- 1School of Basic Medical, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2College of Education, Chengdu College of Arts and Sciences, Chengdu, China
- 3Department of Endocrinology, Chongqing Traditional Chinese Medicine Hospital, Chongqing, China
- 4Department of Pharmacy, Emergency General Hospital, Beijing, China
Introduction: This study aims to explore the risk factors in the progression of gestational diabetes mellitus (GDM) to type 2 diabetes mellitus (T2DM).
Material and methods: Relevant studies were comprehensively searched from PubMed, Web of Science, Cochrane Library, and Embase up to March 12. Data extraction was performed. Differences in risk factors were presented as odds ratios (OR) and corresponding 95% confidence intervals (CI). The quality of the included studies was assessed through the Newcastle-Ottawa Scale and the Agency for Healthcare Research and Quality scale.
Results: This meta-analysis encompassed 46 studies involving a total of 196,494 patients. The factors most strongly associated with the risk of developing T2DM following GDM were the use of progestin-only contraceptives (odds ratio [OR]: 2.12, 95% confidence interval [CI] = 1.00–4.45, P = 0.049), recurrence of GDM (OR: 2.63, 95% CI = 1.88–3.69, P < 0.001), insulin use during pregnancy (OR: 4.35, 95% CI = 3.17–5.96, P < 0.001), pre-pregnancy body mass index (BMI) (OR: 2.97, 95% CI = 2.16–4.07, P < 0.001), BMI after delivery (OR: 4.17, 95% CI = 2.58–6.74, P < 0.001), macrosomia (OR: 3.30, 95% CI = 1.45–7.49, P = 0.04), hypertension (OR: 5.19, 95% CI = 1.31–20.51, P = 0.019), and HbA1c levels (OR: 3.32, 95% CI = 1.81–6.11, P < 0.001). Additionally, age (OR: 1.71, 95% CI = 1.23–2.38, P = 0.001), family history of diabetes (OR: 1.47, 95% CI = 1.27–1.70, P < 0.001), BMI during pregnancy (OR: 1.06, 95% CI = 1.00–1.12, P = 0.056), fasting blood glucose (FBG) (OR: 1.58, 95% CI = 1.36–1.84, P < 0.001), 1-hour oral glucose tolerance test (OGTT) (OR: 1.38, 95% CI = 1.02–1.87, P = 0.037), and 2-hour OGTT (OR: 1.54, 95% CI = 1.28–1.58, P < 0.001) were identified as moderate-risk factors for the development of T2DM.
Conclusion: The systematic review and meta-analysis identified several moderate- to high-risk factors associated with the progression of T2DM in individuals with a history of GDM. These risk factors include the use of progestin-only contraceptives, pre-pregnancy BMI, BMI after delivery, macrosomia, hypertension, persistently elevated levels of HbA1c, fasting blood glucose (FBG), 1-hour and 2-hour oral glucose tolerance tests (OGTT), age, and family history of diabetes. Our findings serve as evidence for the early prevention and clinical intervention of the progression from GDM to T2DM and offer valuable insights to guide healthcare professionals in formulating customized management and treatment strategies for female patients with diverse forms of GDM.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024545200.
Highlights
● This meta-analysis identified multiple moderate- to high-risk factors for T2DM in GDM patients: progestin-only contraceptives, BMI, macrosomia, hypertension, and HbA1c levels, among others, and provided substantial evidence to inform early preventive measures and clinical interventions.
1 Introduction
Gestational diabetes mellitus (GDM) is defined as a state of hyperglycemia during pregnancy that resolves after delivery in women who have never been diagnosed with diabetes. This condition involves glucose intolerance. It was conceptualized by Carrington in 1957 (1) and gained widespread recognition in the 1960s. Due to the lack of standardized diagnostic criteria, the prevalence of GDM ranges from 1% to over 30% globally (2). The median prevalence is the highest in the Middle East and North Africa (15.2%), and the lowest (6.1%) is in Europe. Although earlier studies posited GDM as a benign condition (3), recent evidence suggests that it heightens the risk of various complications like macrosomia and preeclampsia for both babies and mothers during pregnancy. GDM may lead to poor pregnancy outcomes (4) and have long-term effects on the health of mothers and children, such as elevating the risk of obesity and premature cardiovascular disease (5, 6).
Due to changes in maternal demographics and rising global obesity rates in recent years, there is a significant risk of the progression from GDM to type 2 diabetes mellitus (T2DM), which poses a pressing threat to public health. Plenty of studies have indicated a 6-10 times risk of progressing to T2DM in women with GDM in contrast to the general pregnant population (7–10). A 2016 global review has estimated that the cumulative probability of developing T2DM after GDM ranges from 2% to 66%, with substantial regional variations; however, the risk remains significantly higher in women with GDM than in the general female population (11). A 2020 meta-analysis, encompassing 129 studies, reported a relative risk (RR) of 8.3 for the development of T2DM following GDM, with nearly 17% of women with GDM progressing to T2DM (12).
Despite the proven correlation between GDM and T2DM, there is no comprehensive analysis of relevant risk factors for the progression from GDM to T2DM. A meta-analysis and systematic review of risk factors are necessary. Therefore, this study aims to thoroughly evaluate the risk of T2DM among women with GDM through a meta-analysis, and offer evidence-based references for clinicians in the development of postpartum screening plans and intervention strategies during pregnancy, thereby improving T2DM prevention and intervention in female GDM patients.
2 Materials and methods
Our research adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) Declaration (13). It has been registered with PROSPERO under registration number CRD42024545200.
2.1 Search strategy
A thorough and systematic search was carried out across PubMed, EMBASE, Cochrane Library, and Web of Science up to March 12, 2024. Medical subject headings (MeSH) and free-text terms were used for the search. The keywords were: “diabetes mellitus, type 2”, “diabetes, gestational”, “ketosis resistant diabetes mellitus”, “non-insulin dependent diabetes”, “stable diabetes mellitus”, “MODY”, “diabetes mellitus”, “maturity onset diabetes”, “type 2 diabetes”, “adult onset diabetes”, “diabetes type ii”, “insulin independent diabetes”, “DM 2”, “T2DM”, and “pregnancy diabetes”. The details are presented in Supplementary Table S1.
2.2 Inclusion criteria
1. The study population comprised women who were diagnosed with GDM by a physician, aged 18 or more, and subsequently developed T2DM.
2. The original study employed multivariate logistic regression to pinpoint one or more T2DM-associated risk factors, including demographic and lifestyle characteristics (e.g., age, family history of diabetes), pregnancy-related factors (e.g., insulin use during pregnancy, inter-pregnancy intervals), and laboratory analyses (e.g., FBG, OGTT).
3. The studies are prospective or retrospective cohort studies, cross-sectional or case-control studies.
4. The research offered data such as odds ratios (OR), RR, hazard ratios (HR) with 95% CI, or sufficient data for calculation.
5. Studies were published in English.
2.3 Exclusion criteria
1. Patients who were not diagnosed with GDM were excluded.
2. Duplicates, animal studies, reviews, letters, conference abstracts, case reports, case series, and editorials were removed.
3. Studies were excluded if they did not report endpoints related to risk factors or if the full text could not be accessed.
2.4 Data extraction and quality assessment
Data extraction was independently completed by two experienced reviewers. Discrepancies were addressed through discussion or consultation with a third reviewer. A standardized Microsoft Excel spreadsheet, provided by Cochrane, was used for data collection. The extracted information included the first author, publication year, country or region, study design, total sample size, prevalence of T2DM, diagnostic criteria for GDM and T2DM, and other pertinent factors. Furthermore, risk factors for T2DM identified after multifactorial logistic regression analysis, such as OR and 95% CI, were extracted. The quality and methodological rigor of all selected studies were evaluated through the Newcastle-Ottawa Scale (NOS) (14) for cohort and case-control studies, and through the Agency for Healthcare Research and Quality (AHRQ) (15) guidelines for cross-sectional studies. The NOS includes two primary components: one for cohort studies and one for case-control studies, each of which has three domains: selection, comparability, and outcome or exposure. The maximum score for each domain is nine points. The scores are categorized into three levels: low (four points or fewer), moderate (five or six points), and high (seven points or more).
2.5 Statistical analyses
The meta-analysis was performed with the help of STATA. The risk factors for T2DM were analyzed via OR values and their associated 95% CI. A fixed-effects model (FEM) was constructed for the meta-analysis when statistical heterogeneity was minimal (P > 0.10 and I² ≤ 50%); otherwise, a random-effects model (REM) was employed. In the case of observed heterogeneity, the results of the fixed-effects model and the random-effects model were compared (16). If significant discrepancies were identified, a sensitivity analysis was performed by systematically excluding each study to explore potential sources of heterogeneity. Moreover, subgroup analyses were performed to further elucidate the sources of heterogeneity. The primary outcome of this study was the risk factors for T2DM among patients with GDM. Subgroup analyses based on geographic location, study design, and other relevant variables were carried out to enhance the robustness of our findings. Risk factors demonstrating statistical significance were classified as high risk (OR≥2), moderate risk (1< OR < 2), or protective factor(OR< 1). Publication bias was assessed via funnel plots and Egger’s test. A p-value of less than 0.05 signifies statistical significance.
3 Results
3.1 Search results and study characteristics
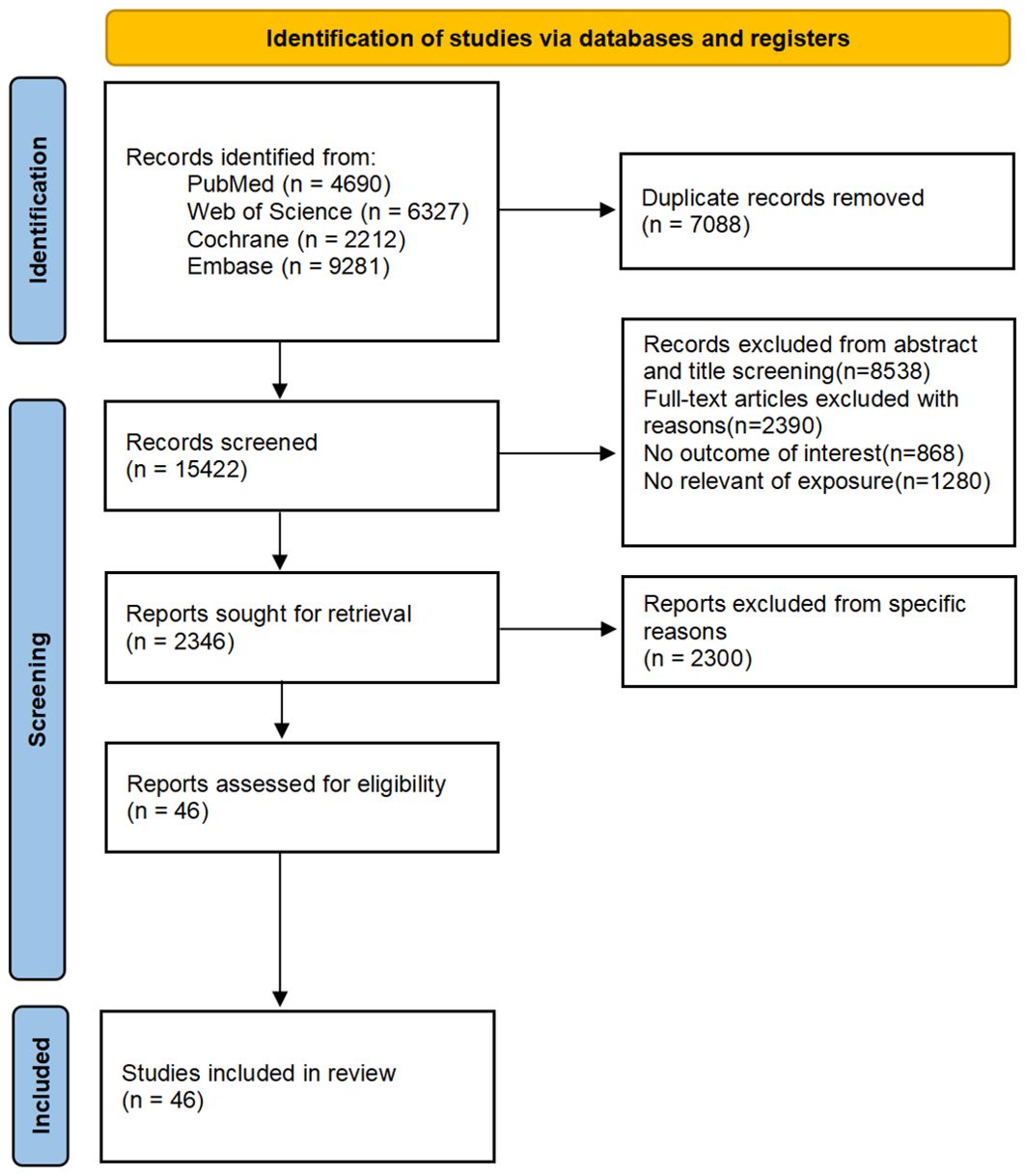
Our initial search yielded 22,510 articles, with 4,690 from PubMed, 6,327 from Web of Science, 9,281 from Embase, and 2,212 from Cochrane. After the exclusion of duplicates and irrelevant studies based on titles and abstracts, the full texts of the remaining 2,346 studies were reviewed. Ultimately, 46 studies were selected for inclusion. The study selection process is outlined in Figure 1.
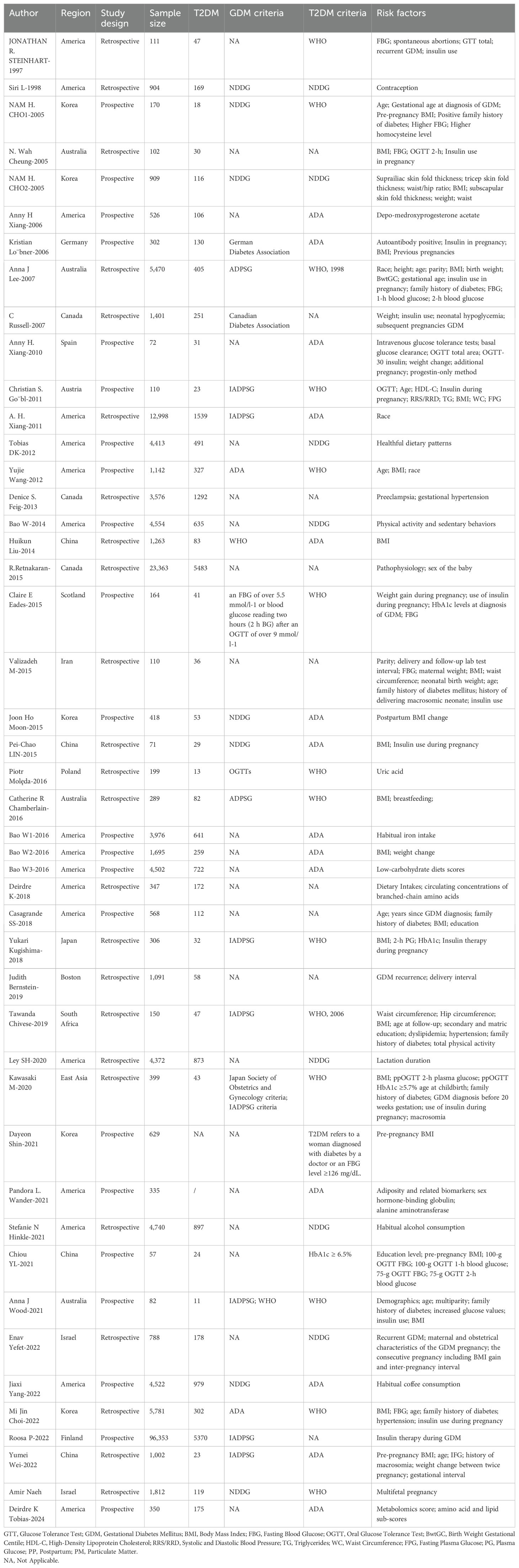
Table 1 presents the characteristics of the included articles, along with an assessment of the quality of each study. The studies were published between 1997 and 2024. Among the studies, six (17–22) were in Europe, 15 in Asia (23–36), 21 in North America (18, 37–56), four in Oceania (57–60), and one in Africa (61). Of the ultimately selected 46 studies, 58 distinct risk factors were identified. Demographic and lifestyle factors included: age, family history of diabetes, use of progestin-only contraceptives, breastfeeding practices, higher education, ethnicity (Asian, White, Hispanic, African American), healthy dietary patterns, physical activity and sedentary behaviors, habitual iron intake, low-carbohydrate diet scores, dietary intakes, habitual alcohol consumption, and habitual coffee consumption. Pregnancy-related factors encompassed: early diagnosis of GDM, recurrence of GDM, insulin use during pregnancy, pre-pregnancy BMI, BMI during pregnancy, BMI postpartum, weight change, gestational interval, parity, waist circumference, macrosomia, neonatal birth weight, hypertension, spontaneous abortions, skin fold thickness measurements (suprailiac, tricep, subscapular), waist/hip ratio, preeclampsia, sex of the baby, and exposure to particulate matter (PM). Laboratory indicators included: HbA1c, FBG, 1-hour and 2-hour OGTT values, elevated homocysteine levels, positive autoantibodies, neonatal hypoglycemia, basal glucose clearance, OGTT total area, 30-minute insulin response during OGTT, high-density lipoprotein cholesterol, triglycerides, uric acid, circulating concentrations of branched-chain amino acids, sex hormone-binding globulin (SHBG), alanine aminotransferase (ALT), metabolomics score, and amino acid and lipid sub-scores.
Due to the absence of sufficient studies, a meta-analysis on 31 risk factors was impossible. Hence, only 26 (17–20, 22–35, 37–40, 42–44, 50–52, 57–61) of the 58 identified risk factors were meta-analyzed. The results of this analysis, as well as those from original outcome studies, are presented in Table 2. We examined the impact of these 26 risk factors on the incidence of T2DM in women with GDM. Notably, publication bias was detected for several factors, including insulin use during pregnancy, hypertension, and the 2-hour OGTT. Detailed information on publication bias can be found in Supplementary Table S2.
3.2 Risk of bias assessment
The quality of the 42 included cohort studies was assessed through NOS, with scores ranging from five to eight stars, indicating a relatively low risk of bias. A summary of the quality assessment is presented in Supplementary Table S3. The four cross-sectional studies were evaluated using the AHRQ assessment tool, and their results also indicated reliable quality. More details are provided in Supplementary Table S3.
3.3 Meta-analysis
3.3.1 Demographic and lifestyle characteristics
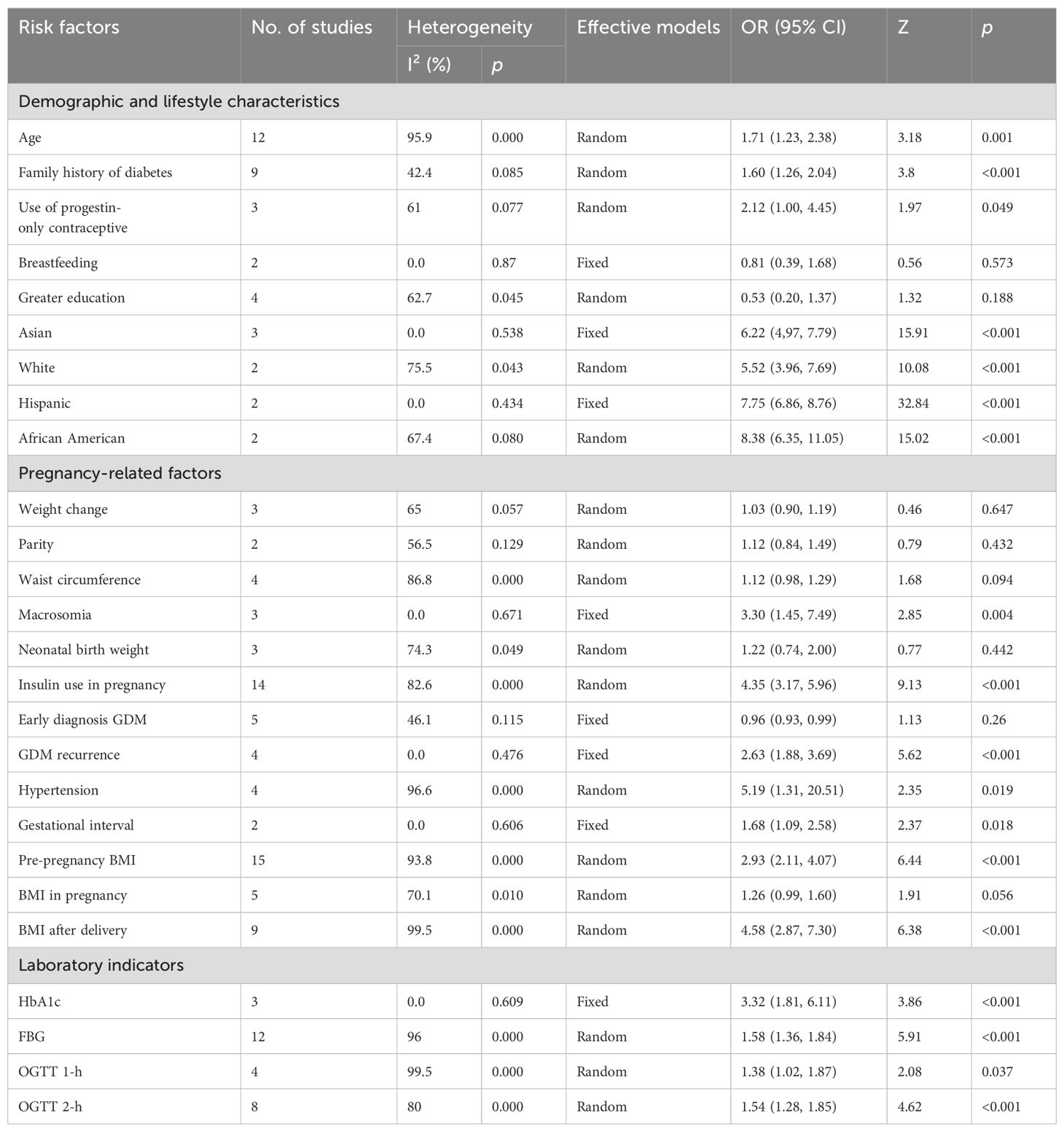
A meta-analysis was performed to examine the influence of age (19, 20, 23, 26, 30, 34, 35, 42, 50, 58, 60), family history of diabetes (20, 23, 26, 30, 34, 50, 58, 60, 61), use of progestin-only contraceptives (18, 38, 39), breastfeeding (59, 60), higher educational attainment (32, 50, 60, 61), and race [Asian (18, 42, 58), White (18, 42), Hispanic (18, 42) African American (18, 42)] on the progression to T2DM from GDM. Of particular note, the use of progestin-only contraceptives (OR: 2.12, 95% CI: 1.00-4.45, P=0.049) was identified to be a high-risk factor. Age (OR: 1.71, 95% CI: 1.23-2.38, P=0.001) and a family history of diabetes (OR: 1.47, 95% CI: 1.27-1.70, P<0.001) were deemed moderate-risk factors for T2DM. Breastfeeding (OR: 0.81, 95% CI: 0.39-1.68, P=0.573) and higher educational attainment (OR: 0.53, 95% CI: 0.20-1.37, P=0.188) were considered protective factors against T2DM, though the results did not reach statistical significance. In terms of race, all four groups had an elevated risk for progression from GDM to T2DM; White individuals had a relatively lower risk (OR: 5.52, 95% CI: 3.96-7.69, P<0.001) and African Americans had a relatively higher risk (OR: 8.38, 95% CI: 6.35-11.05, P<0.001).
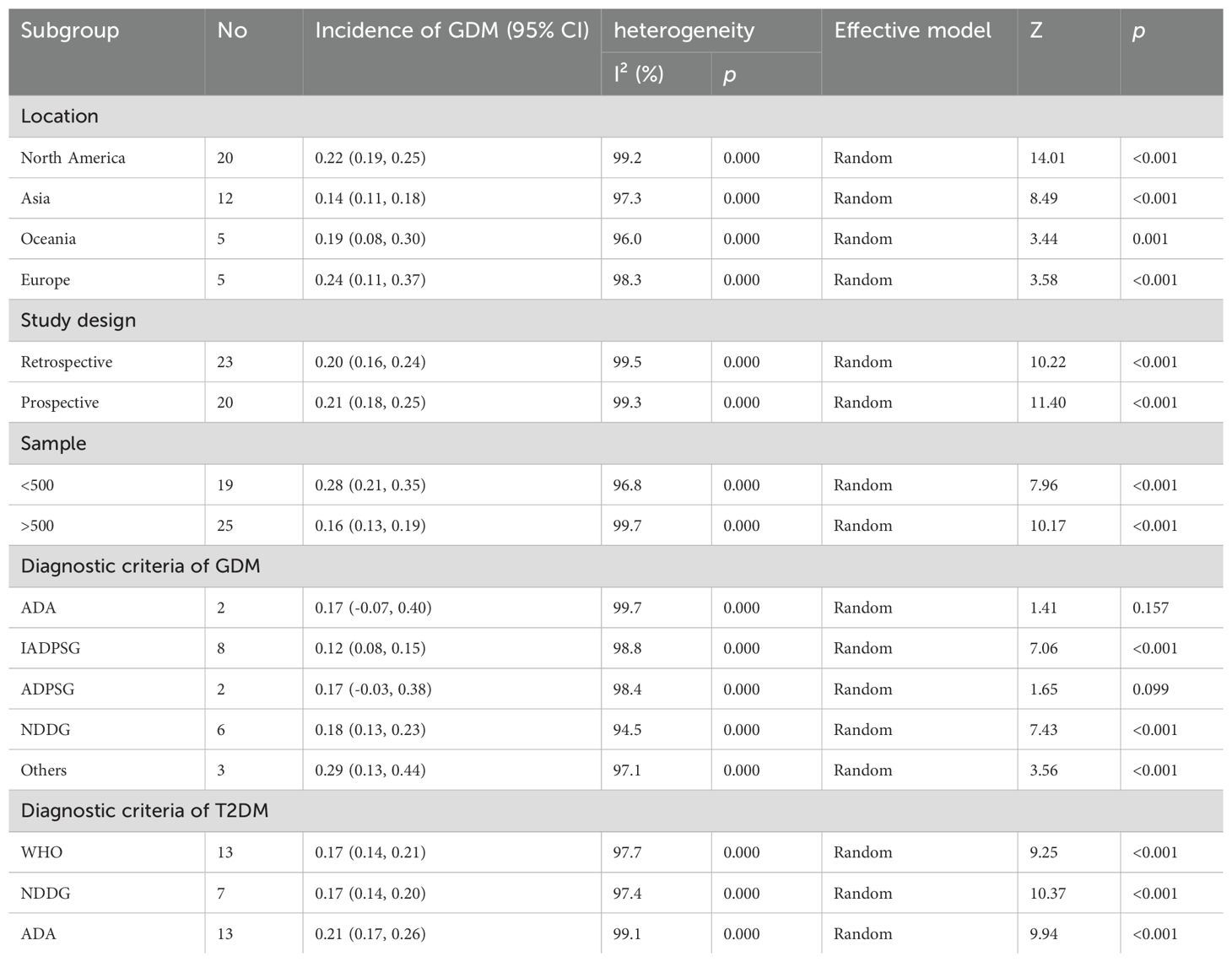
Significant heterogeneity was observed among the studies in terms of age, use of progestin-only contraceptives, higher educational attainment, and race (White and African American), with I² values of 95.9%, 61%, 62.7%, 75.5%, and 67.4%, respectively. However, a comparison of results through both fixed-effect and random-effect models (61) revealed no significant differences for the remaining factors, excluding education and the use of progestin-only contraceptives. This suggests that the results were stable. To further assess this, sensitivity analyses were conducted by sequentially excluding studies that considered educational attainment and progestin-only contraceptives as risk factors. The analysis revealed that the study by Casagrande SS-2018 had a notable influence on the heterogeneity observed for educational attainment. Many of the other studies included were retrospective, which could have contributed to the observed heterogeneity. However, the source of heterogeneity in studies involving progestin-only contraceptives remained unclear. Subgroup analysis based on region, study design, sample size, and diagnostic criteria for T2DM and GDM were subsequently conducted to find potential sources of heterogeneity (Tables 3, 4). No significant publication bias was noted for relevant factors (P > 0.05).
3.3.2 Pregnancy-related factors
A meta-analysis was carried out on 13 pregnancy-related variables: early diagnosis of GDM (20, 23, 30, 33, 60), recurrence of GDM (20, 33, 37, 51), insulin use during pregnancy (17, 19, 20, 22, 26, 28–30, 34, 37, 40, 57, 58, 60), pre-pregnancy BMI (24, 25, 28–32, 47, 57, 59, 61), BMI during pregnancy (17, 19, 23, 35, 60), BMI after delivery (26, 34, 42, 47, 50, 58), weight change (18, 35, 60), gestational interval (35, 51), parity (26, 58), waist circumference (19, 24, 26, 61), macrosomia (26, 30, 35), neonatal birth weight (26, 33, 58), and hypertension (19, 34, 43, 61), which were reported in 5, 4, 14, 18, 3, 8, 3, 2, 2, 4, 3, 3, and 4 studies, respectively. Among the factors examined, six were identified as high-risk factors for the development of Type 2 diabetes (T2DM). These included: recurrence of gestational diabetes mellitus (GDM) (OR: 2.63, 95% CI: 1.88-3.69, P < 0.001), insulin use during pregnancy (OR: 4.35, 95% CI: 3.17-5.96, P < 0.001), pre-pregnancy BMI (OR: 2.97, 95% CI: 2.16-4.07, P < 0.001), BMI after delivery (OR: 4.17, 95% CI: 2.58-6.74, P < 0.001), macrosomia (OR: 3.30, 95% CI: 1.45-7.49, P = 0.004), and hypertension (OR: 5.19, 95% CI: 1.31-20.51, P = 0.019). Among the pregnancy-related variables, BMI during pregnancy was identified as a moderate-risk factor (OR: 1.06, 95% CI: 1.00-1.12, P = 0.056), though its significance was borderline. In contrast, several factors - weight change (OR: 1.03, 95% CI: 0.90-1.19, P = 0.647), gestational interval (OR: 1.68, 95% CI: 1.09-2.58, P = 0.018), parity (OR: 1.12, 95% CI: 0.84-1.49, P = 0.432), waist circumference (OR: 1.12, 95% CI: 0.98-1.29, P = 0.094), and neonatal birth weight (OR: 1.22, 95% CI: 0.74-2.00, P = 0.442) - were also classified as moderate-risk factors but did not reach statistical significance. An early diagnosis of GDM (OR: 0.96, 95% CI: 0.93-0.99, P = 0.26), as reported in five studies, was identified as a protective factor. For the meta-analysis, four factors - macrosomia, early diagnosis of GDM, recurrence of GDM, and gestational interval - were analyzed via a fixed-effects model due to low heterogeneity. The remaining nine factors were analyzed using a random-effects model due to significant heterogeneity. However, when comparing results from both models, only BMI during pregnancy and waist circumference showed significant differences, suggesting stability for the other factors. Even when studies related to BMI during pregnancy and waist circumference were excluded individually, the source of heterogeneity remained unclear. In this meta-analysis, weight change, parity, waist circumference, and neonatal birth weight were not significantly associated with the development of T2DM (P > 0.05). Notably, insulin use during pregnancy and hypertension were significantly correlated with publication bias (P < 0.05).
3.3.3 Laboratory indicators
A meta-analysis was carried out on four laboratory parameters: HbA1c (20, 29, 30) (OR: 3.32, 95% CI=1.81-6.11, P<0.001), FBG (19, 20, 23, 26, 32–34, 37, 57, 58, 60) (OR: 1.58, 95% CI=1.36-1.84, P<0.001), OGTT 1-hour (32, 33, 58, 60) (OR: 1.38, 95% CI=1.02-1.87, P=0.037), and OGTT 2-hour (19, 20, 29, 30, 32, 57, 58, 60) (OR: 1.54, 95% CI=1.28-1.58, P<0.001). These parameters were analyzed across three, twelve, four, and eight studies, respectively. HbA1c was identified as a high-risk factor for T2DM, while the other three parameters were classified as moderate-risk factors. Given the low heterogeneity observed for HbA1c, a fixed-effects model was applied. In contrast, the other three factors demonstrated high heterogeneity (I² = 96%, 99.5%, 80%), prompting the use of a random-effects model. Our findings remained consistent and robust after adjusting for the fixed-effects model, with all four factors showing a significant association with the occurrence of T2DM (P < 0.05). Furthermore, a significant publication bias was identified for the 2-hour OGTT (P < 0.01).
3.3.4 Subgroup analysis and sensitivity analyses
Subgroup analyses suggested that regional differences, diagnostic criteria, sample size, and study design may contribute to the observed heterogeneity in factors such as age, insulin use during pregnancy, and FBG. Specifically, sample size appeared to be a key source of heterogeneity for hypertension and the 1-hour OGTT, while variations in study design could explain the heterogeneity observed in waist circumference and the early diagnosis of GDM. Despite these sources of heterogeneity, sensitivity analyses confirmed the robustness and reliability of our findings. The results of the sensitivity analysis are presented in Supplementary Figure S1.
4 Discussion
46 studies encompassing 196,494 patients were ultimately included in our research. Multiple risk factors in the progression from GDM to T2DM were systematically evaluated. Our findings reveal that several factors significantly contribute to this progression, including age, family history of diabetes, use of progestin-only contraceptives, recurrence of GDM, insulin use during pregnancy, pre- and post-pregnancy BMI, macrosomia, hypertension, and persistently elevated levels of HbA1c, FBG, one-hour and two-hour OGTT results. These results highlight the importance of continuous monitoring and early intervention for high-risk GDM patients in clinical practice.
More evidence suggests that the progression of GDM to T2DM may be significantly correlated with insulin β-cell dysfunction (62). In the forthcoming discussion, this study will delve into the mechanism underlying the role of three distinct types of risk factors in the transition from GDM to T2DM.
4.1 Demographic and lifestyle characteristics
Our study revealed that women with GDM who used progestin-only contraceptives were of advanced maternal age, or had a family history of diabetes were more likely to develop T2DM. These findings are consistent with those of Rayanagoudar et al., who also identified family history and advanced maternal age as significant risk factors for T2DM (63). They reported an RR of 1.70 for a positive family history of T2DM, which is consistent with the OR of 1.47 observed in our study. This indicates that familial factors contribute to a higher incidence of T2DM among women with a history of GDM, to some extent. The potential underlying reasons may include shared lifestyles and life philosophies within families (63). Moreover, our findings indicated a lower risk of T2DM in Caucasian women, aligning with those of Rayanagoudar et al. and You et al., who reported higher risks in Black and non-Hispanic White women after GDM (10, 63). It is important to note that, while race was not identified as a significant risk factor in our study, the observed heterogeneity in the analysis may still reflect variations in lifestyle and genetic factors across different racial groups.
Overall, patient age is a significant risk factor. As individuals age, the function of pancreatic β-cells typically declines, which directly impacts the synthesis and secretion of insulin, thereby influencing glucose regulation (64, 65). For older patients with GDM, β-cell function may have already been impaired due to aging (66, 67). The hyperglycemic stress experienced during pregnancy can further accelerate this decline, which increases their risk of developing T2DM after childbirth. Aging is also associated with heightened insulin resistance (68, 69). As individuals age, they typically experience a reduction in muscle mass and changes in visceral fat distribution, both of which contribute to systemic insulin resistance (70–72). Moreover, advancing age often coexists with inflammaging, which is a state of chronic low-grade inflammation (73, 74). Elevated levels of inflammatory markers, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), exacerbate insulin resistance and further impair β-cell function (75, 76). Lastly, although there remains ongoing debate about the link between progestin-only contraceptives and the development of diabetes, this study has found that women who use these contraceptives appear to have an increased risk of developing T2DM. Progestin-only contraceptives may contribute to this risk by inducing apoptosis in pancreatic β-cells (77), which affects blood glucose levels and disrupts glucose metabolism. This disruption can, in turn, lead to increased insulin resistance and facilitate the progression from GDM to T2DM (78, 79).
4.2 Pregnancy-related factors
Our findings suggest that several pregnancy-related factors significantly elevate the risk of developing T2DM in women with GDM. These factors include GDM recurrence, insulin use during pregnancy, higher BMI before or after pregnancy, the delivery of macrosomic infants, and the presence of hypertension. These results are consistent with those of Rayanagoudar et al., particularly regarding BMI and insulin use during pregnancy (63). Both studies demonstrate that a high BMI substantially increases the risk of T2DM. Rayanagoudar et al. reported a progressive increase in T2DM risk with rising BMI, particularly when BMI reaches overweight or obese levels (63). This underscores the importance of managing weight before, during, and after pregnancy to prevent the progression from GDM to T2DM. Moreover, insulin use during pregnancy and GDM recurrence were identified as significant independent risk factors for T2DM. Rayanagoudar et al. found that women with GDM who required insulin therapy had a notably higher risk of developing T2DM (63). This may reflect the degree of β-cell dysfunction and the dependency on insulin during and after pregnancy. Lastly, in line with the findings of Rayanagoudar et al., our study also indicates no significant association between breastfeeding, neonatal birth weight, and the risk of T2DM in female GDM patients.
Increased insulin requirements in GDM are indicative of β-cell dysfunction. However, when β-cells cannot meet the heightened demand, GDM may develop (80). Notably, GDM patients who require exogenous insulin therapy may already have significant β-cell impairment (80). The compromised β-cell function may not fully recover postpartum, thereby raising the risk of progressing to T2DM. Additionally, a high BMI plays a crucial role in the development of insulin resistance. Obesity leads to an overproduction of inflammatory cytokines, such as TNF-α and IL-6, in adipose tissue, further exacerbating insulin resistance (81–83). Therefore, a high BMI is a significant risk factor in the progression from T2DM to GDM. Pregnancy-related complications, such as hypertension, are also important high-risk factors. Hypertension is often a marker of underlying endothelial dysfunction and systemic inflammation, both of which are closely linked to insulin resistance and the development of diabetes (84, 85). Lastly, macrosomia is another significant risk factor for the transition from GDM to T2DM, involving complex biological and physiological mechanisms. Macrosomia is typically the result of poor glycemic control during pregnancy. In GDM, insulin resistance and/or insufficient β-cell secretion lead to elevated maternal glucose levels (86, 87). These elevated glucose levels can cross the placenta, stimulate fetal growth, and lead to excessive fetal weight, or macrosomia (87). Macrosomia not only reflects the increased fetal size but also mirrors the mother’s metabolic state and insulin sensitivity (86, 87). Furthermore, the development of GDM and macrosomia is correlated with inflammation and oxidative stress (88–90). A hyperglycemic environment can promote the production of free radicals and the release of inflammatory cytokines, which in turn may damage β-cells and impair their function (88–90).
4.3 Laboratory indicators
In terms of laboratory indicators, this study examined the correlation of HbA1c, FBG, as well as the one-hour and two-hour values of OGTT with the progression from GDM to T2DM. All of these indicators were proven to be significantly linked to an increased risk of developing T2DM. These parameters are crucial for monitoring glycemic control in diabetic patients, and the study’s findings further highlight their importance in predicting the progression from GDM to T2DM.
HbA1c, as a critical marker of long-term glycemic control, is particularly crucial in managing GDM. Its low heterogeneity across studies suggests that HbA1c consistently predicts T2DM risk, and can serve as a stable and reliable potential risk assessment tool. For GDM patients, persistently elevated HbA1c levels reflect prolonged hyperglycemia and may indicate further deterioration of pancreatic β-cell function (91, 92). This sustained hyperglycemic state can enhance insulin resistance, impose a greater burden on β-cells, and ultimately lead to β-cell exhaustion (91, 92). Furthermore, the analysis of FBG suggests a moderate increase in the risk of developing T2DM (OR = 1.58) and shows the importance of continued monitoring of FBG levels after pregnancy. As a tool for routine monitoring, FBG immediately reflects glycemic control and aids in the early identification of GDM patients who may develop T2DM. FBG is primarily regulated by the balance between hepatic glucose production and insulin release from pancreatic β-cells (93). For GDM patients, if β-cells fail to manage the persistent hyperglycemic stress after childbirth, their function may continue declining and lead to sustained elevations in FBG levels, which may result in the development of T2DM from GDM (94). Finally, the ORs for OGTT at one hour and two hours were 1.38 and 1.54, respectively, indicating the potential of OGTT in predicting T2DM risk. OGTT can be employed to assess insulin secretion and sensitivity by measuring an individual’s glycemic response to oral glucose (95, 96). Elevated OGTT values at one and two hours generally indicate insufficient insulin secretion or impaired insulin action (95, 96). These elevated test results physiologically reflect a diminished β-cell response to glucose and inadequate peripheral tissue response to insulin, serving as an early warning signal for T2DM development (95, 96). Therefore, systematic postpartum glycemic monitoring is essential for GDM patients, particularly those with high HbA1c and FBG levels. Regular OGTTs complement routine FBG monitoring by identifying glycemic abnormalities that may otherwise go unnoticed, facilitating early detection and timely intervention for T2DM risk.
4.4 Subgroup analysis and sensitivity analyses
Lastly, subgroup and sensitivity analyses were performed to delve into the prevalence and risk factors for T2DM in female GDM patients. The subgroup analysis of prevalence revealed that the incidence of T2DM among GDM patients in Asia is slightly lower compared to Europe, the Americas, and Oceania. This discrepancy may be attributed to differences in sample sizes and diagnostic criteria across regions. Additionally, the subgroup analysis of risk factors highlighted that regional variations and differences in diagnostic standards could explain the observed heterogeneity in age, insulin use during pregnancy, and FBG levels. These findings are consistent with the results of Rayanagoudar et al., who identified that follow-up duration significantly influences the risk assessment of FBG, BMI, and insulin use (63). This suggests that researchers should consider region-specific medical practices and diagnostic criteria when studying T2DM risk factors in different global regions. The sample size was identified as a source of heterogeneity for hypertension and the 1-hour OGTT, while the study design contributed to the heterogeneity observed in waist circumference and early GDM diagnosis. This highlights the critical role of study design in interpreting research findings, as variations in sampling and data collection methods can lead to biased conclusions.
Despite the aforementioned heterogeneity, sensitivity analysis confirms the stability of our findings, which are consistent with those of Rayanagoudar et al. They reported that the impact of certain key variables, such as FBG and BMI, remained significant despite variations in follow-up duration (63). This shows the reliability of the identified risk factors for the progression from GDM to T2DM, even in the presence of potential biases. Additionally, the study highlighted the potential influence of various factors on T2DM development in pregnant women, including healthful dietary patterns, physical activity, sedentary behaviors, habitual iron intake, alcohol consumption, coffee consumption, and multiple pregnancies. However, due to the limited number of original studies, the existing data are insufficient to conduct a meta-analysis on the precise effects of these factors on T2DM risk. Therefore, further research is needed to delve into these associations. It is worth noting that, in terms of the prevention of T2DM, integrative medicine research has indicated that natural remedies such as ginger and Ganoderma lucidum (lingzhi), along with their extracts, may be therapeutic and safe in modulating human metabolism (97–99). These natural agents merit further exploration as promising research foci in future studies.
5 Limitations
Although our meta-analysis aimed to integrate and analyze data from multiple studies, significant differences existed in the diagnostic criteria for GDM and T2DM across the selected studies. This heterogeneity may have affected the consistency and generalizability of the results, thereby limiting our ability to statistically assess these risk factors. Additionally, some studies may not report all essential statistical data, such as CIs, standard deviations, or specific p-values, which could have introduced imprecision in the analyses. Despite our efforts to include as many studies as possible, the sample size in some subgroup analyses remained relatively small, which potentially increases the influence of chance factors. Moreover, the geographic distribution and demographic characteristics of the included studies may not fully reflect the broader population, further limiting the generalizability of our findings.
6 Conclusion
This study has identified several significant risk factors correlated with the development of T2DM in female GDM patients. These factors include the use of progestin-only contraceptives, recurrence of GDM, insulin use during pregnancy, pre- and post-pregnancy BMI, macrosomia, hypertension, and persistently elevated levels of HbA1c, FBG, as well as 1-hour and 2-hour OGTT readings. These findings offer robust and reliable evidence that can guide the management of T2DM in this population. The results have significant implications for health management, as well as for clinical T2DM prevention and intervention in pregnant women. Clinicians can tailor interventions to address these risk factors, ultimately reducing the incidence of T2DM and improving the clinical outcomes in women with GDM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
KC: Writing – original draft, Writing – review & editing. LT: Methodology, Writing – review & editing. XW: Methodology, Writing – review & editing. YL: Writing – review & editing. XZ: Supervision, Writing – review & editing. SC: Conceptualization, Writing – review & editing. WC: Methodology, Writing – review & editing. ZJ: Formal analysis, Funding acquisition, Investigation, Writing – review & editing. DZ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This systematic review was funded by the Chinese medicine service research project, retrospective investigation and promotion of optimization scheme of Chinese medicine service for obesity (KJzX2023-JWO07-08) and the major science and technology project of Sichuan Province, research on the effect of Shenqi compound series based on the protective effect of large blood vessels on cardiovascular benefit of diabetes mellitus (2022ZDZX0022) and by inheritance, innovation and development, School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine(No.CCCXYB202203).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1486861/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis of forest plots of comparison of the two models.
Supplementary Table 1 | Literature search strategy.
Supplementary Table 2 | Publication bias.
Supplementary Table 3 | Study quality assessment using the Newcastle-Ottawa scale tool and AHRQ assessment tool.
Abbreviations
GDM, Gestational diabetes mellitus; T2DM, type 2 diabetes mellitus; MeSH, relative risk RR medical subject headings; HR, hazard ratios; NOS, Newcastle-Ottawa Scale; AHRQ, Agency for Healthcare Research and Quality; FEM, fixed-effects model; REM, random-effects model; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; PM, Particulate Matter.
References
1. Carrington ER, Shuman CR, Reardon HS. Evaluation of the prediabetic state during pregnancy. Obstet Gynecol. (1957) 9:664–9. doi: 10.1097/00006250-195706000-00008
2. Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, et al. Epidemiology and management of gestational diabetes. Lancet. (2024) 404:175–92. doi: 10.1016/S0140-6736(24)00825-0
3. Jarrett RJ. Gestational diabetes: a non-entity? Bmj. (1993) 306:37–8. doi: 10.1136/bmj.306.6869.37
4. Mack LR, Tomich PG. Gestational diabetes: diagnosis, classification, and clinical care. Obstet Gynecol Clin North Am. (2017) 44:207–17. doi: 10.1016/j.ogc.2017.02.002
5. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. (2009) 94:2464–70. doi: 10.1210/jc.2009-0305
6. Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PloS Med. (2018) 15:e1002488. doi: 10.1371/journal.pmed.1002488
7. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5
8. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. Bmj. (2020) 369:m1361. doi: 10.1136/bmj.m1361
9. Juan J, Sun Y, Wei Y, Wang S, Song G, Yan J, et al. Progression to type 2 diabetes mellitus after gestational diabetes mellitus diagnosed by IADPSG criteria: Systematic review and meta-analysis. Front Endocrinol (Lausanne). (2022) 13:1012244. doi: 10.3389/fendo.2022.1012244
10. You H, Hu J, Liu Y, Luo B, Lei A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian J Med Res. (2021) 154:62–77. doi: 10.4103/ijmr.IJMR_852_18
11. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diabetes Rep. (2016) 16:7. doi: 10.1007/s11892-015-0699-x
12. Dennison RA, Chen ES, Green ME, Legard C, Kotecha D, Farmer G, et al. The absolute and relative risk of type 2 diabetes after gestational diabetes: A systematic review and meta-analysis of 129 studies. Diabetes Res Clin Pract. (2021) 171:108625. doi: 10.1016/j.diabres.2020.108625
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Shekelle PG, Ortiz E, Rhodes S, Morton SC, Eccles MP, Grimshaw JM, et al. Validity of the Agency for Healthcare Research and Quality clinical practice guidelines: how quickly do guidelines become outdated? Jama. (2001) 286:1461–7. doi: 10.1001/jama.286.12.1461
16. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). United Kingdom: Cochrane. (2023). Available online at: https://www.cochrane-handbook.org.
17. Löbner K, Knopff A, Baumgarten A, Mollenhauer U, Marienfeld S, Garrido-Franco M, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. (2006) 55:792–7. doi: 10.2337/diabetes.55.03.06.db05-0746
18. Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. (2010) 59:2625–30. doi: 10.2337/db10-0521
19. Göbl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A. Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol. (2011) 118:71–8. doi: 10.1097/AOG.0b013e318220e18f
20. Eades CE, Styles M, Leese GP, Cheyne H, Evans JM. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: an observational follow-up study. BMC Pregnancy Childbirth. (2015) 15:11. doi: 10.1186/s12884-015-0457-8
21. Molęda P, Fronczyk A, Safranow K, Majkowska L. Is uric acid a missing link between previous gestational diabetes mellitus and the development of type 2 diabetes at a later time of life? PloS One. (2016) 11:e0154921. doi: 10.1371/journal.pone.0154921
22. Hakkarainen H, Huopio H, Cederberg H, Voutilainen R, Heinonen S. Delivery of an LGA infant and the maternal risk of diabetes: A prospective cohort study. Prim Care Diabetes. (2018) 12:364–70. doi: 10.1016/j.pcd.2018.04.002
23. Cho NH, Lim S, Jang HC, Park HK, Metzger BE. Elevated homocysteine as a risk factor for the development of diabetes in women with a previous history of gestational diabetes mellitus: a 4-year prospective study. Diabetes Care. (2005) 28:2750–5. doi: 10.2337/diacare.28.11.2750
24. Cho NH, Jang HC, Park HK, Cho YW. Waist circumference is the key risk factor for diabetes in Korean women with history of gestational diabetes. Diabetes Res Clin Pract. (2006) 71:177–83. doi: 10.1016/j.diabres.2005.06.003
25. Liu H, Zhang C, Zhang S, Wang L, Leng J, Liu D, et al. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obes (Silver Spring). (2014) 22:1560–7. doi: 10.1002/oby.20722
26. Valizadeh M, Alavi N, Mazloomzadeh S, Piri Z, Amirmoghadami H. The risk factors and incidence of type 2 diabetes mellitus and metabolic syndrome in women with previous gestational diabetes. Int J Endocrinol Metab. (2015) 13:e21696. doi: 10.5812/ijem.21696
27. Moon JH, Kwak SH, Jung HS, Choi SH, Lim S, Cho YM, et al. Weight gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. (2015) 100:3548–55. doi: 10.1210/JC.2015-1113
28. Lin PC, Hung CH, Huang RD, Chan TF. Predictors of type 2 diabetes among Taiwanese women with prior gestational diabetes mellitus. Jpn J Nurs Sci. (2016) 13:3–9. doi: 10.1111/jjns.2016.13.issue-1
29. Kugishima Y, Yasuhi I, Yamashita H, Sugimi S, Umezaki Y, Suga S, et al. Risk factors associated with the development of postpartum diabetes in Japanese women with gestational diabetes. BMC Pregnancy Childbirth. (2018) 18:19. doi: 10.1186/s12884-017-1654-4
30. Kawasaki M, Arata N, Sakamoto N, Osamura A, Sato S, Ogawa Y, et al. Risk factors during the early postpartum period for type 2 diabetes mellitus in women with gestational diabetes. Endocr J. (2020) 67:427–37. doi: 10.1507/endocrj.EJ19-0367
31. Shin D, Lee KW. High pre-pregnancy BMI with a history of gestational diabetes mellitus is associated with an increased risk of type 2 diabetes in Korean women. PloS One. (2021) 16:e0252442. doi: 10.1371/journal.pone.0252442
32. Chiou YL, Hung CH, Yu CY, Chan TF, Liu MG. Risk factors for women with gestational diabetes mellitus developing type 2 diabetes and the impact on children’s health. J Clin Nurs. (2022) 31:1005–15. doi: 10.1111/jocn.v31.7-8
33. Yefet E, Schwartz N, Nachum Z. Characteristics of pregnancy with gestational diabetes mellitus and the consecutive pregnancy as predictors for future diabetes mellitus type 2. Diabetes Res Clin Pract. (2022) 186:109826. doi: 10.1016/j.diabres.2022.109826
34. Choi MJ, Choi J, Chung CW. Risk and risk factors for postpartum type 2 diabetes mellitus in women with gestational diabetes: A korean nationwide cohort study. Endocrinol Metab (Seoul). (2022) 37:112–23. doi: 10.3803/EnM.2021.1276
35. Wei Y, Juan J, Su R, Song G, Chen X, Shan R, et al. Risk of gestational diabetes recurrence and the development of type 2 diabetes among women with a history of gestational diabetes and risk factors: a study among 18 clinical centers in China. Chin Med J (Engl). (2022) 135:665–71. doi: 10.1097/CM9.0000000000002036
36. Naeh A, Maor-Sagie E, Hallak M, Toledano Y, Gabbay-Benziv R. Greater risk of type 2 diabetes progression in multifetal gestations with gestational diabetes: the impact of obesity. Am J Obstet Gynecol. (2024) 231:259.e1–259.e10. doi: 10.1016/j.ajog.2023.11.1246
37. Steinhart JR, Sugarman JR, Connell FA. Gestational diabetes is a herald of NIDDM in Navajo women. High rate of abnormal glucose tolerance after GDM. Diabetes Care. (1997) 20:943–7. doi: 10.2337/diacare.20.6.943
38. Kjos SL, Peters RK, Xiang A, Thomas D, Schaefer U, Buchanan TA. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. Jama. (1998) 280:533–8. doi: 10.1001/jama.280.6.533
39. Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care. (2006) 29:613–7. doi: 10.2337/diacare.29.03.06.dc05-1940
40. Russell C, Dodds L, Armson BA, Kephart G, Joseph KS. Diabetes mellitus following gestational diabetes: role of subsequent pregnancy. Bjog. (2008) 115:253–9. doi: 10.1111/j.1471-0528.2007.01459.x
41. Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. (2012) 172:1566–72. doi: 10.1001/archinternmed.2012.3747
42. Wang Y, Chen L, Horswell R, Xiao K, Besse J, Johnson J, et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Womens Health (Larchmt). (2012) 21:628–33. doi: 10.1089/jwh.2011.3318
43. Feig DS, Shah BR, Lipscombe LL, Wu CF, Ray JG, Lowe J, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PloS Med. (2013) 10:e1001425. doi: 10.1371/journal.pmed.1001425
44. Bao W, Tobias DK, Bowers K, Chavarro J, Vaag A, Grunnet LG, et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. (2014) 174:1047–55. doi: 10.1001/jamainternmed.2014.1795
45. Retnakaran R, Shah BR. Sex of the baby and future maternal risk of Type 2 diabetes in women who had gestational diabetes. Diabetes Med. (2016) 33:956–60. doi: 10.1111/dme.2016.33.issue-7
46. Bao W, Yeung E, Tobias DK, Hu FB, Vaag AA, Chavarro JE, et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia. (2015) 58:1212–9. doi: 10.1007/s00125-015-3537-4
47. Bao W, Chavarro JE, Tobias DK, Bowers K, Li S, Hu FB, et al. Long-term risk of type 2 diabetes in relation to habitual iron intake in women with a history of gestational diabetes: a prospective cohort study. Am J Clin Nutr. (2016) 103:375–81. doi: 10.3945/ajcn.115.108712
48. Bao W, Li S, Chavarro JE, Tobias DK, Zhu Y, Hu FB, et al. Low carbohydrate-diet scores and long-term risk of type 2 diabetes among women with a history of gestational diabetes mellitus: A prospective cohort study. Diabetes Care. (2016) 39:43–9. doi: 10.2337/dc15-1642
49. Tobias DK, Clish C, Mora S, Li J, Liang L, Hu FB, et al. Dietary intakes and circulating concentrations of branched-chain amino acids in relation to incident type 2 diabetes risk among high-risk women with a history of gestational diabetes mellitus. Clin Chem. (2018) 64:1203–10. doi: 10.1373/clinchem.2017.285841
50. Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res Clin Pract. (2018) 141:200–8. doi: 10.1016/j.diabres.2018.05.010
51. Bernstein J, Lee-Parritz A, Quinn E, Ameli O, Craig M, Heeren T, et al. After gestational diabetes: impact of pregnancy interval on recurrence and type 2 diabetes. Biores Open Access. (2019) 8:59–64. doi: 10.1089/biores.2018.0043
52. Ley SH, Chavarro JE, Li M, Bao W, Hinkle SN, Wander PL, et al. Lactation duration and long-term risk for incident type 2 diabetes in women with a history of gestational diabetes mellitus. Diabetes Care. (2020) 43:793–8. doi: 10.2337/dc19-2237
53. Wander PL, Christophi CA, Araneta MRG, Boyko EJ, Enquobahrie DA, Dabelea D, et al. Adiposity, related biomarkers, and type 2 diabetes after gestational diabetes: The Diabetes Prevention Program. Obes (Silver Spring). (2022) 30:221–8. doi: 10.1002/oby.23291
54. Hinkle SN, Bao W, Wu J, Sun Y, Ley SH, Tobias DK, et al. Association of habitual alcohol consumption with long-term risk of type 2 diabetes among women with a history of gestational diabetes. JAMA Netw Open. (2021) 4:e2124669. doi: 10.1001/jamanetworkopen.2021.24669
55. Yang J, Tobias DK, Li S, Bhupathiraju SN, Ley SH, Hinkle SN, et al. Habitual coffee consumption and subsequent risk of type 2 diabetes in individuals with a history of gestational diabetes - a prospective study. Am J Clin Nutr. (2022) 116:1693–703. doi: 10.1093/ajcn/nqac241
56. Tobias DK, Hamaya R, Clish CB, Liang L, Deik A, Dennis C, et al. Type 2 diabetes metabolomics score and risk of progression to type 2 diabetes among women with a history of gestational diabetes mellitus. Diabetes Metab Res Rev. (2024) 40:e3763. doi: 10.1002/dmrr.v40.1
57. Cheung NW, Helmink D. Gestational diabetes: the significance of persistent fasting hyperglycemia for the subsequent development of diabetes mellitus. J Diabetes Complications. (2006) 20:21–5. doi: 10.1016/j.jdiacomp.2005.05.001
58. Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. (2007) 30:878–83. doi: 10.2337/dc06-1816
59. Chamberlain CR, Oldenburg B, Wilson AN, Eades SJ, O’Dea K, Oats JJ, et al. Type 2 diabetes after gestational diabetes: greater than fourfold risk among Indigenous compared with non-Indigenous Australian women. Diabetes Metab Res Rev. (2016) 32:217–27. doi: 10.1002/dmrr.v32.2
60. Wood AJ, Boyle JA, Barr ELM, Barzi F, Hare MJL, Titmuss A, et al. Type 2 diabetes after a pregnancy with gestational diabetes among first nations women in Australia: The PANDORA study. Diabetes Res Clin Pract. (2021) 181:109092. doi: 10.1016/j.diabres.2021.109092
61. Chivese T, Norris SA, Levitt NS. Progression to type 2 diabetes mellitus and associated risk factors after hyperglycemia first detected in pregnancy: A cross-sectional study in Cape Town, South Africa. PloS Med. (2019) 16:e1002865. doi: 10.1371/journal.pmed.1002865
62. Golden SH, Bennett WL, Baptist-Roberts K, Wilson LM, Barone B, Gary TL, et al. Antepartum glucose tolerance test results as predictors of type 2 diabetes mellitus in women with a history of gestational diabetes mellitus: a systematic review. Gend Med. (2009) 6 Suppl 1:109–22. doi: 10.1016/j.genm.2008.12.002
63. Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. (2016) 59:1403–11. doi: 10.1007/s00125-016-3927-2
64. Tudurí E, Soriano S, Almagro L, Montanya E, Alonso-Magdalena P, Nadal Á, et al. The pancreatic β-cell in ageing: Implications in age-related diabetes. Ageing Res Rev. (2022) 80:101674. doi: 10.1016/j.arr.2022.101674
65. Chaudhary R, Khanna J, Rohilla M, Gupta S, Bansal S. Investigation of pancreatic-beta cells role in the biological process of ageing. Endocr Metab Immune Disord Drug Targets. (2024) 24:348–62. doi: 10.2174/1871530323666230822095932
66. Aguayo-Mazzucato C. Functional changes in beta cells during ageing and senescence. Diabetologia. (2020) 63:2022–9. doi: 10.1007/s00125-020-05185-6
67. Li N, Liu F, Yang P, Xiong F, Yu Q, Li J, et al. Aging and stress induced β cell senescence and its implication in diabetes development. Aging (Albany NY). (2019) 11:9947. doi: 10.18632/aging.102432
68. Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Dordr). (2016) 38:23. doi: 10.1007/s11357-016-9884-3
69. Krentz AJ, Viljoen A, Sinclair A. Insulin resistance: a risk marker for disease and disability in the older person. Diabetes Med. (2013) 30:535–48. doi: 10.1111/dme.2013.30.issue-5
70. Lekva T, Bollerslev J, Godang K, Roland MC, Friis CM, Voldner N, et al. [amp]]beta;-cell dysfunction in women with previous gestational diabetes is associated with visceral adipose tissue distribution. Eur J Endocrinol. (2015) 173:63–70. doi: 10.1530/EJE-15-0153
71. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. (2017) 127:43–54. doi: 10.1172/JCI88880
72. Saponaro C, Sabatini S, Gaggini M, Carli F, Rosso C, Positano V, et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. (2022) 42:2418–27. doi: 10.1111/liv.v42.11
73. Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, et al. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol. (2023) 64:109–22. doi: 10.1007/s12016-021-08899-6
74. Uyar B, Palmer D, Kowald A, Murua Escobar H, Barrantes I, Möller S, et al. Single-cell analyses of aging, inflammation and senescence. Ageing Res Rev. (2020) 64:101156. doi: 10.1016/j.arr.2020.101156
75. Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. (2018) 119:105–10. doi: 10.1002/jcb.v119.1
76. Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. (2017) 27:229–36. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712
77. Nunes VA, Portioli-Sanches EP, Rosim MP, Araujo MS, Praxedes-Garcia P, Valle MM, et al. Progesterone induces apoptosis of insulin-secreting cells: insights into the molecular mechanism. J Endocrinol. (2014) 221:273–84. doi: 10.1530/JOE-13-0202
78. Rebarber A, Istwan NB, Russo-Stieglitz K, Cleary-Goldman J, Rhea DJ, Stanziano GJ, et al. Increased incidence of gestational diabetes in women receiving prophylactic 17alpha-hydroxyprogesterone caproate for prevention of recurrent preterm delivery. Diabetes Care. (2007) 30:2277–80. doi: 10.2337/dc07-0564
79. Waters TP, Schultz BAH, Mercer BM, Catalano PM. Effect of 17alpha-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstet Gynecol. (2009) 114:45–9. doi: 10.1097/AOG.0b013e3181a9454b
80. Rabhi N, Salas E, Froguel P, Annicotte JS. Role of the unfolded protein response in β cell compensation and failure during diabetes. J Diabetes Res. (2014) 2014:795171. doi: 10.1155/2014/795171
81. Said MA, Nafeh NY, Abdallah HA. Spexin alleviates hypertension, hyperuricaemia, dyslipidemia and insulin resistance in high fructose diet induced metabolic syndrome in rats via enhancing PPAR-γ and AMPK and inhibiting IL-6 and TNF-α. Arch Physiol Biochem. (2023) 129:1111–6. doi: 10.1080/13813455.2021.1899242
82. Mirzoyan Z, Valenza A, Zola S, Bonfanti C, Arnaboldi L, Ferrari N, et al. A Drosophila model targets Eiger/TNFα to alleviate obesity-related insulin resistance and macrophage infiltration. Dis Model Mech. (2023) 16. doi: 10.1242/dmm.050388
83. Shi C, Zhu L, Chen X, Gu N, Chen L, Zhu L, et al. IL-6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J Interferon Cytokine Res. (2014) 34:342–8. doi: 10.1089/jir.2013.0078
84. Mancusi C, Izzo R, di Gioia G, Losi MA, Barbato E, Morisco C. Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev. (2020) 27:515–26. doi: 10.1007/s40292-020-00408-8
85. Wang S, Wang Q, Yan X. Association between triglyceride-glucose index and hypertension: a cohort study based on the China Health and Nutrition Survey (2009-2015). BMC Cardiovasc Disord. (2024) 24:168. doi: 10.1186/s12872-024-03747-9
86. Li J, Leng J, Li W, Zhang C, Feng L, Wang P, et al. Roles of insulin resistance and beta cell dysfunction in macrosomia among Chinese women with gestational diabetes mellitus. Primary Care Diabetes. (2018) 12:565–73. doi: 10.1016/j.pcd.2018.07.010
87. Yang W, Liu J, Li J, Liu J, Liu H, Wang Y, et al. Interactive effects of prepregnancy overweight and gestational diabetes on macrosomia and large for gestational age: a population-based prospective cohort in Tianjin, China. Diabetes Res Clin Practice. (2019) 154:82–9. doi: 10.1016/j.diabres.2019.06.014
88. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. (2020) 21:1835. doi: 10.3390/ijms21051835
89. Zhao M, Wang S, Zuo A, Zhang J, Wen W, Jiang W, et al. HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury. Cell Mol Biol Lett. (2021) 26:40. doi: 10.1186/s11658-021-00283-8
90. Zhao N, Yu X, Zhu X, Song Y, Gao F, Yu B, et al. Diabetes mellitus to accelerated atherosclerosis: shared cellular and molecular mechanisms in glucose and lipid metabolism. J Cardiovasc Transl Res. (2024) 17:133–52. doi: 10.1007/s12265-023-10470-x
91. Yin B, Ding L, Chen Z, Chen Y, Zhu B, Zhu Y. Combining HbA1c and insulin resistance to assess the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Res Clin Pract. (2023) 199:110673. doi: 10.1016/j.diabres.2023.110673
92. Zhu HC, Tao Y, Li YM. Correlations of insulin resistance and HbA1c with cytokines IGF-1, bFGF and IL-6 in the aqueous humor of patients with diabetic cataract. Eur Rev Med Pharmacol Sci. (2019) 23:16–22. doi: 10.26355/eurrev_201901_16742
93. Papakonstantinou E, Oikonomou C, Nychas G, Dimitriadis GD. Effects of diet, lifestyle, chrononutrition and alternative dietary interventions on postprandial glycemia and insulin resistance. Nutrients. (2022) 14:823. doi: 10.3390/nu14040823
94. Eickhoff H, Guimarães A, Louro TM, Seiça RM, Castro ESF. Insulin resistance and beta cell function before and after sleeve gastrectomy in obese patients with impaired fasting glucose or type 2 diabetes. Surg Endosc. (2015) 29:438–43. doi: 10.1007/s00464-014-3675-7
95. Kuo FY, Cheng KC, Li Y, Cheng JT. Oral glucose tolerance test in diabetes, the old method revisited. World J Diabetes. (2021) 12:786–93. doi: 10.4239/wjd.v12.i6.786
96. Lages M, Barros R, Moreira P, Guarino MP. Metabolic effects of an oral glucose tolerance test compared to the mixed meal tolerance tests: A narrative review. Nutrients. (2022) 14:2032. doi: 10.3390/nu14102032
97. Ghoreishi PS, Shams M, Nimrouzi M, Zarshenas MM, Lankarani KB, Fallahzadeh Abarghooei E, et al. The effects of ginger (Zingiber officinale roscoe) on non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: A randomized double-blinded placebo-controlled clinical trial. J Diet Suppl. (2024) 21:294–312. doi: 10.1080/19390211.2023.2263788
98. Alvianto S, Widjanarko ND, Lionardi SK, Arifin ES. Unveiling the metabolic effects of ganoderma lucidum in humans: A systematic review and meta-analysis. Traditional Integr Med. (2024), 318–38. doi: 10.18502/tim.v9i3.16536
Keywords: diabetes, epidemiology, endocrinology, prenatal care, high-risk pregnancy, gestational diabetes mellitus, women’s health
Citation: Chen K, Tang L, Wang X, Li Y, Zhang X, Cui S, Chen W, Jin Z and Zhu D (2024) Prevalence and risk factors for type 2 diabetes mellitus in women with gestational diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 15:1486861. doi: 10.3389/fendo.2024.1486861
Received: 27 August 2024; Accepted: 02 December 2024;
Published: 23 December 2024.
Edited by:
A. Seval Ozgu-Erdinc, Ankara Bilkent City Hospital University, TürkiyeReviewed by:
Brianne Guilford, Southern Illinois University Edwardsville, United StatesMohammad Hashem Hashempur, Shiraz University of Medical Sciences, Iran
Copyright © 2024 Chen, Tang, Wang, Li, Zhang, Cui, Chen, Jin and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danping Zhu, emRwNzkwMjAzQDE2My5jb20=; Zhao Jin, ZHIuamluemhhb0BjZHV0Y20uZWR1LmNu
 Kaiqi Chen
Kaiqi Chen Lichao Tang1
Lichao Tang1 Yunhua Li
Yunhua Li Zhao Jin
Zhao Jin