
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 January 2025
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1499735
This article is part of the Research Topic Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) - Pathogenesis, Prevention and Treatment View all 10 articles
 Ruli Wang1†
Ruli Wang1† Ningxi Wu1†
Ningxi Wu1† Huan Qu2†
Huan Qu2† Xiaowei Zheng3
Xiaowei Zheng3 Haoyang Zhang1
Haoyang Zhang1 Lihong Zhu1
Lihong Zhu1 Xiaolei Wang1*
Xiaolei Wang1* Xiaodie Yao1*
Xiaodie Yao1* Le Zhang1*
Le Zhang1*Background: Previous research has indicated that long working hours are connected to a variety of health conditions, including nonalcoholic fatty liver disease (NAFLD). However, this association which has been observed in more population is limited. Our research is designed to evaluate the association between working hours, working type, and NAFLD.
Methods: The study comprised adults with complete details on working hours, working type, and NAFLD from the NHANES 1999-2014. We employed the hepatic steatosis index (HSI) to evaluate NAFLD and examined the relationship between working hours or working type and hepatic steatosis using weighted multiple-variable regression models and restricted cubic spline (RCS) analysis. In addition, further subgroup analysis was performed based on sex, age, ratio of family income to poverty (PIR), education, and diabetes.
Results: Long working hours were significantly linked to an elevated risk of NAFLD (OR: 1.57, 95%CI: 1.21-2.05), even after controlling for confounding factors. RCS analysis suggested that there was no nonlinear relationship between them. When weekly working hours > 50, the likelihood of NAFLD among the population heightened to 57% and this risk increased to 99% in the female population. As for working type, increasing physical intensity of work was associated with higher NAFLD risk, but only heavy manual labor continued to show significance after adjustment (OR:1.39, 95%CI: 1.06-1.81). We observed that the relationship between heavy manual labor and NAFLD was more significant in the older and male populations.
Conclusion: Our results indicate that long working hours and engaging in heavy physical labor are independent risk factors for NAFLD. As working hours increase and individuals engage in heavy physical labor for extended periods, the risk of developing NAFLD significantly rises.
Long working hours can have negative health consequences (1), and research has indicated that long working hours may lead to an heightened risk of hypertension (2), diabetes (3), cardiovascular disease (4, 5), obesity (6), even depression and suicidal tendencies (7). Therefore, it is crucial to plan work hours reasonably. The statutory limit on weekly work hours is less than 48 hours in most European nations (8), and about half of these nations have set a 40-hour workweek cap. Nevertheless, approximately one-third of the global labor force still works more than 48 hours per week.
It is beneficial for health to engage in exercise during free time, and it is also essential for sustaining and enhancing physical strength and work performance (9, 10). One might think that physically demanding work has an advantageous impact on health (11). However, the physical demands of work may actually be detrimental. Reports suggest that jobs with high physical demands are linked to greater levels of disability, reduced body function, and decreased in muscle power (12–14).The differences in physical activity patterns between work and leisure time might be a crucial factor in clarifying this occurrence (15). Furthermore, intense manual labor could contribute to a lack of exercise during leisure time (16).
Non-alcoholic fatty liver disease (NAFLD) is a prevalent metabolic condition with an increasing global incidence and has become a significant factor in chronic liver disease in many parts of the world (17, 18). As people’s understanding of the disease deepens, it has been discovered that NAFLD is actually a disease related to metabolic dysfunction. It was renamed metabolic dysfunction-associated fatty liver disease (MAFLD) in 2020 and subsequently renamed metabolic dysfunction-associated steatotic liver disease (MASLD) in 2023, and a new category, metabolic dysfunction and alcohol-associated liver disease (MetALD), was proposed (19–21). The natural course of NAFLD includes a wide range of pathological conditions, from simple steatosis to steatohepatitis (NASH), as well as with varying levels of fibrosis and cirrhosis (22). Many factors are associated with NAFLD, including obesity, diabetes, hypertension, and dyslipidemia (23, 24). The main cause of death in patients with NAFLD is cardiovascular disease (25). NAFLD is becoming a more significant public health challenge (17), making prevention crucially important.
Both long working hours and heavy physical labor may impact people’s health, therefore we aim to analyze the connection between working hours, working type, and NAFLD. Previous studies have shown that working long hours is strongly linked to NAFLD (26), but they did not indicate whether the association is linear or a dose-response. Additionally, the Korean data may not be representative of the situation in other regions, as it only represents a subset of the Asian population. This study aims to expand the population to include the United States to understand whether the link between long working hours and NAFLD exists in this context. Additionally, no one has explicitly studied the relationship between working type and NAFLD. We will classify occupations based on work intensity to investigate whether different types of occupations are associated with NAFLD.
Data were obtained from the National Health and Nutrition Examination Survey (NHANES), which aims to evaluate the health and nutritional status of both children and adults in the United States (27, 28). We screened 43793 participants aged 20 years and older, which was collected from the NHANES 1999-2014. Exclusion criteria included: (1) other current hepatic disorders or factors leading to chronic liver disease, including hepatitis resulting from hepatitis B virus (HBV), hepatitis c virus (HCV), and liver damage caused by iron overload and liver tumors (n = 5976); (2) individuals who consume more than 1 alcoholic drink per day for women or 2 for men (n = 3711); (3) missing data on the hepatic steatosis index (HSI) and alcohol (n = 459), (4) pregnant participants (n = 222). (5) other missing values (n = 1067). Finally, we filtered out 5210 participants with working hours data and 5116 participants with working type. Figure 1 shows the flow of participants. The protocol was approved by National Center for Health Statistics (NCHS) Research Ethics Review Board, and all participants provided informed consent. Detailed information can be found at https://www.cdc.gov/nchs/nhanes/.
Basic demographic features included age, sex, race (non-Hispanic White, non-Hispanic Black, Mexican American, or other), ratio of family income to poverty (PIR), education level (less than high school, high school and some college or above), body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking status, and diabetes. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), and triglycerides (TG) were extracted. The SBP and DBP data were obtained as the average of three measurements, while data on smoking and diabetes were collected from questionnaires. Detailed laboratory testing methods such as TC, TG, ALT, AST can be found at: https://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. The PIR was used to categorize family income levels into low (PIR< 1.3), middle (PIR: 1.3–3.5), and high (PIR > 3.5) groups. The definition of smoking was established as having smoked 100 or more cigarettes in one’s lifetime or being a current smoker. Diabetes was defined as being informed by a doctor of having diabetes or having a fasting blood glucose concentration of 126 mg/dL or more. We classified working hours into the following categories: less than 30 hours per week, 30-40 hours, 40-50 hours, and more than 50 hours. Working type was classified into four categories: mental labor, light physical labor, medium physical labor, and heavy physical labor (Supplementary Table S1). We used the HSI (29) to diagnose NAFLD in this study due to the absence of abdominal ultrasound data:
+body mass index (BMI) +2 (if diabetic) + 2 (if female).
Prior research has shown a strong correlation between NAFLD and the extent of hepatic steatosis; therefore, we define NAFLD as an HSI greater than 36 (30).
In descriptive analysis, data for categorical variables were reported as frequency (percentages), while continuous variables were reported as medians (IQR) due to their skewed distribution. Kruskal-Wallis tests were conducted to compare differences in continuous variables among groups, while categorical variable differences were examined using chi-square tests. Multivariate logistic regression incorporating weights was used to evaluate the association between working hours or working type and NAFLD across different models. Model 1: No adjustment was made for confounding variables. Model 2: Consideration was given to age, sex, race, PIR, and education in the adjustments. Model 3: Based on model 2, additional factors were considered, including ALT, TC, TG, DBP, SBP, smoking status, and diabetes. We employed restricted cubic spline (RCS) analysis to examine the potential non-linear relationship between working hours and NAFLD. Subgroup analysis was conducted to explore the relationship between working hours or type and NAFLD. Stratification factors included gender (male/female), age (≤ 40/40-60/> 60years), PIR (≤ 1.3/1.3-3.5/> 3.5), education level (less than high school, high school and some college or above), and diabetes (yes/no). We conducted interaction analysis to examine the heterogeneity of the relationship among various subgroups. All of our data analyses were conducted using R version 4.3.2. A two-sided P value less than 0.05 was considered statistically significant.
After excluding participants with significant alcohol intake, viral hepatitis, other liver conditions, and those who missed key parameters, we identified 5210 participants for the working hours analysis and 5116 participants for the working type analysis. Our study found that for working hours, the average age of the total population was 45.4 years, with males accounting for 62.7%. In terms of working type, the average age of participants was 57.0 years, with males accounting for 57.3%.
The working hours showed significant differences among participants based on sex, age, race, education level, PIR, and ALT. As working hours increased, the proportion of males gradually increased. The median age was 50.0 years in the ≤ 30 hours group, 44.0 years in the 30-40 hours group, 45.0 years in the 40-50 hours group, and 44.0 years in the > 50 hours group. This indicates that younger participants may work longer hours than middle-aged and older participants. In the ≤ 30 hours group, 24.6% of participants were from low-income households, 32.2% from middle-income households, and 43.2% from high-income households. In the > 50 hours group, only 13.6% of participants were from low-income households, 27.9% from middle-income households, and 58.4% from high-income households. The proportion of smokers and individuals with diabetes was higher in the< 30 hours work duration group (Table 1).
Compared to the reference group (≤ 30 hours), the risk of NAFLD increased with longer working hours after adjusting for confounding factors, with the significant increase in the > 50 hours group (OR: 1.57, 95%CI: 1.21-2.05, P = 0.006) (Table 2). We found that even after adjusting for age, gender, race, education level, household income, TC, TG, SBP, DBP, ALT, smoking, and diabetes, the relationship between working hours and NAFLD still persisted. In the RCS analysis, no significant nonlinear association of working hours with NAFLD was found (P nonlinear = 0.162), but a linear correlation may exist (Figure 2).
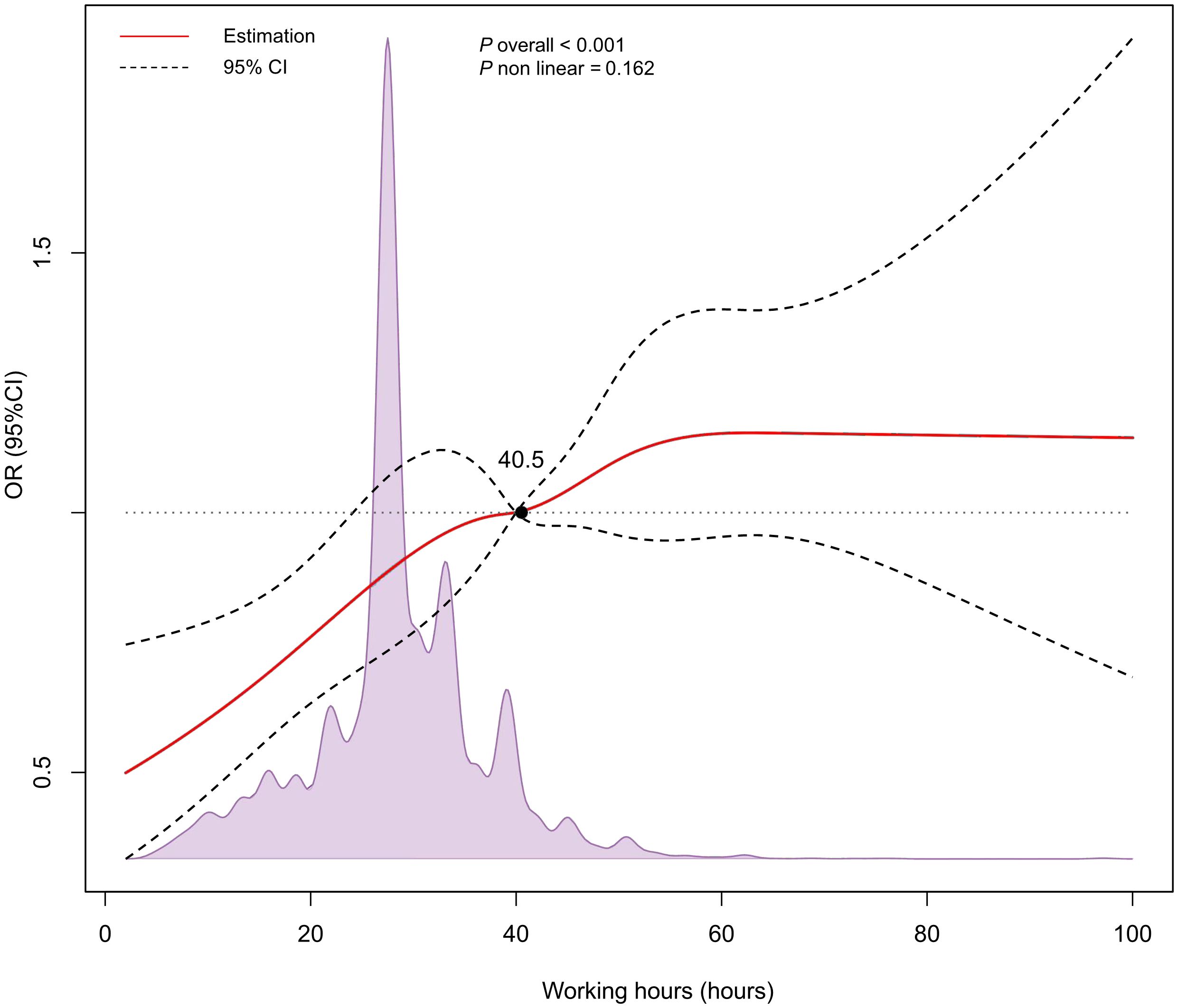
Figure 2. Restricted cubic spline (RCS) analysis for the relationship between working hours and NAFLD.
The type of work was significantly associated with NAFLD before adjusting for confounding factors, and the risk of developing NAFLD increased with higher work intensity. In light physical labor, the probability of NAFLD heightened by 33% (OR: 1.33, 95%CI: 1.08-1.65, P = 0.008). In medium physical labor, the risk heightened by 62% (OR: 1.62, 95%CI: 1.26-2.08, P< 0.001). For heavy physical labor, the risk heightened by 76% (OR: 1.76, 95%CI: 1.44-2.51, P< 0.001). After correcting for age, sex, race, education level, and PIR, no significant connection existed between light physical labor and NAFLD (P = 0.299). Medium physical labor and heavy physical labor were significantly associated with NAFLD. Heavy physical labor showed a 37% increased risk (OR: 1.37, 95%CI: 1.07-1.76, P = 0.014). Further adjustment for TC, TG, SBP, DBP, ALT, smoking status, and diabetes showed that this significant association was only observed in heavy physical labor (OR: 1.39, 95%CI: 1.06-1.81, P = 0.018). Additionally, there was a tendency toward an association between medium physical labor and NAFLD (P = 0.087) (Table 3).
In subgroup analyses stratified by sex, our findings indicated a notable positive correlation between working hours and NAFLD in females (P< 0.050), but no statistically association was detected in male models. There was no statistically association between working hours and NAFLD in the age-stratified analysis. In subgroup analyses stratified by family income, the risk of NAFLD increased in medium-income and high-income populations. In analysis stratified by education level, it is apparent that longer working hours are independently correlated with NAFLD in individuals with a high school education or above (P< 0.050), but not in those with lower educational levels. Among people without diabetes, there is a significant association between working hours and NAFLD (Table 4).
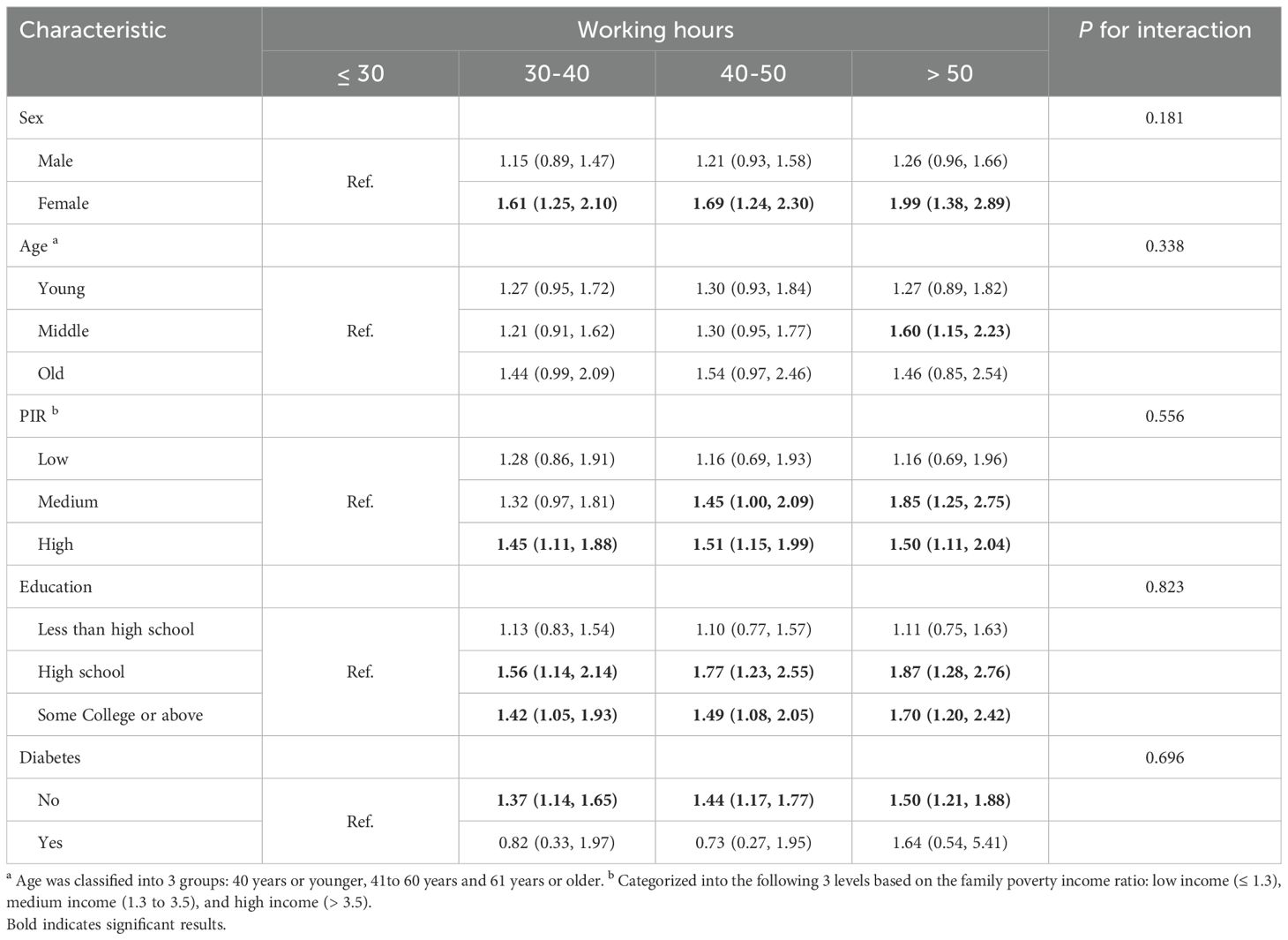
Table 4. Associations of working hours in various subgroups among participants with NAFLD in NHANES 1999-2014.
In subgroup analyses stratified by sex, men were at greater risk for NAFLD with medium physical labor and heavy physical labor, while no significant difference was observed in females. In subgroup analyses stratified by age, NAFLD risk increased among older individuals with higher work intensity (OR: 1.49, 95%CI: 1.11-2.00). In the family income layered analysis, we did not detect a correlation between the type of work and NAFLD. There was no significant difference in the risk of NAFLD among individuals with a high school education or below in light and moderate physical labor. Finally, in the diabetes stratified analysis, there was no meaningful connection between the type of work and NAFLD. Furthermore, the results of the interaction analysis indicated no notable interaction between working hours and the various subgroups (Table 5).
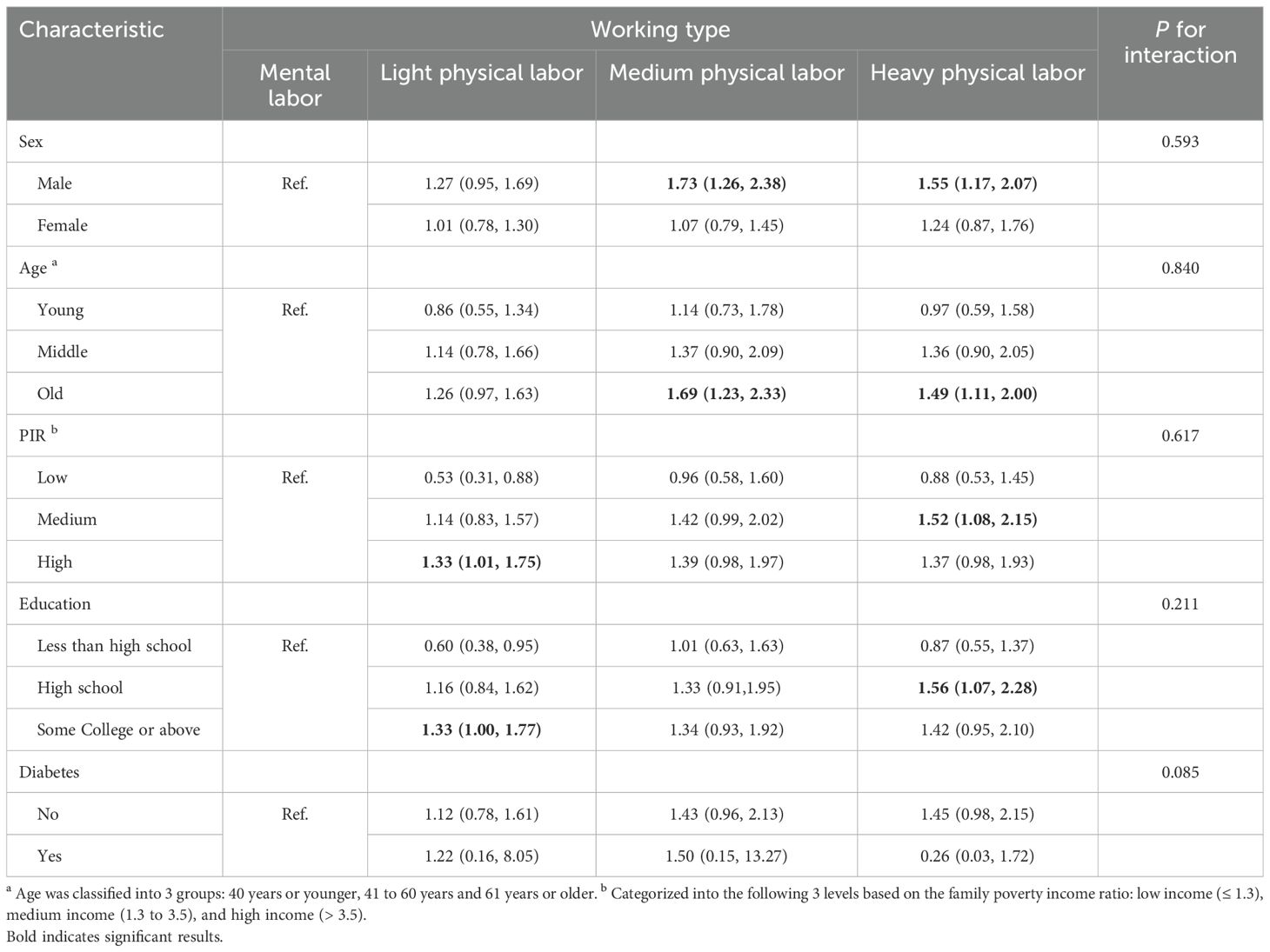
Table 5. Associations of working type in various subgroups among participants with NAFLD in NHANES 1999-2014.
In this study, we found that long working hours significantly increased the risk of NAFLD even after adjusting for confounding factors. Previous research indicated that long working hours raise the likelihood of NAFLD among Koreans (26). In our study, we expanded the population to the United States and found that working hours continued to show a significant relationship with NAFLD even after adjusting for confounding variables, which is consistent with the findings in Koreans. The risk in the US population (before correcting for confounding variables) was relatively higher (OR: 1.57, 95%CI: 1.21-2.05) compared to Korea. This may be attributed to the higher prevalence of NAFLD in North America (31.2%) compared to East Asia (29.7%) (31). Using unconstrained cubic splines based on the Korean study, we demonstrated a linear relationship between working hours and NAFLD (P nonlinear = 0.162) (26). A Chinese study showed that prolonged and high-frequency night shift work increases the risk of NAFLD in male steel workers by 27% (32). Furthermore, the 2024 study by Robert Maidstone et al. on biobanks in the UK demonstrates that long-term night shift work elevates the risk of fatty degeneration by 8% (33). These findings are consistent with our research. Night shift work has been shown to be associated with various liver diseases. The study by Wang Feng et al. indicates that night shift work among Chinese workers is positively correlated with liver function abnormalities (34). Similarly, a study involving the Korean population found that night shift work is positively correlated with NAFLD among young female workers with poor sleep quality (35). Moreover, prolonged night shift work increases the risk of dyslipidemia and liver and kidney function abnormalities among nurses (36). Since there is no data on night shifts in the NHANES database, we used weekly working hours to investigate its association with NAFLD. These studies consistently indicate that poor work patterns can lead to the occurrence of various liver diseases.
Currently, there is no research indicating that heavy physical labor increases the risk of NAFLD. Existing studies have shown that heavy physical labor raises the likelihood of work absence (37, 38), musculoskeletal diseases (39), hypertension (40), cardiovascular disorders (41, 42), and even the risk of mortality from all causes in men (43). In addition, moderate-intensity aerobic exercise can lead to a 2-4% absolute reduction in liver fat degeneration in adults with MAFLD (44). We considered that heavy physical labor may affect the risk of NAFLD through some underlying mechanisms. To verify this hypothesis, we categorized participants into groups based on work intensity: mental labor, light manual work, moderate manual work, and heavy manual work. We observed that the risk of NAFLD increased with greater work intensity. This indirectly suggests that work-related exercise does not provide equivalent health benefits compared to free-time physical activity (45). When adjusting for age, sex, race, education level, PIR, TC, TG, SBP, DBP, ALT, smoking, and diabetes, only heavy physical labor was notably linked to NAFLD (OR: 1.39, 95%CI: 1.06-1.81). One possible explanation for this phenomenon is that while appropriate physical activity can improve mood, excessive physical activity may not only fail to enhance mood, but can also lead to mood deterioration (46, 47). This deterioration can manifest as sleep disturbances, weight and appetite loss, fatigue, irritability, emotional instability, and even depression. Sleep disturbances (48), emotional instability, and depression (49, 50) have been established as risk factors for NAFLD. Additionally, heavy manual work may contribute to a lack of leisure exercise, which is vital for promoting health and enhancing physical fitness and work capacity (9, 51). Furthermore, heavy physical labor can increase the cardiovascular burden on male construction workers, significantly raising the incidence of NAFLD (52). The specific mechanisms still need to be confirmed through extensive research.
Our subgroup analysis showed that the relationship between working hours and NAFLD was more significant in women and high-income individuals. The distribution of body fat in women changes with hormonal cycles compared to men (53), which results in a relatively higher probability of obesity in women (54). This physiological difference may contribute to an increased risk of NAFLD in women. The connection between working type and NAFLD was more pronounced in men and the elderly. This may be due to the higher proportion of male workers in heavy physical labor, as age increases, the consequences of prolonged heavy physical work become more apparent (55, 56).
Long working hours and heavy physical labor are associated with the development of various diseases. Research indicates that for every 10-hour increase in weekly working hours, the likelihood of sleep deprivation increases by approximately 50%, and the risk of difficulty falling asleep also rises significantly (57). Long working hours can lead to an increased probability of obesity (6), which may be caused by the impact of prolonged work on metabolic response mechanisms (58). Existing evidence indicates that long working hours contribute to coronary heart disease, stroke, hypertension, depression, and other chronic diseases (59). The cumulative effect of these factors greatly increases the risk of developing NAFLD. Therefore, it is crucial to properly arrange working hours and avoid intense physical labor.
However, certain limitations exist. Firstly, because this study employed a cross-sectional design, the observed connection does not inherently indicate a cause-and-effect connection. Secondly, we employed a non-direct method (the HSI assessment tool) instead of imaging studies or pathological assessments to determine NAFLD. However, the HSI has been thoroughly confirmed and can be used to predict the existence and severity of NAFLD in numerous extensive studies (29). Thirdly, since our research only consisted of the demographic in the United States, our findings ought to be corroborated in various racial groups. Ultimately, even though we tried to adjust for several potential risk variables, there could still be unaccounted confounding factors or biases beyond our control, such as participants’ specific sleep habits, daily exercise time, and dietary habits. Finally, we investigated the relationship between working hours and NAFLD; however, the associations of other steatotic liver disease categories, such as MASLD and MetALD, remain unexplored. We hope to further explore the connections between working hours, working type and other liver diseases in future research. Regardless of these limitations, this study offers important strengths, such as a large representative sample from across the nation, standardized exceptional clinical and laboratory data gathering, and thorough details on different confounding influences.
The findings of this cross-sectional study suggest significant connection between long working hours and heavy physical labor with NAFLD. Furthermore, our research indicates that these factors heighten the likelihood of developing NAFLD, which may offer insights into innovative interventions and approaches for reducing the risk of NAFLD.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by The NCHS Research Ethics Review Board approved the questionnaire, ensuring that all participants consented with full awareness. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
RW: Formal analysis, Methodology, Writing – original draft. NW: Data curation, Methodology, Writing – original draft. QH: Data curation, Methodology, Project administration, Writing – original draft. XZ: Methodology, Writing – review & editing. HZ: Project administration, Supervision, Writing – original draft. LiZ: Resources, Writing – review & editing. XW: Project administration, Resources, Writing – review & editing. XY: Conceptualization, Methodology, Project administration, Writing – review & editing. LeZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Top medical expert team of Wuxi Taihu Talent Plan (Grant No. DJTD202106, GDTD202105, YXTD202101), Medical Key Discipline Program of Wuxi Health Commission (Grant No. ZDXK2021007, CXTD2021005), Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (Grant No.BJ2023090), Scientific Research Program of Wuxi health Commission (Grant No. Z202109, M202208), and Wuxi Science and Technology Development Fund (Grant No. N20202003, Y20222001, K20241001).
All authors thank NHANES for providing the publicly available data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1499735/full#supplementary-material
1. Bannai A, Tamakoshi A. The association between long working hours and health: a systematic review of epidemiological evidence. Scand J Work Environ Health. (2014) 40:5–18. doi: 10.5271/sjweh.3388
2. Trudel X, Brisson C, Gilbert-Ouimet M, Vézina M, Talbot D, Milot A. Long working hours and the prevalence of masked and sustained hypertension. Hypertension. (2020) 75:532–8. doi: 10.1161/HYPERTENSIONAHA.119.12926
3. Kivimäki M, Virtanen M, Kawachi I, Nyberg ST, Alfredsson L, Batty GD, et al. Long working hours, socioeconomic status, and the risk of incident type 2 diabetes: a meta-analysis of published and unpublished data from 222 120 individuals. Lancet Diabetes Endocrinol. (2015) 3:27–34. doi: 10.1016/S2213-8587(14)70178-0
4. Jeong I, Rhie J, Kim I, Ryu I, Jung PK, Park YS, et al. Working hours and cardiovascular disease in Korean workers: a case-control study. J Occup Health. (2014) 55:385–91. doi: 10.1539/joh.12-0245-oa
5. Virtanen M, Heikkilä K, Jokela M, Ferrie JE, Batty GD, Vahtera J, et al. Long working hours and coronary heart disease: a systematic review and meta-analysis. Am J Epidemiol. (2012) 176:586–96. doi: 10.1093/aje/kws139
6. Kim BM, Lee BE, Park HS, Kim YJ, Suh YJ, Kim JY, et al. Long working hours and overweight and obesity in working adults. Ann Occup Environ Med. (2016) 28:36. doi: 10.1186/s40557-016-0110-7
7. Han S, Ko Y, Moon JE, Cho YS. Working hours are closely associated with depressive mood and suicidal ideation in Korean adults: a nationwide cross-sectional study. Sci Rep. (2021) 11:23102. doi: 10.1038/s41598-021-02574-8
8. Rodriguez-Jareño MC, Demou E, Vargas-Prada S, Sanati KA, Skerjanc A, Reis PG, et al. European Working Time Directive and doctors’ health: a systematic review of the available epidemiological evidence. BMJ Open. (2014) 4:e004916. doi: 10.1136/bmjopen-2014-004916
9. Arvidson E, Börjesson M, Ahlborg G Jr., Lindegård A, Jonsdottir IH. The level of leisure time physical activity is associated with work ability-a cross sectional and prospective study of health care workers. BMC Public Health. (2013) 13:855. doi: 10.1186/1471-2458-13-855
10. Schibye B, Hansen AF, Søgaard K, Christensen H. Aerobic power and muscle strength among young and elderly workers with and without physically demanding work tasks. Appl Ergon. (2001) 32:425–31. doi: 10.1016/S0003-6870(01)00034-5
11. Torgén M, Punnett L, Alfredsson L, Kilbom A. Physical capacity in relation to present and past physical load at work: a study of 484 men and women aged 41 to 58 years. Am J Ind Med. (1999) 36:388–400. doi: 10.1002/(SICI)1097-0274(199909)36:3<388::AID-AJIM6>3.0.CO;2-3
12. Russo A, Onder G, Cesari M, Zamboni V, Barillaro C, Capoluongo E, et al. Lifetime occupation and physical function: a prospective cohort study on persons aged 80 years and older living in a community. Occup Environ Med. (2006) 63:438–42. doi: 10.1136/oem.2005.023549
13. Cassou B, Derriennic F, Iwatsubo Y, Amphoux M. Physical diability after retirement and occupational risk factors during working life: a cross sectional epidemiological study in the Paris area. J Epidemiol Community Health. (1992) 46:506–11. doi: 10.1136/jech.46.5.506
14. Li CY, Wu SC, Wen SW. Longest held occupation in a lifetime and risk of disability in activities of daily living. Occup Environ Med. (2000) 57:550–4. doi: 10.1136/oem.57.8.550
15. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
16. Fransson EI, Heikkilä K, Nyberg ST, Zins M, Westerlund H, Westerholm P, et al. Job strain as a risk factor for leisure-time physical inactivity: an individual-participant meta-analysis of up to 170,000 men and women: the IPD-Work Consortium. Am J Epidemiol. (2012) 176:1078–89. doi: 10.1093/aje/kws336
17. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
18. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
19. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
20. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. et al: A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. (2023) 78:1966–86. doi: 10.1097/HEP.0000000000000520
21. Zhang H, Targher G, Byrne CD, Kim SU, Wong VW, Valenti L, et al. A global survey on the use of the international classification of diseases codes for metabolic dysfunction-associated fatty liver disease. Hepatol Int. (2024) 18:1178–201. doi: 10.1007/s12072-024-10702-5
22. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
23. Zhang L, El-Shabrawi M, Baur LA, Byrne CD, Targher G, Kehar M, et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med (New York NY). (2024) 5:797–815.e792. doi: 10.1016/j.medj.2024.03.017
24. Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. (2024). doi: 10.3350/cmh.2024.0431
25. Lindenmeyer CC, McCullough AJ. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin Liver Dis. (2018) 22:11–21. doi: 10.1016/j.cld.2017.08.003
26. Song E, Kim JA, Roh E, Yu JH, Kim NH, Yoo HJ, et al. Long working hours and risk of nonalcoholic fatty liver disease: korea national health and nutrition examination survey VII. Front Endocrinol. (2021) 12:647459. doi: 10.3389/fendo.2021.647459
27. Wang X, Seo YA, Park SK. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011-2016. Environ Res. (2021) 197:111190. doi: 10.1016/j.envres.2021.111190
28. Tian T, Zhang J, Xie W, Ni Y, Fang X, Liu M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017-2018. Nutrients. (2022) 14:4505. doi: 10.3390/nu14214505
29. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. (2010) 42:503–8. doi: 10.1016/j.dld.2009.08.002
30. Shih KL, Su WW, Chang CC, Kor CT, Chou CT, Chen TY, et al. Comparisons of parallel potential biomarkers of 1H-MRS-measured hepatic lipid content in patients with non-alcoholic fatty liver disease. Sci Rep. (2016) 6:24031. doi: 10.1038/srep24031
31. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
32. Zhang S, Wang Y, Wang Z, Wang H, Xue C, Li Q, et al. Rotating night shift work and non-alcoholic fatty liver disease among steelworkers in China: a cross-sectional survey. Occup Environ Med. (2020) 77:333–9. doi: 10.1136/oemed-2019-106220
33. Huang H, Liu Z, Xie J, Xu C. Association between night shift work and NAFLD: a prospective analysis of 281,280 UK Biobank participants. BMC Public Health. (2023) 23:1282. doi: 10.1186/s12889-023-16204-7
34. Wang F, Zhang L, Wu S, Li W, Sun M, Feng W, et al. Night shift work and abnormal liver function: is non-alcohol fatty liver a necessary mediator? Occup Environ Med. (2019) 76:83–9. doi: 10.1136/oemed-2018-105273
35. Lee Y, Lee W. Shift work and non-alcoholic fatty liver disease in young, healthy workers. Sci Rep. (2024) 14:19367. doi: 10.1038/s41598-024-70538-9
36. Zhao Y, Lu X, Wang Y, Cheng Y, He Q, Qin R, et al. Peripheral blood lipid and liver and kidney function test results in long-term night shift nurses: a cross-sectional study in South China. Front Endocrinol (Lausanne). (2023) 14:1237467. doi: 10.3389/fendo.2023.1237467
37. Andersen LL, Clausen T, Persson R, Holtermann A. Dose-response relation between perceived physical exertion during healthcare work and risk of long-term sickness absence. Scand J Work Environ Health. (2012) 38:582–9. doi: 10.5271/sjweh.3310
38. Andersen LL, Fallentin N, Thorsen SV, Holtermann A. Physical workload and risk of long-term sickness absence in the general working population and among blue-collar workers: prospective cohort study with register follow-up. Occup Environ Med. (2016) 73:246–53. doi: 10.1136/oemed-2015-103314
39. Nordander C, Hansson G, Ohlsson K, Arvidsson I, Balogh I, Strömberg U, et al. Exposure-response relationships for work-related neck and shoulder musculoskeletal disorders–Analyses of pooled uniform data sets. Appl Ergon. (2016) 55:70–84. doi: 10.1016/j.apergo.2016.01.010
40. Clays E, De Bacquer D, Van Herck K, De Backer G, Kittel F, Holtermann A. Occupational and leisure time physical activity in contrasting relation to ambulatory blood pressure. BMC Public Health. (2012) 12:1002. doi: 10.1186/1471-2458-12-1002
41. Holtermann A, Mortensen OS, Burr H, Søgaard K, Gyntelberg F, Suadicani P. Physical demands at work, physical fitness, and 30-year ischaemic heart disease and all-cause mortality in the Copenhagen Male Study. Scand J Work Environ Health. (2010) 36:357–65. doi: 10.5271/sjweh.2913
42. Petersen CB, Eriksen L, Tolstrup JS, Søgaard K, Grønbaek M, Holtermann A. Occupational heavy lifting and risk of ischemic heart disease and all-cause mortality. BMC Public Health. (2012) 12:1070. doi: 10.1186/1471-2458-12-1070
43. Coenen P, Huysmans MA, Holtermann A, Krause N, van Mechelen W, Straker LM, et al. Do highly physically active workers die early? A systematic review with meta-analysis of data from 193 696 participants. Br J Sports Med. (2018) 52:1320–6. doi: 10.1136/bjsports-2017-098540
44. Keating SE, Sabag A, Hallsworth K, Hickman IJ, Macdonald GA, Stine JG, et al. Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: A position statement from exercise and sport science Australia. Sports Med. (2023) 53:2347–71. doi: 10.1007/s40279-023-01918-w
45. Holtermann A, Krause N, van der Beek AJ, Straker L. The physical activity paradox: six reasons why occupational physical activity (OPA) does not confer the cardiovascular health benefits that leisure time physical activity does. Br J Sports Med. (2018) 52:149–50. doi: 10.1136/bjsports-2017-097965
46. Peluso MA, Guerra de Andrade LH. Physical activity and mental health: the association between exercise and mood. Clinics (Sao Paulo). (2005) 60:61–70. doi: 10.1590/S1807-59322005000100012
47. Morgan WP, Brown DR, Raglin JS, O’Connor PJ, Ellickson KA. Psychological monitoring of overtraining and staleness. Br J Sports Med. (1987) 21:107–14. doi: 10.1136/bjsm.21.3.107
48. Marjot T, Ray DW, Williams FR, Tomlinson JW, Armstrong MJ. Sleep and liver disease: a bidirectional relationship. Lancet Gastroenterol Hepatol. (2021) 6:850–63. doi: 10.1016/S2468-1253(21)00169-2
49. Shea S, Lionis C, Kite C, Atkinson L, Chaggar SS, Randeva HS, et al. Non-alcoholic fatty liver disease (NAFLD) and potential links to depression, anxiety, and chronic stress. Biomedicines. (2021) 9:1697. doi: 10.3390/biomedicines9111697
50. Ruan X, Chen J, Sun Y, Zhang Y, Zhao J, Wang X, et al. Depression and 24 gastrointestinal diseases: a Mendelian randomization study. Transl Psychiatry. (2023) 13:146. doi: 10.1038/s41398-023-02459-6
51. Alavinia SM, van Duivenbooden C, Burdorf A. Influence of work-related factors and individual characteristics on work ability among Dutch construction workers. Scand J Work Environ Health. (2007) 33:351–7. doi: 10.5271/sjweh.1151
52. Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. Bmj. (2021) 373:n604. doi: 10.1136/bmj.n604
53. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. Jama. (2002) 288:1723–7. doi: 10.1001/jama.288.14.1723
54. Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab. (2021) 50:101122. doi: 10.1016/j.molmet.2020.101122
55. Stocks SJ, Turner S, McNamee R, Carder M, Hussey L, Agius RM. Occupation and work-related ill-health in UK construction workers. Occup Med (Lond). (2011) 61:407–15. doi: 10.1093/occmed/kqr075
56. Savinainen M, Nygård CH, Ilmarinen J. A 16-year follow-up study of physical capacity in relation to perceived workload among ageing employees. Ergonomics. (2004) 47:1087–102. doi: 10.1080/00140130410001686357
57. Virtanen M, Ferrie JE, Gimeno D, Vahtera J, Elovainio M, Singh-Manoux A, et al. Long working hours and sleep disturbances: the Whitehall II prospective cohort study. Sleep. (2009) 32:737–45. doi: 10.1093/sleep/32.6.737
58. Flier JS, Elmquist JK. A good night’s sleep: future antidote to the obesity epidemic? Ann Intern Med. (2004) 141:885–6. doi: 10.7326/0003-4819-141-11-200412070-00014
59. Lunde LK, Skare Ø, Mamen A, Sirnes PA, Aass HCD, Øvstebø R, et al. et al: cardiovascular health effects of shift work with long working hours and night shifts: study protocol for a three-year prospective follow-up study on industrial workers. Int J Environ Res Public Health. (2020) 17:589. doi: 10.3390/ijerph17020589
Keywords: working hours, working type, hepatic steatosis index, NAFLD, NHANES
Citation: Wang R, Wu N, Qu H, Zheng X, Zhang H, Zhu L, Wang X, Yao X and Zhang L (2025) The association between working hours and working type with non-alcoholic fatty liver disease: results from the NHANES 1999-2014. Front. Endocrinol. 15:1499735. doi: 10.3389/fendo.2024.1499735
Received: 21 September 2024; Accepted: 20 December 2024;
Published: 14 January 2025.
Edited by:
Stanisław Surma, Medical University of Silesia, PolandReviewed by:
Tatsuya Sato, Sapporo Medical University, JapanCopyright © 2025 Wang, Wu, Qu, Zheng, Zhang, Zhu, Wang, Yao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Zhang, emhhbmdsZUBqaWFuZ25hbi5lZHUuY24=; Xiaodie Yao, eGlhb2RpZXlhb0AxNjMuY29t; Xiaolei Wang, ZG9jdG9yd2FuZzkyNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.