- 1Clinical Research Center for Reproduction and Genetics in Hunan Province, Reproductive & Genetic Hospital of CITIC-Xiangya, Changsha, China
- 2College of Information Science and Engineering, Hunan University, Changsha, China
- 3NHC Key Laboratory of Human Stem Cell and Reproductive Engineering, School of Basic Medical Sciences, Central South University, Hunan University, Changsha, China
Objective: To clarify whether intrauterine adhesions (IUAs) affect endometrial receptivity (ER) on the day of ovulation and to compare patients with mild and moderate-severe adhesions.
Methods: This prospective cohort study included 592 infertile women with IUAs who underwent frozen-thawed embryo transfer (FET). Patients were divided into groups with or without IUAs; and pregnant and nonpregnant populations based on whether a clinical pregnancy was achieved. The ultrasound ER parameters on the ovulation day were compared. Patients with IUAs were then divided into mild or moderate-severe IUA subgroups according to IUA degree.
Results: The proportions of patients with Type B plus Type C endometrial morphology (94% vs. 75%, P<0.001), an endometrial thickness≥8mm (97% vs. 81%, P<0.001), an endometrial volume≥2ml (94% vs. 67%, P<0.001), a frequency of endometrial peristalsis≥2 times/min (84% vs. 53%, P<0.001), low subendometrial volume (11.54 ± 2.94 vs. 9.57 ± 2.35, P<0.001) and subendometrial vascularization flow index (VFI) values (2.70 ± 3.10 vs. 2.23 ± 2.23, P=0.033) and a low live birth rate (65% vs. 56%, P=0.039) were significantly higher in the group without IUAs than in the group with IUAs. The group with moderate-severe IUAs had lower proportion of patients with an endometrial thickness≥8mm (73% vs. 89%, P=0.008) and an endometrial volume ≥2ml (58% vs. 78%, P=0.005), a lower frequency of endometrial peristalsis≥2 times/min (42% vs. 65%, P=0.003), and low subendometrial volume (9.22 ± 2.29 vs. 9.97 ± 2.36, P=0.023) and subendometrial flow index (FI) (31.48 ± 3.64 vs. 33.43 ± 4.17, P=0.002) values than the group with mild IUAs; a high antral follicle count (AFC), basal follicle-stimulating hormone (FSH), and anti-Müllerian hormone (AMH) levels and an endometrial thickness≥8mm were independent predictors of clinical pregnancy.
Conclusion: IUAs can affect ER on the ovulation day and the live birth rate during natural cycles. Moderate-severe IUAs have a greater impact on ER than mild adhesions do; however, if these adhesions are treated properly, they do not have adverse effects on the clinical pregnancy rate. A high AFC, basal FSH and AMH levels and an endometrial thickness ≥8 mm were found to be independent predictors of clinical pregnancy.
Introduction
Intrauterine adhesions (IUAs) are considered one of the main reproductive system diseases in women worldwide and are characterized by endometrial fibrosis with partial to complete obliteration of the uterine cavity and/or cervical canal (1, 2). The reported prevalence of IUAs varies between 0.3% and 21.5% (3). Any event that causes damage to the endometrium may lead to the development of IUAs, resulting in menstrual disturbances, infertility and (recurrent) pregnancy loss (4).
Transvaginal sonography (TVS) has been adopted as a routine method to assess endometrial receptivity (ER) (5). Hysteroscopic adhesiolysis is the standard treatment for removing IUAs, restoring the uterine architecture and facilitating communication among the uterine cavity, cervical canal and fallopian tubes to allow both normal menstrual flow and adequate sperm transportation (6). While B Pecorino et al. (7) reported that endometrial scratching in the patients with repeated implantation failure had no significant improvement in implantation and clinical pregnancy rates.
The relationship between IUAs and reproductive performance has been frequently described in the literature; IUAs, especially moderate and severe IUAs, may strongly impact fertility, predisposing individuals to pregnancy disorders and obstetric complications in subsequent pregnancies (8–10). Is this because IUAs have a negative impact on ER? The aim of the present study was to clarify whether IUAs affect ER on the day of ovulation and to further compare patients with mild and moderate–severe adhesions. To our knowledge, this is the first prospective study comparing ER on the day of ovulation during natural cycles between patients with and without a history of adhesions.
Materials and methods
Study design and participants
This prospective cohort study was conducted at the Reproductive and Genetic Hospital of CITIC-Xiangya from March 2019 to September 2023, and 592 infertile women (417 with a history of IUAs and 175 without no IUAs) who underwent frozen-thawed embryo transfer (FET) were included. Written informed consent was obtained from all participants. The institutional review board approved this study (date of approval: 11 September 2019; reference number: LL-SC-2019-023; Changsha, China).
The inclusion criteria for patients with a history of IUAs were as follows (1): underwent FET after adhesiolysis (2); underwent a natural FET cycle (3); aged 20–35 years (4); had a body mass index of 18–24 kg/m2; and (5) had at least 1 high-quality embryo. The exclusion criteria were as follows (1): endometriosis, adenomyosis, or adenomyoma (2); endometritis (3); congenital uterine malformations (4); untreated hydrosalpinx; or (5) uterine cavity fluid (diameter ≥2 mm) caused by caesarean section diverticulum.
The inclusion criteria for the control group of patients with no IUAs were as follows (1): underwent FET (2); underwent a natural FET cycle (3); age 20-35 years (4); body mass index 18-24 kg/m2 (5) had at least 1 high-quality embryo. The exclusion criteria were (1): endometriosis, adenomyosis, or adenomyoma (2); intrauterine adhesion or endometritis (3);congenital uterine malformations (4); untreated hydrosalpinx; or (5) uterine cavity fluid caused by cesarean section diverticulum and diameter ≥2 mm.
Adhesiolysis
IUAs were graded on the basis of the American Fertility Society classification (11): mild IUA was indicated by 1-4, moderate was indicated by 5-8, and severe was indicated by 9-12. All the included patients underwent adhesiolysis in our center before the in vitro fertilization (IVF) procedure, which was performed by an experienced surgeon under general anesthesia. The adhesions were dissected by using bipolar energy (Olympus) and/or hysteroscopic scissors (Karl Storz, Tuttlingen, Germany) until the uterine cavity was achieved to restore uterine morphology. Then, based on patients’ uterine width, a heart-shaped intrauterine balloon (Cook Medical, Bloomington, IN, USA) or Foley catheter was inserted into the uterine cavity, and all patients received crosslinked hyaluronan gel (MateRegen; BioRegen Biomedical, Changzhou, China) to prevent adhesion reformation.
In vitro fertilization procedure
Depending on the cause of infertility, IVF or intracytoplasmic sperm injection (ICSI) was applied for fertilization. In this study, all patients underwent FET during natural cycles. Thawed cleavage-stage embryos and blastocysts were transferred on the 3rd day and the 5th day after ovulation. Embryo morphology was scored on the basis of the Alpha Scientists in Reproductive Medicine and European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group of Embryology (ASEBIR) consensus (12). A maximum of two embryos were transferred.
Ultrasound evaluation
Ultrasound diagnosis of IUAs
GE VOLUSON E8 ultrasound instrument (GE VOLUSON, E8, General Electric Tech Co., Ltd., New York, USA) equipped with a 5–9 MHz transvaginal three-dimensional (3D) probe was used for the preoperative evaluation of uterine cavity. 2D ultrasound was used routinely as a first-line diagnostic tool for the assessment of the endometrial integrity to look for disruptions of the endometrial–myometrial junction. Adhesions are seen as bands of myometrial tissue traversing the endometrial cavity and adjoining the opposing uterine walls. For suspected patients, 3D ultrasound was used to further clarify adhesions. During 3D TVS, the morphological characteristics suggesting IUA were classified into six categories: marginal irregularity, thinning (< 2 mm), defect, obliteration, fibrosis, or calcification (13, 14).
Assessment of ER
TVS was performed on the day of ovulation for all included patients to evaluate ER. Ultrasound ER parameters, including endometrial thickness, morphology, volume, movement and blood perfusion, were assessed by the same senior ultrasonic doctor (Dr. Li) using the same ultrasound machine.
The maximum diameter of the endometrium was measured in the longitudinal plane. The Gonen classification criteria (15) were adopted to classify endometrial morphology: Type A: the increase in reflectivity leads to a completely homogeneous, hyperechoic endometrium, with the central echo line not visible; Type B: the endometrium has the same reflectivity compared to the surrounding myometrium, and the central echo line is not obvious or missing; and Type C: a “triple-line” endometrium is present, consisting of a prominent outer and central hyperechogenic line and inner hypoechogenic or black regions.
The movement of the endometrium was observed and recorded within 3 minutes and was divided into 5 types according to Ljland et al. (16) (1): positive wave: the peristaltic wave from the cervix to fundus (2); negative wave: the peristaltic wave from the fundus to the cervix (3); static wave: the endometrium is in a static state (4); bidirectional wave: the endometrium of both the uterine fundus and cervix contract simultaneously; and (5) random wave: irregular motion types with an uncertain direction or multiple starting points.
Endometrial blood perfusion was evaluated based on the Applebaum classification standard (17): I: vessels penetrate the outer hypoechoic area around the endometrium but do not enter the outer edge of the hyperechoic area; II: vessels penetrate the outer edge of the endometrium with high echogenicity but do not enter the internal area of low echogenicity; and III: vessels enter the hypoechogenic inner area of the endometrium.
The ultrasound machine was switched to 3D mode with power Doppler. Virtual organ computer-aided analysis (VOCAL) software was used to outline the endometrium, and the endometrial volume, endometrial vascularization index (VI), vascularization flow index (VFI) and flow index (FI) were obtained (18, 19).
Outcome measures
Serum human chorionic gonadotropin (HCG) levels were measured 14 days (12 days after blastocyst transfer), and TVS was performed 4 weeks after transfer. The primary outcome was live birth, defined as the complete expulsion or extraction of a product of fertilization after 20 completed weeks of gestation that, after separation from the woman, breathed or showed other evidence of life, irrespective of whether the umbilical cord had been cut or the placenta was attached. The secondary outcome was clinical pregnancy, which was confirmed if a gestational sac was observed, and a viable pregnancy was diagnosed when fetal cardiac activity was detected (20).
The included patients were divided into groups according to the presence or absence of IUAs: the group with IUAs and the group without IUAs. All enrolled patients were further divided into two groups according to whether a clinical pregnancy was achieved: the pregnant group and the nonpregnant group. Patients with IUAs were then divided into mild or moderate-severe IUA subgroups according to the degree of IUAs. The ER parameters on the day of ovulation were compared between these groups.
Statistical analysis
The distribution of patient demographics was analyzed via the Kolmogorov–Smirnov test. Continuous variables are expressed as means ± standard deviations (SDs). Categorical variables are described as frequencies and percentages. The Mann−Whitney U test or Student’s t test was used to assess continuous variables, and the chi-square test or Fisher’s exact test was used to assess differences in categorical variables between the pregnant group and the nonpregnant group. Univariate and multivariate logistic regression analyses were used to identify independent predictors of clinical pregnancy; odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All the statistical analyses were performed via R software (version 4.3.2), and a two-sided p value < 0.05 was considered statistically significant.
Results
Characteristics and ultrasound parameters on the day of ovulation for patients without IUAs
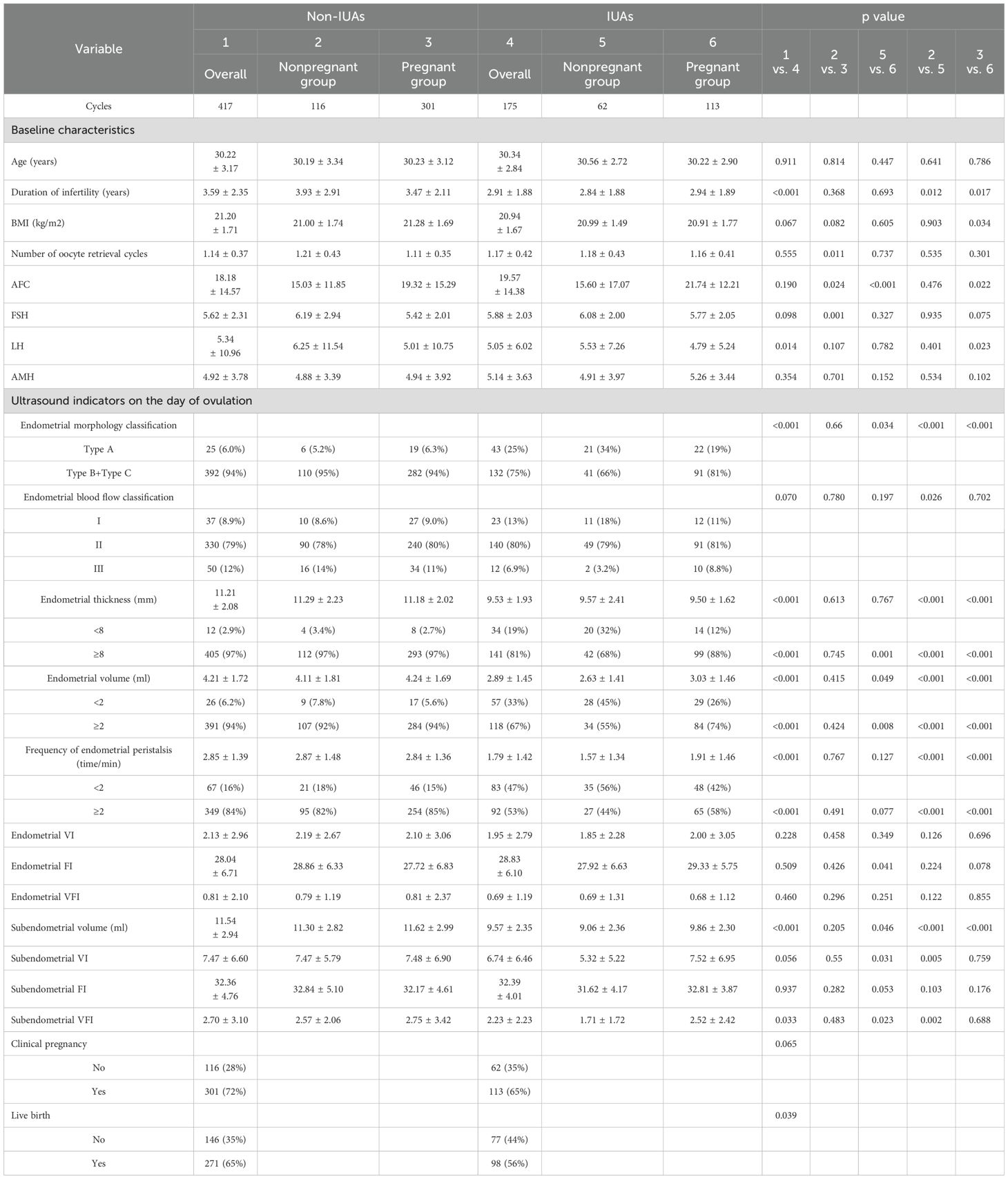
During the study period, there were a total of 191 patients with a history of IUAs, after excluding 10 patients who were dropped and 6 patients of lost to follow up, 175 patients with previous IUAs (93 patients had moderate–severe IUAs, and 82 patients had mild IUAs) were included; A total of 447 patients who did not have a history of IUAs were enrolled, after excluding 19 patients who were dropped and 11 patients of lost to follow up, 417 patients were ultimately included as the control group. The overall clinical pregnancy rates were 69.9% (414/592) and 72.2% (301/417) for patients without IUAs, 64.6% (113/175) for patients with a history of IUAs [63.4% (59/93) for moderate-severe IUAs, 65.9% (54/82) for mild IUAs]. The basic, clinical and endometrial ultrasound characteristics of the overall group, pregnant group and nonpregnant group of patients without or with IUAs are displayed in Table 1.
Patients in the nonpregnant group had greater numbers of oocytes retrieved (1.21 ± 0.43 vs. 1.11 ± 0.35, P=0.011), higher basal follicle-stimulating hormone (FSH, 6.19 ± 2.94 vs. 5.42 ± 2.01, P=0.001) levels and lower antral follicle counts (AFCs, 15.03 ± 11.85 vs. 19.32 ± 15.29, P=0.024) than did those in the pregnant group. There were no significant differences in any endometrial or subendometrial ultrasound indicators on the day of ovulation between the nonpregnant group and the pregnant group.
Characteristics and ultrasound parameters on the day of ovulation for patients with IUAs
For patients with IUAs, the nonpregnant group had a lower AFC (15.60 ± 17.07 vs. 21.74 ± 12.21, P<0.001), lower proportions of patients with Type B plus Type C endometrial morphology (66% (41/62) vs. 81% (91/113), P=0.034), an endometrial thickness ≥8 mm (68% (42/62) vs. 88% (99/113), P=0.001) and an endometrial volume ≥2 ml (55% (34/62) vs. 74% (84/113), P=0.008), and lower endometrial FI (27.92 ± 6.63 vs. 29.33 ± 5.75, P=0.041), subendometrial volume (9.06 ± 2.36 vs. 9.86 ± 2.30, P=0.046), subendometrial VI (5.32 ± 5.22 vs. 7.52 ± 6.95, P=0.031), and subendometrial VFI (1.71 ± 1.72 vs. 2.52 ± 2.42, P=0.023) values than those in the pregnant group. No significant differences in other ultrasound indicators were found.
Characteristics and ultrasound parameters on the day of ovulation between patients with and without IUAs
Patients without IUAs generally had a longer duration of infertility (3.59 ± 2.35 years vs. 2.91 ± 1.88 years, P<0.001) and higher basal luteinizing hormone (LH) levels (5.34 ± 10.96 vs. 5.05 ± 6.02, P=0.014) than those with IUAs did; other basic characteristics, such as female age, BMI, number of oocyte retrieval cycles, AFC, FSH level, and anti-Müllerian hormone (AMH) level, were not significantly different between the two groups (P>0.05). In the comparison of the ER ultrasound parameters between the two groups, the proportions of patients with Type B plus Type C endometrial morphology (94% (392/417) vs. 75% (132/175), P<0.001), an endometrial thickness ≥8 mm (97% (405/417) vs. 81% (141/175), P<0.001), an endometrial volume ≥2 ml (94% (391/417) vs. 67% (118/175), P<0.001), a frequency of endometrial peristalsis ≥2 times/min (84% (349/417) vs. 53% (92/175), P<0.001), low subendometrial volume (11.54 ± 2.94 vs. 9.57 ± 2.35, P<0.001) and subendometrial VFI (2.70 ± 3.10 vs. 2.23 ± 2.23, P=0.033) values on the day of ovulation and a low live birth rate (65% (271/417) vs. 56% (98/175), P=0.039) were significantly higher in the group without IUAs than that in the group with IUAs.
For nonpregnant patients, the group without IUAs had a longer duration of infertility, a greater proportion of patients with Type B plus Type C endometrial morphology, greater proportions of patients with an endometrial thickness ≥8 mm, an endometrial volume ≥2 ml, a frequency of endometrial peristalsis ≥2 times/min, a subendometrial volume, a greater proportion of patients with type III endometrial blood flow, and greater subendometrial VFI and VI values than did the group with IUAs.
Compared with patients with IUAs, patients without IUAs also had a longer duration of infertility, a greater BMI, a greater basal LH level, a greater likelihood of having Type B plus Type C endometrial morphology, an endometrial thickness ≥8 mm, and an endometrial volume ≥2 ml, a greater frequency of endometrial peristalsis ≥2 times/min, a greater subendometrial volume, and a lower AFC.
Characteristics and ultrasound parameters on the day of ovulation between patients with moderate-severe IUAs and patients with mild IUAs
There were no significant differences in any basic characteristics between patients with moderate-severe IUAs and patients with mild IUAs, but patients with moderate-severe IUAs had a lower likelihood of having an endometrial thickness ≥8 mm (73% (68/93) vs. 89% (73/82), P=0.008) and an endometrial volume ≥2 ml (58% (54/93) vs. 78% (64/82), P=0.005), a lower frequency of endometrial peristalsis ≥2 times/min (42% (39/93) vs. 65% (53/82), P=0.003), and lower subendometrial volume (9.22 ± 2.29 vs. 9.97 ± 2.36, P=0.023) and a subendometrial FI (31.48 ± 3.64 vs. 33.43 ± 4.17, P=0.002) values than did patients with mild IUAs (Table 2).
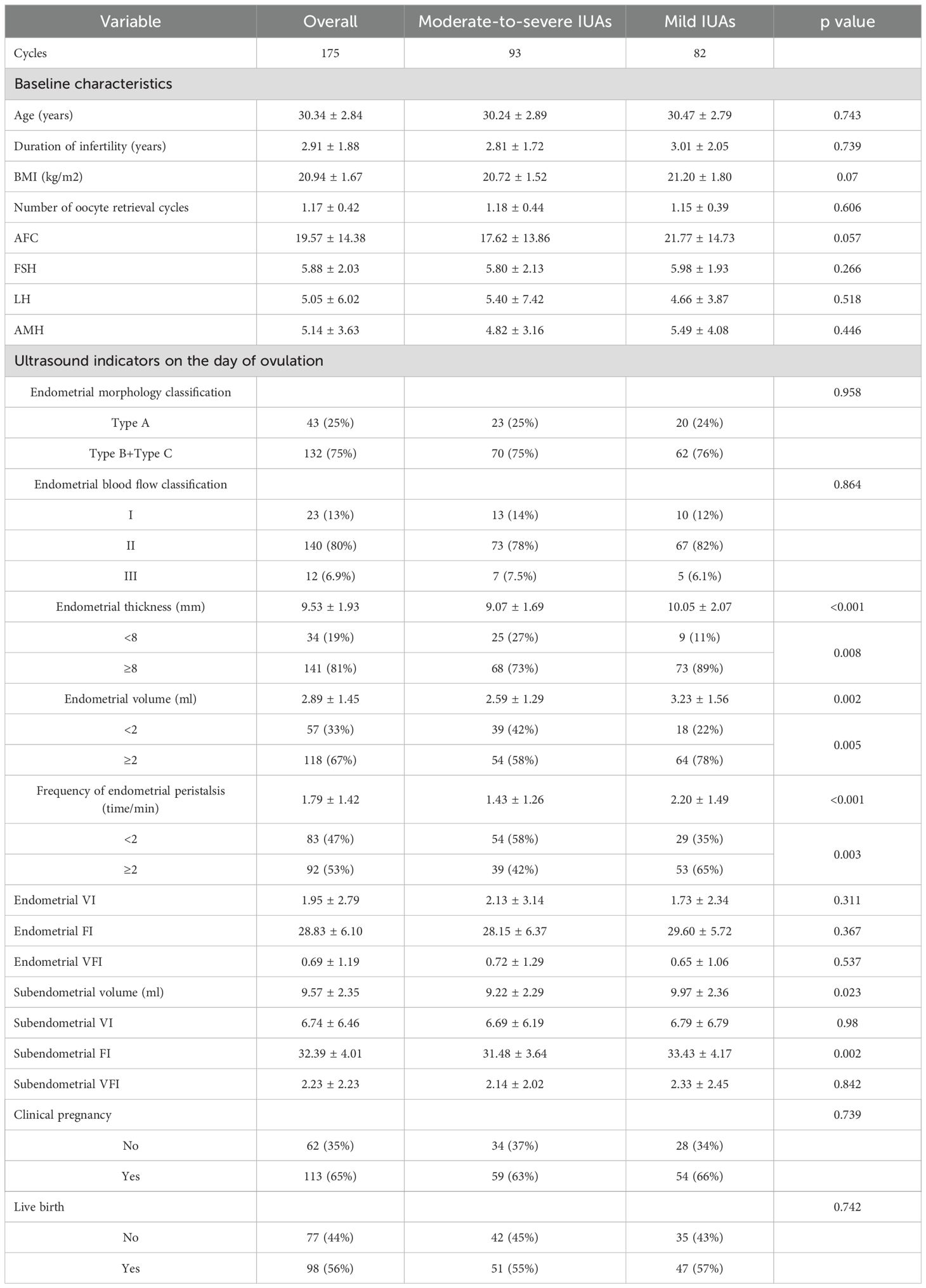
Table 2. Comparisons of characteristics and ultrasound parameters on the day of ovulation between the mild- and moderate-severe IUA groups.
Univariate and multivariate logistic regression analyses
Univariate and multivariate logistic regression analyses were used to obtain the ORs and 95% CIs of the independent risk factors contributing to clinical pregnancy. The results revealed that a high AFC, basal FSH level, and AMH level and an endometrial thickness ≥8 mm were independent predictors of clinical pregnancy after FET. Protective factors for clinical pregnancy after FET cycles include a greater AFC (aOR, 1.04; 95% CI, 1.02–1.06; P<0.001) and an endometrial thickness ≥8 mm on the day of ovulation (aOR, 2.31; 95% CI, 1.09–4.93; P=0.029); risk factors for clinical pregnancy after FET cycles include a greater basal FSH level (aOR, 0.90; 95% CI, 0.81–0.99; P=0.038) and a greater AMH level (aOR, 0.94; 95% CI, 0.88–1.00; P=0.044); and other endometrial and subendometrial ultrasound indicators on the day of ovulation and the presence of uterine adhesions do not affect the clinical pregnancy rate (Table 3).
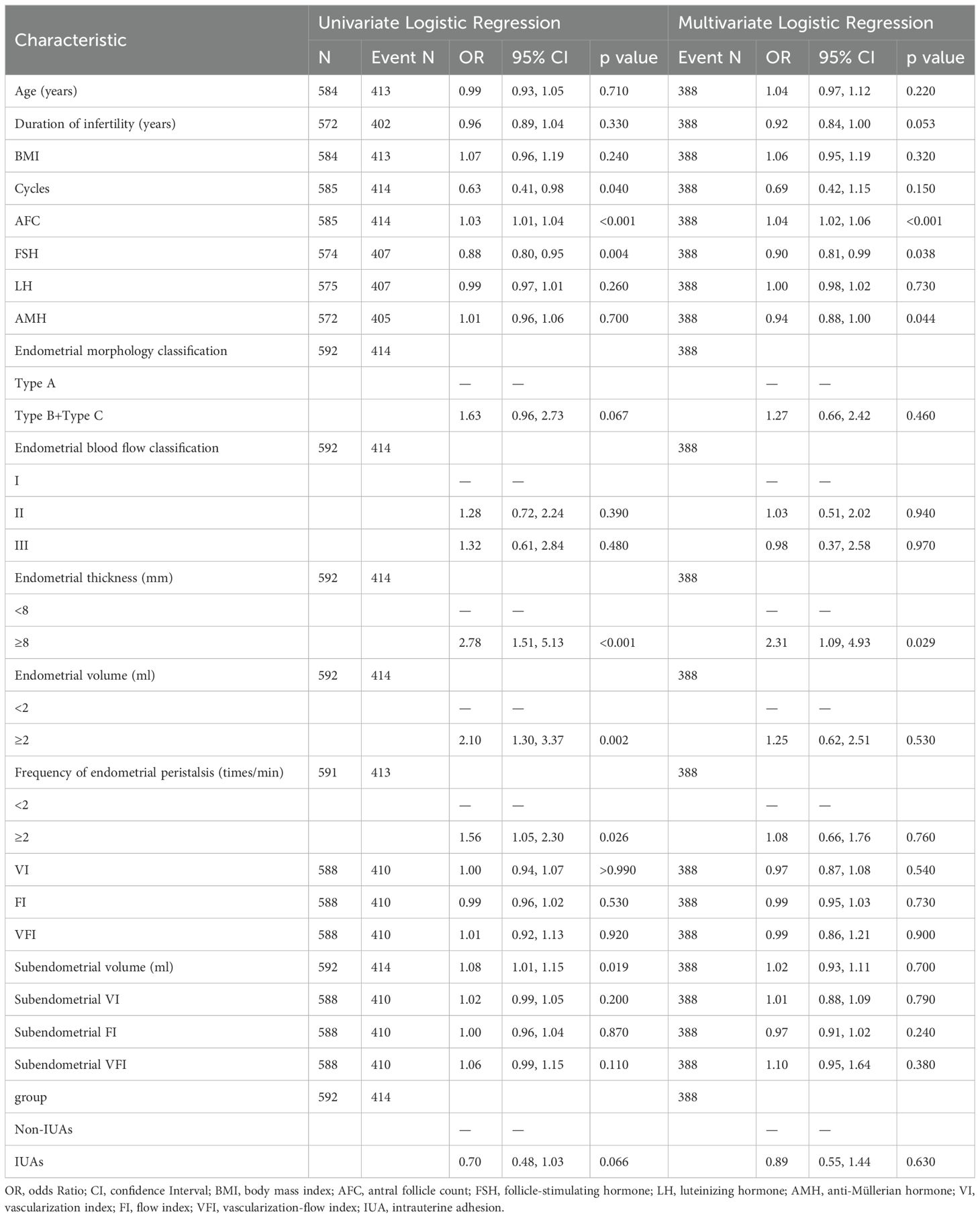
Table 3. Univariate and multivariate logistic regression analyses of risk factors contributing to clinical pregnancy.
Discussion
In this study, we compared ER on the day of ovulation between patients with and without a history of IUAs and reported that the live birth rate was significantly higher in patients without IUAs than in patients with IUAs. Compared with patients without IUAs, patients with IUAs (regardless of pregnancy status) had a thinner endometrium, a lower likelihood of having Type B+C endometrial morphology, a smaller volume, a lower frequency of endometrial peristalsis, a smaller subendometrial volume, and less subendometrial blood perfusion. However, there was no significant difference in the live birth rate between patients with mild and moderate–severe adhesions. Compared with patients with moderate–severe adhesions, those with mild adhesions had greater endometrial thickness and volume, peristaltic frequency, subendometrial volume and subendometrial blood perfusion. A high AFC and an endometrial thickness ≥8 mm are protective factors for clinical pregnancy, while high levels of FSH and AMH are risk factors for clinical pregnancy.
IUA development is multifactorial with multiple predisposing and causal factors, which often cause subfertility or infertility (21). Currently, hysteroscopic adhesiolysis is widely considered the gold standard for the diagnosis and treatment of IUAs (22). TVS is a first-line diagnostic method for IUAs and a noninvasive modality for analyzing ER (23).
In this study, IUAs affected the endometrial thickness, volume, morphology, subendometrial blood perfusion and endometrial peristalsis. Some previous studies have suggested that the “triple line sign” on the day of ovulation is related to clinical pregnancy and that adhesion disrupts the morphology of the endometrium (24, 25); thus, the proportion of patients with the triple line sign also decreases significantly (Types B+C). The live birth rate of patients with IUAs was lower, which was consistent with the results of previous studies (26, 27), but there was no significant difference in the clinical pregnancy rates, indicating that IUAs may have little effect on embryo implantation but may impair the ability to tolerate pregnancy in the long term.
Notably, there was no significant difference in the ultrasound parameters of patients without IUAs, regardless of pregnancy status. Among patients with IUAs, pregnant patients had significantly better endometrial ultrasound indicators (thickness, volume, blood perfusion) than nonpregnant patients did. However, there was no significant difference in peristaltic waves between pregnant and nonpregnant patients with adhesions, which is likely due to adhesion affecting the flexibility of endometrial peristalsis. However, we observed that there was no significant difference in endometrial blood perfusion between patients with adhesions and those without adhesions. This may be because, in this study, relatively few patients had moderate–severe adhesions, and most of these patients had moderate adhesions; thus, there was no significant difference in blood perfusion between patients with mild and moderate–severe adhesions.
Studies generally suggest that moderate to severe IUAs have a greater impact on pregnancy outcomes (27, 28). Although the ER indicators of moderate–severe adhesions were better than those of mild adhesions in this study, there was no significant difference in the live birth rate or clinical pregnancy rate between these two groups. This may be related to the high proportion of moderate adhesions, or it may indicate that patients with adhesions can achieve satisfactory pregnancy outcomes with proper treatment before transfer.
When ovarian reserve function and endometrial development are good, the probability of clinical pregnancy is relatively high. Similarly, decreased ovarian function (elevated FSH) is a risk factor for clinical pregnancy, which is consistent with previous research results (29, 30). Notably, the results of this study indicate that a high AMH level is a risk factor for clinical pregnancy.
The present work has several significant strengths. First, this was the first prospective study comparing ER on the day of ovulation during natural cycles between patients with and without a history of IUAs. Second, the degree of influence of mild and moderate–severe adhesions was also analyzed. However, several limitations remain. First, this study analyzed only the ER status of the endometrium on the day of ovulation in natural FET cycles, and the impact of adhesions on other populations and nonovulation days remains to be verified. Second, among patients with moderate-severe IUAs, the proportion of those with severe adhesions is relatively small, which may have a certain impact on the observation results. Third, no comparison of ER was made preadhesiolysis and postadhesiolysis.
In conclusion, IUAs can affect ER on the day of ovulation and the live birth rate during natural cycles. Moderate–severe IUAs have a greater impact on ER than mild IUAs do; however, if these adhesions are treated properly, they do not have adverse effects on the clinical pregnancy rate. A high AFC and an endometrial thickness ≥8 mm are protective factors for clinical pregnancy, whereas high FSH and AMH levels are risk factors for clinical pregnancy. However, the conclusions and ideas of such reviews need to be validated in other populations, as well as in studies with larger sample sizes and longer follow-up periods.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YO: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. YP: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MZ: Data curation, Writing – review & editing. YM: Data curation, Writing – review & editing. FG: Data curation, Funding acquisition, Writing – review & editing. YL: Data curation, Writing – review & editing. HC: Data curation, Writing – review & editing. XL: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grant from the National Key Research and Development Program of China (2022YFC2702500), the Research Grant of CITIC-Xiangya (No. YNXM-201908), and the Hunan Provincial Grant for Innovative Province Construction (2019SK4012).
Acknowledgments
The authors would like to thank the staff at the Reproductive Center and Imaging Department of the Reproductive and Genetic Hospital of CITIC-Xiangya for the data collection in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bosteels J, Weyers S, Mol BWJ, D’Hooghe T. Anti-adhesion barrier gels following operative hysteroscopy for treating female infertility: A systematic review and meta-analysis. Gynecological Surg. (2014) 11(2):113–27. doi: 10.1007/s10397-014-0832-x
2. Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minimally Invasive Gynecology. (2014) 21(1):44–54. doi: 10.1016/j.jmig.2013.07.018
3. Schenker JG, Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertility Sterility. (1982) 37(5):593–610. doi: 10.1016/s0015-0282(16)46268-0
4. Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome-one century later. Fertility Sterility. (2008) 89(4):759–79. doi: 10.1016/j.fertnstert.2008.02.096
5. Meirzon D, Jaffa AJ, Gordon Z, Elad D. A new method for analysis of non-pregnant uterine peristalsis using transvaginal ultrasound. Ultrasound Obstet Gynecol. (2011) 38(2):217–24. doi: 10.1002/uog.8950
6. Abbott JA, Munro MG, Singh SS, Scheib S, Jackson TR, Jansen F, et al. AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE). Gynecol Surg. (2017) 14(1):6. doi: 10.1186/s10397-017-1007-3
7. Pecorino B, Scibilia G, Rapisarda F, Borzì P, Scollo P. Evaluation of implantation and clinical pregnancy rates after endometrial scratching in women with recurrent implantation failure. . Ital J Gynaecology Obstetrics. (2018).
8. Myers EM, Hurst BS. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril. (2012) 97(1):160–4. doi: 10.1016/j.fertnstert.2011.10.036
9. Cai H, Qiao L, Song KJ, He Y. Oxidized, regenerated cellulose adhesion barrier plus intrauterine device prevents recurrence after adhesiolysis for moderate to severe intrauterine adhesions. J Minim Invasive Gynecol. (2017) 24(1):80–8. doi: 10.1016/j.jmig.2016.09.021
10. Amer MI, Abd-El-Maeboud KHI, Abdelfatah I, Salama FA, Abdallah AS. Human amnion as a temporary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. J Minim Invasive Gynecol. (2010) 17(5):605–11. doi: 10.1016/j.jmig.2010.03.019
11. Buttram VC Jr, Siegler A, DeCherney A, Gibbons W, March CGV, American Fertility Society. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil Steril. (1988) 49:944–55. doi: 10.1016/s0015-0282(16)59942-7
12. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum Reprod. (2011) 22(6):632–46. doi: 10.1016/j.rbmo.2011.02.001
13. Burjoo A, Zhao XP, Zou LX, Liu XY, Lei L, Zhang BY, et al. The role of preoperative 3D-ultrasound in intraoperative judgement for hysteroscopic adhesiolysis. Ann Transl Med. (2020) 8(4):55. doi: 10.21037/atm.2020.01.06
14. Amin TN, Saridogan E, Jurkovic D. Ultrasound and intrauterine adhesions: a novel structured approach to diagnosis and management. Ultrasound Obstet Gynecol. (2015) 46(2):131–9. doi: 10.1002/uog.2015.46.issue-2
15. Gonen Y, Casper RF. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). J Vitr Fertil Embryo Transf. (1990) 7(3):146–52. doi: 10.1007/BF01135678
16. Ijland MM, Evers JLH, Dunselman GA, van Katwijk C, Lo CR, Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril. (1996) 65(4):746–9. doi: 10.1016/S0015-0282(16)58207-7
17. Applebaum M. The uterine biophysical profile. Ultrasound Obstetrics Gynecology. (1995) 5(1):67–8. doi: 10.1046/j.1469-0705.1995.05010067.x
18. Arya S, Kupesic Plavsic S. Preimplantation 3D ultrasound: current uses and challenges. J Perinatal Med. (2017) 45(6):745–58. doi: 10.1515/jpm-2016-0361
19. Pandey H, Guruvare S, Kadavigere R, Rao CR. Utility of three dimensional (3-D) ultrasound and power Doppler in identification of high risk endometrial cancer at a tertiary care hospital in southern India: A preliminary study. Taiwan J Obstet Gynecol. (2018) 57(4):522–527. doi: 10.1016/j.tjog.2018.06.007
20. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009*. Fertil Steril. (2009) 92:1520–4. doi: 10.1016/j.fertnstert.2009.09.009
21. Hooker AB, de Leeuw RA, Twisk JWR, Brölmann HAM, Huirne JAF. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: Long-term follow-up of a randomized controlled trial. Hum Reprod. (2021) 36(1):70–81. doi: 10.1093/humrep/deaa289
22. Wenzhi X, Xin X, Ping Z, Hanglin W, Xiaona L. A meta-analysis of obstetric and neonatal outcomes in patients after treatment of hysteroscopic adhesiolysis. Front Endocrinol. (2023) 14:1126740. doi: 10.3389/fendo.2023.1126740
23. Arora A, Goel JK, Goel R, Arya SB, Prajapati N. Ultrasonography and doppler study to predict uterine receptivity in infertile patients undergoing embryo transfer and its correlation with pregnancy rate. J South Asian Fed Obstet Gynaecol. (2021). doi: 10.1007/s13224-015-0742-5
24. Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum Reprod Update. (2019) 25(2):202–23. doi: 10.1093/humupd/dmy044
25. Hou Z, Zhang Q, Zhao J, Xu A, He A, Huang X, et al. Value of endometrial echo pattern transformation after hCG trigger in predicting IVF pregnancy outcome: a prospective cohort study. Reprod Biol Endocrinol. (2019) 17(1):74. doi: 10.1186/s12958-019-0516-5
26. Hooker AB, Mansvelder FJ, Elbers RG, Frijmersum Z. Reproductive outcomes in women with mild intrauterine adhesions; a systematic review and meta-analysis. J Maternal-Fetal Neonatal Med. (2022) 35(25):6933–41. doi: 10.1080/14767058.2021.1931103
27. He M, Chen Q, He J, Zhao Q, Jiang H, Xia Y. Reproductive outcomes of women with moderate to severe intrauterine adhesions after transcervical resection of adhesion: A systematic review and meta-analysis. Med (United States). (2023) 102(11):e33258. doi: 10.1097/MD.0000000000033258
28. Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynecology Obstetrics. (2014) 125(2):121–4. doi: 10.1016/j.ijgo.2013.10.026
29. Busnelli A, Somigliana E, Cirillo F, Levi-Setti PE. Is diminished ovarian reserve a risk factor for miscarriage? Results of a systematic review and meta-analysis. Hum Reprod Update. (2021) 27(6):973–88. doi: 10.1093/humupd/dmab018
Keywords: endometrial receptivity, intrauterine adhesion, frozen-thawed embryo transfer, natural cycle, ovulation day, transvaginal sonography
Citation: Ouyang Y, Peng Y, Zheng M, Mao Y, Gong F, Li Y, Chen H and Li X (2025) The impact of intrauterine adhesions on endometrial receptivity in patients undergoing in vitro fertilization-embryo transfer. Front. Endocrinol. 15:1489839. doi: 10.3389/fendo.2024.1489839
Received: 02 September 2024; Accepted: 27 December 2024;
Published: 21 January 2025.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Basilio Pecorino, Kore University of Enna, ItalyTolga Karacan, Bagcilar Education and Research Hospital, Türkiye
Copyright © 2025 Ouyang, Peng, Zheng, Mao, Gong, Li, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xihong Li, eGlob25nbGl5eGtAMTYzLmNvbQ==
 Yan Ouyang
Yan Ouyang Yangqin Peng
Yangqin Peng Mingxiang Zheng
Mingxiang Zheng Yuyao Mao1
Yuyao Mao1 Xihong Li
Xihong Li