- 1Department of Endocrinology and Diabetes, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 2Center for Advanced Medicine and Clinical Research, Nagoya University Hospital, Nagoya, Japan
- 3Research Center of Health, Physical Fitness and Sports, Nagoya University, Nagoya, Japan
- 4Department of Respiratory Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 5Department of Clinical Oncology and Chemotherapy, Nagoya University Hospital, Nagoya, Japan
Background: Immune-related adverse events (irAEs) are reported to be associated with better overall survival (OS) in non-small cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors. However, there may be a bias in that patients who develop irAEs must survive long enough to experience the irAEs, and no prospective studies adjusting for immortal time bias (ITB) have examined the relationship between OS and pituitary dysfunction or the two different types of thyroid dysfunction: destructive thyroiditis and hypothyroidism without prior thyrotoxicosis (isolated hypothyroidism).
Methods: Patients with NSCLC who received nivolumab or pembrolizumab at Nagoya University Hospital between November 2, 2015 and February 1, 2023 were enrolled. Endocrine irAEs were prospectively assessed during scheduled evaluations of hormone levels. The association between irAE development and survival when considering ITB was examined by time-dependent Cox regression analysis.
Results: Of the 194 patients included, 11 (5.7%), 10 (5.2%), and 5 (2.6%) developed pituitary dysfunction, destructive thyroiditis, and isolated hypothyroidism, respectively. The development of pituitary dysfunction (HR 0.36, 95% CI 0.13–0.98, p = 0.045) and destructive thyroiditis (HR 0.31, 95% CI 0.10–0.97, p = 0.044), but not isolated hypothyroidism (HR 1.15, 95% CI 0.42–3.20, p = 0.786), was significantly associated with longer OS.
Conclusion: NSCLC patients developing pituitary dysfunction and destructive thyroiditis showed better OS even after adjusting for ITB, suggesting that these irAEs indicate a better prognosis.
Introduction
Immune checkpoint inhibitors (ICIs) have shown striking efficacy against many types of cancer and currently play a crucial role in cancer therapy. However, despite their benefits, ICIs can trigger a diverse range of adverse events known as immune-related adverse events (irAEs), which occur in various organs including endocrine glands (1–4). Several studies have reported a relationship between the development of irAEs and better survival outcomes in patients treated with ICIs (5, 6). Recently, we reported that the development of pituitary and thyroid dysfunction after ICI treatment was associated with longer overall survival (OS) in non-small cell lung cancer (NSCLC) patients (7). However, this study may have been subjected to immortal time bias (ITB) because patients who develop irAEs must survive long enough to experience the irAEs. This bias may lead to overestimation of the survival benefits associated with irAEs, especially in the case of late-onset irAEs such as pituitary dysfunction (8). Using a time-dependent Cox model, several retrospective studies reported that the development of overall irAEs remained associated with improved OS in NSCLC patients even after taking into account ITB (9, 10). Furthermore, a subgroup analysis revealed associations of thyroid dysfunction and skin toxicity with longer OS (10). In addition, one prospective study in patients with concurrent NSCLC, renal cell carcinoma, and melanoma reported that thyroid dysfunction was associated with improved progression-free survival and OS when considering ITB (11). However, to our knowledge, no prospective studies have shown that the onset of pituitary dysfunction, which generally occurs several months after ICI initiation, has a survival benefit in the setting of ITB. Moreover, no prospective studies have examined the survival benefits of the two different types of thyroid dysfunction, destructive thyroiditis and hypothyroidism without prior thyrotoxicosis (isolated hypothyroidism), while taking into account ITB. In this prospective observational real-world study, we examined the relationship between the development of irAEs and survival in NSCLC patients treated with anti-programmed cell death-1 (PD-1) immunotherapy, taking into account ITB.
Methods
Study population
In this prospective study analyzing endocrine irAEs in patients treated with ICIs (UMIN000019024), patients with NSCLC who had received at least one cycle of treatment with nivolumab or pembrolizumab at Nagoya University Hospital between November 2, 2015 and February 1, 2023 (Figure 1) were included. This study focused exclusively on patients treated with anti-PD-1 antibody therapy for the analyses, because the incidence of irAEs and the prognosis may vary depending on the class of ICIs. Patients with a history of any prior ICI treatment were excluded. This study was conducted following the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of Nagoya University Hospital (Approval No. 2015-0273). Written informed consent was obtained from all patients prior to study enrollment.
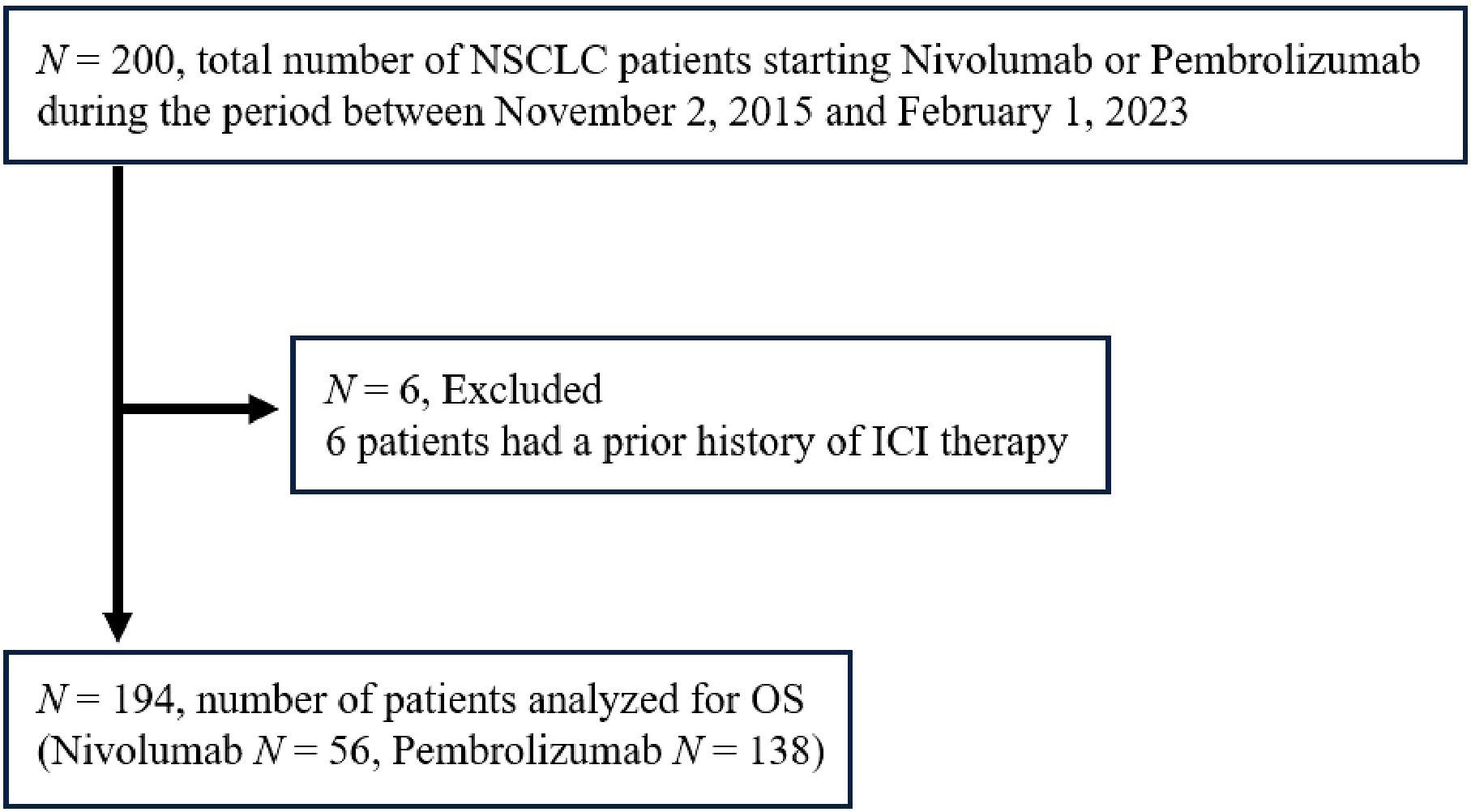
Figure 1. Enrollment of the study subjects. NSCLC, non-small cell lung cancer; ICI, immune checkpoint inhibitor; OS, overall survival.
Data collection and definition of irAEs
Baseline clinical data, including age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), and presence of distant metastasis (i.e. bone, liver, and brain), were collected through electronic medical records. To evaluate endocrine irAEs, the levels of adrenocorticotropic hormone, cortisol, and blood glucose were assessed at baseline and every 6 weeks after the initial ICI administration for 48 weeks, as described previously (12). Free triiodothyronine, free thyroxine, and thyroid-stimulating hormone (TSH) levels were assessed at baseline and 6, 12, 18, 24, 36, and 48 weeks after the first administration of ICIs based on a minor change to the study protocol. These measurements were also conducted when clinically needed. Each endocrine irAE was defined according to the Japan Endocrine Society clinical guidelines for endocrine irAEs (13). Thyroid dysfunction was classified into two types: 1) destructive thyroiditis (development of transient thyrotoxicosis in the absence of TSH receptor antibody (TRAb) positivity and 2) isolated hypothyroidism (development of hypothyroidism without preceding thyrotoxicosis). Non-endocrine irAEs were defined based on the judgment of attending physicians and/or clinical improvement after treatment with corticosteroids or other immunosuppressive agents. Non-endocrine irAEs were categorized based on the organ involved and were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Patients who developed multiple irAEs were assigned to the irAE group for analysis of the relationships between OS and each individual irAE. Patients were followed up until November 1, 2023.
Statistical analysis
The study endpoint was OS, which was defined as the time from initial ICI treatment to death from any cause. To address ITB, univariate and multivariate Cox regression analyses were performed using each irAE as the time-dependent covariate to evaluate the correlation between OS and any irAE that occurred in at least five patients. In the analysis of overall irAEs, the time of onset was defined as the time from ICI initiation to the first irAE development. Other covariates included in the multivariate Cox regression analysis were age, sex, ECOG-PS, histology, and presence of metastasis. Continuous and categorical variables were compared between patients with and those without irAEs using the Mann–Whitney U test and Fisher exact test, respectively. All statistical tests were two sided, and significance was defined as a p value < 0.05. Statistical analyses were conducted using IBM SPSS Statistics, version 29.
Results
Patient characteristics
A total of 194 NSCLC patients treated with nivolumab or pembrolizumab were included in the analysis (Figure 1). The patient baseline characteristics are presented in Table 1. The median age at the initiation of ICI therapy was 70 years. Of the 194 patients, 75.3% were male, and 30.4% had squamous cell carcinoma. Regarding the ICI agent, 56 patients received nivolumab and 138 patients pembrolizumab (of whom 59 received it as combination therapy with chemotherapy [cisplatin, carboplatin, pemetrexed, or nab-paclitaxel]). The median follow-up period for the whole cohort was 410 (interquartile range [IQR] 179–882) days.
irAE profiles
During the follow-up period, 82 (42.3%) patients experienced 115 irAEs of all grades. The most common irAEs were skin toxicity (n = 27, 13.9%), pneumonitis (n = 24, 12.4%), thyroid dysfunction (n = 15, 7.7%), gastrointestinal toxicity (n = 12, 6.2%), hepatic toxicity (n = 11, 5.7%), and pituitary dysfunction (n = 11, 5.7%) (Table 1). Among the patients with thyroid dysfunction, 10 (5.2%) and 5 (2.6%) developed destructive thyroiditis and isolated hypothyroidism, respectively (Table 1). The median time of onset of each irAE type is shown in Figure 2. The median time to develop the first irAE was 5.1 (IQR 1.3–14.0) weeks. Pituitary dysfunction developed significantly later than did the other irAEs (median 29.6 [IQR 21.9–34.1] vs. 4.4 [IQR 1.9–10.9] weeks, p < 0.001). Of the 82 patients, 53 developed only one irAE, whereas 29 presented with two or more irAEs (Table 1). All cases of pituitary dysfunction were managed with hospitalization for treatment and pituitary function assessment and were classified as grades 3–5 (Supplementary Table 1). However, none of the patients with pituitary dysfunction were treated with high-dose glucocorticoids (Supplementary Table 1). Skin toxicity and thyroid dysfunction comprised mainly grades 1–2 irAEs, while hepatic toxicity, gastrointestinal toxicity, and pneumonitis tended to be more severe, with grades 3–5 irAEs accounting for 33.3–55.5% of cases (Supplementary Table 1). A substantial number of patients who developed severe irAEs discontinued ICI therapy and were treated with high-dose glucocorticoids (Supplementary Table 1). Of the 82 patients who developed any irAE, 31 were treated with systemic glucocorticoids. Among these 31 patients, 6 discontinued glucocorticoids, whereas the remaining 25 were still on glucocorticoids at the end of the study period.
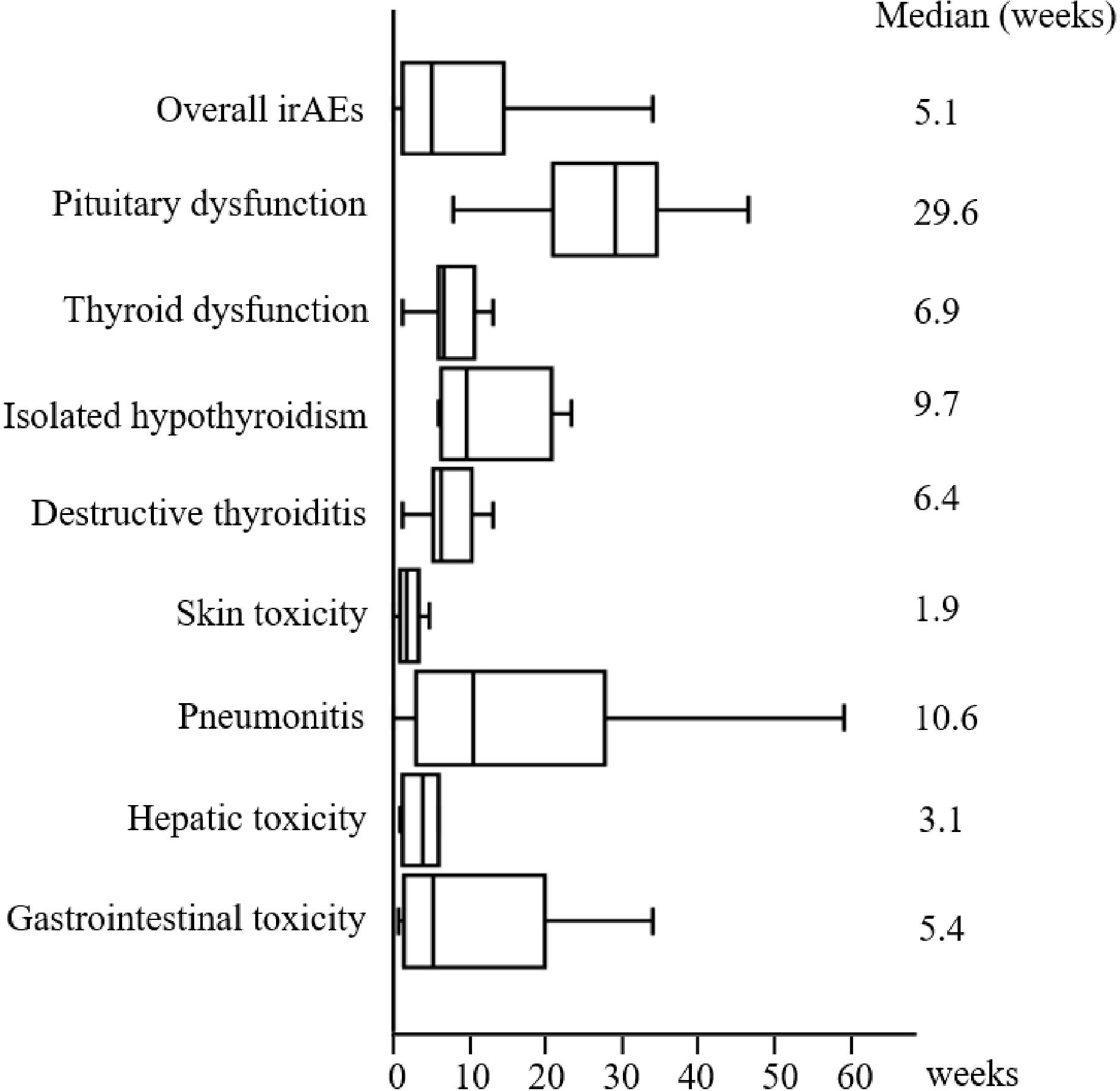
Figure 2. Time of irAE onset. The median time (in weeks) from the first administration of ICI therapy to the development of each irAE. The left, middle, and right lines of the boxes correspond to the 25th, 50th, and 75th percentiles, respectively. The whiskers extend from the minimum to maximum times. irAE, immune-related adverse event.
Univariate analysis of the association between irAE development and survival
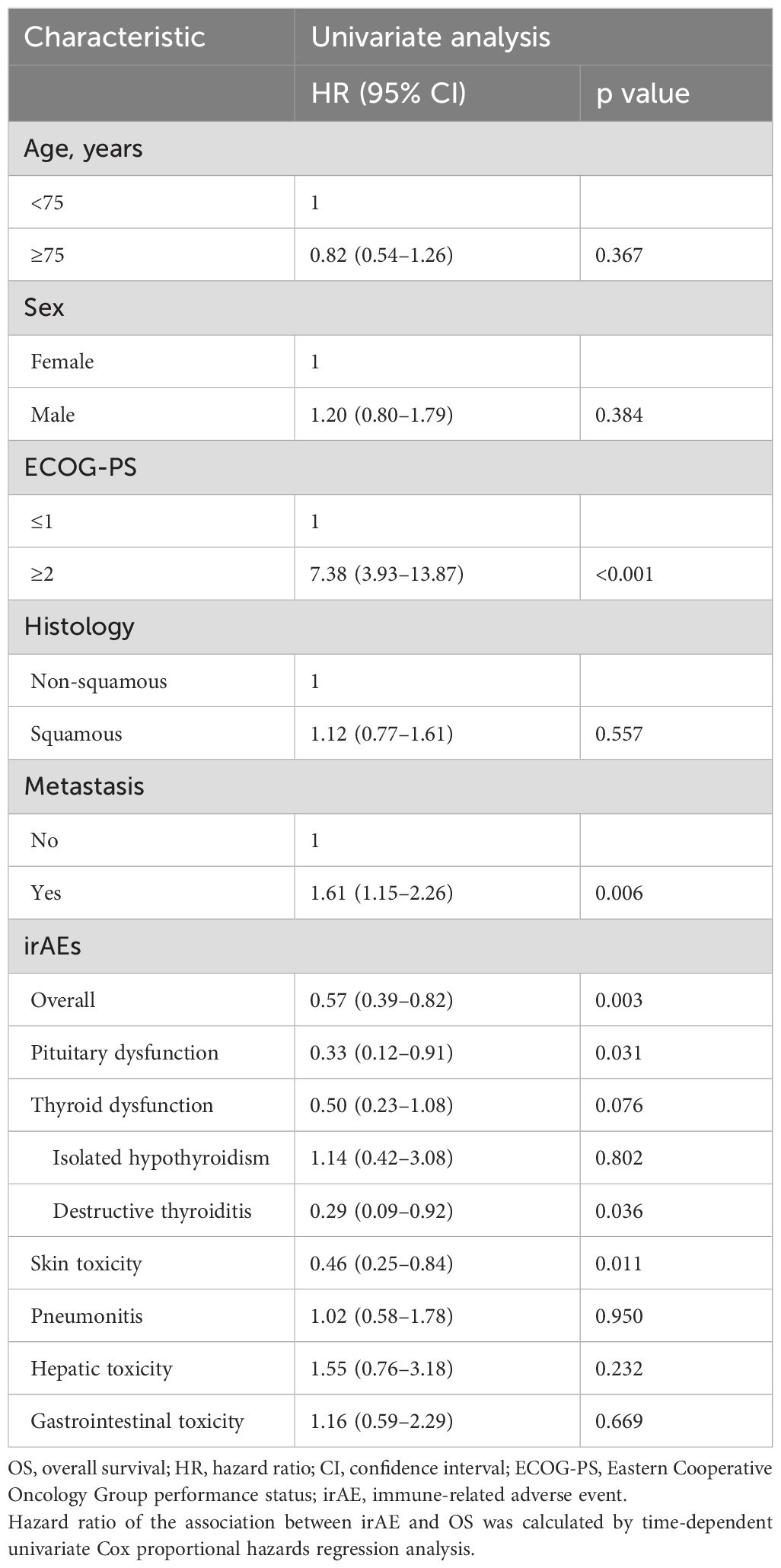
To evaluate the association between irAE development and OS taking into account ITB, we performed time-dependent Cox proportional hazards regression analysis using irAEs as the time-dependent covariate. In addition, regarding thyroid dysfunction, subgroup analyses were performed separately for patients with isolated hypothyroidism versus destructive thyroiditis. The univariate analyses showed that ECOG-PS ≥ 2 (hazard ratios [HR] 7.38, 95% confidence interval [CI] 3.93–13.87, p < 0.001) and presence of metastasis (HR 1.61, 95% CI 1.15–2.26, p = 0.006) were significantly associated with shorter OS, whereas the developments of overall irAEs (HR 0.57, 95% CI 0.39–0.82, p = 0.003), pituitary dysfunction (HR 0.33, 95% CI 0.12–0.91, p = 0.031), destructive thyroiditis (HR 0.29, 95% CI 0.09–0.92, p = 0.036), and skin toxicity (HR 0.46, 95% CI 0.25–0.84, p = 0.011) were significantly associated with longer OS (Table 2). On the other hand, there was no significant association between OS and the development of overall thyroid dysfunction, isolated hypothyroidism, hepatic toxicity, gastrointestinal toxicity, or pneumonitis (Table 2).
Multivariate analysis of the associations of pituitary dysfunction and destructive thyroiditis with OS
To identify the irAEs that are independent prognostic factors for OS, we performed time-dependent multivariate Cox regression analyses using irAEs as the time-dependent covariate, with adjustments for clinical variables (age, sex, ECOG-PS, histology, and metastasis). In addition to an ECOG-PS of 0–1 and absence of metastasis, pituitary dysfunction was independently associated with prolonged OS (HR 0.36, 95% CI 0.13–0.98, p = 0.045) (Table 3). There were no significant differences in the baseline characteristics (age, sex, ECOG-PS, histology, and metastasis) between patients who developed pituitary dysfunction and those who did not (Supplementary Table 2). Similarly, destructive thyroiditis was independently associated with prolonged OS (HR 0.31, 95% CI 0.10–0.97, p = 0.044) (Table 4). There were no significant differences in baseline characteristics between patients who developed destructive thyroiditis and those who did not (Supplementary Table 3). Overall irAEs (Supplementary Table 4) and skin toxicity (Supplementary Table 5) were also identified as independent prognostic factors for OS in the multivariate analyses. There were no significant differences in baseline characteristics between patients who developed skin toxicity and those who did not (Supplementary Table 6), whereas the prevalence of ECOG-PS ≥ 2 was significantly higher among patients who developed overall irAEs than those who did not (2/82 [2.4%] vs. 11/112 [9.8%], p = 0.046, Supplementary Table 7).
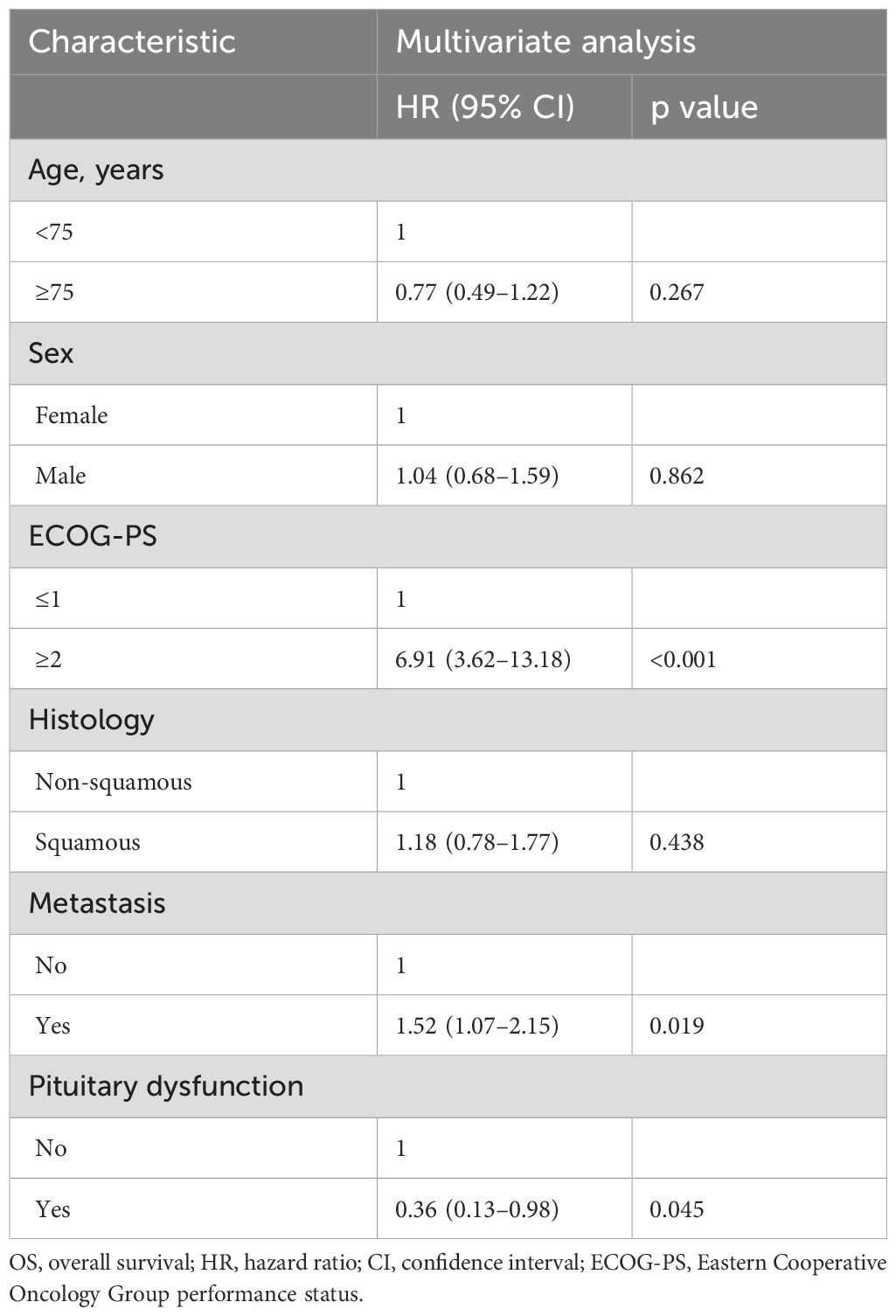
Table 3. Time-dependent multivariate Cox regression analysis of the association between pituitary dysfunction and OS.
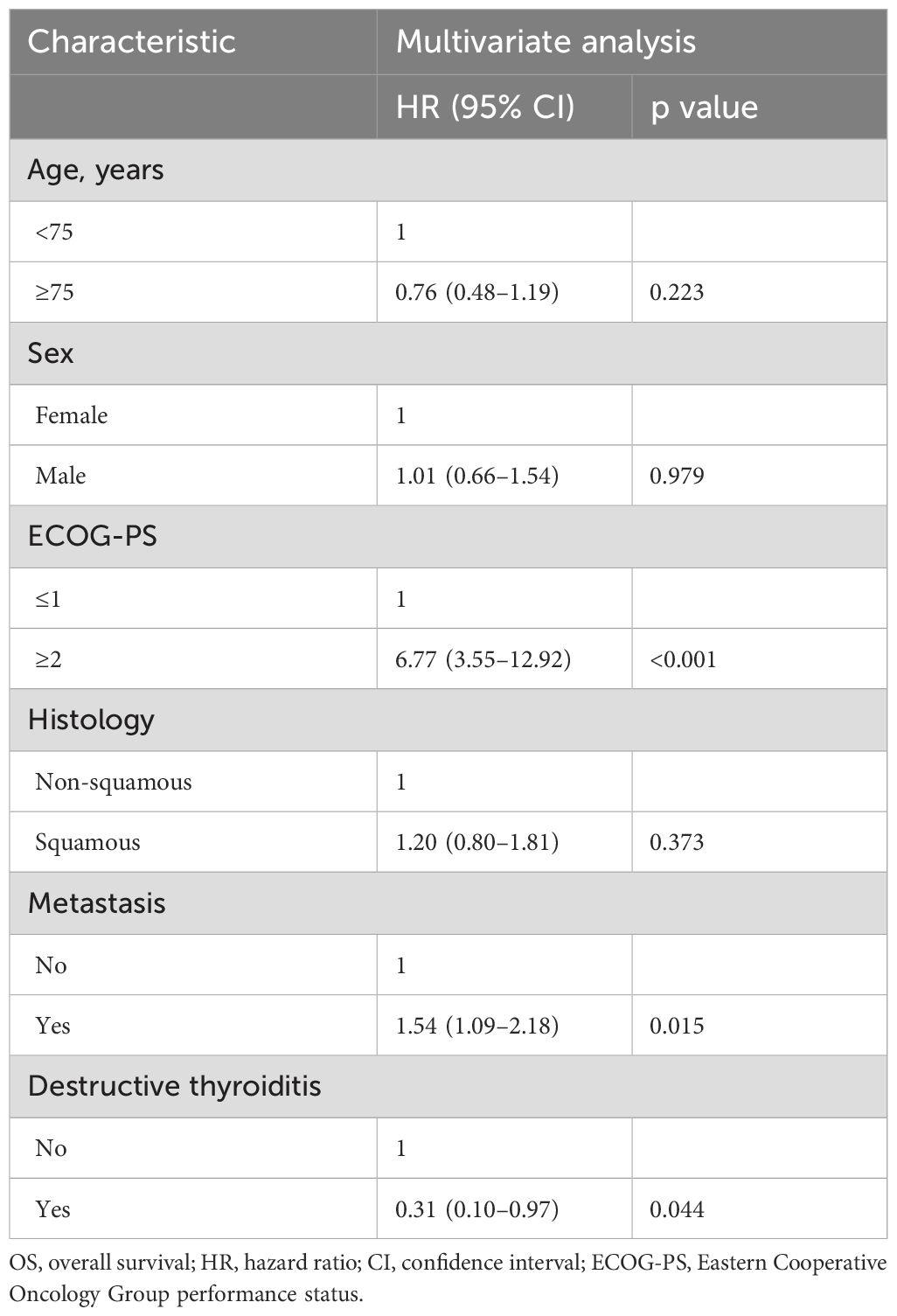
Table 4. Time-dependent multivariate Cox regression analysis of the association between destructive thyroiditis and OS.
Discussion
To our knowledge, this is the first prospective study to demonstrate that pituitary dysfunction and destructive thyroiditis are significantly associated with prolonged OS in NSCLC patients treated with anti-PD-1 antibodies, even after accounting for ITB, in real-world clinical practice. We also confirmed that the occurrences of skin toxicity and overall irAEs were significantly associated with favorable outcomes, consistent with a previous retrospective study (10). Overall irAEs were significantly associated with prolonged OS because of the contribution of three irAEs (pituitary dysfunction, destructive thyroiditis, and skin toxicity) to improved OS. Attending physicians should be aware that patients with any of these three irAEs will have a better prognosis compared with patients without these irAEs.
Although many studies have explored the relationship between the development of irAEs and survival, few have evaluated the association between pituitary dysfunction and OS while taking into account ITB. One retrospective study demonstrated that the development of hypophysitis was associated with improved OS by univariate analysis, but this association disappeared after utilizing multivariate landmark survival analysis (14). Similarly, in another retrospective study, the survival benefit of pituitary dysfunction was not significant after accounting for ITB (15). One possible explanation for the discordant results between those two studies and ours is that pituitary dysfunction could have been overlooked, and the impact of pituitary dysfunction on OS might not have been accurately reflected in the previous retrospective studies. In fact, whereas 5.2% (10/194) of patients developed pituitary dysfunction after the initiation of anti-PD-1 antibodies in this study, only 1.1% (7/656) (14) and 1.2% (7/610) (15) of patients experienced pituitary dysfunction after anti-PD-1 antibodies in the other two studies, respectively.
Thyroid dysfunction is another frequent endocrine irAE. Most thyroid irAEs manifest as destructive thyroiditis with transient thyrotoxicosis, but they can also manifest as isolated hypothyroidism without preceding thyrotoxicosis (12, 16–21). In a previous retrospective study and a prospective study, thyroid dysfunction, including both destructive thyroiditis and isolated hypothyroidism, was correlated with longer OS after addressing ITB (10, 11). However, in our study, while the development of destructive thyroiditis was significantly associated with longer OS, isolated hypothyroidism and overall thyroid dysfunction were not. It is possible that the previous studies overlooked cases of isolated hypothyroidism, which develops significantly later compared with thyrotoxicosis (19). If this were the case, thyroid dysfunction in previous studies might have comprised mainly destructive thyroiditis. Consistent with our findings, Muir et al. reported in a retrospective study that the development of overt thyrotoxicosis, but not hypothyroidism, was associated with both prolonged OS and progression-free survival in patients with malignant melanoma, although their study did not take ITB into account (22). We recently reported greater changes in thyroid autoantibody (anti-thyroglobulin and anti-thyroid peroxidase antibodies) titers from baseline to irAE onset in patients with thyrotoxicosis than in those with isolated hypothyroidism (19). This finding suggests that the thyroid autoimmune response is greater in destructive thyroiditis than in isolated hypothyroidism. Therefore, it is possible that the anti-tumor immune response induced by ICIs may also be stronger in patients with destructive thyroiditis than in those with isolated hypothyroidism.
The reason why the impact on the outcomes varies among the different types of irAEs is still unknown. One hypothesis is that there may be common epitopes between irAE-associated antigens and tumor-associated antigens. Previous studies showed that the development of vitiligo as an irAE was associated with a favorable prognosis in melanoma patients (23). This is explained by activation of cytotoxic T lymphocytes targeting the common antigen between melanoma cells and normal melanocytes (24). Therefore, there might be shared antigens between pituitary or thyroid cells and NSCLC cells. Another possibility is that there might be some common germline genetic variants such as human leukocyte antigen (HLA) genotypes or single nucleotide polymorphisms related to both organ-specific irAEs and ICI efficacy. Recently, Jiang et al. reported that HLA-DRB4 was correlated with both the development of endocrine irAEs and improved OS in NSCLC patients receiving ICIs (25). In addition, the differences in survival impact depending on the type of irAE may be partially attributed to the differential management of irAEs according to the organ involved. ICI therapies are often discontinued and replaced with high-dose systemic corticosteroids in patients with severe non-endocrine irAEs such as hepatic toxicity, gastrointestinal toxicity, and pneumonitis (4, 26, 27). Several studies showed that high-dose corticosteroids can negatively affect the anti-tumor efficacy of ICIs (28, 29). Naqash et al. reported that ICI discontinuation after irAE development can also have a detrimental effect on OS (30). In contrast, patients with endocrine irAEs should not be treated with high-dose systemic corticosteroids and should continue ICIs under hormone replacement therapies according to most guidelines from academic societies (4, 13, 26, 27). Further studies are needed to understand the mechanism underlying the association between organ specific irAEs and ICI efficacy.
There are some limitations to this study. First, this was a single institutional study with a relatively small sample size. Second, it is possible that destructive thyroiditis occurred during the 6-week intervals between the thyroid function tests; the destructive thyroiditis might have gone undetected if the patient quickly progressed to hypothyroidism after preceding thyrotoxicosis. Third, while endocrine irAEs were evaluated prospectively as primary outcomes during scheduled endocrine evaluations during the follow-up period, non-endocrine irAEs were diagnosed clinically by the attending physicians and were not evaluated during the regularly scheduled evaluations in advance, which could lead to underestimation of their incidence. Fourth, the development of pituitary dysfunction may have been masked in patients treated with systemic glucocorticoids until the end of the study period.
Conclusion
This prospective study clarified that the development of the specific irAEs (pituitary dysfunction, destructive thyroiditis, and skin toxicity) was associated with prolonged OS in NSCLC patients treated with anti-PD-1 antibodies even after accounting for ITB, suggesting that these irAEs could be prognostic biomarkers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethical Committee of Nagoya University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KS: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. TK: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. TI: Data curation, Writing – review & editing. KO: Data curation, Writing – review & editing. MA: Formal analysis, Writing – review & editing. THan: Data curation, Writing – review & editing. TM: Data curation, Writing – review & editing. MS: Data curation, Writing – review & editing. TO: Data curation, Writing – review & editing. DH: Data curation, Writing – review & editing. HS: Data curation, Writing – review & editing. RB: Data curation, Writing – review & editing. THas: Data curation, Writing – review & editing. MIn: Data curation, Writing – review & editing. MIs: Data curation, Writing – review & editing. HA: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SI: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We sincerely thank Yuichi Ando (Professor and Director of Department of Clinical Oncology and Chemotherapy, Nagoya University Hospital) for scientific advice on this study.
Conflict of interest
HA received grants from Ono Pharmaceutical Co., Ltd. and MSD K.K., and personal fees from Ono Pharmaceutical Co., Ltd. and MSD K.K. outside of this study. SI received personal fees from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb, and MSD K.K. outside of this study. THas received personal fees from AstraZeneca KK, Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Taiho Pharmaceutical Co. Ltd., MSD K.K., Merck Biopharma Co. Ltd., Pfizer Inc., and Eli Lilly Japan KK, and grants from Novartis Pharma KK, AstraZeneca KK, BeiGene Inc., AbbVie Inc., Amgen Co. Ltd., and Chugai Pharmaceutical Co. Ltd., outside of this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1490042/full#supplementary-material
Abbreviations
irAEs, immune-related adverse events; OS, overall survival; NSCLC, non-small cell lung cancer; ICIs, immune checkpoint inhibitors; ITB, immortal time bias; PD-1, programmed cell death-1; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; TSH, thyroid-stimulating hormone; TRAb, TSH receptor antibody; IQR, interquartile range; HR, hazard ratio; CI, confidence interval; HLA, human leukocyte antigen.
References
1. Iwama S, Kobayashi T, Arima H. Clinical characteristics, management, and potential biomarkers of endocrine dysfunction induced by immune checkpoint inhibitors. Endocrinol Metab (Seoul). (2021) 36:312–21. doi: 10.3803/EnM.2021.1007
2. Iwama S, Kobayashi T, Yasuda Y, Arima H. Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab. (2022) 36:101660. doi: 10.1016/j.beem.2022.101660
3. Kobayashi T, Iwama S, Arima H. Clinical characteristics and potential biomarkers of thyroid and pituitary immune-related adverse events. Endocr J. (2024) 71:23–9. doi: 10.1507/endocrj.EJ23-0524
4. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9:e002435. doi: 10.1136/jitc-2021-002435
5. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. (2018) 4:374–8. doi: 10.1001/jamaoncol.2017.2925
6. Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. (2018) 19:e893–900. doi: 10.1016/j.cllc.2018.08.008
7. Kobayashi T, Iwama S, Yasuda Y, Okada N, Okuji T, Ito M, et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both Malignant melanoma and non-small cell lung carcinoma: a prospective study. J Immunother Cancer. (2020) 8:e000779. doi: 10.1136/jitc-2020-000779
8. Kfoury M, Najean M, Lappara A, Voisin AL, Champiat S, Michot JM, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias. Cancer Treat Rev. (2022) 110:102452. doi: 10.1016/j.ctrv.2022.102452
9. Socinski MA, Jotte RM, Cappuzzo F, Nishio M, Mok TSK, Reck M, et al. Association of immune-related adverse events with efficacy of atezolizumab in patients with non-small cell lung cancer: pooled analyses of the phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials. JAMA Oncol. (2023) 9:527–35. doi: 10.1001/jamaoncol.2022.7711
10. Yu Y, Chen N, Yu S, Shen W, Zhai W, Li H, et al. Association of immune-related adverse events and the efficacy of anti-PD-(L)1 monotherapy in non-small cell lung cancer: adjusting for immortal-time bias. Cancer Res Treat. (2024) 56:751–64. doi: 10.4143/crt.2023.1118
11. Basak EA, van der Meer JWM, Hurkmans DP, Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, et al. Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. (2020) 30:966–73. doi: 10.1089/thy.2019.0726
12. Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: A prospective study. J Endocr Soc. (2018) 2:241–51. doi: 10.1210/js.2017-00432
13. Arima H, Iwama S, Inaba H, Ariyasu H, Makita N, Otsuki M, et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J. (2019) 66:581–6. doi: 10.1507/endocrj.EJ19-0163
14. Kotwal A, Rouleau SG, Dasari S, KottsChade L, Ryder M, Kudva YC, et al. Immune checkpoint inhibitor-induced hypophysitis: lessons learnt from a large cancer cohort. J Investig Med. (2022) 70:939–46. doi: 10.1136/jim-2021-002099
15. Johnson J, Goldner W, Abdallah D, Qiu F, Ganti AK, Kotwal A. Hypophysitis and secondary adrenal insufficiency from immune checkpoint inhibitors: diagnostic challenges and link with survival. J Natl Compr Canc Netw. (2023) 21:281–7. doi: 10.6004/jnccn.2022.7098
16. Okada N, Iwama S, Okuji T, Kobayashi T, Yasuda Y, Wada E, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. (2020) 122:771–7. doi: 10.1038/s41416-020-0736-7
17. Iwama S, Kobayashi T, Yasuda Y, Okuji T, Ito M, Ando M, et al. Increased risk of thyroid dysfunction by PD-1 and CTLA-4 blockade in patients without thyroid autoantibodies at baseline. J Clin Endocrinol Metab. (2022) 107:e1620–e30. doi: 10.1210/clinem/dgab829
18. Zhou X, Iwama S, Kobayashi T, Ando M, Arima H. Risk of thyroid dysfunction in PD-1 blockade is stratified by the pattern of TgAb and TPOAb positivity at baseline. J Clin Endocrinol Metab. (2023) 108:e1056–e62. doi: 10.1210/clinem/dgad231
19. Yamagami A, Iwama S, Kobayashi T, Zhou X, Yasuda Y, Okuji T, et al. Changes in TgAb and TPOAb titers are greater in thyrotoxicosis than isolated hypothyroidism induced by PD-1 blockade. Endocr J. (2024) 71:515–26. doi: 10.1507/endocrj.EJ23-0480
20. Kobayashi T, Iwama S, Yamagami A, Yasuda Y, Okuji T, Ito M, et al. Elevated TSH level, TgAb, and prior use of ramucirumab or TKIs as risk factors for thyroid dysfunction in PD-L1 blockade. J Clin Endocrinol Metab. (2022) 107:e4115–e23. doi: 10.1210/clinem/dgac467
21. Kobayashi T, Iwama S, Yamagami A, Izuchi T, Suzuki K, Otake K, et al. Combined use of tyrosine kinase inhibitors with PD-(L)1 blockade increased the risk of thyroid dysfunction in PD-(L)1 blockade: a prospective study. Cancer Immunol Immunother. (2024) 73:146. doi: 10.1007/s00262-024-03733-2
22. Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. (2021) 106:e3704–e13. doi: 10.1210/clinem/dgab263
23. Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. (2015) 33:773–81. doi: 10.1200/JCO.2014.57.4756
24. Kubo T, Hirohashi Y, Tsukahara T, Kanaseki T, Murata K, Morita R, et al. Immunopathological basis of immune-related adverse events induced by immune checkpoint blockade therapy. Immunol Med. (2022) 45:108–18. doi: 10.1080/25785826.2021.1976942
25. Jiang CY, Zhao L, Green MD, Ravishankar S, Towlerton AMH, Scott AJ, et al. Class II HLA-DRB4 is a predictive biomarker for survival following immunotherapy in metastatic non-small cell lung cancer. Sci Rep. (2024) 14:345. doi: 10.1038/s41598-023-48546-y
26. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
27. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
28. Verheijden RJ, van Eijs MJM, May AM, van Wijk F, Suijkerbuijk KPM. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. NPJ Precis Oncol. (2023) 7:41. doi: 10.1038/s41698-023-00380-1
29. Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. (2015) 21:749–55. doi: 10.1158/1078-0432.CCR-14-2353
30. Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. (2020) 69:1177–87. doi: 10.1007/s00262-020-02536-5
Keywords: pituitary, destructive thyroiditis, PD-1, immune checkpoint inhibitors, immune-related adverse events
Citation: Suzuki K, Kobayashi T, Izuchi T, Otake K, Ando M, Handa T, Miyata T, Sugiyama M, Onoue T, Hagiwara D, Suga H, Banno R, Hase T, Inoue M, Ishii M, Arima H and Iwama S (2024) Development of pituitary dysfunction and destructive thyroiditis is associated with better survival in non-small cell lung cancer patients treated with programmed cell death-1 inhibitors: a prospective study with immortal time bias correction. Front. Endocrinol. 15:1490042. doi: 10.3389/fendo.2024.1490042
Received: 02 September 2024; Accepted: 14 October 2024;
Published: 07 November 2024.
Edited by:
Hidenori Fukuoka, Kobe University, JapanReviewed by:
Shinobu Takayasu, Hirosaki University, JapanCheol Ryong Ku, Yonsei University, Republic of Korea
Copyright © 2024 Suzuki, Kobayashi, Izuchi, Otake, Ando, Handa, Miyata, Sugiyama, Onoue, Hagiwara, Suga, Banno, Hase, Inoue, Ishii, Arima and Iwama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shintaro Iwama, aXdhbWEuc2hpbnRhcm8ubTFAZi5tYWlsLm5hZ295YS11LmFjLmpw; Hiroshi Arima, YXJpbWEuaGlyb3NoaS50NkBmLm1haWwubmFnb3lhLXUuYWMuanA=; Tomoko Kobayashi, a29iYXlhc2hpLnRvbW9rby5qMkBmLm1haWwubmFnb3lhLXUuYWMuanA=
†ORCID: Tomoko Kobayashi, orcid.org/0000-0003-3151-1287
Hiroshi Arima, orcid.org/0000-0003-3746-1997
Shintaro Iwama, orcid.org/0000-0002-3281-0337
 Koji Suzuki
Koji Suzuki Tomoko Kobayashi1*†
Tomoko Kobayashi1*† Megumi Inoue
Megumi Inoue Makoto Ishii
Makoto Ishii Shintaro Iwama
Shintaro Iwama