- 1Department of Neurology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 2Department of Neurosurgery, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Objectives: Thyroid hormone levels have been indicated to be associated with the functional outcome in critical illness. However, the studies on thyroid hormones and status epilepticus (SE) are rare. This study aimed to evaluate the predictive value of serum thyroid hormone levels on admission for unfavorable outcome in adult patients with SE.
Methods: We investigated and validated the predictive value of serum thyroid hormone levels on admission for the prognosis of adult SE patients. We extracted the clinical information and outcomes of patients. Modified Rankin scale (mRS) scores were applied to assess the patients’ functional outcome, and mortality at 30 days after SE onset was identified. Serum levels of thyroid hormones including free thyroxin (FT4), free triiodothyronine (FT3) and thyroid-stimulating hormone (TSH) were detected on admission.
Results: We first analyzed the discovery cohort of 87 patients with SE. We found that 35.6% (31/87) of the patients had a poor outcome at discharge, and 18.4% (16/87) of the patients died during hospital stay and at 30-day follow up. The serum FT3 levels in the non-survivors group were significantly lower than those in the survivors group. Low T3 syndrome occurred in 29.9% (26/87) of SE cases and patients with low T3 syndrome were more likely to have unfavorable outcomes. Furthermore, we observed similar results in the external cohort, which validated our findings.
Conclusions: Serum FT3 levels measured on admission are independently associated with 30-day mortality in SE patients. Additionally, low T3 syndrome may be a promising candidate for predicting SE prognosis.
Introduction
Status epilepticus (SE) is a life-threatening neurological emergency with considerable mortality and morbidity (1). The mortality rate of SE is reported to range from 5 to 46% (2). Furthermore, survivors of SE may have long-term adverse sequelae (3). Predicting the prognosis of SE is challenging due to the heterogeneous etiologies and diverse clinical manifestations. Prior studies have proposed some scales, such as status epilepticus severity score (STESS) and epidemiology-based mortality score in status epilepticus (EMSE), to help clinicians to predict SE outcome. STESS scale consists of four variables: history of seizures, age, seizure type and consciousness level, which can be used to assess the risk of mortality early in SE (4). Thereafter, the EMSE scale, a clinical-electrophysiological tool, was reported to predict mortality in SE, which applied a complicated scoring system with a combination of etiology, age, comorbidity, and characteristics on electroencephalography (EEG) (5). However, these scales have some limitations under specific conditions. SE patients with consciousness impairment may not be able to provide the previous history of epilepsy, especially when their relatives are not present. Additionally, EEG examination is not available for every SE patient in every region. An ideal tool for predicting SE outcome should be simple and easily available markers, which could be useful to help clinicians to identify SE patients with high risk of poor prognosis, and thus early and adequate interventions could be taken to improve these patients’ functional outcomes.
Thyroid hormones are crucial for brain development and maturation, and also play an important role in maintaining brain functions (6). The thyroid gland produces and releases thyroxin (T4) and triiodothyronine (T3), which is modulated by thyroid-stimulating hormone (TSH) (7). Both T4 and T3 have a bound form and a free form, and the free form is bioactive (8). Critical illness may lead to a reduced peripheral conversion of free T4 (FT4) to free T3 (FT3), which contributes to the development of low T3 syndrome (decreased serum T3, low or normal serum T4, and normal TSH levels) (7, 9). Previous studies have indicated that thyroid hormone alterations as well as low T3 syndrome are associated with the prognosis of various neurological diseases, such as ischemic stroke (10) and autoimmune encephalitis (9). Of note, thyroid hormones have been indicated to be involved in the pathogenesis of epilepsy (11). Thyroid hormones affect neurotransmission, as well as regulate the development and function of γ-aminobutyric acid (GABAergic) interneurons, and thus impact inhibitory and excitatory neuronal circuits, further participating in triggering or sustaining epileptic activity (12, 13). However, the prognostic value of thyroid hormones in SE patients remains unclear.
In the present study, we aimed to explore the predictive value of serum levels of thyroid hormones for unfavorable outcome at discharge as determined by the modified Rankin scale (mRS) score as well as 30-day mortality in adult patients with SE. Furthermore, we sought to investigate whether low T3 syndrome could predict the prognosis of adult patients with SE.
Methods
Study design and ethics
Two independent cohorts were included in the present study. Cohort 1 served as the discovery cohort, and we reviewed the medical records of patients with SE admitted to the affiliated hospital of Southwest Medical University from January 2020 to December 2023. Cohort 2 was an external validation cohort, which included a dataset of SE patients from the affiliated Traditional Chinese Medicine hospital of Southwest Medical University between January 2021 and December 2023. The present study was approved by the ethics committees of the affiliated hospital of Southwest Medical University and the affiliated Traditional Chinese Medicine hospital of Southwest Medical University. Informed consent was obtained from all patients or family members.
Definitions and criteria
SE patients aged at least 18 years old without previous history of thyroid diseases were included. SE was defined as prolonged clinical and/or electrographic seizure activity which lasted for more than five minutes, or recurrent seizure activity with no complete functional recovery in between (14). Refractory SE was defined as no response to first-line and second-line antiepileptic drugs (AEDs) (15). Super-refractory SE is that seizure activity continues for 24 hours or longer after the initiation of anesthetic treatment (16). Patients with SE caused by hypoxic-ischemic encephalopathy after cardiac arrest were excluded. SE etiology was classified into four groups: acute symptomatic SE, remote symptomatic SE, symptomatic SE due to progressive brain disorders, or unprovoked SE of unknown etiology, which was suggested by the International League Against Epilepsy (ILAE) (17). Status epilepticus severity score (STESS) scale consists of four variables: history of seizures, age, seizure type and consciousness level (4). Low T3 syndrome was defined as serum FT3 below the lower limit of the reference interval along with normal TSH levels (9). Clinical outcomes at discharge were evaluated using the mRS score. An mRS score of less than 3 was regarded a good outcome, while an mRS score of equal to or above 3 (including death) was regarded a poor outcome.
Data collection
Clinical information was collected, including age; sex; etiology of SE; previous history of seizures; STESS at SE onset; SE duration; the number of AEDs. Data of laboratory tests including white blood cell count, neutrophil count, lymphocyte count, monocyte count, red blood cell count, red cell distribution width, platelet count, albumin, FT3, FT4 and TSH within 24 hours after admission were collected. Data of outcomes included mRS scores at hospital discharge and mortality at 30-day follow up after SE onset. The follow-up information was obtained from the hospital records or by interviewing (in person or by telephone) the patients and their relatives.
Statistical analysis
The Shapiro-Wilk test was conducted to distinguish between normal and non-normal distributions. Continuous variables are presented as means (standard deviation) or medians (interquartile range, IQR) for normally distributed and skewly distributed variables, respectively, while categorical variables were described as frequency and percentage. For comparative analysis of three or more groups, analysis of variance or Kruskal-Wallis test was used for normally distributed and skewly distributed data, respectively. Univariate analyses were performed by using the t-test, the Mann-Whitney U-test and the chi-square (χ2) test. The t-test or Mann-Whitney U-test were employed to compare continuous data, and the chi-square (χ2) test was applied to compare categorical variables. Variables with P < 0.10 in the univariate analysis were screened to enter into the multivariate logistic regression model. Multicollinearity was evaluated with the variance inflation factor (VIF). The variable selected for subsequent multivariate analysis was tolerance > 0.1 and VIF < 5. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive value of FT3 for 30-day mortality in SE patients. The cutoff point of FT3 was set by the Youden index. All statistical analyses were carried out by using GraphPad Prism 9.0 and SPSS 26.0 softwares, and P values < 0.05 were regarded significant.
Results
Baseline characteristics of patients with SE
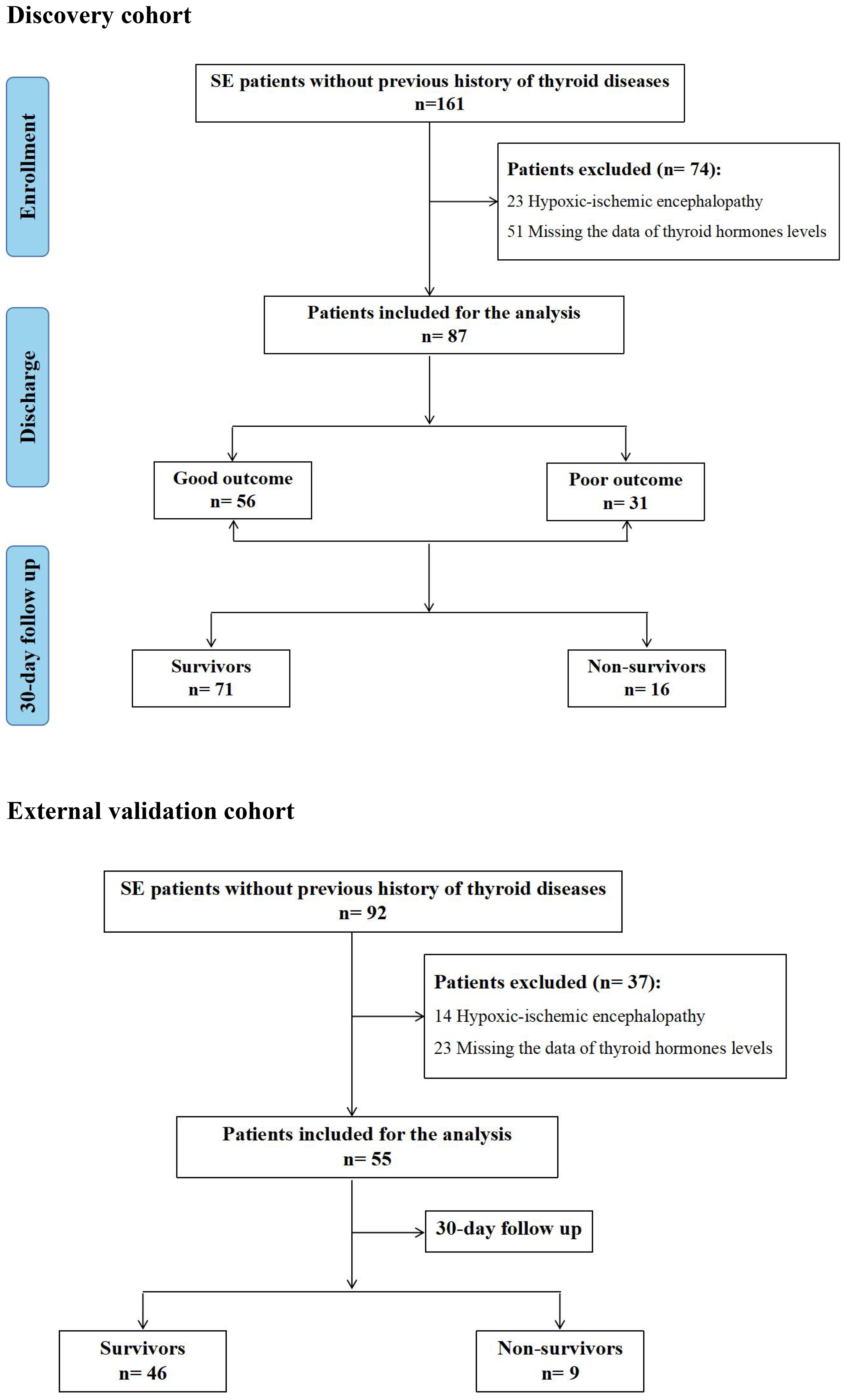
In the Discovery cohort, Out of 161 patients with SE, 23 patients with hypoxic-ischemic encephalopathy and 51 patients without measurements of thyroid hormones within 24 hours after admission were ruled out. Of the 87 SE patients included in the analysis, 31 patients (35.6%) suffered from poor outcomes at discharge, and 16 patients (18.4%) died during hospital stay and at 30-day follow up after SE onset. Additionally, 26 of the 87 patients (29.9%) met the diagnostic criteria of low T3 syndrome. In the external validation cohort, a total of 55 SE patients meeting the inclusion criteria were included. Out of 55 SE patients, 9 patients (16.4%) died during hospital stay and at 30-day follow up after SE onset, and 15 patients (27.3%) met the diagnostic criteria of low T3 syndrome. The flowchart of the study patients was shown in Figure 1.
Thyroid hormones in SE patients
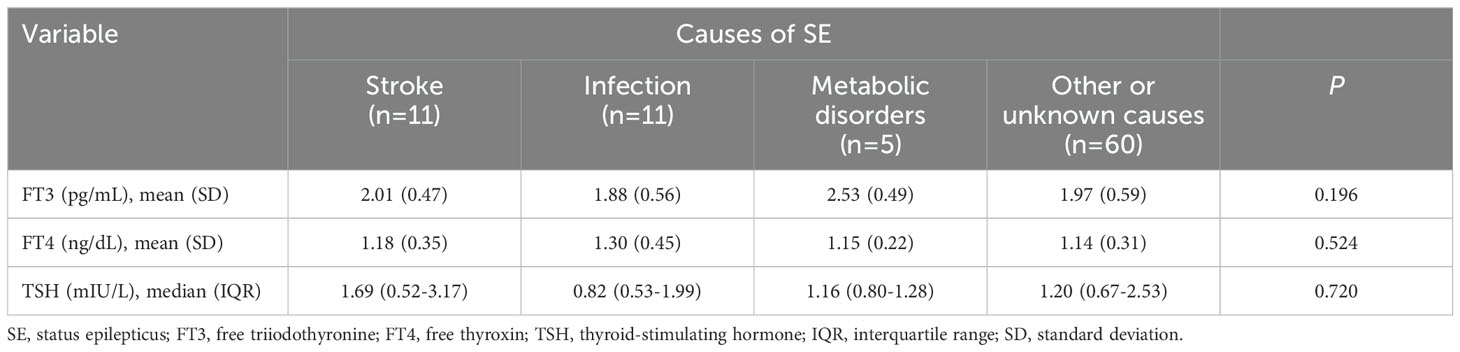
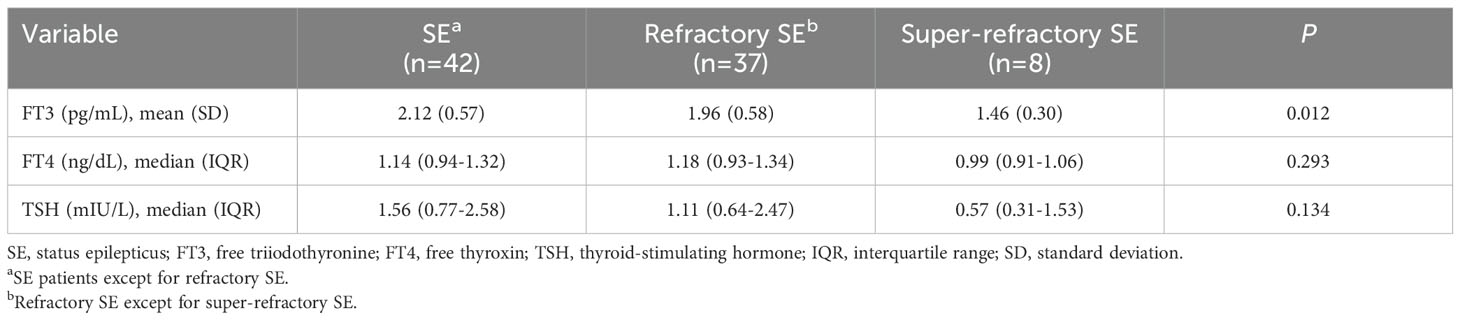
The results of thyroid hormones testing among SE patients with different etiologies and different classes of SE severity were summarized in Tables 1, 2, respectively. No significant difference was observed in serum FT3, FT4 and TSH levels among SE patients with different causes (all P > 0.05, Table 1). In addition, serum TSH and FT4 levels were comparable among patients with different classes of SE severity, while FT3 levels were remarkably decreased as the SE severity increased, and patients with super-refractory SE had the lowest FT3 levels (P = 0.012, Table 2).
Thyroid hormones and poor outcome at discharge
Univariable comparisons of clinical characteristics and data of laboratory tests at admission between SE patients with good outcome and poor outcome at discharge were shown in Table 3. SE patients with poor outcome at discharge were older as compared to patients with good outcome (patients with poor outcome had median age 60 years, IQR 49-69 versus median age 47 years, IQR 28-58 in patients with good outcome; P = 0.006). Moreover, SE patients with poor outcome had significantly greater proportions of super-refractory SE and abnormal thyroid function, higher baseline red cell distribution width and STESS, as well as lower baseline red blood cell count, platelet count and albumin.
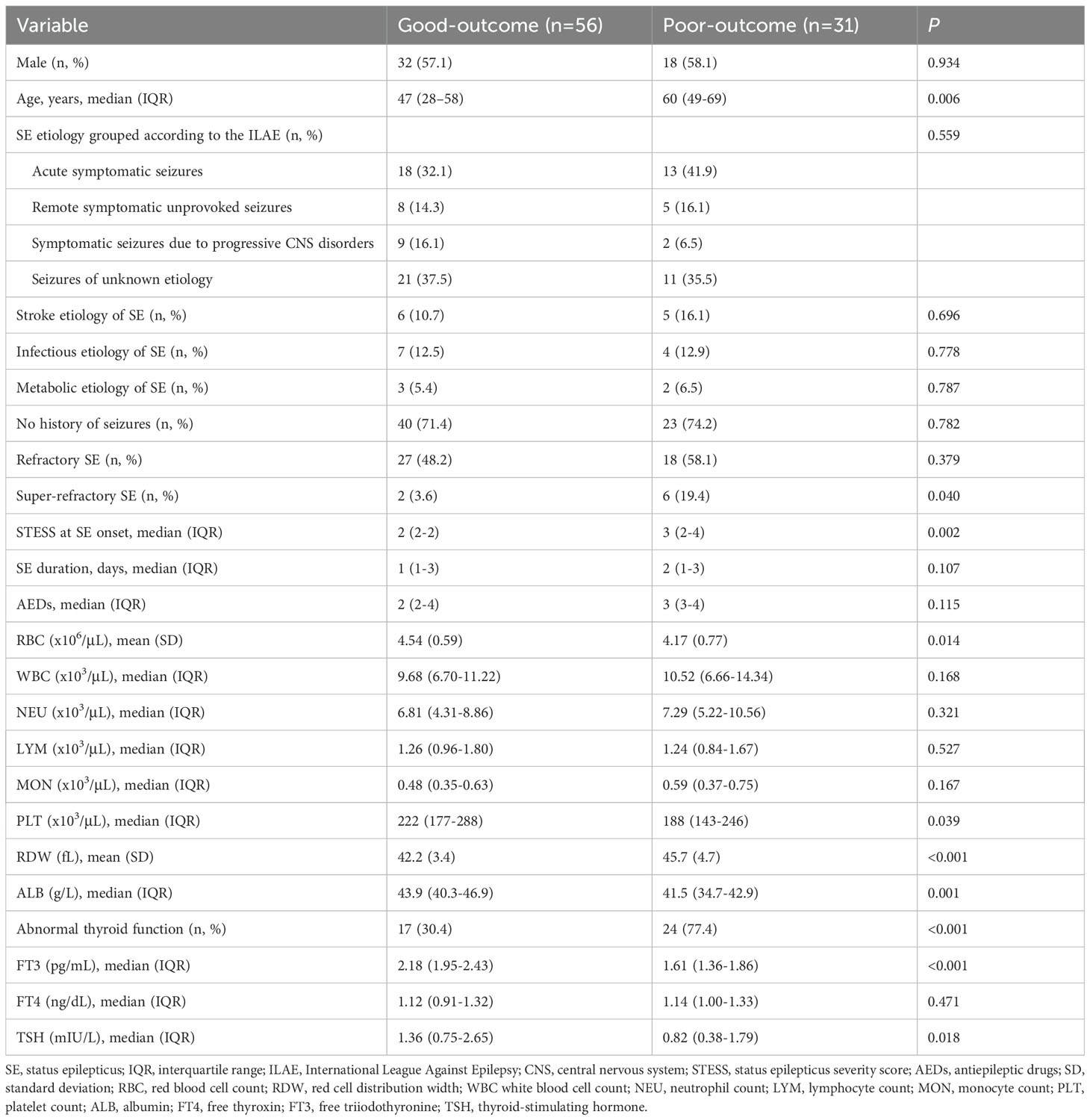
Table 3. Univariate analysis of clinical characteristics and laboratory data between SE patients with good or poor outcomes at discharge.
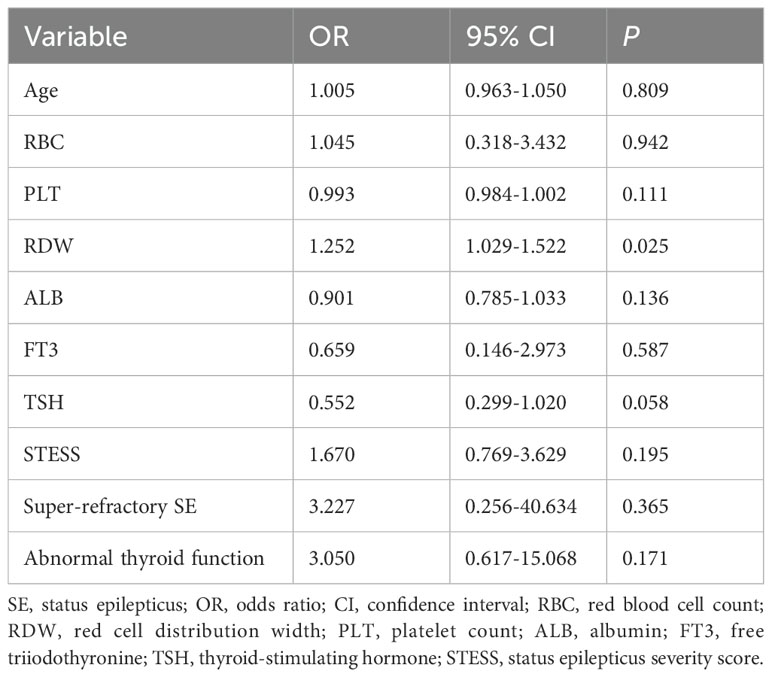
Serum levels of FT3 and TSH were remarkably lower in patients with poor outcome than in patients with good outcome (Table 3). Serum FT4 levels were higher in patients with poor outcome, but the difference was not statistically significant (P = 0.471). Furthermore, a multivariate logistic regression analysis was conducted including age, super-refractory SE, abnormal thyroid function, STESS, red blood cell count, red cell distribution width, platelet count, albumin, FT3 and TSH (Table 4). These variables were selected as they were observed to be significantly different between patients with good outcome and poor outcome at discharge in the univariable analysis above (all P < 0.10, Table 3). The multivariate analysis indicated no significant association between serum FT3 and TSH levels and poor outcome (FT3: odds ratio (OR) = 0.659; 95% confidence interval, 0.146-2.973; P = 0.587; TSH: OR = 0.552; 95% confidence interval, 0.299-1.020; P = 0.058).
Thyroid hormones and mortality at 30-day follow up
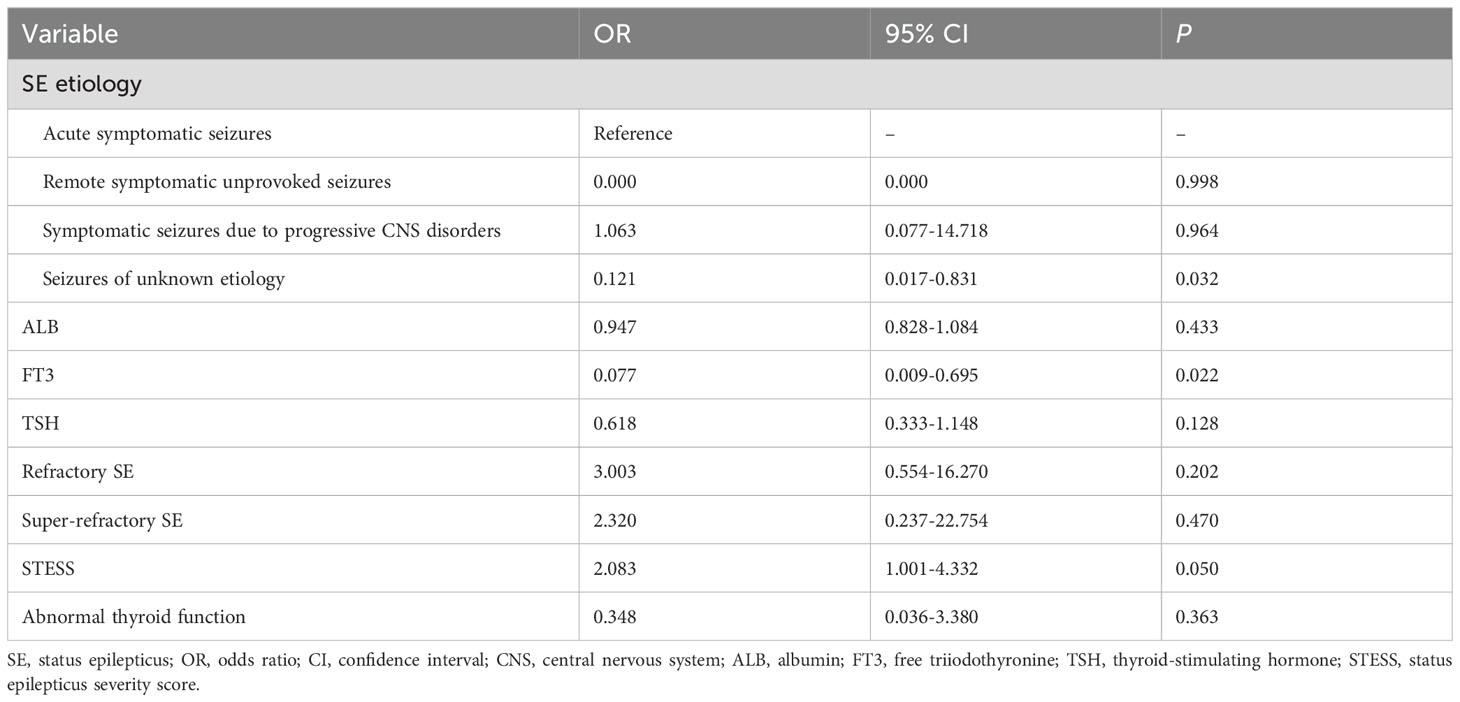
Univariable comparisons of clinical characteristics and data of laboratory tests at admission between survivors and non-survivors were shown in Table 5. Non-survivors had significantly greater proportions of refractory SE and abnormal thyroid function, higher baseline neutrophil count and STESS, and lower albumin levels compared to survivors. Serum levels of FT3 and TSH were remarkably lower in non-survivors than in survivors. Serum FT4 levels were lower in non-survivors, but the difference was not statistically significant (P = 0.132). SE etiology, refractory SE, super-refractory SE, abnormal thyroid function, STESS, albumin, FT3 and TSH were screened for the further multivariate logistic regression analysis (Table 6) as these variables were associated with 30-day mortality in the univariate analysis (all P < 0.10, Table 5). The multivariate analysis demonstrated that FT3 was an independent predictor of 30-day mortality (OR = 0.077; 95% confidence interval, 0.009-0.695; P = 0.022).
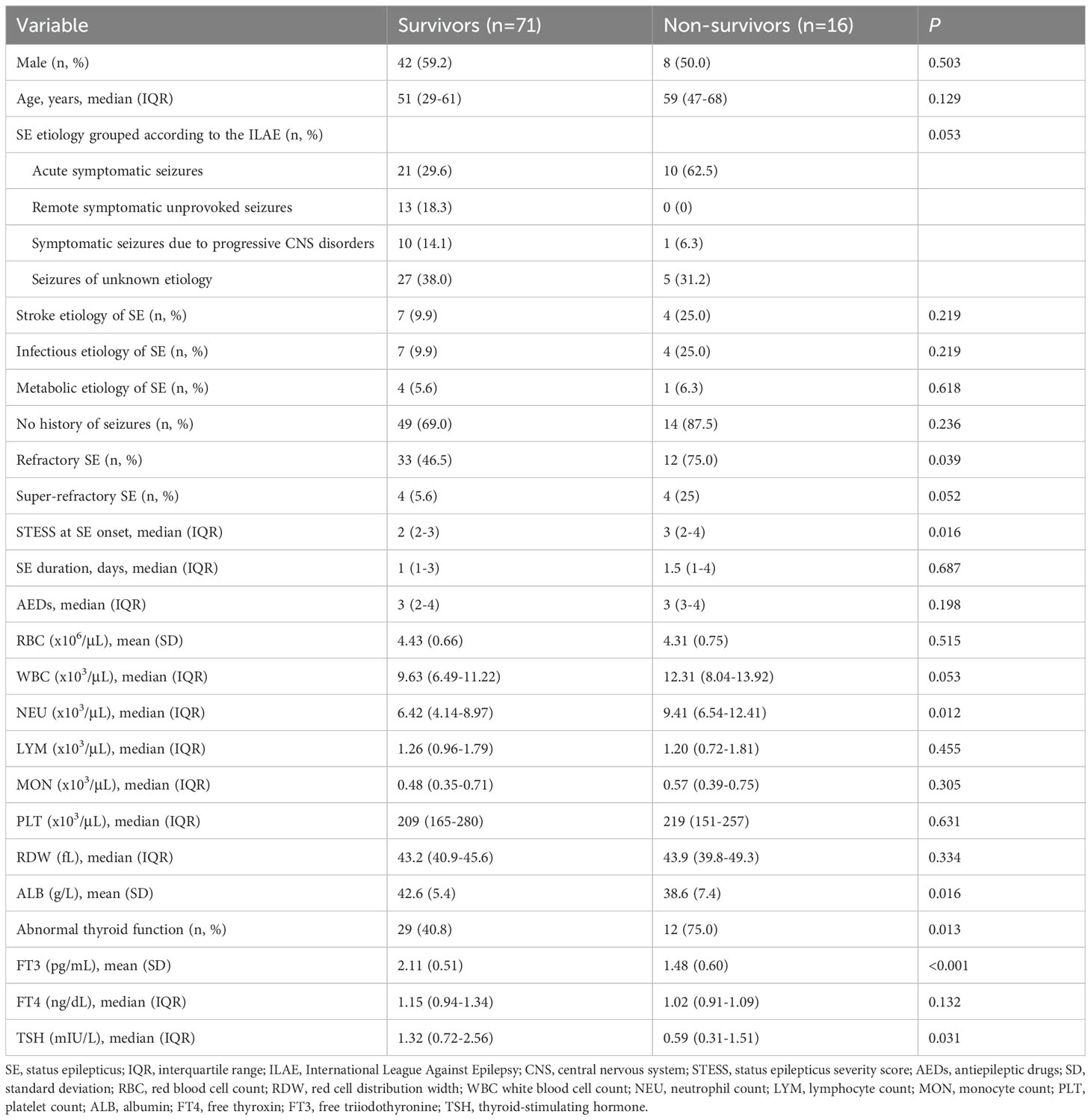
Table 5. Univariate analysis of clinical characteristics and laboratory data between survivors and non-survivors.
The predictive power of FT3 for mortality at 30-day follow up
Given that FT3 was suggested to be independently related to 30-day mortality, we further carried out the receiver operating characteristic (ROC) curve to evaluate the predictive ability of FT3 for 30-day mortality in SE patients. The area under the ROC curve of FT3 was 0.780 (95% confidence interval: 0.635-0.925, P < 0.001) for 30-day mortality (Figure 2). The optimal predictive cutoff value for 30-day mortality by FT3 was 1.64 (sensitivity 68.75%, specificity 83.10%).
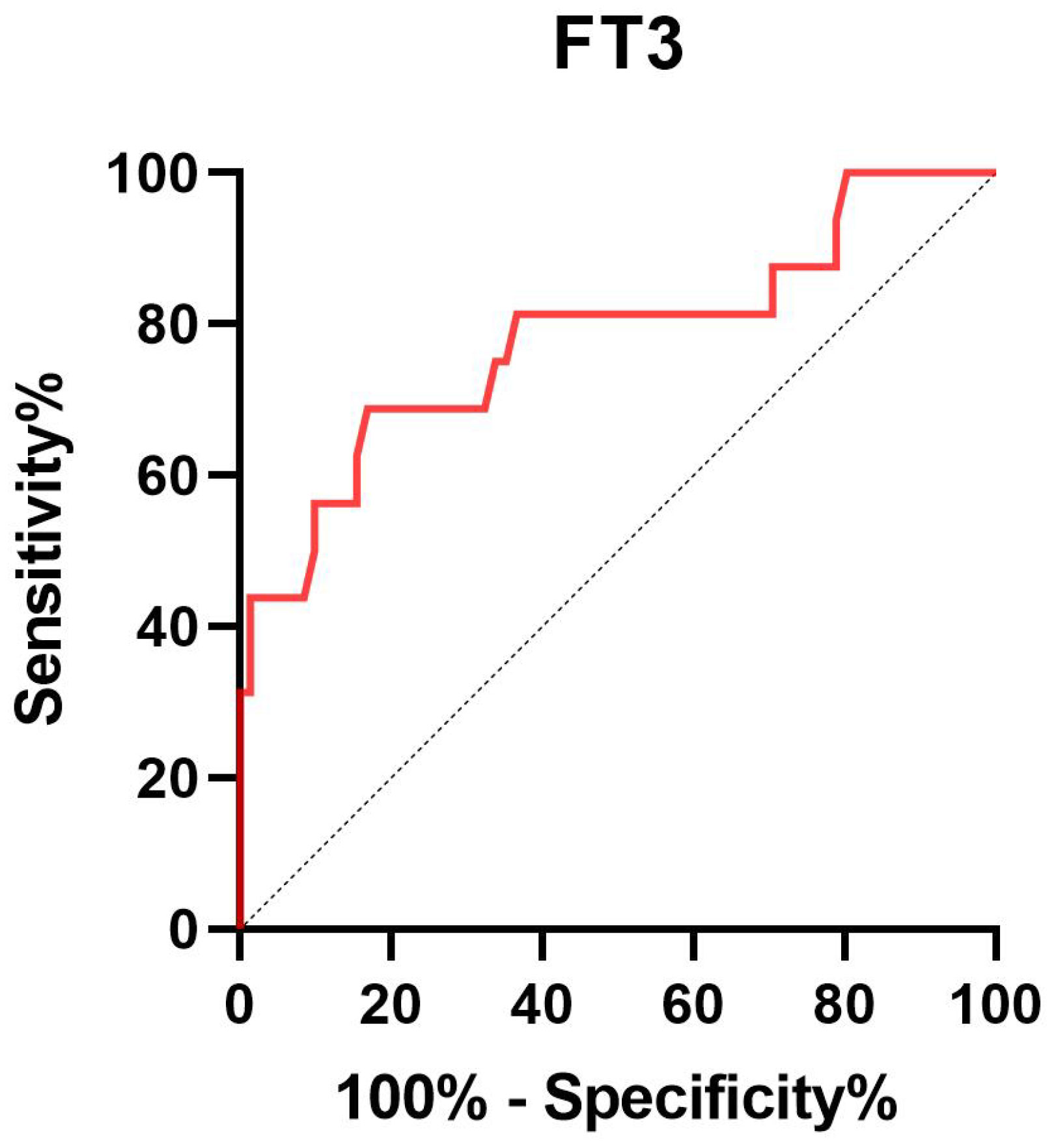
Figure 2. Receiver operating characteristic curve of FT3 to predict 30-day mortality in SE patients. FT3 free triiodothyronine; SE status epilepticus.
Poor outcome at discharge and 30-day mortality stratified by T3 status
The clinical characteristics and outcomes of patients with and without low T3 syndrome were presented in Table 7. Poor outcome at discharge was observed more frequently among patients with low T3 syndrome than among patients without low T3 syndrome (P < 0.001). Likewise, the proportion of 30-day mortality was remarkably higher in patients with low T3 syndrome than in those without low T3 syndrome (P = 0.025).
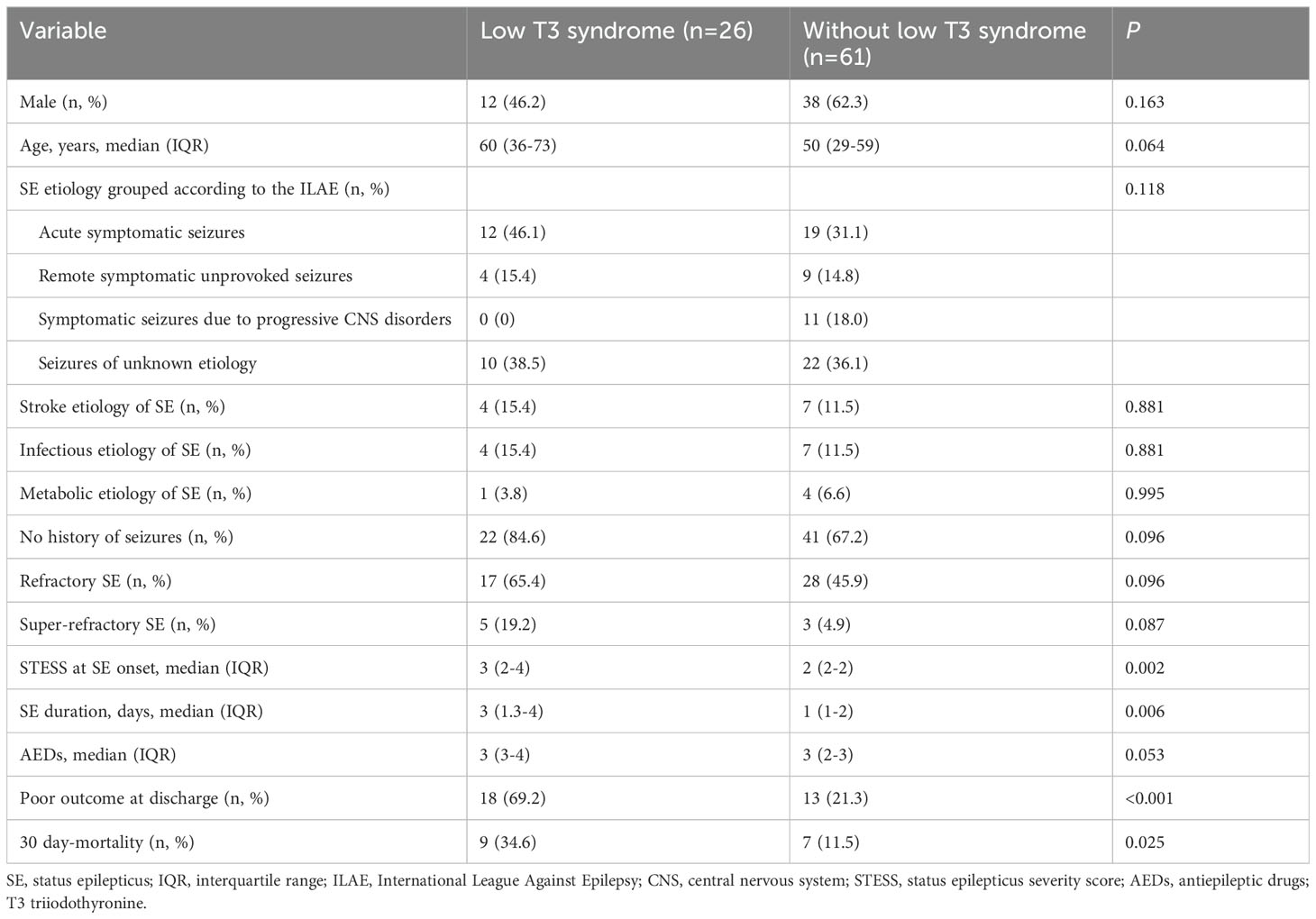
Table 7. Comparisons of outcomes at discharge and 30-day mortality in SE patients with or without low T3 syndrome.
External cohort validated the predictive value of FT3 for SE prognosis
We further employed an external cohort to validate our findings. Univariable and multivariable analyses performed in the external cohort were displayed in Tables 8, 9, respectively. Univariable analysis identified SE etiology, FT3, albumin, STESS and red cell distribution width as prognostic factors for 30-day mortality in SE patients (all P < 0.10, Table 8), and these markers were entered into the multivariable analysis (Table 9). The multivariate analysis showed that only FT3 was independently associated with 30-day mortality (OR = 0.002; 95% confidence interval, 0.000-0.791; P = 0.041). Additionally, SE patients with low T3 syndrome had higher proportion of 30-day mortality compared to those without low T3 syndrome (P = 0.013, Table 10). Collectively, the results from the external cohort were consistent with the findings of the discovery cohort, which further validated the predictive value of FT3 for the prognosis of SE patients.
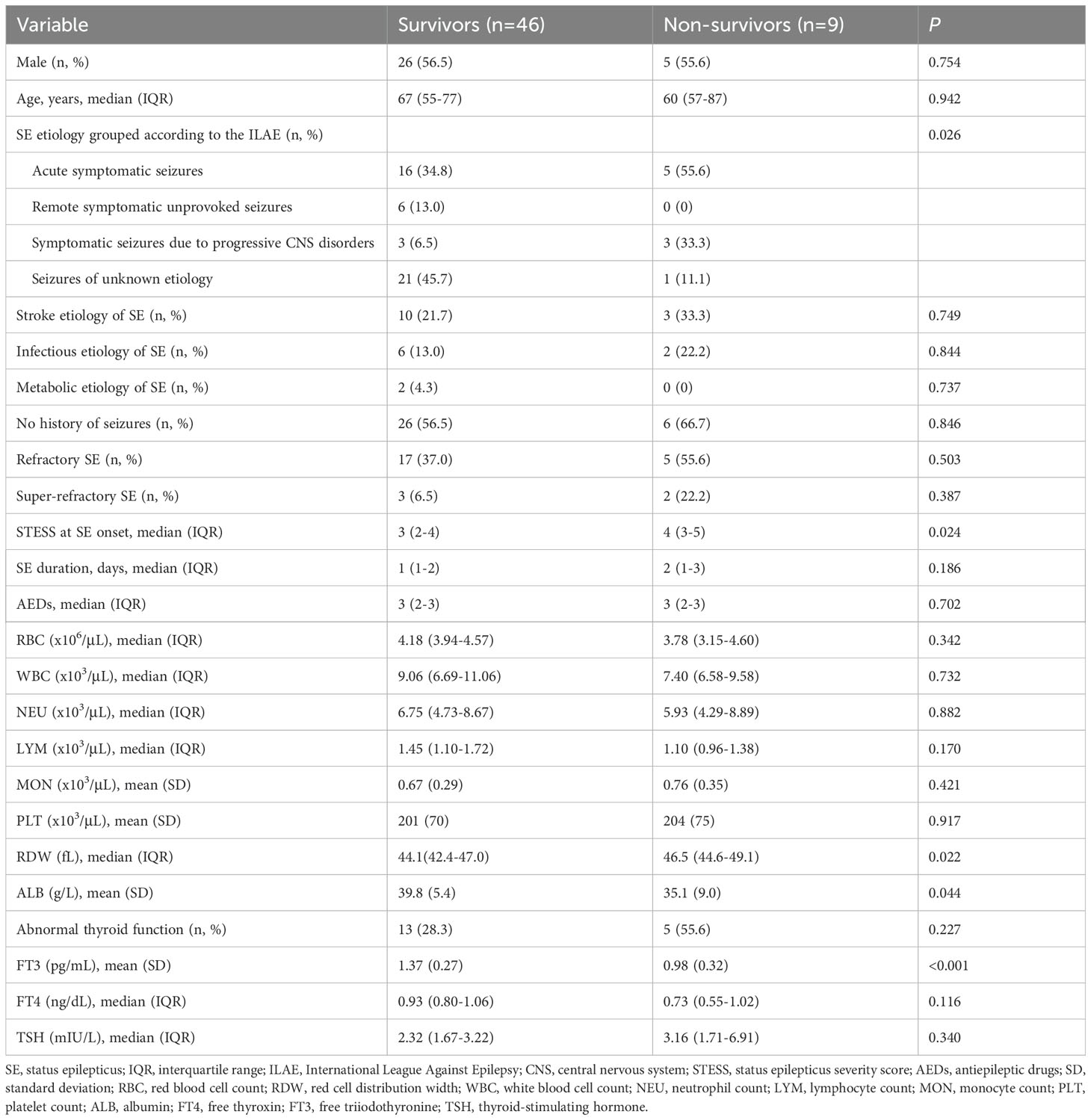
Table 8. Univariate analysis of clinical characteristics and laboratory data between survivors and non-survivors in the external validation cohort.
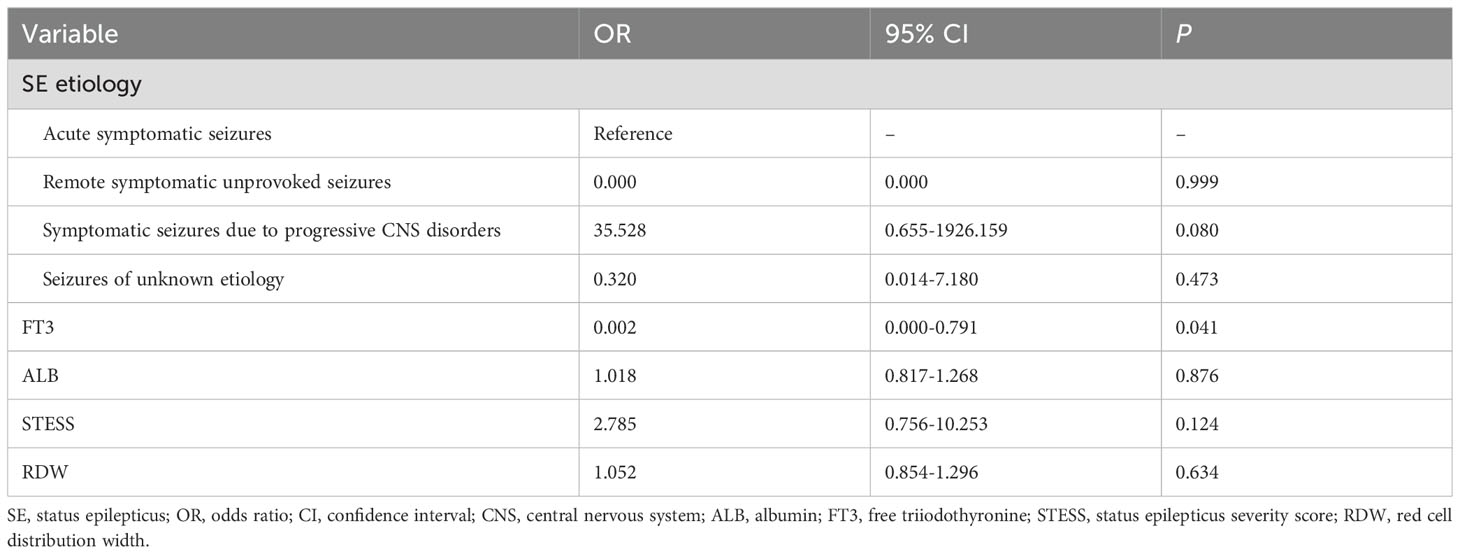
Table 9. Multivariate analysis of predictors for 30-day mortality in SE patients in the external validation cohort.
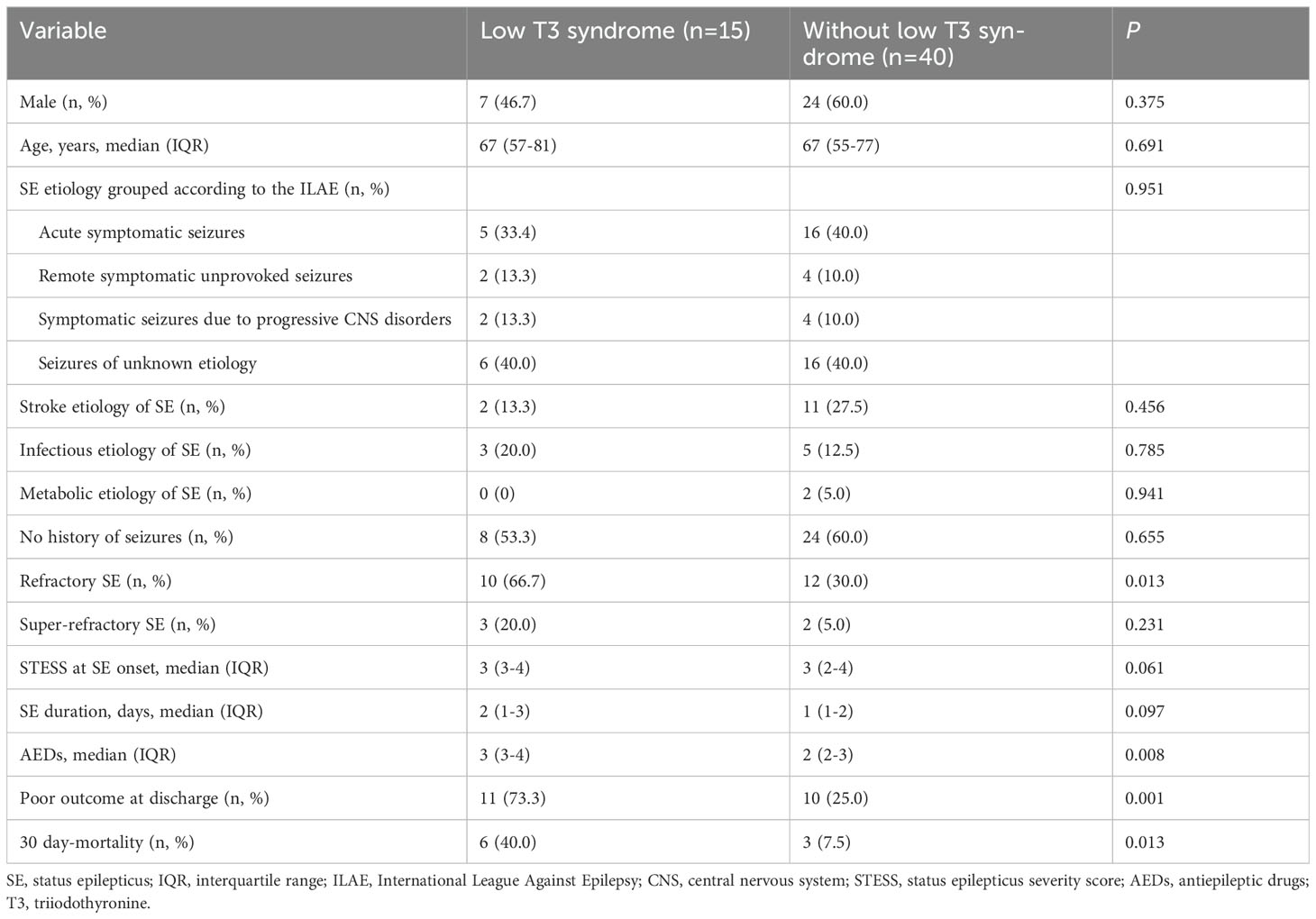
Table 10. Comparisons of SE outcomes in patients with or without low T3 syndrome in the external validation cohort.
Discussion
Our study indicated that serum levels of FT3 measured on admission were independently associated with 30-day mortality in SE patients. Our findings supported that serum FT3 level was an independent predictor for the prognosis of SE patients. Additionally, we also observed that low T3 syndrome was associated with poor outcome at hospital discharge and higher risk of mortality in SE patients. Of note, this study for the first time explored the correlation between thyroid hormones and SE prognosis.
Thyroid hormones, including T3 and T4, are synthesized and released by the thyroid gland and play a vital role in brain development as well as the maintenance of brain function (18). Thyroid hormone disorders can lead to dysregulated energy metabolism of the central nervous system (CNS), further destroy biological processes including survival and differentiation of neurons, cytoskeleton dynamics and neurotransmission, etc. (19). Thyroid hormones have been suggested to be involved in the pathogenesis of epilepsy. Thyroid hormones exert genomic and non-genomic effects on mitochondrial biogenesis and function, and the decreased activity of thyroid hormones has been indicated to be associated with mitochondrial dysfunction as well as the resulting oxidative stress, thus promoting the generation of epileptic seizures and the development of chronic epilepsy (11). Moreover, thyroid hormones can regulate the development and function of GABAergic neurons (12, 20). As we know, GABA, as the principal inhibitory neurotransmitter, exerts a crucial role in seizure suppression (21). Therefore, it can be speculated that thyroid hormone abnormalities may result in dysregulation of GABAergic system, further disrupting the excitatory-inhibitory balance, ultimately triggering seizures. Of note, the association of thyroid function with epilepsy might be not causal. A recent study applied a Mendelian randomization analysis to investigate the potential causal relationships between thyroid disorders and various types of epilepsy, which indicated no significant causal relationships therein (22). Actually, a bidirectional interplay may exist between thyroid hormones and seizure. In addition to the involvement of thyroid hormones in epileptogenesis as mentioned above, seizure could also result in the variations in thyroid hormone levels, which may be involved in its impact on the hypothalamic-pituitary-thyroid axis. Neuronal hyperactivity during a seizure activates the hypothalamus by specific neurotransmitter alterations or by the release of other mediators, and thus affects the secretion of thyrotropin releasing hormone (TRH), further modulating TSH secretion as well as subsequent thyroid hormone biosynthesis and secretion (23). Thus, it seems that the correlation between thyroid hormones and seizure is complex and diverse.
Despite that the potential relationship between thyroid hormones and seizure has been proposed, studies on thyroid hormones and prognosis of seizure are rare. Our study for the first time investigated the predictive value of thyroid hormones for SE prognosis. We first observed that serum FT3 levels were remarkably decreased as the SE severity increased, and patients with super-refractory SE had the lowest FT3 levels, which indicated the potential relationship between thyroid hormones and SE outcome. Furthermore, our results revealed that serum FT3 level was an independent predictor for 30-day mortality in SE patients. It is suggested that T3 could promote astrocytic glutamate uptake and thus protect astrocytes and neurons against glutamate toxicity (24), which may explain the correlation between low serum FT3 levels and poor SE prognosis. Similar to our results, lower serum FT3 levels have been found to be associated with poor prognosis of multiple CNS diseases. The study by O’Keefe et al. (25) revealed that lower FT3 levels were related to worse outcomes after ischemic stroke. Another investigation indicated that declined FT3 levels as well as increased TSH levels were associated with worse functional outcome and higher risk of mortality within 6 months after traumatic brain injury (13). However, several studies have shown a different result. The research by Ray et al. (26) investigated TSH, T3, and FT4 levels in 180 seriously ill patients after 3 hours of ICU admission, which demonstrated no statistically significant differences in T3 and FT4 levels between survivors and non-survivors. The possible explanations for the inconsistent results may be different disease backgrounds, the differences in the timing of blood sample collecting and the methods of thyroid hormones tested, etc.
Low T3 syndrome is characterized by declined serum T3, decreased or normal serum T4, and normal TSH levels, which has been reported to be closely associated with poor functional outcomes, unfavorable prognosis, and higher risk of mortality in serious diseases (27, 28). In our study, we observed that SE patients with low T3 syndrome had poor outcome at discharge and higher 30-day mortality. Critical diseases cause a declined peripheral conversion of FT4 to FT3, which may lead to the development of low T3 syndrome (29). Therefore, we presumed that SE patients with low T3 syndrome may have relatively severe disease states, and thus suffer from worse outcome. Although low T3 syndrome is reported to be associated with adverse outcomes of various severe acute and chronic diseases, it is controversial whether patients with serious illness accompanied by low T3 syndrome can benefit from thyroid hormone supplementation in the acute stage of diseases, since a decreased serum T3 level may be a compensatory mechanism to conserve energy and diminish protein consumption (30).
Of note, long-term treatment with AEDs has been reported to be associated with changes in thyroid hormone metabolism. The underlying mechanism remains unclear, which may be that AEDs affect hepatic microsomal enzyme systems or uridine diphosphate glucuronosyltransferase, and thus interfere the metabolism of thyroid hormones (31). Several previous studies investigated the effects of different AEDs on thyroid hormones. Adhimoolam et al. (32) compared thyroid hormone levels between adult epileptic patients receiving conventional or newer AEDs and healthy adults, and they found that epileptic patients receiving conventional AEDs such as sodium valproate, carbamazepine and phenytoin had significantly lower FT4 and TSH levels, while no significant difference in thyroid hormones was observed between epileptic patients treated with newer AEDs and healthy adults. Thus, they proposed that conventional AEDs may have remarkable alteration in the thyroid hormone levels compared to the newer AEDs. However, a recent meta-analysis from Han et al. (33) indicated that both conventional and newer AEDs could affect thyroid function. Their results suggested that conventional AEDs including carbamazepine and phenytoin were closely associated with decreased T4 and T3 levels, and newer AEDs like topiramate may result in elevated TSH levels. Additionally, both levetiracetam and valproic acid may lead to subclinical hypothyroidism. In our study, since most of enrolled patients received sodium valproate, we did not evaluate and compare the effects of conventional and newer AEDs on thyroid hormones as well as on the association between thyroid hormones and SE prognosis, and future studies are needed to explore this issue.
Although our results have shed light on the value of thyroid hormones, particularly FT3 for the prediction of SE prognosis, there are some limitations in our study. First, despite that this study included two independent cohorts, each cohort had a relatively small sample size. Second, the blood samples detected in our study were obtained within 24 hours after admission, and dynamically detecting changes in thyroid hormone levels is lacking. Third, some AEDs could affect the function of thyroid hormones, and our study only compared the difference in the number of AEDs between SE patients with good outcome and poor outcome, which indicated no significant differences therein. However, the present study did not yet assess the impact of different types of AEDs, and future investigations are needed to clarify whether various AEDs could affect the correlation between thyroid hormones and SE prognosis. Fourth, the current study only assessed SE outcome at discharge and 30 days after SE onset, future studies are needed to explore the association between thyroid hormone levels and the long-term prognosis of SE.
Conclusions
Lower serum FT3 level was independently associated with 30-day mortality in SE patients. Low T3 syndrome could be a potential biomarker for predicting the prognosis of SE. Future large sample, multi-center, prospective studies are needed to validate our results and to evaluate the potential of thyroid function and low T3 syndrome as reliable markers for predicting the long-term prognosis of SE.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the ethics committees of the affiliated hospital of Southwest Medical University and the affiliated Traditional Chinese Medicine hospital of Southwest Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JF: Conceptualization, Funding acquisition, Investigation, Writing – original draft. XC: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. JL: Investigation, Project administration, Validation, Writing – review & editing. LP: Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University (No. 21024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. (2015) 14:615–24. doi: 10.1016/S1474-4422(15)00042-3
2. Hanin A, Demeret S, Lambrecq V, Rohaut B, Marois C, Bouguerra M, et al. Clinico-biological markers for the prognosis of status epilepticus in adults. J Neurol. (2022) 269:5868–82. doi: 10.1007/s00415-022-11199-4
3. Lattanzi S, Trinka E, Brigo F, Meletti S. Clinical scores and clusters for prediction of outcomes in status epilepticus. Epilepsy Behav. (2023) 140:109110. doi: 10.1016/j.yebeh.2023.109110
4. Rossetti AO, Logroscino G, Bromfield EB. A clinical score for prognosis of status epilepticus in adults. Neurology. (2006) 66:1736–8. doi: 10.1212/01.wnl.0000223352.71621.97
5. Leitinger M, Höller Y, Kalss G, Rohracher A, Novak HF, Höfler J, et al. Epidemiology-based mortality score in status epilepticus (EMSE). Neurocrit Care. (2015) 22:273–82. doi: 10.1007/s12028-014-0080-y
6. Talhada D, Santos CRA, Gonçalves I, Ruscher K. Thyroid hormones in the brain and their impact in recovery mechanisms after stroke. Front Neurol. (2019) 10:1103. doi: 10.3389/fneur.2019.01103
7. Wang Y, Sun F, Hong G, Lu Z. Thyroid hormone levels as a predictor marker predict the prognosis of patients with sepsis. Am J Emerg Med. (2021) 45:42–7. doi: 10.1016/j.ajem.2021.02.014
8. Fu J, Zhao Q, Li J, Chen X, Peng L. Association between thyroid hormone levels in the acute stage of stroke and risk of poststroke depression: A meta-analysis. Brain Behav. (2024) 14:e3322. doi: 10.1002/brb3.v14.1
9. Qiao S, Zhang SC, Zhang RR, Wang L, Wang ZH, Jiang J, et al. Thyroid function and low free triiodothyronine in Chinese patients with autoimmune encephalitis. Front Immunol. (2022) 13:821746. doi: 10.3389/fimmu.2022.821746
10. Jiang X, Xing H, Wu J, Du R, Liu H, Chen J, et al. Prognostic value of thyroid hormones in acute ischemic stroke - a meta analysis. Sci Rep. (2017) 7:16256. doi: 10.1038/s41598-017-16564-2
11. Tamijani SM, Karimi B, Amini E, Golpich M, Dargahi L, Ali RA, et al. Thyroid hormones: Possible roles in epilepsy pathology. Seizure. (2015) 31:155–64. doi: 10.1016/j.seizure.2015.07.021
12. Westerholz S, de Lima AD, Voigt T. Regulation of early spontaneous network activity and GABAergic neurons development by thyroid hormone. Neuroscience. (2010) 168:573–89. doi: 10.1016/j.neuroscience.2010.03.039
13. Mele C, Pagano L, Franciotta D, Caputo M, Nardone A, Aimaretti G, et al. Thyroid function in the subacute phase of traumatic brain injury: a potential predictor of post-traumatic neurological and functional outcomes. J Endocrinol Invest. (2022) 45:379–89. doi: 10.1007/s40618-021-01656-8
14. Lin CH, Ho CJ, Lu YT, Shih FY, Chuang YC, Tsai MH. Predicting the functional outcome of adult patients with status epilepticus. J Clin Med. (2019) 8:992. doi: 10.3390/jcm8070992
15. Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol. (2011) 10:922–30. doi: 10.1016/S1474-4422(11)70187-9
16. Kantanen AM, Reinikainen M, Parviainen I, Ruokonen E, Ala-Peijari M, Bäcklund T, et al. Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. (2015) 49:131–4. doi: 10.1016/j.yebeh.2015.04.065
17. Sutter R, Valença M, Tschudin-Sutter S, Rüegg S, Marsch S. Procalcitonin and mortality in status epilepticus: an observational cohort study. Crit Care. (2015) 19:361. doi: 10.1186/s13054-015-1072-9
18. de Escobar GM, Obregón MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. (2004) 18:225–48. doi: 10.1016/j.beem.2004.03.012
19. Sawicka-Gutaj N, Zawalna N, Gut P, Ruchała M. Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions. Pharmacol Rep. (2022) 74:847–58. doi: 10.1007/s43440-022-00377-w
20. Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, et al. Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology. (2007) 148:92–102. doi: 10.1210/en.2006-0164
21. Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. (2001) 42 Suppl 3:8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x
22. Lu D, Wang Y, Yang Y, Zhang H, Fan X, Chen S, et al. Thyroid function and epilepsy: a two-sample Mendelian randomization study. Front Hum Neurosci. (2024) 17:1295749. doi: 10.3389/fnhum.2023.1295749
23. Han JY, Lee IG, Shin S, Park J. Seizure duration may increase thyroid-stimulating hormone levels in children experiencing a seizure. J Int Med Res. (2020) 48:300060519888401. doi: 10.1177/0300060519888401
24. Mendes-de-Aguiar CB, Alchini R, Decker H, Alvarez-Silva M, Tasca CI, Trentin AG. Thyroid hormone increases astrocytic glutamate uptake and protects astrocytes and neurons against glutamate toxicity. J Neurosci Res. (2008) 86:3117–25. doi: 10.1002/jnr.v86:14
25. O’Keefe LM, Conway SE, Czap A, Malchoff CD, Benashski S, Fortunato G, et al. Thyroid hormones and functional outcomes after ischemic stroke. Thyroid Res. (2015) 8:9. doi: 10.1186/s13044-015-0021-7
26. Ray DC, Drummond GB, Wilkinson E, Beckett GJ. Relationship of admission thyroid function tests to outcome in critical illness. Anaesthesia. (1995) 50:1022–5. doi: 10.1111/j.1365-2044.1995.tb05943.x
27. Gao R, Chen RZ, Xia Y, Liang JH, Wang L, Zhu HY, et al. Low T3 syndrome as a predictor of poor prognosis in chronic lymphocytic leukemia. Int J Cancer. (2018) 143:466–77. doi: 10.1002/ijc.v143.3
28. Rothberger GD, Valestra PK, Knight K, Desai AK, Calixte R, Shapiro LE. Low free T(3) is associated with worse outcomes in patients in the ICU requiring invasive mechanical ventilation. J Intensive Care Med. (2021) 36:313–8. doi: 10.1177/0885066619890822
29. Bunevicius A, Deltuva V, Tamasauskas S, Tamasauskas A, Laws ER Jr, Bunevicius R. Low triiodothyronine syndrome as a predictor of poor outcomes in patients undergoing brain tumor surgery: a pilot study: clinical article. J Neurosurg. (2013) 118:1279–87. doi: 10.3171/2013.1.JNS121696
30. Perez AC, Jhund PS, Stott DJ, Gullestad L, Cleland JG, van Veldhuisen DJ, et al. Thyroid-stimulating hormone and clinical outcomes: the CORONA trial (controlled rosuvastatin multinational study in heart failure). JACC Heart Fail. (2014) 2:35–40. doi: 10.1016/j.jchf.2013.07.008
31. Zhang YX, Shen CH, Lai QL, Fang GL, Ming WJ, Lu RY, et al. Effects of antiepileptic drug on thyroid hormones in patients with epilepsy: A meta-analysis. Seizure. (2016) 35:72–9. doi: 10.1016/j.seizure.2016.01.010
32. Adhimoolam M, Arulmozhi R. Effect of antiepileptic drug therapy on thyroid hormones among adult epileptic patients: An analytical cross-sectional study. J Res Pharm Pract. (2016) 5:171–4. doi: 10.4103/2279-042X.185717
Keywords: status epilepticus, thyroid hormones, free triiodothyronine, low T3 syndrome, prognosis
Citation: Fu J, Chen X, Li J and Peng L (2024) Thyroid hormones and prognosis in adults with status epilepticus: a retrospective study. Front. Endocrinol. 15:1452299. doi: 10.3389/fendo.2024.1452299
Received: 04 July 2024; Accepted: 23 October 2024;
Published: 08 November 2024.
Edited by:
Takayoshi Ubuka, International Cancer Laboratory Co., Ltd., JapanReviewed by:
U. K. Misra, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaKenan Zhang, Duke University, United States
Copyright © 2024 Fu, Chen, Li and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglun Li, amluZ2x1bmxpMTIzQDE2My5jb20=; Lilei Peng, bGlsZWkucGVuZ0Bzd211LmVkdS5jbg==
 Jie Fu
Jie Fu Xiu Chen1
Xiu Chen1 Lilei Peng
Lilei Peng