- 1Ministry of Education-Shanghai Key Laboratory of Children’s Environmental Health, Early Life Health Institute, and Department of Pediatrics, Xinhua Hospital, Shanghai Jiao-Tong University School of Medicine, Shanghai, China
- 2Department of Obstetrics and Gynecology, Mount Sinai Hospital, and Institute of Health Policy, Management and Evaluation, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 3Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Introduction: Gestational diabetes mellitus (GDM) is a common pregnancy complication with potential short- and long-term adverse consequences for both mothers and fetuses. It is unclear whether GDM affects linear growth in the offspring; research data are limited and inconsistent.
Methods: In a prospective birth cohort in Shanghai (n=2055 children; 369 born to mothers with GDM). We sought to evaluate the impact of GDM on longitudinal linear growth in early childhood. Length/height was measured in children at birth, 6 weeks, 6 months, 1, 2 and 4 years of age. Multivariate linear regression and generalized estimating equation models were employed to assess the impact of GDM on length/height for age Z score (LAZ/HAZ).
Results: Average birth length was similar in infants of GDM vs. euglycemic mothers. Adjusting for maternal and child characteristics, the children of mothers with GDM had consistently lower LAZ/HAZ compared to children of mothers without diabetes at ages 6 weeks, 6 months, 1, 2 and 4 years. GDM was associated with a 0.12 (95% confidence intervals 0.04-0.21) deficit in LAZ/HAZ in the growth trajectory from birth to age 4 years after adjusting for maternal and child characteristics.
Discussion: GDM was associated with impaired longitudinal linear growth in early childhood. Further studies are warranted to understand the long-term impact on stature and health.
Introduction
Gestational diabetes mellitus (GDM) is a common pregnancy complication characterized by de novo glucose intolerance during gestation affecting both the fetuses and the mothers (1, 2). GDM has been associated with macrocosmic birth and increased risks of obesity and glucose intolerance in the offspring in later life (3–5). It is unclear whether GDM affects linear growth in the offspring. Basic science studies indicate that the osteogenic capability of bone marrow mesenchymal stem cells is impaired under high glucose conditions (6, 7), suggesting that a high-glucose environment may affect bone’s growth potential. However, research data are limited and inconsistent concerning whether GDM affects linear growth in the offspring at birth (8–19), during infancy (0-2 years) (8, 9, 13, 14, 18–24), early childhood (2-5 years) (8, 13, 25–27), late childhood (6-10 years) (13, 28–30) and adolescence (11-18 years) (29, 31, 32). Some studies reported lower height or reduced linear growth in the offspring of mothers with GDM (9, 28, 31, 32), while others reported increased height (8, 13, 33) or no significant differences (12, 26, 27, 29). Common limitations in previous studies included inadequate sample sizes (18–20, 28), inaccurate length/height data based on routine childcare records rather than standardized measurements (13, 21, 27, 31), and lack of adjustment for major confounding factors (such as maternal height) (8, 13, 18, 19, 21, 22, 26). Longitudinal data are scanty concerning the impact of GDM on linear growth in early childhood. In the present study, we sought to assess the impact of GDM on longitudinal linear growth in early childhood (from birth to age 4 years) in a large prospective birth cohort.
Methods
Study design and population
This was a prospective follow-up study of children in the Shanghai Birth Cohort (SBC) (34). The SBC is a prospective cohort involving 4127 pregnancies in six tertiary obstetric care hospitals in Shanghai between 2013 and 2016. The study was approved by the research ethics boards of Shanghai Xinhua Hospital (the coordination center) and all participating hospitals. Written informed consent was obtained from all study participants.
There was a total of 3692 live births (3331 singletons) in the SBC, and 2207 singleton children remained in follow-ups at age 4 years. We excluded children whose mothers had pre-gestational diabetes (n=7) or preeclampsia/eclampsia (n=27), and children with birth defects (n=16), born at gestational age <34 weeks (n=13), or missing information on maternal GDM (n=34), child’s sex or gestational age at delivery (n=55). The final study sample included 2055 children with at least one follow-up length/height measurement between ages 6 weeks and 4 years. The numbers of children with data available on length/height were 1772 (86%) at birth, 1801 (88%) at 6 weeks, 1739 (85%) at 6-months, 1805 (88%) at 12 months, 1874 (91%) at 2 years, and 1951 (95%) at 4 years of age, respectively. There were 1139 children with complete data on length/height measurements at all the six time points. Figure 1 illustrates the selection of study subjects and follow-up length/height measurements from birth to 4 years of age.

Figure 1. Flowchart in the selection of study subjects and child’s length/height measurements in the Shanghai Birth Cohort. GDM, gestational diabetes mellitus.
GDM was diagnosed by a 75g oral glucose tolerance test at 24-28 weeks of gestation according to International Association of Diabetes and Pregnancy Study Groups (IADPSG)’ criteria (35): if any blood glucose value reaches or exceeds the following thresholds: fasting 5.1 mmol/L, 1- hour 10.0 mmol/L, and 2-hour 8.5 mmol/L. There were 369 mothers with GDM in the study cohort.
Maternal/pregnancy characteristics
Available maternal/pregnancy characteristics included age, ethnicity, parity, education, smoking or alcohol use during pregnancy, hypertensive disorders in pregnancy (chronic or gestational, excluding preeclampsia or eclampsia), height, pre-pregnancy weight, gestational weight gain, mode of delivery, infant’s sex, birth weight and gestational age. Pre-pregnancy body mass index (BMI) was calculated as weight (kg)/(height [m]) ^2. Gestational weight gain was calculated as the difference between the weight measured within one week before delivery and pre-pregnancy weight, and was classified as inadequate, adequate, and excessive if it was below, within, or above the recommendations of the 2009 Institute of Medicine guidelines (36): 12.5-18.0 kg for underweight; 11.5-16.0 kg for normal weight; 7.0-11.5 kg for overweight; and 5.0-9.0 kg for obese women. Women were classified by pre-pregnancy BMI as underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2) or obese (30.0+ kg/m2). Gestational age was determined by the date of last menstruation period and confirmed by first-trimester ultrasound dating. If the difference between the 2 estimates was more than 2 weeks, the ultrasound dating-based estimate was used.
Length/height measurements in children
Length/height was measured at birth and postnatal follow-ups at ages 6 weeks (42 days), 6 months, 1, 2 and 4 years by trained research staff. Length (birth to age 2 years) and standing height (age 4 years) were measured following standardized operating protocols. Length (birth to 2 years of age) were measured in supine position using a Seca 416 Infantometer (Seca Netherlands, Hamburg). At age 4 years, standing height was measured by a wall-mounted stadiometer (Seca Netherlands, Hamburg). Length and height measurements were measured twice to the closest 0.1 cm, and the averages were taken as the final values.
Length/height-for-age Z scores were calculated according to the World Health Organization (WHO) growth references (37) using the ‘anthro’ and ‘anthroplus’ package in R.
Statistical analysis
Data are presented as mean ± SD for continuous variables or frequency (percentage) for categorical variables. The independent t-test and Pearson’s chi-square test were used to compare differences in continuous and categorical variables between GDM and euglycemic pregnancy groups, respectively. Generalized linear models were employed to evaluate the association between GDM and LAZ/HAZ at each follow-up time point. Generalized estimating equation (GEE) models were used to assess the association between GDM and linear growth trajectory from birth to 4 years of age overall accounting for the correlations in length/height measurements over ages within the same subjects. In the adjusted models, we included pre-specified known factors affecting length/height (maternal height, infant’s sex, gestational age at birth). Other co-variables, including maternal age, ethnicity, parity, smoking in pregnancy, alcohol drinking in pregnancy, pre-pregnancy BMI, gestational weight gain, hypertensive disorders in pregnancy, mode of delivery and breastfeeding were subject to a stepwise regression selection process. Only co-variables with P<=0.20 would be retained in the final parsimonious models.
Since pre-pregnancy BMI may be considered an upstream risk factor of GDM, gestational weight gain may be influenced by maternal GDM, and GDM may affect postnatal linear growth through affecting gestational age at birth, we repeated the stepwise regression models in sensitivity analyses excluding gestational age, or gestational weight gain, or both pre-pregnancy BMI and gestational weight gain. To disentangle the potential confounding effects of maternal hypertension from GDM in the associations with offspring’s linear growth, we excluded those exposed to maternal hypertensive disorders in a sensitivity analysis. Considering the potential impact of paternal height, we also conducted a sensitivity analysis to include paternal height (with a high frequency of missing values) in the adjusted models in a sensitivity analysis.
Effect sizes in regression coefficients (β) with 95% confidence intervals (CIs) were presented. Missing values in continuous co-variables were not imputed. Missing values in categorical co-variables were taken as a valid category in regression models. P<0.05 was considered statistically significant in assessing the primary association of interest between GDM and linear growth from birth to age 4 years in a GEE model. P values in other comparisons were for exploratory information only, and multiple comparisons were not accounted for. All statistical analyses were conducted using R version 4.2.3 and STATA V.15.
Results
Of the 3331 singleton births in Shanghai birth cohort, 2207 children remained in follow-ups at age 4 years, 2055 children were included in the final study cohort (Figure 1). Comparing children who lost to vs. remained in follow-ups at age 4 years (Supplementary Table S1), their mothers tended to be younger (mean: 29.1 vs. 29.5 years), had higher pre-pregnancy weight (mean: 59.5 kg vs. 56.4 kg) and BMI (mean: 22.6 vs. 21.5 kg/m2) and were likely to have GDM (18.1% vs. 12.7%), while the children had shorter gestational age (mean: 38.9 vs. 39.1 weeks) and were more likely to be delivered by cesarean section (52.4% vs. 45.6%). Other maternal and infant characteristics were similar.
Characteristics of study subjects
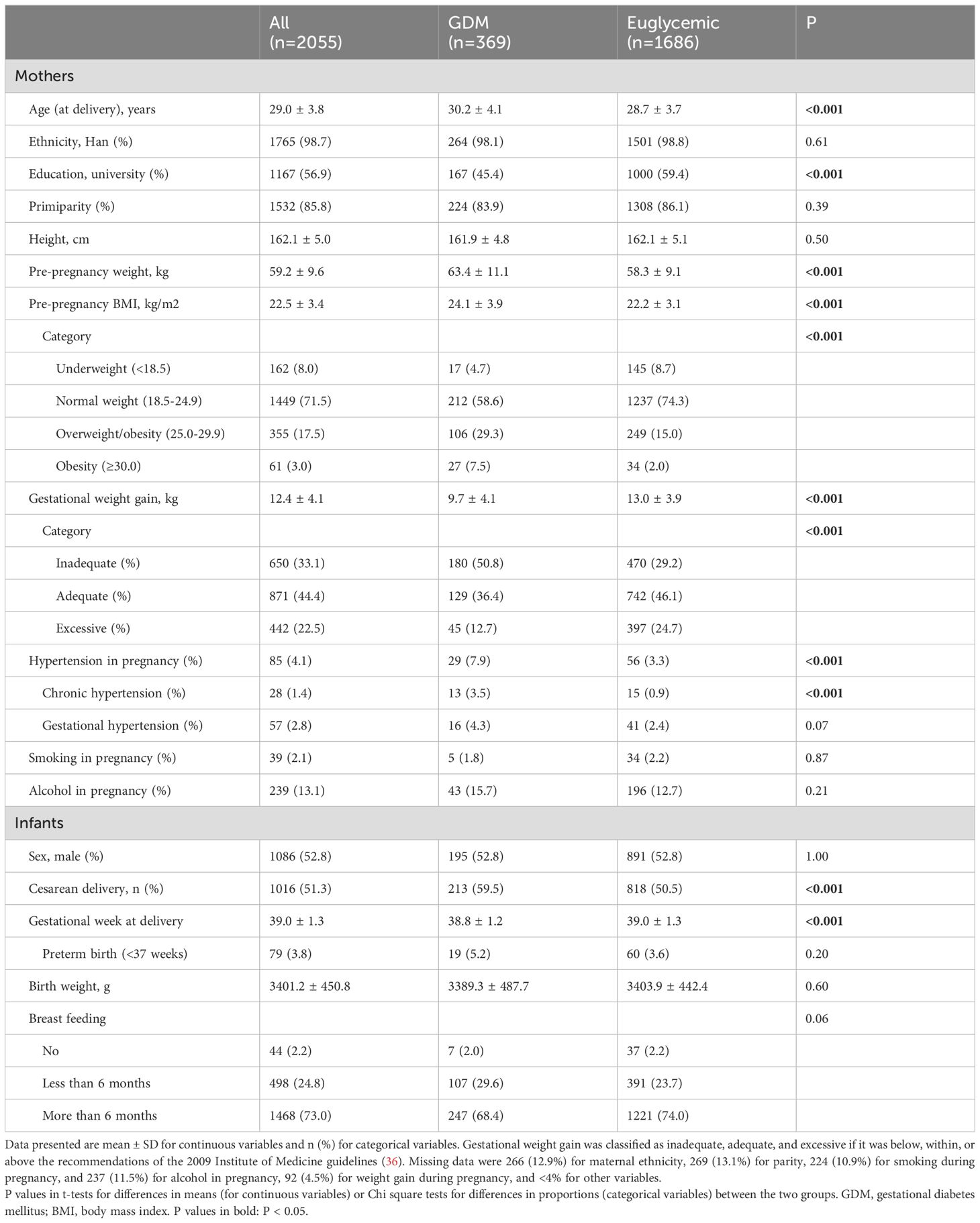
Table 1 presents the characteristics of subjects in the final study cohort. The average age of mothers at delivery was 29 years, and all mothers were over 20 years of age. There were 369 mothers (18%) with GDM. Compared to mothers with a euglycemic pregnancy, mothers with GDM were older (mean: 30.2 vs. 28.7 years), less likely to have completed college or higher education, had higher pre-pregnancy BMI (mean: 24.1 vs. 22.2 kg/m2), but were less likely to have excessive weight gain during pregnancy (12.7% vs. 24.7%). GDM mothers were more likely to be affected by hypertensive disorders in pregnancy (7.9% vs. 3.3%). There were no significant differences in maternal height, smoking or alcohol consumption during pregnancy between the two groups. Among mothers with GDM and information available on treatments (n=243), 24 (9.9%) mothers required the use of insulin in the management of hyperglycemia, the rest took dietary and life style interventions only. The infants of mothers with GDM had a slightly shorter average gestational age at delivery (mean: 38.8 vs. 39.0 weeks), and were more likely to be delivered via cesarean section (59.5% vs. 50.5%), while birth weights were comparable. A slightly higher proportion of infants of GDM mothers had breastfeeding less than 6 months (29.6% vs. 23.7%).
Length/height from birth to age 4 years
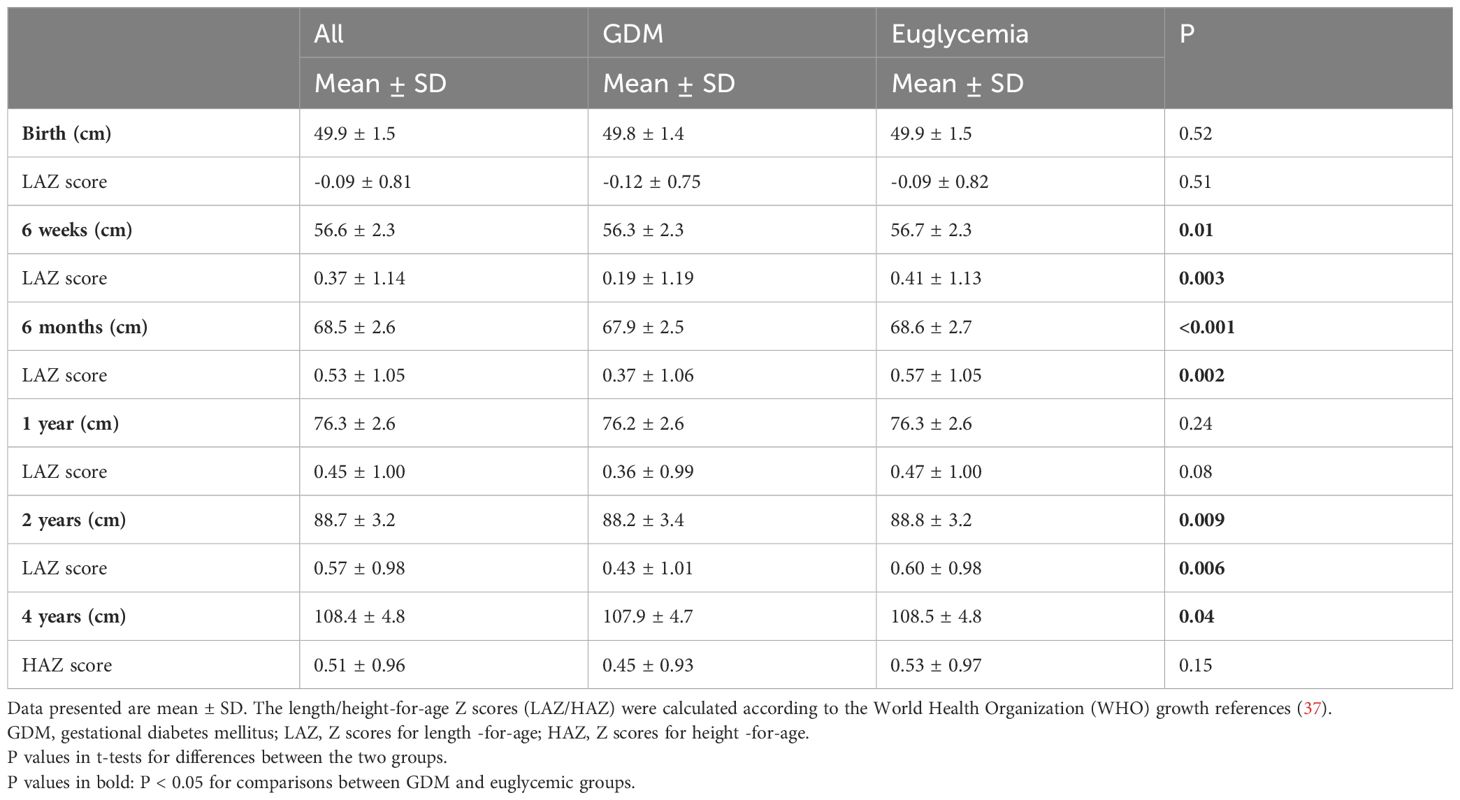
Table 2 presents the data on length/height in centimeter and for age z scores (LAZ/HAZ) comparing the offspring of GDM vs. euglycemic mothers. Average birth length was similar, but average LAZ/HAZ scores were consistently lower in the offspring of GDM mothers in all postnatal follow-up age points (6 weeks, 6 months, 1, 2 and 4 years), and statistically significantly so at 6 weeks, 6 months and 2 years. On average, crude LAZ/HAZ values were 0.08 to 0.22 lower in the offspring of GDM vs. euglycemic mothers at 6 weeks to 4 years of age. The mean differences in Z scores between the two groups showed a narrowing trend over increasing ages (Z score differences: 6 weeks -0.22, 6 months -0.20, 1 year -0.11, 2 years -0.17, 4 years -0.08).
Adjusting for maternal and child characteristics, the offspring of mothers with GDM had consistently lower LAZ/HAZ than the offspring of euglycemic mothers, with mean differences (regression coefficients) in the range of -0.11 to -0.18 between ages 6 weeks and 4 years (adjusted P<0.05 at all the five age points: 6 weeks, 6 months, 1, 2 and 4 years) (Table 3). Accounting for within-subject correlations in length//height, the children born to mothers with GDM exhibited a significantly reduced linear growth from birth to age 4 years overall, with a mean linear growth deficit of 0.12 (95% CI: 0.04, 0.21) in LAZ/HAZ after adjusting for maternal height, pre-pregnancy BMI, gestational weight gain, alcohol drinking status in pregnancy, gestational age at delivery and child’s sex (other maternal and child factors did not affect the comparisons and were excluded at P>0.2).
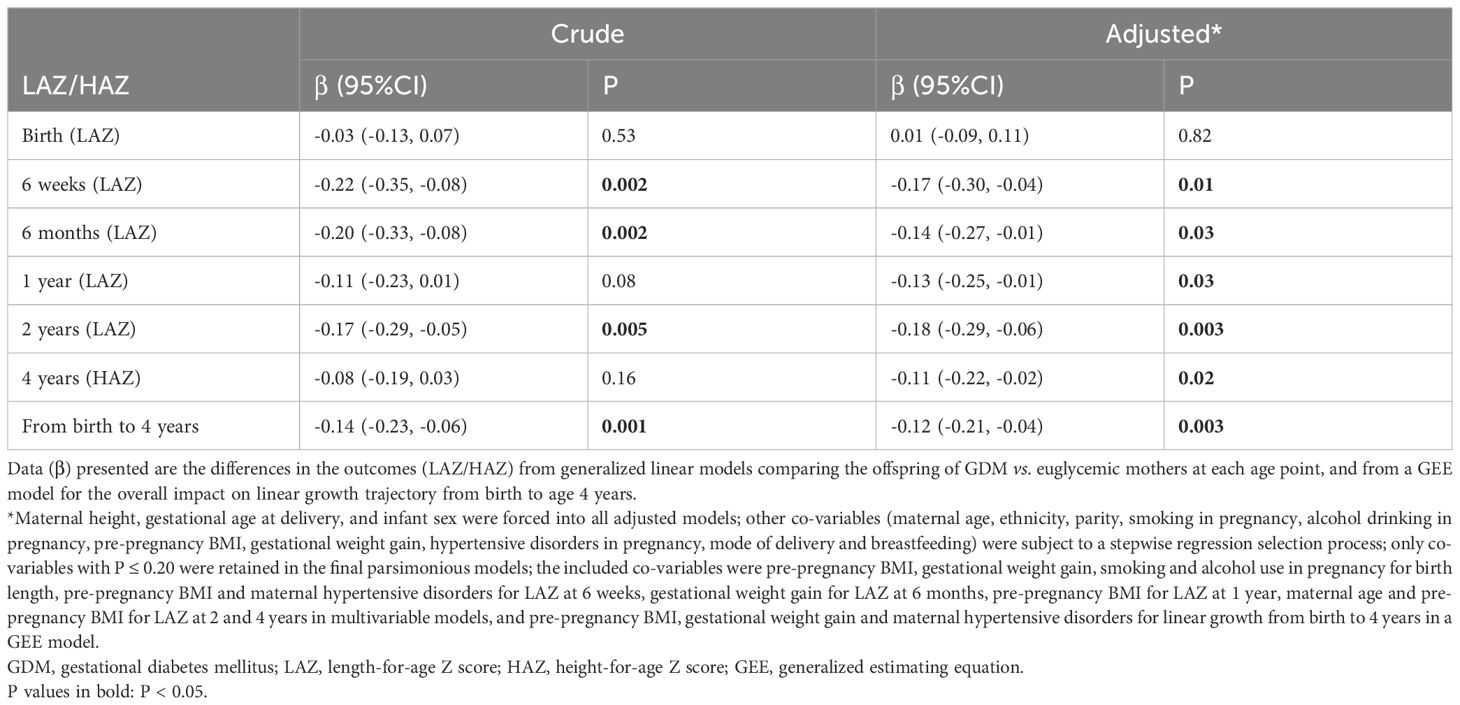
Table 3. The associations between GDM and longitudinal linear growth from birth to age 4 years in the offspring.
Sensitivity analyses excluding gestational age, gestational weight gain, or pre-pregnancy BMI and gestational weight gain as covariates, or excluding children born to mothers with hypertensive disorders in pregnancy in the analyses showed similar results as in the main analysis (Supplementary Tables S2, S3). With further adjustment for paternal height, the associations showed a similar pattern, but could not detect a statistically significant effect of GDM on linear growth due to the relatively small sample size (data available on paternal height: n=36 for children of GDM mothers) (Supplementary Table S4).
Among children of mothers with GDM, there were no significant differences comparing children of mothers who received insulin treatment versus those who received dietary and lifestyle interventions only in LAZ/HAZ scores at birth (mean ± SD: -0.33 ± 0.93 vs. -0.06 ± 0.80, P = 0.20) or age 4 years (0.56 ± 1.02 vs. 0.44 ± 0.93, P = 0.60). There was no significant difference in linear growth from birth to age 4 years overall in children of GDM mothers who received insulin treatment or not (Supplementary Table S5).
Discussion
In a large birth cohort with longitudinal follow-up data on measured length/height, the offspring exposed to GDM exhibited consistently shorter stature in early childhood from age 6 weeks to 4 years as compared to the offspring of euglycemic mothers, although no significant differences were observed in birth length. The finding suggests an adverse impact of GDM on linear growth in early childhood. We speculated that this could be due to “impaired” bone growth potential that could manifest shortly in early postnatal life, but towards a gradual recovery path during early childhood.
We did not detect any significant differences in birth length (although numerically lower average birth length z score in GDM), consistent with a study in northern Chinese children (16) as well as in two studies in European populations (UK and Germany) (9, 20). In contrast, some studies reported greater birth length in the offspring of mothers with GDM (8, 13). These discrepant findings could be partly due to the differences in the diagnostic criteria of GDM, and the quality of care in the management of hyperglycemia in GDM (38). We used the recent IADPSG criteria with lower glucose cutoffs than the commonly used diagnostic criteria in earlier years. Therefore, women with the diagnosed of GDM nowadays tend to have less severe hyperglycemia than those GDM diagnosed in earlier years. In the study cohort, mothers with GDM experienced less frequent excessive weight gain during pregnancy compared to mothers without diabetes, and their infants have similar birth weights as infants of euglycemic mothers, suggesting high-quality care in the management of hypoglycemia in GDM. In our study, birth length was similar in the offspring of GDM and euglycemic mothers (although numerically slightly lower in the mean birth length z score in the GDM group). However, in all subsequent follow ups during infancy, the infants of GDM mothers had consistently shorter length at ages 6 months, 6 months, 1 and 2 years. This finding is consistent with the observations in a northern Chinese study population (n=1420 infants of mothers with impaired glucose tolerance or impaired fasting glucose or newly diagnosed diabetes) at ages 3, 6, 9, and 12 months (21, 33), as well as with a study of 3-month-old infants (n=90 infants of mothers with GDM) in Canada (22). A reduced length gain in the offspring of GDM mothers was noted from 3 to 24 months in another previous study (9). These findings were corroborated in a meta-analysis encompassing 4 studies including 25,736 infants (39). However, we also noted that another study in a Chinese population did not detect any changes in LAZ trajectories in the offspring of GDM mothers (n=205) from birth to 12 months (23). Taken together, most studies suggest an adverse impact of GDM on linear growth in early postnatal life. We speculated that this could probably be due to “impaired” bone growth potential in utero.
There have been only a few longitudinal studies on the impact of GDM on linear growth from birth to early childhood by age 4-5 years. In the PANDORA study of children aged 0-5 years with 25% of children follow-up at age 5 years, no differences in height trajectories were detected in the offspring of GDM (n= 228) and euglycemic mothers (n=95) (25). A study in Indian children (n=41 children of mothers with diabetes) using length measurements without considering other covariates reported no differences in length/height at ages 1 and 5 years (8). In contrast, a Swedish study reported increased height at age 4-5 years in female offspring of GDM mothers (n=110) (13). These differences may be partly due to limited sample sizes and different adjustments in the analyses. In the present longitudinal study on linear growth during early childhood with the largest sample size (369 children in GDM and 1686 in euglycemic pregnancy), we have accounted for important confounders such as maternal height which may help to better delineate the longitudinal impact of GDM on linear growth.
The observed adverse impact of GDM on linear growth is consistent with two studies in adolescents. Grunnet et al. reported decreased height in the offspring of mothers with GDM (n=561) at age 9-16 years (32). Similarly, a study of Dutch children reported a slight decrease in height z score in early adolescence in the offspring of GDM (n=104) (31). Taken together, the body of evidence suggests a negative impact of GDM on linear growth from early childhood to adolescence. It remains unclear whether this may be translated into an adverse impact on adult stature.
The mechanisms underlying the negative impact of GDM on postnatal linear growth are unknown. Insulin plays a pivotal role in bone and muscle growth (40), and studies have shown that preterm infants exposed to higher glucose and insulin concentrations have shorter leg length at term corrected age (41), and shorter stature at school ages (42). A similar response might be expected in linear growth in the offspring of mothers with GDM who might have been exposed to hyperinsulinemia during fetal life. However, basic science studies have shown that insulin can directly acts on insulin receptors in the growth plate, leading to chondrocyte proliferation and differentiation (43). Insulin can regulate GH receptor expression in the liver and stimulate IGF-1 production promoting bone growth (44). Cell and animal studies indicate that the osteogenic capability of bone marrow mesenchymal stem cells could be impaired under high glucose conditions (6, 7). Hyperglycemia could activate the non-canonical Wnt/PKC pathway, increasing adipogenesis while inhibiting osteogenic differentiation, leading to reduced bone mass and impaired skeletal development (45–47). Bone marrow stem cells show signs of senescence under high glucose conditions (6, 48). Therefore, high glucose and insulin could have opposite effects on bone growth. The hyperglycemia in GDM may engender a plethora of alterations in the fetus which might impinge upon the differentiation of bone marrow mesenchymal stem cells, leading to “impaired” bone growth potential, and consequently manifested dampened linear growth in early postnatal life, although the intrauterine impact might not be severe enough for a discernable impact on birth length. However, this interpretation is largely speculative. The mechanisms remain elusive and necessitate further investigations.
Our study has limitations. Paternal height may influence linear growth in the offspring. Paternal height was missing in the majority of study subjects in the study cohort. However, we observed a similar result pattern in a sensitivity analysis incorporating paternal height. There were some differences in maternal age, pre-pregnancy BMI, GDM, child’s gestational age and mode of delivery between children who remained in vs. lost to follow up at age 4 years. The potential impact on the findings could not be evaluated. All study subjects were Chinese from a metropolitan city in China, more studies in other regions and populations are warranted in understanding the generalizability of the study findings.
Conclusions
Our study data suggest that GDM may impair linear growth in early childhood in the offspring. Further studies are warranted to understand the potential long-term impact on stature and health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Access to the deidentified participant research data must be approved by the research ethics board on a case-by-case basis, please contact the corresponding authors (Z-C Luo, emMubHVvQHV0b3JvbnRvLmNh; Fei Li, ZmVpbGlAc2hzbXUuZWR1LmNu) for assistance in data access request.
Ethics statement
The studies involving humans were approved by Ethics committees of Xinhua Hospital (the coordination center, reference number M2013-010). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
Z-LC: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing, Data curation. XL: Data curation, Formal analysis, Writing – original draft. M-YT: Data curation, Validation, Writing – original draft. M-NY: Data curation, Methodology, Writing – review & editing. HH: Data curation, Project administration, Writing – review & editing. FF: Data curation, Project administration, Writing – review & editing. TW: Writing – original draft. FO: Project administration, Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Investigation. JZ: Data curation, Funding acquisition, Project administration, Supervision, Writing – review & editing, Conceptualization, Investigation. FL: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing, Investigation. Z-CL: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing, Formal analysis, Investigation, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82125032, 81930095), the Ministry of Science and Technology of China (2019YFA0802501), the China Brain Initiative Grant (STI2030-Major Projects 2021ZD0200800), the Science and Technology Commission of Shanghai Municipality (19410713500, 23Y21900500 and 2018SHZDZX01), the Shanghai Municipal Commission of Health and Family Planning (GWVI-11.1-34, 2020CXJQ01, 2018YJRC03), Innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20211100), and the Guangdong Key Project (2018B030335001). The funders have no role in all aspects of the study, including study design, data collection and analysis, the preparation of the manuscript, and the decision for publication.
Acknowledgments
We gratefully acknowledged all research staff who had contributed to patient recruitment and data collection in the Shanghai Birth Cohort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1470678/full#supplementary-material
References
1. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
2. Saravanan P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. (2020) 8:793–800. doi: 10.1016/s2213-8587(20)30161-3
3. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
4. Aberg A, Rydhstroem H, Frid A. Impaired glucose tolerance associated with adverse pregnancy outcome: A population-based study in southern Sweden. Am J Obstet Gynecol. (2001) 184:77–83. doi: 10.1067/mob.2001.108085
5. Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. (2003) 111:e221–6. doi: 10.1542/peds.111.3.e221
6. Luo M, Zhao Z, Yi J. Osteogenesis of bone marrow mesenchymal stem cell in hyperglycemia. Front Endocrinol (Lausanne). (2023) 14:1150068. doi: 10.3389/fendo.2023.1150068
7. Hankamolsiri W, Manochantr S, Tantrawatpan C, Tantikanlayaporn D, Tapanadechopone P, Kheolamai P. The effects of high glucose on adipogenic and osteogenic differentiation of gestational tissue-derived mscs. Stem Cells Int. (2016) 2016:9674614. doi: 10.1155/2016/9674614
8. Krishnaveni GV, Hill JC, Leary SD, Veena SR, Saperia J, Saroja A, et al. Anthropometry, glucose tolerance, and insulin concentrations in Indian children: relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care. (2005) 28:2919–25. doi: 10.2337/diacare.28.12.2919
9. Prentice PM, Olga L, Petry CJ, Simmons D, Murphy HR, Hughes IA, et al. Reduced size at birth and persisting reductions in adiposity in recent, compared with earlier, cohorts of infants born to mothers with gestational diabetes mellitus. Diabetologia. (2019) 62:1977–87. doi: 10.1007/s00125-019-4970-6
10. Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord. (1997) 21:451–6. doi: 10.1038/sj.ijo.0800429
11. Jang HC, Cho NH, Min YK, Han IK, Jung KB, Metzger BE. Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in korean women with gdm. Diabetes Care. (1997) 20:1582–8. doi: 10.2337/diacare.20.10.1582
12. Ahlsson F, Lundgren M, Tuvemo T, Gustafsson J, Haglund B. Gestational diabetes and offspring body disproportion. Acta Paediatrica. (2009) 99:89–93. doi: 10.1111/j.1651-2227.2009.01532.x
13. Nilsson C, Carlsson A, Landin-Olsson M. Increased risk for overweight among swedish children born to mothers with gestational diabetes mellitus. Pediatr Diabetes. (2014) 15:57–66. doi: 10.1111/pedi.12059
14. Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: A longitudinal study. Br J Nutr. (2018) 120:1380–7. doi: 10.1017/s0007114518002933
15. Hojaji E, Aghajani M, Zavoshy R, Noroozi M, Jahanihashemi H, Ezzeddin N. Household food insecurity associations with pregnancy hypertension, diabetes mellitus and infant birth anthropometric measures: A cross-sectional study of Iranian mothers. Hypertension Pregnancy. (2021) 40:109–17. doi: 10.1080/10641955.2021.1874010
16. Zhao X, Li N, Jia R, Chen S, Wang L. The factors affecting the physical development of neonates in pregnant women with or without gestational diabetes mellitus. PloS One. (2021) 16:e0251024. doi: 10.1371/journal.pone.0251024
17. Liu S, Guan L, Liu X, Fan P, Zhou M, Wu Y, et al. Atp-binding cassette transporter G1 (Abcg1) polymorphisms in pregnant women with gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. (2023) 287:20–8. doi: 10.1016/j.ejogrb.2023.05.033
18. Vohr BR, McGarvey ST. Growth patterns of large-for-gestational-age and appropriate-for-gestational-age infants of gestational diabetic mothers and control mothers at age 1 year. Diabetes Care. (1997) 20:1066–72. doi: 10.2337/diacare.20.7.1066
19. Logan KM, Emsley RJ, Jeffries S, Andrzejewska I, Hyde MJ, Gale C, et al. Development of early adiposity in infants of mothers with gestational diabetes mellitus. Diabetes Care. (2016) 39:1045–51. doi: 10.2337/dc16-0030
20. Uebel K, Pusch K, Gedrich K, Schneider KT, Hauner H, Bader BL. Effect of Maternal Obesity with and without Gestational Diabetes on Offspring Subcutaneous and Preperitoneal Adipose Tissue Development from Birth up to Year-1. BMC Pregnancy Childbirth. (2014) 14:138. doi: 10.1186/1471-2393-14-138
21. Dong L, Liu E, Guo J, Pan L, Li B, Leng J, et al. Relationship between maternal fasting glucose levels at 4-12 gestational weeks and offspring growth and development in early infancy. Diabetes Res Clin Pract. (2013) 102:210–7. doi: 10.1016/j.diabres.2013.10.017
22. Kramer CK, Hamilton JK, Ye C, Hanley AJ, Connelly PW, Sermer M, et al. Antepartum determinants of rapid early-life weight gain in term infants born to women with and without gestational diabetes. Clin Endocrinol (Oxf). (2014) 81:387–94. doi: 10.1111/cen.12437
23. Hu J, Liu Y, Wei X, Li L, Gao M, Liu Y, et al. Association of gestational diabetes mellitus with offspring weight status across infancy: A prospective birth cohort study in China. BMC Pregnancy Childbirth. (2021) 21. doi: 10.1186/s12884-020-03494-7
24. Titmuss A, Barzi F, Barr ELM, Webster V, Wood A, Kelaart J, et al. Association between maternal hyperglycemia in pregnancy and offspring anthropometry in early childhood: the pandora wave 1 study. Int J Obes (Lond). (2023) 47:1120–31. doi: 10.1038/s41366-023-01366-6
25. Titmuss A, Longmore DK, Barzi F, Barr ELM, Webster V, Wood A, et al. Association between hyperglycaemia in pregnancy and growth of offspring in early childhood: the pandora study. Pediatr Obes. (2022) 17. doi: 10.1111/ijpo.12932
26. Kaul P, Bowker SL, Savu A, Yeung RO, Donovan LE, Ryan EA. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia. (2018) 62:249–58. doi: 10.1007/s00125-018-4758-0
27. Wang J, Wang L, Liu H, Zhang S, Leng J, Li W, et al. Maternal gestational diabetes and different indicators of childhood obesity: A large study. Endocrine Connections. (2018) 7:1464–71. doi: 10.1530/ec-18-0449
28. Antikainen L, Jääskeläinen J, Nordman H, Voutilainen R, Huopio H. Prepubertal children exposed to maternal gestational diabetes have latent low-grade inflammation. Hormone Res Paediatrics. (2018) 90:109–15. doi: 10.1159/000491938
29. Hockett CW, Bedrick EJ, Zeitler P, Crume TL, Daniels S, Dabelea D. Exposure to diabetes in utero is associated with earlier pubertal timing and faster pubertal growth in the offspring: the epoch study. J Pediatr. (2019) 206:105–12. doi: 10.1016/j.jpeds.2018.10.053
30. Zeng J, Shen F, Zou Z-Y, Yang R-X, Jin Q, Yang J, et al. Association of maternal obesity and gestational diabetes mellitus with overweight/obesity and fatty liver risk in offspring. World J Gastroenterol. (2022) 28:1681–91. doi: 10.3748/wjg.v28.i16.1681
31. Hammoud NM, Visser GHA, van Rossem L, Biesma DH, Wit JM, de Valk HW. Long-term bmi and growth profiles in offspring of women with gestational diabetes. Diabetologia. (2018) 61:1037–45. doi: 10.1007/s00125-018-4584-4
32. Grunnet LG, Hansen S, Hjort L, Madsen CM, Kampmann FB, Thuesen ACB, et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: A clinical study within the danish national birth cohort. Diabetes Care. (2017) 40:1746–55. doi: 10.2337/dc17-0514
33. Liu G, Li N, Sun S, Wen J, Lyu F, Gao W, et al. Maternal ogtt glucose levels at 26–30 gestational weeks with offspring growth and development in early infancy. BioMed Res Int. (2014) 2014:1–11. doi: 10.1155/2014/516980
34. Zhang J, Tian Y, Wang W, Ouyang F, Xu J, Yu X, et al. Cohort profile: the shanghai birth cohort. Int J Epidemiol. (2019) 48:21–g. doi: 10.1093/ije/dyy277
35. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
36. Moore Simas TA, Waring ME, Sullivan GM, Liao X, Rosal MC, Hardy JR, et al. Institute of medicine 2009 gestational weight gain guideline knowledge: survey of obstetrics/gynecology and family medicine residents of the United States. Birth. (2013) 40:237–46. doi: 10.1111/birt.12061
37. Bloem M. The 2006 WHO child growth standards. BMJ. (2007) 334:705–6. doi: 10.1136/bmj.39155.658843.BE
38. Wahabi HA, Alzeidan RA, Bawazeer GA, Alansari LA, Esmaeil SA. Preconception care for diabetic women for improving maternal and fetal outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth. (2010) 10:63. doi: 10.1186/1471-2393-10-63
39. Manerkar K, Harding J, Conlon C, McKinlay C. Maternal gestational diabetes and infant feeding, nutrition and growth: A systematic review and meta-analysis. Br J Nutr. (2020) 123:1201–15. doi: 10.1017/s0007114520000264
40. Lecka-Czernik B. Diabetes, bone and glucose-lowering agents: basic biology. Diabetologia. (2017) 60:1163–9. doi: 10.1007/s00125-017-4269-4
41. Alsweiler JM, Harding JE, Bloomfield FH. Tight glycemic control with insulin in hyperglycemic preterm babies: A randomized controlled trial. Pediatrics. (2012) 129:639–47. doi: 10.1542/peds.2011-2470
42. Tottman AC, Alsweiler JM, Bloomfield FH, Gamble G, Jiang Y, Leung M, et al. Long-term outcomes of hyperglycemic preterm infants randomized to tight glycemic control. J Pediatr. (2018) 193:68–75.e1. doi: 10.1016/j.jpeds.2017.09.081
43. Wu S, Aguilar AL, Ostrow V, De Luca F. Insulin resistance secondary to a high-fat diet stimulates longitudinal bone growth and growth plate chondrogenesis in mice. Endocrinology. (2011) 152:468–75. doi: 10.1210/en.2010-0803
44. Gat-Yablonski G, Yackobovitch-Gavan M, Phillip M. Which dietary components modulate longitudinal growth? Curr Opin Clin Nutr Metab Care. (2017) 20:211–6. doi: 10.1097/mco.0000000000000364
45. Keats EC, Dominguez JM 2nd, Grant MB, Khan ZA. Switch from canonical to noncanonical wnt signaling mediates high glucose-induced adipogenesis. Stem Cells. (2014) 32:1649–60. doi: 10.1002/stem.1659
46. Lerner UH, Ohlsson C. The wnt system: background and its role in bone. J Intern Med. (2015) 277:630–49. doi: 10.1111/joim.12368
47. Bao K, Jiao Y, Xing L, Zhang F, Tian F. The role of wnt signaling in diabetes-induced osteoporosis. Diabetol Metab Syndr. (2023) 15:84. doi: 10.1186/s13098-023-01067-0
Keywords: early childhood, gestational diabetes mellitus, linear growth, length/height for age z score, birth cohort
Citation: Chen Z-L, Liu X, Tao M-Y, Yang M-N, He H, Fang F, Wu T, Ouyang F, Zhang J, Li F and Luo Z-C (2024) Gestational diabetes mellitus and linear growth in early childhood. Front. Endocrinol. 15:1470678. doi: 10.3389/fendo.2024.1470678
Received: 25 July 2024; Accepted: 05 November 2024;
Published: 27 November 2024.
Edited by:
Li Ming Wen, The University of Sydney, AustraliaReviewed by:
Xinyue Liu, University of California, Los Angeles, United StatesGalia Gat-Yablonski, Schneider Children’s Medical Center, Israel
Copyright © 2024 Chen, Liu, Tao, Yang, He, Fang, Wu, Ouyang, Zhang, Li and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-Cheng Luo, emMubHVvQHV0b3JvbnRvLmNh; Fei Li, ZmVpbGlAc2hzbXUuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Zhong-Cheng Luo, orcid.org/0000-0002-1794-1312
 Zi-Lin Chen
Zi-Lin Chen Xin Liu1†
Xin Liu1† Min-Yi Tao
Min-Yi Tao Hua He
Hua He Fengxiu Ouyang
Fengxiu Ouyang Jun Zhang
Jun Zhang Fei Li
Fei Li Zhong-Cheng Luo
Zhong-Cheng Luo