- 1Ahfad Centre for Science and Technology, Ahfad University for Women, Khartoum, Sudan
- 2Faculty of Medicine, International University of Africa, Khartoum, Sudan
- 3Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, University of Khartoum, Khartoum, Sudan
- 4Faculty of Medicine, Sudan International University, Khartoum, Sudan
Background: Africa, like the rest of the world, is experiencing an increasing prevalence of diabetes mellitus. Diabetes increases the risk for coronary artery disease (CAD) by fourfold compared to people without diabetes. C677T polymorphism in methylenetetrahydrofolate reductase (MTHFR) and hyperhomocysteinemia were reported by many studies as risk factors for CAD among patients with type 2 diabetes mellitus (T2DM). Early detection of modifiable risk factors for CAD is an important aspect of management of diabetes. This is the only study in Sudan which investigates the association between MTHFR genotypes and plasma homocysteine levels, and their role in premature CAD (PCAD) among patients with T2DM.
Methods: This study is a comparative study. We enrolled 226 Sudanese patients with T2DM, age range 25-60 years, recruited from Alshaab and Omdurman teaching hospitals in Khartoum State. 113 patients had CAD confirmed by angiography and electrocardiography (ECG) and 113 had no evidence of CAD. Polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP), using Hinf1 restriction enzyme, were used to determine MTHFR genotypes. Plasma homocysteine levels were determined by enzymatic assay on the Hitachi Cobas Integra® 400 plus. Data was analyzed using statistical package for Social Sciences (SPSS) 23, using Mann-Whtney U test, general linear model, Chi-square test and logistic regression analysis.
Results: The frequencies of TT, CT, and CC genotypes were 16,40 and 44% among T2DM patients with PCAD. In T2DM patients without PCAD, the frequencies of TT, CT, and CC genotypes were 00,19 and 83%. The T allele showed strong association with PCAD among T2DM patients, p <0.001, odds ratio (OR) 6.2, 95% CI (3.4-11.6). Patients with PCAD showed higher plasma homocysteine levels than patients without PCAD (13.5 µmol/L versus 10 µmol/L, p < 0.001). The T allele had significant effect on homocysteine level, (p <0.001). Plasma homocysteine levels were higher in individuals with TT genotype than those with CT or CC genotypes in patients with PCAD (16.2 + 5.3, 14.3 + 5.7 and 12.9 + 5.02 µmol/L, p=0.017). Homocysteine levels showed a significant association with CAD, p<0.001, OR 3.2, 95% CI (1.9—5.5).
Conclusions: Our study suggests that C677T polymorphism of MTHFR gene and hyperhomocysteinemia are risk factors for PCAD in Sudanese population with T2DM.
Introduction
Globally, the burden of diabetes mellitus (DM) continues to increase. The International Diabetes Federation (IDF) estimates that in 2022, 537 million adults live with diabetes worldwide (1).
Africa, like the rest of the world, is experiencing an increasing prevalence of DM, 1 in 22 adults live with DM in Africa (1, 2). In Sudan, previous studies have shown that there is high prevalence of diabetes in the adult populations (1, 3). The IDF reported, in 2021, that Sudan is one of the top five countries in the prevalence of diabetes in the Middle East and North Africa (4).
Diabetes increases the risk for coronary artery disease (CAD) by fourfold compared to people without diabetes (5, 6). CAD leads to significant morbidity and mortality. Identification and management of risk factors for CAD is an important aspect of management of DM.
Hyperhomocysteinemia has been identified as an independent risk factor for CAD (7, 8). Hyperhomocysteinemia has been associated with genetic defects in enzymes involved in its metabolism and/or with nutritional deficiencies of vitamin B6, B12 and folic acid (9, 10). Numerous studies have documented the influences of a common polymorphism (C677T) of methylenetetrahydrofolate reductase (MTHFR) on homocysteine levels. However, the relationship between this mutation and cardiovascular diseases (CVD) has remained a controversial issue (11–13).
Homocysteine and diabetes appear to have a negative synergistic effect on the cardiovascular system, and it is therefore important to investigate this effect in patients with diabetes who have higher risk for CAD than the normal population (14).
Homocysteine (Hcy) is metabolized by either remethylation to methionine or transsulphuration to cysteine. The former reaction is catalyzed by the vitamin B12-dependent methionine synthase. The transsulfuration pathway is catalyzed by cystathionine-β-synthase (CβS) which is a vitamin B6 dependent process (13, 15).
MTHFR catalyzes the reduction of 5,10-methylene tetrahydrofolate to 5 methyltetrahydrofolate, which is the methyl donor in the remethylation of Hcy to methionine. The most common mutation in the MTHFR gene is the 677→T variant which has been shown to encode a thermolabile enzyme with reduced activity (16, 17).
Several studies investigated possible association between MTHFR genotypes and plasma homocysteine levels and the incidence of different MTHFR genotypes in CAD patients (18–22). The results of these studies have been controversial. Many studies failed to show association between MTHFR genotypes and plasma homocysteine levels and their role in CAD. The variation was thought to be caused by ethnic or geographical differences, at least in some of these studies (23–25).
There is paucity in the studies which included patients with diabetes, who are already at increased risk for CAD (2, 5).
In Sudan, there is a gap in our knowledge about the most important modifiable risk factors for PCAD; therefore, identifying these factors in our population can improve prevention of these serious diabetic complications.
In this study, we investigated the association between MTHFR genotypes and plasma homocysteine (Hcy) levels and their role in PCAD in Sudanese patients with type 2 diabetes mellitus.
Materials, methods and study subjects
This is a comparative study, using a non-probability, selective sampling technique. 226 patients with diabetes were enrolled in this study according to statistical power formula.
113 patients had CAD and 113 had no evidence of CAD.
CAD was confirmed by angiography (≥ 50% stenosis of at least one of coronary arteries) and electrocardiography (ST segment depression or ST segment elevation) (26).
T2DM was diagnosed according to the WHO criteria (Glycated hemoglobin (HbA1c), ≥ 6.5%, 48 mmol/mol) or fasting plasma glucose ≥7.0 mmol/L (126 mg/dL) (27). Type1diabetus mellitus patients (T1DM) were excluded from the study. None of our patient was on insulin treatment. The age range of our study population was 25-60 years. The study was conducted in 2 hospitals (Alshaab and Oumdurman teaching hospitals) in Khartoum State, which treats Sudanese patients from different ethnic backgrounds.
Smoking was defined as the use of any tobacco at the time of conducting the study, while hypertension was defined with as systolic blood pressure >130 mmHg and diastolic blood pressure > 90 mmHg or using anti-hypertensive medication. The study protocol was approved by the Sudanese ministry of health ethics committee. Informed consent was obtained from all patients prior to data collection; investigation was conducted in accordance with the Declaration of Helsinki.
Biochemical analysis
Venous blood was obtained in Ethylenediamine tetraaetate (EDTA) after an overnight fast. Plasma Hcy level was determined by enzymatic assay on Hitachi Cobas Integra® 400 plus, the principle of the test is based on measuring the co-substrate conversion product, NAD spectrophotometrically at 340 nm. The lipid profile was estimated using Roche Cobas c311 based on enzymatic method. Glycated hemoglobin (HbA1c) was measured by a turbidimetric inhibition immunoassay (TINA) using Roche Cobas c311 analyzer. Authentic reagents were obtained from Roche diagnostics dealer in Sudan. The biochemical analysis was performed in East Model hospital in Khartoum.
DNA preparation and genotyping
The PCR procedure was performed in the centre of Sudanese association for supporting patients with kidney disease.
Buffy coat was used for DNA extraction following the manufacturer’s guide of QIAquick Gel Extraction Kit (1000) From QIAGEN (Manchester, United Kingdom). The MTHFR C677T single-nucleotide polymorphism (SNP) was determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method using the Hinf I restriction endonuclease enzyme (BioLabs, Frankfurt, Germany). The primers used were forward (5’- TGA AGG AGA AGG TGT CTG CGG GA -3’, and reverse, 5’- AGG ACG GTG CGG TGA GAG TG -3’). The PCR mixture used contained 18.5 µl 5xFIREPoL ® Master Mix from SOLIS BIODYNE company, Tartu, Estonia, 5 µl of DNA template and 0.5µl of each primer were added.
PCR was performed under the following cycling conditions: 94°C for 3 min (initial denaturation), followed by 94°C for 1 min, 61°C for 1 min (annealing), 72°C for 2 min, and 72°C for 3 min (final extension), for a total of 40 cycles using MULTI GENE OPTI MAX from Cleaver Scientific Ltd (Rugby, United Kingdom).
PCR products were electrophoresed on agarose gel (2%, 0.5 mg/mL ethidium bromide) and visualized using Gel documentation system CSL-MICRODOC System from Cleaver Scientific (Rugby, United Kingdom).
PCR-RFLP was used to determine the MTHFR C677T genotypes. PCR products were subjected to overnight restriction digestion by HinfI (5Units) at 37°C. Restriction products were electrophoresed on agarose gel (2%, 0.5 mg/mL ethidium bromide) and visualized as was the PCR product.
Presence of the C-allele resulted in no cleavage of the PCR product. Heterozygote C677T yielded two fragments of 198 bp, 175 bp. Homozygote C677T yielded two fragments of 175 bp (Figure 1).
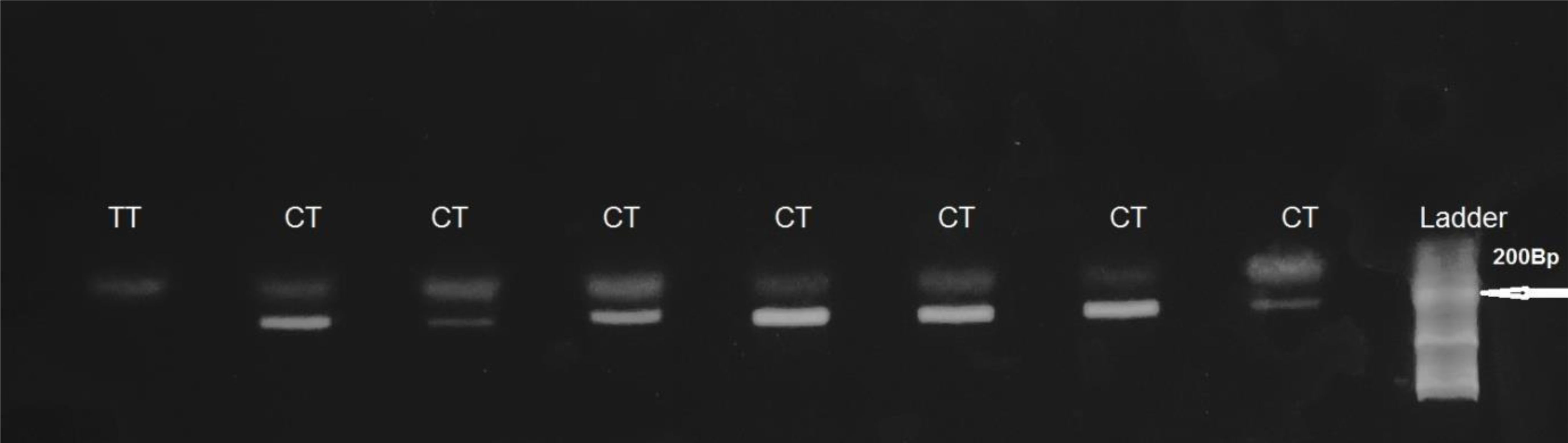
Figure 1. HinfI-treated MTHFR gene PCR fragments separated on 2% agarose gel. Presence of the C-allele resulted in no cleavage of the PCR product. Heterozygote C677T, (CT genotype) yielded two fragments of 198 bp and 175 bp. Homozygote C677T (TTgenotype) yielded two fragments of 175 bp.
Statistical analysis
The data collected was analyzed using Statistical Package for the Social Sciences (SPSS) 23. Categorical variables were expressed as numbers and percentages. Mann-Whitney-U test was used to compare differences between quantitative variables among the two groups and expressed as median and Interquartile Range (IQR), general linear model was used to determine the significant differences in homocysteine levels according to genotypes, chi square test was used to detect the association of MTHFR polymorphisms with CAD and logistic regression analyses was used to test the association between (selected significant independent variables) and CAD among T2DM patients, p < 0.05 was considered statistically significant.
Results
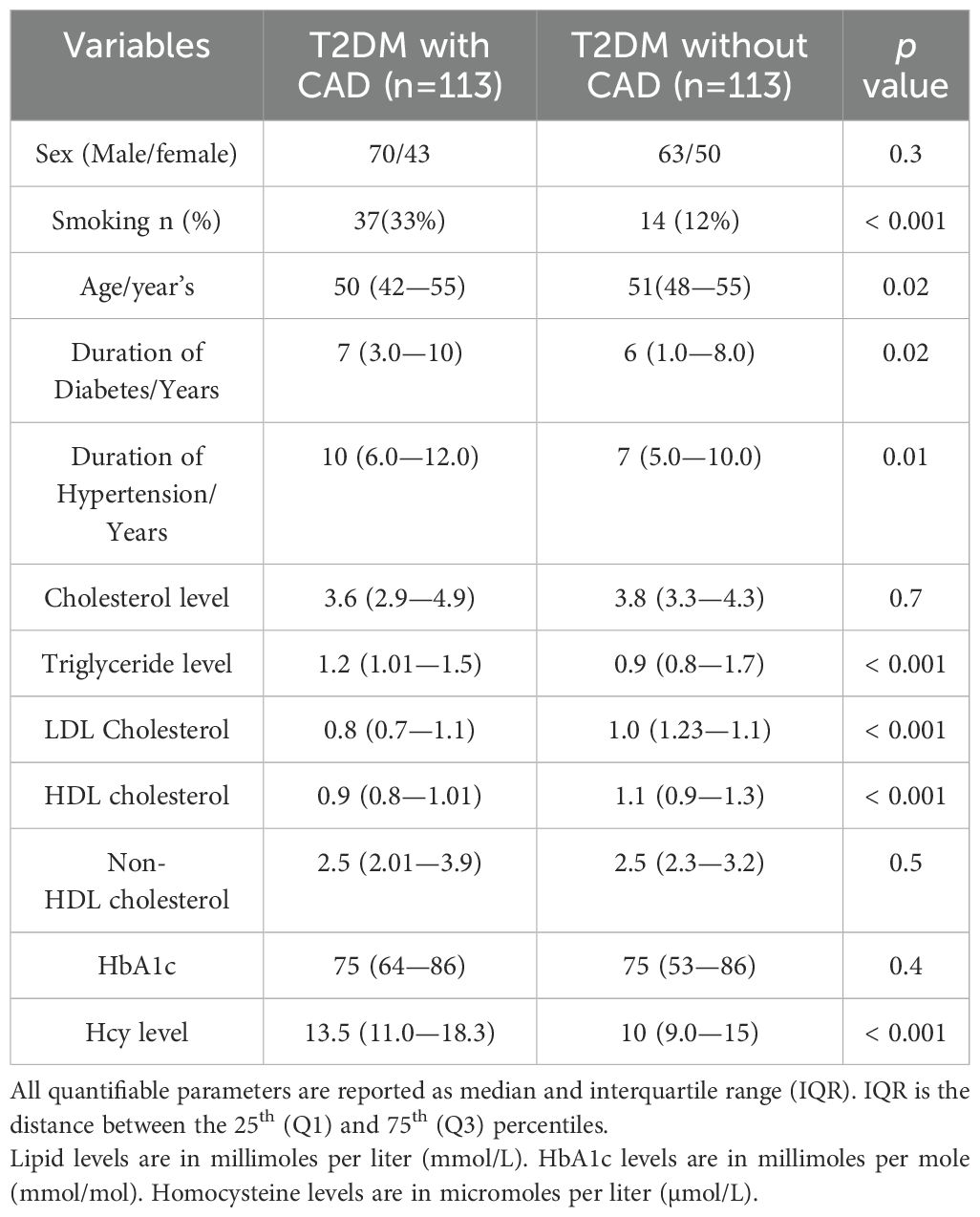
The clinical and biochemical characteristics of patients were summarized in Table 1.
Gender had no significant effect on CAD, p > 0.05(0.3). Age, smoking, duration of diabetes and hypertension were significantly different between the two groups, p < 0.02, < 0.001, 0.02 and 0.01 respectively, Diabetic patients with CAD have significantly higher levels of plasma triglycerides, LDL cholesterol and lower levels of HDL cholesterol, p < 0.001 but no difference noted in total cholesterol, and non-HDL cholesterol levels between the two groups, p > 0.05, 0.7 and 0.5 respectively. Plasma homocysteine levels were significantly higher in diabetic patients with P CAD, p < 0.001.
Association of MTHFR gene polymorphisms with PCAD in patients with T2DM
The frequencies of TT, CT and CC genotypes among patients with T2DM and CAD were 16, 40 and 44%, and were 00, 19 and 83% in T2DM patients without CAD, (p < 0.001). The frequency of the T allele was higher in patients with PCAD than without CAD (0.36 versus 0.08%, p < 0.001). The odds ratio (OR) for CAD in T2DM patients who carry the T allele was 6.2, CI 95% (3.4-11.6) (Table 2).
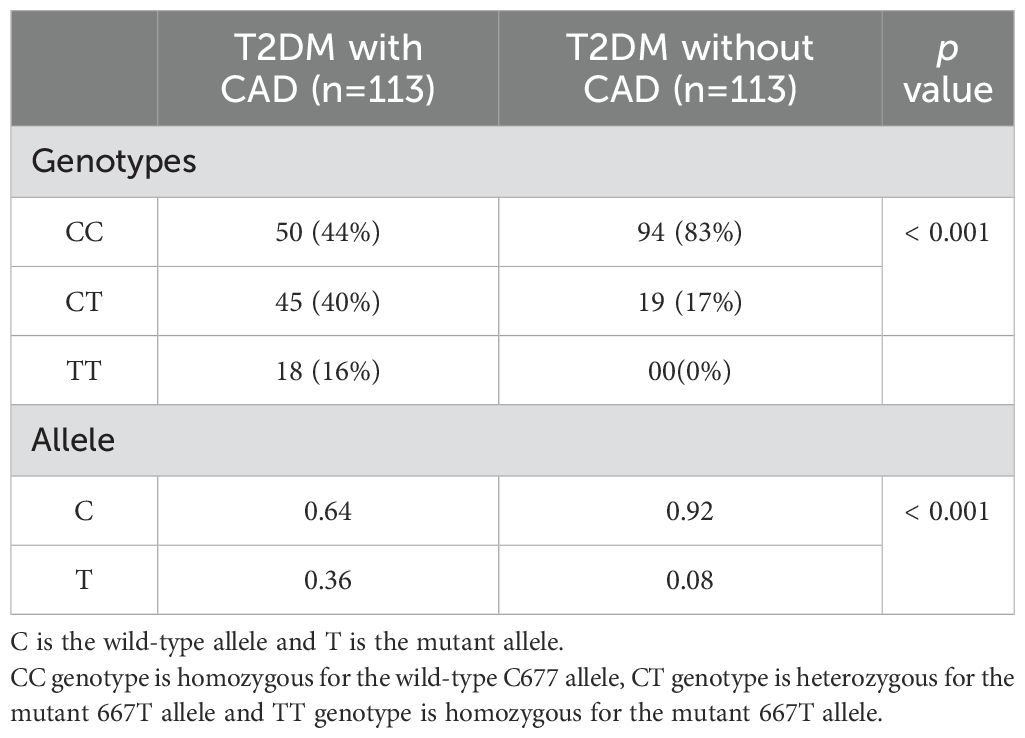
Table 2. Distribution of MTHFR genotypes and alleles in type 2 diabetic patients with and without CAD.
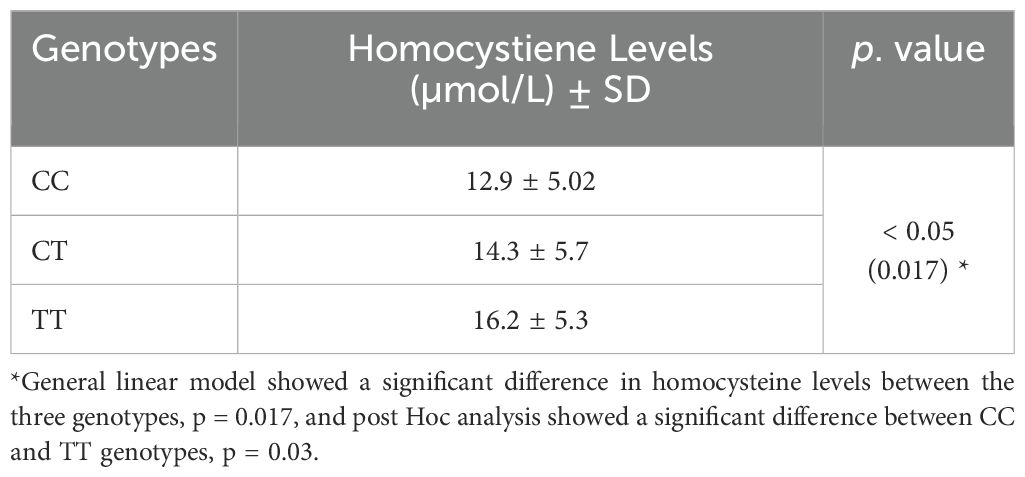
Correlation between MTHFR genotypes and plasma homocysteine levels
Plasma homocysteine levels were significantly different between MTHFR genotypes:
16.2 ± 5.3, 14.3 ± 5.7 and 12.9 ± 5.02 µmol/L in TT, CT and CC genotypes respectively, p = 0.017. Post hoc analysis showed significantly higher levels of homocysteine in the TT genotype than CC genotype, p = 0.03. Homocysteine levels showed significant association with CAD, p < 0.001, OR 3.2, 95% CI (1.9-5.5), (Table 3).
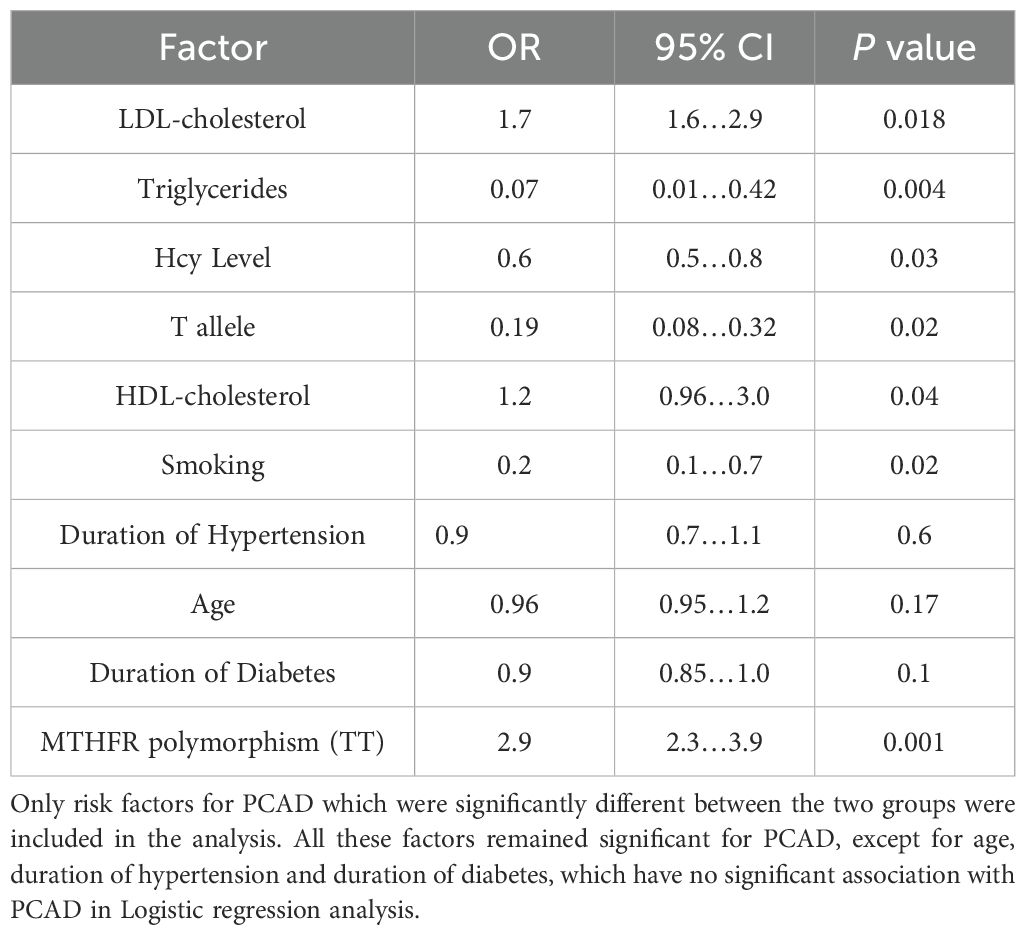
Logistic regression analysis
Logistic regression analysis was performed to ascertain the effects of selected significant risk factors in the study on likelihood that patients have PCAD.
It showed that age, duration of hypertension and duration of diabetes were not associated with PCAD, p = 0.17, 0.6 and 0.1 respectively, while other significant factors remained associated with PCAD (Table 4).
Discussion
Worldwide, the frequency of MTHFR gene mutations varies among racial and ethnic groups (28, 29). In Africa, MTHFR gene polymorphism is markedly low (below 10%) for 677 T allele (30, 31). In Europeans and North Americans, the frequency ranges from 10-18%, in contrast, the frequency of T allele is markedly high among Chinese populations at around 45% (32). In our study, the mutant T allele frequency was 36% in cases and 8% in controls (Table 2). In a study done on Zambian population, the T allele frequency was 1%, and no subject had the TT genotype (31). However, it is known that Sudanese population have a high level of genetic diversity and therefore are unlikely to be representative of African populations south of the Sahara (33, 34).
The frequency of the T allele was significantly different between T2DM patients with and without CAD, p <0.001. This finding was consistent with other findings done in diabetic patients from different populations (30, 35).
Elevated homocysteine level (hyperhomocysteinemia) seems to predict cardiovascular events among diabetic patients (6, 36–41). However, many other studies failed to establish the connection and claimed that hyperhomocysteinemia is an innocent bystander among diabetic patients (42, 43). The genetic basis of hyperhomocysteinemia has also been known (44, 45) and MTHFR polymorphism MTHFR 677T allele has been associated with high homocysteine levels (46–48), while others failed to detect such a relationship (49, 50). Ethnicity differences could be a responsible parameter for at least some of these controversial results (28, 29). Hcy levels are also influenced by various environmental factors and, vitamin B12 and folate (51, 52). The limitations of this study include the lack of data on drug treatments, not measuring lipoprotein (a) and that folate status and vitamin B12 levels were not measured.
Plasma homocysteine levels are influenced by age, diet, genetic background and the use of medications like metformin and fibrates which are used frequently in T2DM patients (53). In the presence of these confounding factors, a single test of plasma homocysteine, in a relatively small number of study subjects, cannot provide enough information for us to establish a causal relationship between homocysteine and PCAD. In addition, the participants in the study came from one city capital Khartoum and might not be representative of the whole country. We therefore cannot recommend universal measurement of homocysteine levels in our T2DM patients especially that homocysteine test is relatively expensive for our patients who are mostly working class. However, health professionals are encouraged to advise patients with T2DM to increase intake of vegetables and fruits, within a balanced diabetic diet. Improvement of lifestyle factors like cessation of smoking, physical exercise and maintaining ideal body weight will decrease the overall risk for cardiovascular disease and may have a positive effect on plasma total homocysteine levels (54).
In conclusion, homocysteine levels are increased in our T2DM with PCAD compared to diabetic patients without CAD. The increase in the homocysteine levels is associated with T allele in MTHFR gene, presence of T allele will increase risk for CAD significantly.
Our study suggested that hyperhomocysteinemia is an important factor for PCAD in our patients with T2DM and more studies are required to confirm these findings and to study the effect of nutritional advice and supplementation with the relevant vitamins on homocysteine levels and prevention of PCAD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The National Research Ethics Committee, Ministry of Health, Sudan. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. NM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft. IA: Methodology, Supervision, Validation, Writing – review & editing. BE: Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are grateful for the laboratory of the East Model hospital in Khartoum for donating reagents and allowing use of their analyzers for biochemical measurements. The Sudanese association for supporting patients with kidney disease, a registered charity, allowed the use of their molecular suite for PCR studies and donated reagents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Kibirige D, Chamba N, Andia-Biraro I, Kilonzo K, Laizer SN, Sekitoleko I, et al. Indicators of optimal diabetes care and burden of diabetes complications in Africa: a systematic review and meta-analysis. BMJ Open. (2022) 12:e060786. doi: 10.1136/bmjopen-2022-060786
3. Charani E, Cunnington AJ, Yousif AH, Ahmed MS, Ahmed AE, Babiker S, et al. In transition: current health challenges and priorities in Sudan. BMJ Global Health. (2019) 4:e001723. doi: 10.1136/bmjgh-2019-001723
4. Aguirre F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, et al. IDF diabetes atlas: Sixth edition. International Diabetes Federation.
5. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. (2014) 57:1542–51. doi: 10.1007/s00125-014-3260-6
6. Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. (2020) 141:e779–806. doi: 10.1161/CIR.0000000000000766
7. Ji J, Liu Y, Liu H, Hao Z, Liu J, Chen Q. Relationship between procalcitonin, homocysteine and severity of coronary artery disease in type 2 diabetic patients. Int J Gerontology. (2019) 3:226–30. doi: 10.6890/IJGE.201909_13(3).0009
8. De Bree A, Verschuren WM, Kromhout D, Kluijtmans LA, Blob HJ. Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. Pharmacol Rev. (2002) 54:599–618. doi: 10.1124/pr.54.4.599
9. Kullo IJ, Ding K, Boerwinkle E, Turner ST, Mosley TH, Kardia SL, et al. Novel genomic loci influencing plasma homocysteine levels. Stroke. (2006) 37:1703–9. doi: 10.1161/01.STR.0000225929.96190.b3
10. Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. (2018) 41:372–83. doi: 10.1007/s12272-018-1016-4
11. Settin A, El-Baz R, Ismaeel A, Tolba W, Abdallah WA. Association of ACE and MTHFR genetic polymorphisms with type 2 diabetes mellitus: susceptibility and complications. J Renin Angiotensin Aldosterone Syst. (2015) 16:838–43. doi: 10.1177/1470320313516172
12. Wu K, Zhang S, Guan Z, Li X, Li R, Yin Y, et al. Methylenetetrahydrofolate reductase gene polymorphism C677T is associated with increased risk of coronary heart disease in chinese type 2 diabetic patients. Chin Med Sci J. (2021) 36:103–7. doi: 10.24920/003792
13. Miao L, Deng GX, Yin RX, Nie RJ, Yang S, Wang Y, et al. No causal effects of plasma homocysteine levels on the risk of coronary heart disease or acute myocardial infarction: A Mendelian randomization study. Eur J Prev Cardiol. (2021) 28:227–39. doi: 10.1177/2047487319894679
14. Van Guldener C, Stehouwer CD. Diabetes mellitus and hyperhomocysteinemia. In: Seminars in vascular medicine 2002, vol. 2. Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA (2002). p. 087–96.
15. Park W-C, Chang J-H. Clinical implications of methylenetetrahydrofolate reductase mutations and plasma homocysteine levels in patients with thromboembolic occlusion. Vasc specialist Int. (2014) 30:113–20. doi: 10.5758/vsi.2014.30.4.113
16. Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, Singh LR. Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med. (2019) 51:1–13. doi: 10.1038/s12276-019-0216-4
17. Ramkaran P, Phulukdaree A, Khan S, Moodley D, Chuturgoon AA. Methylenetetrahydrofolate reductase C677T polymorphism is associated with increased risk of coronary artery disease in young South African Indians. Gene. (2015) 571:28–32. doi: 10.1016/j.gene.2015.06.044
18. Bouzidi N, Hassine M, Fodha H, Ben Messaoud M, Maatouk F, Gamra H, et al. Association of the methylene-tetrahydrofolate reductase gene rs1801133 C677T variant with serum homocysteine levels, and the severity of coronary artery disease. Sci Rep. (2020) 10:10064. doi: 10.1038/s41598-020-66937-3
19. Bazmpani MA, Karvounis H, Kassimis G. Compound heterozygous MTHFR (C677T and A1298C) variants and anterior STEMI: cause or bystander? Future Cardiol. (2020) 17:841–5. doi: 10.2217/fca-2020-0144
20. Shaukat MHS, Toledo-Garcia A, Torosoff M. Recurrent myocardial infarction despite normal C-reactive protein in a patient with Behcet’s disease and compound heterozygous methylenetetrahydrofolate reductase (MTHFR) mutations (C677T and A1298C). Cureus. (2019) 11:e5344. doi: 10.7759/cureus.5344
21. Chehadeh SWEH, Jelinek HF, Al Mahmeed WA, Tay GK, Odama UO, Elghazali GE, et al. Relationship between MTHFR C677T and A1298C gene polymorphisms and complications of type 2 diabetes mellitus in an Emirati population. Meta Gene. (2016) 17:70–5. doi: 10.1016/j.mgene.2016.04.002
22. Shivkar RR, Gawade GC, Padwal MK, Diwan AG, Mahajan SA, Kadam CY. Association of MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms with serum homocysteine, folate and vitamin B12 in patients with young coronary artery disease. Indian J Clin Biochem. (2021) 37:224–31. doi: 10.1007/s12291-021-00982-1
23. Kavrakova JB, Cekovska S, Panov S, Petkovska L, Spasovski D, Krstevska M. Homocystinemia and polymorphism of the gene for methylene tetrahydrofolate reductase (C677T) in patient with coronary artery disease. MEDICUS. (2019) 4:136141. Available at: http://hdl.handle.net/20.500.12188/10375
24. Dilley A, Hooper WC, El-Jamil M, Renshaw M, Wenger NK, Evatt BL. Mutations in the genes regulating methylene tetrahydrofolate reductase (MTHFR C→ T677) and Cystathione β-synthase (CBS G→ A919, CBS T→ c833) are not associated with myocardial infarction in African Americans. Thromb Res. (2001) 103:109–15. doi: 10.1016/S0049-3848(01)00278-X
25. Xu T, Chen S, Yang F, Wang Y, Zhang K, Fu G, et al. The impact of homocysteine on the risk of coronary artery disease in individuals with diabetes: a Mendelian randomization study. Acta Diabetol. (2021) 58:301–7. doi: 10.1007/s00592-020-01608-3
26. Writing Committee Members, Weintraub WS, Karlsberg RP, Tcheng JE, Boris JR, Buxton AE, et al. ACCF/AHA 2011 key data elements and definitions of a base cardiovascular vocabulary for electronic health records: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards. Circulation. (2011) 124:103–23. doi: 10.1161/CIR.0b013e31821ccf71
27. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO consultation. Geneva: World Health Organization (2006).
28. Reyes L, Godfrey D, Ming LJ, MacLean C, Gonzalez FJ, Madrigal L. The distribution in native populations from Mexico and Central America of the C677T variant in the MTHFR gene. Am J Hum Biol. (2021) 33:e23567. doi: 10.1002/ajhb.23567
29. Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. (2000) 151:862–77. doi: 10.1093/oxfordjournals.aje.a010290
30. Raghubeer S, Matsha TE. Methylenetetrahydrofolate (MTHFR), the one carbon cycle, and cardiovascular risks. Nutrients. (2021) 13:4562. doi: 10.3390/nu13124562
31. Atadzhanov M, Mwaba MH, Mukomena PN, Lakhi S, Mwaba P, Rayaprolu S, et al. Frequency of APOE, MTHFR and ACE polymorphisms in the Zambian population. BMC Res Notes. (2014) 7:194–204. doi: 10.1186/1756-0500-7-194
32. Zhang X, Hou C, Liu P, Chen L, Liu Y, Tang P, et al. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and subacute combined degeneration: revealing a genetic predisposition. Front Neurol. (2019) 9:1162. doi: 10.3389/fneur.2018.01162
33. Alfonso C, Alshamali F, Pereira JB, Fernandesa V, Costaa M, Pereira L. mtDNA diversity in Sudan (EAST africa). Forensic Sci International: Genet Supplement Series. (2008) 1:257–8. doi: 10.1016/j.fsigss.2007.10.118
34. Ibrahim ME. Genetic diversity in the Sudanese: insight on origin and implications for health. Hum Mol Genet. (2021) 30:37–41. doi: 10.1093/hmg/ddab028
35. Zhang D, Zhou Y, Han L, Ji H, Li J. The effect of MTHFR C677T polymorphism on type 2 diabetes mellitus with vascular complications in Chinese Han population: a meta-analysis. Endocr J. (2014) 61:717–26. doi: 10.1507/endocrj.EJ14-0071
36. Mallhi TH, Shahid M, Rehman K, Khan YH, Alanazi AS, Alotaibi NH, et al. Biochemical association of MTHFR C677T polymorphism with myocardial infarction in the presence of diabetes mellitus as a risk factor. Metabolites. (2023) 13:251–67. doi: 10.3390/metabo13020251
37. Sun J, Xu Y, Xue J, Zhu Y, Lu H. Methylenetetrahydrofolate reductase polymorphism associated with susceptibility to coronary heart disease in Chinese type 2 diabetic patients. Mol Cell Endocrinol. (2005) 229:95–101. doi: 10.1016/j.mce.2004.09.003
38. Bahadır A, Eroz R, Türker Y. Does the MTHFR C677T gene polymorphism indicate cardiovascular disease risk in type 2 diabetes mellitus patients? Anatol J Cardiol. (2015) 15:524–30. doi: 10.5152/akd.2014.5555
39. Rahimi Z, Nomani H, Mozafari H, Vaisi-Raygani A, Madani H, MalekKhosravi S, et al. Factor V G1691A, prothrombin G20210A and methylenetetrahydrofolate reductase polymorphism C677T are not associated with coronary artery disease and type 2 diabetes mellitus in western Iran. Blood Coagul Fibrinolysis. (2009) 20:252–6. doi: 10.1097/MBC.0b013e3283255487
40. Ma L, Liu Q, Jiang Y, Zhao H, Zhao T, Cao Y, et al. Genetically elevated circulating homocysteine concentrations increase the risk of diabetic kidney disease in Chinese diabetic patients. J Cell Mol Med. (2019) 23:2794–800. doi: 10.1111/jcmm.2019.23.issue-4
41. Rajabi M, Razzaghof MR, Kashani HH. Hyperhomocysteinemia and increased risk of coronary artery disease in Iranian patients with diabetes mellitus type II: a cross-sectional study. Comp Clin Path. (2020) 29:223–30. doi: 10.1007/s00580-019-03027-5
42. Lu J, Chen K, Chen W, Liu C, Jiang X, Ma Z, et al. Association of serum homocysteine with cardiovascular and all-cause mortality in adults with diabetes: A prospective cohort. Oxid Med Cell Longev. (2022) 2022:1–11. doi: 10.1155/2022/2156483
43. Rudy A, Kowalska I, Strączkowski M, Kinalska I. Homocysteine concentrations and vascular complications in patients with type 2 diabetes. Diabetes Metab. (2005) 31:112–7. doi: 10.1016/S1262-3636(07)70176-3
44. Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. (2011) 34:93–9. doi: 10.1007/s10545-010-9151-1
45. Muzurović E, Kraljević I, Solak M, Dragnić S, Mikhailidis DP. Homocysteine and diabetes: role in macrovascular and microvascular complications. J Diabetes Complicat. (2021) 35:107834. doi: 10.1016/j.jdiacomp.2020.107834
46. Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardiocerebrovascular risk. J Appl Genet. (2008) 49:267–82. doi: 10.1007/BF03195624
47. Ma T, Sun XH, Yao S, Chen ZK, Zhang JF, Xu WD, et al. Genetic variants of homocysteine metabolism, homocysteine, and frailty-rugao longevity and ageing study. J Nutr Health Aging. (2020) 24:198–204. doi: 10.1007/s12603-019-1304-9
48. Cirillo M, Coccia ME, Attanasio M, Fatini C. Homocysteine, vitamin B status and MTHFR polymorphisms in Italian infertile women. Eur J Obstet Gynecol Reorod Biol. (2021) 263:72–8. doi: 10.1016/j.ejogrb.2021.06.003
49. Zetterberg H, Coppala A, D'Angelo A, Palmér M, Rymo L, Blennow . No association between the MTHFR A1298C and transcobalamin C776G genetic polymorphisms and hyper-homocysteinemia in thrombotic disease. Thromb Res. (2003) 108:127–31.
50. Friso S, Girelli D, Trabetti E, Stranieri C, Olivieri O, Tinazzi E, et al. A1298C methyleneteetrahydrofolate reductase mutation and coronary artery disease: relationships with C677T polymorphism and homocysteine/folate metabolism. Clin Exp Med. (2002) 2:7–12
51. Komorniak N, Szczuko M, Kowalewski B, Stachowska E. Nutritional deficiencies, bariatric surgery, and serum homocysteine level: review of current literature. Obes Surgy. (2019) 29:3735–42. doi: 10.1007/s11695-019-04100-2
52. Kaye AD, Jeha GM, Pham AD, Fuller MC, Lerner ZI, Sibley GT, et al. Folic acid supplementation in patients with elevated homocysteine levels. Adv Ther. (2020) 37:4149–64. doi: 10.1007/s12325-020-01474-z
53. Desouza C, Keebler M, McNamara DB, Fonseca V. Drugs affecting homocysteine metabolism: impact on cardiovascular risk. Drugs. (2002) 62:605–16. doi: 10.2165/00003495-200262040-00005
Keywords: diabetes mellitus, hyperhomocysteinemia, MTHFR, CAD, polymorphism
Citation: Mohammed NO, Ali IA, Elamin BK and Saeed BO (2025) 5,10-methylenetetrahydrofolate reductase C677T gene polymorphism as a risk factor for premature coronary artery disease in patients with type 2 diabetes mellitus. Front. Endocrinol. 15:1502497. doi: 10.3389/fendo.2024.1502497
Received: 26 September 2024; Accepted: 23 December 2024;
Published: 22 January 2025.
Edited by:
Eddie-Williams Owiredu, University of Alabama at Birmingham, United StatesReviewed by:
Stephen Opoku, University of Alabama at Birmingham, United StatesSimeon Babarinde, The Scripps Research Institute, United States
Copyright © 2025 Mohammed, Ali, Elamin and Saeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bakri Osman Saeed, c2FlZWRiYWtyaUBob3RtYWlsLmNvbQ==
 Nisreen O. Mohammed
Nisreen O. Mohammed Ibtisam A. Ali
Ibtisam A. Ali Bahaelddin K. Elamin
Bahaelddin K. Elamin Bakri Osman Saeed
Bakri Osman Saeed