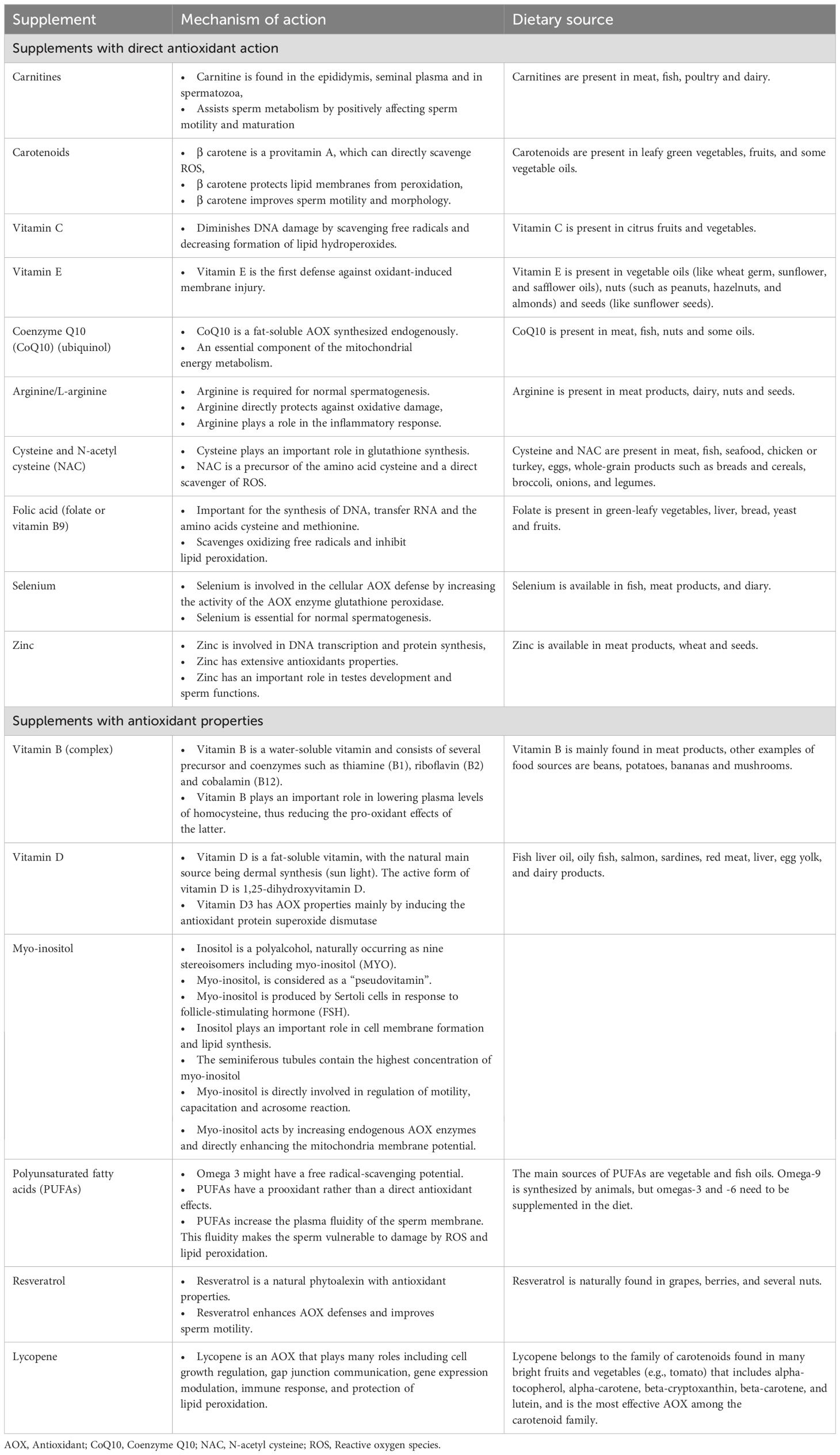
Enzymatic AOXs include glutathione (GSH) peroxidase, glutathione reductase, catalase (CAT) and superoxide dismutase (SOD) (19). Nonenzymatic AOXs provide protection against ROS by binding to enzymatic AOXs and ions that helps subsequent suppression of enzymes involved in the oxidation process (16). They function by scavenging free radicals, stabilizing enzymatic AOXs, and chelating metal ions, thereby mitigating OS. Prominent non-enzymatic AOXs include vitamins such as Vitamin C and Vitamin E (17, 20, 21), glutathione (22, 23), coenzyme Q10 (24–27), carotenoids (e.g., beta-carotene, lycopene) (28), polyphenols (29), and trace elements like selenium and zinc (24, 30). AOXs are available in the market as individual or combined products. Generally, AOXs utilized in clinical practice are categorized as substances with direct AOX actions or substances with AOX properties (16). Table 1 summarizes the types, natural dietary sources and the mechanism(s) of action of AOXs.
Safety profile of antioxidants
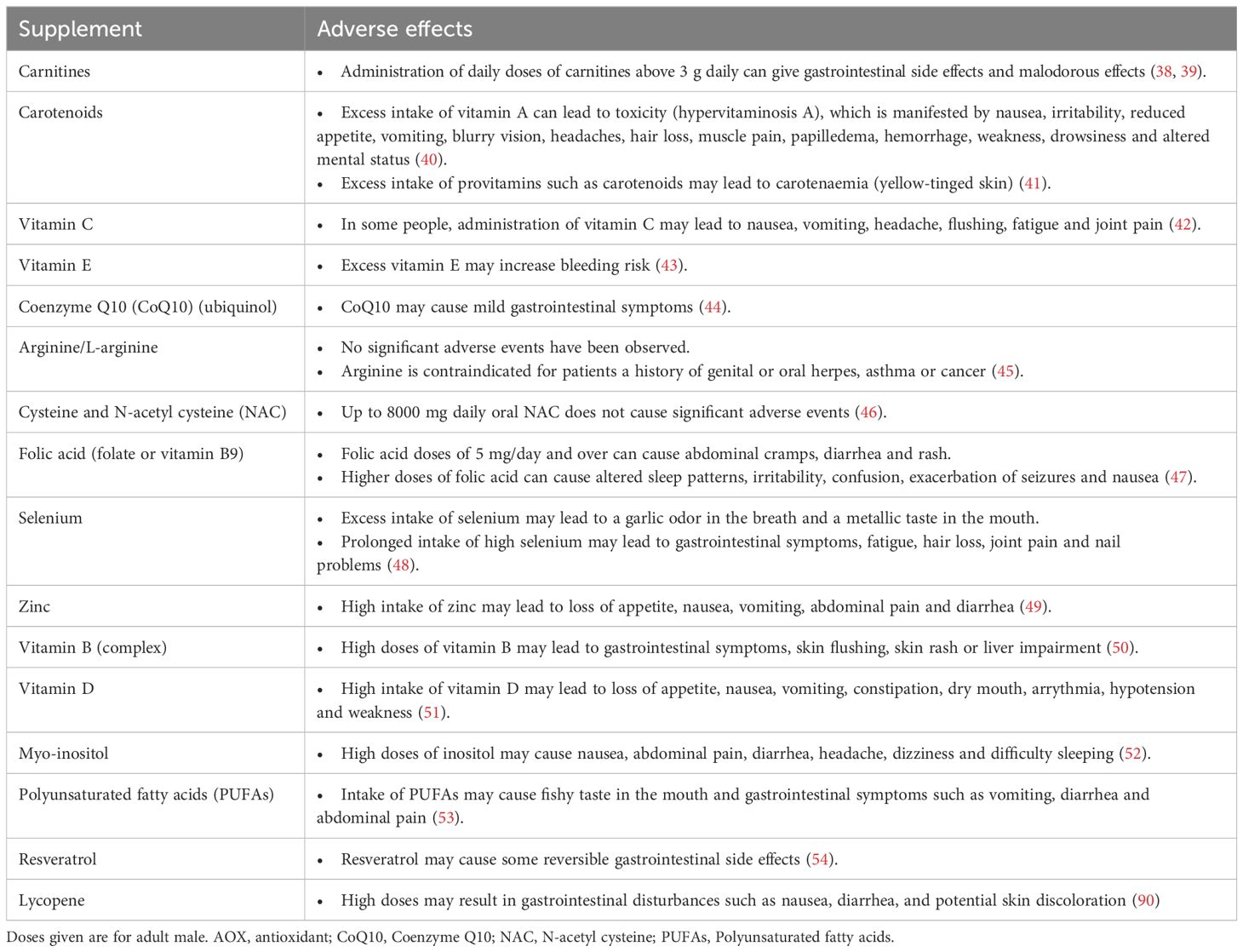
AOXs are generally well tolerated and have a good safety profile. However, the fact that AOXs are largely available over-the-counter with a reasonable price increases the risk that some patients can overmedicate with AOXs leading to detrimental effects. It has been suggested that administration of large amounts or improper combinations of AOXs may lead to shift of the redox balance towards a ‘reductive stress’ status (31). The term ‘antioxidant paradox’ highlights the potential negative impact of over-utilization of AOX supplementation (32, 33). Recent reports indicate that the intake of excess AOXs may even cause impairment of male fertility potential due to reductive stress (34, 35). However, there is no clear understanding of the exact mechanism of action of reductive stress on male reproduction, and further research is required. Additionally, high doses of AOXs may be associated with some adverse effects. Furthermore, it is imperative to consider the role of excipients or fillers in commercially available supplements. For instance, titanium dioxide, previously regarded as a safe additive, has recently been implicated in genotoxicity, raising concerns about its safety. These findings underscore the need for a judicious and evidence-based approach in the formulation and administration of AOX supplements to ensure both their safety and therapeutic efficacy (36, 37). A summary of potential adverse effects of AOXs frequently used in the treatment of infertile couples is provided in Table 2 (38–54).
Antioxidant therapy of male infertility
Increased public awareness of the positive impact of nutrition on different body functions highlights the significance of dietary modification as an important strategy to enhance fertility potential (Figure 1). A healthy diet or an extra dietary intake of AOXs has been correlated with high sperm quality in healthy individuals (55–58).
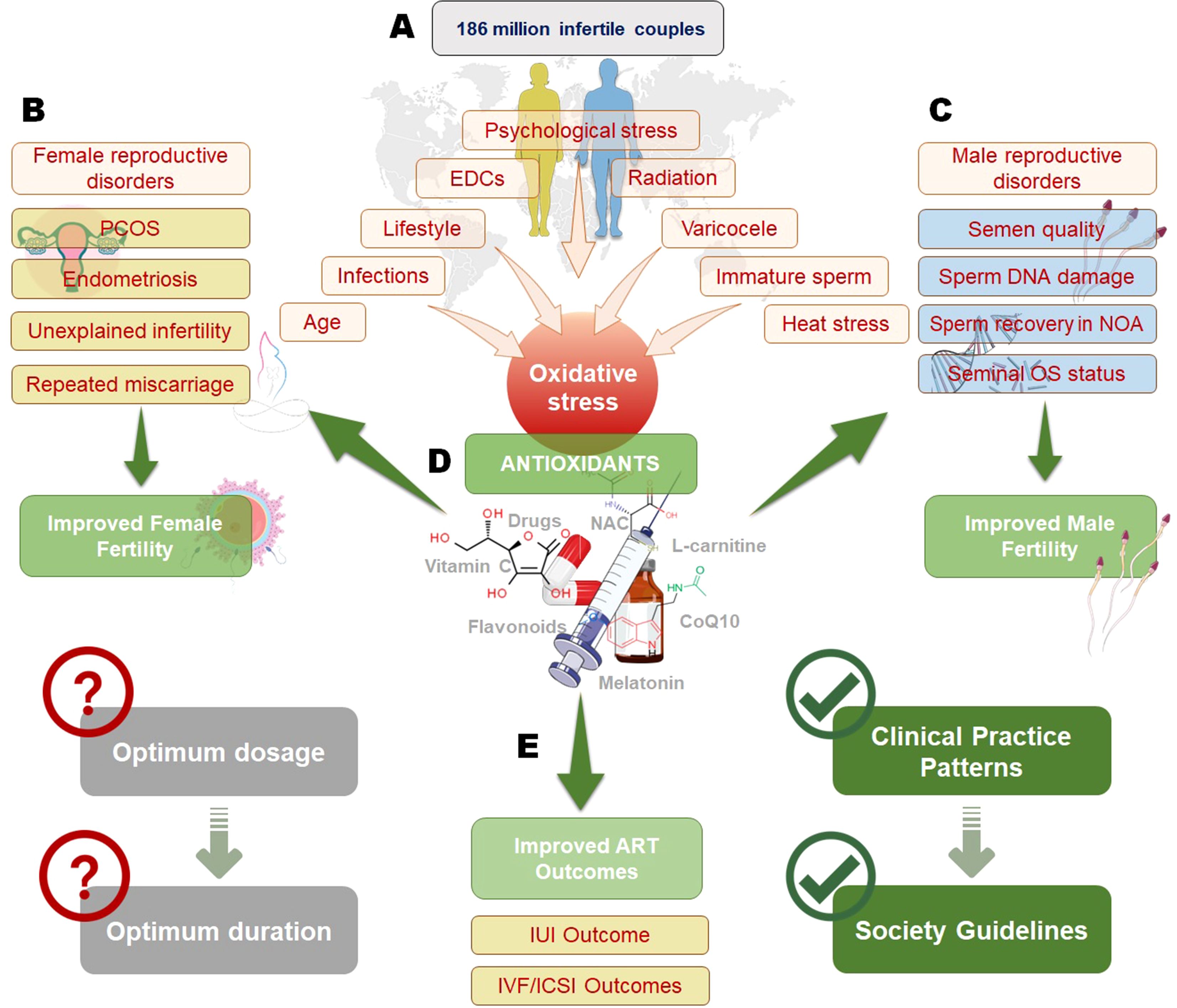
Figure 1. Impact of oxidative stress on infertility and the role of antioxidants in improving reproductive outcomes. Oxidative stress, influenced by factors such as psychological stress, endocrine-disrupting chemicals (EDCs), radiation, varicocele, infections, age, and lifestyle, affects both male and female fertility (A). In females (B), oxidative stress contributes to conditions like polycystic ovary syndrome (PCOS), endometriosis, unexplained infertility, and recurrent miscarriage, while in males (C), it impacts semen quality, sperm DNA integrity, non-obstructive azoospermia (NOA), and seminal oxidative stress status. Antioxidant therapy, including vitamins, flavonoids, N-acetylcysteine (NAC), L-carnitine, and coenzyme Q10 (CoQ10), plays a crucial role in mitigating oxidative stress and improving both male and female fertility (D). However, determining the optimum dosage and duration of antioxidant therapy remains a challenge. Antioxidants have also been shown to enhance outcomes in assisted reproductive technologies (ART), such as intrauterine insemination (IUI) and in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), contributing to the development of clinical guidelines for infertility management (E).
Rationale of antioxidant therapy of infertile men: role of oxidative stress
In the male genital tract, seminal ROS are produced by sperm, white blood cells, and as by- products during various metabolic processes. Small amounts of ROS are required for normal sperm functions such as sperm capacitation and acrosome reaction (2, 59). Excess ROS production may occur under different circumstances such as smoking (60), leukocytospermia (61), and varicocele (62). Male Oxidative Stress Infertility (MOSI) defines a group of infertile men with abnormal semen analysis results and excess ROS in their semen (63). This category of patients was previously classified as having idiopathic male infertility (IMI). The high prevalence of IMI highlights the significance of MOSI diagnosis during infertility workup. OS significantly impairs male fertility through disruption of sperm capacitation, induction of DNA damage such as sperm DNA fragmentation (SDF), reduced motility, and diminished sperm membrane fluidity. These detrimental effects are further exacerbated by a marked decline in sperm vitality, resulting from plasma membrane peroxidation and loss of membrane integrity. Together, these factors compromise overall sperm quality and hinder the fertilization process (64, 65).
AOX supplementation has demonstrated significant potential in managing MOSI, as highlighted by a systematic review encompassing 97 clinical trials. These studies revealed the beneficial effects of AOX supplementation in men with (a) abnormal semen quality, (b) varicocele, and (c) idiopathic or unexplained infertility (66). However, the efficacy of AOX therapy relies heavily on the accurate identification of OS through reliable diagnostic testing. Assessing OS biomarkers, including ROS levels, total antioxidant capacity (TAC), lipid peroxidation products and DNA fragmentation, is critical for detecting redox imbalances. Advanced diagnostic techniques, such as chemiluminescence, ELISA, and sperm-specific OS tests, provide precise evaluations of OS in semen (67–69). By employing this targeted diagnostic approach, AOX therapy can be personalized for individuals with confirmed OS, avoiding the risks associated with empirical or excessive supplementation, such as reductive stress (7). Integrating OS testing into clinical protocols ensures optimized therapeutic outcomes, personalized interventions, and minimizes potential adverse effects related to inappropriate AOX use.
The impact of different AOX supplementations to improve fertility outcomes in infertile men has been the subject of extensive research in the last few decades. Additionally, the growing interest in AOX therapy for male infertility has led to the development of variety of AOX combinations. In the male, AOXs may enhance fertility through preserving sperm DNA integrity and mitochondrial transport (70). The AOXs that are commonly used in published studies include vitamin E, vitamin C, carotenoids, carnitine, cysteine, coenzyme Q10 (CoQ10), selenium, zinc, and folic acid (16, 24–26, 30, 71–90).
Potential indications of antioxidant supplementation to enhance male fertility
Beyond MOSI, several clinical conditions and lifestyle factors warrant consideration for AOX therapy in male infertility treatment. Chronic diseases, specifically diabetes mellitus and obesity, have been associated with increased seminal OS and subsequent fertility impairment (91–93). In diabetic men, hyperglycemia-induced OS leads to increased SDF and reduced sperm motility, making them potential candidates for AOX supplementation (93).
Environmental and occupational exposures represent another significant indication for AOX therapy. Men exposed to heavy metals, pesticides, or electromagnetic radiation often exhibit elevated OS markers in seminal plasma (94). Cancer treatments significantly elevate OS through multiple mechanisms: chemotherapeutic agents, particularly alkylating agents and platinum compounds, generate excessive ROS directly damaging sperm DNA and membranes (87, 88), while radiation therapy induces both acute and chronic OS states in testicular tissue, leading to prolonged spermatogenic dysfunction (95).
Recently, attention has focused on men recovering from Coronavirus disease-19 (COVID-19) infection as potential candidates for AOX therapy. It has been suggested that COVID-19 infection may impact male reproductive function through OS-mediated mechanisms (96). While more research is needed, AOX supplementation might play a role in reproductive recovery post-infection (97).
Antioxidant therapy of infertile men: recommendations of professional societies
To date, results of studies on AOX therapy of infertile men are controversial, and no firm conclusion could be reached. Therefore, the guidelines of professional societies do not recommend the routine use AOXs in treatment of male infertility, mainly due to the heterogeneity of the available data. This should not be construed as a contraindication to the use of AOXs; rather, it underscores the necessity for more robust, high-quality, and standardized evidence to substantiate their routine application in clinical practice. The latest guidelines by the European Association of Urology state that “no conclusive data are available regarding the beneficial treatment with AOXs in men with idiopathic infertility, although they may improve semen parameters” (98). Similarly, the current American Urological Association and American Society for Reproductive Medicine guidelines question the benefits of AOXs in treating male infertility, and indicate lack of data to recommend the use of specific agents for this purpose (Moderate Recommendation; Evidence Level: Grade B)” (99).
Antioxidant therapy of infertile men: impact of on fertility outcomes
Basic semen parameters
Results emerging from the review by Agarwal et al. (2022) indicated a significant increase of basic sperm parameters including sperm concentration, total motility, progressive motility and normal forms following AOX therapy versus placebo or no treatment (100). However, these results should be interpreted with caution due to high heterogeneity that was observed among the studies included in this meta-analysis. Data of another meta-analysis also indicate better semen quality in infertile men who received AOX supplementation after varicocelectomy (101). A previous systematic review also reported that administration of AOXs such as vitamin E, vitamin C, N-acetyl cysteine (NAC), carnitines, CoQ10, lycopene, selenium, and zinc, resulted in significant improvement of sperm concentration, motility, and morphology (102). Further, a recent meta-analysis examined the impact of supplementation with L-Carnitine/ALC or NAC on semen quality and had the same conclusion (81). Further, in vitro glycine supplementation has been found to improve the quality of cryopreserved sperms in an animal experiment (103).
According to data from the Cochrane review, administration of carnitines and combined AOX formulations had positive effect of sperm motility, and supplementation with polyunsaturated fats and zinc resulted in increase of sperm concentration (16). Zinc supplementation plays a pivotal role in enhancing spermatogenesis through its diverse functions in male reproductive physiology. It is indispensable for spermatogenesis, as it supports the functionality of Sertoli cells and maintains the structural integrity of the seminiferous epithelium, which is essential for effective sperm production. Zinc also serves as a cofactor for critical enzymes involved in DNA synthesis, repair, and cellular division, thereby facilitating the proliferation and differentiation of spermatogenic cells. Additionally, its antioxidant properties help counteract OS, shielding germ cells from damage induced by ROS. Moreover, zinc regulates testosterone secretion, a hormone essential for sustaining optimal spermatogenic activity and promoting healthy sperm concentration. Despite abundance of studies reporting positive outcome of AOX therapy on basic sperm parameters, it is difficult to get a firm conclusion due to the high heterogeneity among published studies.
Sperm recovery in patients with non-obstructive azoospermia
A study investigated the outcome of three-month supplementation with multivitamins, micronutrients and CoQ10 in infertile men with non-obstructive azoospermia (n=24) as compared to no treatment (n=11) (104). Histological evaluation of testicular biopsies of azoospermic patients showed maturation arrest mostly at spermatid level. At monthly follow up visits, the semen analysis of the treated group showed recovery of motile sperm in the ejaculate at concentration of 4.0 million/ml, while the untreated groups remained unchanged. Authors of the latter study reported further increase of sperm density on subsequent visits, and 2 pregnancies within 3 months. However, these results should be taken with caution due to poor study design and high drop-out ratio (105). More importantly, these results were not replicated in further studies.
Sperm DNA fragmentation
Folic acid supplementation resulted in lowering the levels of SDF in carriers of MTHFR gene TT polymorphism (106). However, a meta-analysis found no significant difference in SDF levels in infertile men following AOX supplementation as compared to placebo or no treatment (100). Of note, only small number of sudies investigated this outcome with heterogenity in inclusion criteria among study populations, and differences in AOX combinations and SDF assays used. Future studies are warranted to examine the effacacy of AOX therapy in men with high SDF.
Seminal oxidative stress indices
Few studies demonstrated improvement in OS as determined by reduction of levels of malonaldehyde (MDA), a lipid peroxidation byproduct following therapy with Vitamin C, vitamin E, beta-carotene, zinc, selenium, and NAC when used alone or in combination (107–111). A recent meta-analysis indicates a significant increase in seminal AOX and reduction of MDA following AOX therapy although significant inter-study heterogeneity was detected (112). MDA, a byproduct of lipid peroxidation, serves as a key biomarker of OS and exhibits cytotoxic effects on spermatozoa. Elevated MDA levels compromise the structural integrity and fluidity of the sperm membrane, resulting in diminished motility and reduced viability (113, 114). Furthermore, MDA can directly interact with DNA, causing strand breaks and base modifications, thereby adversely affecting sperm DNA integrity and reducing fertilization potential (115, 116). Consequently, the observed reduction in MDA levels following AOX therapy highlights its therapeutic significance in alleviating OS and improving outcomes in OS-related male infertility.
Clinical pregnancy rate and live-birth rate
Recent years have wetnessed a growing interest in the study of the impact of AOX therapy on clinical pregnancy rate (CPR) and live-birth rate (LBR). In a Cochrane review investigated AOX supplementation protocols for male subfertility, focusing on active substances including carnitines, coenzyme Q10, selenium, zinc, vitamins C and E, and carotenoids. Dosages varied according to the specific AOX, with some studies incorporating combinations of more than three AOXs. The duration of supplementation ranged from three to nine months, with outcomes evaluated at defined intervals of three, six, or nine months. The latter Cochrane review reported that AOX supplementation in infertile men resulted in a significant increase of CPR (odds ratio (OR) 2.97, 95% confidence interval (CI) 1.91-4.63) although the level of evidence was found to be low (16). A similar finding was reported by a recent meta-analysis where AOX therapy of infertile men was associated increased spontaneous CPR (OR 1.97, 95% CI: 1.28, 3.04; p<0.01) (100). In a third meta-analysis, CPR was significantly higher in infertile men with IMI who received combined L-carnitine and acetyl L-carnitine (ALC) supplementation (OR=3.76, p=0.002). The study evaluated the efficacy of combined L-carnitine and ALC supplementation in men diagnosed with idiopathic oligoasthenoteratozoospermia (iOAT). The treatment protocols consisted of LC administered at a dose of 2 g/day and LAC at 1 g/day, with intervention durations spanning 12 to 24 weeks across the included trials. This combination therapy demonstrated potential to improve key semen parameters, particularly forward motility and total motile sperm counts, while also contributing to enhanced pregnancy rates in affected patients (73).
Older reviews have also reported higher CPR in infertile men treated with AOXs whether couples were trying to oncieve naturally or through one of the medically assisted reproduction (MAR) procedures (102, 117, 118). However, a study did not find better pregnancy rates in infertile couples in whom the male partner received CoQ10 supplementation, although sperm parameters were found better (26, 27, 119).
On the other hand, the results of systematic reviews by Cochrane (16) and Agarwal et al. (2022) were unable to show improvement of LBR despite higher pregnancy rates possibly due to scarcity of data available on this outcome (100). This may be attributed to methodological difficulties that preclude performing this kind of studies as they require longer durations of follow up.
Miscarriage rate
According to the systematic reviews by Cochrane (16) and Agarwal et al. (2022) (100), AOX therapy of infertile men was not correlated with miscarriage rates mainly due to small number of studies addressing this parameter.
Dose and duration of antioxidant therapy in male infertility
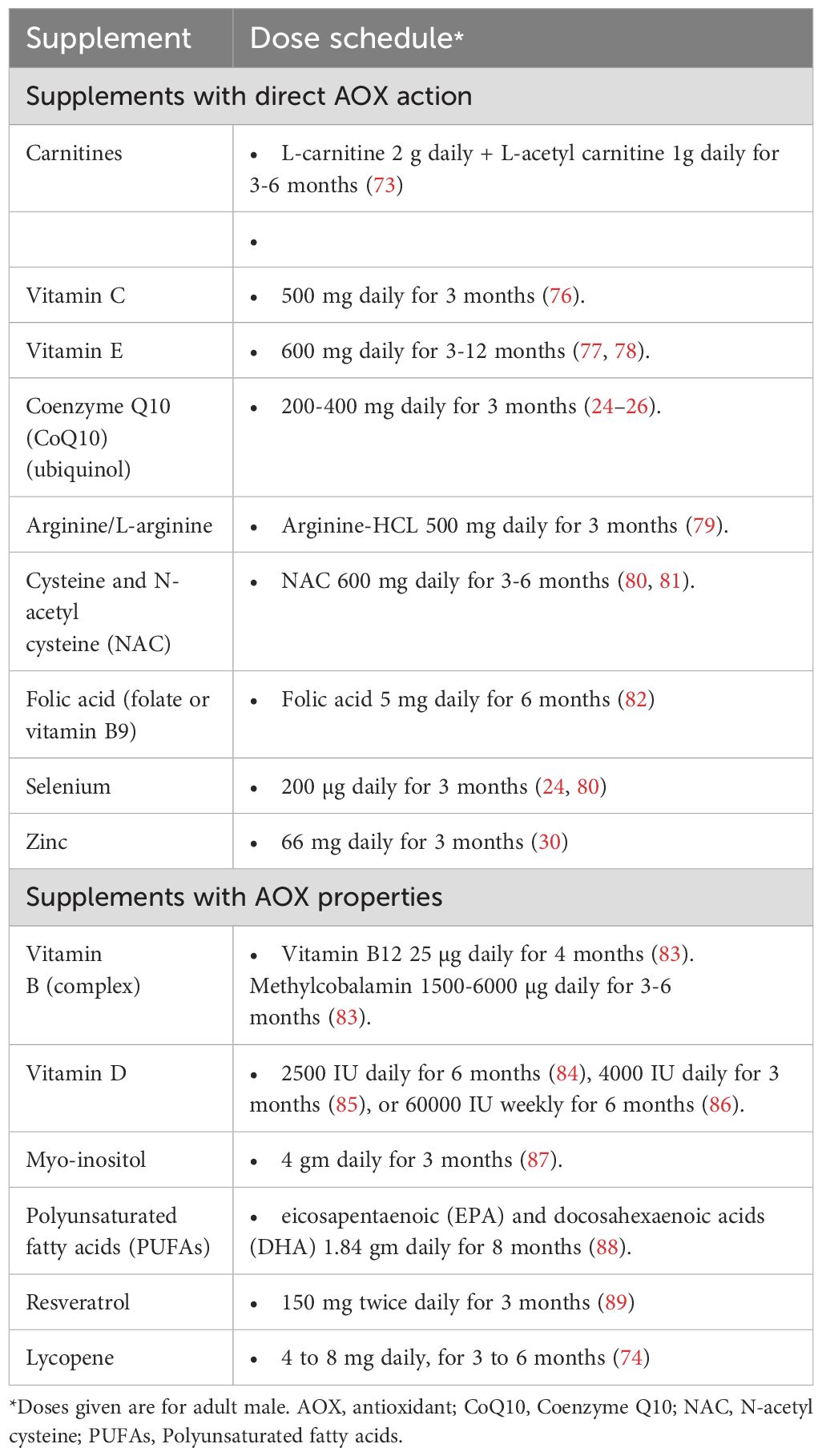
To date, there is no clear guidance as to the optimum dose or duration of AOX therapy for infertile men. The duration reported by different studies examining the effects of AOX therapy in male infertility is variable ranging from 1 month (120) to 12 months (78). A minimum of 26 weeks is recommended by the US Food and Drug Administration to evaluate the therapeutic potential of an intervention for male factor infertility (121). Based on this recommendation, it is assumed that a period of 6.5 months is needed for AOX therapy in infertile men. For instance, L-carnitine, L-acetyl carnitine, NAC, and vitamin E have been reported to require a duration of 6-12 months (52, 56, 57, 62, 63). The dose of AOX supplementation is also variable and is mainly decided by the manufacturer and according to the experience of the individual practitioner (66). A summary of dose schedules of AOXs frequently used in the treatment of infertile men is provided in Table 3 (24–26, 30, 73, 74, 76–89).
Practitioners’ attitude towards antioxidant therapy of male infertility
Reproductive specialists continue to prescribe AOXs in their daily practice of male infertility treatment despite lack of clear guidelines (102). The results of a global survey including 1,327 reproductive physicians indicated that 43.7% of the survey participants prescribe AOXs to all patients and another 41.9% prescribe AOXs for selected categories of patients (66). Interestingly, 80% of the clinicians indicated that they prescribe AOXs for treatment of infertility without testing for seminal OS. The results of the survey showed lack of consensus among practitioners regarding the desired treatment outcome. While 18.8% of clinicians suggested basic semen parameters as their primary outcome measure, an equal number selected LBR, and others selected either SDF or CPR. Additionally, a significant diversity was observed among practitioners regarding the AOXs they prescribe with 36 different AOXs being listed. It may be appropriate to use a combination of AOXs that act by different mechanisms to protect spermatozoa against oxidative damage (66). However, no clear guideline is currently available to recommend the use of specific AOX or AOX combinations for male infertility treatment.
Antioxidant therapy of female infertility
Rationale of antioxidant therapy of role of female infertility: role of oxidative stress
Although the evaluation of OS in male reproduction can be easily done by examining the semen, the same evaluation is more complicated in women as OS indices in the serum do not accurately reflect the situation in the female reproductive system. Accordingly, more invasive procedures are needed such as examining the follicular fluid obtained during in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) or examining the peritoneal fluid obtained by laparoscopy. Consequently, much of the information on OS in female reproduction has been obtained from animal studies. Despite this limitation, OS was shown to be involved in many aspects of female reproduction (122–124). ROS in the ovaries is mainly produced in the macrophages, leukocytes, and cytokines present in the follicular fluid. On the other hand, the ovary contains two types of the AOX enzymes; SOD: copper-zinc SOD (Cu/Zn SOD), mainly present in the Graafian follicle and manganese SOD (MnSOD) concentrated mainly in the corpus luteum (124). ROS are involved in many physiological functions of the ovary such as steroidogenesis, oocyte maturation, ovulation, formation of blastocysts, implantation, luteolysis and maintenance of the luteal phase (124, 125). More specifically, in small doses, they are involved in folliculogenesis, dominant follicle selection and corpus luteum formation in part by regulating angiogenesis (126). In addition, they play a role in dominant follicle selection, as regressing follicles undergo a process of apoptosis which is promoted by ROS. This delicate balance is an interplay between ROS production and the up-regulation of the AOX pathways CAT and GSH which opposes the apoptotic process (124).
ROS are also involved in ovulation, follicular rupture and corpus luteum formation. Besides cytokines, and vascular endothelial growth factor (VEGF), the local production of prostaglandin F2α (PG2 α) leading to vascular ischemia at the site of follicular rupture (i.e. ovulation) stimulates the production of the ROS (122). On the other hand, following ovulation, the increased activity of Cu/Zn-SOD during the early and mid-luteal phase supports corpus luteum function while its diminished activity during the regression phase is associated with luteolysis and cessation of corpus luteum function, ushering menstruation (127).
OS, resulting from increased ROS production or diminished AOX capacity, can negatively impact female reproductive processes. It can damage oocytes, impair follicular development, reduce endometrial receptivity, and disrupt early embryo development, potentially leading to fertilization failure, and implantation issues. Additionally, OS can cause hormonal imbalances that further compromise fertility, indicating the role of ROS-AOX balance in maintaining reproductive health (128). OS was shown to be associated to a varying extend with many reproductive diseases such as the PCOS (9), endometriosis (11–13), POF (129), unexplained infertility (122) and even RM (15). Consequently, AOXs are used with varying degrees of success in the treatment of these conditions (Figure 1).
Antioxidant therapy of polycystic ovary syndrome
The PCOS is the most common female reproductive disorders and affects 5% to 10% of US women (130). The updated 2018 diagnostic criteria for PCOS require the presence of at least two of the following three features: (1) oligo/anovulation, (2) clinical and/or biochemical hyperandrogenism, and (3) PCO identified by the presence of ≥20 antral follicles per ovary on transvaginal ultrasound, with or without increased ovarian volume. These criteria are more stringent than the previous Rotterdam criteria, aiming to identify women with higher metabolic risks associated with PCOS (131).
About two thirds of PCOS patients suffer from insulin resistance often leading to the development of the metabolic syndrome (130). Although many theories have been proposed to explain the pathogenesis of PCOS, recent studies have increasingly linked OS to various aspects of the condition (124, 132). A study demonstrated that hyperglycemia induces excess ROS production from mononuclear cells, which subsequently triggers the release of tumor necrosis factor-α (TNF-α) (105). The resulting inflammatory cascade of events exacerbates OS and insulin resistance. This, in turn, contributes to the formation of multiple ovarian cysts, chronic anovulation, and infertility (133, 134). Another study showed that elevated insulin levels promote the proliferation of thecal cells and increase the secretion of androgens, including luteinizing hormone (LH), while also upregulating the expression of cytochrome P450 and insulin growth factor-1 (IGF-1) receptors (107). The activity of cytochrome P450 is known to generate ROS, perpetuating a vicious cycle of OS. Additionally, plasma levels of L-carnitine were found to be significantly lower in obese PCOS patients compared to their non-obese counterparts (135). Despite these findings, it remains unclear whether OS is a primary factor in the development of PCOS or a secondary consequence of hyperglycemia, insulin resistance, and associated cardiovascular and necrotic complications in these women (124, 132).
Based on these and other findings, AOXs were proposed as a treatment of PCOS either on their own, as a combination or as adjuvants to other agents for ovulation induction in the infertile PCOS patients. For example, Sahelpour et al. (2019) treated 80 PCOS patients with L-carnitine (3g/day) for 3 months (109). The authors found that insulin sensitivity improved significantly and that the serum levels of low-density lipoprotein (LDL) decreased. Additionally, there was a decrease in the body mass index (BMI) and the patients reported that their menstrual cycles became more regular and that their hirsutism diminished (136). In a randomized controlled trial (RCT), 60 overweight PCOS patients were treated with 250 mg of carnitine supplements for 12 weeks, while the control group received a placebo (110). They found that carnitine administration was associated with a significant reduction in BMI, and waist and hip circumferences compared to placebo. They also found a significant reduction in fasting plasma glucose, serum insulin levels, homoeostasis model of assessment-insulin resistance and dehydroepiandrosterone sulphate (DHEAS) in the treated patients. However, in this latter study, carnitine administration had no effect on lipid profiles or free testosterone. NAC is another popular agent used for the treatment of infertile PCOS patients with encouraging results (137). However, a meta-analysis reported that the use of NAC showed no significant improvement in pregnancy rate, serum LH level, fasting insulin, and LH/follicle-stimulating hormone (FSH) ratio despite that the BMI and total testosterone were significantly reduced (138).
Zinc and selenium were also used in the treatment of PCOS based on the fact that serum zinc and selenium levels in women with PCOS are significantly lower than in healthy controls (139–141). Consequently, PCOS women were treated with the daily administration of 220 mg zinc sulfate (containing 50 mg zinc) for 8 weeks and found that this regimen resulted in the improvement of their metabolic profiles (142). Similarly, the administration of 200 micrograms per day of selenium for 8 weeks in PCOS patients had beneficial effects on their insulin metabolism parameters, triglycerides and very low-density lipoprotein-C (VLDL-C) levels (143).
Combinations of AOXs are now increasingly being recommended for PCOS treatment. For example, a combination of NAC (1200 mg/day) and L-arginine (1600 mg/day) for 6 months to treat 8 oligo-menorrhoeic patients with PCOS helped restoring the menstrual function, as evidenced by the increased number of menstrual cycles, and significantly decreased the homeostatic model assessment (HOMA) index (144).
The potential for AOXs to replace metformin in the treatment of PCOS has also been explored. In a study by Oner and Muderris (2011), NAC was compared with metformin in 100 patients with PCOS (119). Both treatments resulted in significant reductions in BMI, hirsutism scores, fasting insulin levels, HOMA index, free testosterone levels, and menstrual irregularity compared to baseline values, demonstrating equal efficacy. Additionally, NAC significantly lowered both total cholesterol and LDL levels, while metformin only reduced total cholesterol (145).
Antioxidants were also used as adjuvant therapies in combination with ovulation induction agents in infertile patients with PCOS (146). In 2014, a RCT was conducted to evaluate the effectiveness of adding 3g of L-carnitine daily to a regimen of 250 mg clomiphene citrate, administered from day 3 to day 7 of the menstrual cycle, in anovulatory women with PCOS (147). The combination treatment significantly improved both ovulation rates and cumulative pregnancy rates compared to clomiphene citrate alone. Additionally, the L-carnitine group showed significant improvements in patient tolerability, lipid profiles, and BMI. Vitamin D is often used as a supplement in the treatment of PCOS and a meta-analysis showed that this may be beneficial for follicular development and menstrual cycle regulation in the PCOS patients (148). Chen et al. found that using vitamin E (100 mg/day during the follicular and luteal phases of the cycle) did not result in significant improvements in ovulation induction, clinical pregnancy, or ongoing pregnancy rates in infertile women with PCOS (149). In the latter study, a significantly lower dose of human menopausal gonadotropin (HMG) was required in these women, which is expected given that women with PCOS typically have high serum anti-mullerian hormone (AMH) levels and are more prone to developing ovarian hyperstimulation syndrome (OHSS).
In conclusion, while AOXs have shown promising potential in the treatment of PCOS, further research is essential to validate these findings. Additional studies are needed to determine which specific AOX or combination of AOXs offers the most benefit for managing this complex syndrome. New research can also help development of targeted therapies that can effectively address the underlying OS and other related factors in PCOS.
Antioxidant therapy of endometriosis-associated infertility
Endometriosis affects about 10% of reproductive age women (150). Patients usually present with menstrual pain (dysmenorrhea) and/or infertility problems (12). OS has been implicated in the pathogenesis of endometriosis (12, 151–153). In these patients, OS results from excess ROS produced by the erythrocytes and apoptotic endometrial cells present in the endometriosis implants. ROS are also produced by the activated macrophages responsible for phagocytizing these apoptotic cells (11). Cells are damaged by OS-induced lipid peroxidation, which is proportional to the severity of the disease (154). It was also found that the peritoneal fluid of endometriosis patients contains high concentrations of various OS markers such as MDA, pro-inflammatory cytokines (interleukin-6 (IL-6), TNF-α, transforming growth factor- β (TGF-β) and IL-1β), angiogenic factors (IL-8 and VEGF), neural growth factor and oxidized LDL (ox-LDL) (155, 156). Besides their role in inflammation, these cytokines are detrimental to the processes of fertilization and embryo development: TNF-α increases the free implants adhesions and their invasiveness through its effect on matrix metallo-proteinases (157) while TGF-β hinders the local immune activity by inhibiting the natural killer cells which in turn impede the development of embryos (158). Further, mitochondria of ectopic endometrial stromal cells (ESCs) was found to generate excess ROS (159). A delicate redox balance between iron and heme oxygenase-1 (HO-1) has been identified, and this balance could be responsible for iron-induced OS in endometriotic cyst fluid (152). Additionally, a genetic role for OS in endometriosis has been proposed (22). A study investigated the genetic polymorphisms of four genes encoding AOX enzymes involved in OS and found that the variant genotypes for the CAT-262C, T polymorphism were significantly more frequent in individuals with endometriosis (160).
Consequently, AOXs have been utilized in the treatment of endometriosis, particularly for managing endometriosis-associated pain, with varying degrees of success (161–163). For instance, daily administration of a combination of vitamin E (1200 IU) and vitamin C (1000 mg) for eight weeks led to significant improvements in chronic pain, dysmenorrhea, and dyspareunia compared to placebo (164). Similarly, the severity of pelvic pain, dysmenorrhea and dyspareunia significantly decreased after 8 weeks of supplementation with vitamin C (1000 mg/day, 2 tablets of 500 mg each) and vitamin E (800 IU/day, 2 tablets of 400 IU each) compared to placebo (165). A recent study observed similar improvements following the administration of vitamin D (50,000 IU) every two weeks for 12 weeks in patients with endometriosis (166). Furthermore, a systematic review concluded that AOXs show promising results in treating endometriosis-related pain (167). However, the authors of the latter study emphasized the need for further studies to draw more definitive conclusions.
Few studies were conducted to evaluate AOX therapy in women with endometriosis-associated infertility (168, 169). Encouraging results were obtained following vitamin C supplementation in a group of women with endometriosis-associated infertility undergoing IVF treatment (170). In addition to human studies, animal research has also shown promise. For instance, studies conducted on rats demonstrated positive effects of AOX therapy on endometriosis-related infertility (146). However, these findings are preliminary and need to be confirmed through rigorous clinical trials in humans.
It is vital to take into account that AOXs should be given in proper dose and duration as excessive intake can lead to reductive stress (35). The delicate oxidant/AOX balance function is a double-edged blade. Although OS is implicated in cell death and apoptosis in endometriosis, upregulation of AOX functions in endometriotic cysts may result in restoration of cell survival (171, 172).
Antioxidant therapy of premature ovarian failure and poor ovarian reserve
POF or premature menopause is the cessation of ovarian function before the age of 40 years. In POF, the ovaries lose their germinative and hormonal functions because of the exhaustion of the number of ovarian follicles before the typical age for physiological menopause which is around 50 years of age. Women with POF present with amenorrhea, hypergonadotropinism, and hypoestrogenism (173).
OS is increasingly recognized as a key factor influencing reproductive health, with effects that extend beyond fertility into broader physiological systems. Estrogen, owing to its intrinsic AOX properties, plays a critical role in mitigating OS. Its chemical structure enables the direct scavenging of free radicals while simultaneously enhancing the expression and activity of various AOX enzymes, thereby offering substantial protection against oxidative damage (174, 175). Elevated OS has been associated with systemic manifestations, including heightened lipid peroxidation, which correlates with mood disturbances, sleep disorders, and other health concerns (152). Dietary patterns enriched with AOX-rich components, such as the Mediterranean diet, have shown effectiveness in alleviating OS-related symptoms, underscoring the potential of dietary interventions in reducing oxidative damage (176). Similarly, hormone replacement therapy highlights the protective effects of estradiol in combating OS (177). These observations underscore the critical role of AOX-based strategies in advancing reproductive health and improving broader health outcomes, including those related to infertility.
OS has also been implicated in the process of ovarian ageing and an increased incidence of aneuploidy in ageing oocytes. In ageing oocytes, ROS attack the 8th carbon atom of guanine leading to base mutations and mismatches in DNA replication (178). Accordingly, AOXs were explored as a method to delay this ageing process. Studies in mice have shown that peroxiredoxin-4 can be used to protect against ovarian ageing (179, 180). Similar results were reported with quercetin (181, 182). Ochiai et al. used resveratrol as an adjuvant therapy in women with poor ovarian reserve in an attempt to prevent premature menopause (183). Similarly, melatonin was used as an adjuvant for the prevention of POF during chemotherapy (184, 185), given its experimentally proven role in the female reproductive tract (186). Obviously, these promising reports need to be confirmed by prospective randomized trials.
Antioxidant therapy of unexplained infertility
OS has been frequently associated with unexplained infertility. High levels of MDA, a marker of lipid peroxidation, were found in the peritoneal fluid of women with unexplained infertility, suggesting a potential role of OS in these cases (116). More recently, a study identified increased activity of prolidase, a type of matrix metalloprotease, in the plasma of women with unexplained infertility, proposing that this could contribute to implantation defects (187).
Given these findings, several attempts have been made to treat women with unexplained infertility using AOXs. Studies suggest that AOXs, such as CoQ10, vitamins C and E, melatonin, NAC, and alpha-lipoic acid (ALA), may improve oocyte quality, enhance endometrial receptivity, and restore hormonal balance by mitigating OS (188, 189). The evaluation often incorporates indirect markers such as improved fertilization rates, successful embryo development, and clinical pregnancy rates as proxies for oocyte quality. For example, CoQ10 has been shown to improve oocyte quality by enhancing mitochondrial function (190), while melatonin, may support both oocyte quality and endometrial receptivity (191).
Although some studies have reported positive outcomes when using AOX therapy in conjunction with other fertility treatments, the evidence remains mixed, and large-scale RCTs are needed to confirm its efficacy and safety. A study evaluated the role of adding NAC to clomiphene citrate in treating unexplained infertility but found the combination ineffective (192). Vitamin E supplementation in women with unexplained infertility undergoing controlled ovarian hyperstimulation and intrauterine insemination (IUI) was only effective in increasing the thickness of the endometrium without any effect on the pregnancy rate (193). In a more recent RCT, the addition of daily oral multivitamins and minerals to the stimulation regimen of patients with unexplained infertility treated with IVF/ICSI did not improve oocyte quality (metaphase II oocytes) or pregnancy rates in these women (194). These studies indicate the need for further research to evaluate the efficacy of AOX therapy in unexplained infertility and to determine whether OS is a causative factor or merely an associated finding in this condition.
Antioxidant therapy of repeated miscarriages
RM or recurrent pregnancy loss is defined as the spontaneous loss of two or more pregnancies (195). It affects 1% of couples trying to conceive and in 50% of instances the cause is unknown (196). The relationship between OS and early pregnancy failure was first suggested by Jauniaux et al. (197). Subsequently, OS was explored as a cause of RM (198). Total oxidant level and OS index were significantly increased, and total antioxidant capacity, were significantly decreased in the serum of women with RM (199). Similarly, Ghneim et al. studied 25 women with RM and compared them to non-pregnant women and women with healthy pregnancies (200). They found significant reduction of SOD activities and serum levels of zinc, copper and manganese in healthy pregnant women compared to non-pregnant women with further reduction in RM patients. They suggested that dietary supplementation of Zn, Cu and Mn may have positive impact on these patients pre- and post-conception. The association between OS and RM was also supported by other studies (198, 201, 202). To the contrary, no statistically significant difference was found in the pre-gestational serum levels of OS indices in women with RM compared to controls (203).
Despite these conflicting data, AOXs were used in the treatment of RM with various claims of success. For example, a study compared the effects of administration of NAC 0.6 g + folic acid 500 μg/day in a group of 80 patients with a history of RM with an aged-matched group of 86 patients treated with folic acid 500 μ/day alone (204). They found a significantly increased rate of continuation of a living pregnancy and the take-home baby rate in the NAC supplementation group (P < 0.047, RR 1.98, 95% CI 1.3-4.0). However, a Cochrane review conducted in 2016 showed that AOXs did not help in preventing pregnancy loss (RR 1.12, 95% CI 0.24 to 5.29) but the authors suggested that more studies are needed to further explore this outcome (205).
Antioxidant therapy and the outcome of medically assisted reproduction
OS in an important factor in the success of medically assisted reproduction (IUI, IVF and ICSI) (112, 128, 154). In these techniques, OS can result from endogenous as well as exogenous sources. These factors and the effect of AOXs on ART are discussed in this section (Figure 1).
Impact of antioxidant therapy on intrauterine insemination outcomes
One of the main indications of IUI is the presence of oligozoospermia, asthenozoospermia or teratozoospermia or a combination of the three parameters in the male partner of the infertile couple. As mentioned previously, male partners with these defects exhibit OS in their semen (206–208). In addition, culture media used in the preparation of the semen as well as those used in cell cultures have varying amounts of ROS which adds to the OS burden to which spermatozoa are exposed (209, 210). Moreover, the process of sperm preparation deprives the spermatozoa from the natural AOXs present in the seminal plasma leading to accumulation of ROS. This is even more pronounced when centrifugation is used in the preparation (e.g. Percoll gradient separation). Cryopreservation and thawing are extra sources of OS when frozen semen is used for IUI (211).
Accordingly, AOXs are used to treat the male partners of the infertile couples as mentioned previously (16, 66). In addition, AOX supplementation of the sperm preparation medium for IUI was used in an attempt to counteract the effects of OS. Pentoxifylline exerts several AOX activities, such as the maintenance of GSH levels and mitochondrial viability (212). The addition of pentoxifylline to the sperm preparation medium resulted in improvement of semen characteristics in asthenozoospermic patients (213). Additionally, CPR was significantly increased by adding pentoxifylline to the semen preparation medium for IUI (27.5% versus 11.5% in the control group) (214).
Similarly, the addition of caffeine to the semen preparation medium for IUI was described (215). Finally, adjusting the sperm and embryo cryopreservation media by adding AOXs is used to mitigate the effect of OS (216).
Impact of antioxidant therapy on IVF and ICSI outcomes
The techniques of IVF and ICSI exert a considerable amount of OS on the gametes and embryos. In addition to the endogenous and exogenous sources of OS mentioned previously to which spermatozoa are subjected, the oocytes and embryos are also subjected to similar OS (217). For example, oocytes lose their AOX protection contained in the cumulus cells during the denudation process. The changes of temperature and the osmotic and pH changes during the oocyte and embryo handling (denudation, pipetting, etc.) are all sources of OS (100). In addition, exogenous sources of OS to which the gametes and embryos are subjected include exposure to the atmospheric O2, the high oxygen concentration in the incubator (compared to the 2% concentration in the fallopian tube), volatile organic compounds present in the laboratory air or released from the consumables (plastic ware), visible light, humidity in the incubator and the type of mineral oil used. The processes of cryopreservation and thawing are also sources of OS (100). Consequently, many steps are taken in the IVF laboratory in order to counteract or diminish the effect of this OS (128).
Accordingly, men and women undergoing IVF or ICSI can be treated with AOXs in an attempt to improve the clinical results. Treatment of infertile men with the AOX “Menevit” for 3 months prior to IVF resulted in higher viable pregnancy rate (38.5%) compared to the control group (16%). The study enrolled 60 couples undergoing IVF-ICSI treatment, who were randomly assigned into two groups in a 2:1 ratio: one receiving the AOX Menevit and the other a placebo. Male participants were included based on evidence of OS, indicated by impaired sperm parameters such as poor morphology, reduced motility, or compromised membrane integrity, in conjunction with significant SDF (>25% TUNEL positivity). Female partners were excluded if they exhibited diminished ovarian reserve, defined as fewer than five oocytes retrieved in a prior IVF cycle or elevated early follicular phase FSH levels, or were aged over 39 years (218). The Cochrane review published in 2019 showed that although the use of AOXs for men prior to IVF did not improve the CPR (OR = 2.64, 95% CI 0.94 to 7.41), the LBR increased significantly (OR = 3.61, 95% CI 1.27). However, the authors of the review noted that these data were based on 2 RCTs only and that further studies are needed (11).
Women undergoing IVF and ICSI were also treated with AOXs prior to the procedure with variable claims of success. These treatments include NAC, melatonin, coQ10, thiamine, riboflavin, niacin B3, vitamins B6 and B12, folate, vitamins A, C, D and K, calcium, phosphorus, magnesium, sodium, potassium, chloride, iron, zinc, copper, selenium, iodine, vitamin E, vitamin K, L-arginine, inositol, biotin, pantothenic acid, eicosatetraenoic acid (EPA), docosahexaenoic acid (DHA) and various combinations of the above (188). The treatment is usually given for 3 to 6 months prior to the procedure. Pre-treatment of IVF patients with AOXs improved embryo quality, particularly in poor responders (219). Indeed, a significantly lower glutathione-S-transferase (GST) enzyme activity and significantly high MDA levels have been shown in the cumulus cells of poor responders compared to high responders (220). However, the Cochrane review conducted by Showell et al. in 2020 showed no improvement in the CPR (OR = 1.15, 95% CI = 0.95 – 140) or LBR (0.94, 95% CI = 0.41- 2.15) when AOXs were given to women treated with IVF or ICSI (188). In this Cochrane review, the LBR was calculated from 2 small RCTs and it is therefore clear that more studies are needed, particularly in view of the multifactorial nature of IVF and ICSI.
Finally, supplementation of embryo culture media with AOXs was shown to improve the quality of the embryos, the implantation rate as well as the CPR in IVF/ICSI cycles (221, 222). Furthermore, the addition of a combination of AOXs (ALC, NAC and α-lipoic acid) to the cryoprotectant medium of mice embryos resulted in increased blastocyst cryo-survival and viability post-vitrification (216).
The outcome of ICSI is known to be influenced by a myriad of factors. In a recent Cochrane review, it was stated that the chance of live birth with ICSI is between 30% and 41% (223). Consequently, efforts are directed to maximize ICSI outcomes. Increased OS has been identified as an important factor governing success of ICSI (112). Thus, it is plausible to use AOX in an endeavor to optimize the outcome of ICSI. Although the list of publications on the use of AOX in couples undergoing ICSI is steadily growing, knowledge on the relationship between AOX and ICSI outcomes is lacking (224). An RCT including 85 women with different indications for ICSI who have been supplemented with AOX daily from the cycle preceding ICSI cycle, versus 85 women who were not given any AOX acting as controls, concluded that AOX therapy did not change clinical or laboratory outcomes (225). However, A recent systematic review indicated a favorable impact of AOX on pregnancy outcomes following ICSI (224). In a recent systematic review and meta-analysis of RCTs, it was concluded that oral supplementation of CoQ10 may increase clinical pregnancy rates, without an effect on LBR, and MR compared with placebo or no-treatment in women with infertility undergoing ICSI (226). Moreover, a recent meta-analysis concluded that melatonin treatment significantly increases the CPR but not LBR in ART cycles. Melatonin treatment also increased the number of oocytes collected, maturate oocytes, and good quality embryos. There was no evidence suggesting that melatonin treatment increases the adverse events in ART cycles. However, the findings of this meta-analysis should be explained cautiously due to remarkable heterogeneity of the included IVF patients (227). AOXs such as inositol, L-carnitine, CoQ10, and ALC may be helpful for women undergoing ICSI or in cases of previous ICSI failure (226). However, the role of AOXs in increasing LBR is limited by low-quality evidence (188). Indeed, conducting large controlled clinical trials using AOXs supplementation is mandatory to evaluate their influence on ICSI outcome.
Future directions
Available research on AOX therapy is surrounded by several limitations and biases, including small sample sizes, heterogenous patient populations and inclusion criteria, poor study designs, and inconsistent reporting of clinical outcomes. Additionally, the use of a wide range of AOXs (either single or in combinations) and the lack of standardized dosages in different studies preclude the ability to draw a firm conclusion on the efficacy of AOX supplementation in solving infertility problem. This has led to the skepticism of the current guidelines by professional societies regarding AOX therapy for infertility. This may also explain the considerable variability that currently exists in practitioners’ attitude towards the prescription of AOX therapy for the purpose of overcoming infertility issues. Such variability surrounds many aspects of AOX therapy including pre-testing the patient for OS, the choice of a specific AOX or AOX combination, proper dose, optimum duration of therapy and the desired outcome. Therefore, there is a great demand for high-quality research to clarify the exact role of AOXs in the context of infertility treatment.
Future studies should focus on careful diagnostic work-up to assess OS indices as an essential pre-request before starting AOX therapy (206). Such test would be helpful for selecting patients with high levels of OS who are candidates for AOX therapy, and for monitoring the treatment response. This in turn requires the availability of reliable tools for confirming the diagnosis of OS and evaluation its severity. Indeed, this is technically difficult in women. However, assessment of seminal OS in men is feasible using some tools such as the MiOXSYS System that can help assess oxidation-reduction potential (ORP) levels in semen and seminal plasma (207). The test would help personalize treatment for cases with high seminal ORP such as idiopathic infertility, varicocele or those with genital tract infection. The 6th Edition of the World Health Organization (WHO) manual for human semen analysis classifies seminal OS testing as a research tool, emphasizing the lack of robust evidence to confirm its clinical utility (208). Despite this designation, OS testing is extensively utilized in andrology research, clinical laboratories, and ART centers, underscoring its recognized importance in advancing the understanding of male fertility.
Future studies are also warranted to investigate the impact of AOX on LBR, MR, and SDF since only few well-designed studies have analyzed these outcomes in infertile patients following AOX therapy. Additionally, future studies are needed to determine the proper treatment duration, optimum dosage as well as the ideal supplement (single vs. combined AOX) (206). Furthermore, it is essential to ensure that there are no negative consequences of utilizing AOXs as a therapeutic option for infertile men.
Conclusion and take-home message
OS plays a significant role in couples’ infertility. The theoretical basis for AOX therapy to counteract the harmful effects of ROS on fertility is feasible. In infertile men, it is hypothesized that cases with elevated seminal OS may benefit from AOX therapy. Hence, several AOXs, including vitamins E and C, carotenoids, carnitine, cysteine, CoQ10, selenium, zinc, and folate, have been employed as potential therapeutic interventions for male infertility. However, results of clinical trials remain controversial, and current guidelines from major professional societies offer no definitive recommendations regarding AOX use in male infertility.
AOXs have shown promise in the management of PCOS, endometriosis, poor ovarian reserve and unexplained infertility. Also, AOX supplements such as inositol, L-carnitine, ALC and CoQ10 have shown potential for women undergoing ICSI, particularly those with previous ICSI failure. However, current evidence supporting AOX in improving important reproductive outcomes such as LBR remains low, necessitating further studies. Additionally, further research is needed to evaluate the exact role of AOX therapy and to identify the most effective single AOX or AOX combinations in these conditions.
Author contributions
RS: Writing – original draft, Writing – review & editing. HS: Writing – original draft, Writing – review & editing. ME: Writing – original draft, Writing – review & editing. SD: Writing – original draft, Writing – review & editing. PS: Writing – original draft, Writing – review & editing. AN: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALA, Alpha-Lipoic Acid; ALC, Acetyl-L-Carnitine; AOX, Antioxidant; ARG, L-Arginine; ART, Assisted Reproductive Technology; BMI, Body Mass Index; CAT, Catalase; CoQ10, Coenzyme Q10; COVID-19, Coronavirus disease-19; CPR, Clinical Pregnancy Rate; DHA, Docosahexaenoic Acid; DHEAS, Dehydroepiandrosterone Sulphate; ESCs, Ectopic Endometrial Stromal Cells; EPA, Eicosatetraenoic Acid; FSH, Follicle-Stimulating Hormone; GSH, Glutathione; GST, Glutathione-S-Transferase; HMG, Human Menopausal Gonadotropin; HO-1, Heme Oxygenase-1; HOMA, Homeostatic Model Assessment; ICSI, Intracytoplasmic Sperm Injection; IL, Interleukin; IMI, Idiopathic Male Infertility; IUI, Intrauterine Insemination; IVF, In Vitro Fertilization; LBR, Live Birth Rate; LDL, Low-Density Lipoprotein; LH, Luteinizing Hormone; MAR, Medically Assisted Reproduction; MDA, Malonaldehyde; MOSI, Male Oxidative Stress Infertility; MR, Miscarriage Rate; NAC, N-Acetyl Cysteine; NOA, Non-Obstructive Azoospermia; OHSS, Ovarian Hyperstimulation Syndrome; OS, Oxidative Stress; PCOS, Polycystic Ovary Syndrome; PG2α, Prostaglandin F2α; POF, Premature Ovarian Failure; RCT, Randomized Controlled Trial; RM, Recurrent Miscarriages; ROS, Reactive Oxygen Species; SDF, Sperm DNA Fragmentation; SOD, Superoxide Dismutase; TAC, Total Antioxidant Capacity; TGF-β, Tumor Growth Factor-β; TNF-α, Tumor Necrosis Factor-α; VEFG, Vascular Endothelial Growth Factor; VLDL-C, Very-Low-Density Lipoprotein-C.
References
1. Takeshima T, Usui K, Mori K, Asai T, Yasuda K, Kuroda S, et al. Oxidative stress and male infertility. Reprod Med Biol. (2021) 20:41–52. doi: 10.1002/rmb2.12353
2. Agarwal A, Sengupta P. Oxidative stress and its association with male infertility. Male infertility: Contemp Clin approaches andrology ART antioxidants. (2020) Parekattil SJ, Esteves SC, Agarwal A. (Springer, Cham: Springer Nature), 57–68. doi: 10.1007/978-3-030-32300-4
3. Bui AD, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. (2018) 50:e13012. doi: 10.1111/and.2018.50.issue-8
4. Walczak–Jedrzejowska R, Wolski JK, Slowikowska–Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent Eur J urology. (2013) 66:60. doi: 10.5173/ceju.2013.01.art19
5. Dutta S, Sengupta P, Samrot AV. Physiological and pathological functions of reactive nitrogen species (RNS) and reactive sulphur species (RSS) on male reproductive functions. J Integrated Sci Technology. (2024) 12:755–. doi: 10.62110/sciencein.jist.2024.v12.755
6. Dutta S, Henkel R, Sengupta P, Agarwal A. Physiological role of ROS in sperm function. Male infertility: Contemp Clin approaches Andrology ART antioxidants. (2020) Parekattil SJ, Esteves SC, Agarwal A. (Springer, Cham: Springer Nature) 337–45. doi: 10.1007/978-3-030-32300-4_27
7. Sengupta P, Dutta S, Irez T. Oxidants and antioxidants in male reproduction: roles of oxidative and reductive stress. J Integrated Sci Technology. (2024) 12:753–. doi: 10.62110/sciencein.jist.2024.v12.753
8. Sengupta P, Roychoudhury S, Nath M, Dutta S. Oxidative stress and idiopathic male infertility. Oxid Stress Toxicity Reprod Biol Medicine: A Compr Update Male Infertility-Volume One. (2022) 1358:181–204. doi: 10.1007/978-3-030-89340-8_9
9. Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. (2019) 10:86. doi: 10.4103/ijpvm.IJPVM_576_17
10. Sengupta P, Dutta S, Hassan MF. Polycystic ovary syndrome (PCOS) and oxidative stress. J Integrated Sci Technology. (2024) 12:752–. doi: 10.62110/sciencein.jist.2024.v12.752
11. Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod biomedicine online. (2006) 13:126–34. doi: 10.1016/S1472-6483(10)62026-3
12. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell longevity. (2017) 2017:7265238. doi: 10.1155/2017/7265238
13. Carvalho LFP, Samadder AN, Agarwal A, Fernandes LFC, Abrao MS. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecology Obstetrics. (2012) 286:1033–40. doi: 10.1007/s00404-012-2439-7
14. Wang Y, Li N, Zeng Z, Tang L, Zhao S, Zhou F, et al. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol Hum Reprod. (2021) 27:gaaa081. doi: 10.1093/molehr/gaaa081
15. Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstetrical gynecological survey. (2007) 62:335–47. doi: 10.1097/01.ogx.0000261644.89300.df
16. Smits R, Mackenzie-Proctor R, Yazdani A, Stankiewicz M, Jordan V, Showell M. Antioxidants for male subfertility. Cochrane Database Syst Rev. (2019) 3(3):CD007411. doi: 10.1002/14651858.CD007411.pub4
17. Agarwal A, Finelli R, Selvam MKP, Leisegang K, Majzoub A, Tadros N, et al. A global survey of reproductive specialists to determine the clinical utility of oxidative stress testing and antioxidant use in male infertility. World J Men's Health. (2021) 39:470. doi: 10.5534/wjmh.210025
18. Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. (2006) 160:1–40. doi: 10.1016/j.cbi.2005.12.009
19. Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. (2018) 63:68–78. doi: 10.1016/j.advms.2017.05.005
20. Sengupta P, Dutta S, Slama P, Roychoudhury S. COVID-19, oxidative stress, and male reproductive dysfunctions: is vitamin C a potential remedy? Physiol Res. (2022) 71:47. doi: 10.33549/physiolres
21. Bolle P, Evandri MG, Saso L. The controversial efficacy of vitamin E for human male infertility. Contraception. (2002) 65:313–5. doi: 10.1016/S0010-7824(02)00277-9
22. Huang Y-Y, Wu C-H, Liu C-H, Yang S-F, Wang P-H, Lin L-Y, et al. Association between the genetic variants of glutathione peroxidase 4 and severity of endometriosis. Int J Environ Res Public Health. (2020) 17:5089. doi: 10.3390/ijerph17145089
23. Naher Z, Biswas S, Mollah F, Ali M, Arslan M. Role of glutathione in male infertility. Bangladesh J Med Biochem. (2011) 4:20–5. doi: 10.3329/bjmb.v4i2.13772
24. Alahmar AT, Sengupta P. Impact of coenzyme Q10 and selenium on seminal fluid parameters and antioxidant status in men with idiopathic infertility. Biol Trace element Res. (2021) 199:1246–52. doi: 10.1007/s12011-020-02251-3
25. Alahmar AT, Sengupta P, Dutta S, Calogero AE. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin Exp Reprod Med. (2021) 48:150. doi: 10.5653/cerm.2020.04084
26. Alahmar AT, Calogero AE, Sengupta P, Dutta S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J men's Health. (2021) 39:346. doi: 10.5534/wjmh.190145
27. Alahmar AT, Calogero AE, Singh R, Cannarella R, Sengupta P, Dutta S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin Exp Reprod Med. (2021) 48:97. doi: 10.5653/cerm.2020.04175
28. Pasquariello R, Anipchenko P, Pennarossa G, Crociati M, Zerani M, Brevini TA, et al. Carotenoids in female and male reproduction. Phytochemistry. (2022) 204:113459. doi: 10.1016/j.phytochem.2022.113459
29. Silberstein T, Har-Vardi I, Harlev A, Friger M, Hamou B, Barac T, et al. Antioxidants and polyphenols: concentrations and relation to male infertility and treatment success. Oxid Med Cell Longevity. (2016) 2016:9140925. doi: 10.1155/2016/9140925
30. Ebisch I, Pierik F, De Jong F, Thomas C, Steegers-Theunissen R. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int J andrology. (2006) 29:339–45. doi: 10.1111/j.1365-2605.2005.00598.x
31. Dutta S, Sengupta P, Roychoudhury S, Chakravarthi S, Wang CW, Slama P. Antioxidant paradox in male infertility:‘A blind eye’on inflammation. Antioxidants. (2022) 11:167. doi: 10.3390/antiox11010167
32. Gutteridge JM, Halliwell B. Reoxygenation injury and antioxidant protection: a tale of two paradoxes. Arch Biochem biophysics. (1990) 283:223–6. doi: 10.1016/0003-9861(90)90635-C
33. Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol. (2013) 75:637–44. doi: 10.1111/j.1365-2125.2012.04272.x
34. Selvam MKP, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radical Biol Med. (2020) 152:375–85. doi: 10.1016/j.freeradbiomed.2020.03.008
35. Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia. (2019) 51:e13162. doi: 10.1111/and.2019.51.issue-1
36. Bischoff NS, de Kok TM, Sijm DT, van Breda SG, Briedé JJ, Castenmiller JJ, et al. Possible adverse effects of food additive E171 (titanium dioxide) related to particle specific human toxicity, including the immune system. Int J Mol Sci. (2020) 22:207. doi: 10.3390/ijms22010207
37. Alaee S, Ilani M. Effect of titanium dioxide nanoparticles on male and female reproductive systems. J Advanced Med Sci Appl Technologies. (2017) 3:3–8. doi: 10.18869/nrip.jamsat.3.1.3
38. Stadler DD, Bale JF, Chenard CA, Rebouche CJ. Effect of long-term valproic acid administration on the efficiency of carnitine reabsorption in humans. Metabolism. (1999) 48:74–9. doi: 10.1016/S0026-0495(99)90013-6
39. Alesci S, Manoli I, Costello R, Coates P, Gold PW, Chrousos GP, et al. Carnitine: Lessons from one hundred years of research. Ann New York Acad Sci. (2004) 1033:ix–xi. doi: 10.1196/annals.1320.019
40. Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. (2006) 83:191–201. doi: 10.1093/ajcn/83.2.191
41. Aşkın Ö, Uzunçakmak TKÜ, Altunkalem N, Tüzün Y. Vitamin deficiencies/hypervitaminosis and the skin. Clinics Dermatol. (2021) 39:847–57. doi: 10.1016/j.clindermatol.2021.05.010
42. Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, et al. Vitamin C—sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. (2021) 13:615. doi: 10.3390/nu13020615
43. Rafeeq H, Ahmad S, Tareen MBK, Shahzad KA, Bashir A, Jabeen R, et al. Biochemistry of fat soluble vitamins, sources, biochemical functions and toxicity. Haya: Saudi J Life Sci. (2020) 5:188–96. doi: 10.36348/sjls.2020.v05i09.007
44. Hathcock JN, Shao A. Risk assessment for coenzyme Q10 (Ubiquinone). Regul Toxicol Pharmacol. (2006) 45:282–8. doi: 10.1016/j.yrtph.2006.05.006
45. Holeček M. Side effects of amino acid supplements. Physiol Res. (2022) 71:29. doi: 10.33549/physiolres
46. Tenório MCDS, Graciliano NG, Moura FA. Oliveira ACMd, Goulart MOF. N-acetylcysteine (NAC): impacts on human health. Antioxidants. (2021) 10:967. doi: 10.3390/antiox10060967
47. Devnath GP, Kumaran S, Rajiv R, Shaha KK, Nagaraj A. Fatal folic acid toxicity in humans. J forensic Sci. (2017) 62:1668–70. doi: 10.1111/jfo.2017.62.issue-6
48. Hadrup N, Ravn-Haren G. Toxicity of repeated oral intake of organic selenium, inorganic selenium, and selenium nanoparticles: A review. J Trace Elements Med Biol. (2023) 79:127235. doi: 10.1016/j.jtemb.2023.127235
49. Hussain S, Khan M, Sheikh TMM, Mumtaz MZ, Chohan TA, Shamim S, et al. Zinc essentiality, toxicity, and its bacterial bioremediation: A comprehensive insight. Front Microbiol. (2022) 13:900740. doi: 10.3389/fmicb.2022.900740
50. Uebanso T, Shimohata T, Mawatari K, Takahashi A. Functional roles of B-vitamins in the gut and gut microbiome. Mol Nutr Food Res. (2020) 64:2000426. doi: 10.1002/mnfr.202000426
51. Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients. (2013) 5:3605–16. doi: 10.3390/nu5093605
52. Carlomagno G, Unfer V. Inositol safety: clinical evidences. Eur Rev Med Pharmacol Sci. (2011) 15:931–6.
53. Quispe R, Alfaddagh A, Kazzi B, Zghyer F, Marvel FA, Blumenthal RS, et al. Controversies in the use of omega-3 fatty acids to prevent atherosclerosis. Curr Atheroscl Rep. (2022) 24:571–81. doi: 10.1007/s11883-022-01031-9
54. Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: A literature review. Int J Mol Sci. (2020) 21:2084. doi: 10.3390/ijms21062084
55. Eskenazi B, Kidd S, Marks A, Sloter E, Block G, Wyrobek A. Antioxidant intake is associated with semen quality in healthy men. Hum reproduction. (2005) 20:1006–12. doi: 10.1093/humrep/deh725
56. Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod update. (2017) 23:371–89. doi: 10.1093/humupd/dmx006
57. Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jørgensen N, Mendiola J, et al. Semen quality in relation to antioxidant intake in a healthy male population. Fertility sterility. (2013) 100:1572–9. doi: 10.1016/j.fertnstert.2013.08.032
58. Sengupta P. Current trends of male reproductive health disorders and the changing semen quality. Int J Prev Med. (2014) 5:1.
59. Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. (1996) 48:835–50. doi: 10.1016/S0090-4295(96)00313-5
60. Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertility sterility. (2002) 78:491–9. doi: 10.1016/S0015-0282(02)03294-6
61. Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ Jr., Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertility sterility. (2002) 78:1215–24. doi: 10.1016/S0015-0282(02)04237-1
62. Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertility sterility. (2010) 94:1531–4. doi: 10.1016/j.fertnstert.2009.12.039
63. Agarwal A, Parekh N, Selvam MKP, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J men's Health. (2019) 37:296–312. doi: 10.5534/wjmh.190055
64. Agarwal A, Cho C-L, Esteves SC, Majzoub A. Reactive oxygen species and sperm DNA fragmentation. Trans andrology urology. (2017) 6:S695. doi: 10.21037/tau.2017.05.40
65. Aitken RJ. DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Trans andrology urology. (2017) 6:S761. doi: 10.21037/tau.2017.09.13
66. Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Selvam MKP, et al. Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. World J men's Health. (2021) 39:233. doi: 10.5534/wjmh.200196
67. Sengupta P, Dutta S, Samrot AV. Sperm DNA fragmentation testing in infertility. In: Genetic Testing in Reproductive Medicine, Singapore: Springer (2024). p. 47–66.
68. Sengupta P, Dutta S, Saleh R. Assessment of seminal oxidative stress. In: Human Semen Analysis: From the WHO Manual to the Clinical Management of Infertile Men. Cham: Springer (2024). p. 247–65.
69. Agarwal A, Qiu E, Sharma R. Laboratory assessment of oxidative stress in semen. Arab J Urology. (2018) 16:77–86. doi: 10.1016/j.aju.2017.11.008
70. Ali M, Martinez M, Parekh N. Are antioxidants a viable treatment option for male infertility? Andrologia. (2021) 53:e13644. doi: 10.1111/and.13644
71. Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian J Urology. (2017) 33:207–14. doi: 10.4103/iju.IJU_15_17
72. Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci. (2020) 77:93–113. doi: 10.1007/s00018-019-03253-8
73. Zhang X, Cui Y, Dong L, Sun M, Zhang Y. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: A systematic review and meta-analysis. Andrologia. (2020) 52:e13470. doi: 10.1111/and.13470
74. Durairajanayagam D, Agarwal A, Ong C, Prashast P. Lycopene and male infertility. Asian J andrology. (2014) 16:420–5. doi: 10.4103/1008-682X.126384
75. Margiana R, Pakpahan C, Pangestu M. A systematic review of retinoic acid in the journey of spermatogonium to spermatozoa: From basic to clinical application. F1000Research. (2022) 11:1–14. doi: 10.12688/f1000research.110510.2
76. Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J urol. (2015) 41:230–8. doi: 10.1590/S1677-5538.IBJU.2015.02.07
77. Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertility sterility. (1995) 64:825–31. doi: 10.1016/S0015-0282(16)57861-3
78. Ener K, Aldemir M, Işık E, Okulu E, Özcan MF, Uğurlu M, et al. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: a randomised controlled study. Andrologia. (2016) 48:829–34. doi: 10.1111/and.2016.48.issue-7
79. Appleton J. Arginine: clinical potential of a semi-essential amino acid. Altern Med Rev. (2002) 7:512–23.
80. Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J urology. (2009) 181:741–51. doi: 10.1016/j.juro.2008.10.015
81. Zhou Z, Cui Y, Zhang X, Zhang Y. The role of N-acetyl-cysteine (NAC) orally daily on the sperm parameters and serum hormones in idiopathic infertile men: A systematic review and meta-analysis of randomised controlled trials. Andrologia. (2021) 53:e13953. doi: 10.1111/and.13953
82. Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertility sterility. (2002) 77:491–8. doi: 10.1016/S0015-0282(01)03229-0
84. Bartl I, Ondrušová M, Kužma M, Jackuliak P, Gažová A, Kyselovič J, et al. Treatment with cholecalciferol leads to increase of selected semen parameters in young infertile males: results of a 6-month interventional study. Physiol Res. (2021) 70:S99. doi: 10.33549/physiolres.934781
85. Maghsoumi-Norouzabad L, Zare Javid A, Mansoori A, Dadfar M, Serajian A. The effects of Vitamin D3 supplementation on Spermatogram and endocrine factors in asthenozoospermia infertile men: a randomized, triple blind, placebo-controlled clinical trial. Reprod Biol Endocrinology. (2021) 19:1–16. doi: 10.1186/s12958-021-00789-y
86. Wadhwa L, Priyadarshini S, Fauzdar A, Wadhwa SN, Arora S. Impact of vitamin D supplementation on semen quality in vitamin D-deficient infertile males with oligoasthenozoospermia. J Obstetrics Gynecology India. (2020) 70:44–9. doi: 10.1007/s13224-019-01251-1
87. Calogero A, Gullo G, La Vignera S, Condorelli R, Vaiarelli A. Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double-blind randomized placebo-controlled study. Andrology. (2015) 3:491–5. doi: 10.1111/andr.2015.3.issue-3
88. Safarinejad M. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. (2011) 43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x
89. Illiano E, Trama F, Zucchi A, Iannitti RG, Fioretti B, Costantini E. Resveratrol-based multivitamin supplement increases sperm concentration and motility in idiopathic male infertility: a pilot clinical study. J Clin Med. (2020) 9:4017. doi: 10.3390/jcm9124017
90. Trumbo PR. Are there adverse effects of lycopene exposure? 1. J Nutr. (2005) 135:2060S–1S. doi: 10.1093/jn/135.8.2060S
91. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J andrology. (2012) 33:145–53. doi: 10.2164/jandrol.111.013193
92. Cannarella R, Crafa A, Curto R, Condorelli RA, La Vignera S, Calogero AE. Obesity and male fertility disorders. Mol Aspects Med. (2024) 97:101273. doi: 10.1016/j.mam.2024.101273
93. Huang R, Chen J, Guo B, Jiang C, Sun W. Diabetes-induced male infertility: potential mechanisms and treatment options. Mol Med. (2024) 30:11. doi: 10.1186/s10020-023-00771-x
94. Wdowiak N, Wójtowicz K, Wdowiak-Filip A, Pucek W, Wróbel A, Wróbel J, et al. Environmental factors as the main hormonal disruptors of male fertility. J Clin Med. (2024) 13:1986. doi: 10.3390/jcm13071986
95. Duffin K, Mitchell RT, Brougham MF, Hamer G, van Pelt AM, Mulder CL. Impacts of cancer therapy on male fertility: Past and present. Mol Aspects Med. (2024) 100:101308. doi: 10.1016/j.mam.2024.101308
96. Falahieh FM, Zarabadipour M, Mirani M, Abdiyan M, Dinparvar M, Alizadeh H, et al. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reproduction Fertility Dev. (2021) 33:683–90. doi: 10.1071/RD21153
97. Sengupta P, Dutta S, Roychoudhury S, D’Souza UJA, Govindasamy K, Kolesarova A. COVID-19, oxidative stress and male reproduction: possible role of antioxidants. Antioxidants. (2022) 11:548. doi: 10.3390/antiox11030548
98. Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur urology. (2021) 80:603–20. doi: 10.1016/j.eururo.2021.08.014
99. Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. J urology. (2021) 205:36–43. doi: 10.1097/JU.0000000000001521
100. Agarwal A, Maldonado Rosas I, Anagnostopoulou C, Cannarella R, Boitrelle F, Munoz LV, et al. Oxidative stress and assisted reproduction: a comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment. Antioxidants. (2022) 11:477. doi: 10.3390/antiox11030477
101. Wang J, Wang T, Ding W, Wu J, Wu G, Wang Y, et al. Efficacy of antioxidant therapy on sperm quality measurements after varicocelectomy: A systematic review and meta-analysis. Andrologia. (2019) 51:e13396. doi: 10.1111/and.v51.10
102. Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J urology. (2018) 16:113–24. doi: 10.1016/j.aju.2017.11.013
103. Nazif MS, Rehman ZU, Khan H, Khan FA, Hussain T, Ahmad A, et al. Glycine improved cryopreserved spermatozoa quality in Achai bull. BioMed Res Int. (2022) 2022:8282387. doi: 10.1155/2022/8282387
104. Singh AK, Tiwari AK, Singh PB, Dwivedi US, Trivedi S, Singh SK, et al. Multivitamin and micronutrient treatment improves semen parameters of azoospermic patients with maturation arrest. Indian J Physiol Pharmacol. (2010) 54:157–63.
105. Kumar R. Medical management of non-obstructive azoospermia. Clinics (Sao Paulo). (2013) 68 Suppl 1:75–9. doi: 10.6061/clinics/2013(sup01)08
106. Huang WJ, Lu XL, Li JT, Zhang JM. Effects of folic acid on oligozoospermia with MTHFR polymorphisms in term of seminal parameters, DNA fragmentation, and live birth rate: a double-blind, randomized, placebo-controlled trial. Andrology. (2020) 8:110–6. doi: 10.1111/andr.12652
107. Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. (2009) 74:73–6. doi: 10.1016/j.urology.2009.02.034
108. Comhaire F, Christophe A, Zalata A, Dhooge W, Mahmoud A, Depuydt C. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukotrienes Essential Fatty Acids (PLEFA). (2000) 63:159–65. doi: 10.1054/plef.2000.0174
109. Omu A, Al-Azemi M, Kehinde E, Anim J, Oriowo M, Mathew T. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Principles Practice. (2008) 17:108–16. doi: 10.1159/000112963
110. Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch andrology. (2003) 49:83–94. doi: 10.1080/01485010390129269
111. Suleiman SA, Ali ME, Zaki Z, El-Malik E, Nasr M. Lipid peroxidation and human sperm motility: protective role of vitamin E. J andrology. (1996) 17:530–7. doi: 10.1002/j.1939-4640.1996.tb01830.x
112. Agarwal A, Cannarella R, Saleh R, Harraz AM, Kandil H, Salvio G, et al. Impact of antioxidant therapy on natural pregnancy outcomes and semen parameters in infertile men: a systematic review and meta-analysis of randomized controlled trials. World J Men's Health. (2023) 41:14. doi: 10.5534/wjmh.220067
113. Chandra AK, Sengupta P, Goswami H, Sarkar M. Effects of dietary magnesium on testicular histology, steroidogenesis, spermatogenesis and oxidative stress markers in adult rats. Indian J Exp Biol. (2013) 51(1):37–47.
114. Chandra AK, Sengupta P, Goswami H, Sarkar M. Excessive dietary calcium in the disruption of structural and functional status of adult male reproductive system in rat with possible mechanism. Mol Cell Biochem. (2012) 364:181–91. doi: 10.1007/s11010-011-1217-3
115. Montjean D, Ménézo Y, Benkhalifa M, Cohen M, Belloc S, Cohen-Bacrie P, et al. Malonaldehyde formation and DNA fragmentation: two independent sperm decays linked to reactive oxygen species. Zygote. (2010) 18:265–8. doi: 10.1017/S0967199409990311
116. Polak G, Rola R, Gogacz M, Kozioł-Montewka M, Kotarski J. Malonyldialdehyde and total antioxidant status in the peritoneal fluid of infertile women. Ginekologia polska. (1999) 70:135–40. doi: 10.1016/s0301-2115(00)00352-3
117. Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. BioMed Res Int. (2014) 2014:426951. doi: 10.1155/2014/426951
118. Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod biomedicine online. (2010) 20:711–23. doi: 10.1016/j.rbmo.2010.03.008
119. Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, et al. Coenzyme Q10 and male infertility: a meta-analysis. J assisted Reprod Genet. (2013) 30:1147–56. doi: 10.1007/s10815-013-0047-5
120. Stanislavov R, Nikolova V, Rohdewald P. Improvement of seminal parameters with Prelox®: a randomized, double-blind, placebo-controlled, cross-over trial. Phytotherapy Research: Int J Devoted to Pharmacol Toxicological Eval Natural Product Derivatives. (2009) 23:297–302. doi: 10.1002/ptr.v23:3
121. FDA. Testicular toxicity: evaluation during drug development. Food and Drug Administration 5630 Fishers Lane, Rm 1061, Rockville, MD 20852: Center for Drug Evaluation and Research (2018). Available online at: www.fda.gov/regulatory-information/search-fda-guidance-documents/testicular-toxicity-evaluation-during-drug-development (Accessed December 19, 2024).
122. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol endocrinology. (2012) 10:1–31. doi: 10.1186/1477-7827-10-49
123. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility sterility. (2003) 79:829–43. doi: 10.1016/S0015-0282(02)04948-8
124. Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinology. (2018) 16:1–18. doi: 10.1186/s12958-018-0391-5
125. Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci. (2011) 108:1462–7. doi: 10.1073/pnas.1017213108
126. Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD (P) H oxidase. Mol Cell Biochem. (2004) 264:85–97. doi: 10.1023/B:MCBI.0000044378.09409.b5
127. Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecologic Invest. (2001) 8:S40–S2. doi: 10.1177/1071557601008001S13
128. Henkel R, Morris A, Vogiatzi P, Saleh R, Sallam H, Boitrelle F, et al. Predictive value of seminal oxidation-reduction potential analysis for reproductive outcomes of ICSI. Reprod biomedicine online. (2022) 45:1007–20. doi: 10.1016/j.rbmo.2022.05.010
129. Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. (2021) 236:7966–83. doi: 10.1002/jcp.v236.12
130. Homburg R. Polycystic ovary syndrome. Best Pract Res Clin Obstetrics Gynaecology. (2008) 22:261–74. doi: 10.1016/j.bpobgyn.2007.07.009
131. Kostroun KE, Goldrick K, Mondshine JN, Robinson RD, Mankus E, Reddy S, et al. Impact of updated international diagnostic criteria for the diagnosis of polycystic ovary syndrome. F&S Rep. (2023) 4:173–8. doi: 10.1016/j.xfre.2022.12.003
132. Dabravolski SA, Nikiforov NG, Eid AH, Nedosugova LV, Starodubova AV, Popkova TV, et al. Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci. (2021) 22:3923. doi: 10.3390/ijms22083923
133. Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N. Increased prolidase activity and oxidative stress in PCOS. Clin endocrinology. (2013) 79:105–10. doi: 10.1111/cen.2013.79.issue-1
134. Mancini A, Bruno C, Vergani E, d’Abate C, Giacchi E, Silvestrini A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: controversies and new insights. Int J Mol Sci. (2021) 22:1667. doi: 10.3390/ijms22041667
135. Celik F, Kose M, Yilmazer M, Köken GN, Arioz DT, Kanat Pektas M. Plasma L-carnitine levels of obese and non-obese polycystic ovary syndrome patients. J Obstetrics Gynaecology. (2017) 37:476–9. doi: 10.1080/01443615.2016.1264375
136. Salehpour S, Nazari L, Hoseini S, Moghaddam PB, Gachkar L. Effects of L-carnitine on polycystic ovary syndrome. JBRA assisted reproduction. (2019) 23:392. doi: 10.5935/1518-0557.20190033
137. Sandhu JK, Waqar A, Jain A, Joseph C, Srivastava K, Ochuba O, et al. Oxidative stress in polycystic ovarian syndrome and the effect of antioxidant N-acetylcysteine on ovulation and pregnancy rate. Cureus. (2021) 13:1–7. doi: 10.7759/cureus.17887
138. Song Y, Wang H, Huang H, Zhu Z. Comparison of the efficacy between NAC and metformin in treating PCOS patients: a meta-analysis. Gynecological Endocrinology. (2020) 36:204–10. doi: 10.1080/09513590.2019.1689553
139. Abedini M, Ghaedi E, Hadi A, Mohammadi H, Amani R. Zinc status and polycystic ovarian syndrome: A systematic review and meta-analysis. J Trace Elements Med Biol. (2019) 52:216–21. doi: 10.1016/j.jtemb.2019.01.002
140. Coskun A, Arikan T, Kilinc M, Arikan DC, Ekerbiçer HÇ. Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur J Obstetrics Gynecology Reprod Biol. (2013) 168:183–6. doi: 10.1016/j.ejogrb.2013.01.021
141. Jamilian M, Mansury S, Bahmani F, Heidar Z, Amirani E, Asemi Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. (2018) 11:1–7. doi: 10.1186/s13048-018-0457-1
142. Foroozanfard F, Jamilian M, Jafari Z, Khassaf A, Hosseini A, Khorammian H, et al. Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol diabetes. (2015) 123:215–20. doi: 10.1055/s-0035-1548790
143. Jamilian M, Razavi M, Fakhrie Kashan Z, Ghandi Y, Bagherian T, Asemi Z. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin endocrinology. (2015) 82:885–91. doi: 10.1111/cen.2015.82.issue-6
144. Masha A, Manieri C, Dinatale S, Bruno G, Ghigo E, Martina V. Prolonged treatment with N-acetylcysteine and L-arginine restores gonadal function in patients with polycistic ovary syndrome. J endocrinological Invest. (2009) 32:870–2. doi: 10.1007/BF03345763
145. Oner G, Muderris II. Clinical, endocrine and metabolic effects of metformin vs N-acetyl-cysteine in women with polycystic ovary syndrome. Eur J Obstetrics Gynecology Reprod Biol. (2011) 159:127–31. doi: 10.1016/j.ejogrb.2011.07.005
146. Dubey P, Reddy S, Boyd S, Bracamontes C, Sanchez S, Chattopadhyay M, et al. Effect of nutritional supplementation on oxidative stress and hormonal and lipid profiles in PCOS-affected females. Nutrients. (2021) 13:2938. doi: 10.3390/nu13092938
147. Ismail AM, Hamed AH, Saso S, Thabet HH. RETRACTED: Adding l-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur J Obstetrics Gynecology Reprod Biol. (2014) 180:148–52. doi: 10.1016/j.ejogrb.2014.06.008
148. Fang F, Ni K, Cai Y, Shang J, Zhang X, Xiong C. Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complementary therapies Clin practice. (2017) 26:53–60. doi: 10.1016/j.ctcp.2016.11.008
149. Chen J, Guo Q, Y-H P, Ren Q-L, Chi L, Hu R-K, et al. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: a retrospective cohort study. BMC women's Health. (2020) 20:1–9. doi: 10.1186/s12905-020-00930-w
150. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstetrics gynecology Clinics North America. (1997) 24:235–58. doi: 10.1016/S0889-8545(05)70302-8
151. Kokot I, Piwowar A, Jędryka M, Kratz EM. Is there a balance in oxidative-antioxidant status in blood serum of patients with advanced endometriosis? Antioxidants. (2021) 10:1097. doi: 10.3390/antiox10071097
152. Imanaka S, Yamada Y, Kawahara N, Kobayashi H. A delicate redox balance between iron and heme oxygenase-1 as an essential biological feature of endometriosis. Arch Med Res. (2021) 52:641–7. doi: 10.1016/j.arcmed.2021.03.006
153. Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Arabipoor A. Oxidative stress statues in serum and follicular fluid of women with endometriosis. Cell J (Yakhteh). (2017) 18:582. doi: 10.22074/cellj.2016.4724
154. Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin obstetrics gynecology. (2006) 18:325–32. doi: 10.1097/01.gco.0000193003.58158.4e
155. Bedaiwy MA, Falcone T, Sharma R, Goldberg J, Attaran M, Nelson D, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum reproduction. (2002) 17:426–31. doi: 10.1093/humrep/17.2.426
156. Tosti C, Pinzauti S, Santulli P, Chapron C, Petraglia F. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod Sci. (2015) 22:1053–9. doi: 10.1177/1933719115592713
157. Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertility sterility. (2008) 90:247–57. doi: 10.1016/j.fertnstert.2008.02.093
158. Young VJ, Ahmad SF, Duncan WC, Horne AW. The role of TGF-β in the pathophysiology of peritoneal endometriosis. Hum Reprod update. (2017) 23:548–59. doi: 10.1093/humupd/dmx016
159. Chen C, Zhou Y, Hu C, Wang Y, Yan Z, Li Z, et al. Mitochondria and oxidative stress in ovarian endometriosis. Free Radical Biol Med. (2019) 136:22–34. doi: 10.1016/j.freeradbiomed.2019.03.027
160. Irimia T, Puşcaşiu L, Mitranovici M-I, Crişan A, Budianu MA, Bănescu C, et al. Oxidative-stress related gene polymorphism in endometriosis-associated infertility. Medicina. (2022) 58:1105. doi: 10.3390/medicina58081105
161. Clower L, Fleshman T, Geldenhuys WJ, Santanam N. Targeting oxidative stress involved in endometriosis and its pain. Biomolecules. (2022) 12:1055. doi: 10.3390/biom12081055
162. Ansariniya H, Yavari A, Javaheri A, Zare F. Oxidative stress-related effects on various aspects of endometriosis. Am J Reprod Immunol. (2022) 88:e13593. doi: 10.1111/aji.13593
163. Fadin M, Nicoletti MC, Pellizzato M, Accardi M, Baietti MG, Fratter A. Effectiveness of the integration of quercetin, turmeric, and N-acetylcysteine in reducing inflammation and pain associated with endometriosis. In-vitro and in-vivo studies. Minerva Ginecologica. (2020) 72:285–91. doi: 10.23736/S0026-4784.20.04615-8
164. Santanam N, Kavtaradze N, Murphy A, Dominguez C, Parthasarathy S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Trans Res. (2013) 161:189–95. doi: 10.1016/j.trsl.2012.05.001
165. Amini L, Chekini R, Nateghi MR, Haghani H, Jamialahmadi T, Sathyapalan T, et al. The effect of combined vitamin C and vitamin E supplementation on oxidative stress markers in women with endometriosis: a randomized, triple-blind placebo-controlled clinical trial. Pain Res Management. (2021) 2021:5529741. doi: 10.1155/2021/5529741
166. Mehdizadehkashi A, Rokhgireh S, Tahermanesh K, Eslahi N, Minaeian S, Samimi M. The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis. Gynecological Endocrinology. (2021) 37:640–5. doi: 10.1080/09513590.2021.1878138
167. Sukan B, Akdevelioğlu Y, Sukan VN. Effect of antioxidant supplementation on endometriosis-related pain: A systematic review. Curr Nutr Rep. (2022) 11:753–64. doi: 10.1007/s13668-022-00432-1
168. Máté G, Bernstein LR, Török AL. Endometriosis is a cause of infertility. Does reactive oxygen damage to gametes and embryos play a key role in the pathogenesis of infertility caused by endometriosis? Front endocrinology. (2018) 9:725. doi: 10.3389/fendo.2018.00725
169. Ferreira EM, Giorgi VSI, Rodrigues JK, de Andrade AZ, Junior AAJ, Navarro PA. Systemic oxidative stress as a possible mechanism underlying the pathogenesis of mild endometriosis-related infertility. Reprod BioMedicine Online. (2019) 39:785–94. doi: 10.1016/j.rbmo.2019.06.011
170. Lu X, Wu Z, Wang M, Cheng W. Effects of vitamin C on the outcome of in vitro fertilization–embryo transfer in endometriosis: A randomized controlled study. J Int Med Res. (2018) 46:4624–33. doi: 10.1177/0300060518786918
171. Iwabuchi T, Yoshimoto C, Shigetomi H, Kobayashi H. Oxidative stress and antioxidant defense in endometriosis and its Malignant transformation. Oxid Med Cell longevity. (2015) 2015:848595. doi: 10.1155/2015/848595
172. Shigetomi H, Imanaka S, Kobayashi H. Effects of iron-related compounds and bilirubin on redox homeostasis in endometriosis and its Malignant transformations. Hormone Mol Biol Clin Invest. (2022) 43:187–92. doi: 10.1515/hmbci-2021-0065
173. Rebar RW. Premature ovarian failure. Obstetrics Gynecology. (2009) 113:1355–63. doi: 10.1097/AOG.0b013e3181a66843
174. Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM. Menopause as risk factor for oxidative stress. Menopause. (2012) 19:361–7. doi: 10.1097/gme.0b013e318229977d
175. Cervellati C, Bergamini CM. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin Chem Lab Med (CCLM). (2016) 54:739–53. doi: 10.1515/cclm-2015-0807
176. Silva TR, Oppermann K, Reis FM, Spritzer PM. Nutrition in menopausal women: a narrative review. Nutrients. (2021) 13:2149. doi: 10.3390/nu13072149
177. Leal M, Díaz J, Serrano E, Abellán J, Carbonell LF. Hormone replacement therapy for oxidative stress in postmenopausal women with hot flushes. Obstetrics gynecology. (2000) 95:804–9. doi: 10.1016/s0029-7844(00)00822-x
178. Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J pineal Res. (2008) 44:280–7. doi: 10.1111/j.1600-079X.2007.00524.x
179. Jiang Y, Zhang Z, Cha L, Li L, Zhu D, Fang Z, et al. Resveratrol plays a protective role against premature ovarian failure and prompts female germline stem cell survival. Int J Mol Sci. (2019) 20:3605. doi: 10.3390/ijms20143605
180. Liang X, Yan Z, Ma W, Qian Y, Zou X, Cui Y, et al. Peroxiredoxin 4 protects against ovarian ageing by ameliorating D-galactose-induced oxidative damage in mice. Cell Death Disease. (2020) 11:1053. doi: 10.1038/s41419-020-03253-8
181. Zheng S, Ma M, Chen Y, Li M. Effects of quercetin on ovarian function and regulation of the ovarian PI3K/Akt/FoxO3a signalling pathway and oxidative stress in a rat model of cyclophosphamide-induced premature ovarian failure. Basic Clin Pharmacol Toxicology. (2022) 130:240–53. doi: 10.1111/bcpt.v130.2
182. Chen Y, Zhao Y, Miao C, Yang L, Wang R, Chen B, et al. Quercetin alleviates cyclophosphamide-induced premature ovarian insufficiency in mice by reducing mitochondrial oxidative stress and pyroptosis in granulosa cells. J Ovarian Res. (2022) 15:138. doi: 10.1186/s13048-022-01080-3
183. Ochiai A, Kuroda K. Preconception resveratrol intake against infertility: Friend or foe? Reprod Med Biol. (2020) 19:107–13. doi: 10.1002/rmb2.12303
184. Jang H, Hong K, Choi Y. Melatonin and fertoprotective adjuvants: prevention against premature ovarian failure during chemotherapy. Int J Mol Sci. (2017) 18:1221. doi: 10.3390/ijms18061221
185. Xu H, Bao X, Kong H, Yang J, Li Y, Sun Z. Melatonin protects against cyclophosphamide-induced premature ovarian failure in rats. Hum Exp Toxicology. (2022) 41:09603271221127430. doi: 10.1177/09603271221127430
186. Talpur H, Chandio I, Brohi R, Worku T, Rehman Z, Bhattarai D, et al. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod Domest animals. (2018) 53:831–49. doi: 10.1111/rda.2018.53.issue-4
187. Isbilen E, Kulaksizoglu S, Kirmizioglu M, Karuserci Komurcu O, Tabur S. Role of prolidase activity and oxidative stress biomarkers in unexplained infertility. Int J Gynecology Obstetrics. (2022) 156:430–5. doi: 10.1002/ijgo.v156.3
188. Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database systematic Rev. (2020) 8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4
189. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol endocrinology. (2005) 3:1–21. doi: 10.1186/1477-7827-3-28
190. Rodríguez-Varela C, Labarta E. Does coenzyme Q10 supplementation improve human oocyte quality? Int J Mol Sci. (2021) 22:9541. doi: 10.3390/ijms22179541
191. de Almeida Chuffa LG, Lupi LA, Cucielo MS, Silveira HS, Reiter RJ, Seiva FRF. Melatonin promotes uterine and placental health: potential molecular mechanisms. Int J Mol Sci. (2019) 21:300. doi: 10.3390/ijms21010300
192. Rizk AY, Bedaiwy MA, Al-Inany HG. N-acetyl-cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate–resistant patients with polycystic ovary syndrome. Fertility sterility. (2005) 83:367–70. doi: 10.1016/j.fertnstert.2004.07.960
193. Cicek N, Eryilmaz OG, Sarikaya E, Gulerman C, Genc Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J assisted Reprod Genet. (2012) 29:325–8. doi: 10.1007/s10815-012-9714-1
194. Youssef MA, Abdelmoty HI, Elashmwi HA, Abduljawad EM, Elghamary N, Magdy A, et al. Oral antioxidants supplementation for women with unexplained infertility undergoing ICSI/IVF: randomized controlled trial. Hum Fertility. (2015) 18:38–42. doi: 10.3109/14647273.2014.927595
195. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility Sterility. (2013) 99(1):63. doi: 10.1016/j.fertnstert.2012.09.023
196. Royal College of Obstetrics and Gynecology. The Investigation and Treatment of Couples with Recurrent First trimester and Second-trimester Miscarriage. Green Top Guidelines No. 17. RCOG (2011). London, UK: RCOG (2011). Available online at: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/recurrent-miscarriage-green-top-guideline-no-17/ (Accessed December 19, 2024).
197. Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. Am J pathology. (2000) 157:2111–22. doi: 10.1016/S0002-9440(10)64849-3
198. Zejnullahu VA, Zejnullahu VA, Kosumi E. The role of oxidative stress in patients with recurrent pregnancy loss: a review. Reprod Health. (2021) 18:1–12. doi: 10.1186/s12978-021-01257-x
199. Yiyenoğlu ÖB, Uğur MG, Özcan HÇ, Can G, Öztürk E, Balat Ö, et al. Assessment of oxidative stress markers in recurrent pregnancy loss: a prospective study. Arch gynecology obstetrics. (2014) 289:1337–40. doi: 10.1007/s00404-013-3113-4
200. Ghneim HK, Al−Sheikh YA, Alshebly MM, Aboul−Soud MA. Superoxide dismutase activity and gene expression levels in Saudi women with recurrent miscarriage. Mol Med Rep. (2016) 13:2606–12. doi: 10.3892/mmr.2016.4807
201. Bogavac M, Jakovljević A, Nikolić A, Milošević Tošić M, Perić T, Belopavlović Z. Biomarkers of oxidative stress in pregnant women with recurrent miscarriages. J Lab Med. (2019) 43:101–14. doi: 10.1515/labmed-2018-0148
202. Sumitha Prabhu P, Aneesh P, Jiju J, Reshma P, Dinesh Roy D. Evidence of increased oxidative stress and DNA damages in women with recurrent abortions. Int J Sci Eng Res. (2015) 6:15–9.
203. Namlı Kalem M, Akgun N, Kalem Z, Bakirarar B, Celik T. Chemokine (CC motif) ligand-2 (CCL2) and oxidative stress markers in recurrent pregnancy loss and repeated implantation failure. J assisted Reprod Genet. (2017) 34:1501–6. doi: 10.1007/s10815-017-0992-5
204. Amin AF, Shaaban OM, Bediawy MA. N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reprod biomedicine online. (2008) 17:722–6. doi: 10.1016/S1472-6483(10)60322-7
205. Balogun OO, da Silva Lopes K, Ota E, Takemoto Y, Rumbold A, Takegata M, et al. Vitamin supplementation for preventing miscarriage. Cochrane Database Systematic Rev. (2016) 2016(5):CD004073. doi: 10.1002/14651858.CD004073.pub4
206. Agarwal A, Selvam MKP, Arafa M, Okada H, Homa S, Killeen A, et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J Andrology. (2019) 21:565–9. doi: 10.4103/aja.aja_5_19
207. Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod biomedicine online. (2017) 34:48–57. doi: 10.1016/j.rbmo.2016.10.008
208. Agarwal A, Roychoudhury S, Bjugstad KB, Cho C-L. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. (2016) 8:302–18. doi: 10.1177/1756287216652779
209. Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. (2014) 37:99. doi: 10.4103/2319-4170.128725
210. Panner Selvam M, Henkel R, Sharma R, Agarwal A. Calibration of redox potential in sperm wash media and evaluation of oxidation–reduction potential values in various assisted reproductive technology culture media using MiOXSYS system. Andrology. (2018) 6:293–300. doi: 10.1111/andr.2018.6.issue-2
211. Gualtieri R, Kalthur G, Barbato V, Longobardi S, Di Rella F, Adiga SK, et al. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants. (2021) 10:1025. doi: 10.3390/antiox10071025
212. Chen YM, Tu CJ, Hung KY, Wu KD, Tsai TJ, Hsieh BS. Inhibition by pentoxifylline of TNF-α-stimulated fractalkine production in vascular smooth muscle cells: evidence for mediation by NF-κB down-regulation. Br J Pharmacol. (2003) 138:950–8. doi: 10.1038/sj.bjp.0705088
213. Tarlatzis B, Kolibianakis E, Bontis J, Tousiou M, Lagos S, Mantalenakis S. Effect of pentoxifylline on human sperm motility and fertilizing capacity. Arch andrology. (1995) 34:33–42. doi: 10.3109/01485019508987828
214. Negri P, Grechi E, Tomasi A, Fabbri E, Capuzzo A. Effectiveness of pentoxifylline in semen preparation for intrauterine insemination. Hum Reproduction. (1996) 11:1236–9. doi: 10.1093/oxfordjournals.humrep.a019363
215. del Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem. (2002) 50:3698–703. doi: 10.1021/jf011702q
216. Truong TT, Gardner DK. Antioxidants increase blastocyst cryosurvival and viability post-vitrification. Hum reproduction. (2020) 35:12–23. doi: 10.1093/humrep/dez243
217. Martín-Romero FJ, Miguel-Lasobras EM, Domínguez-Arroyo JA, González-Carrera E, Álvarez IS. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod biomedicine online. (2008) 17:652–61. doi: 10.1016/S1472-6483(10)60312-4
218. Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust New Z J Obstetrics Gynaecology. (2007) 47:216–21. doi: 10.1111/j.1479-828X.2007.00723.x
219. Xu Y, Nisenblat V, Lu C, Li R, Qiao J, Zhen X, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinology. (2018) 16:1–11. doi: 10.1186/s12958-018-0343-0
220. Tural R, Karakaya C, Erdem M, Aykol Z, Karabacak RO, Kavutcu M. Investigation of oxidative stress status in cumulus cells in patıents with in vitro fertilization. Turkish J Med Sci. (2021) 51:1969–75. doi: 10.3906/sag-2104-188
221. Gardner DK, Kuramoto T, Tanaka M, Mitzumoto S, Montag M, Yoshida A. Prospective randomized multicentre comparison on sibling oocytes comparing G-Series media system with antioxidants versus standard G-Series media system. Reprod Biomedicine Online. (2020) 40:637–44. doi: 10.1016/j.rbmo.2020.01.026
222. Placidi M, Di Emidio G, Virmani A, D’Alfonso A, Artini PG, D’Alessandro AM, et al. Carnitines as mitochondrial modulators of oocyte and embryo bioenergetics. Antioxidants. (2022) 11:745. doi: 10.3390/antiox11040745
223. Cutting E, Horta F, Dang V, Van Rumste MM, Mol BWJ. Intracytoplasmic sperm injection versus conventional in vitro fertilisation in couples with males presenting with normal total sperm count and motility. Cochrane Database Systematic Rev. (2023) 8(8):CD001301. doi: 10.1002/14651858.CD001301.pub2
224. Arhin SK, Zhao Y, Lu X, Chetry M, Lu J. Effect of micronutrient supplementation on IVF outcomes: a systematic review of the literature. Reprod biomedicine online. (2017) 35:715–22. doi: 10.1016/j.rbmo.2017.08.018
225. Aboulfoutouh I, Youssef M, Khattab S. Can antioxidants supplementation improve ICSI/IVF outcomes in women undergoing IVF/ICSI treatment cycles? Randomised controlled study. Fertility Sterility. (2011) 96:S242. doi: 10.1016/j.fertnstert.2011.07.928
226. Florou P, Anagnostis P, Theocharis P, Chourdakis M, Goulis DG. Does coenzyme Q 10 supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J assisted Reprod Genet. (2020) 37:2377–87. doi: 10.1007/s10815-020-01906-3
Keywords: antioxidants, female infertility, male infertility, reactive oxygen species, oxidative stress
Citation: Saleh R, Sallam H, Elsuity MA, Dutta S, Sengupta P and Nasr A (2025) Antioxidant therapy for infertile couples: a comprehensive review of the current status and consideration of future prospects. Front. Endocrinol. 15:1503905. doi: 10.3389/fendo.2024.1503905
Received: 29 September 2024; Accepted: 09 December 2024;
Published: 09 January 2025.
Edited by:
Weibin Bai, Jinan University, ChinaReviewed by:
Zia Ur Rehman, University of Agriculture, PakistanKamil Gill, Pomeranian Medical University, Poland
Copyright © 2025 Saleh, Sallam, Elsuity, Dutta, Sengupta and Nasr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramadan Saleh, c2FsZWhyMjAxMEB5YWhvby5jb20=; cmFtYWRhbl9zYWxlaEBtZWQuc29oYWcuZWR1LmVn
†ORCID: Ramadan Saleh, orcid.org/0000-0003-0503-3533
Sulagna Dutta, orcid.org/0000-0002-7893-5282
Pallav Sengupta, orcid.org/0000-0002-1928-5048
 Ramadan Saleh
Ramadan Saleh Hassan Sallam
Hassan Sallam Mohamad AlaaEldein Elsuity1,2
Mohamad AlaaEldein Elsuity1,2 Sulagna Dutta
Sulagna Dutta Pallav Sengupta
Pallav Sengupta