- 1Department of Geriatrics, Tianjin Medical University General Hospital, Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in the Central Nervous System, Ministry of Education, State Key Laboratory of Experimental Hematology, Tianjin Key Laboratory of Elderly Health, Tianjin Geriatrics Institute, Tianjin, China
- 2Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China
- 3Department of Toxicology and Sanitary Chemistry, School of Public Health, Tianjin Medical University, Tianjin, China
Aims: Few prior studies have explored the relationship between phenylalanine and diabetic small vessel disease (SVD) in patients with different durations of type 2 diabetes mellitus(T2DM). Our study aimed to explore whether phenylalanine is associated with the risk of SVD and to further explore whether phenylalanine interacted with the duration of T2DM to alter the risk of SVD.
Materials and methods: A total of 1,032 T2DM patients were enrolled using the Liaoning Medical University First Affiliated Hospital (LMUFAH) system. SVD was defined as patients with diabetic nephropathy (DN) or diabetic retinopathy (DR) alone, or both. Serum amino acids were measured by mass spectrometry (MS) technology. A binary logistic regression model was used to examine associations of phenylalanine with SVD risk. Restricted cubic spline (RCS) regression was used to draw the odds ratio curves of plasma phenylalanine for SVD. Additive interaction analysis was employed to test the interaction of low phenylalanine with a long duration of T2DM for SVD.
Results: Among the 1,032 T2DM patients, 286 (27.7%) had SVD. Phenylalanine <42μmol/L was associated with a markedly increased risk of SVD (OR 1.76, 95%CI 1.23 to 2.51), which was enhanced by a duration of T2DM of ≥5 years to 4.83 (95%CI 2.97-7.87) with significant additive interactions. The inclusion of phenylalanine and duration of T2DM into a traditional risk factor model substantially increased the area under the receiver operating characteristic curve from 0.67 to 0.71 (95% CI 0.70 to 0.75) (P <0.05).
Conclusions: In Chinese patients with T2DM, phenylalanine <42μmol/L was associated with an increased risk of SVD, which was further amplified by a duration of T2DM of ≥5 years.
1 Introduction
The prevalence of type 2 diabetes mellitus (T2DM) and its complications has resulted in an enormous burden of death and disability worldwide. Complications from T2DM are very common, with half of the patients with T2DM in an observational study of 28 countries in Asia, Africa, South America, and Europe presenting with small vessel disease (SVD) and 27% with macrovascular diseases (1). It is therefore clear that SVD is more prevalent than macrovascular diseases. Diabetic vascular complicating diseases are the primary cause of mortality in diabetes sufferers, the most common of which are diabetic nephropathy (DR) and diabetic retinopathy (DN) (2). According to the epidemiology survey of T2DM in large Chinese cities, DR and DN account for 39.7% and 31.5% of microangiopathy in diabetes, respectively (2). Furthermore, a comparatively low frequency of macrovascular disease in Chinese individuals with diabetes was confirmed in the follow-up study of incident complications (3).
Although standardized treatments based on the strict control of blood glucose, blood pressure, and blood lipids have been verified to be able to slow the progression of diabetic microangiopathy, they have not completely blocked or reversed the disease (2, 4, 5). Hence, expanding the current knowledge on the physiopathology of SVD and identifying novel potential biomarkers may help to facilitate the detection and management of SVD.
In recent years, amino acids and related metabolites in the blood have been intensely studied as some of the most promising biomarker candidates for T2DM and its complications. It is worth noting that a substantial number of studies have shown that circulating amino acid levels are altered in patients with T2DM as well as SVD. Aromatic amino acids (AAAs), i.e., tyrosine, phenylalanine, and tryptophan, play an important role in energy metabolism as substrates of oxaloacetate (6, 7), which can indirectly promote gluconeogenesis and affect the secretion of insulin and glucagon (8). A 6-year-long cohort study found that AAAs were predictors of insulin resistance (9). In cross-sectional studies, phenylalanine shows the most consistent positive associations with prediabetes and insulin resistance (10, 11).
Currently, the association of phenylalanine with SVD in T2DM patients remains controversial. A case-cohort study involving 11,140 participants with T2DM found no prospective association between phenylalanine and SVD (12). Nevertheless, some studies have also found that phenylalanine may be related to SVD to a certain extent by regulating vascular endothelial function and the production of neurotransmitters (13, 14). Furthermore, dysregulation of phenylalanine and impaired conversion to tyrosine (15) may also contribute to the pathogenesis of SVD (16). Beyond this, whether the duration of diabetes affects the association between phenylalanine and SVD in T2DM is currently unknown.
Therefore, we conducted a retrospective study to explore the association between phenylalanine and SVD in T2DM and further tested whether there is an interaction between phenylalanine and the duration of diabetes on the risk of SVD.
2 Materials and methods
2.1 Study design and population
We used a retrospective study design to evaluate the association between phenylalanine and small vessel disease in T2DM. We retrieved electronic medical records of 1,898 patients with a diagnosis of T2DM at the Liaoning Medical University First Affiliated Hospital (LMUFAH), Jinzhou, China who were admitted to the hospital from May 2015 to August 2016 (17). The inclusion criteria were as follows: age ≥18 years; complete information for height, weight, and blood pressure was available. Based on these criteria, 1,032 patients with T2DM who were diagnosed according to the 1999 World Health Organization’s criteria (18) or who were treated with antidiabetic drugs remained in our current analysis. The patients were divided into two groups: SVD and non-SVD. The LMUFAH Clinical Research Ethics Committee approved the study, and informed consent was waived due to the retrospective character of the study, which is consistent with the Helsinki Declaration.
2.2 Data collection
We retrospectively extracted clinical information from the electronic medical system (EMS), including demographics, anthropometrics, biochemical test parameters, medications, and disease status. Age was automatically determined by the EMS as the gap between admission year and birth year. Body weight, height, and blood pressure were measured by specially trained doctors and nurses using standardized methods. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Venous blood samples were taken in the morning after fasting for at least 8 hours. Lipids, i.e., high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and glycated hemoglobin (HbA1c) were examined in the central laboratory of the hospital. Disease status included duration of diabetes, DR, DN, coronary heart disease (CHD), and stroke.
Use of oral anti-diabetic drugs (OAD), insulin, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and beta-blockers, statins, and other lipid-lowering drugs in hospital was documented.
2.3 Clinical definitions
BMI was classified into four categories: underweight (<18.5 kg/m2), normal weight (18.5-23.9 kg/m2), overweight (24-27.9 kg/m2), and obese (≥28 kg/m2) as recommended by the National Health Commission in China (19). According to the criteria recommended by the American Diabetes Association, the reasonable HbA1c goal was<7.0% (20). We divided the duration of diabetes into two categories according to the median (5 years).
Stroke was defined as subarachnoid hemorrhage, cerebral venous thrombosis, spinal cord stroke, or ischemic stroke. CHD was defined as having a history of angina with an abnormal electrocardiogram or on a stress test, myocardial infarction, angina coronary artery bypass graft surgery, or angioplasty. DR was detected by bilateral fundus photography and was diagnosed by clinical manifestations of vascular abnormalities in the retina including microaneurysms, retinal hemorrhages, hard exudates, or vitreous hemorrhage. The clinical diagnosis of DN was defined as persistent albuminuria or progressive reduction in estimated glomerular filtration rate (eGFR) with or without retinopathy and was judged by clinicians (21). In our study, SVD was defined as patients with DN or DR alone, or both (12).
2.4 Measurement of amino acids
A previous study described the measurement of amino acids in detail (22). Briefly, the metabolomics approach was based on mass spectrometry (MS) technology. We collected whole blood after at least 8h fasting which was stored as a dried blood spot (DBS) and used in the metabolomic assay. Metabolites in the DBS were measured by direct infusion MS technology equipped with AB Sciex 4000 QTrap system (AB Sciex, Framingham, MA, USA). High-purity water and acetonitrile from Thermo Fisher (Waltham, MA, USA) were used as the diluting agent and mobile phase, respectively. 1-Butanol and acetyl chloride from Sigma-Aldrich (St Louis, MO, USA) were used to derive samples. Isotope-labeled internal standard samples of 12 amino acids (NSK-A) were purchased from Cambridge Isotope Laboratories (Tewksbury, MA, USA) while standard samples of the amino acids were purchased from Chrom systems (Grafelfing, Germany).
2.5 Statistical analysis
Normally distributed continuous data were expressed as mean ± standard deviation (SD), while skewed data were expressed as median (interquartile range). A Q-Q plot was used to test the normality. Qualitative variables were reported as frequencies (%). To determine significant differences between the two groups, Student’s t-test or the Mann–Whitney U-test was used for the continuous data, while the Chi-square test (or Fisher’s test when appropriate) was used for categorical variables.
A binary logistic regression model was used to obtain the odds ratio (OR) at a 95% confidence interval (CI) of phenylalanine for the risk of SVD. A structured adjustment scheme was established to adjust for the effect of traditional risk factors on T2DM patients with SVD. We obtained univariable OR values and the ORs after adjusting for age, gender, duration of diabetes, BMI, smoking, drinking alcohol, systolic blood pressure (SBP), TG, HDL-C, HbA1c, tyrosine and the use of anti-diabetes drugs, lipid-lowering drugs, and antihypertensive drugs. Restricted cubic spline (RCS) nested in the logistic regression was used to check potential non-linear associations between amino acid levels with the risk of SVD. An RCS curve is a smoothing curve that was used to provide an intuitive non-linear association between amino acid levels with the risk of SVD. In our analysis, we chose four knots (23). As previously (17), we selected a cut-off point by visually checking the curve where the OR of phenylalanine for SVD started to rise rapidly.
We repeated the logistic regression analysis in groups with different diabetes durations to obtain the OR values. Additive interaction analysis was used to verify the relationship between the duration of diabetes (≥5 or <5 years) and phenylalanine for SVD. We calculated the relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and synergy index (S) to estimate additive interactions; RERI>0, AP>0, or S>1 indicated a significant additive interaction (24).
Areas under the receiver operating characteristic curves were compared to provide an estimate of the potential predictive value of phenylalanine and the duration of diabetes beyond the traditional risk factors for SVD.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and a two-sided p-value of <0.05 in all results was accepted as statistically significant.
3 Results
3.1 Characteristics of the study participants
The clinical and biochemical characteristics of the study participants are shown in Tables 1, 2. The mean age of the 1,032 participants was 57.2 (SD 13.8) years, the mean BMI was 25.3 (SD 3.9) kg/m2, and the mean duration of T2DM was 5 (0-10) years. Of the 1,032 patients, 53.2% were male and 286 had SVD. There were 210 (20.4%) and 199 (19.3%) individuals diagnosed with CHD and stroke, respectively. Patients with SVD were older, comprised more women, and had a longer duration of diabetes, higher SBP, and greater use of insulin and ACEIs. Furthermore, patients with SVD had lower β-blockers use, and CHD and stroke were less prevalent compared with those without SVD. Phenylalanine was lower in SVD than in non-SVD.
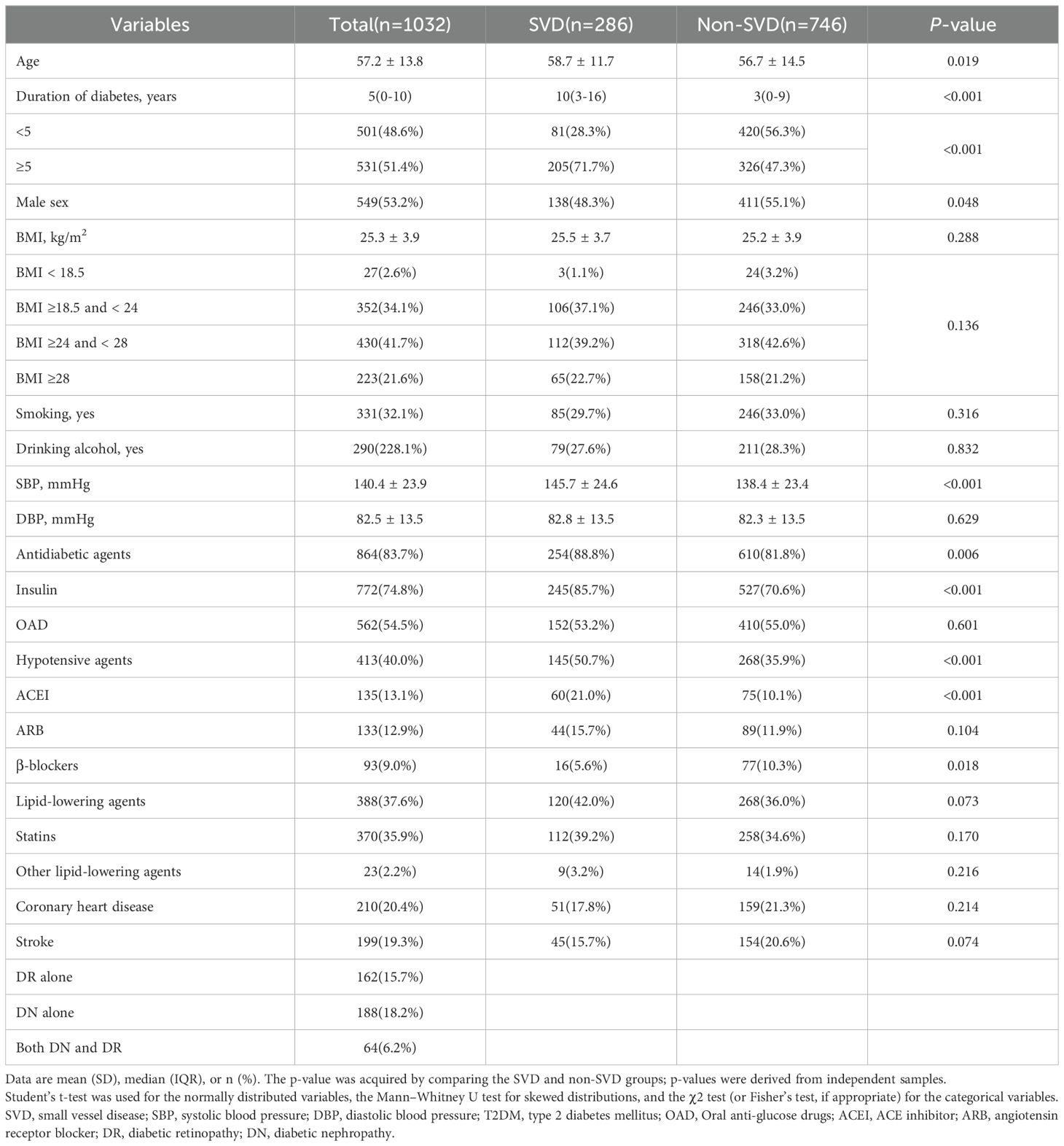
Table 1. Clinical characteristics of patients with T2DM divided into groups according to the presence of small vessel disease.
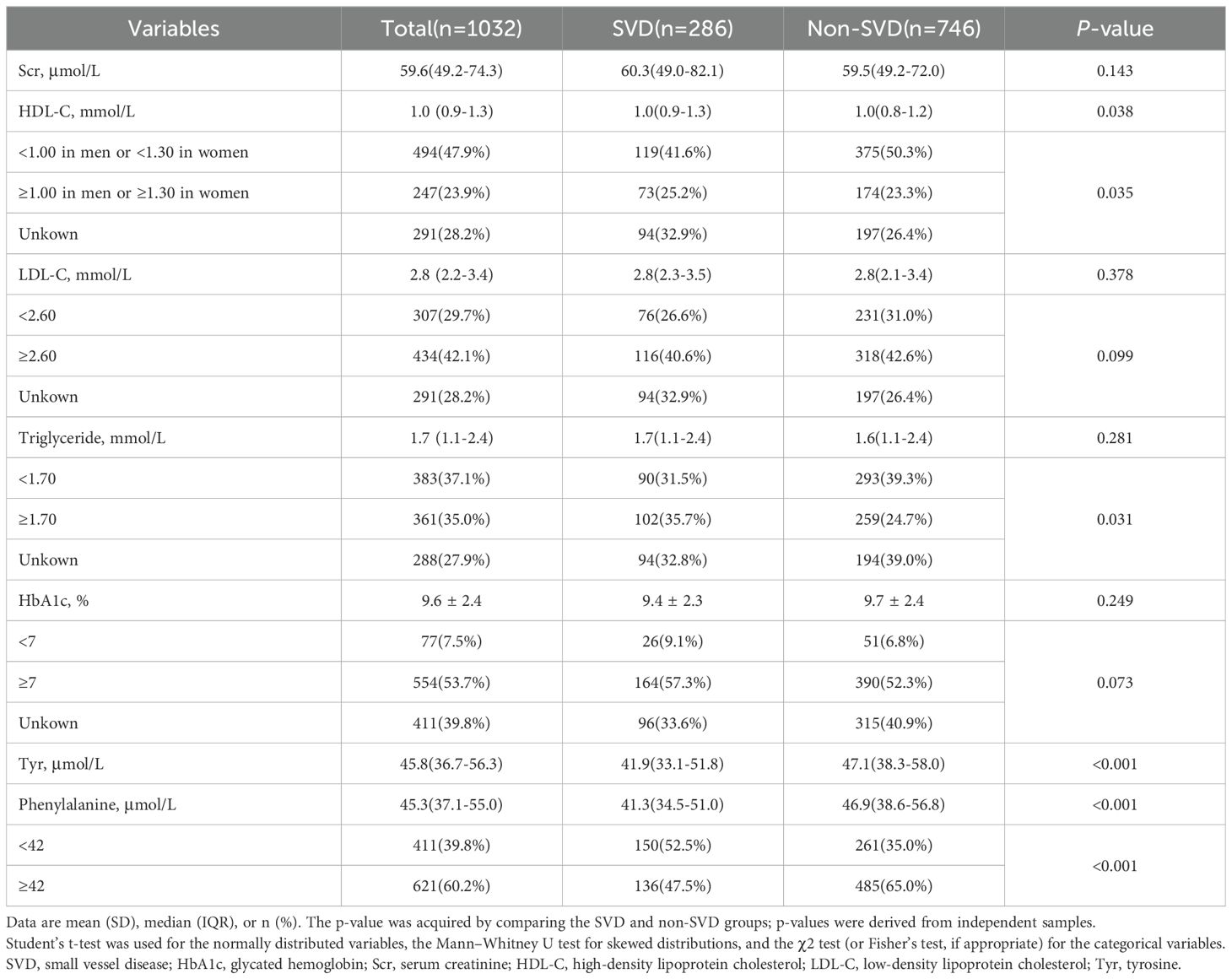
Table 2. Biochemical characteristics of patients with T2DM divided into groups according to the presence of small vessel disease.
3.2 The relationship between phenylalanine and small vessel disease
Plasma phenylalanine concentration and SVD were negatively correlated in a non-linear relationship. A phenylalanine level below 42μmol/L was associated with a markedly increased risk of SVD (Figure 1). In a univariable analysis, low levels of plasma phenylalanine were positively associated with the risk of SVD (OR: 2.05, 95% CI: 1.55, 2.70). After adjusting for traditional risk factors, i.e., age, gender, BMI, duration of diabetes, drinking alcohol, smoking, SBP, HDL-C, TG, HbA1c, antidiabetic drugs, lipid-lowering drugs, antihypertensive drugs, and tyrosine, the positive association was strengthened in multivariable model 2 (OR: 1.76, 95% CI: 1.23, 2.51) (Table 3).
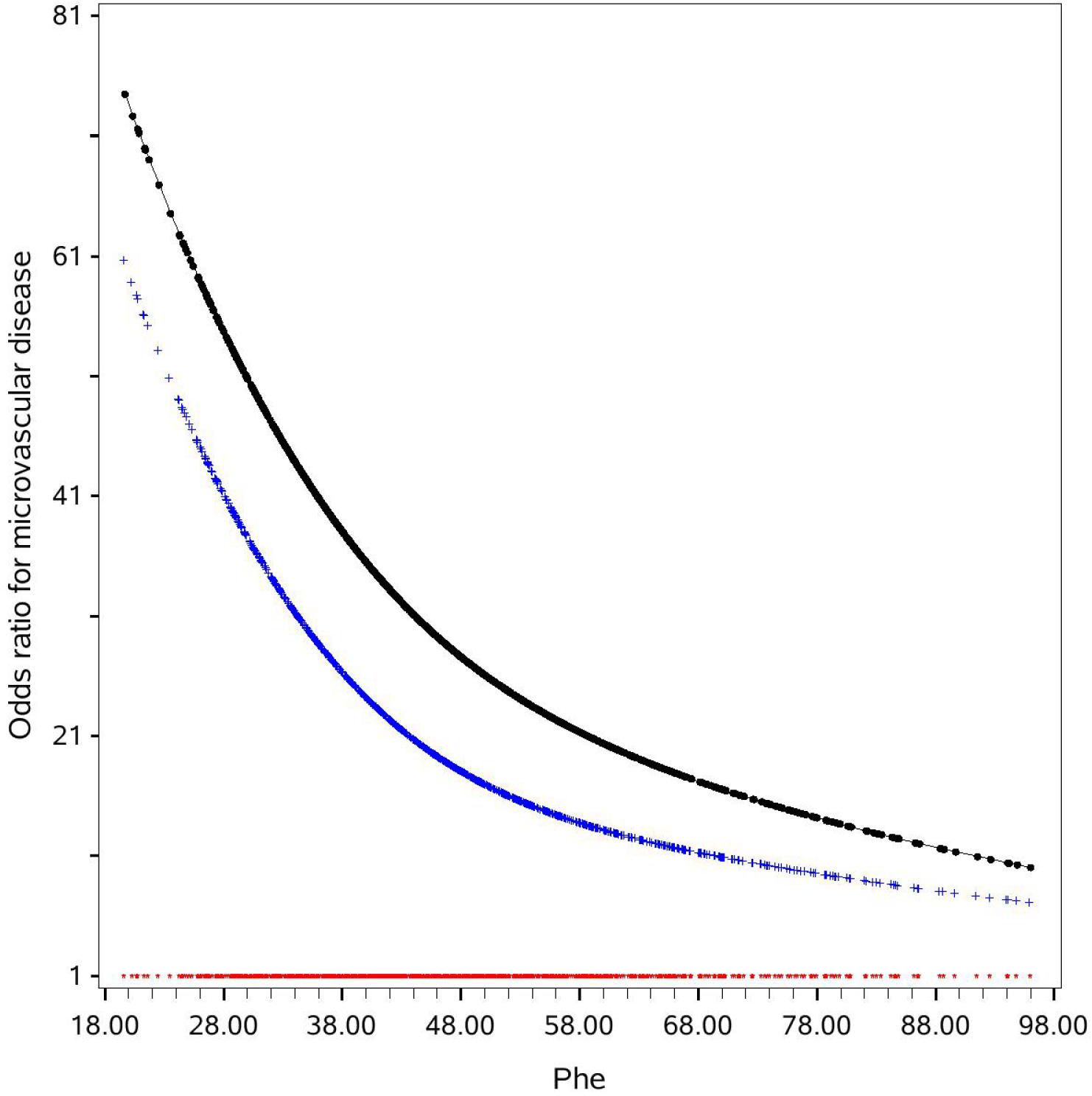
Figure 1. OR curves of phenylalanine for small vessel disease in Chinese patients with T2DM. The black curve was derived from a univariable analysis and the blue curve was derived from a multivariate analysis that adjusted for age, gender, body mass index (<18.5, 18.5-23.9, 24-27.9 and ≥28 kg/m2), smoking, drinking alcohol, duration of diabetes, systolic blood pressure, high-density lipoprotein cholesterol (<1.0 mmol/L in men or <1.3 mmol/L in women, ≥1 mmol/L in men or ≥1.3 mmol/L in women, unknown), low-density lipoprotein cholesterol (<2.6 and ≥2.6 mmol/L, unknown), HbA1c (<7%, 7%~8%, and ≥8%, unknown), triglyceride (<1.7 and ≥1.7 mmol/L, unknown), antidiabetic drugs, lipid-lowering drugs, and antihypertensive drugs use. The red curve indicates the reference level (i.e., the OR for small vessel disease was 1). The missing values of HbA1c and lipids were presented as one category. HbA1c, glycated hemoglobin; Phe, phenylalanine; T2DM, type 2 diabetes mellitus.
3.3 Additive interaction between phenylalanine and duration of diabetes for SVD
The associations of plasma phenylalanine levels with SVD at different diabetic durations are shown in Table 4. In patients with a longer duration of diabetes (≥5 years), low levels of phenylalanine had a statistically significant higher odds ratio for SVD risk in both the univariate and multivariate analyses compared to the patients with a shorter duration of diabetes (<5 years) (OR: 2.36, 95% CI: 1.65, 3.39; OR: 2.05, 95% CI: 1.32, 3.18, respectively). Compared with high phenylalanine (i.e., ≥42μmol/L, the cutoff point of the RCS) and short duration of diabetes (i.e., <5 years, the median), a concurrent presence of both low phenylalanine (<42μmol/L) and long duration of diabetes (≥5 years) markedly increased the risk of SVD [OR: 4.83 (95% CI, 2.97-7.87] compared to low phenylalanine alone (multivariable OR: 1.48, 95% CI: 0.88-2.49) and long duration of diabetes alone (multivariable OR: 2.59, 95% CI: 1.67, 4.02). The additive interaction was significant (RERI: 1.76, 95% CI: 0.05-3.47; AP: 0.37, 95% CI: 0.10-0.63 and S: 1.85, 95% CI: 1.03-3.35) (Table 5).
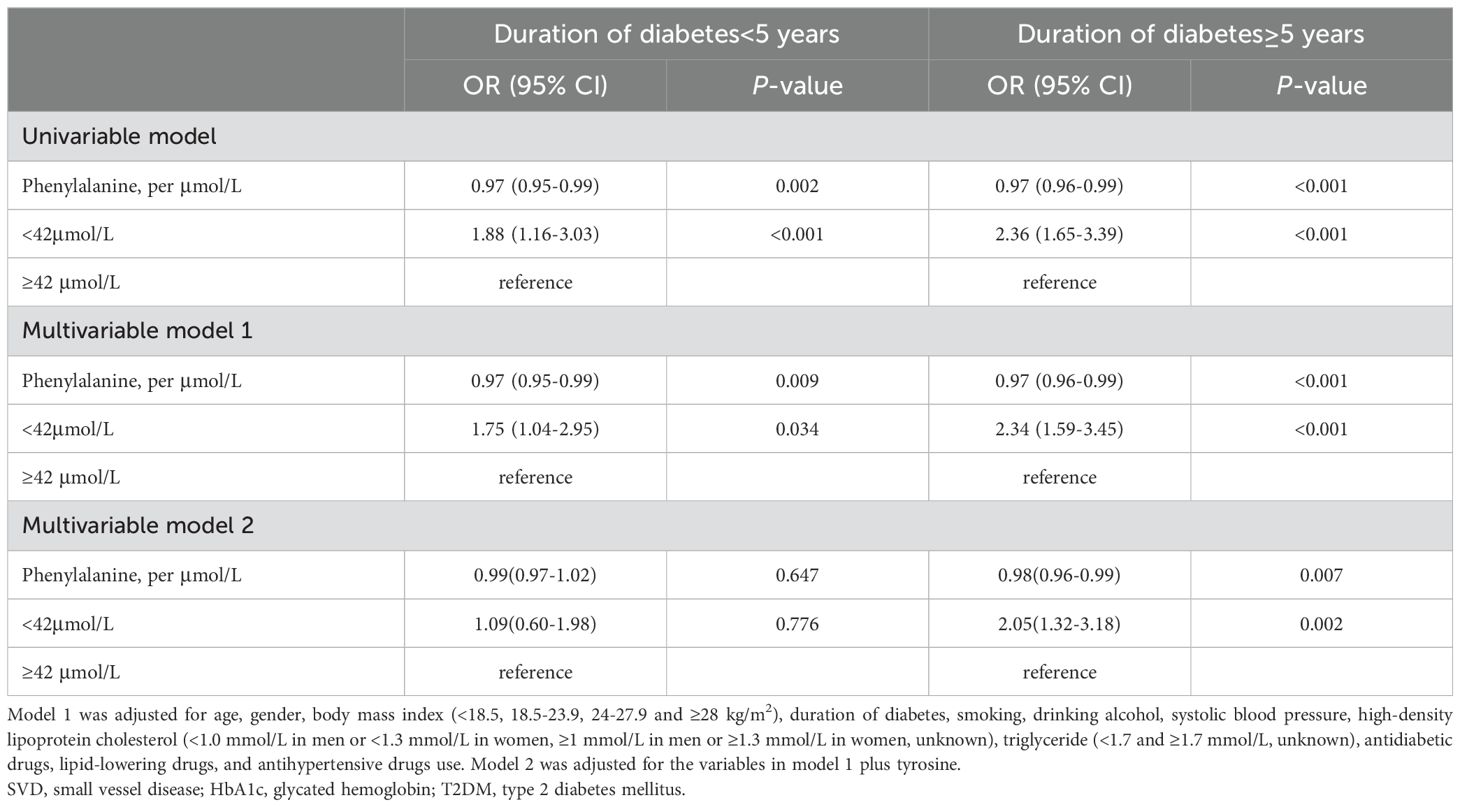
Table 4. Odds ratio of phenylalanine levels and different durations of diabetes for the risk of small vessel diseases.
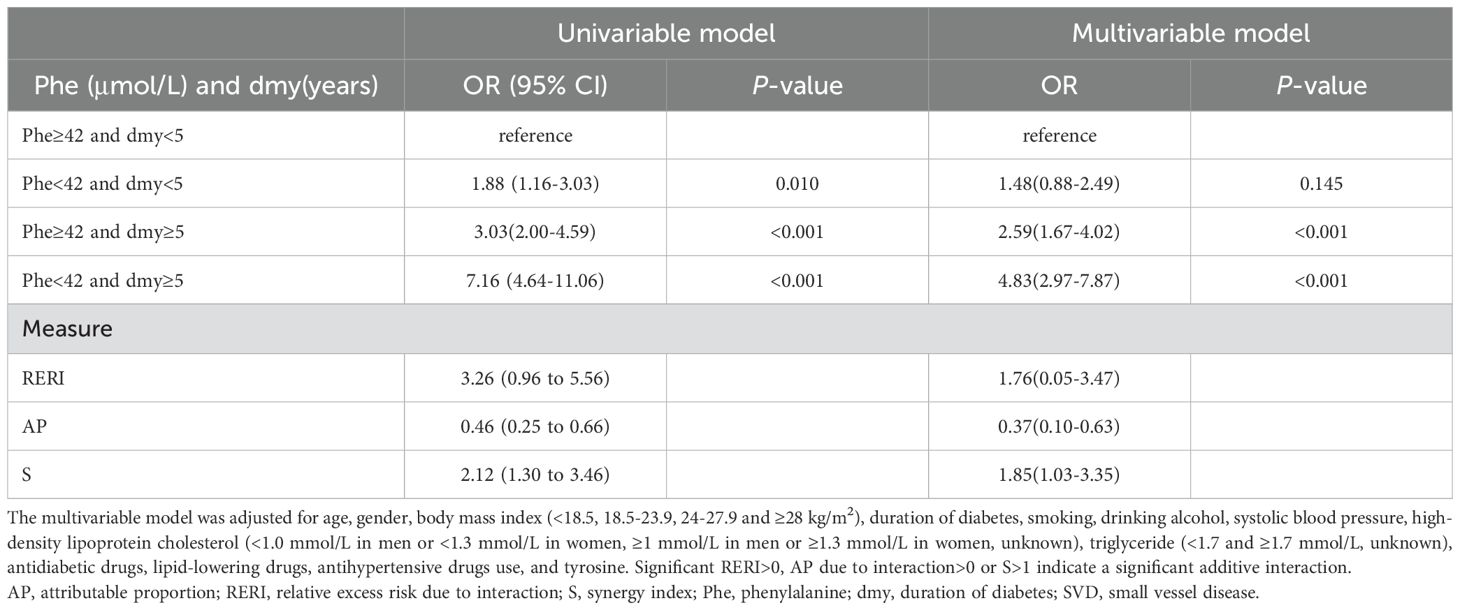
Table 5. Additive interaction of phenylalanine and duration of diabetes for SVD in type 2 diabetes mellitus.
3.4 Potential discriminative values of phenylalanine and duration of diabetes for SVD
The inclusion of phenylalanine and duration of diabetes in the traditional risk factor model significantly increased the area under the curve (AUC) from 0.67 to 0.71 (p<0.05) (data not shown).
4 Discussion
In this retrospective study, we found that plasma phenylalanine concentration was negatively associated with the risk of SVD in T2DM. We also detected an additive interaction between phenylalanine and duration of diabetes: the OR of low-level plasma phenylalanine for the risk of SVD was magnified in patients with a duration of diabetes >5 years. Furthermore, phenylalanine and the duration of diabetes significantly increased the discriminative values of the traditional risk factors for SVD in T2DM.
The progression of SVD, i.e., DN and DR, increases the risk of all-cause mortality and blindness (2, 25), posing a significant threat to the quality of life of T2DM patients. Unfortunately, SVD in T2DM develops silently until severe damage has occurred. There is, therefore, a strong need to explore new markers to detect SVD earlier. Our study found that low-level plasma phenylalanine was associated with the risk of SVD in T2DM patients. Similarly, a study based on the GenodiabMar cohort found that phenylalanine was a common risk factor not only for DN but also for both DR and proteinuria (26). In addition, as a downstream product of phenylalanine, low tyrosine also appears to be a marker of microvascular risk in individuals with T2DM independently of other fundamental markers of kidney function (12), which might be relevant to our findings. However, our results were inconsistent with the findings of other studies (27–29), such as that by Zhao et al. which found that phenylalanine modifies insulin receptor beta (IRβ) and inactivates insulin signaling and glucose uptake and was positively correlated with T2DM onset. Previous studies have shown that an association pattern between amino acids and SVD is often influenced by differences in genetics, pathophysiology, culture, and lifestyle among different ethnicities.
In recent years, multiple common pathogenetic mechanisms of DN and DR have been proposed (30, 31), in which microvascular endothelial dysfunction plays a critical role in both kinds of SVD (2). It is well known that nitric oxide (NO) biosynthesis, catalyzed by endothelial nitric oxide synthase (NOS), determines homeostasis of the endothelial structure and function (32, 33). Appropriate levels of NO are able to protect renal endothelial and mesangial cells from apoptosis and fibrosis by inducing the expression of antioxidant genes (34). Tetrahydrobiopterin (BH4) is an important cofactor in the catalytic activity of NOS (35), and its synthesis is mediated by GTP cyclohydrolase-1 (GCH1) (36). When BH4 is insufficient, NOS may become “uncoupled” and produce a large amount of superoxide such reactive oxygen species (ROS), which aggravates oxidative stress and impairs vascular function (37). Furthermore, oxidative stress could cause the disruption of tight junction proteins (TJs), resulting in impaired endothelial barrier function (38).
Phenylalanine is an essential aromatic amino acid obtained through dietary intake in the body and it is mainly used in protein and tyrosine synthesis and, as a substrate, is involved in the synthesis of important neurotransmitters and hormones (39). Our study showed that low-concentration phenylalanine was associated with an increased risk of SVD in T2DM, which may be related to endothelial dysfunction caused by NO synthesis dysfunction which was in turn caused by insufficient phenylalanine. Manasi Nandi et al. found that oral supplementation of L-phenylalanine was capable of enhancing endogenous biosynthesis of BH4 by activating the GCH1-GFRP protein complex, while restoring NO content, reducing ROS levels, and improving vascular endothelial function (14). The role of L-phenylalanine in binding pockets on GCH1-GFRP complexes in the treatment of endothelial dysfunction was also validated in a separate study (40). Furthermore, phenylalanine protects TJs that are located on endothelial cells from damage by inhibiting NF-KB (41). We speculate that disruption of phenylalanine metabolism affects the balance between NO and ROS, thereby affecting endothelial barrier function.
Another potential mechanism was the protective effect of dopamine on vascular endothelium. Peripheral dopamine can directly regulate glucose uptake and lipid metabolism (42), which indirectly reduces endothelial injury caused by hyperglycemia. As a substrate for tyrosine hydroxylase, phenylalanine is indirectly involved in dopamine synthesis and is considered to be a secondary precursor of dopamine (43). Kinya Kuriyama et al. found that dopamine consumption in diabetic rats increased significantly (44), suggesting more substrate may be needed in diabetic patients. Furthermore, dopamine D2-like receptors, especially the D4 receptor, located primarily on the arterial endothelium, have been shown to ameliorate hyperglycemia-induced endothelial dysfunction through the PI3K/eNOS pathway (45). Taken together, we hypothesize that the relative dopamine deficiency in diabetes partially mediates the association between low phenylalanine and a higher risk of small vessel disease.
It is well known that the duration of diabetes is a risk factor for diabetic complications. With the progression of diabetes, oxidative stress and microvascular endothelial dysfunction gradually worsen, eventually triggering vascular-related diseases such as arteriosclerosis and diabetic small vessel disease (46, 47). Our study found that the cutoff value of diabetes duration was 5 years, which could contribute to earlier identification of SVD compared with the 10 years (48) proposed in previous studies. This cut-off point has also been shown to be a risk factor for SVD in multiple studies (49, 50). However, few studies have explored whether there is an interaction between the duration of diabetes and phenylalanine on SVD. In our study, we found a significant additive interaction between the duration of diabetes and low-level phenylalanine on SVD, which indicated that the duration of diabetes and disturbed phenylalanine metabolism may together contribute to the development of SVD in T2DM. However, due to the nature of the retrospective study, we were unable to determine whether SVD caused abnormal amino acid metabolism, which needs to be confirmed in a large cohort study.
Our study has important clinical and public health implications. We found that low-level phenylalanine was associated with an increased risk of SVD, which indicated that SVD, to some extent, can be improved by modulating phenylalanine metabolism. In addition, our study showed an additive interaction between the duration of diabetes (≥5 years) and low-level phenylalanine on SVD, which dramatically widens our appreciation of the etiological mechanisms of diabetic small vessel disease.
This study had some limitations. First, the retrospective design of this study could not verify true causality. Large population-based prospective cohort studies and basic research are required to validate our findings. Second, considering the heterogeneity between Chinese and other ethnicities, caution is required in extending our findings to other populations. Third, there are different degrees of data missing for factors such as LDL-C, HDL-C, TG, and HbA1c in our database. Given that the core of our research was phenylalanine rather than glycolipid metabolism in this study, we regarded the missing values as a category. Fourth, considering that phenylalanine cannot be synthesized by itself and can only be obtained from diet, diet can thus affect phenylalanine levels. Unfortunately, information on diet was not available, thus diet cannot be integrated into the statistical analysis. Finally, the diagnosis of DN and DR in this study only reflected the existing conditions of patients at that time and was not confirmed through long-term monitoring. For example, classification and staging data of DR were not collected, which is one of the limitations of our study.
In summary, our findings detected that low plasma phenylalanine was associated with a high risk of SVD in Chinese patients with T2DM. This association was further amplified in patients with the duration of diabetes ≥5 years. The potential discriminative value of phenylalanine and duration of diabetes for SVD risk was also reflected in the increased area under the receiver operating characteristic curve. Additional prospective cohort studies are required to verify these findings in different ethnic groups with T2DM. Further mechanistic explorations are also needed to reveal the underlying molecular mechanisms of serum phenylalanine in the pathogenesis of SVD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Liaoning Medical University First Affiliated Hospital Clinical Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JZ: Data curation, Formal analysis, Supervision, Investigation, Validation, Writing – original draft, Project administration. WZ: Formal analysis, Investigation, Validation, Writing – original draft. Y-YM: Investigation, Writing – original draft, Validation. X-RL: Writing – review & editing, Data curation, Formal analysis, Validation. W-ML: Writing – review & editing, Investigation. XY: Validation, Writing – review & editing. Z-ZF: Conceptualization, Validation, Writing – review & editing. QZ: Conceptualization, Supervision, Validation, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was supported by National Natural Science Foundation of China (Grant No.92163213); National Key Research and Development Program of China (Grant No. 2023YFC3605200); Tianjin science and technology plan project (Grant No. 21JCZDJC00940); Tianjin Key Laboratory of Elderly Health; National Key Research and Development Program of China (Grant No. 2021YFA1301202); National Natural Science Foundation of China (Grant No. 82273676); Liaoning Province Scientific and Technological Project (Grant No. 2021JH2/10300039).
Acknowledgments
The authors thank all the physicians, nurses, and research staff at the Liaoning Medical University First Affiliated Hospital who participated in this study and assisted in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SVD, Small vessel disease; DN, Diabetic nephropathy; DR, Diabetic retinopathy; MS, Mass spectrometry; RCS, Restricted cubic spline; T2DM, Type 2 diabetes mellitus; AAAs, Aromatic amino acids; eGFR, estimated glomerular filtration rate; BMI, Body mass index; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; TG, Triglyceride; HbA1c, Glycated hemoglobin; CHD, Coronary heart disease; OAD, Oral anti-diabetic drugs; ACEIs, Angiotensin-converting enzyme inhibitors; ARBs, Angiotensin receptor blockers; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; NO, Nitric oxide; eNOS, Endothelial nitric oxide synthase; BH4, Tetrahydrobiopterin; ROS, Reactive oxygen species; TJs, Tight junction proteins; Phe, Phenylalanine.
References
1. Litwak L, Goh SY, Hussein Z, Malek R, Prusty V, Khamseh ME. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol Metab syndrome. (2013) 5:57. doi: 10.1186/1758-5996-5-57
2. Yang J, Liu Z. Mechanistic pathogenesis of endothelial dysfunction in diabetic nephropathy and retinopathy. Front Endocrinol. (2022) 13:816400. doi: 10.3389/fendo.2022.816400
3. Chi ZS, Lee ET, Lu M, Keen H, Bennett PH. Vascular disease prevalence in diabetic patients in China: standardised comparison with the 14 centres in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. (2001) 44 Suppl 2:S82–6. doi: 10.1007/PL00002944
4. Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. BioMed Res Int. (2021) 2021:1497449. doi: 10.1155/2021/1497449
5. Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, et al. The progress in understanding and treatment of diabetic retinopathy. Prog retinal eye Res. (2016) 51:156–86. doi: 10.1016/j.preteyeres.2015.08.001
6. Schutz Y. Protein turnover, ureagenesis and gluconeogenesis. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J Int vitaminologie Nutr. (2011) 81:101–7. doi: 10.1024/0300-9831/a000064
7. Bender DA. The metabolism of "surplus" amino acids. Br J Nutr. (2012) 108 Suppl 2:S113–21. doi: 10.1017/S0007114512002292
8. Gar C, Rottenkolber M, Prehn C, Adamski J, Seissler J, Lechner A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit Rev Clin Lab Sci. (2018) 55:21–32. doi: 10.1080/10408363.2017.1414143
9. Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. (2013) 36:648–55. doi: 10.2337/dc12-0895
10. Zhang X, Wang Y, Hao F, Zhou X, Han X, Tang H, et al. Human serum metabonomic analysis reveals progression axes for glucose intolerance and insulin resistance statuses. J Proteome Res. (2009) 8:5188–95. doi: 10.1021/pr900524z
11. Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. (2010) 53:757–67. doi: 10.1007/s00125-009-1637-8
12. Welsh P, Rankin N, Li Q, Mark PB, Würtz P, Ala-Korpela M, et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: results from the ADVANCE trial. Diabetologia. (2018) 61:1581–91. doi: 10.1007/s00125-018-4619-x
13. Kim MK, Aung MH, Mees L, Olson DE, Pozdeyev N, Iuvone PM, et al. Dopamine deficiency mediates early rod-driven inner retinal dysfunction in diabetic mice. Invest Ophthalmol Vis Sci. (2018) 59:572–81. doi: 10.1167/iovs.17-22692
14. Heikal L, Starr A, Hussein D, Prieto-Lloret J, Aaronson P, Dailey LA, et al. l-phenylalanine restores vascular function in spontaneously hypertensive rats through activation of the GCH1-GFRP complex. JACC Basic Transl Sci. (2018) 3:366–77. doi: 10.1016/j.jacbts.2018.01.015
15. Kopple JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. (2007) 137:1586S–1590S; discussion 1597S-1598S. doi: 10.1093/jn/137.6.1586S
16. Wang H, Li S, Wang C, Wang Y, Fang J, Liu K. Plasma and vitreous metabolomics profiling of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. (2022) 63:17. doi: 10.1167/iovs.63.2.17
17. Li J, Cao YF, Sun XY, Han L, Li SN, Gu WQ, et al. Plasma tyrosine and its interaction with low high-density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J Diabetes Invest. (2019) 10:491–8. doi: 10.1111/jdi.2019.10.issue-2
18. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
19. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci BES. (2002) 15:83–96.
20. American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes Publ Am Diabetes Assoc. (2019) 37:11–34. doi: 10.2337/cd18-0105
21. Selby NM, Taal MW. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. (2020) 22 Suppl 1:3–15. doi: 10.1111/dom.14007
22. Wang Q, Sun T, Cao Y, Gao P, Dong J, Fang Y, et al. A dried blood spot mass spectrometry metabolomic approach for rapid breast cancer detection. OncoTargets Ther. (2016) 9:1389–98. doi: 10.2147/OTT.S95862
23. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.v29:9
24. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. (2005) 20:575–9. doi: 10.1007/s10654-005-7835-x
25. Cataldi S, Tramontano M, Costa V, Aprile M, Ciccodicola A. Diabetic retinopathy: are lncRNAs new molecular players and targets? Antioxidants (Basel Switzerland). (2022) 11(10). doi: 10.3390/antiox11102021
26. Barrios C, Zierer J, Würtz P, Haller T, Metspalu A, Gieger C, et al. Circulating metabolic biomarkers of renal function in diabetic and non-diabetic populations. Sci Rep. (2018) 8:15249. doi: 10.1038/s41598-018-33507-7
27. Zhou Q, Sun WW, Chen JC, Zhang HL, Liu J, Lin Y, et al. Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRβ. Nat Commun. (2022) 13:4291. doi: 10.1038/s41467-022-32000-0
28. Qiu G, Zheng Y, Wang H, Sun J, Ma H, Xiao Y, et al. Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int J Epidemiol. (2016) 45:1507–16. doi: 10.1093/ije/dyw221
29. Stancáková A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes. (2012) 61:1895–902. doi: 10.2337/db11-1378
30. Jonas JB, Wang YX, Wei WB, Xu J, You QS, Xu L. Chronic kidney disease and eye diseases: the Beijing eye study. Ophthalmology. (2017) 124:1566–9. doi: 10.1016/j.ophtha.2017.04.024
31. Xie Z, Xiao X. Novel biomarkers and therapeutic approaches for diabetic retinopathy and nephropathy: Recent progress and future perspectives. Front Endocrinol. (2022) 13:1065856. doi: 10.3389/fendo.2022.1065856
32. Endemann DH, Schiffrin EL. Nitric oxide, oxidative excess, and vascular complications of diabetes mellitus. Curr hypertension Rep. (2004) 6:85–9. doi: 10.1007/s11906-004-0081-x
33. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. (2012) 33:829–37. doi: 10.1093/eurheartj/ehr304
34. Nishad R, Mukhi D, Tahaseen SV, Mungamuri SK, Pasupulati AK. Growth hormone induces Notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy. J Biol Chem. (2019) 294:16109–22. doi: 10.1074/jbc.RA119.008966
35. Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. (1989) 264:20496–501. doi: 10.1016/S0021-9258(19)47089-0
36. Nichol CA, Lee CL, Edelstein MP, Chao JY, Duch DS. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proc Natl Acad Sci United States America. (1983) 80:1546–50. doi: 10.1073/pnas.80.6.1546
37. Li L, Chen W, Rezvan A, Jo H, Harrison DG. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arteriosclerosis thrombosis Vasc Biol. (2011) 31:1547–54. doi: 10.1161/ATVBAHA.111.226456
38. Mazzon E, Puzzolo D, Caputi AP, Cuzzocrea S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol Med (Cambridge Mass.). (2002) 8:353–66.
39. Alamshah A, Spreckley E, Norton M, Kinsey-Jones JS, Amin A, Ramgulam A, et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int J Obes. (2017) 41:1693–701. doi: 10.1038/ijo.2017.164
40. Hussein D, Starr A, Heikal L, McNeill E, Channon KM, Brown PR, et al. Validating the GTP-cyclohydrolase 1-feedback regulatory complex as a therapeutic target using biophysical and in vivo approaches. Br J Pharmacol. (2015) 172:4146–57. doi: 10.1111/bph.2015.172.issue-16
41. Feng L, Li W, Liu Y, Jiang WD, Kuang SY, Wu P, et al. Protective role of phenylalanine on the ROS-induced oxidative damage, apoptosis and tight junction damage via Nrf2, TOR and NF-κB signalling molecules in the gill of fish. Fish shellfish Immunol. (2017) 60:185–96. doi: 10.1016/j.fsi.2016.11.048
42. Tavares G, Marques D, Barra C, Rosendo-Silva D, Costa A, Rodrigues T, et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol Metab. (2021) 51:101241. doi: 10.1016/j.molmet.2021.101241
43. Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. (2007) 137:1539S–1547S; discussion 1548S. doi: 10.1093/jn/137.6.1539S
44. Nishimura C, Kuriyama K. Alterations in the retinal dopaminergic neuronal system in rats with streptozotocin-induced diabetes. J neurochemistry. (1985) 45:448–55. doi: 10.1111/j.1471-4159.1985.tb04008.x
45. Wang H, Yao Y, Liu J, Cao Y, Si C, Zheng R, et al. Dopamine D(4) receptor protected against hyperglycemia-induced endothelial dysfunction via PI3K /eNOS pathway. Biochem Biophys Res Commun. (2019) 518:554–9. doi: 10.1016/j.bbrc.2019.08.080
46. Zhu DD, Wang YZ, Zou C, She XP, Zheng Z. The role of uric acid in the pathogenesis of diabetic retinopathy based on Notch pathway. Biochem Biophys Res Commun. (2018) 503:921–9. doi: 10.1016/j.bbrc.2018.06.097
47. He Y, Zhang M, Wu Y, Jiang H, Fu H, Cai Y, et al. Aberrant activation of Notch-1 signaling inhibits podocyte restoration after islet transplantation in a rat model of diabetic nephropathy. Cell Death Dis. (2018) 9:950. doi: 10.1038/s41419-018-0985-z
48. American Diabetes A. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S151–67. doi: 10.2337/dc21-S011
49. Zhao Y, Liu L, Zuo L, Zhou X, Wang S, Gao H, et al. A novel risk score model for the differential diagnosis of type 2 diabetic nephropathy: A multicenter study. J Diabetes Res. (2023) 2023:5514767. doi: 10.1155/2023/5514767
50. Sen S, Ramasamy K, Vignesh TP, Kannan NB, Sivaprasad S, Rajalakshmi R, et al. Identification of risk factors for targeted diabetic retinopathy screening to urgently decrease the rate of blindness in people with diabetes in India. Indian J Ophthalmol. (2021) 69:3156–64. doi: 10.4103/ijo.IJO_496_21
Keywords: type 2 diabetes mellitus, small vessel disease, phenylalanine, duration of diabetes, T2DM
Citation: Zheng J, Zhang W, Miao Y-Y, Li X-R, Luo W-M, Yang X-L, Fang Z-Z and Zhang Q (2025) Interactive effect of phenylalanine with duration of diabetes on the risk of small vessel disease in Chinese patients with T2DM. Front. Endocrinol. 15:1472967. doi: 10.3389/fendo.2024.1472967
Received: 30 July 2024; Accepted: 04 October 2024;
Published: 03 January 2025.
Edited by:
Gulali Aktas, Abant Izzet Baysal University, TürkiyeReviewed by:
Amela Dervisevic, University of Sarajevo, Bosnia and HerzegovinaAyse Kevser Demir, Samsun University, Türkiye
Copyright © 2025 Zheng, Zhang, Miao, Li, Luo, Yang, Fang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zhang, emhhbmdxaWFuZ3l1bHZAMTYzLmNvbQ==; Zhong-Ze Fang, ZmFuZ3pob25nemVAdG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jun Zheng
Jun Zheng Wei Zhang
Wei Zhang Yu-Yang Miao
Yu-Yang Miao Xue-Rui Li
Xue-Rui Li Wei-Ming Luo
Wei-Ming Luo Xi-Lin Yang
Xi-Lin Yang Zhong-Ze Fang
Zhong-Ze Fang Qiang Zhang
Qiang Zhang