- 1Department of Metabolic Diseases, Jagiellonian University Medical College, Krakow, Poland
- 2Internal Medicine, Metabolic Diseases and Diabetology Clinical Department, University Hospital in Krakow, Krakow, Poland
- 3Department of Internal Medicine and Diabetology, Poznan University of Medical Sciences, Poznan, Poland
- 4Department of Internal Medicine, Diabetology and Cardiometabolic Disorders, Faculty of Medical Sciences Zabrze, Medical University of Silesia, Katowice, Poland
- 5Department of Internal Medicine and Metabolic Diseases, Medical University of Bialystok, Bialystok, Poland
Background: Continuous glucose monitoring (CGM) improves glycemic control and quality of life. Data on glycemic indices and fear of hypoglycemia (FoH) in newly diagnosed T1DM patients are limited.
Aim: To assess the impact of initiating intermittently scanned CGM (isCGM) within 1–6 months of diagnosis on glycemic control and FoH in adults with T1DM.
Subjects and methods: After wearing a blinded sensor for 14 days, participants were randomized (1:1) to either isCGM (intervention) or self-monitoring blood glucose (SMBG) with glucometers and blinded CGM (control). Primary outcomes were changes in time below 70 mg/dl (TB70) and FoH, assessed in the Hypoglycemia Fear Survey (HFS). Main secondary outcomes included changes in mean glucose and time in range (TIR) from baseline to 4 weeks after randomization.
Results: The full analysis set included 23 patients (12 from the intervention group and 11 from the control group), aged 25.6 ± 5.1 years (14 men, 9 women). All participants were on multiple daily insulin injections. TB70 changed from 2.42% to 2.25% in the intervention, and from 2.81% to 1.82% in the control group, and the between-therapy difference of 0.83% was insignificant. No difference between intervention and control groups in change in HFS-worry and HFS-behavior subscales between baseline and after 4 weeks was found (−1.6 ± 3.2 and 1.0 ± 2.2, respectively). The mean glucose levels changed from 7.03 mmol/l to 6.73 mmol/l and from 7.07 mmol/l to 7.43 mmol/l, in the intervention and control groups, respectively, which resulted in a between-therapy significant glucose difference of −0.66 mmol/l. The mean TIR changed from 88.0% to 90.0% in the intervention group and from 85.2 to 84.1% in the control group—the between-therapy difference was insignificant (3,1%). The study ended early due to CGM reimbursement policy changes, after which most patients eligible for the study could have isCGM reimbursed.
Conclusions: In newly diagnosed T1DM adults, TIR is high and hypoglycemia risk is low. The study group was small; however, the data suggest that the use of isCGM soon after T1DM diagnosis could result in mean glucose decrease, but not in change in TB70 and FoH.
Introduction
Use of continuous glucose monitoring (CGM) systems has become the standard of glucose control in most patients with type 1 diabetes (T1DM) (1). It is recommended by the American Diabetes Association (ADA) to initiate CGM early in T1DM, even at time of diagnosis (2). There are two types of CGM—intermittently scanned CGM (isCGM) and real-time CGM (rtCGM) (3). It is well proven that CGM systems increase time in range (TIR), reduce the number and duration of hypoglycemic episodes, reduce time spent in hyperglycemia, and improve the quality of life (QoL) (4–6). QoL in people with T1DM is decreased due to a need of performing insulin injections, frequent glucose measurements, and carbohydrate intake counting needed to prevent development of chronic complications (7, 8). The first few months after the diagnosis can be particularly difficult as the newly diagnosed patients need to adapt to the reality of a chronic and challenging disease. The diagnosis of T1DM is usually followed by a transient improvement in blood glucose levels and a drop in insulin requirement, which sometimes meets the criteria of a so-called “partial clinical remission” (PCR) (9–11). The phenomenon of PCR occurs in approximately 25%–60% of adult T1DM patients (11–13). In some rare cases of a total diabetes remission, insulin treatment can be even temporarily interrupted (14). PCR typically starts shortly after diagnosis, usually no later than by the end of the 12th month, and lasts for several months (12, 15). During PCR, due to a substantial drop in the insulin requirements and a challenge to adjust its dose, the higher risk of mild hypoglycemia is observed (16). While limited data on severe hypoglycemia episodes in adults with T1DM during this period were published, such patients were seen in the authors’ clinical practice. Possible severe hypoglycemic events may cause dramatic psychological trauma and affect the future treatment efficacy and quality of life for years to come in this population. Clinical data on the initial period of T1DM in adults regarding this initial drop in insulin requirement, particularly in terms of hypoglycemia, fear of hypoglycemic episodes, and the quality of life, are limited, especially on an effect of isCGM on hypoglycemic episodes and psychological well-being of newly diagnosed patients with T1DM.
The aim of this study was to evaluate the impact of the isCGM system on glycemic control and assess the fear of hypoglycemic episodes as well as quality of life in young adults with newly diagnosed T1DM.
Materials and methods
This prospective, randomized, non-masked study was conducted in four academic centers in Poland—Krakow, Poznan, Zabrze, and Bialystok (ClinicalTrials.gov reg. no. NCT06414824). Patients were eligible for the study if aged 18–35 years, newly diagnosed T1DM (1–6 months), treated with multiple daily injections of insulin (MDI), and in the investigator’s opinion technically capable of using isCGM. Patients were not included if they had used any CGMS or were on pump therapy, were pregnant or were planning pregnancy or breast feeding, were participating in another clinical trial that could affect glucose measurements or glucose management, had known allergy to medical adhesives, had severe end-organ damage (kidney, liver), and were diagnosed with psychiatric disorders.
After 14 days of wearing a blinded sensor, participants were randomly assigned by the central interactive web response system in a ratio 1:1 to isCGM (intervention group) or to self-monitoring of blood glucose (SMBG) with glucometers and blinded CGM (control group) using Randomizer for Clinical Trials developed by the Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz (www.randomizer.at). The big stick randomization method was used, with equal probabilities assigned to each group, until a prespecified maximum tolerated imbalance of 3 was reached. Neither site nor sex was used as a factor for subgroup stratification. Participants needed to achieve a minimum of 70% wear time of the blinded glucose sensor prior to being randomized. Neither participants nor investigators were masked, which is a common feature of similar studies (4). The primary outcomes were change in time below 70 mg/dl (TB70) and fear of hypoglycemia assessed (FoH) in Hypoglycemia Fear Survey (HFS) from baseline to 4 weeks after randomization between study groups, and secondary outcomes were change in CGM-derived metrics: mean glucose, glucose management indicator (GMI), time in range (TIR), time above 180 mg/dl (TA180), time above 250 mg/dl (TA250), time below 54 mg/dl (TB54), coefficient of variation (CV) from baseline to 4 weeks after randomization, and a difference in the Diabetes Distress Scale (DDS) and in the Diabetes Treatment Satisfaction Questionnaire (DTSQ) at the end of study between study groups (17–20). The DDS questionnaire contains 17 items across four fields: emotional burden (five items), physician distress (four items), regimen distress (five items), and interpersonal distress (three items). Each item is scored on a 6-point scale: forms 1 (“not a problem”) to 6 (“very significant problem”). The average score of <2.0 is no or little distress, 2.0–3.0 reflects moderate distress, and >3.0 high distress. A score of >2.0 is considered as clinically significant distress. DTSQs (status) contains eight items scored on a 7-point scale ranging from 0 (“very dissatisfied,” “very inconvenient”) to 6 (“very satisfied,” “very convenient”) and indicates patients’ satisfaction at baseline. DTSQc (change) contains seven items scored on a 7-point scale ranging from −3 to 3. High scores indicate much more satisfied, convenient, or likely to recommend, whereas low scores indicate dissatisfied, inconvenient, and unlikely to recommend new therapy. HFS contains two subscales: behavior (15 items) and worry (17 items). All items are rated on a 5-point scale (0 = “never” to 4 = “almost always”). Higher scores indicate higher fear of hypoglycemia.
In a large study on isCGM in adults with well-controlled type 1 diabetes, the baseline TB70 was 14.2% ± 10%, and the FoH-adjusted mean score was approximately 30 ± 5 (4). Our prediction was that the study population would likely have lower TB70, which we estimated at 8% ± 5%. One of the main reasons we estimated a lower TB70 is that the current isCGM system has a lower mean absolute relative difference. To detect a difference to a mean improvement of 40% TB70 (to the target TB70 of 5% set by the International Consensus (17)), at 80% power and α of 0.05, we would need 45 participants in each arm. Similarly, assuming a 10% improvement in HFS, 45 participants in each arm would also be required.
The study was approved by the local bioethics committee. Participants gave written informed consent prior to entering the study. Statistical analyses were performed using IBM SPSS Statistics (version 29). Final analyses were performed using per-protocol treatment population. A significance level of p=0.05 was used in the analyses. Quantitative variables were checked for normal distribution. Parametric t test or non-parametric U tests were performed, where applicable, to describe clinical characteristics and differences between the study groups. For nominal variables, the Fisher’s exact test was used.
Results
There were 28 patients enrolled between May 2022 and December 2023. After completing the baseline 14-day phase of wearing a blinded sensor, the patients were randomly assigned to the intervention group (n=13) and control group (n=15). The full analysis set included 23 patients (12 from the intervention group and 11 from the control group). One patient withdrew consent to participate in the study, and four patients were excluded from analysis due to sensor failure or early sensor detachment (Figure 1).
The study was terminated early due to changes in reimbursement policy in Poland, as during the study groups of patients eligible for reimbursement were significantly expanded; particularly all patients who would be eligible for the study could have isCGM reimbursed. Moreover, the next generation of studied device, Libre 2, formally still isCGMS, but having features of rtCGMS, was introduced on the Polish market (21).
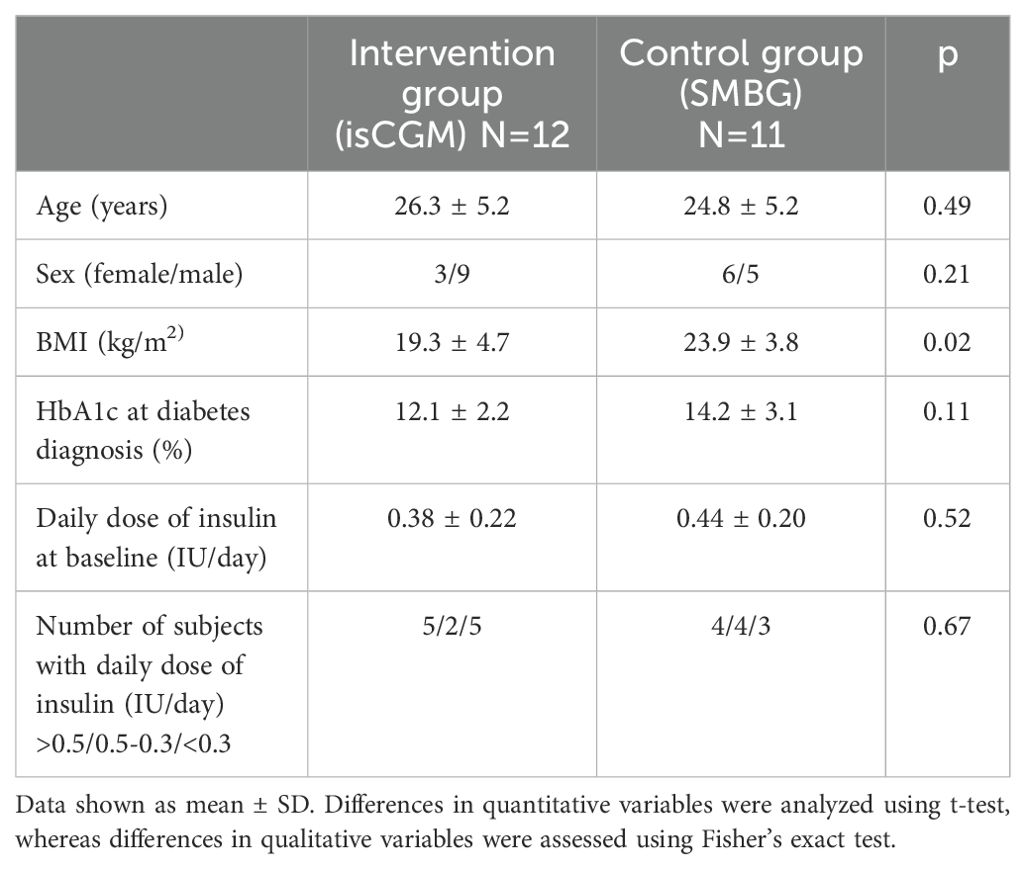
There was no difference between the intervention and control groups in terms of sex (men: 9 in 12 vs. 5 in 11) and age (26.3 ± 5.2 vs. 24.8 ± 5.2 years). At diabetes diagnosis, HbA1c% was similar between intervention and control groups (12.1 ± 2.2% vs. 14.2 ± 3.1%). At baseline, the mean BMI was lower in the intervention than control group (19.3 ± 4.7 vs. 23.9 ± 3.8 kg/m2). The mean daily dose of insulin did not differ between intervention and control groups (0.38 ± 0.22 vs. 0.44 ± 0.20 IU/kg), and the number of patients with daily insulin requirement less than 0.5 IU/kg was 7 in each group (Table 1).
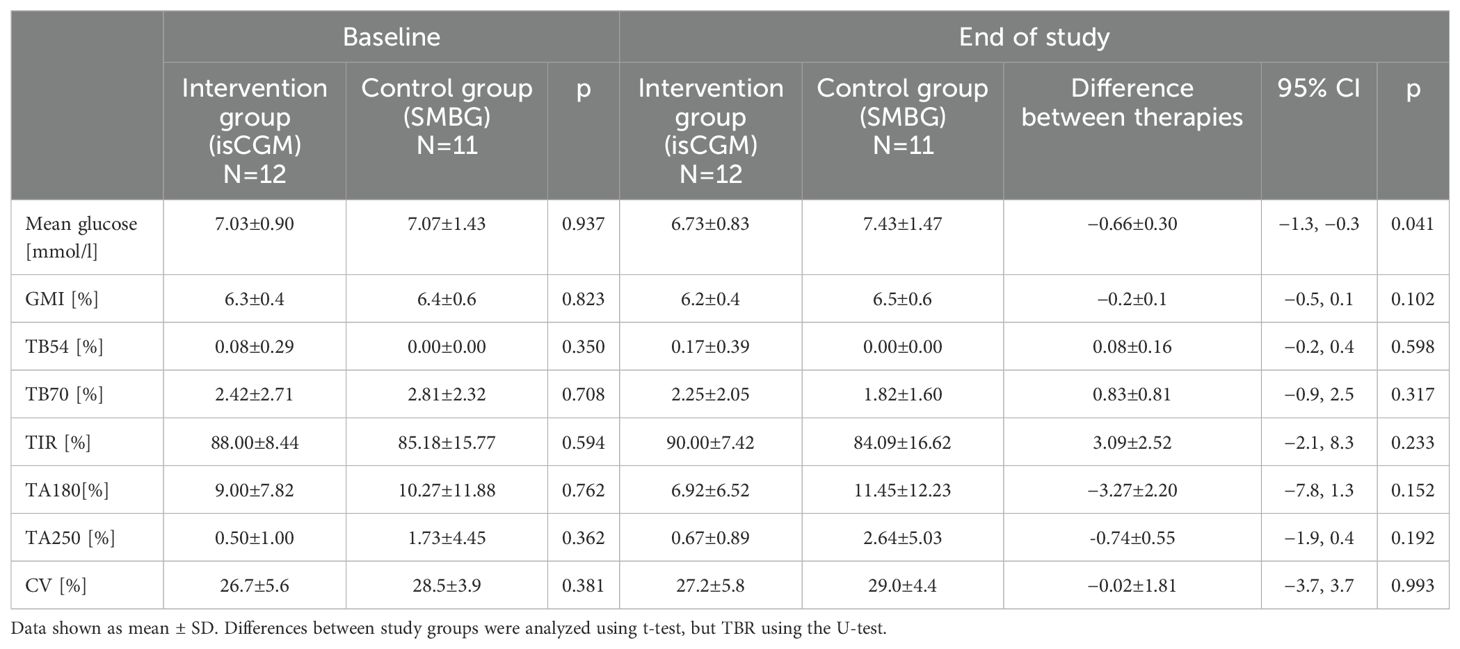
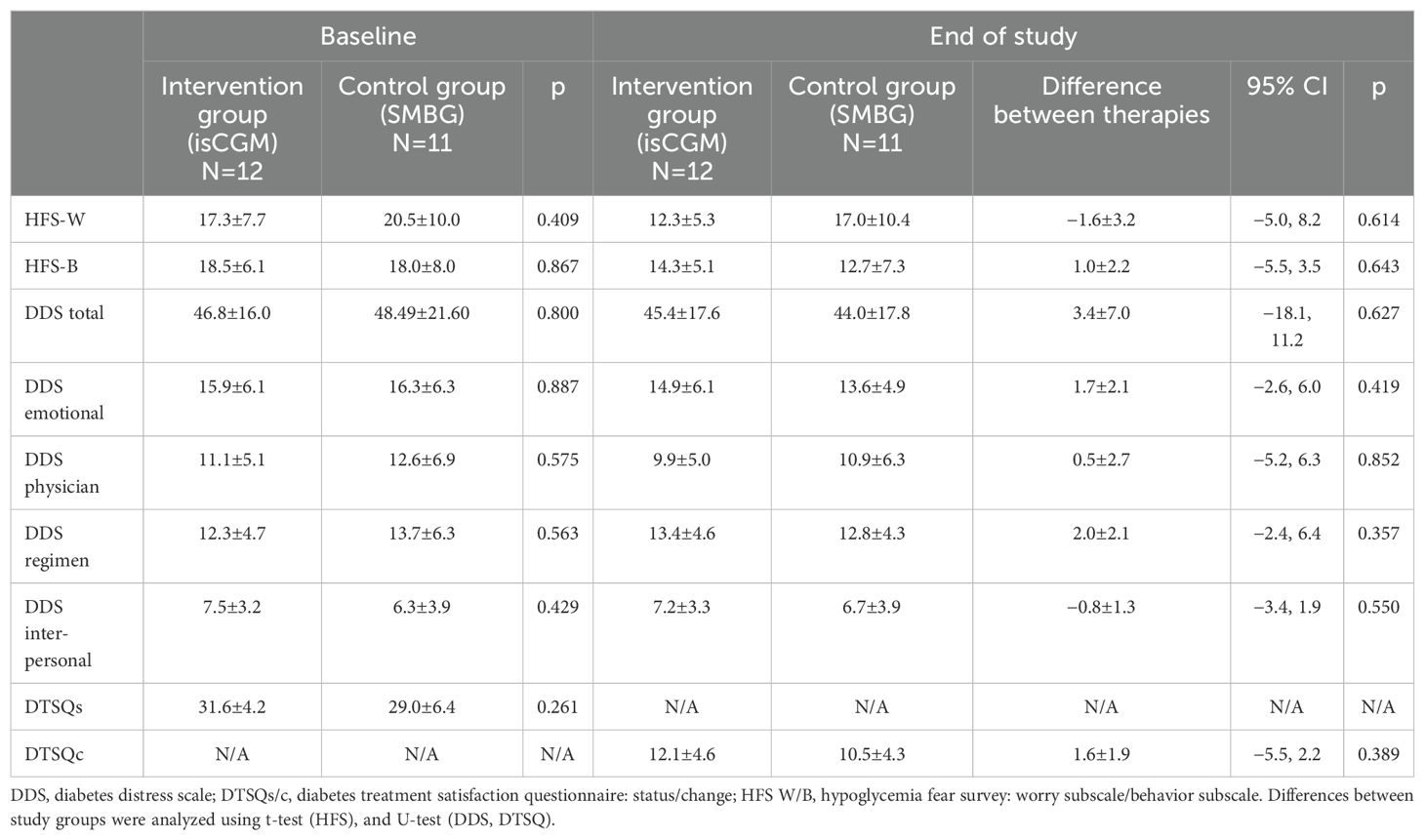
The mean time spent in hypoglycemia <70 mg/dl changed from 2.42% to 2.25% in the intervention group, and from 2.81% to 1.82% in the control group, and the between-therapy difference of 0.83% was insignificant (Figure 2; Table 2). No significant difference between intervention and control groups in change in HFS-worry and HFS-behavior subscales between baseline and after 4 weeks was found (−1.6 ± 3.2 and 1.0 ± 2.2, respectively) (Table 3). However, due to a smaller than planned sample size, the analysis was underpowered.

Figure 2. Mean glycemic indices among the study groups by week of the study: (A) Mean glucose; (B) GMI; (C) Time in range 70-180 mg/dl; (D) Time below 70 mg/dl; (E) Time below 54 mg/dl; (F) Time above 180 mg/dl; (G) Time above 250 mg/dl; (H) Coefficient of variation. Number in boxes indicate differences in mean change between therapies (intervention vs control) after 4 weeks. Bold values denote statistical significance.
The mean glucose level changed from 7.03 mmol/l to 6.73 mmol/l in the intervention group and from 7.07 mmol/l to 7.43 mmol/l in the control group, which resulted in a between-therapy significant difference of −0.66 mmol/l. No statistically significant difference was found in between-group change in GMI, TA180, TA250, TB54, and CV. Detailed data on glycemic indices are shown in Figure 2 and Table 2. Patient satisfaction with treatment did not change significantly when compared intervention with control (Table 3). Similarly, no difference in change in diabetes distress was found (Table 3).
No event of severe hypoglycemia (requiring hospitalization or third-party intervention) and no event of diabetic ketoacidosis were reported during the study.
Discussion
In this multicenter randomized controlled trial, we have examined the impact of soon-after T1DM diagnosis introduction of isCGM in adults on glycemic indices, diabetes treatment satisfaction, diabetes distress, and FOH.
First, we have shown that within first months after T1DM diagnosis, patients maintain good glycemic control, as baseline TIR was very high. This likely depends, at least partially, on PCR of T1DM, during which daily insulin requirement drops. Different criteria are proposed to diagnose PCR; most are based on insulin requirements, eq. daily dose of insulin of <0.5 IU/kg or <0.3 IU/kg, or an insulin dose-adjusted A1C (A1c [%]*4*DDI [IU/kg] <9.0) (9, 22). In our study, most patients’ daily insulin requirement was less than 0.5 IU/kg. Analyzed glycemic indices seem to be much better if compared with published data of the general T1DM population of longer diabetes duration, as in the presented study the mean baseline TIR was close to 90% and in different T1DM populations varies widely from 50% to 85% (23–26). In recently published studies, where glycemic control was analyzed in children and adolescents with newly diagnosed T1DM, TIR was ca. 70% (27, 28). To the best of our knowledge, no similar study in adults with newly diagnosed T1DM was published within the last years.
Second, we have shown that even in patients with so high a percentage of TIR, introducing CGM significantly decreased mean glucose. While TIR and GMI appeared to improve, the changes were not statistically significant. This lack of significance is likely due to underpowered analyses resulting from the early termination of the study and a lower than planned number of subjects examined. Use of CGM resulted in improvement in mean glucose, GMI, TIR, time above range (TAR), and time below range (TBR), which was well documented in many studies so far. However, in none of these studies, baseline TIR was close to 90% (4, 27–30).
Next, in our study, no change in TBR and patients’ FoH was observed. However, findings related to TBR and FOH should be considered preliminary due to the early termination of the study and reduced statistical power. Such change could likely be observed in patients with high baseline FoH, such as those with a history of severe hypoglycemic events. Moreover, in a longer study, with more hypoglycemic events, the effect of isCGM could be more clearly demonstrated. Data from some observational studies suggest lower FoH in patients using isCGM, especially in subjects with impaired awareness of hypoglycemia (31–34). Worth noting is that, in our previous study, we have shown that lower FoH is observed in patients performing more daily scans compared with those who perform fewer (23). Results of RCT provided divergent data. A large RCT investigating isCGM in adults with well-controlled T1DM, performed in 2016, did not show a positive effect of isCGM on hypoglycemia worry and hypoglycemia behavior (4, 34, 35). However, in that experiment, a positive effect of isCGM on treatment satisfaction score was seen (4). This finding is confirmed by other studies in which isCGM was tested versus SMBG (31, 33). In our study, we have not shown improvement in results of DDS and DTSQ.
We must acknowledge that our study has some limitations. First, we terminated the study early; thus, sample size is smaller than it was planned, so some expected effects of isCGM use might not be seen. Among eligible patients, some percentage did not provide consent, primarily because they did not want to be assigned to the control group. Additionally, this was the reason one patient withdrew consent. Moreover, the study was rather short-term, and some effects could need a longer study duration to be proofed. The strength of the study is its randomized nature in a specific group of patients with short-term duration of diabetes. However, despite randomization, an imbalance between the study groups was found in terms of BMI, which could lead to bias in the estimates of the treatment effects, in particular the observed difference in change in mean glucose.
The results of our study should be interpreted in the context of the long-term effects of isCGM, as its use leads to sustained improvements in glycemic control (30, 36). Use of isCGM improves not only glycemic indices but other important clinical outcomes as well. In real-world settings of FUTURE study, use of isCGM was associated with fewer hospitalizations due to hypoglycemia and/or diabetic ketoacidosis and less workplace absenteeism (31).
Accessibility and reimbursement policies of CGM systems differ among countries limiting common use of CGM worldwide. Thus, results of the present study, particularly improvement in mean glucose, support recommendation on early use of CGM, even from a day of diagnosis.
Since the use of CGM is considered the standard for glucose monitoring in T1DM and early use is recommended by the ADA, future research could focus on which features and what settings of CGM would provide most benefits to a specific group of patients.
Conclusions
In adults, the first months after T1DM diagnosis are associated with good glycemic control, high TIR, and low hypoglycemia risk. Early initiation of isCGM use soon after T1DM diagnosis was not associated with either TB70 or FoH reduction; however, the mean glucose level was decreased. Additionally, a trend to further increase TIR was seen. Given the early termination of the study and the small sample size, the results of the study should be considered as preliminary.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the Bioethics Committee of the Medical Chamber in Krakow, Poland. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. AG-W: Investigation, Writing – review & editing. MW: Investigation, Writing – review & editing. MK: Investigation, Writing – review & editing. LL: Investigation, Writing – review & editing. DR: Investigation, Writing – review & editing. DS: Investigation, Writing – review & editing. IK: Supervision, Writing – review & editing. KS: Supervision, Writing – review & editing. DZ-K: Supervision, Writing – review & editing. MM: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Diabetes Poland Grant was awarded to JH.
Conflict of interest
DZ-Z and MM have received lecture honoraria and serve on advisory boards for Abbott Diabetes Care. IK serves on advisory boards for Abbott Diabetes Care.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, et al. The management of type 1 diabetes in adults. A consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care. (2021) 44:2589–625. doi: 10.2337/dci21-0043
2. American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S126–44. doi: 10.2337/dc24-S007
3. Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet. (2019) 394:1265–73. doi: 10.1016/S0140-6736(19)31142-0
4. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. (2016) 388:2254–63. doi: 10.1016/S0140-6736(16)31535-5
5. Karges B, Tittel SR, Bey A, Freiberg C, Klinkert C, Kordonouri O, et al. Continuous glucose monitoring versus blood glucose monitoring for risk of severe hypoglycaemia and diabetic ketoacidosis in children, adolescents, and young adults with type 1 diabetes: a population-based study. Lancet Diabetes Endocrinol. (2023) 11:314–23. doi: 10.1016/S2213-8587(23)00061-X
6. Polonsky WH, Hessler D, Ruedy KJ, Beck RW, DIAMOND Study Group. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. (2017) 40:736–41. doi: 10.2337/dc17-0133
7. Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME, DCCT/EDIC Research Group. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care. (2013) 36:3131–8. doi: 10.2337/dc12-2109
8. Nielsen HB, Ovesen LL, Mortensen LH, Lau CJ, Joensen LE. Type 1 diabetes, quality of life, occupational status and education level - A comparative population-based study. Diabetes Res Clin Pract. (2016) 121:62–8. doi: 10.1016/j.diabres.2016.08.021
9. Mortensen HB, Hougaard P, Swift P, Hansen L, Holl RW, Hoey H, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. (2009) 32:1384–90. doi: 10.2337/dc08-1987
10. Nagl K, Hermann JM, Plamper M, Schroder C, Dost A, Kordonouri O, et al. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3657 children and adolescents from Germany and Austria. Pediatr Diabetes. (2017) 18:428–34. doi: 10.1111/pedi.12413
11. Zhong T, Tang R, Xie Y, Liu F, Li X, Zhou Z. Frequency, clinical characteristics, and determinants of partial remission in type 1 diabetes: Different patterns in children and adults. J Diabetes. (2020) 12:761–8. doi: 10.1111/1753-0407.13044
12. Humphreys A, Bravis V, Kaur A, Walkey HC, Godsland IF, Misra S, et al. Individual and diabetes presentation characteristics associated with partial remission status in children and adults evaluated up to 12 months following diagnosis of type 1 diabetes: An ADDRESS-2 (After Diagnosis Diabetes Research Support System-2) study analysis. Diabetes Res Clin Pract. (2019) 155:107789. doi: 10.1016/j.diabres.2019.107789
13. Pilacinski S, Adler AI, Zozulinska-Ziolkiewicz DA, Gawrecki A, Wierusz-Wysocka B. Smoking and other factors associated with short-term partial remission of Type 1 diabetes in adults. Diabetes Med. (2012) 29:464–9. doi: 10.1111/j.1464-5491.2011.03467.x
14. Karges B, Durinovic-Belló I, Heinze E, Debatin KM, Boehm B, Karges W. Immunological mechanisms associated with long-term remission of human type 1 diabetes. Diabetes Metab Res Rev. (2006) 22:184–9. doi: 10.1002/dmrr.600
15. Zhong T, Tang R, Gong S, Li J, Li X, Zhou Z. The remission phase in type 1 diabetes: Changing epidemiology, definitions, and emerging immuno-metabolic mechanisms. Diabetes Metab Res Rev. (2020) 36:e3207. doi: 10.1002/dmrr.3207
16. Addala A, Gu A, Zaharieva D, Prahalad P, Buckingham BA, Scheinker D, et al. Clinically significant hypoglycemia is rare in youth with T1D during partial clinical remission. Diabetes. (2020) 69. doi: 10.2337/db20-1294-P
17. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. (2019) 42:1593–603. doi: 10.2337/dci19-0028
18. Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care. (2011) 34:801–6. doi: 10.2337/dc10-1343
19. Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. (2005) 28:626–31. doi: 10.2337/diacare.28.3.626
20. Bradley C. Diabetes treatment satisfaction questionnaire. In: Bradley C, editor. Handbook of Psychology and Diabetes. Harwood Academic Publishers, Chur, Switzerland (1994).
21. Rozporzadzenie Ministra Zdrowia z dnia 27 pazdziernika 2022 r. zmieniajace rozporzadzenie w sprawie wykazu wyrobow medycznych wydawanych na zlecenie (Dz.U. 2022 poz. 2319). Available online at: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20220002319 (Accessed September 29, 2024).
22. Podolakova K, Barak L, Jancova E, Tarnokova S, Podracka L, Dobiasova Z, et al. Complete remission in children and adolescents with type 1 diabetes mellitus-prevalence and factors. Sci Rep. (2023) 13:6790. doi: 10.1038/s41598-023-34037-7
23. Hohendorff J, Witek P, Kania M, Sudol M, Hajduk K, Stepien A, et al. Higher scanning frequency is correlated with less fear of hypoglycemia in type 1 diabetes patients using isCGM. Front Endocrinol (Lausanne). (2022) 13:996933fdv. doi: 10.3389/fendo.2022.996933fdv
24. Hohendorff J, Gumprecht J, Mysliwiec M, Zozulinska-Ziolkiewicz D, Malecki MT. Intermittently scanned continuous glucose monitoring data of polish patients from real-life conditions: more scanning and better glycemic control compared to worldwide data. Diabetes Technol Ther. (2021) 23:577–85. doi: 10.1089/dia.2021.0034
25. Sandig D, Grimsmann J, Reinauer C, Melme A, Zimny S, Muller-Korbsch M, et al. Continuous glucose monitoring in adults with type 1 diabetes: real-world data from the german/Austrian prospective diabetes follow-up registry. Diabetes Technol Ther. (2020) 22:602–12. doi: 10.1089/dia.2020.0019
26. Matejko B, Juza A, Kieć-Wilk B, Cyranka K, Krzyzowska S, Chen X, et al. Transitioning of people with type 1 diabetes from multiple daily injections and self-monitoring of blood glucose directly to miniMed 780G advanced hybrid closed-loop system: A two-center, randomized, controlled study. Diabetes Care. (2022) 45:2628–35. doi: 10.2337/dc22-0470
27. Franceschi R, Cauvin V, Stefani L, Berchielli F, Soffiati M, Maines E. Early initiation of intermittently scanned continuous glucose monitoring in a pediatric population with type 1 diabetes: A real world study. Front Endocrinol (Lausanne). (2022) 13:907517. doi: 10.3389/fendo.2022.907517
28. Addala A, Zaharieva DP, Gu AJ, Prahalad P, Scheinker D, Buckingham B, et al. Clinically serious hypoglycemia is rare and not associated with time-in-range in youth with new-onset type 1 diabetes. J Clin Endocrinol Metab. (2021) 106:3239–47. doi: 10.1210/clinem/dgab522
29. Yan J, Zhou Y, Zheng X, Zheng M, Lu J, Luo S, et al. Effects of intermittently scanned continuous glucose monitoring in adult type 1 diabetes patients with suboptimal glycaemic control: A multi-centre randomized controlled trial. Diabetes Metab Res Rev. (2023) 39:e3614. doi: 10.1002/dmrr.3614
30. Duarte DB, Fonseca L, Santos T, Silva VB, Puga FM, Saraiva M, et al. Impact of intermittently scanned continuous glucose monitoring on quality of life and glycaemic control in persons with type 1 diabetes: A 12-month follow-up study in real life. Diabetes Metab Syndr. (2022) 16:102509. doi: 10.1016/j.dsx.2022.102509
31. Charleer S, De Block C, Van Huffel L, Broos B, Fieuws S, Nobels F, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): A prospective observational real-world cohort study. Diabetes Care. (2020) 43:389–97. doi: 10.2337/dc19-1610
32. Charleer S, De Block C, Bolsens N, Van Huffel L, Nobels F, Mathieu C, et al. Sustained impact of intermittently scanned continuous glucose monitoring on treatment satisfaction and severe hypoglycemia in adults with type 1 diabetes (FUTURE): an analysis in people with normal and impaired awareness of hypoglycemia. Diabetes Technol Ther. (2023) 25:231–41. doi: 10.1089/dia.2022.0452
33. Boscari F, Ferretto S, Cavallin F, Fadini GP, Avogaro A, Bruttomesso D. Effectiveness of adding alarms to flash glucose monitoring in adults with type 1 diabetes under routine care. Acta Diabetol. (2022) 59:921–8. doi: 10.1007/s00592-022-01884-1
34. Talbo MK, Katz A, Hill L, Peters TM, Yale JF, Brazeau AS. Effect of diabetes technologies on the fear of hypoglycaemia among people living with type 1 diabetes: a systematic review and meta-analysis. EClinicalMedicine. (2023) 62:102119. doi: 10.1016/j.eclinm.2023.102119
35. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. (2018) 61:539–50. doi: 10.1007/s00125-017-4527-5
36. Jensen MH, Cichosz SL, Gustenhoff P, Nikontovic A, Hejlesen O, Vestergaard P. Long-term glucose-lowering effect of intermittently scanned continuous glucose monitoring for type 1 diabetes patients in poor glycaemic control from Region North Denmark: An observational real-world cohort study. PloS One. (2022) 17:e0274626. doi: 10.1371/journal.pone.0274626
Keywords: isCGM, fear of hypoglycemia, TIR, TBR, type 1 diabetes
Citation: Hohendorff J, Grzelka-Wozniak A, Wrobel M, Kania M, Lapinska L, Rokicka D, Stoltny D, Kowalska I, Strojek K, Zozulinska-Ziolkiewicz D and Malecki MT (2025) Impact of the initiation of isCGM soon after type 1 diabetes mellitus diagnosis in adults on glycemic indices and fear of hypoglycemia: a randomized controlled trial. Front. Endocrinol. 15:1503891. doi: 10.3389/fendo.2024.1503891
Received: 29 September 2024; Accepted: 12 December 2024;
Published: 09 January 2025.
Edited by:
Yun Shen, Pennington Biomedical Research Center, United StatesReviewed by:
Yunzhe Qian, Harvard University, United StatesXinhang Wang, Stanford University, United States
Copyright © 2025 Hohendorff, Grzelka-Wozniak, Wrobel, Kania, Lapinska, Rokicka, Stoltny, Kowalska, Strojek, Zozulinska-Ziolkiewicz and Malecki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maciej T. Malecki, bWFjaWVqLm1hbGVja2lAdWouZWR1LnBs
 Jerzy Hohendorff
Jerzy Hohendorff Agata Grzelka-Wozniak
Agata Grzelka-Wozniak Marta Wrobel4
Marta Wrobel4 Lidia Lapinska
Lidia Lapinska Dorota Zozulinska-Ziolkiewicz
Dorota Zozulinska-Ziolkiewicz Maciej T. Malecki
Maciej T. Malecki