- 1Department of Surgery, Community Health Service Center of Suzhou Science & Technology Town, Suzhou, Jiangsu, China
- 2Department of General Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 3Department of Rehabilitation, The People’s Hospital of Suzhou New District, Suzhou, Jiangsu, China
Introduction: Radioactive iodine (RAI) is commonly used in the management of differentiated thyroid cancers (DTCs). However, the long-term efficacy and the risk of tumor recurrence associated with it remain unclear. In particular, the comparison between recombinant human thyrotropin (rhTSH) and thyroid hormone withdrawal (THW) in terms of medium- and long-term recurrence rate in DTC patients has not been fully elucidated.
Methods: A systematic search was carried out to identify articles comparing medium- and long-term outcomes (> 2 years) based on treatment with either rhTSH or THW. Ten studies, consisting of six randomized controlled trials (RCTs) and four retrospective studies with a total of 2,833 patients, were included in the analysis.
Results: There was no significant difference in the medium- and long-term recurrence rates between the rhTSH group and the THW group. This was also the case in subgroup analyses of only RCTs or only retrospective studies. The structural incomplete response (SIR) rate was slightly higher in the rhTSH group, but a subgroup analysis of RCTs alone showed no significant difference in SIR between the two groups.
Discussion: rhTSH is comparable to THW in achieving successful ablation of residual disease and maintaining low recurrence rates. However, further RCTs are required to investigate whether rhTSH can increase the risk of SIR.
1 Introduction
Thyroid cancer is the most common endocrine tumor, and its incidence has been increasing globally in recent decades (1). Approximately 614,000 and 206,000 new cases in females and males, respectively, were reported worldwide in 2022 (2). The most common type, differentiated thyroid cancer (DTC), constitutes about 90% and has a good prognosis (3). With standardized treatment, clinical remission, which has a 10-year cancer-specific mortality rate of less than 10%, is typically achievable. However, when distant metastasis occurs, the overall survival rate of DTC decreases to 40% (4). The main treatment modality for DTC includes total or near-total thyroidectomy, radioactive iodine (RAI) therapy, and thyroid hormone suppression therapy for at least six decades (5). RAI treatment refers to the clinical use of iodine-131 (131I), a radioisotope of iodine. This practice is widely accepted in the management of DTC and is often used post-surgery for the ablation of residual thyroid tissue (6). For RAI to be effective, a heightened concentration of thyroid stimulating hormone (TSH) is necessary to maximize the uptake of 131I in normal or neoplastic thyroid cells. This can be achieved through thyroid hormone therapy withdrawal (THW) or the administration of recombinant human TSH (rhTSH) (5).
The European Medical Association first approved rhTSH in 2005 to be used as an adjunctive tool of RAI for diagnostic or therapeutic purposes in patients who have undergone thyroidectomy for DTC (7). This was followed by an FDA approval in 2007. A meta-analysis and multiple other studies have confirmed that the ablation rate of residual tissue after rhTSH administration is similar to that obtained after THW (8–11). In contrast to THW, patients can continue levothyroxine (LT4) supplementation, which aids in avoiding short-term hypothyroidism, preserving quality of life, maintaining normal renal iodine clearance, and reducing potential side effects (12). Therefore, rhTSH has garnered recommendations in multiple guidelines for use alongside RAI in patients with DTC (5, 13).
Despite the long-term clinical use of rhTSH, most studies have focused on its short-term efficacy. Hence, there is still a lack of research on long-term efficacy and recurrence, a gap that persists even in the most recent guidelines (13). This meta-analysis summarized previous studies, with the main objective of evaluating the long-term efficacy of RAI therapy using various preparation methods. The overarching goal was to aid decision making based on the efficacy results.
2 Methods
This meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and is registered in the PROSPERO database (CRD42023437018).
2.1 Search strategy
Two investigators (L.S. and Z.W.) performed independent searches in electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, and ClinicalTrial, for articles published in English from inception until May 2023. There was no restriction based on country of origin or article type. The reference list of each article was independently screened to identify additional studies not obtained from the initial search. The following search terms were used: “thyrotropin alfa,” “rhTSH,” “thyroid neoplasms,” “follow-up,” and “long-term.”
2.2 Study selection and inclusion/exclusion criteria
Studies were screened for appropriateness before retrieval of the full article by two reviewers (Q.Y. and L.S.), and disagreements were resolved by consensus. Studies were included for analysis if all the following criteria were met:
(1) Randomized controlled clinical trials (RCTs) or observational studies with a retrospective or prospective design that evaluated patients with DTC;
(2) Studies evaluating clinical outcomes from adult patients (at least age 16 years);
(3) Patients must have undergone total or near-total thyroidectomy as a primary treatment for DTC;
(4) Median duration of follow-up of more than 2 years.
2.3 Risk of bias and quality assessment
Each eligible RCT was assessed by two independent reviewers (Q.Y. and L.S.) using the Cochrane risk of bias tool 2.0, which reviewed the following seven areas: random sequence generation, allocation concealment, masking of participants and personnel, masking of outcome assessment, incomplete outcome data, selective reporting, and other biases (14). The quality of observational studies was also assessed using the Newcastle-Ottawa scale (15). A risk of bias graph was drawn, and a risk of bias summary was compiled.
2.4 Statistical analysis
Statistical analysis was performed using the Review Manager software (version 5.3; The Nordic Cochrane Center). Forest plots were generated for dichotomous variables. Dichotomous variables were analyzed using the Mantel-Haenszel risk ratios (RRs) with 95% confidence intervals (CIs), while continuous variables were analyzed using weighted-mean differences. Heterogeneity between studies was assessed using the Cochrane χ2 value, and the degree of heterogeneity was measured by the I2 value. A fixed-effects model was used for calculations unless significant heterogeneity existed (I2 >50%), in which case a random-effects model was used. A P value of <0.05 was considered statistically significant. Sensitivity analysis was designed to exclude one study at a time, RCTs or observation studies only, studies with a long median follow-up time (>5 years), and studies after 2015. Secondary outcomes were considered successful ablation, structure incomplete response, and biochemical incomplete response (5).
3 Results
3.1 Findings from the literature search
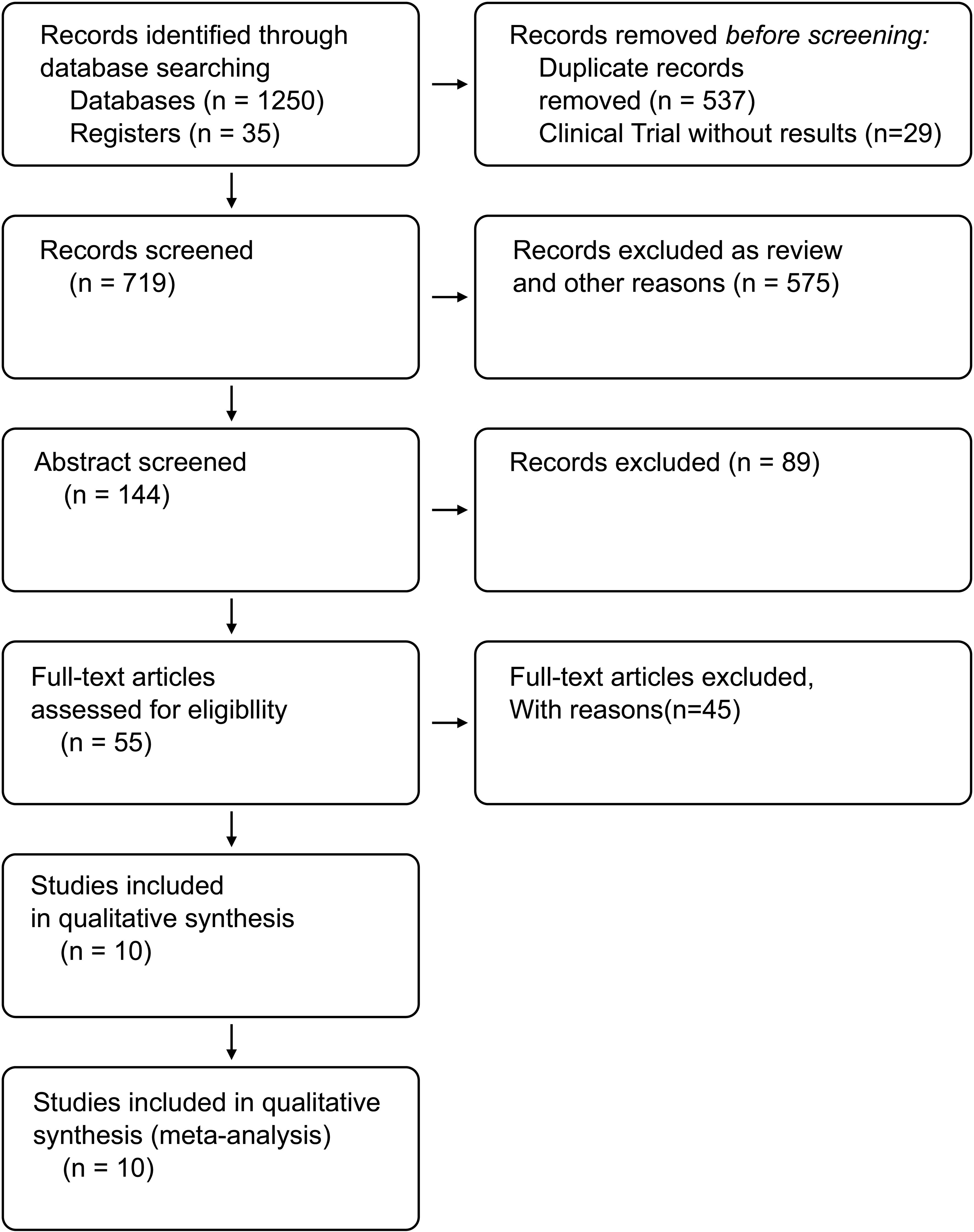
A total of 1,285 studies were identified following the comprehensive search, of which 1,230 were excluded based on titles and abstracts. A total of ten studies were considered eligible, including six RCTs (16–21) and four retrospective studies (22–25). Figure 1 shows the flowchart of our search strategy.
3.2 Study characteristics
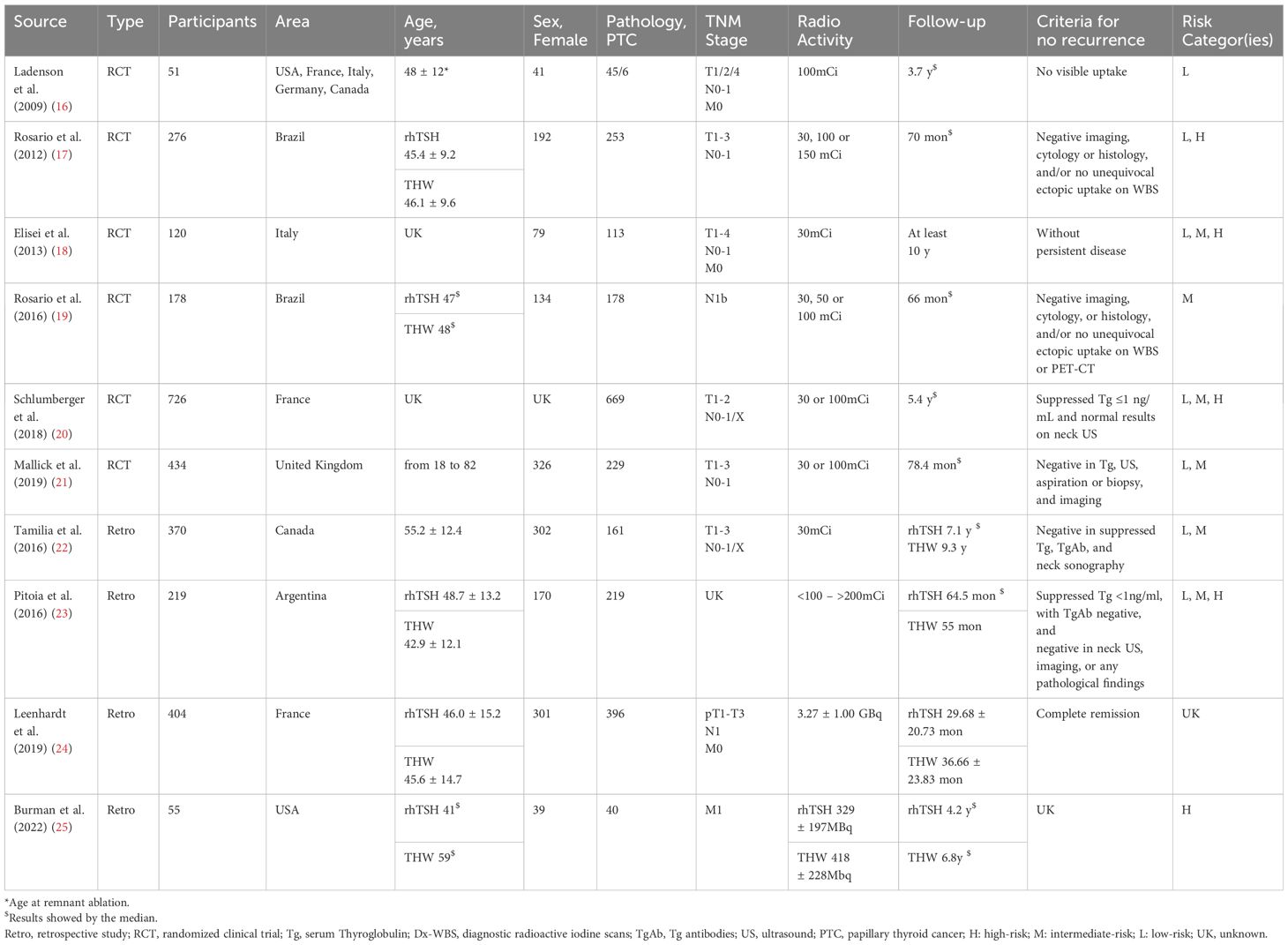
The ten included studies recruited 2,833 patients and were conducted in Europe, South America, Canada, and the US. Doses of 131I ranged from 30 mCi to more than 200 mCi. One study focused on patients with low-risk DTC (16), and another examined patients with intermediate-risk DTC (19). Additionally, several other studies investigated patients across multiple risk levels of DTC. The median follow-up time was at least 3.7 years (16). The characteristics of the included RCTs and observational studies are illustrated in Table 1.
3.3 Patients’ characteristics
The rhTSH group included 1,303 participants (46%), and the THW group included 1,530 (54%) participants. There were 1,584 and 513 female and male participants, respectively, and the gender of the remaining 726 participants was not mentioned (20). A total of 2,303 participants (81.3%) received a diagnosis of papillary thyroid cancer (PTC), and 530 (18.7%) had other subtypes of thyroid cancer.
3.4 Quality assessment
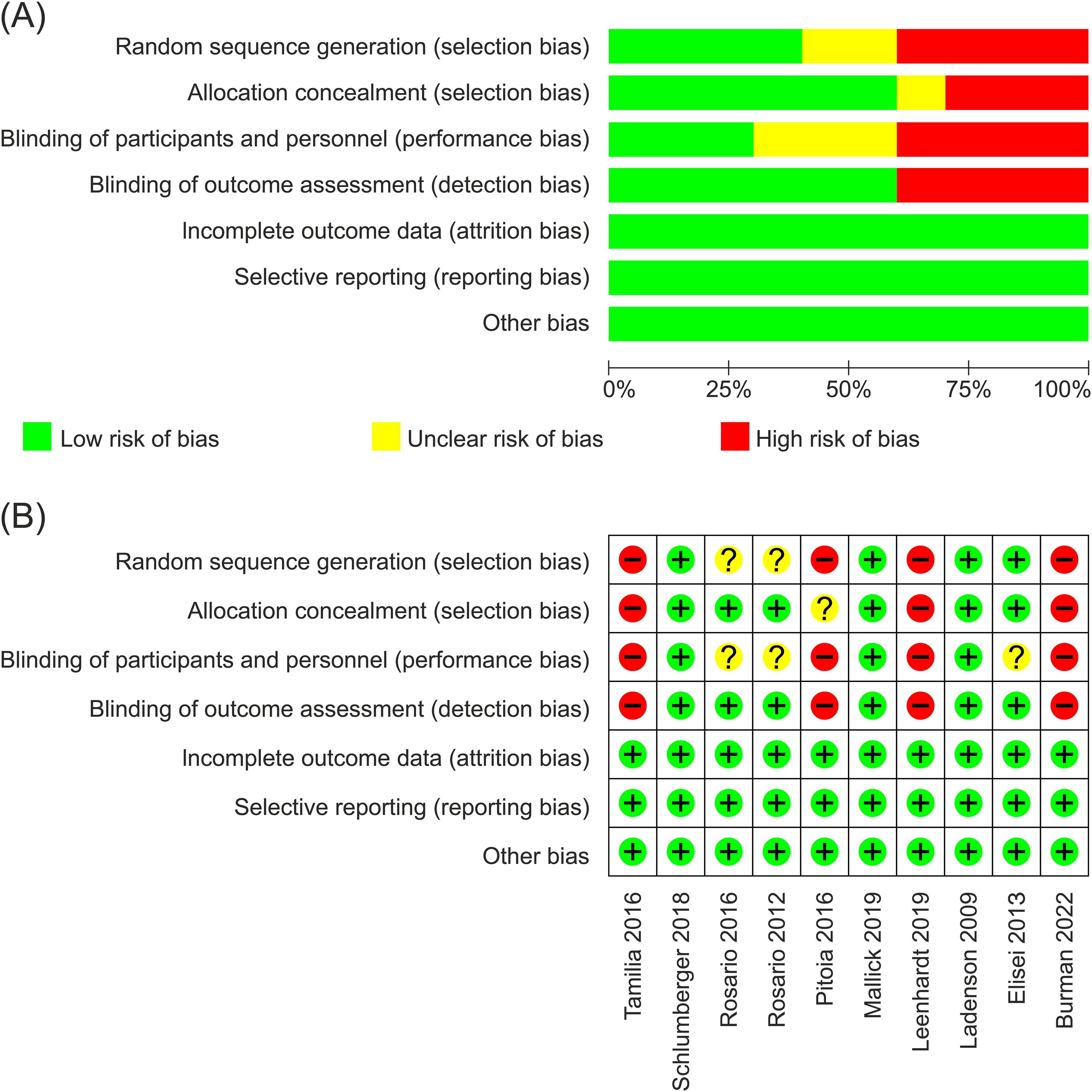
The methodological quality of the studies assessed with Newcastle-Ottawa scale was generally good. Figures 2A, B provide a risk of bias summary and graph for the included studies. Studies were all open-label because of different methods to stimulate TSH in two groups (the rhTSH group using rhTSH for two consecutive days and the THW group discontinuing thyroid hormone for 3–6 weeks). Three studies employed independent imaging reviews to exhibit an approach aimed at minimizing bias resulting from the non-blind nature of all included studies (16, 20, 21).
3.5 Primary outcomes
3.5.1 Recurrence rates
One hundred and twenty-three participants (9.5%) experienced recurrence in the rhTSH group, while 167 individuals (11.0%) in the THW group had a recurrence. There was no heterogeneity among the included studies (I2 = 0%; P=0.68), so a fixed-effects model was used. No statistical difference in recurrence rates was observed between the two groups (RR=1.07; 95% CI, 0.87–1.32; P = 0.53) (Figure 3).
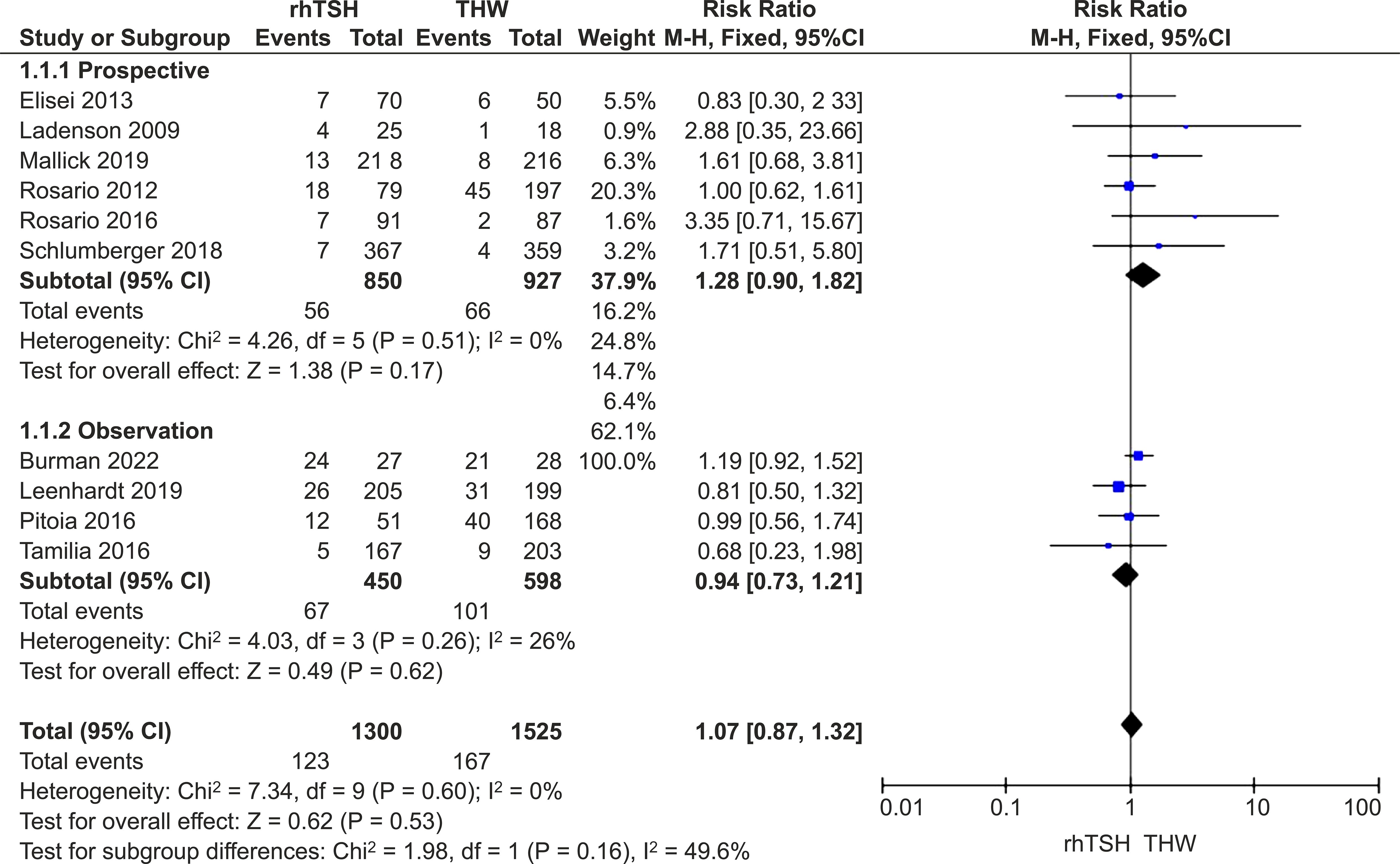
Figure 3. Forest plots comparing the recurrence rates between groups receiving rhTSH and THW, including subgroup analyses of RCTs and retrospective studies. rhTSH, recombinant human thyroid stimulating hormone; THW, thyroid hormone withdrawal; RCT, randomized controlled trial.
3.5.2 Sensitivity analysis and subgroup analysis based on study design
Exclusion of any study did not significantly alter the findings from the main analysis. Analysis of only RCTs (RR=1.28; 95% CI: 0.90–1.82; P=0.17) or observation studies (RR=0.94; 95% CI: 0.73–1.21; P=0.62) showed no difference in recurrence rates between the groups (Figure 3).
3.5.3 Subgroup analysis of studies with 5-year follow-up
Seven studies provided long-term (at least 5-year) follow-up data (16, 17, 19–23). There was no difference in recurrence rates between the two groups in this subset of studies (RR=1.14; 95% CI, 0.81–1.60; P = 0.46; I2 = 0%; P=0.60).
3.5.4 Subgroup analysis of studies conducted after 2015
Seven studies conducted after 2015 were included in our analysis (19–25). There was no difference in recurrence rates between the two groups in this subset of studies (RR=1.12; 95% CI, 0.80–1.56; P = 0.51; I2 = 14%; P=0.32).
3.5.5 Subgroup analysis based on 131I dose
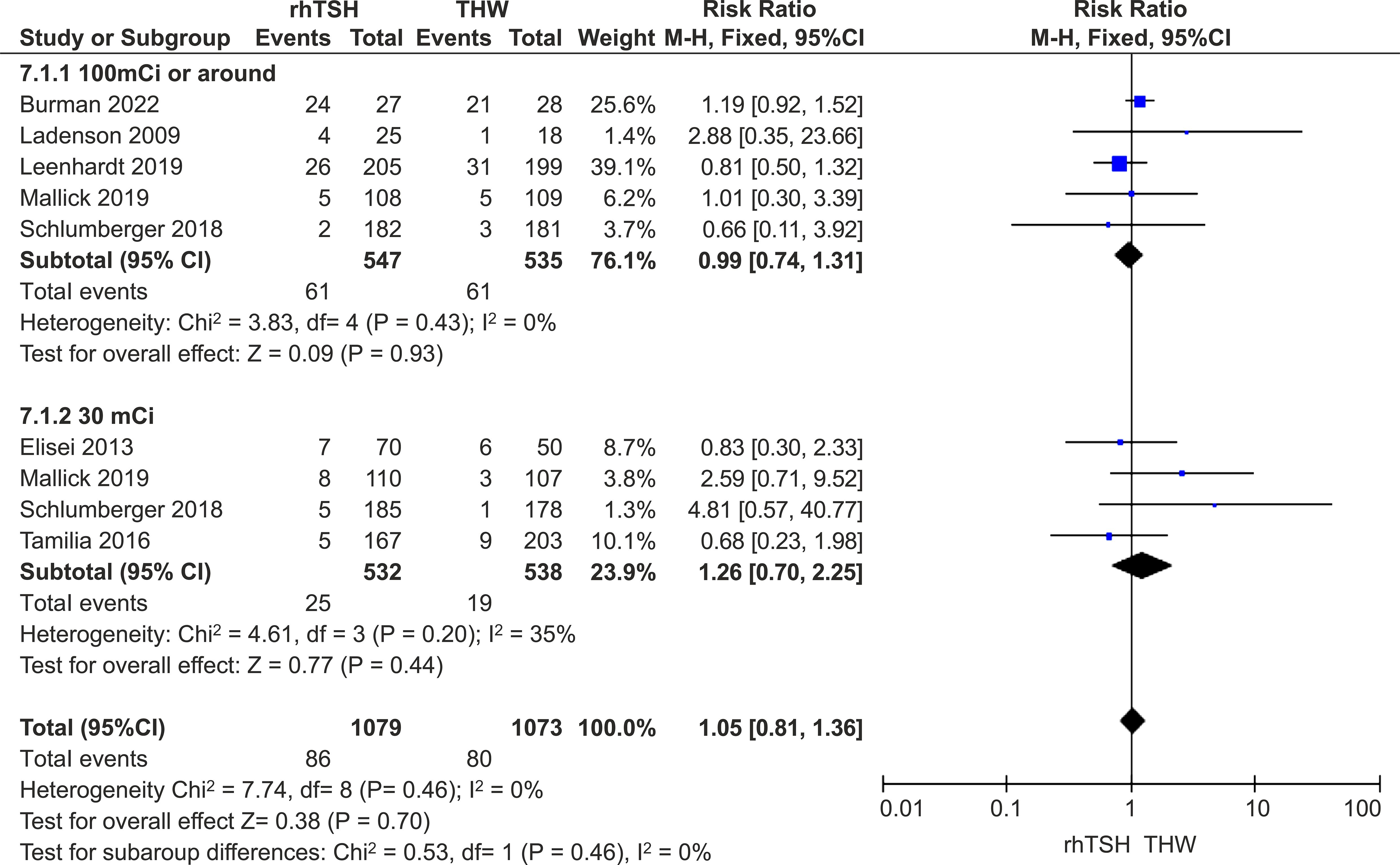
Five studies provided medium- and long-term follow-up data on patients who received approximately 100 mCi of RAI (16, 20, 21, 24, 25), and four studies provided data for those who received approximately 30 mCi (18, 20–22). The other studies did not clearly present the situation of patients with different doses (17, 19, 23). There was no difference between the two groups in this subset of studies (RR=1.05; 95% CI, 0.81–1.36; P=0.46; I2 = 0%; P=0.46) (Figure 4).
3.5.6 Subgroup analysis based on risk strata
Only three studies provided data on low-risk patients (16, 18, 23), while four studies provided detailed data on intermediate- and high-risk patients (16, 17, 23, 25). Other studies did not detail the specific data of each risk level (19–22, 24). There was no significant difference (RR=1.33; 95% CI, 0.96–1.84; P=0.09; I2 = 45%; P=0.12). Furthermore, detailed data showed only 229 low-risk patients and 337 intermediate- and high-risk patients, so the results may not be reliable.
3.6 Secondary outcomes
3.6.1 Successful ablation
Nine studies were included in this analysis (16–24), and the rate of successful ablation was similar in both groups (80.5% vs. 81.2%). Our meta-analysis demonstrated no significant difference in successful ablation between studies that utilized different preparations for RAI (RR=0.81; 95% CI, 0.66–1.00; P=0.05; I2 = 0%; P=0.75). In an analysis of RCTs only, there was no significant intergroup difference in ablation rates (RR=0.88; 95% CI, 0.65–1.20; P=0.41).
3.6.2 Structural incomplete response
There was no heterogeneity of studies (I2 = 0%; P = 0.61); hence, a fixed-effects model was used. Our meta-analysis of nine studies (16–24) demonstrated that the SIR rate was a little higher in the rhTSH group (RR=1.51; 95% CI, 1.04–2.20; P=0.03). However, a subgroup analysis of RCTs showed no significant difference between the two groups (RR=1.64; 95% CI, 0.98–2.73; P=0.06) (Figure 5).
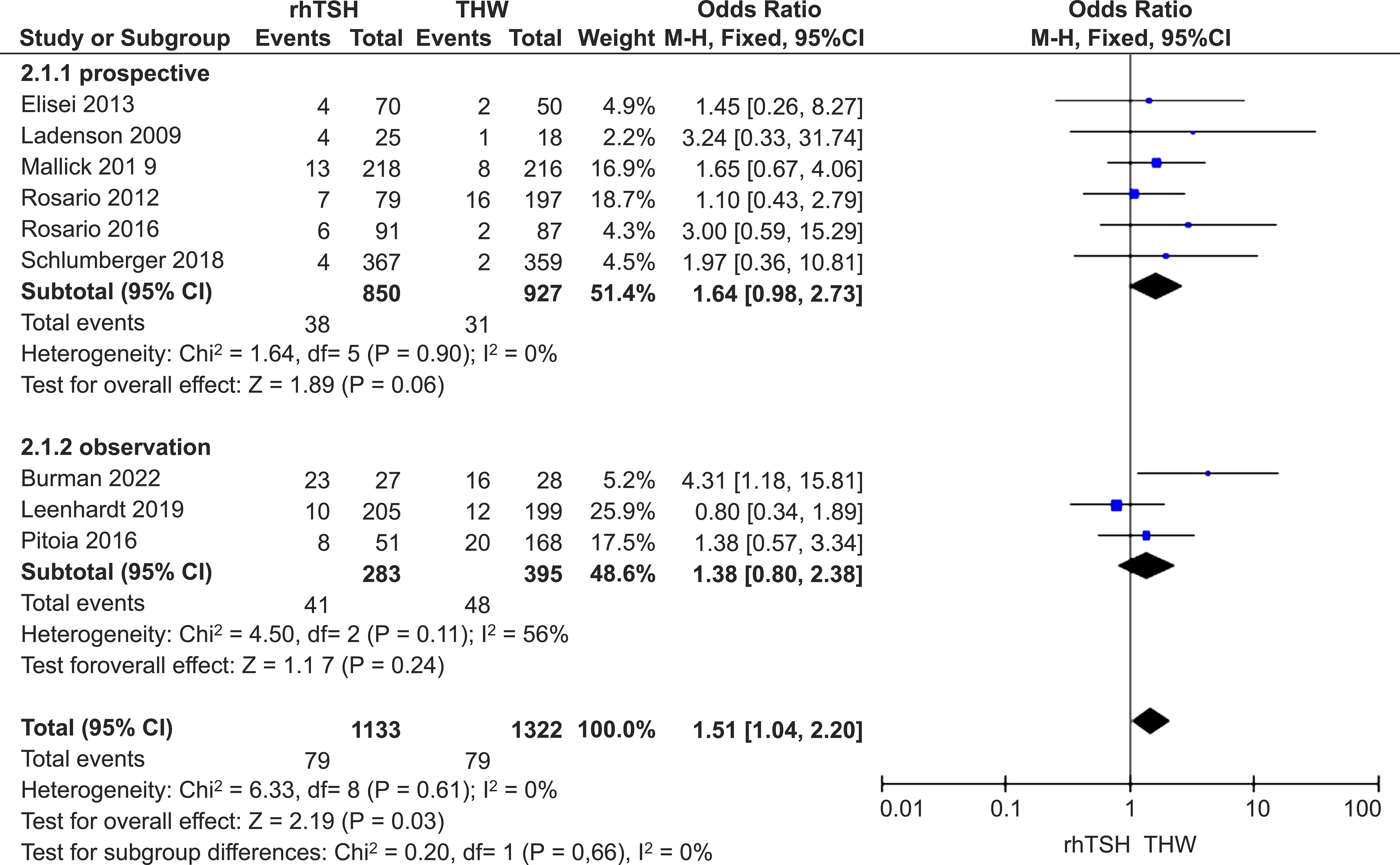
Figure 5. Forest plots comparing SIR rates between groups receiving rhTSH and THW, including subgroup analyses of RCTs and retrospective studies. SIR, structural incomplete response; rhTSH, recombinant human thyroid stimulating hormone; THW, thyroid hormone withdrawal; RCT, randomized controlled trial.
3.6.3 Biochemical incomplete response
This analysis included five studies (16, 20, 23–25) with no heterogeneity (I2 = 0%; P=0.61). Meta-analysis demonstrated no significant difference between the two groups of participants (RR=0.76; 95% CI, 0.47–1.25; P=0.28). Only two RCTs provided BIR data, so the analysis of RCTs seemed insignificant (16, 20).
4 Discussion
For over two decades, rhTSH has been available and included in recommendations by several guidelines to replace THW for use alongside RAI (5, 13). However, some issues regarding its use require further clarification. One is the medium- and long-term efficacy of RAI combined with rhTSH. DTCs are known to grow slowly and have a good prognosis, with a 5-year overall survival rate of over 95%, while the recurrence rate ranges from 14% to 30%. Therefore, the risk of recurrence and the medium- and long-term persistence of DTC are worthy of attention. However, the long-term follow-up data with a duration exceeding 5 years is relatively scant. Two years can act as an intermediate milestone to preliminarily gauge the treatment effectiveness. Future research will be dedicated to obtaining more long-term follow-up data to more accurately assess the recurrence situation. The most important finding from our meta-analysis comparing the medium- and long-term efficacy of rhTSH and THW in patients undergoing RAI for DTC showed no difference between the two methods, and this was consistent with the results of RCTs.
Notably, the definition of recurrence adopted in various studies was inconsistent. Some articles defined recurrence as “clinical evidence of tumor recurrence after a disease-free period, such as suspicious lymph nodes, thyroid bed nodules, or highly suspicious metastatic lesions,” which is similar to SIR (20, 21). Other studies added biochemical indicators for recurrence assessment (16, 23), although the criteria were disparate. This limitation and discrepancy in the employed criteria affected recurrence results.
A release by the American Thyroid Association in 2015 (ATA 2015) advocated for tailored management of DTC based on prognosis. The guideline provided evidence that tumors identified with RAI imaging can be used as criteria for structural assessment and detection of stimulating thyroglobulin levels (> 1ng/mL) for biochemical recurrence. The disease outcome at any point during follow-up in the guideline is described by four categories: excellent response (ER), BIR, SIR, and indeterminate response (IDR). This guideline also provided a favorable evaluation framework rather than methods to evaluate the prognosis of DTC, which is a more convenient and accurate approach. Of these four outcomes, BIR has a 20% probability of progressing into SIR (26, 27). Also, 50% to 85% of patients with SIR continue to have persistent disease (28–30), further lending credence to the fact that these two outcomes warrant attention. According to the specific situation of disease recurrence, SIR and BIR were analyzed in this meta-analysis. The results showed that the SIR rate was slightly higher in the rhTSH group than in the THW group, with similar results in RCTs, while there was no difference in the BIR rate of both groups. SIR deserves attention in clinical practice because it indicates a poor prognosis. The results of this meta-analysis show that the medium- and long-term outcomes of rhTSH in SIR require further investigation. More RCTs may be needed to confirm the specific results.
Due to the importance of the ATA 2015 guideline, a subgroup analysis of studies published after 2015 was conducted. Additionally, the guideline clarified that low-risk patients can be exempted from RAI treatment, and a subgroup analysis of different patient categories was also conducted. The results of these two analyses showed no significant difference between patients receiving rhTSH and THW. However, it is worth noting that no RCTs were launched after 2015, and the statistics of intermediate- and high-risk patients were unclear in the studies of this meta-analysis. This constitutes a major limitation of our study.
The participants included in this study were mainly intermediate-risk and low-risk patients, with a few being high-risk. It is not clearly indicated whether rhTSH can be administered to high-risk patients. Several retrospective studies have shown that for 131I adjuvant therapy, the therapeutic efficacy and medium- and long-term prognosis observed in the rhTSH and THW groups are similar (31, 32). However, no prospective studies have corroborated this finding. For the risk analysis, we acknowledge that the limited number of low-risk patients (only 229 cases) and the unclear statistical data of intermediate- and high-risk patients in the subgroup analysis are indeed limitations of this study. In future research, we plan to more strictly define the risk criteria and increase the sample size, especially for medium- and long-term follow-up studies of patients at different risk levels, to improve the reliability of the risk analysis.
The “Martinique Principles” propose that 131I therapy can be used for residual ablation, adjuvant treatment, and treatment of known disease (33). They also recognize that residual ablation and adjuvant treatment cannot be accurately distinguished. Otherwise, subgroup analysis based on the dose makes the comparison among different studies more feasible and meaningful as the dose is a more relatively objective and quantifiable indicator than indication. Therefore, we conducted a subgroup analysis based on the dose of 131I rather than the indication of use (residual ablation or adjuvant treatment). Although the difference is not significant, the choice of 131I dose remains a debatable issue, particularly in situations where residual ablation is recommended only in intermediate- and high-risk patients and also combined with adjuvant treatment. More studies specifically focusing on medium- and long-term recurrence with different 131I doses are needed to provide clarity on this matter.
Since 2015, many guidelines have updated the clinical application of rhTSH beyond residual ablation (13, 34, 35) in the absence of corresponding follow-up data. Our subgroup analysis of studies with a 5-year follow-up showed no significant difference between groups. As DTC can relapse 20 to 30 years after initial treatment (36, 37), patients should be observed for longer follow-up durations.
5 Conclusion
The cumulative data of our systematic review and meta-analysis revealed no significant difference in the medium- and long-term recurrence rate of patients receiving rhTSH or THW in preparation for RAI. More extensive RCTs, especially based on intermediate-risk and high-risk patients are needed to explore whether rhTSH can increase the risk of SIR.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Zhongliang Wu, d3psMTU1NUBzaW5hLmNvbQ==.
Author contributions
ZW: Conceptualization, Methodology, Writing – review & editing. QY: Data curation, Investigation, Writing – original draft, Writing – review & editing. LS: Data curation, Investigation, Writing – review & editing. JX: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge Di Yan for his guidance in software during the preparation of this work. We also would like to acknowledge Editage (https://www.editage.com/) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3. Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: a systematic review and meta-analysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. (2004) 89:3668–76. doi: 10.1210/jc.2003-031167
4. Magarey MJR, Freeman JL. Recurrent well-differentiated thyroid carcinoma. Oral Oncol. (2013) 49:689–94. doi: 10.1016/j.oraloncology.2013.03.434
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
6. Woodrum DT, Gauger PG. Role of 131I in the treatment of well differentiated thyroid cancer. J Surg Oncol. (2005) 89:114–21. doi: 10.1002/jso.20185
7. Haugen BR, Cooper DS, Emerson CH, Luster M, Maciel RMB, Biscolla RPM, et al. Expanding indications for recombinant human TSH in thyroid cancer. Thyroid. (2008) 18:687–94. doi: 10.1089/thy.2008.0162
8. Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. (2006) 91:926–32. doi: 10.1210/jc.2005-1651
9. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. (2012) 366:1674–85. doi: 10.1056/NEJMoa1109589
10. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. (2012) 366:1663–73. doi: 10.1056/NEJMoa1108586
11. Xu G, Wu T, Ge L, Li W. A systematic review of adjuvant interventions for radioiodine in patients with thyroid cancer. Oncol Res Treat. (2015) 38:368–72. doi: 10.1159/000433486
12. Duntas LH, Biondi B. Short-term hypothyroidism after levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences. Eur J Endocrinol. (2007) 156:13–9. doi: 10.1530/eje.1.02310
13. Wadsley J, Armstrong N, Bassett-Smith V, Beasley M, Chandler R, Cluny L, et al. Patient preparation and radiation protection guidance for adult patients undergoing radioiodine treatment for thyroid cancer in the UK. Clin Oncol (R Coll Radiol). (2023) 35:42–56. doi: 10.1016/j.clon.2022.07.002
14. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.4. UK: Cochrane (2023). Available at: http://www.training.cochrane.org/handbook (accessed August 22, 2023).
15. Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. (2003) 7:1–173. doi: 10.3310/hta7270
16. Elisei R, Schlumberger M, Driedger A, Reiners C, Kloos RT, Sherman SI, et al. Follow-up of low-risk differentiated thyroid cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after either recombinant human thyrotropin or thyroid hormone withdrawal. J Clin Endocrinol Metab. (2009) 94:4171–9. doi: 10.1210/jc.2009-0869
17. Rosario PW, Mineiro Filho AFC, Lacerda RX, Calsolari MR. Long-term follow-up of at least five years after recombinant human thyrotropin compared to levothyroxine withdrawal for thyroid remnant ablation with radioactive iodine. Thyroid. (2012) 22:332–3. doi: 10.1089/thy.2011.0242
18. Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, et al. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity ¹³¹I after either recombinant human tsh or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up. J Clin Endocrinol Metab. (2013) 98:2693–700. doi: 10.1210/jc.2012-4137
19. Rosario PW, Mourão GF, Calsolari MR. Recombinant human tsh versus thyroid hormone withdrawal in adjuvant therapy with radioactive iodine of patients with papillary thyroid carcinoma and clinically apparent lymph node metastases not limited to the central compartment (cN1b). Arch Endocrinol Metab. (2017) 61:167–72. doi: 10.1590/2359-3997000000247
20. Schlumberger M, Leboulleux S, Catargi B, Deandreis D, Zerdoud S, Bardet S, et al. Outcome after ablation in patients with low-risk thyroid cancer (estimabl1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol. (2018) 6:618–26. doi: 10.1016/S2213-8587(18)30113-X
21. Dehbi HM, Mallick U, Wadsley J, Newbold K, Harmer C, Hackshaw A. Recurrence after low-dose radioiodine ablation and recombinant human thyroid-stimulating hormone for differentiated thyroid cancer (hilo): Long-term results of an open-label, non-inferiority randomised controlled trial. Lancet Diabetes Endocrinol. (2019) 7:44–51. doi: 10.1016/S2213-8587(18)30306-1
22. Mujammami M, Hier MP, Payne RJ, Rochon L, Tamilia M. Long-term outcomes of patients with papillary thyroid cancer undergoing remnant ablation with 30 millicuries radioiodine. Thyroid. (2016) 26:951–8. doi: 10.1089/thy.2016.0036
23. Pitoia F, Abelleira E, Cross G. Thyroglobulin levels measured at the time of remnant ablation to predict response to treatment in differentiated thyroid cancer after thyroid hormone withdrawal or recombinant human TSH. Endocrine. (2017) 55:200–8. doi: 10.1007/s12020-016-1104-5
24. Leenhardt L, Leboulleux S, Bournaud C, Zerdoud S, Schvartz C, Ciappuccini R, et al. Recombinant thyrotropin vs levothyroxine withdrawal in 131I therapy of N1 thyroid cancer: a large matched cohort study (ThyrNod). J Clin Endocrinol Metab. (2019) 104:1020–8. doi: 10.1210/jc.2018-01589
25. Gomes-Lima CJ, Chittimoju S, Wehbeh L, Dia S, Pagadala P, Al-Jundi M, et al. Metastatic differentiated thyroid cancer survival is unaffected by mode of preparation for 131I administration. J Endocr Soc. (2022) 6:bvac032. doi: 10.1210/jendso/bvac032
26. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. (2010) 20:1341–9. doi: 10.1089/thy.2010.0178
27. Vaisman F, Momesso D, Bulzico DA, Pessoa CHCN, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). (2012) 77:132–8. doi: 10.1111/j.1365-2265.2012.04342.x
28. Vaisman F, Shaha A, Fish S, Michael Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). (2011) 75:112–9. doi: 10.1111/j.1365-2265.2011.04002.x
29. Hsieh CJ, Wang PW. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid. (2014) 24:488–93. doi: 10.1089/thy.2012.0611
30. Schuff KG, Weber SM, Givi B, Samuels MH, Andersen PE, Cohen JI. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope. (2008) 118:768–75. doi: 10.1097/MLG.0b013e318162cae9
31. Iizuka Y, Katagiri T, Ogura K, Inoue M, Nakamura K, Mizowaki T. Comparison of thyroid hormone withdrawal and recombinant human thyroid-stimulating hormone administration for adjuvant therapy in patients with intermediate- to high-risk differentiated thyroid cancer. Ann Nucl Med. (2020) 34:736–41. doi: 10.1007/s12149-020-01497-0
32. Hugo J, Robenshtok E, Grewal R, Larson S, Tuttle RM. Recombinant human thyroid stimulating hormone–assisted radioactive iodine remnant ablation in thyroid cancer patients at intermediate to high risk of recurrence. Thyroid. (2012) 22:1007–15. doi: 10.1089/thy.2012.0183
33. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. (2019) 29:461–70. doi: 10.1089/thy.2018.0597
34. Avram AM, Giovanella L, Greenspan B, Lawson SA, Luster M, Van Nostrand D, et al. SNMMI procedure standard/EANM practice guideline for nuclear medicine evaluation and therapy of differentiated thyroid cancer: abbreviated version. J Nucl Med. (2022) 63:15N–35N.
35. Pacini F, Fuhrer D, Elisei R, Handkiewicz-Junak D, Leboulleux S, Luster M, et al. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur Thyroid J. (2022) 11:e210046. doi: 10.1530/ETJ-21-0046
36. Leung AM, Dave S, Lee SL, Campion FX, Garber JR, Pearce EN. Factors determining the persistence or recurrence of well-differentiated thyroid cancer treated by thyroidectomy and/or radioiodine in the boston, Massachusetts area: a retrospective chart review. Thyroid Res. (2011) 4:9. doi: 10.1186/1756-6614-4-9
Keywords: recombinant human thyroid stimulating hormone, differentiated thyroid carcinoma, long-term recurrence, thyroid hormone withdrawal, radioactive iodine
Citation: Yao Q, Song L, Xu J and Wu Z (2024) Medium- and long-term recurrence after radioiodine therapy for differentiated thyroid carcinoma with recombinant human thyrotropin: a meta-analysis. Front. Endocrinol. 15:1474121. doi: 10.3389/fendo.2024.1474121
Received: 01 August 2024; Accepted: 03 December 2024;
Published: 17 December 2024.
Edited by:
Yuebang Yin, Erasmus Medical Center, NetherlandsReviewed by:
Flávia Dornelas Kurkowski, Pontifical Catholic University of Rio Grande do Sul, BrazilQianqian Lu, Peking University, China
Diego Bromfman Pianta, Pontifical Catholic University of Rio Grande do Sul, Brazil
Copyright © 2024 Yao, Song, Xu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongliang Wu, d3psMTU1NUBzaW5hLmNvbQ==; Jun Xu, bW8xMDRAc2luYS5jb20=
†These authors share first authorship
 Qixian Yao1†
Qixian Yao1† Zhongliang Wu
Zhongliang Wu