
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 23 September 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1452192
Introduction: Through a network meta-analysis, we compared different treatment measures for patients with diabetic foot ulcers (DFU), assessing their impact on the healing of DFU and ranking them accordingly.
Methods: We searched the PubMed, the China National Knowledge Infrastructure (CNKI), Embase, the WanFang and the WeiPu database. The retrieval time was from database establishment to January 2024, and retrieval entailed subject and free words. Randomized controlled trials (RCTs) with different treatment measures for DFU were included. Data extraction and evaluation were based on the PRISMA guidelines. Meta-analyses using pairwise and network methods were employed to compare and rank the effectiveness of different treatments for DFU.
Results: Ultimately, we included 57 RCTs involving a total of 4,826 patients with DFU. When it comes to ulcer healing rates, compared to standard of care(SOC),platelet-rich plasma(PRP), hyperbaric oxygen therapy(HBOT), topical oxygen therapy(TOT), acellular dermal matrix(ADM), and stem cells(SCs) in both direct meta-analysis(DMA) and network meta-analysis(NMA) can effectively increase the complete healing rate. For Scs+PRP, a statistically significant improvement was only observed in the NMA. Moreover, when compared to the negative pressure wound therapy(NPWT) group, the PRP+NPWT group was more effective in promoting the complete healing of ulcers. In terms of promoting the reduction of ulcer area, no statistical differences were observed among various treatment measures. When it comes to ulcer healing time, both PRP and NPWT can effectively shorten the healing time compared to SOC. Furthermore, when compared to the NPWT group, the combined treatment of PRP and ultrasonic debridement(UD) with NPWT is more effective in reducing healing time. In terms of amputation rates and adverse reactions, the PRP group effectively reduced the amputation rate and adverse reactions for patients with DFU. Additionally, compared to the NPWT group, the combined treatment of PRP and UD with NPWT reduced the incidence of adverse reactions. However, no significant differences were observed among other treatment measures in terms of amputation rates and adverse reactions. The ranking results showed that the efficacy of PRP+NPWT and UD+NPWT in promoting ulcer healing, reducing ulcer area, shortening healing time, decreasing amputation rates and adverse reactions is superior to that of the alone PRP group, NPWT group, and UD group. Conversely, the SOC group demonstrates the least effective performance in all aspects.
Conclusion: Due to the particularity of the wound of DFU, the standard of care is not effective, but the new treatment scheme has a remarkable effect in many aspects. And the treatment of DFU is not a single choice, combined with a variety of methods often achieve better efficacy, and will not bring more adverse reactions.
Diabetes (DM) is a rapidly spreading disease worldwide, posing a significant health challenge globally (1). It is estimated that there were 451 million patients with diabetes in 2017, and this number is projected to rise to 693 million by 2045 (2). Diabetic foot ulcer (DFU) is one of the clinical manifestations of diabetic nerve lesions, defined as structural or functional changes in the foot associated with diabetic nerve lesions and varying degrees of peripheral vascular disease, such as ulcers, infections, or gangrene (3). DFU is one of the most common, most severe, and most costly complications of diabetes (4–7), with approximately 19% to 34% of patients with diabetes experiencing a DFU in their lifetime (8). It is characterized by complex management, high incidence rate, and high mortality rate (9). The global incidence rate of diabetes foot ulcers is approximately 6.3%, occurring predominantly in patients with type 2 diabetes(T2DM), the elderly, and those with a prolonged history of diabetes (10). According to predictions from the World Health Organization (WHO), by 2030, DFU will affect more than 19% of the world’s adult population (11). DFU are also the primary reason for hospitalizations among diabetes patients worldwide. Reports indicate that nearly 88% of lower leg amputations are associated with DFU, often resulting in disability and severely compromising the quality of life (12, 13). The 5-year mortality rate for patients with DFU is 30%, while the 5-year mortality rate for those undergoing amputations exceeds 70% (14). Furthermore, the annual cost associated with DFU treatment and amputations is extremely high, approximately 10.9 billion USD worldwide (15). From this, it is evident that DFU are associated with significant incidence rates and mortality rates, as well as imposing a substantial economic, social, and public health burden.
Currently, the standard treatments for DFU includes debridement, dressing, offloading, vascular assessment, infection management, and blood glucose control
(16). However, these treatments are not satisfactory. It has been reported that the complete healing rates for DFU patients after 12 weeks and 20 weeks of standard therapy are only 24% and 31% respectively (17). Therefore, in recent years, several adjunctive techniques have been developed for the debridement treatment of DFU. These include ultrasonic debridement (UD), negative pressure wound therapy (NPWT) [including vacuum-assisted closure (UAC) and vacuum sealing drainage (VSD)], and oxygen therapies [such as hyperbaric oxygen therapy (HBOT) and topical oxygen therapy (TOT)]. Additionally, studies have found that using stem cells (SCs), growth factors, or tissue-engineering dressings can form the basis for a new treatment approach. Among these, fat-derived SCs, platelet-rich plasma(PRP), and acellular dermal matrix(ADM) have emerged as focal points of research. These treatment methods aim to restore the body’s natural healing process (18). Direct meta-analysis(DMA) have been employed to compare the efficacy and safety of these therapeutic measures in the treatment of DFU (19–26), yet divergent opinions persist, such as:Tasmania et al. concluded that the use of PRP in DFU promoted wound healing, reduced ulcer volume, reduced the time to complete wound healing, and reduced the incidence of adverse events, with no difference in the probability of wound complications (27). This is consistent with previous findings (23, 25, 28). However, Ajay et al. concluded that PRP had no significant effect on promoting ulcer healing (29); Zhao et al. found no difference in ulcer incidence, risk of amputation, or adverse events with HBOT compared to standard treatment (ST) (30). Sharma et al. believe that HBOT has significant effect on the complete healing of diabetic foot ulcers, and can shorten the healing time and reduce the incidence of major amputations (24). Moreover, there have been no studies that directly compared the therapeutic outcomes of these varied treatments for DFU. In contrast, network meta-analysis(NMA) can utilize both direct and indirect data to compare various interventions, and by ranking the therapeutic effects of all interventions, they can identify the most effective treatment method. Consequently, to further evaluate the impact of different therapeutic methods on the outcome of DFU efficacy, we included relevant randomized controlled trials (RCTs) in a NMA, aiming to provide stronger evidence for the effectiveness and safety of various treatments for DFU.
Our study follows the recommendations of the assessing the methodological quality of systematic reviews(AMSTAR)guidelines (31) and is consistent with the preferred reporting items for systematic reviews and meta-analyses(PRISMA)statement (32).
We searched the PubMed, the China National Knowledge Infrastructure (CNKI), Embase, the WanFang and the WeiPu database. The retrieval time was from database establishment to January 2024, and retrieval entailed subject and free words. The search terms were included in the abstract or title, include “diabetic foot ulcers,” “platelet-rich plasma,” “negative pressure wound therapy,” “hyperbaric oxygen therapy,” “topical oxygen therapy,” “ultrasonic debridement,” “acellular dermal matrix,” “stem cells,” and “randomized controlled trials.” The publication type of the studies is restricted to randomized controlled trials (without language or location limitations).
Studies included in the NMA must meet the following criteria: (1) The subjects are patients with DFU, regardless of age, gender, race or nationality. (2) The study type is a RCT. (3) The study divided the participants into two or more groups, and patients in each group were treated with one treatment or a combination of treatment measures. The therapeutic measures include: platelet-rich plasma, negative pressure wound therapy, hyperbaric oxygen therapy, topical oxygen therapy, ultrasonic debridement, acellular dermal matrix, stem cell transplantation and standard treatments, so as to compares the efficacy and safety of different treatment measures in patients with DFU. (4) The study provides at least one effective efficacy indicator: complete healing rate, healing time required, reduction in ulcer area, amputation rate, and adverse reactions (infection, allergy, pain, etc.).
Exclusion criteria: (1) Studies that do not meet the criteria for RCTs, including: reviews, commentaries, letters, etc. (2) Exclusion of studies that did not include relevant outcome measures (3) Exclusion of subjects who do not meet the diagnostic criteria for DFU. (4) Repeated publications. (5) Documents with incomplete or insufficient data.
The primary outcome measures in the NMA studies mainly include ulcer healing rate, time required for ulcer healing, reduction in ulcer area, amputation rate, and adverse reactions (infection, allergy, pain, etc.).
Two evaluators independently searched the database based on inclusion and exclusion criteria, searching the full text of the initially included articles. They used a uniform form to extract data, including: author name, publication year, country, research subjects (sample size, gender ratio, average age, and smoking history), interventions (experimental group and control group), duration of the study, duration and area of DFU, and main research results. The methodological quality of the study was evaluated in accordance with the Cochrane Risk Bias tool. Evaluated aspects included the following: random sequence generation, hidden distribution concealment, the blinding of subjects and intervention providers, the blinding of result evaluation, the integrity of the outcome data, selective result reporting, and other sources of bias. When there was inconsistency, judgment was reached through public discussion.
For DMA, we used STATA12.0 software for statistical analysis, using odds ratio (OR) and 95% confidence interval (CI) as the evaluation index of the results, represented by mean difference and 95% CI. First, heterogeneity was assessed using the X^2 test (a=0.05) and a quantitative analysis of I^2 for heterogeneity (I^2 ≥ 50%) conducted. In cases of no heterogeneity between the research results, the meta-analysis was conducted. In cases of statistical heterogeneity between the research results, the source of heterogeneity was further analyzed, and the random heterogeneity model was used after excluding the influence of obvious clinical heterogeneity. Funnel maps created using the STATA software were employed to detect publication bias.
We performed a Bayesian NMA using R and STATA software. NMA can combine direct and indirect comparisons to further analyze the effects of different treatment options on DFU. The results of the comparison effect are expressed as OR and its 95% CI. Moreover, we built a network diagram using the mtc.network”command of the gemtc”package in the R software. Furthermore, we calculated the percentage area under the cumulative ranking (SUCRA) curve, ranking the different interventions. One intervention had a higher SUCRA value than others, indicating that the better the treatment effect, the lower the incidence of adverse reactions. A node splitting method was used to evaluate the consistency hypothesis of direct and circumstantial evidence. When direct evidence of the results was consistent with circumstantial evidence (P> 0.05), the consistency model was adopted.
Based on a pre-designed literature search strategy, a total of 1,737 articles were identified, of which 1,501 were not classified as RCTs. After reviewing the titles and abstracts, 147 articles were excluded, allowing for a detailed review of 89 articles. Ultimately, we included 57 RCTs involving a total of 4,826 patients with DFU. Other studies were excluded: 18 did not have clear efficacy outcome indicators, 8 were repetitive studies, and 6 did not meet the criteria for diabetes foot. The literature screening process and results are shown in Figure 1.
We have summarized the fundamental characteristics of the included studies, as shown in Table 1. The studies were published between 2009 and 2024, with 34 originating from Asia (29, 38, 40, 42, 45, 48–50, 57–63, 65–74, 76, 77, 81–87), 11 from North America (33, 35–37, 39, 51–56), 6 from Europe (34, 41, 43, 46, 47, 64), and 6 from Africa (44, 75, 78–80). The included studies encompassed 11 distinct interventions: PRP, NPWT (including UAC and VSD), HBOT, TOT, UD,ADM, SCs, SOC, PRP+SCs, PRP+NPWT, and NPWT +UD. Among them, there are 15 studies comparing PRP vs SOC, 6 studies on NPWT vs SOC, 7 studies on HBOT vs SOC, 5 studies on TOT vs SOC, 2 studies on UD vs SOC, 6 studies on ADM vs SOC, 3 studies on SCs vs SOC, 1 study on PRP vs HBOT vs SOC, 3 studies on PRP+NPWT vs NPWT, 2 studies on PRP+NPWT vs SOC, 5 studies on NPWT+UD vs NPWT, and 2 studies on SCs vs PRP +SCs vs SOC.
The quality of the included studies was evaluated, and the results showed that the blinding of the subjects and intervention providers was the main source of potential bias (Figure 2). This may be due to the fact that different treatment measures have entirely different approaches, and both patients and researchers are aware of the nature of the study and the allocation of the study group, making blinding impossible.
Figure 3 shows the mesh map included in the study, and we included the following 11 interventions in the NMA. Each letter represents a different treatment: A=PRP、B=NPWT、C=HBOT、D=TOT、E=UD、F=ADM、G=SCs、H=SOC、I=SCs+PRP、J=UD+NPWT、K=PRP+NPWT.The size of the nodes is proportional to the number of patients, the line between points represents direct comparative evidence, the thickness of the edges is proportional to the number of studies evaluated per intervention, and the edge color represents the average bias risk per head-to-head comparison.
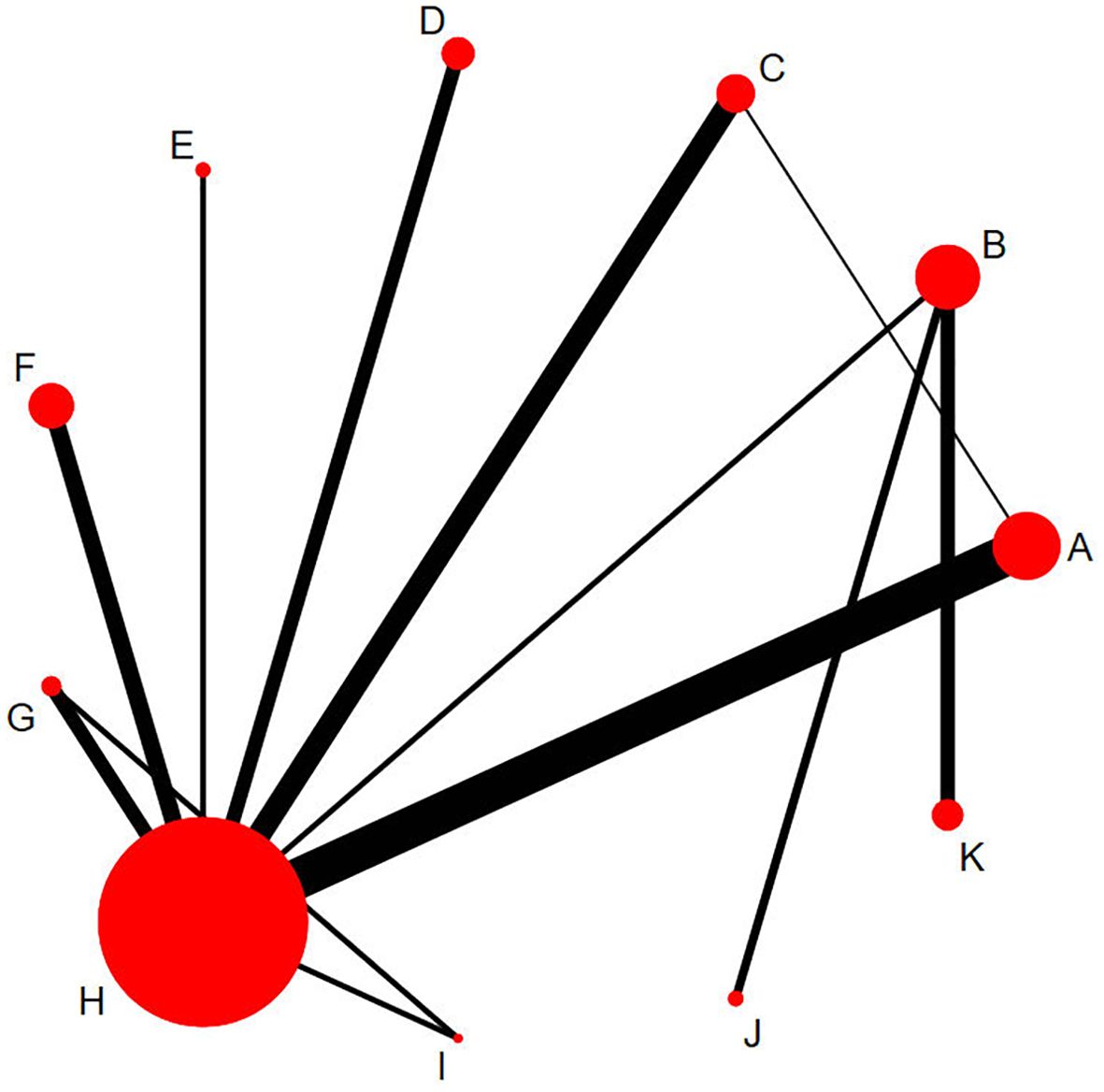
Figure 3. Network plots of eligible comparisons for different treatment strategies. The cirdes represent intervention arms in an RCT-larger cirdes represent presence in more RCTs. The lines connect interventions that were compared in an RCT and thicker connecting lines indicate more direct RCT comparisons. Intervention (A), Platelet-rich plasma; (B), Negative pressure wound therapy; (C), Hyperbaric oxygen therapy; (D), Topical oxygen therapy; (E), Ultrasonic debridement; (F), Acellular dermal matrix; (G), Stem cells; (H), Standard of care; (I), Stem cells+Platelet-rich plasma; (J), Ultrasonic debridement+Negative pressure wound therapy; (K), Platelet-rich plasma+Negative pressure wound therapy.
Table 2 presents the results of DMA and NMA regarding the efficacy outcomes of various therapeutic measures for DFU. Below, the analysis results of different efficacy indicators are described.
Forty-nine studies involving 4,010 patients with DFU patients reported on the impact of various interventions on complete wound healing.In both the DMA and NMA groups, we observed that compared to SOC, PRP (0.15, 95% CI, 0.07-0.28), HBOT (0.37, 95% CI, 0.14-0.92), TOT (0.25, 95% CI, 0.078-0.69), ADM (0.19, 95% CI, 0.07-0.5), and Scs (0.10, 95% CI, 0.022-0.40) showed higher ulcer healing rates. When compared to NPWT, the PRP+NPWT group (3.5, 95% CI, 1.20-10.0) had a significantly higher ulcer healing rate. Furthermore, in the NMA, we noted that the complete wound healing in the PRP+stem cell transplantation group (22.0, 95% CI, 2.5-23.0) was significantly higher than that in the SOC. No significant differences were observed among other interventions.
Nineteen studies involving 1626 DFU patients reported effects on ulcer area. It was found that in both the DMA and NMA groups, different interventions had no significant advantage in the incidence of reduced ulcer area.
The impact on ulcer healing time was reported in 15 studies involving 1,135 patients with DFU. Research found that in both the DMA and NMA groups, PRP and NPWT effectively reduced the time to complete healing compared to SOC. Moreover, compared to NPWT, UD combined with NPWT and PRP combined with NPWT were more effective.
Seventeen studies involving 1,706 patients with DFU reported on the impact of various interventions on the amputation rate. In the DMA, the PRP group had a lower amputation rate compared to the SOC (6.8, 95% CI, 2.2-41.0), with no significant difference observed among other interventions. Similarly, in the NMA group, we also observed a lower amputation rate in the PRP group compared to the SOC (7.0, 95% CI, 2.2-41.0), with no other notable findings.
Research involving 2755 patients with DFU reported on the impact of different interventions on adverse events. In both the DMA and NMA groups, we observed that compared to SOC, PRP (3.5, 95% CI, 1.4-8.9) resulted in a lower rate of adverse events. When compared to NPWT, the PRP+NPWT group (0.19, 95% CI, 0.032-0.91) and the UD+NPWT group (0.099, 95% CI, 0.0082-0.73) had lower adverse events, while no significant differences were observed among other interventions.
NMA can evaluate the best effect of each intervention for different results and sort each intervention by SUCRA values, with a higher SUCRA value indicating a better intervention or a lower incidence of adverse reactions. Table 3 displays the detailed ranking results. Based on Table 3, it can be observed that the UD combined with NPWT group is most effective in reducing the ulcer area (91.59%), shortening the ulcer healing time (72.98%), and decreasing the amputation rate (92.15%) and adverse reactions (90.98%). This is followed by the PRP combined with NPWT group. When considering the complete wound healing, the SCs combined with PRP group (83.56%) demonstrates the best efficacy, followed by the PRP combined with NPWT group (80%), and then the UD combined with NPWT group (73.58%). In comparison to other groups, the SOC group is the least effective in promoting ulcer healing (1.52%), reducing the ulcer area (28.06%), shortening the ulcer healing time (0%), and decreasing the amputation rate (12.86%) and adverse reactions (18.45%). Furthermore, it is noted that the treatments of SCs, PRP, and ADM are generally more effective in promoting ulcer healing and reducing amputations compared to NPWT, HBOT, TOT, and UD groups. The efficacy of PRP+NPWT and UD+NPWT in promoting ulcer healing, reducing the ulcer area, shortening the healing time, and decreasing the amputation rate and adverse reactions are superior to that of the alone PRP, NPWT, and UD groups.
Figure 4 displays a comparison-adjusted funnel diagram. Most studies on the funnel map are symmetrically distributed across the vertical lines of X=0, indicating that there were no significant small-sample effects and publication bias.
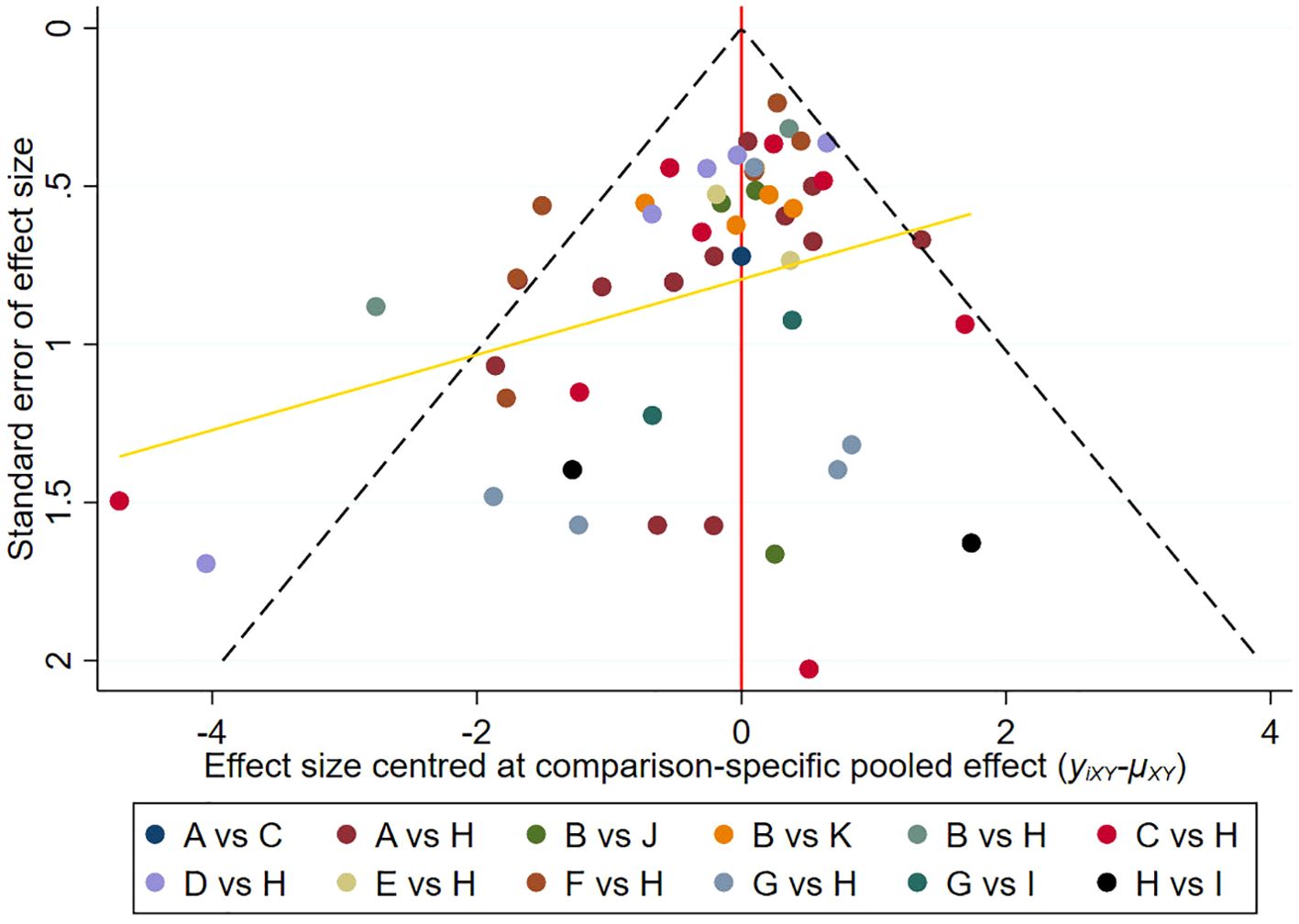
Figure 4. Comparison-adjusted funnel plot. Points of different colors represent different interventions.Each dot represents a direct comparison of different interventions in the study. (A), Platelet-rich plasma; (B), Negative pressure wound therapy; (C), Hyperbaric oxygen therapy; (D), Topical oxygen therapy; (E), Ultrasonic debridement; (F), Acellular dermal matrix; (G), Stem cells; (H), Standard of care; (I), Stem cells+Platelet-rich plasma; (J), Ultrasonic debridement+Negative pressure wound therapy; (K), Platelet-rich plasma+Negative pressure wound therapy.
As a post-hoc sensitivity analysis, we evaluated the results after removing studies that had scored highly on the Cochrane Risk of Bias Tool. The results showed that after removing the study of Brigido et al., there was no significant difference between the current results and those before the elimination, and the ranking order remained consistent, which proved that the results of network meta-analysis were reliable.
DFU form the basis for 40 - 70% of non-traumatic lower extremity amputations in DM, often leading to disability and severely impeding the quality of life (88, 89). The Wound Healing Society (WHS) pointed out in its 2013 DFU treatment guidelines that “promoting decompression, reducing bacterial and cellular burden through adequate debridement, while using moist dressings for local wound care, absorbing wound exudate, and local and systemic antibiotic treatment when necessary, as well as treating osteomyelitis.” Moreover, the guidelines also stated that cell and non-cell equivalents improve DFU healing by releasing growth factors, cytokines, and proteins that stimulate the wound bed (5). Therefore, besides the first-line conventional treatments, in recent years, several new treatment modalities such as NPWT, TOT, HBOT, growth factors, bioengineered skin substitutes, and electrophysical therapy have brought new opportunities to DFU patients, yet consensus has not been reached (18). Numerous studies have currently been conducted to compare these treatments and analyze their impact on DFU outcomes, thereby evaluating the efficacy and safety of these drugs. However, DMA mainly focuses on the effects of two different interventions, and few studies have compared the effects of multiple different treatments. Therefore, we have collected relevant published RCTs and used NMA to directly and indirectly compare the effects of different treatments on DFU outcomes, including complete healing rate, ulcer area reduction, time required for ulcer healing, amputation rate, and adverse reactions. Our aim is to provide clinicians with information on the risks and benefits of different treatment options when choosing different DFU treatment strategies.
Normal wound healing is a meticulously orchestrated process, involving a series of complex and continuous interactions between regulatory cytokines. The four main stages of wound healing are the coagulation phase, inflammation phase, proliferation phase, re-epithelialization, and remodeling phase, which result in the restoration of tissue functional integrity (90, 91). Most wounds heal within 2-4 weeks. If the healing process is interrupted and the skin structure and functional integrity cannot be restored within 3 months, it is usually referred to as a chronic or refractory ulcer (92). However, due to changes in the microenvironment caused by DM, including changes in oxygen levels, chemokines, growth factor synthesis, extracellular matrix, and oxidative stress, which alter normal cell recruitment and activation, and lead to impaired or delayed wound healing (89). Such wounds deviate from the normal process, remain unhealed for long periods, forming chronic refractory ulcers in DM, making the treatment riskier, more time-consuming, and more expensive. Currently, it is widely believed that peripheral nerve lesions and peripheral vascular lesions are the two main factors causing DFU in patients with DM (93). Secondly, the high glucose environment and inflammation disorder in diabetes patients are also major factors leading to the difficulty in healing DFU (94–96). Therefore, how to promote the healing of DFU has become a challenge.
Traditional wound care employs various enhanced dressings to promote wound healing, yet these dressings often adhere to the wound scab, altering the process and damaging new granulation tissue, thereby impeding wound healing (97). NPWT is a state-of-the-art noninvasive adjunct therapy system. It leverages VSD or VAC devices to maintain negative pressure. By connecting to a sealed dressing and tube from an open wound to a collection vessel, it removes fluid, thereby facilitating wound healing (98–100). Previous meta-analyses have indicated that NPWT treatment for DFU has a higher ulcer healing rate, shorter healing time, and lower amputation rate compared to SOC (101, 102). In a meta-analysis by Lin et al., it was observed that the healing rate in the NPWT group was significantly higher than in the SOC, while the granulation tissue formation time was significantly shorter. However, there was no statistically significant difference in the incidence of adverse events or amputations between the two groups (21). This aligns with our findings, where the results from DMA and NMA indicate that NPWT does not offer superiority in terms of complete ulcer healing or reduction in ulcer area compared to SOC. However, it does reduce the time required for ulcer healing without increasing the incidence of adverse reactions or amputations. The SUCRA ranking suggests that NPWT is superior to SOC in all aspects of the study. In addition, UD is also a non-contact wound treatment method. Both Singh and Tehrani have found that the use of UD in patients with DFU can significantly accelerate the healing process (103, 104), although its long-term complete healing response seems less favorable. In the latest meta-analysis by Chen and others, it was found that compared to SOC, the use of UD significantly increases the healing rate of wound ulcers and results in a higher percentage reduction in wound ulcers (20). In our study, it was observed that compared to SOC, the UD group showed no significant advantages in complete healing rate or reduction in ulcer area, possibly due to the insufficient number of included studies. However, in terms of adverse reactions, UD is as safe as standard treatment. Ruran and others conducted a meta-analysis comparing NPWT and UD in the treatment of DFU, and found that NPWT is similar to UD in treating DFU, but surpasses standard wound care in terms of efficacy and safety (105). In our study, we further analyzed the efficacy of UD combined with NPWT in treating DFU. The study found that compared to alone NPWT treatment, UD combined with NPWT treatment shows similar efficacy in terms of complete healing rate and reduction in ulcer area, but has an advantage in terms of shortening the healing time of ulcers and reducing adverse reactions. The SUCRA ranking results suggest that UD combined with NPWT is more effective than alone UD, NPWT, or SOC in terms of complete healing rate, reduction in ulcer area, and shortening the healing time of ulcers, while also having lower adverse reaction and amputation rates. In diabetic patients, under the state of elevated blood glucose, the peripheral nerves and blood vessels are subjected to abnormal cell proliferation, vascular endothelial cell disorder, the micro-environment change, and inflammatory response, which are the main reasons why DFU is difficult to heal (106). By using different internal and external pressures, NPWT can drain deep necrotic tissue and secretions, reduce wound infection, keep the wound moist, and promote wound healing (107). The mechanisms of NPWT at the tissue and cellular level include: promoting granulation and angiogenesis, wound boundary epithelialization, and promoting cell migration and proliferation. The large strain mechanism includes wound edge closure and clearance of infectious material exudate (108). UD can remove bacteria, fungi, and necrotic tissue through the effects of cavitation and hemostasis during high-frequency and high-energy ultrasonic jet irrigation. Compared to traditional debridement techniques, UD not only helps to control infections but also promotes ulcer surface healing through thermal and biological effects. The thermal effect is manifested by increased skin temperature and improved blood supply, which facilitates tissue repair (109), while the biological effect is observed in low-frequency ultrasonic waves that indirectly promote the release of growth factors, accelerating ulcer healing more quickly (110). Therefore, when the two are used in combination, it can not only improve the wound microenvironment, change the microvascular hemodynamics, control the wound infection, but also promote the regeneration of endothelial cells and promote faster healing of ulcers. There was no increased incidence of adverse effects, and amputation rates were lower than with conventional treatment.
In addition to the combined treatment with UD, NPWT can also be used in conjunction with PRP. PRP is a preparation rich in platelets, with a concentration higher than that of whole blood, and its platelet concentration in the plasma is 4-5 times higher than that of whole blood (111, 112). PRP can be applied alone to DFU or in combination with other treatments. In the two recent meta-analyses, Gong and Peng et al. found that PRP treatment for DFU increased the likelihood of wound healing, reduced the ulcer volume, and decreased the time required for complete wound healing (25, 26). Tasmania et al.’s meta-analysis also showed that in terms of safety, there was no difference or recurrence in the probability of wound complications between PRP and SOC, but it significantly reduced the incidence of adverse events overall (27). In addition, multiple studies have found that PRP combined with fat-derived stem cell transplantation can promote angiogenesis and increase transplantation rates (113, 114). Yin et al.’s meta-analysis showed that compared to the control group, NPWT combined with PRP had significant advantages in terms of reducing healing time, improving ulcer healing rates, and shortening hospitalization duration, but there was no significant difference in dressing change time and hospitalization costs (22). However, whether PRP treatment for DFU results in later amputation and the extent of amputation is still unclear. Our study found that both DMA and NMA results showed that when applied alone, PRP could improve the complete healing rate, reduce healing time, and decrease the amputation rate and adverse reaction rate compared to the standard treatment group, but there was no significant difference in reducing the ulcer area. Our study also found that when combined with NPWT treatment, PRP could effectively improve the complete healing rate, reduce healing time, and decrease adverse reactions compared to NPWT treatment alone, but there was no significant difference in reducing the ulcer area. The main mechanism of PRP in wound healing is through the release of various bioactive molecules stored in platelets, including PDGF, transforming growth factor β(TGF-β), VEGF, epithelial growth factor (EGF), and adhesion molecules such as fibrin, fibronectin, and hyalenin (115). These factors are known to regulate cell migration, adhesion, proliferation, and differentiation, and promote the accumulation of extracellular matrix (ECM) by binding to specific cell surface receptors, thereby playing an important role in wound healing and regeneration (115, 116). In addition to growth factors, PRP include many important proteins, such as fibrin and antibacterial proteins, which not only provides scaffolds for tissue regeneration, promotes wound contraction, blood clotting and wound closure, but also inhibits bacterial growth (117–119). Furthermore, our study revealed that compared to standard treatment, when combined with SCs, PRP had no significant statistical difference in complete healing rate as shown in DMA results, but in NMA, it showed a higher complete healing rate.
In chronic wounds, the affected tissues are oxygen-deficient, impeding the healing of ulcers. An increase in oxygen levels in wound tissues often indicates better wound healing and fewer bacterial colonizations (120). Therefore, oxygen plays a significant role in the healing of chronic wounds. HBOT involves breathing 100% oxygen at pressures two to three times higher than normal atmospheric pressure in a hyperbaric chamber, leading to an increase in oxygen tension in both arteries and tissues. It can improve local tissue oxygenation, and further research suggests that HBOT may improve new blood vessel formation, stimulate stem cells and growth factors, inhibit inflammation, and have antibacterial effects on anaerobic bacteria (121). There are different views on the effectiveness and safety of HBOT in DFU. Research by Zhao and colleagues showed no differences in terms of ulcer incidence, amputation risk, or adverse events compared to SOC (30). However, a study by Sharma and colleagues believed that HBOT had a significant effect on the complete healing of DFU, while also reducing healing time and the risk of major amputations. Additionally, this study found no differences in the reduction of ulcer area or average percentages of mortality, and the SOC had fewer adverse events (24). In our study, we found that both HBOT and TOT can improve the complete healing rate of ulcers, but there were no differences in terms of reducing the area of ulcers, amputation rates, or adverse events. The SUCRA ranking results suggest that both HBOT and TOT treatments are superior to SOC in all aspects of the study.
In addition to considering the efficacy and safety of the treatment, when we choose the treatment for diabetic foot patients in the clinic, we also need to further consider the economic benefits of the treatment for patients and whether ethical support is needed. The hospitalization cost of DFU increases with the severity of the disease. It generally depends on the extent of the ulcers and the underlying pathology that caused them in the first place, but also on the interventions used to treat them. Therefore, at the initial visit, it is important that the clinician adequately assess the patient for potential complications as well as the wound itself, and determine whether peripheral artery disease, neuropathy, or both are present. Therefore, cost-effective measures are necessary to reduce intervention costs in treating DFUs and thus decrease the economic burden associated with them (122, 123). When it comes to the use of stem cells to treat diabetic foot patients, we also need to obtain the relevant ethical approval and informed consent of the patient. In addition, DFUs affect multiple areas of a person’s functioning, including both physical and psychological distress. Therefore, it is important to pay attention to the psychosomatic lectures of diabetic patients.
Our study provides a comprehensive analysis of the current primary treatments for DFU, incorporating the latest RCTs and ranking various treatment outcomes. This ensures our results are detailed and robust, offering clinicians a reliable foundation for choosing DFU treatment options. However, our study has its limitations. Firstly, our research population varies in terms of race, background, and age, and there are fewer studies on certain observed indicators and treatment measures. This warrants a future RCTs involving a broader range of regions and populations for further analysis. Secondly, the measurement and timing errors in the observed indicators included in our study may vary across different studies, which could potentially affect clinical efficacy. Lastly, since different treatment approaches have distinct pathways, it is impossible to prevent blindness. This is the main reason for the potential biases in our study. Nevertheless, the conclusions and limitations of this study may offer some guidance for the design of new trials.
The treatment options for DFU are not singular. Research has found that combining multiple methods often yields better outcomes without increased adverse reactions. Therefore, when confronting patients with DFU, clinicians can choose one or multiple methods based on the actual condition of the ulcer, and evaluate the efficacy and risks of different treatment plans according to various scenarios. Future research requires more clinical trials to investigate the effectiveness of combined treatments for DFU.
HO: Writing – original draft, Writing – review & editing. JY: Conceptualization, Investigation, Writing – review & editing. HW: Conceptualization, Investigation, Writing – review & editing. JH: Conceptualization, Data curation, Methodology, Writing – review & editing. YY: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project is supported by the project of Sichuan Medical Association “The effect of Compound Huangbai Liquid combined with Dragon blood exhaustive application on chronic diabetic lower limb ulcer”(No. Q20052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhou B, Lu Y, Hajifathalian K. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population- based studies with 4∗4 million participants. Lancet. (2016) 387:1513–30. doi: 10.1016/S0140-6736(16)00618-8
2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
3. Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. (2013) 8:CD006810. doi: 10.1002/14651858
4. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
5. Lavery LA, Davis KE, Berriman SJ, Braun L, Nichols A, Kim PJ, et al. WHS guidelines update: diabetic foot ulcertreatment guidelines. Wound Repair Regener. (2016) 24:112–26. doi: 10.1111/wrr.12391
6. Zeng X, Tang Y, Hu K, Jiao W, Ying L, Zhu L, et al. Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: a case report. Medicine. (2017) 96:e9212. doi: 10.1097/MD.0000000000009212
7. Bus SA. The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg. (2016) 138:179S–87S. doi: 10.1097/PRS.0000000000002686
8. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers: Diabetic foot ulcers. Ann N.Y. Acad Sci. (2018) 1411:153–65. doi: 10.1111/nyas.13569
9. Dai J, Jiang C, Chen H, Chai Y. Assessment of the risk factors of multidrug-resistant organism infection in adults with type 1 or type 2 diabetes and diabetic foot ulcer. Can J Diabetes. (2020) 44(4):342–9. doi: 10.1016/j.jcjd.2019.10.009
10. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis †. Ann Med. (2017) 49:106–16. doi: 10.1080/07853890.2016.1231932
12. Alvarsson A, Sandgren B, Wendel C, Alvarsson M, Brismar K. A retrospective analysis of amputation rates in diabetic patients: can lower extremity amputations be further prevented? Cardiovasc Diabetol. (2012) 11:1–11. doi: 10.1186/1475-2840-11-18
13. Chinese society of Diabetes, Chinese Society of Infectious Diseases, Chinese society of tissue repair and regeneration. Chinese guidelines for the prevention and treatment of diabetic foot (2019 edition)(I). Chin J Diabetes. (2019) 11:92–108.
14. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabeticfoot complications are comparable to cancer. J Foot Ankle Res. (2020) 13:16. doi: 10.1186/s13047-020-00383-2
15. Jodheea-Jutton A, Hindocha S, Bhaw-Luximon A. Health economics of diabetic foot ulcer and recent trends to accelerate treatment. Foot. (2022) 52:101909. doi: 10.1016/j.foot.2022.101909
16. Riedel U, Schüßler E, Härtel D, Keiler A, Nestoris S, Stege H. Wound treatment in diabetes patients and diabetic foot ulcers. Hautarzt. (2020) 71:835e42. doi: 10.1007/s00105-020-04699-9
17. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A metaanalysis. Diabetes Care. (1999) 22:692–5. doi: 10.2337/diacare.22.5.692
18. Braun LR, Fisk WA, Lev-Tov H, Kirsner RS, Isseroff RR. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol. (2014) 15:267e281. doi: 10.1007/s40257-014-0081-9
19. Huang W, Chen Y, Wang N, Yin G, Wei C, Xu W. The efficacy and safety of acellular matrix therapy for diabetic foot ulcers: A meta-analysis of randomizedClinical trials. J Diabetes Res. (2020) 2020:6245758. doi: 10.1155/2020/6245758
20. Chen H, Yu Z, Liu N, Huang J, Liang X, Liang X, et al. The efficacy of low-frequency ultrasound as an added treatment for chronic wounds: A meta-analysis. Int Wound J. (2023) 20:448–57. doi: 10.1111/iwj.13893
21. Chen L, Zhang S, Da J, Wu W, Ma F, Tang C, et al. A systematic review and meta-analysis of efficacy and safety of negative pressure wound therapy in thetreatment of diabetic foot ulcer. Ann Palliat Med. (2021) 10:10830–9. doi: 10.21037/apm-21-2476
22. Yin X-L, Hu L, Li T, Zou Y, Li H-L. A meta-analysis on the efficacy of vacuum sealing drainage combined with autologous platelet-rich plasma in the treatment of Grade 2 and Grade 3 diabetic foot ulcers. Int Wound J. (2023) 20:1033–41. doi: 10.1111/iwj.13956
23. Elsharkawi M, Ghoneim B, O'Sullivan M, Lowery AJ, Westby D, Tawfick W, et al. Role of adipose derived stem cells in patients with diabetic foot ulcers: systematic review and meta-analysis of randomised controlled trials. Int J Low Extrem Wounds. (2023) 11:15347346231174554. doi: 10.1177/15347346231174554
24. Sharma R, Sharma SK, Mudgal SK, Jelly P, Thakur K. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcer, a systematic review and meta-analysis of controlled clinical trials. Sci Rep. (2021) 11:2189. doi: 10.1038/s41598-021-81886-1
25. Gong F, Zhang Y, Gao J. Effect of platelet-rich plasma vs standard management for the treatment of diabetic foot ulcer wounds: A meta-analysis. Int Wound J. (2023) 20:155–63. doi: 10.1111/iwj.13858
26. Peng Y, Wang J, Liu X, Zhou Y, Jia S, Xu J, et al. Efficacy of platelet-rich plasma in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. Ann Vasc Surg. (2024) 98:365–73. doi: 10.1016/j.avsg.2023.05.045
27. Pino-Sedeño TD, Trujillo-Martın MM, Andia I. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis. Wound Repair Regener. (2019) 27:170–82. doi: 10.1111/wrr.12690
28. Yammine K, Ghanimeh J, Agopian SJ. PRP versus standard of care for venous leg ulcers: A systematic review and meta-analysis of prospective comparative studies. Int J Low Extrem Wounds. (2022) 14:15347346221094424. doi: 10.1177/15347346221094424
29. Gupta A, Channaveera C, Sethi S, Ranga S, Anand V. Efficacy of intra-lesional platelet rich plasma in diabetic foot ulcer. J Am Podiatr Med Assoc. (2021) 111(3):7. doi: 10.7547/19-149
30. Zhao D, Luo S, Xu W, Hu J, Lin S, Wang N. Efficacy and safety of hyperbaric oxygen therapy used in patients with diabetic foot: a meta-analysis of randomized clinical trials. Clin Therapeut. (2017) 39:2088e2094. doi: 10.1016/j.clinthera.2017.08.014
31. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
33. Driver VR, Reyzelman A, Kawalec J, French M. A prospective, randomized, blinded, controlled trial comparing transdermal continuous oxygen delivery to moist wound therapy for the treatment of diabetic foot ulcers. Ostomy Wound Manage. (2017) 63:12–28.
34. Frykberg RG, Franks PJ, Edmonds M, Brantley JN, Téot L, Wild T, et al. A multinational, multicenter, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy of cyclical topical wound oxygen (TWO2) therapy in the treatment of chronic diabetic foot ulcers: the TWO2 study. Diabetes Care. (2020) 43:616–24. doi: 10.2337/dc19-0476
35. Niederauer MQ, Michalek JE, Armstrong DG. A prospective, randomized, double-blind multicenter study comparing continuous diffusion of oxygen therapy to sham therapy in the treatment of diabetic foot ulcers. J Diabetes Sci Technol. (2017) 11:883–91. doi: 10.1177/1932296817695574
36. Niederauer MQ, Michalek JE, Liu Q, Papas KK, Lavery LA, Armstrong DG. Continuous diffusion of oxygen improves diabetic foot ulcer healing when compared with a placebo control: a randomised, double-blind, multicentre study. J Wound Care. (2018) 27:S30–45. doi: 10.12968/jowc.2018.27
37. Yu J, Lu S, McLaren AM, Perry JA. Cross KM.Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regener. (2016) 24:1066–72. doi: 10.1111/wrr.12490
38. Chen C-Y, Wu R-W, Hsu M-C, Hsieh C-J, Chou M-C. Adjunctive hyperbaric oxygen therapy for healing of chronic diabetic foot ulcers: A randomized controlled trial. J Wound Ostomy Continence Nurs. (2017) 44:536–45. doi: 10.1097/WON.0000000000000374
39. Fedorko L, Bowen JM, Jones W, Oreopoulos G, Goeree R, Hopkins RB, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: A prospective, double-blind, randomized controlled clinical trial. Diabetes Care. (2016) 39:392–9. doi: 10.2337/dc15-2001
40. Ma L, Li P, Shi Z, Hou T, Chen X, Du J. A prospective, randomized, controlled study of hyperbaric oxygen therapy: effects on healing and oxidative stress of ulcer tissue in patients with a diabetic foot ulcer. Ostomy Wound Manage. (2013) 59:18–24.
41. Santema KTB, Stoekenbroek RM, Koelemay MJW, Reekers JA, van Dortmont LMC, Oomen A, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower- extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care. (2018) 41:112–9. doi: 10.2337/dc17-0654
42. Chaudhary P, Khandelwal S, Poddar DD, Saxena N, Singh RAK, Biswal UC. Comparative study of different treatment options of grade III and IV diabetic foot ulcers to reduce theIncidence of amputations. Clin Pract. (2013) 3:e9. doi: 10.4081/cp.2013.e9
43. Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. (2010) 33:998–1003. doi: 10.2337/dc09-1754
44. Salama SE, Eldeeb AE, Elbarbary AH, Abdelghany SE. Adjuvant hyperbaric oxygen therapy enhances healing of nonischemic diabetic foot ulcers compared with standard wound care alone. Int J Low Extrem Wounds. (2019) 18:75–80. doi: 10.1177/1534734619829939
45. Kumar A, Shukla U, Prabhakar T, Srivastava D. Hyperbaric oxygen therapy as an adjuvant to standard therapy in the treatment of diabetic foot ulcers. J Anaesthesiol Clin Pharmacol. (2020) 36:213–8. doi: 10.4103/joacp
46. Lonardi R, Leone N, Gennai S, Trevisi Borsari G, Covic T, Silingardi R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: arandomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res Ther. (2019) 10:223. doi: 10.1186/s13287-019-1328-4
47. Smith OJ, Leigh R, Kanapathy M, Macneal P, Jell G, Hachach-Haram N, et al. Fat grafting and platelet-rich plasma for the treatment of diabetic foot ulcers: A feasibility-randomised controlled trial. Int Wound J. (2020) 17:1578–94. doi: 10.1111/iwj.13433
48. Uzun E, Güney A, Gönen ZB, Özkul Y, Kafadar İH, Günay M, et al. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg. (2021) 27:636–42. doi: 10.1016/j.fas.2020.08.002
49. Han S-K, Kim H-R, Kim W-K. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regener. (2010) 18:342–8. doi: 10.1111/j.1524-475X.2010.00593.x
50. Meamar R, Ghasemi-Mobarakeh L, Norouzi M-R, Siavash M, Hamblin MR, Fesharaki M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int Immunopharmacol. (2021) 101:108282. doi: 10.1016/j.intimp.2021.108282
51. Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J. (2006) 3:181–7. doi: 10.1111/j.1742-481X.2006.00209.x
52. Driver VR, Lavery LA, Reyzelman AM, Dutra TG, Dove CR, Kotsis SV, et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regener. (2015) 23:891–900. doi: 10.1111/wrr.12357
53. Walters J, Cazzell S, Pham H, Vayser D, Reyzelman A. Healing rates in a multicenter assessment of a sterile, room temperature, acellular dermal matrix versus conventional care wound management and an active comparator in the treatment of full-thickness diabetic foot. Eplasty. (2016) 16:e10.
54. Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. (2009) 6:196–208. doi: 10.1111/j.1742-481X.2009.00585.x
55. Zelen CM, Orgill DP, Serena T, Galiano R, Carter MJ, DiDomenico LA, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. (2017) 14:307–15. doi: 10.1111/iwj.12600
56. Zelen CM, Orgill DP, Serena TE, Galiano RE, Carter MJ, DiDomenico LA, et al. An aseptically processed, acellular, reticular, allogenic human dermis improves healing in diabetic foot ulcers: A prospective, randomised, controlled, multicentre follow-up trial. Int Wound J. (2018) 15:731–9. doi: 10.1111/iwj.12920
57. Gao H, Chen J, Zhao Z, Wang G. A combination of ultrasonic debridement and topical cortex phellodendri compound fluid in patients with diabetic foot ulcers. Med (Baltimore). (2022) 101:e29604. doi: 10.1097/MD.0000000000029604
58. Rastogi A, Bhansali A, Ramachandran S. Efficacy and safety of low-frequency, noncontact airborne ultrasound therapy (Glybetac) for neuropathic diabetic foot ulcers: A randomized, double-blind, sham-control study. Int J Low Extrem Wounds. (2019) 18:81–8. doi: 10.1177/1534734619832738
59. Li Y, Zhang J, Song S. Clinical effect of ultrasonic debridement knife therapy combined with negativepressure wound therapy on patients with diabetic foot ulcers. Chongqing Med Sci. doi: 10.3969/j.issn.1671-8348.2016.35.027
60. Wang G, Wu Y. The clinical efficacy of ultrasonic cleaver combined with negative pressure wound in the treatment of diabetic foot ulcer. Foreign Med Sci Section Medgeography. (2018) 39. doi: 10.3969/j.issn.1001-8883.2018.01.015
61. Li X, Song P, Xiong Z, Pei X. Application of the ultrasonic waterjet combined with vacuum sealing drainage in diabetic foot ulcer. J Bengbu Med Coll. (2022) 47. doi: 10.13898/j.enki.issn.1000-2200.2022.08.005
62. Shukui, Diao. The effect f vacuum sealing drainage combined with ultrasonic debridement on refractory wounds of diabetic foot and inflammatory factors. Diabetes New World. doi: 10.16658/j.cnki.1672-4062.2022.09.022
63. Ji Y, Jin Y, Jin W. Effect of ultrasonic debridement machine combined with vacuum sealing drainage in diabetic foot ulcer healing. China J Modern Med. (2019) 29. doi: 10.3969/j.issn.1005-8982.2019.01.020
64. Seidel D, Storck M, Lawall H, Wozniak G, Mauckner P, Hochlenert D, et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: results of the German DiaFu-RCT. BMJ Open. (2020) 10:e026345. doi: 10.1136/bmjopen-2018-026345
65. Maranna H, Lal P, Mishra A, Bains L, Sawant G, Bhatia R, et al. Negative pressure wound therapy in grade 1 and 2 diabetic foot ulcers: A randomized controlled study. Diabetes Metab Syndr. (2021) 15:365–71. doi: 10.1016/j.dsx.2021.01.014
66. Srivastava V, Meena RN, Pratap A, Verma AK, Ansari MA, Mishra SP, et al. Effect of negative pressure wound therapy on wound thermometry in diabetic foot ulcers. J Family Med Prim Care. (2022) 11:7001–7. doi: 10.4103/jfmpc.jfmpc_72_22
67. James SMD, Sureshkumar S, Elamurugan TP, Debasis N, Vijayakumar C, Palanivel C. Comparison of vacuum-assisted closure therapy and conventional dressing on wound healing in patients withDiabetic foot ulcer: A randomized controlled trial. Niger J Surg. (2019) 25:14–20. doi: 10.4103/njs
68. Sukur E, Akar A, Uyar AÇ, Cicekli O, Kochai A, Turker M, et al. Vacuum-assisted closure versus moist dressings in the treatment of diabetic wound ulcers after partial foot amputation: A retrospective analysis in 65 patients. J Orthop Surg (Hong Kong). (2018) 26:2309499018799769. doi: 10.1177/2309499018799769
69. Sajid MT, Mustafa QA, Shaheen N, Hussain SM, Shukr I, Ahmed M, et al. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moistWound therapy in the treatment of diabetic foot ulcers. J Coll Physicians Surg Pak. (2015) 25:789–93.
70. Chen L, Liu J, Zhang Y. Clinical effect of autologous platelet-rich plasma combined with negative pressure on treatment of diabetic foot infection. Chin J Nosocomiol. (2021) 31. doi: 10.11816/cn.ni.2021-202118. Na 7.
71. Wenhua G, Jiangmin Z, Hongliang Z. Autologous platelet-rich gel combined with vacuum sealing drainage in the treatment of diabetic foot ulcers. Int J Geriatr. (2021) 42. doi: 10.3969/j.issn.1674-7593.2021.04.012
72. Xiaojing W, Jiancai L, Cuiyan Z. Therapeutic effect of autologous platelet-rich gel combined with continuous vacuum sealing drainage on diabetic foot ulcer. Nurs Pract Res. (2018) 15. doi: 10.3969/j.issn.1672-9676.2018.11.017
73. Niu R. Effect of autologous platelet rich gel combined with negative pressure drainage on insulin resistance and wound healing in diabetic foot patients. Mod Diagn Treat. (2020) 31.
74. Wang G, Sun Q. Effect of autologous platelet rich gel combined with negative pressure assisted closure technique in the treatment of diabetic foot ulcer. Chin J Clin Rational Drug Use. 14. doi: 10.15887/j.enki.13-1389/r.2021.23.069
75. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. (2017) 38:206–11. doi: 10.1016/j.avsg.2016.04.023
76. Alamdari NM, Shafiee A, Mirmohseni A, Besharat S. Evaluation of the efficacy of platelet-rich plasma on healing of clean diabetic foot ulcers: A randomized clinical trial in Tehran, Iran. Diabetes Metab Syndr. (2021) 15:621–6. doi: 10.1016/j.dsx.2021.03.005
77. Singh SP, Kumar V, Pandey A, Pandey P, Gupta V, Verma R. Role of platelet-rich plasma in healing diabetic foot ulcers: a prospective study. J Wound Care. (2018) 27:550–6. doi: 10.12968/jowc.2018.27.9.550
78. Elsaid A, El-Said M, Emile S, Youssef M, Khafagy W, Elshobaky A. Randomized controlled trial on autologous platelet-rich plasma versus saline dressing in treatment of non-healing diabetic foot ulcers. World J Surg. (2020) 44:1294–301. doi: 10.1007/s00268-019-05316-0
79. Orban YA, Soliman MA, Hegab YH, Alkilany MM. Autologous platelet-rich plasma vs conventional dressing in the management of chronic diabetic foot ulcers. Wounds. (2022) 33:36–42. doi: 10.25270/wnds/2022.3642
80. Hossam EM, Alserr AHK, Antonopoulos CN, Zaki A, Eldaly W. Autologous platelet rich plasma promotes the healing of non-ischemic diabetic foot ulcers. A randomized controlled trial. Ann Vasc Surg. (2022) 82:165–71. doi: 10.1016/j.avsg.2021.10.061
81. Xie J, Fang Y, Zhao Y, Cao D, Lv Y. Autologous platelet-rich gel for the treatment of diabetic sinus tract wounds: A clinical study. J Surg Res. (2020) 247:271–9. doi: 10.1016/j.jss.2019.09.069
82. Li L, Chen D, Wang C, Yuan N, Wang Y, He L, et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regener. (2015) 23:495–505. doi: 10.1111/wrr.12294
83. Li W, Wang QY, Bai XG, Xu J. Autologous platelet-rich gel in the treatment of diabetic foot ulcers: A retrospective study. Med (Baltimore). (2022) 101:e31701. doi: 10.1097/MD.0000000000031701
84. Ullah A, Jawaid SI, Qureshi PNAA, Siddiqui T, Nasim K, Kumar K. Effectiveness of injected platelet-rich plasma in the treatment of diabetic foot ulcer disease. Cureus. (2022) 14:e28292. doi: 10.7759/cureus.28292
85. Du L, Zeng D, Hu X, Ren X, He D. The efficacy of autologous platelet-rich gel and traditional chinese medicine in diabetic foot treatment: A parallel randomized controlled clinical trial. Ann Vasc Surg. (2022) 87:529–37. doi: 10.1016/j.avsg.2022.07.026
86. Jeong SH, Han SK, Kim WK. Treatment of diabetic foot ulcers using a blood bank platelet concentrate. Plast Reconstr Surg. (2010) 125:944–52. doi: 10.1097/PRS.0b013e3181cb6589
87. Tofigh AM, Tajik M. Comparing the standard surgical dressing with dehydrated amnion and platelet-derived growth factor dressings in the healing rate of diabetic foot ulcer: A randomized clinical trial. Diabetes Res Clin Pract. (2022) 185:109775. doi: 10.1016/j.diabres.2022.109775
88. Alves FLMT, Laporta GZ. Prevalence and factors associated with lower limb amputation in individuals with type II diabetes mellitus in a referral hospital in Fortaleza, Ceará, Brazil: A hospital-based cross-sectional study. Heliyon. (2020) 6:e04469. doi: 10.1016/j.heliyon.2020.e04469
89. Sarfo-Kantanka O, Sarfo FS, Kyei I, Agyemang C, Mbanya JC. Incidence and determinants of diabetes-related lower limb amputations in Ghana, 2010-2015- a retrospective cohort study. BMCEndocrine Disord. (2019) 19:27. doi: 10.1186/s12902-019-0353-8
90. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: A cellular perspective. Physiol Rev. (2019) 99:665–. doi: 10.1152/physrev.00067.2017
91. Shah A, Amini-Nik S. The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18051068
92. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regener. (2009) 17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x
93. Jørgensen TS, Hellsten Y, Gottlieb H, Brorson S. Assessment of diabetic foot ulcers based on pictorial material: an interobserver study. J Wound Care. (2020) 29:658–63. doi: 10.12968/jowc.2020.29.11.658
94. Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. BioMed Res Int. (2013) 2013:385641. doi: 10.1155/2013/385641
95. Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharm (Basel). (2020) 13(4):60. doi: 10.3390/ph13040060
96. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. (2013) 93:137–88. doi: 10.1152/physrev.00045.2011
97. Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. (2013) 10:CD010318. doi: 10.1002/14651858.CD010318.pub2
98. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. (2004) 114:917–22. doi: 10.1097/01.PRS.0000133168.57199.E1
99. Schwien T, Gilbert J and Lang C. Pressure ulcer prevalence and the role of negative pressure wound therapy in home health quality outcomes. Ostomy Wound Manage. (2005) 51:47–60.
100. Karatepe O, Eken I, Acet E, Unal O, Mert M, Koc B, et al. Vacuum assisted closure improves the quality of life in patients with diabetic foot. Acta Chir Belg. (2011) 111:298–302. doi: 10.1080/00015458.2011.11680757
101. Zhang J, Hu Z-C, Chen D, Guo D, Zhu J-Y, Tang B. Effectiveness and safety of negative-pressure wound therapy for diabetic foot ulcers: a meta-analysis. Plast Reconstr Surg. (2014) 134:141–51. doi: 10.1097/PRS.0000000000000275
102. Liu S, He C, Cai Y, Xing Q, Guo Y, Chen Z, et al. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis. Ther Clin Risk Manag. (2017) 13:533–44. doi: 10.2147/TCRM.S131193
103. Singh A. Usage of ultrasound in wound management: comparison between ultrasound wound debridement and sharp debridement in diabetic foot ulcers: a randomised clinical trial. Malaysia: University of Malaya, Faculty of Medicine (2006).
104. Tehrani M, Amini S, Hammanmi M. (2011). Low frequency ultrasound debridement in diabetic foot ulcer patients with osteomyelitis, in: DFCon 2011 Diabetic Foot Global Conference, Anaheim, CA.
105. Wang R, Feng Y, Di B. Comparisons of negative pressure wound therapy and ultrasonic debridement for diabetic foot ulcers: a network meta-analysis. Int J Clin Exp Med. (2015) 8:12548–56.
106. Liu Z, Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. (2018) 10:CD010318. doi: 10.1002/14651858.CD010318.pub3
107. Shu X, Shu S, Tang S, Yang L, Liu D, Li K, et al. Efficiency of stem cell based therapy in the treatment of diabetic foot ulcer: a metaanalysis. Endocr J. (2018) 65:403–13. doi: 10.1507/endocrj.EJ17-0424
108. Borys S, Hohendorff J, Frankfurter C. Negative pressure wound therapy use in diabetic foot syndrome-from mechanisms of action to clinical practice. Eur J Clin Invest. (2019) 49:e13067. doi: 10.1111/eci.13067
109. Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, doubleblind, controlled, multicenter study. Ostomy Wound Manage. (2005) 51:24–39.
110. Demir H, Yaray S, Kirnap M, Yaray K. Comparison of the effects of laser and ultrasound treatments on experimental wound healing in rats. J Rehabil Res Dev. (2004) 41:721–8. doi: 10.1682/JRRD.2003.08.0131
111. Malanga GA, Goldin M. PRP: review of the current evidence for musculoskeletal conditions. Curr Phys Med Rehab Rep. (2014) 2:1–15. doi: 10.1007/s40141-013-0039-5
112. Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg. (2006) 118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf
113. Findikcioglu F, Findikcioglu K, Yavuzer R, Lortlar N, Atabay K. Effect of intraoperative platelet-rich plasma and fibrin glue application on skin flap survival. J Craniofac Surg. (2012) 23:1513–7. doi: 10.1097/SCS.0b013e3182597ce6
114. Sönmez TT, Vinogradov A, Zor F, Kweider N, Lippross S, Liehn EA, et al. The effect of platelet rich plasma on angiogenesis in ischemic flaps in VEGFR2-luc mice. Biomaterials. (2013) 34:2674–82. doi: 10.1016/j.biomaterials.2013.01.016
115. Franchini M, Cruciani M, Mengoli C, Marano G, Pupella S, Veropalumbo E, et al. Effificacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. (2018) 16:502–13. doi: 10.2450/2018.0111-18
116. Martínez CE, Smith PC, Palma Alvarado VA. The inflfluence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front Physiol. (2015) 6:290. doi: 10.3389/fphys.2015.00290
117. Romano F, Paolino FM, Rizzo BA, Russo A, Southworth S, Serra R, et al. The use of growth factors, CD34(+) cells and fibrin for the management of chronic venous ulcers. Int Wound J. (2016) 13:1011–3. doi: 10.1111/iwj.12500
118. Houdek MT, Wyles CC, Stalboerger PG, Terzic A, Behfar A, Moran SL. Collagen and fractionated platelet-rich plasma scaffold for dermal regeneration. Plast Reconstr Surg. (2016) 137:1498–506. doi: 10.1097/PRS.0000000000002094
119. Burnouf T, Chou ML, Wu YW, Su CY, Lee LW. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/ detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion. (2013) 53:138–46. doi: 10.1111/j.1537-2995.2012.03668.x
120. Guo S, DiPietro LA. Critical review in oral biology & medicine: Factors afecting wound healing. J Dent. Res. (2010) 89:219–29.
121. Camporesi EM, Bosco G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. (2014) 41:247–52.
122. Woods TJ, Tesfay F, Speck P, Kaambwa B. Economic evaluations considering costs and outcomes of diabetic foot ulcer infections: a systematic review. PloS One. (2020) 15:e0232395. doi: 10.1371/journal.pone.0232395
Keywords: diabetic foot ulcers, ultrasonic debridement, negative pressure wound therapy, stem cells, hyperbaric oxygen therapy, topical oxygen therapy, platelet-rich plasma, acellular dermal matrix
Citation: OuYang H, Yang J, Wan H, Huang J and Yin Y (2024) Effects of different treatment measures on the efficacy of diabetic foot ulcers: a network meta-analysis. Front. Endocrinol. 15:1452192. doi: 10.3389/fendo.2024.1452192
Received: 20 June 2024; Accepted: 29 August 2024;
Published: 23 September 2024.
Edited by:
Nazarii Kobyliak, Bogomolets National Medical University, UkraineReviewed by:
Ajay Vikram Singh, Federal Institute for Risk Assessment (BfR), GermanyCopyright © 2024 OuYang, Yang, Wan, Huang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong OuYang, MTY2ODg0NjczNEBxcS5jb20=; Jing Yang, eWp3eXluZm1AMTYzLmNvbQ==; Haiyan Wan, NTQzMzY2MjNAcXEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.