- 1Department of Microbiology and Microbial Pathogenesis, Washington University Medical School, St. Louis, MO, United States
- 2QuidelOrtho Corporation, San Diego, CA, United States
- 3Molecular Thyroid Research Laboratory, Department of Medicine I, Johannes Gutenberg University (JGU) Medical Center, Mainz, Germany
Background: A novel and rapid cell-based bioassay, Turbo TSI, for measurement of thyroid-stimulating immunoglobulins (TSI) was recently reported. An assessment of the analytical performance of this TSI bioassay is described herein.
Methods: Thawed cells from Turbo TSI kits were treated with different concentrations of a World Health Organization (WHO) international standard (IS) TSI-positive serum. TSI was measured as a function of luciferase activity measured as relative light units (RLU) and converted into international units per liter (IU/L). Analytical performance studies were performed on numerous samples, over multiple days, by two users at two sites.
Results: The limit of blank, limit of detection and limit of quantitation were determined to be 0.007 IU/L, 0.014 IU/L, and 0.021 IU/L, respectively. Receiver operator characteristics (ROC) analysis determined the cut-off to be 0.0241 IU/L with an area under the curve of 0.984. The linear range was shown to be from 0.015 to 11.958 IU/L. The intra-laboratory precision was ≤15%CV. The overall reproducibility of the assay was ≤20%CV for five concentrations (0.06 to 5.16 IU/L). Interference and cross reactivity studies with a variety of substances showed that the assay was robust. The Turbo TSI bioassay demonstrated 95.2% (95% CI 83.3-98.1) positive percent agreement and 94.8% (95% CI 90.9-97.1) negative percent agreement with an FDA-cleared bioassay (Thyretain® TSI) using serum from 295 patients with autoimmune thyroid disease.
Conclusions: The Turbo TSI bioassay exhibits excellent analytical performance and a high level of reproducibility. The performance compared well with Thyretain® TSI, an FDA-cleared TSI bioassay.
Introduction
Both guidelines of the European (ETA) and American (ATA) thyroid associations for the management of thyrotoxicosis in general and autoimmune hyperthyroidism in particular, as well as the guidelines for the management of Graves’ eye disease, emphasize the role of the thyrotropin receptor autoantibodies (TSH-R-Ab) in the management of patients with subclinical and overt hyperthyroidism (1–3). These guidelines also recommend the measurement of these disease-specific autoantibodies for the accurate diagnosis and monitoring of the clinical activity and severity of the autoimmune thyroid and extrathyroidal diseases. Indeed, the role of stimulatory anti-TSH-R-Ab in the pathogenesis of autoimmune hyperthyroidism is well established (4–6). Only bioassays can differentiate the functionality of and measure the stimulatory activity of anti-TSH-R-Ab (7, 8). Many bioassays for TSI have been described including an FDA-cleared TSI bioassay (Thyretain®, QuidelOrtho, San Diego, CA) (9). Validation and standardization of this established and reproducible bioassay have been reported (10, 11). Furthermore, dilution analysis of TSI positive samples yielded predictive values pertaining to response to antithyroid treatment and proved to be able to differentiate between various Graves’ phenotypes (12–14).
Recently, a novel bioassay was developed which greatly reduces the complexity and time required to measure TSI. This new bioassay, referred to as Turbo TSI, avoids the need for cell culture, has no wash or lysis steps, and has a total turn-around-time of less than 2 hours (15). Preliminary performance data show that it performed well in detecting and measuring levels of TSI. The present study aimed to evaluate the analytical performance of the Turbo TSI bioassay for the detection of TSI.
Materials and equipment
Cells
The GS-TSH-R Mc4 cell line used in the Turbo TSI bioassay in the present study were obtained from QuidelOrtho Corporation, San Diego, CA. The GS-TSH-R Mc4 cells are Chinese hamster ovary (CHO) cells genetically engineered to express a chimeric form of the human TSH-R and a modified luciferase enzyme, Glosensor (GS), that is active only in the presence of cyclic AMP (15). Frozen cells are thawed immediately prior to use in the bioassay.
Turbo TSI stimulating reporter bioassay kit reagents
● Turbo TSI Cells.
● Turbo TSI Negative Control (Neutral Cap).
● Turbo TSI Low Positive Control (White Cap).
● Turbo TSI Mid Positive Control (Brown Cap).
● Turbo TSI High Positive Control (Black Cap).
● Turbo TSI cAMP Reagent.
● Turbo TSI Standard A (Red Cap).
● Turbo TSI Standard B (Orange Cap).
● Turbo TSI Standard C (Yellow Cap).
● Turbo TSI Standard D (Blue Cap).
● Turbo TSI Standard E (Purple Cap).
● Turbo TSI Data Analysis Software.
Other materials and equipment required
● –70°C or lower freezer or liquid nitrogen Dewar.
● Luminometer capable of reading a 96 multi-well plate (e.g. GloMax Navigator, Promega, Madison WI)).
● Luminometer Calibrator Plate.
● Sterile Transfer Pipette.
● 96-Well White, Flat Bottom Assay Plate (e.g., Costar, 3912).
● Microplate Adhesive Film (e.g., USA Scientific, 2920-0000).
● Sterile Reagent Reservoirs (e.g., VWR, 41428-954).
● Water Bath, 35°C to 37°C.
● Vortex Mixer.
● Timer.
● Household Bleach.
Methods
Bioassay protocols
The Thyretain® TSI bioassay was performed according to the manufacturer’s product insert and as previously described (14, 16).
Brief overview of Turbo TSI protocol/procedure
Briefly, TSI standards, controls, and test samples are added to a white 96-well white plate in 5 µL per well. A freezer vial containing Turbo TSI cells at a concentration of 4x106 cells/mL is thawed in a 37°C water bath. These cells are then mixed with 5 mL of cAMP reagent with luciferase substrate (Promega, Madison, WI, USA) and placed in a reagent reservoir. The cell suspension is then dispensed into the wells of the plate at 50 µL/well. The plate is then incubated at ambient temperature for 60 minutes. At the end of the incubation, the plate is read using the GloMax Navigator luminometer (Promega, Madison, WI). The resulting relative light units (RLU) are then converted to TSI quantity in IU/L using the Thyretain®/Turbo TSI Data Analysis Tool.
Detailed steps of Turbo TSI procedure
1. Thaw one vial of each cAMP Reagent, Standard Panel, and Controls for 7 to 10 minutes in a 35°C to 37°C water bath. Ensure these reagents are equilibrated to room temperature (20°C to 25°C).
2. Vortex samples, standards, and controls for 5 to 10 seconds. Add 5-μL Standards, Control, or sample to the bottom corner of each well in a white 96-well plate in singlet.
3. Thaw one vial of Turbo TSI Cells for 3 to 5 minutes in a 35°C to 37°C water bath until just thawed.
4. Transfer the entire volume of the cells with a transfer pipette to the bottle containing 5-mL of cAMP Reagent. (Note: The cell suspension must be added to the cAMP Reagent within 30 minutes of thawing).
5. Mix by inverting the bottle several times.
6. Dispense 50-μL per well of the Turbo TSI cell suspension (Step 4) per well using a 20-μL to 200-μL (or 50-μL to 300-μL) multi-channel pipette. (Note: The cell suspension must be added to the plate within 30 minutes after combining with the cAMP Reagent).
7. Seal the plate with a microplate adhesive film. Gently shake the plate laterally right to left on the benchtop 5 to 6 times.
8. Incubate the plate at room temperature for 60 minutes.
9. Gently remove the adhesive film from the plate once the incubation period is complete and before placing the plate into the luminometer.
10. Read the plate in a GloMax or equivalent luminometer.
11. Transfer the Patient and Raw data to the Turbo TSI Data Analysis Software (DAS) workbook. Results are reported as IU/L calculated based the proprietary algorithm.
Interpretation of results
The Turbo TSI assay quantifies results in IU/L using the Thyretain Turbo TSI Data Analysis Software (DAS) workbook. The assay’s Reportable Range is from 0.021 IU/L to 11 IU/L. Samples with results less than 0.021 IU/L will be reported as “< 0.021 IU/L”. Samples with results greater than 11 IU/L will be reported as “> 11 IU/L”. The dilution of samples with results greater than 11 IU/L is not supported.
Serum samples
Normal serum samples were obtained from Interstate Blood Bank, Inc. (Memphis, TN, USA) or from the serum bank at the Molecular Thyroid Research Laboratory, Johannes Gutenberg University (JGU) Medical Center, Mainz, Germany subsequent to written informed consent and approval of the Ethical Committee of the Rhineland-Palatinate Medical Association, number 837.214.10 (7223). All normal serum samples were tested for TSH, free T4, and free T3 and for autoantibodies to thyroperoxidase, thyroglobulin, and TSH-R. All normal serum samples were shown to be from euthyroid patients and were negative for autoantibodies to thyroid antigens. Serum samples from patients with autoimmune thyroid disease (AITD) and TSI-positive serum samples were obtained from the serum bank at the JGU Thyroid Lab.
limits of blank, detection, and quantitation
The limits of blank (LoB), detection (LoD), and quantitation (LoQ) were determined according to the Clinical and Laboratory Standards Institute (CLSI)-approved guidelines (17, 18). LoB and LoD were calculated according to the following formulas: LoB = Mean blank + 1.64 x SD blank and LoD = LoB + 1.64 × mean SD low concentration sample. The Turbo TSI LOD was verified using WHO IS spiked into normal serum at concentrations of 0.010, 0.012, 0.016 and to 0.018 IU/L. Twenty-six samples at each concentration were tested. The LoQ was determined using World Health Organization (WHO) International Standard (IS) spiked samples at concentrations between 0.02 and 0.03 IU/L. Five spiked samples were tested in 12 replicates each, across two lots of Turbo TSI bioassay over three days. The LoQ was determined from the lowest sample concentration meeting accuracy criteria (%TE ≤ 45%) where Total Error = Bias + 1.96 x Precision (%CV).
Turbo TSI cut-off
The Turbo TSI cut-off was determined using 198 serum samples that were classified as positive or negative for TSI by the Thyretain® TSI bioassay.
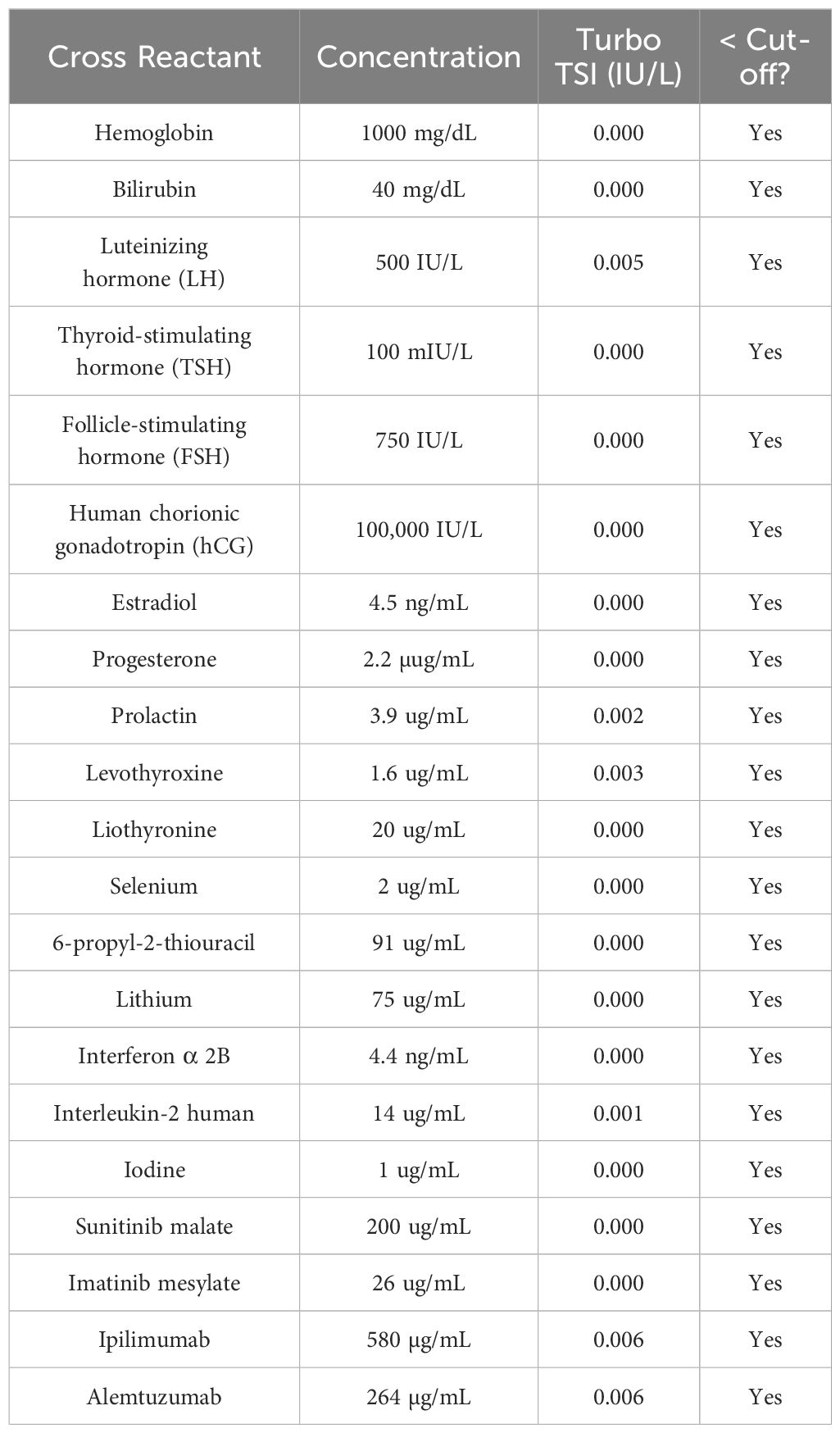
Cross reactivity
Twenty-one blood components, hormones and therapeutic drugs were tested at supraphysiologic concentrations. Serum obtained from patients with 12 disease states were tested (182 total samples).
Interference testing
The same 21 blood components, hormones and therapeutic drugs that were tested for cross reactivity were tested for interference of the Turbo TSI assay. Each substance was mixed with a TSI-positive serum (0.233 IU/L) sample and tested in the Turbo TSI assay. The percent difference between the measurement with and without the substance was determined. Fifty-eight serum samples from patients with various disease states were also tested for Interference in Turbo TSI assay by mixing them with a low TSI positive (0.178 IU/L) serum.
Linearity
The Turbo TSI assay was run at 17 concentrations from 0.19 to 12.00 IU/L using 5 replicates per concentration.
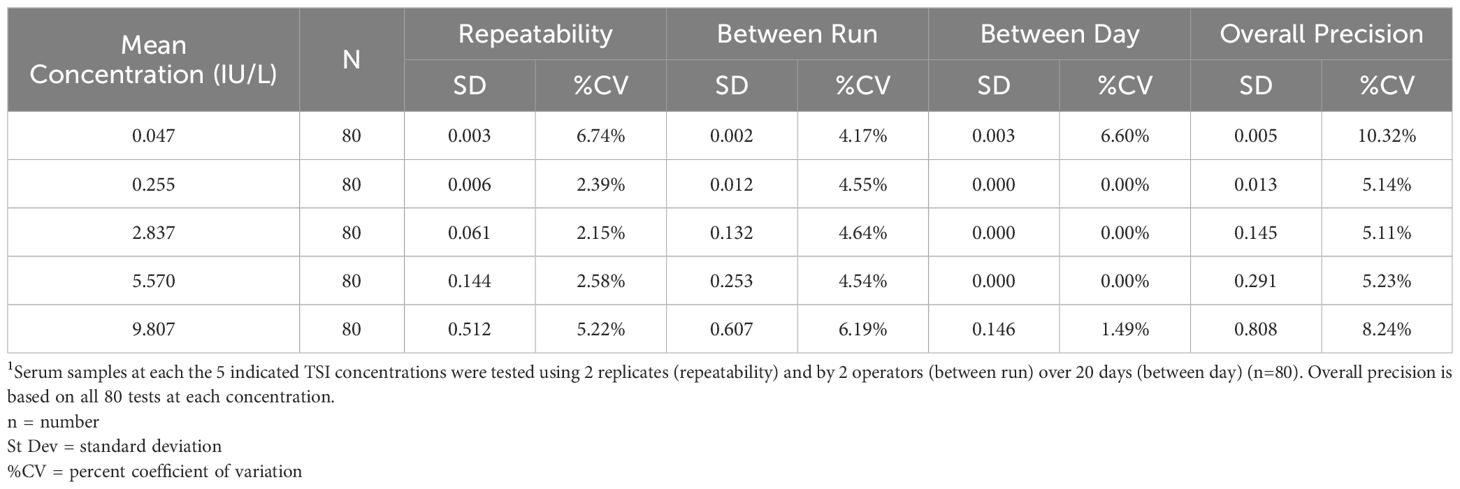
Assay precision
Five concentrations of positive TSI samples from 0.047 to 9.807 IU/L were tested by 2 operators using two replicates over 20 days (n=80).
Reproducibility
A panel of TSI-positive serum samples were tested in the Turbo TSI bioassay by two operators at three locations over 5 days.
Sample and kit stability
TSI-positive samples at five concentrations were stored at the indicated temperatures for the indicated periods of time days before testing. Three different concentrations of TSI serum samples were tested by three lots of Turbo TSI kit once a month up to 16 months. The stability of the TSI kit was evaluated using the recommended approach in the CLSI-EP25 guidelines. A scatter plot containing a linear regression line was generated to visualize the calculated TSI concentration (IU/L) for each sample (y-axis) plotted against time over 16 months (x-axis).
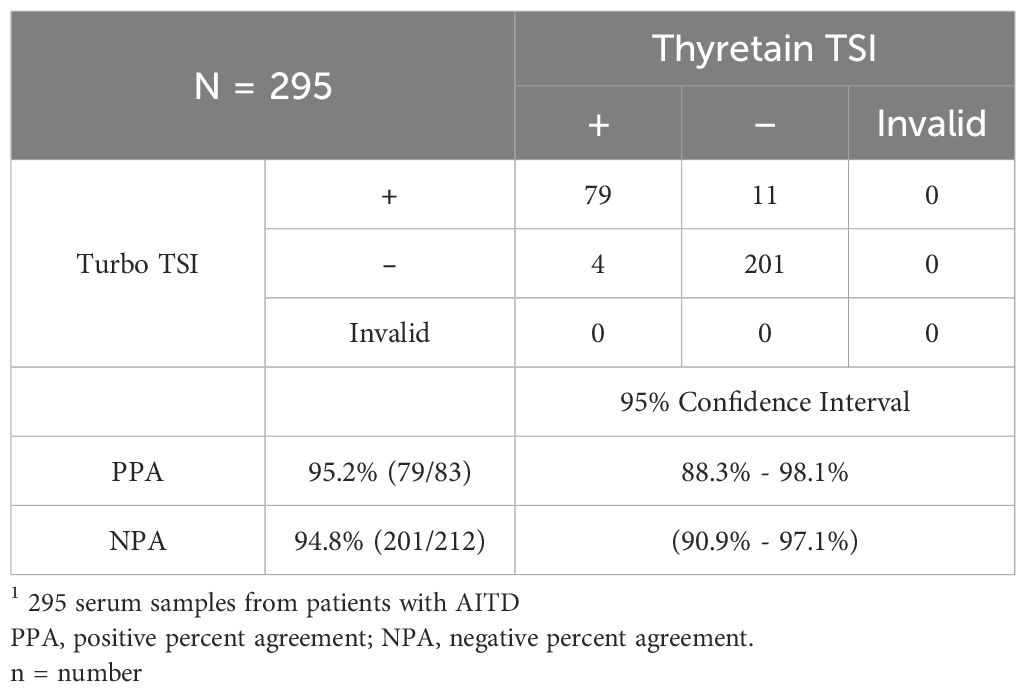
Comparison of Thyretain and Turbo Bioassays
Two-hundred and ninety-five serum samples from patients with AITD were tested with the Thyretain® TSI Bioassay and Turbo TSI bioassay at two sites.
Results
Determination of the limits of blank, detection and quantitation and assay cut-off
The LOB was found to be 0.007 IU/L. The LOD was determined to be 0.016 IU/L (Table 1). The LoQ was determined to be 0.021 IU/L (Supplementary Table 1). The Turbo TSI bioassay cutoff was determined to be 0.0241 IU/L using a ROC curve analysis, which yielded the highest true positive rate (98.7% sensitivity) together with the lowest false positive rate (93.5% specificity) against results from the Thyretain® TSI bioassay (Figure 1).
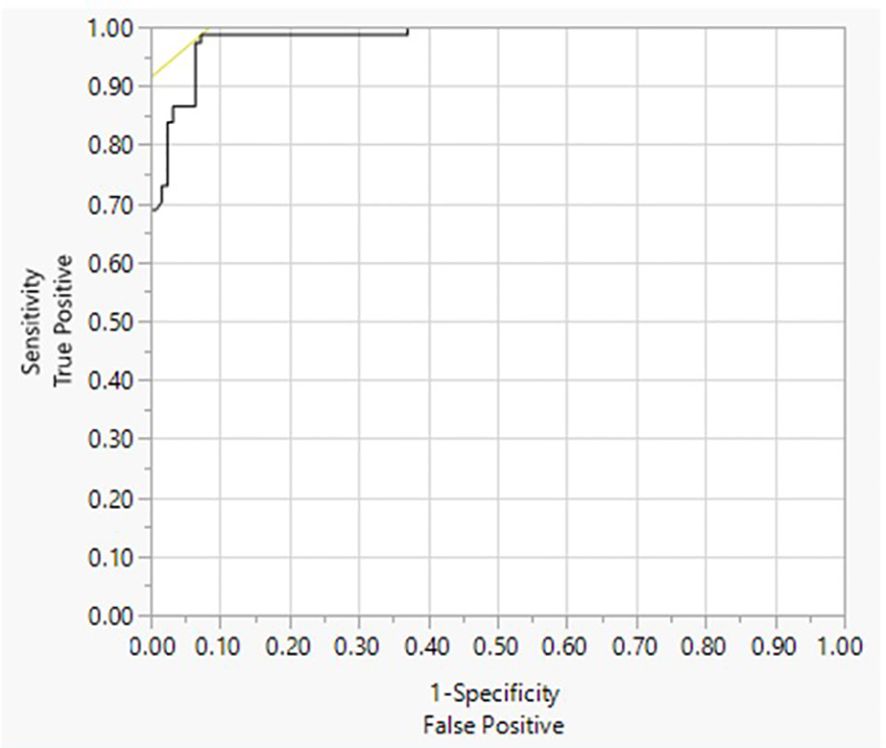
Figure 1. Receiver operating characteristics (ROC) of Turbo TSI. ROC analysis of the Turbo TSI bioassay. Diagnosis = 1 to be positive. Area under the curve (AUC) = 0.98363.
Cross reactivity and interference testing
The TSI concentrations measured for each of the tested blood components, hormones and drugs were all below the Turbo TSI assay cutoff (0.0241 IU/L), indicating there was no cross-reactivity (Table 2). Of the 182 serum samples from patients with various disease states and autoantibodies all but seven were below the Turbo TSI cut-off of 0.0241 IU/L (1/22 with type 1 diabetes; 2/50 celiac disease, and 4/21 thyroid cancer) (Supplementary Table 2). Six of these seven positive samples were positive in the Thyretain® TSI bioassay (data not shown). The same blood components, hormones and drugs were tested in interference studies and the percent change for each tested substance was ≤15% (Supplementary Table 3). None of the sera from patients with various disease states or autoantibodies interfered with the Turbo TSI assay (Supplementary Table 4).
Linearity and precision
The Turbo TSI assay demonstrated linearity from 0.015 to 11.958 IU/L, as all samples showed ±10% deviation from the expected linear relationship (Supplementary Table 5). The overall within-laboratory precision was ≤15%CV for all samples (Table 3).
Sample and kit stability
TSI serum samples stored at different temperatures (15°C, 23°C, and 30°C) are stable for up to 4 days (Supplementary Table 6). TSI serum samples were stable at -70°C for up to at least 14 months. The samples also can be stored at -20°C for 3 months, or at 4°C for 3 weeks (Supplementary Table 7).
Overall quantification (IU/L) for all serum samples measured over 16 months using three different kit lots showed a %CV ≤14% and, based on a regression analysis, no significant measurement drift was observed under the storage conditions for all Turbo TSI kits (Supplementary Table 8).
Reproducibility
The overall reproducibility of Turbo TSI assay was %CV ≤20 for five concentrations (0.06 to 5.16 IU/L) (Supplementary Table 9).
Temperature tolerability
A sample panel containing five clinical TSI positive specimens were tested at different temperatures. The Turbo TSI assay performed in the temperature range between 18°C and 26°C yielded consistent results with %CV ≤20% for all controls and samples (Supplementary Table 10).
Comparison with Thyretain TSI bioassay
Turbo TSI bioassay demonstrated 95.2% positive percent agreement and 94.8% negative percent agreement when compared to the Thyretain® TSI Reporter bioassay using 295 serum samples (Table 4). All samples were tested with a TRAb immunoassay (Kronus, Inc., Star, ID, USA) and there was 89% concordance between the TRAb assay and the two bioassays (data not shown).
Discussion
The present report describes in detail the analytical performance and validation of a novel, rapid, and sensitive cell-based bioassay, Turbo TSI, for the measurement of TSH-R-Ab with functional stimulating activity. This new bioassay demonstrates excellent analytical sensitivity, precision, reproducibility, and linear range. Problems with interference and cross-reactivity were not observed. The assay is robust in that kits were quite stable and serum could be stored for long periods of time at various temperatures. In addition, the assay is performed at ambient temperature and can be performed at 20-250 C (68-770 F), thus obviating the need for an incubator. Another noteworthy feature of the Turbo TSI bioassay is that it is a quantitative assay and results are reported in IU/L.
The Turbo TSI bioassay was compared with the FDA-cleared Thyretain® TSI and exhibited excellent positive and negative percent agreement for detecting TSI. Furthermore, the clinical application of this rapid, sensitive, and user-friendly bioassay was recently demonstrated within a prospective, controlled trial of patients with AITD (19). In this comparative study of TSH-R-Ab binding and bioassays, the TSI results of the Turbo TSI assay closely correlated with the phenotype and clinical activity and severity of patients with Graves’ hyperthyroidism and extrathyroidal manifestations.
TSH-R-Ab are important biomarkers in patients with autoimmune thyroid disease (AITD), e.g. Graves’ thyroidal and extrathyroidal disease (20). These specific autoantibodies are unique in that they can affect the function of the thyroid (4, 21). Guidelines of the European (ETA) and American (ATA) Thyroid associations recommend testing for TSH-R-Ab in patients suspected of having autoimmune hyperthyroidism (3). There are a number of in vitro diagnostic tests available to measure TSH-R-Ab (21). Most of these are different configurations of immunoassays that measure binding of antibodies to the TSH-R. None of these assays the measures the functional activity of TRAb. Many bioassays have been described that measure the thyroid stimulatory activity of TSH-R-Ab (8). Except for one FDA-cleared product, most of these bioassays are research tools and have not been submitted to full validation studies.
Validation studies of TSI and TBI bioassays have been previously reported by our group (14, 22). Turbo TSI is based on an engineered cell line that constitutively expresses both a human TSH-R and a unique cyclic AMP-dependent luciferase (15, 23, 24). The Turbo TSI test has a number of features that offer clinical laboratories the opportunity to measure TSI without the usual complexity and long turn-around-time of bioassays. First, the cells are used immediately after thawing, and thus no cell culturing is necessary. Second, the TSI-induced cyclic AMP activates the luciferase very quickly which shortens the incubation time. Third, the luciferin substrate enters the cells and thus, in contrast to all other TSI bioassays, a lysis step is not required prior to measuring light production. These advantages, however, are moot if the assay does not exhibit the requisite performance in validation studies which the present study has shown.
In conclusion, the development and validation of an easy-to-perform, but sensitive, reproducible, and quantitative TSI bioassay, offers a significant advancement for the evaluation of patients with AITD. There are many clinical circumstances in which knowing the functional activity of TSH-R-Ab is important and actionable. In addition, because of its simple procedure and rapid results, the Turbo TSI bioassay may allow routine assessment of TSI in patients with autoimmune hyperthyroidism.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical approval was not required for the deidentified normal human serum samples which were obtained from Interstate Blood Bank, Inc. (Memphis, TN, USA). Human serum samples from the serum bank at the Molecular Thyroid Research Laboratory, Johannes Gutenberg University (JGU) Medical Center, Mainz, Germany were obtained subsequent to written informed consent and approval of the Ethical Committee of the Rhineland-Palatinate Medical Association, number 837.214.10 (7223).
Author contributions
PO: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. HK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LM: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing – review & editing. JH: Data curation, Funding acquisition, Project administration, Writing – review & editing. GK: Conceptualization, Formal Analysis, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by QuidelOrtho Corp., San Diego, CA, USA.
Acknowledgments
We acknowledge Ron Lollar for organizing and supervising the validation studies.
Conflict of interest
Authors HK, LM, and JAH were employed by the company QuidelOrtho. PO and GK consult for QuidelOrtho, The JGU Medical Center, Mainz, Germany receives research-associated funding from QuidelOrtho Corp, San Diego, CA, USA.
The remaining authors declare that the research was conducted in the absence of anycommercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Quidelortho. The funder had the following involvement in the study: data collection and analysis.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1468768/full#supplementary-material
Abbreviations
AITD, autoimmune thyroid disease; CHO, Chinese hamster ovary; CRE, cAMP response element; CV%, coefficient of variation percentage; DAS, Data analysis software; FSH, follicle stimulating hormone; GS, Glosensor; HCG, human chorionic gonadotropin; LH, luteinizing hormone; LoB, limit of blank; LoD, limit of detection; LoQ, limit of quantitation; RLU, relative light units; TBI, thyroid-blocking immunoglobulin; TRAb, thyroid stimulating hormone receptor antibody; TSH, thyroid stimulating hormone (thyrotropin); TSH-R, thyroid stimulating hormone (thyrotropin) receptor; TSH-R-Ab, thyroid stimulating hormone receptor antibody; TSI, thyroid-stimulating immunoglobulin.
References
1. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. (2021) 185:G43–67. doi: 10.1530/EJE-21-0479
2. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European thyroid association guideline for the management of graves’ Hyperthyroidism. Eur Thyroid J. (2018) 7:167–86. doi: 10.1159/000490384
3. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. (2016) 26:1343–421. doi: 10.1089/thy.2016.0229
4. Ando T, Latif R, Davies TF. Thyrotropin receptor antibodies: new insights into their actions and clinical relevance. Best Pract Res Clin Endocrinol Metab. (2005) 19:33–52. doi: 10.1016/j.beem.2004.11.005
5. Diana T, Ponto KA, Kahaly GJ. Thyrotropin receptor antibodies and Graves’ orbitopathy. J Endocrinol Invest. (2021) 44:703–12. doi: 10.1007/s40618-020-01380-9
6. George A, Diana T, Langericht J, Kahaly GJ. Stimulatory thyrotropin receptor antibodies are a biomarker for graves’ Orbitopathy. Front Endocrinol (Lausanne). (2020) 11:629925. doi: 10.3389/fendo.2020.629925
7. Diana T, Wuster C, Olivo PD, Unterrainer A, Konig J, Kanitz M, et al. Performance and specificity of 6 immunoassays for TSH receptor antibodies: A multicenter study. Eur Thyroid J. (2017) 6:243–9. doi: 10.1159/000478522
8. Olivo PD. Bioassays for thyrotropin receptor autoantibodies. Best Pract Res Clin Endocrinol Metab. (2023) 37:101744. doi: 10.1016/j.beem.2023.101744
9. Lytton SD, Kahaly GJ. Bioassays for TSH-receptor autoantibodies: an update. Autoimmun Rev. (2010) 10:116–22. doi: 10.1016/j.autrev.2010.08.018
10. Diana T, Kanitz M, Lehmann M, Li Y, Olivo PD, Kahaly GJ. Standardization of a bioassay for thyrotropin receptor stimulating autoantibodies. Thyroid. (2015) 25:169–75. doi: 10.1089/thy.2014.0346
11. Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. (2010) 95:2123–31. doi: 10.1210/jc.2009-2470
12. Kahaly GJ. Management of graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab. (2020) 105:3704–20. doi: 10.1210/clinem/dgaa646
13. Kahaly GJ, Wuster C, Olivo PD, Diana T. High titers of thyrotropin receptor antibodies are associated with orbitopathy in patients with graves disease. J Clin Endocrinol Metab. (2019) 104:2561–8. doi: 10.1210/jc.2018-02705
14. Leschik JJ, Diana T, Olivo PD, Konig J, Krahn U, Li Y, et al. Analytical performance and clinical utility of a bioassay for thyroid-stimulating immunoglobulins. Am J Clin Pathol. (2013) 139:192–200. doi: 10.1309/AJCPZUT7CNUEU7OP
15. Miao LY, Kim HJ, Whitlatch K, Jaiswal D, Navarro A, Egan R, et al. A rapid homogenous bioassay for detection of thyroid-stimulating antibodies based on a luminescent cyclic AMP biosensor. J Immunol Methods. (2022) 501:113199. doi: 10.1016/j.jim.2021.113199
16. Li Y, Kim J, Diana T, Klasen R, Olivo PD, Kahaly GJ. A novel bioassay for anti-thyrotrophin receptor autoantibodies detects both thyroid-blocking and stimulating activity. Clin Exp Immunol. (2013) 173:390–7. doi: 10.1111/cei.12129
17. Pierson-Perry JF. Evaluation of detection capability for clinical laboratory measurement procedures. In: CLSI, editor. Clinical and Laboratory Standards Institute1055, 2nd Edition. 2012 Westlakes Drive, Suite 300 Berwyn, PA 193122012: Clinical and Laboratory Standards Institute.
18. NCCLS. Protocols for Determination of Limits of Detection and Limits of Quantitation; Approved Guideline. NCCLS document EP17-A. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA: NCCLS (2004), ISBN: ISBN 1-56238-551-8.
19. Wolf J, Alt S, Kramer I, Kahaly GJ. A novel monoclonal antibody degrades the thyrotropin receptor autoantibodies in graves’ Disease. Endocr Pract. (2023) 29:553–9. doi: 10.1016/j.eprac.2023.04.002
20. Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, et al. Graves’ disease. Nat Rev Dis Primers. (2020) 6:52. doi: 10.1038/s41572-020-0184-y
21. Kahaly GJ, Diana T, Olivo PD. Tsh receptor antibodies: relevance & Utility. Endocr Pract. (2020) 26:97–106. doi: 10.4158/EP-2019-0363
22. Diana T, Li Y, Olivo PD, Lackner KJ, Kim H, Kanitz M, et al. Analytical performance and validation of a bioassay for thyroid-blocking antibodies. Thyroid. (2016) 26:734–40. doi: 10.1089/thy.2015.0447
23. Kumar BA, Kumari P, Sona C, Yadav PN. GloSensor assay for discovery of GPCR-selective ligands. Methods Cell Biol. (2017) 142:27–50. doi: 10.1016/bs.mcb.2017.07.012
Keywords: analytical performance, validation, bioassay, thyroid-stimulating receptor antibody, thyroid-stimulating immunoglobulin
Citation: Olivo PD, Kim H, Miao L, Houtz JA and Kahaly GJ (2025) Analytical validation of a novel bioassay for thyroid-stimulating immunoglobulin. Front. Endocrinol. 15:1468768. doi: 10.3389/fendo.2024.1468768
Received: 11 September 2024; Accepted: 27 November 2024;
Published: 07 January 2025.
Edited by:
Noriyuki Koibuchi, Gunma University, JapanReviewed by:
Jean-Louis Charli, National Autonomous University of Mexico, MexicoRicardo Pujol Borrell, Autonomous University of Barcelona, Spain
Copyright © 2025 Olivo, Kim, Miao, Houtz and Kahaly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul D. Olivo, b2xpdm9wZEB3dXN0bC5lZHU=; George J. Kahaly, Z2VvcmdlLmthaGFseUB1bmltZWRpemluLW1haW56LmRl
 Paul D. Olivo
Paul D. Olivo Hannah Kim
Hannah Kim Lynn Miao2
Lynn Miao2