
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 September 2024
Sec. Cardiovascular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1471548
This article is part of the Research Topic Cardiometabolic diseases in postmenopausal women View all 5 articles
Background: Postmenopausal women are at an increased risk of arterial stiffness, which can be assessed using estimated pulse wave velocity (ePWV). This study aimed to investigate the relationship between serum klotho levels and ePWV in postmenopausal women.
Methods: This cross-sectional study used data from postmenopausal women who participated in the National Health and Nutrition Examination Survey (NHANES) between 2007 and 2016. Participants were divided into two groups based on the presence of hypertension. Weighted multivariate linear regression was used to analyze the relationship between serum Klotho levels and ePWV in each group. Restricted cubic spline models with multivariable adjustments were employed to examine nonlinear associations within each group.
Results: Our analysis included 4,468 postmenopausal women from the NHANES database, with 1,671 in the non-hypertensive group and 2,797 in the hypertensive group. In all regression models, serum Klotho (ln-transformed) levels were significantly and independently negatively correlated with ePWV in the non-hypertensive group. After fully adjusting for confounders, a 1-unit increase in ln(Klotho) was associated with a 0.13 m/s decrease in ePWV (β = −0.13, 95% CI −0.23 to −0.03; p = 0.008). Additionally, in the fully adjusted model, participants in the highest quartile of ln(Klotho) had an ePWV value 0.14 m/s lower than those in the lowest quartile (p for trend = 0.017; 95% CI −0.23 to −0.05; p = 0.002). This negative correlation was consistent across subgroups and was particularly significant among women aged < 60 years, nonsmokers, and non-Hispanic Black women. However, no association was observed between serum Klotho levels and ePWV in the hypertensive group.
Conclusion: Hypertension may affect the relationship between serum Klotho level and ePWV in postmenopausal women. Increased serum Klotho levels may reduce arterial stiffness in postmenopausal women. Further studies are required to confirm these findings.
The risk of cardiovascular diseases (CVDs) increases with age in both men and women; however, in women, this risk accelerates more rapidly after menopause (1, 2). This transition often leads to conditions, such as central obesity, hypertension, diabetes, and dyslipidemia (3–5). Postmenopausal women experience substantial hormonal changes, particularly a reduction in estrogen levels, which are associated with an increased CVD risk. In an aging society, the burden of CVD among postmenopausal women is rising, leading to substantial medical and social costs (6). Thus, addressing the health status of postmenopausal women is of paramount importance.
Arterial stiffness increases with age and is recognized as an independent risk factor for cardiovascular morbidity and mortality (7). Accelerated arterial stiffness is a notable concern for postmenopausal women and is widely considered as an independent predictor of CVDs (8). Understanding the factors influencing arterial stiffness is crucial for identifying potential therapeutic targets to mitigate CVD risk in postmenopausal women. Measurement of arterial stiffness is recommended for the prevention and management of CVDs (9). Carotid-femoral pulse wave velocity (cf-PWV) is the standard method for assessing arterial stiffness; a higher cf-PWV indicates reduced vascular elasticity and increased arterial stiffness (10). However, cf-PWV is not been widely adopted in clinical practice due to the need of specialized personnel and equipment. To address these limitations, estimated pulse wave velocity (ePWV) has been introduced as an alternative method. ePWV, calculated using age and mean blood pressure (MBP), can effectively predict cf-PWV and has demonstrated an excellent correlation with in vivo assessments (11).
Serum Klotho is an anti-aging protein encoded by the Klotho gene (12). It is primarily expressed in the distal convoluted tubules of the kidneys and plays a critical role in various physiological processes, including inflammation regulation, antioxidation, and aging prevention (13, 14). Mice deficient in serum Klotho exhibit a range of syndromes similar to human aging, such as reduced lifespan, arterial stiffness, skin atrophy, and osteoporosis, whereas overexpression of serum Klotho extends the lifespan of transgenic mice by 30% (12, 15). Several cohort studies have indicated that decreased serum Klotho levels are associated with conditions, such as heart failure, hypertension, and atrial fibrillation (16–18). However, the relationship between serum Klotho levels and arterial stiffness in postmenopausal women remains unclear.
Therefore, our study aims to explore the relationship between serum Klotho levels and arterial stiffness in postmenopausal women, as assessed using ePWV.
This cross-sectional study, utilized data from the National Health and Nutrition Examination Survey (NHANES), an ongoing nationwide survey conducted by the National Center for Health Statistics (NCSH) at the U.S. Centers for Disease Control and Prevention. The NHANES adopts a multistage, stratified, subgroup probability sampling design in two-year cycles. Prior to conducting the present study, all participants provided written informed consent, and the study was approved by the National Centre for Health Statistics Ethics Review Board. For our study, we used data from five cycles involving 50,588 participants from 2007 to 2016. First, we excluded 36,284 participants with missing serum Klotho data, resulting in a cohort of 4,690 postmenopausal women. Next, we excluded 222 participants who lacked data on covariates, including heart failure, coronary heart disease, stroke, alcohol consumption, diabetes, smoking, hypertension, body mass index (BMI), estimated glomerular filtration rate (eGFR) and educational level. Ultimately, 4,468 postmenopausal women were included in the final analysis. Given the impact of hypertension and antihypertensive medications on arterial stiffness (19, 20), participants were further divided into hypertension (n=2,797) and non-hypertension groups (n=1,671). The participant selection process is illustrated in Figure 1.
Menopausal status was defined based on the self-reported reproductive health questionnaire. Participants were classified as postmenopausal if they denied having a menstrual period within the past twelve months and subsequently indicated hysterectomy or menopause as the reason for this absence.
Clinical samples available were collected by specialist workers on dry ice and stored at −80 °C. Samples from the participants were tested by enzyme-linked immunosorbent assay according to the manufacturer’s protocol. Each sample was analyzed in duplicate, and the average of the two values was used for the final result. A detailed description of the Klotho detection method is available on the NHANES website.
Blood pressure was measured by a trained health technician. Participants obtained three consecutive sphygmomanometric readings taken after 5 min of sitting still. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined by calculating the average of all blood pressure readings. The outcome variable for arterial stiffness was evaluated using ePWV, which was calculated using the following equation (11): 9.587 − (0.402 × age) + (4.560 × 10−3 × age2) − (2.621 × 10−5 × age2 × MBP) + (3.176 × 10−3 × age × MBP) − (1.832 × 10−2 × MBP). MBP was calculated as DBP + (0.4 × SBP − DBP).
Potential confounding variables that might affect the relationship between serum Klotho levels and arterial stiffness were considered. These included age, race/ethnicity, education level, smoking status, alcohol consumption, BMI, stroke, coronary heart disease, diabetes, hyperlipidemia, albumin level, and eGFR.
Education level was categorized into three groups: college or higher, high school or equivalent, and lower than high school. Race and ethnicity were classified as Mexican American, non-Hispanic Black, non-Hispanic White, or other. Smoking status was categorized according to the NCHS classification: individuals who smoked fewer than 100 cigarettes in their lifetime were considered never-smokers, whereas participants who smoked more than 100 cigarettes in their lifetime were considered smokers. For alcohol consumption, the categories were as follows: none (fewer than 12 drinks in a lifetime), former (more than 12 drinks in a lifetime but none in the past year), moderate (less than one drink per day for women), and heavy (one or more drinks per day for women). BMI was calculated as weight in kilograms (kg) divided by the square of the height in meters (m²). The eGFR was computed using the Chronic Kidney Disease Epidemiology Collaboration equation.
Hypertension was defined according to the 2017 American Heart Association blood pressure guidelines, encompassing individuals with a systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, those who self-reported hypertension, and those using antihypertensive medications (21). Coronary heart disease was identified as self-reported, doctor-diagnosed coronary heart disease, incidence of angina, or a history of heart attack. Hyperlipidemia was defined as total cholesterol levels ≥ 200 mg/dL, triglyceride levels ≥ 150 mg/dL, low-density lipoprotein levels ≥ 130 mg/dL, or high-density lipoprotein levels ≤ 50 mg/dL for women (22). Additionally, individuals who reported the use of cholesterol-lowering medications were categorized as having hyperlipidemia. Diabetes was defined as self-reported, doctor-diagnosed diabetes, or current use of insulin or antidiabetic medications.
Given the complex multistage sampling design of the NHANES, appropriate sample weights (1/5 × 2 Year Mobile Examination Center Weight) were calculated according to the NHANES guidelines. Continuous variables are presented as weighted means ± standard error and compared using weighted independent sample t-tests. Categorical variables are presented as unweighted case numbers and weighted percentages and were compared using weighted chi-square tests. To address the skewed Klotho distributions and facilitate interpretation, the data were log transformed [ln(Klotho)]. Subsequently, ln(Klotho) was divided into four quartiles, with the first quartile serving as a reference. ln(Klotho) was analyzed as both a continuous and categorical variable using weighted multivariate linear regression models to examine the independent association between ln(Klotho) and ePWV in both the hypertensive and non-hypertensive groups. Model 1 did not adjust for covariates, while Model 2 was adjusted for age, race, educational level, and BMI. Model 3 included all the covariates from Model 2, along with adjustments for alcohol consumption, smoking status, diabetes mellitus, coronary heart disease, heart failure, stroke, hyperlipidemia, eGFR, and albumin levels. Multivariate-adjusted restricted cubic spline analysis was conducted to assess the nonlinear relationship between ln(Klotho) and ePWV in both groups. Additionally, stratified analyses were conducted based on age, BMI, hyperlipidemia, diabetes, race, and smoking status to examine the association between ln(Klotho) and ePWV across different subgroups. All analyses were conducted using R version 4.3.2, with p < 0.05 indicating statistical significance.
The study sample comprised 4,468 participants aged 40–79 years, recruited between 2007 and 2016. Among these 2,797 participants had hypertension, while 1,671 participants were normotensive. Table 1 presents the baseline characteristics of the study population. Participants had a mean age of 61.0 ± 9.0 years. The results indicated, hypertensive patients were older than normotensive patients, with a higher prevalence of Black ethnicities, lower educational attainment, and lower family poverty income ratio. They also exhibited increased BMI and waist circumference. Moreover, the hypertensive group exhibited an increased incidence of comorbidities, such as diabetes, coronary heart disease, stroke, and heart failure. Hypertensive patients also exhibited elevated blood glucose and triglyceride levels, but reduced eGFR and total cholesterol levels (p < 0.001). Moreover, the hypertensive group showed increased ePWV (9.96 ± 1.64 vs. 8.72 ± 1.38, p < 0.001) and decreased serum Klotho levels (834 ± 288 vs. 866 ± 311, p = 0.021).
Table 2 presents the β coefficients and corresponding 95% confidence intervals (CIs) for ln(Klotho) and ePWV across various models in the hypertensive and non-hypertensive groups. In the non-hypertensive group, ln(Klotho) and ePWV exhibited significant and independent negative correlations across the various adjusted models. After fully adjusting for confounders, a 1-unit increase in ln(Klotho) was associated with a decrease in ePWV by 0.13 m/s (β = −0.13, 95% CI −0.23 to −0.03; p = 0.008). Additionally, in the fully adjusted model, participants in the highest quartile of ln(Klotho) had an ePWV value 0.14 m/s lower than those in the lowest quartile of ln(Klotho) (p for trend = 0.017; 95% CI: −0.23 to −0.05; p = 0.002). However, this relationship was not observed in the hypertensive group. Restricted cubic spline models were used to further investigate the potential nonlinear relationship between ln(Klotho) and ePWV in both groups (Figure 2). The results suggested no nonlinear correlation in the hypertensive group (p for nonlinearity = 0.280).
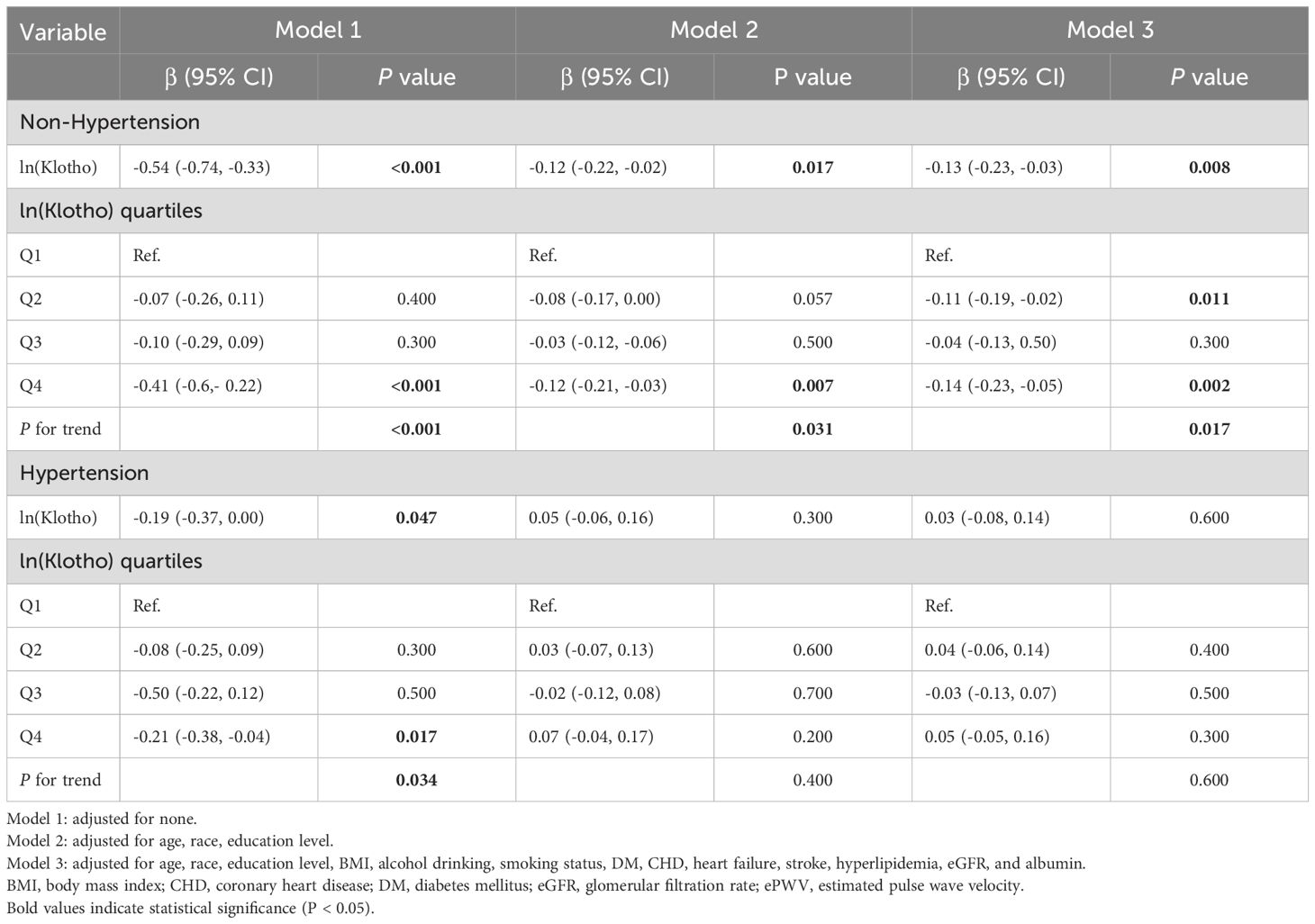
Table 2. Associations between ln(Klotho) and ePWV in postmenopausal women with and without hypertension.
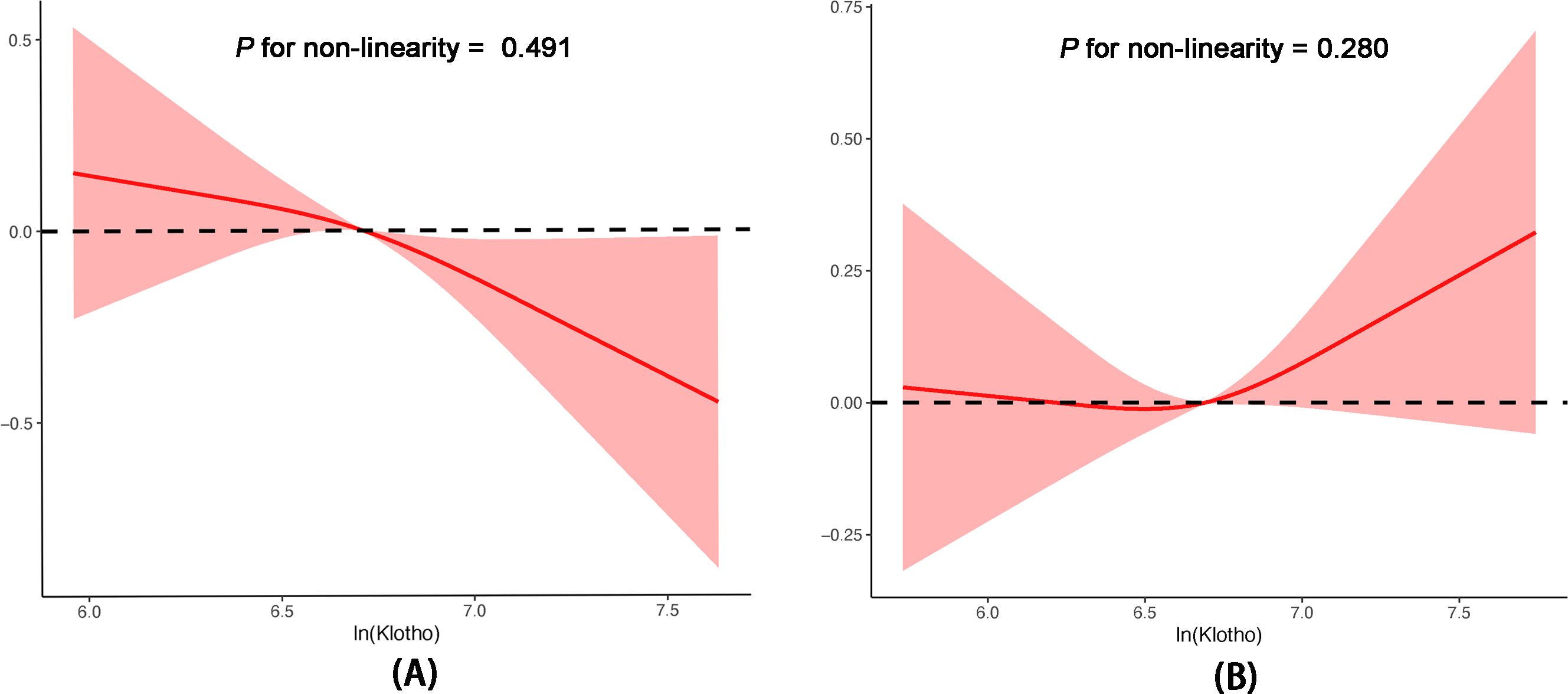
Figure 2. Associations between serum Klotho levels and ePWV in postmenopausal women without hypertension (A) and with hypertension (B) according to multivariable linear regression based on restricted cubic splines. The results were adjusted for age, race, education level, BMI, alcohol drinking, smoking status, DM, CHD, heart failure, stroke, hyperlipidemia, eGFR, and albumin. BMI, body mass index; CHD, coronary heart disease; DM, diabetes mellitus; eGFR, glomerular filtration rate; ePWV, estimated pulse wave velocity.
We performed interaction and subgroup analyses of ln(Klotho) and ePWV in postmenopausal women without hypertension. In the fully adjusted model (Model 3), there was no significant interaction in the subgroup analysis stratified by age, BMI, race, smoking, hyperlipidemia, and diabetes (Figure 3). However, significant statistical differences were observed in patients younger than 60 years, non-smokers, and non-Hispanic Black patients (p = 0.014, p = 0.008, p = 0.007, respectively).
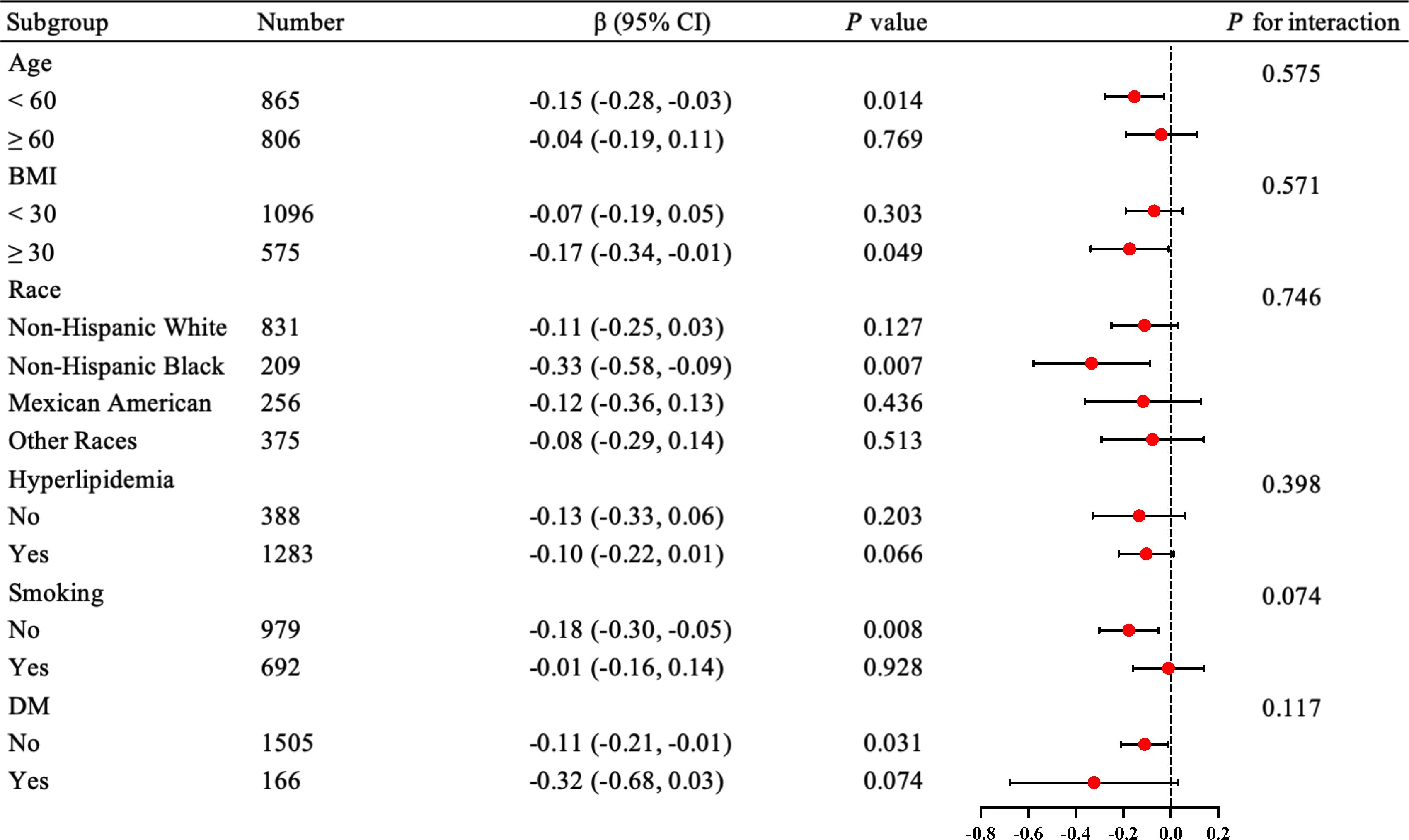
Figure 3. Forest plot for subgroup analysis of the relationship between serum Klotho levels and ePWV in postmenopausal women without hypertension. The results were adjusted for age, race, education level, BMI, alcohol drinking, smoking status, DM, CHD, heart failure, stroke, hyperlipidemia, eGFR, and albumin. BMI, body mass index (kg/m2); CHD, coronary heart disease; DM, diabetes mellitus; eGFR, glomerular filtration rate; ePWV, estimated pulse wave velocity.
To the best of our knowledge, this is the first cross-sectional study exploring the relationship between serum Klotho levels and arterial stiffness in postmenopausal women. After adjusting for confounding factors, ln(Klotho) was found to be significantly negatively correlated with arterial stiffness in postmenopausal women without hypertension. This correlation remained consistent across various subgroups and showed statistically significant differences among women under 60 years of age, non-smokers, and non-Hispanic Black women. These findings suggest that early intervention to increase serum klotho levels and enhance risk factor management may be beneficial in preventing the progression of arterial stiffness in postmenopausal women.
Arterial stiffness is an indicator of vascular aging and is associated with various cardiovascular risk factors and comorbidities (7, 23). The European gold standard for assessing arterial stiffness is cf-PWV (24). However, cf-PWV measurement requires skilled operators and specialized equipment, which limits its application in clinical practice. Currently, ePWV is widely used as a novel index of arterial stiffness, and its accuracy in assessing arterial stiffness has been validated (11, 25). Postmenopausal women are at increased risk of arterial stiffness (26). It is widely recognized that a decline in blood estrogen levels is a key factor that exacerbates arterial stiffness in postmenopausal women (27). Estrogen deficiency reduces the repair capacity of endothelial cells and leads to arterial damage and endothelial dysfunction (28, 29). Although hormone replacement therapy (HRT) is commonly used in postmenopausal women, notable improvements in arterial stiffness have not been observed with standard HRT (30). Recent studies have suggested that age-related factors may play a crucial role in accelerating arterial stiffening in postmenopausal women.
In recent years, serum Klotho has garnered widespread attention as an anti-aging biomarker associated with longevity and various CVDs. Several studies have explored the relationship between serum Klotho levels and arterial stiffness. Animal model studies have demonstrated a causal relationship between serum Klotho deficiency and arterial stiffness measured using PWV (31). In a study of 114 patients with chronic kidney disease (CKD) patients, Kitagawa et al. found that low serum Klotho levels were independently associated with increased brachial-ankle pulse wave velocity (ba-PWV), suggesting that serum Klotho levels are a notable determinant of arterial stiffness in patients with CKD (32). Another cross-sectional cohort study that included 172 patients with early diabetic kidney disease, showed a statistically significant negative correlation between serum Klotho levels and pulse wave velocity (PWV), emphasizing the effect of serum Klotho levels on aortic wall stiffness (33). Analysis of the NHANES cohort from to 2007–2016 revealed an inverse and independent association between serum Klotho concentration and arterial stiffness, as indicated by pulse pressure (34). However, although this cohort was large, the pulse pressure is influenced by both cardiac and arterial functions. A more precise and reliable method for assessing arterial stiffness is PWV, which depends solely on arterial properties (35). Moreover, no relevant subgroup analyses targeting race or comorbidities have been performed.
Recently, conflicting results have been reported regarding the relationship between serum Klotho levels and arterial stiffness. In 2018, the KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) trial analyzed data from 2,101 patients and found no association between serum Klotho levels and ba-PWV in patients with advanced CKD (36). Fountoulakis et al. suggested that this finding could be due to the influence of advanced CKD, diabetes, and the use of renin-angiotensin system inhibitors on serum Klotho levels and arterial stiffness (33). Additionally, Liang et al. conducted a study on 716 Chinese individuals and found no association between serum Klotho levels and cf-PWV (37). The exact cause of these discrepancies remains unclear, but they might be attributed to differences in ethnic populations, patient selection, and study design. Future research should include more diverse populations to further validate these findings.
Increased arterial stiffness has also been observed in postmenopausal women. In a small sample study, Matsubara et al. indicated a negative correlation between plasma Klotho levels and the β-stiffness index in postmenopausal women, suggesting that aerobic exercise training increased plasma Klotho levels and reduced arterial stiffness (38). In 2023, Yu et al. also found a significant negative correlation between serum Klotho concentration and hypertension in postmenopausal women (17). However, these studies had small sample sizes.
In our study, we analyzed a nationally representative sample of 4,468 postmenopausal women, which constitutes a large sample size. Additionally, this is the first study to use ePWV to assess arterial stiffness in postmenopausal women. We found a significant negative correlation between ln(Klotho) and ePWV in postmenopausal women without hypertension, independent of other cardiovascular risk factors. Different from previous studies, we found no correlation between serum Klotho levels and arterial stiffness in postmenopausal women with hypertension. Possible explanations include the following: First, previous studies with smaller sample sizes might have overlooked the impact of hypertension on the relationship between Klotho and ePWV. Second, hypertension interacts with arterial stiffness in a vicious cycle, potentially accelerating its progression (20, 39). Lastly, most participants with hypertension in our cohort received antihypertensive medications, which might have affected arterial stiffness (19).
Serum Klotho levels may be associated with arterial stiffness through several mechanisms. Inflammation and oxidative stress are potential causes of endothelial damage and arterial stiffness. Klotho can modulate inflammation by inhibiting TNF-α-induced expression of adhesion molecules (40) and NF-κB (41) activation. Additionally, decreased serum Klotho levels was significantly associated with a pro-inflammatory state, characterized by reduced serum IL-10 levels and elevated CRP levels and TNF-α/IL-10 ratio (42). In an in vitro experiment, Klotho deficiency increased the production of endogenous reactive oxygen species, promoting oxidative stress injury and apoptosis in mouse kidney cells (43). Recently, Donate-Correa et al. demonstrated that Klotho exerts its antioxidant effects through various pathways, including the regulation of manganese superoxide dismutase, the transcription factors FoxO and Nrf2, and other known antioxidant systems (44). Therefore, Klotho is a potential therapeutic target for oxidative stress. Furthermore, Klotho inhibited vascular calcification by preventing the transformation of muscle cells into osteoblast-like cells (45). Moreover, studies have demonstrated the protective effects of serum Klotho against angiotensin II-mediated oxidative stress, apoptosis, and senescence in human aortic smooth muscle cells (46). Mechanistic studies demonstrated that Klotho knockdown potentiated the development of accelerated calcification through a Runx2 and myocardin-serum response factor-dependent pathway (47). Other mechanisms by which Klotho deficiency leads to arterial stiffness include enhanced autophagic activity, which results in the upregulation of scleraxis, a key transcription factor for collagen synthesis (48). In animal studies, Klotho-deficient mice exhibited impaired gonadotropin regulation, leading to atrophy of the female reproductive system and reduced estrogen synthesis (49). This suggests that Klotho affects arterial stiffness by modulating estrogen levels, which may explain the association between serum Klotho and arterial stiffness in postmenopausal women. Further studies are required to explore these underlying mechanisms.
Finally, we assessed the stability of our results using subgroup and interaction tests; however, no interactions were observed. We found a significant negative correlation between ln(Klotho) and ePWV among women aged < 60 years, non-smokers, and non-Hispanic Black women. Age is a significant factor influencing arterial stiffness and calcification (50). Age-related extracellular matrix stiffening can trigger pathogenic mechanotransductive signaling, leading to Klotho promoter methylation, which in turn downregulates Klotho gene expression and accelerates chondrocyte aging in vitro (51). These mechanisms may explain the impact of age on the predictive value of serum Klotho. These findings suggest that early interventions aimed at increasing serum Klotho levels may be beneficial in preventing the progression of arterial stiffness and hypertension in postmenopausal women.
In recent years, therapies targeting Klotho have shown great potential. Animal studies have demonstrated that enhancing Klotho expression can inhibit the progression of hypertension and mitigate kidney damage (52). Repeated low-dose injections of Klotho (10 μg/kg) significantly inhibited the growth of breast tumors in mice, and Klotho was well tolerated (53). In 2023, Castner et al. discovered for the first time in non-human primates that a single low-dose injection of Klotho (10 μg/kg) significantly improved cognitive function in aging rhesus monkeys (54). Further animal experiments and studies are required to demonstrate the effect of serum Klotho on improving arterial stiffness in postmenopausal women.
The present study explored the complex relationship between serum Klotho levels and ePWV in a nationally representative sample of postmenopausal women. However, our study has several limitations. First, its cross-sectional design precluded the establishment of a causal relationship between serum Klotho levels and arterial stiffness. Second, some data were collected through self-reported measures, which may have affected the accuracy. Finally, despite our efforts to account for various potential confounding factors, the possibility of residual confounding remains, which could have affected the validity of our results.
In non-hypertensive postmenopausal women, serum Klotho levels were significantly negatively correlated with ePWV, particularly among women aged < 60 years, nonsmokers, and non-Hispanic Black women. Future research is necessary to further explore the causal mechanisms with the aim of improving arterial stiffness and decreasing cardiovascular risk in postmenopausal women.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by NHANES Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
BW: Data curation, Formal analysis, Software, Writing – original draft. WX: Data curation, Methodology, Writing – original draft. ZM: Conceptualization, Project administration, Software, Writing – original draft. WY: Data curation, Software, Writing – original draft. XM: Writing – original draft, Writing – review & editing, Funding acquisition. GA: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grants of the National Natural Science Foundation of China (No.81970319) and the Taishan Scholars Program of Shandong Province (No.tsqn202103170).
The authors thank the efforts of all participants and investigators in the NHANES study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rajendran A, Minhas AS, Kazzi B, Varma B, Choi E, Thakkar A, et al. Sex-specific differences in cardiovascular risk factors and implications for cardiovascular disease prevention in women. Atherosclerosis. (2023) 384:117269. doi: 10.1016/j.atherosclerosis.2023.117269
2. Mohanty P, Patnaik L, Nayak G, Dutta A. Gender difference in prevalence of hypertension among Indians across various age-groups: a report from multiple nationally representative samples. BMC Public Health. (2022) 22:1524. doi: 10.1186/s12889-022-13949-5
3. Visniauskas B, Kilanowski-Doroh I, Ogola BO, Mcnally AB, Horton AC, Imulinde Sugi A, et al. Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases. J Hum Hypertens. (2023) 37:609–18. doi: 10.1038/s41371-022-00771-0
4. Mumusoglu S, Yildiz BO. Metabolic syndrome during menopause. Curr Vasc Pharmacol. (2019) 17:595–603. doi: 10.2174/1570161116666180904094149
5. Stefanska A, Bergmann K, Sypniewska G. Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv Clin Chem. (2015) 72:1–75. doi: 10.1016/bs.acc.2015.07.001
6. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. (2021) 397:2385–438. doi: 10.1016/S0140-6736(21)00684-X
7. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. (2014) 63:636–46. doi: 10.1016/j.jacc.2013.09.063
8. O'Neill SM, Travers CM, Otahal P, Khoo SK, Sharman JE. Menopause and accelerated aortic stiffness. Maturitas. (2024) 180:107900. doi: 10.1016/j.maturitas.2023.107900
9. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. (1999) 21:1009–60. doi: 10.3109/10641969909061028
10. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. (2021) 128:864–86. doi: 10.1161/CIRCRESAHA.121.318061
11. Greve SV, Blicher MK, Kruger R, Sehestedt T, Gram-Kampmann E, Rasmussen S, et al. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J Hypertens. (2016) 34:1279–89. doi: 10.1097/HJH.0000000000000935
12. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. (2005) 309:1829–33. doi: 10.1126/science.1112766
13. Kanbay M, Demiray A, Afsar B, Covic A, Tapoi L, Ureche C, et al. Role of klotho in the development of essential hypertension. Hypertension. (2021) 77:740–50. doi: 10.1161/HYPERTENSIONAHA.120.16635
14. Ebert T, Pawelzik SC, Witasp A, Arefin S, Hobson S, Kublickiene K, et al. Inflammation and premature ageing in chronic kidney disease. Toxins (Basel). (2020) 12:227. doi: 10.3390/toxins12040227
15. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. (1997) 390:45–51. doi: 10.1038/36285
16. Cai J, Zhang L, Chen C, Ge J, Li M, Zhang Y, et al. Association between serum Klotho concentration and heart failure in adults, a cross-sectional study from NHANES 2007-2016. Int J Cardiol. (2023) 370:236–43. doi: 10.1016/j.ijcard.2022.11.010
17. Yu J, Li J, Li M, Wang L, Xu X, Li M. Association between serum Klotho concentration and hypertension in postmenopausal women, a cross-sectional study from NHANES 2013-2016. BMC Geriatr. (2023) 23:466. doi: 10.1186/s12877-023-04191-8
18. Mizia-Stec K, Wieczorek J, Polak M, Wybraniec MT, Woźniak-Skowerska I, Hoffmann A, et al. Lower soluble Klotho and higher fibroblast growth factor 23 serum levels are associated with episodes of atrial fibrillation. Cytokine. (2018) 111:106–11. doi: 10.1016/j.cyto.2018.08.005
19. Schettini I, Rios D, Figueiredo RC. Effect of different classes of antihypertensive drugs on arterial stiffness. Curr Hypertens Rep. (2023) 25:61–70. doi: 10.1007/s11906-023-01238-4
20. Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. (2010) 55:9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464
21. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. (2018) 71:e13–e115. doi: 10.1161/HYP.0000000000000065
22. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
23. Wang X, Ma H, Li X, Heianza Y, Manson JE, Franco OH, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern Med. (2023) 183:340–9. doi: 10.1001/jamainternmed.2023.0015
24. Jin W, Chowienczyk P, Alastruey J. Estimating pulse wave velocity from the radial pressure wave using machine learning algorithms. PloS One. (2021) 16:e0245026. doi: 10.1371/journal.pone.0245026
25. Heffernan KS, Stoner L, London AS, Augustine JA, Lefferts WK. Estimated pulse wave velocity as a measure of vascular aging. PloS One. (2023) 18:e0280896. doi: 10.1371/journal.pone.0280896
26. McEniery CM. Transitioning the menopause: A stiff challenge. Arterioscler Thromb Vasc Biol. (2020) 40:850–2. doi: 10.1161/ATVBAHA.120.313980
27. Tsai SS, Lin YS, Hwang JS, Chu PH. Vital roles of age and metabolic syndrome-associated risk factors in sex-specific arterial stiffness across nearly lifelong ages: Possible implication of menopause and andropause. Atherosclerosis. (2017) 258:26–33. doi: 10.1016/j.atherosclerosis.2017.01.023
28. Fu L, Adu-Amankwaah J, Sang L, Tang Z, Gong Z, Zhang X, et al. Gender differences in GRK2 in cardiovascular diseases and its interactions with estrogen. Am J Physiol Cell Physiol. (2023) 324:C505–16. doi: 10.1152/ajpcell.00407.2022
29. Gersh F, O'Keefe JH, Elagizi A, Lavie CJ, Laukkanen JA. Estrogen and cardiovascular disease. Prog Cardiovasc Dis. (2024) 84:60–7. doi: 10.1016/j.pcad.2024.01.015
30. Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. (2009) 53:805–11. doi: 10.1161/HYPERTENSIONAHA.108.126516
31. Lin Y, Chen J, Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension. (2016) 67:564–73. doi: 10.1161/HYPERTENSIONAHA.115.06825
32. Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PloS One. (2013) 8:e56695. doi: 10.1371/journal.pone.0056695
33. Fountoulakis N, Psefteli PM, Maltese G, Gnudi L, Siow RC, Karalliedde J. Reduced levels of the antiaging hormone klotho are associated with increased aortic stiffness in diabetic kidney disease. Kidney Int Rep. (2023) 8:1380–8. doi: 10.1016/j.ekir.2023.04.021
34. Alkalbani M, Prabhu G, Lagbo J, Qayyum R. Serum Klotho and pulse pressure; insight from NHANES. Int J Cardiol. (2022) 355:54–8. doi: 10.1016/j.ijcard.2022.02.021
35. Avolio AP, Kuznetsova T, Heyndrickx GR, Kerkhof P, Li JK. Arterial flow, pulse pressure and pulse wave velocity in men and women at various ages. Adv Exp Med Biol. (2018) 1065:153–68. doi: 10.1007/978-3-319-77932-4_10
36. Kim HJ, Kang E, Oh YK, Kim YH, Han SH, Yoo TH, et al. The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study. BMC Nephrol. (2018) 19:51. doi: 10.1186/s12882-018-0851-3
37. Liang WY, Wang LH, Wei JH, Li QL, Li QY, Liang Q, et al. No significant association of serum klotho concentration with blood pressure and pulse wave velocity in a Chinese population. Sci Rep. (2021) 11:2374. doi: 10.1038/s41598-021-82258-5
38. Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra SG, Tanahashi K, et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. (2014) 306:H348–355. doi: 10.1152/ajpheart.00429.2013
39. Safar ME. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. (2018) 15:97–105. doi: 10.1038/nrcardio.2017.155
40. Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. (2009) 35:341–6. doi: 10.1007/s12020-009-9181-3
41. Guo Y, Zhuang X, Huang Z, Zou J, Yang D, Hu X, et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both. Vitro vivo. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:238–51. doi: 10.1016/j.bbadis.2017.09.029
42. Martín-Núñez E, Donate-Correa J, Ferri C, López-Castillo Á, Delgado-Molinos A, Hernández-Carballo C, et al. Association between serum levels of Klotho and inflammatory cytokines in cardiovascular disease: a case-control study. Aging (Albany NY). (2020) 12:1952–64. doi: 10.18632/aging.v12i2
43. Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. (2005) 101:e67–74. doi: 10.1159/000086500
44. Donate-Correa J, Martín-Carro B, Cannata-Andía JB, Mora-Fernández C, Navarro-González JF. Klotho, oxidative stress, and mitochondrial damage in kidney disease. Antioxid (Basel). (2023) 12:239. doi: 10.3390/antiox12020239
45. Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, Koide M, et al. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol. (2009) 297:H1673–1684. doi: 10.1152/ajpheart.00455.2009
46. Maltese G, Psefteli PM, Rizzo B, Srivastava S, Gnudi L, Mann GE, et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. (2017) 21:621–7. doi: 10.1111/jcmm.12996
47. Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. (2012) 125:2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405
48. Chen K, Sun Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J Mol Med (Berl). (2019) 97:1615–25. doi: 10.1007/s00109-019-01841-6
49. Toyama R, Fujimori T, Nabeshima Y, Itoh Y, Tsuji Y, Osamura RY, et al. Impaired regulation of gonadotropins leads to the atrophy of the female reproductive system in klotho-deficient mice. Endocrinology. (2006) 147:120–9. doi: 10.1210/en.2005-0429
50. Ouyang L, Yu C, Xie Z, Su X, Xu Z, Song P, et al. Indoleamine 2,3-dioxygenase 1 deletion-mediated kynurenine insufficiency in vascular smooth muscle cells exacerbates arterial calcification. Circulation. (2022) 145:1784–98. doi: 10.1161/CIRCULATIONAHA.121.057868
51. Iijima H, Gilmer G, Wang K, Bean AC, He Y, Lin H, et al. Age-related matrix stiffening epigenetically regulates α-Klotho expression and compromises chondrocyte integrity. Nat Commun. (2023) 14:18. doi: 10.1038/s41467-022-35359-2
52. Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. (2009) 54:810–7. doi: 10.1161/HYPERTENSIONAHA.109.134320
53. Ligumsky H, Rubinek T, Merenbakh-Lamin K, Yeheskel A, Sertchook R, Shahmoon S, et al. Tumor suppressor activity of klotho in breast cancer is revealed by structure-function analysis. Mol Cancer Res. (2015) 13:1398–407. doi: 10.1158/1541-7786.MCR-15-0141
Keywords: serum Klotho, estimated pulse wave velocity, postmenopausal women, hypertension, arterial stiffness
Citation: Wang B, Xu W, Mei Z, Yang W, Meng X and An G (2024) Association between serum Klotho levels and estimated pulse wave velocity in postmenopausal women: a cross-sectional study of NHANES 2007–2016. Front. Endocrinol. 15:1471548. doi: 10.3389/fendo.2024.1471548
Received: 27 July 2024; Accepted: 26 August 2024;
Published: 12 September 2024.
Edited by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroReviewed by:
Dana Liana I. Stoian, Victor Babes University of Medicine and Pharmacy, RomaniaCopyright © 2024 Wang, Xu, Mei, Yang, Meng and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guipeng An, Z3VpcGVuZ2FuQGhvdG1haWwuY29t; Xiao Meng, bXg4MWZseUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.