
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 October 2024
Sec. Obesity
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1449374
 Mohammed Dashti1
Mohammed Dashti1 Naser M. Ali2
Naser M. Ali2 Hussain Alsaleh3
Hussain Alsaleh3 Sumi Elsa John1
Sumi Elsa John1 Rasheeba Nizam1
Rasheeba Nizam1 Fahd Al-Mulla1*
Fahd Al-Mulla1* Thangavel Alphonse Thanaraj1*
Thangavel Alphonse Thanaraj1*Background: The Kuwaiti and Qatari populations have a high prevalence of obesity, a major risk factor for various metabolic disorders. Previous studies have independently explored mitochondrial DNA (mtDNA) variations and their association with obesity in these populations. This study aims to investigate the role of mtDNA haplogroups and variants in obesity risk among these Gulf populations.
Methods: Whole exome sequencing data from 1,112 participants (348 Kuwaitis and 764 Qataris) were analyzed for mtDNA variants. Participants were classified as obese or non-obese based on body mass index (BMI). Association analyses were performed to examine the relationship between mtDNA haplogroups and obesity, adjusting for covariates such as age and sex.
Results: Haplogroup R was found to be protective against obesity, with an odds ratio (OR) of 0.69 (p = 0.045). This association remained significant after adjusting for age and sex (OR = 0.694; 95% CI: 0.482-0.997; p = 0.048). Several mtDNA variants, particularly those involved in mitochondrial energy metabolism, showed nominal associations with obesity, but these did not remain significant after correcting for multiple testing.
Conclusion: Haplogroup R consistently demonstrates a protective association against obesity in both Kuwaiti and Qatari populations, highlighting its potential as a biomarker for obesity risk in the Gulf region. However, further research with larger sample sizes is needed to validate these findings and clarify the role of mtDNA variants in obesity.
Mitochondria are membrane-bound organelles that play a critical role in the generation of the metabolic energy in cells through the process of oxidative phosphorylation. Additionally, mitochondria are key components in calcium buffering, heme biosynthesis, iron homeostasis and regulation of cellular apoptosis (1). Hence, the mitochondrial functions play an important role in cellular and metabolic health and homeostasis. Mitochondrial proteins are encoded by both the nuclear DNA and the mitochondrial DNA (mtDNA). mtDNA is a 16.6 kb circular double-stranded DNA that encodes 37 genes that are important for normal mitochondrial function (2).
Altered mitochondrial function has been implicated in multiple disorders, including dementia, epilepsy, strokes, Parkinson disease, ataxia, cardiomyopathy, coronary artery disease, chronic fatigue syndrome, primary biliary cirrhosis, and ovarian dysfunction (3). Moreover, many studies have connected mitochondrial dysfunction with metabolic diseases, including type 2 diabetes and obesity (4–7). Obesity is a chronic inflammatory disease that recognized as a major public health concern. The pivotal role of mitochondria in metabolic signaling processes, such as ATP levels, oxidative stress, ER stress and inflammation, underscores their influence on obesity (6). In vitro experiments conducted by Yin et al. (8) revealed that adipocyte mitochondrial oxidative capacity is reduced in obese compared to lean adults, suggesting that obesity is related to mitochondrial dysfunction (8). A more recent study by Bordoni et al. (9) illustrated changes in mtDNA copy number in overweight/obese group compared to those with normal-weight (9).
Pathogenic changes in either mtDNA or nuclear DNA, following patterns of autosomal dominant, autosomal recessive, X-linked, de novo, or maternal inheritance, are linked to mitochondrial disorders (47). Historically, the identification of such changes was pursued using Sanger sequencing. However, with the emergence of advanced high-throughput sequencing technologies, next-generation sequencing (NGS) has become the preferred method for examining mitochondrial variations (10–12). In recent work, we showcased the application of whole exome sequencing (WES) to evaluate entire mitochondrial haplogroups and their potential connection to obesity risk in both Kuwaiti and Qatari populations (7, 13).
Growing evidence in literature suggests association of mitochondrial DNA variants and haplogroups with obesity in various ethnic populations (2, 14–16, 48, 17). In the current study, we aim to investigate the contribution of mitochondrial variants and haplogroups to the risk of obesity in Arabs from the Gulf region which is marked by high prevalence of obesity (18). To this end, we employed WES data from Kuwait and Qatar. These nations are not only geographically and ethnically aligned but also showcase significant overlap in the genetic makeup of their population subgroups (19–23) and in their maternal lineage structures (7, 13).
The institutional Ethical Review Committee at Dasman Diabetes Institute reviewed and approved this study in accordance with the declaration of Helsinki (2008 amendments). The human whole-exome sequence data used in this study were previously published as separate studies in 7, 13, 24, 25. The original studies obtained written informed consent of participants who were recruited under protocols approved by the Institutional Review Boards of Hamad Medical Corporation and Weill Cornell Medical College in Qatar for Qatari samples.
The study cohort comprised a total of 1,112 Kuwaiti and Qatari individuals. Whole exomes of the 348 Kuwaiti participants of the cohort were sequenced on Illumina Hiseq platform (Illumina Inc. USA) using Illumina capture kits as described in (25) and available at Sequence Read Archive (accession: SUB12360411). A subset of these samples (288 individuals) was previously published in 13. Whole exomes of the 764 Qatari participants with available clinical characteristic were used in the present research. These samples (Supplementary Table 1) were previously sequenced on Illumina Hiseq platform (Illumina Inc. USA) using Agilent capture kits that captured the whole mitochondria genome (24). The combined dataset of 1,112 Kuwaiti and Qatari individuals was divided into obese and non-obese groups based on body mass index (BMI); 667 individuals BMI of ≥ 30 kg/m2 were considered obese and 445 with BMI < 30 kg/m2 were considered non-obese.
The whole exome sequencing data were aligned to the GRCh37 human genome assembly using the Burrows-Wheeler Aligner (BWA-MEM) with default parameters (26). After removing duplicated reads, mtDNA sequences (NC_012920.1), which correspond to the Revised Cambridge Reference Sequence (rCRS), were extracted using Picard tool version 2.20.2 (http://broadinstitute.github.io/picard) and SAMtools tool version 0.1.19 (27). mtDNA coverage and Genomic Variant Call Format (GVCF) were created for each sample using Genome Analysis Tool Kit (GATK) version v3.8-1-0 (28). GATK haplocaller was used to genotype a combined 287 GVCF files to identify mtDNA variants in Variant Calling Format (VCF) file. By way of using HaploGrep 2 tool (29) with phylotree build 17 (accessed on 16 January 2022), the mitochondrial haplogroup profiling mtDNA VCF files was carried out for each of the 1,112 samples. mtDNA annotation was carried out using Ensembl Variant Effect Predictor (VEP) (49) and Mitomap (30).
In this work, the power calculation was conducted based on the proportions of obese and non-obese individuals within the combined cohort from Kuwait and Qatar. We observed that 59.98% (p1 = 0.5998) of the population were obese, while 40.02% (p2 = 0.4002) were non-obese. The effect size determined for this dataset was 0.4020. Our statistical power analysis, conducted at a significance level of α=0.05, revealed a power of 99.99% (0.9999), indicating a highly reliable probability of detecting the observed variance in obesity rates between classifications.
Descriptive statistics for the demographic and anthropometric data were carried out using R software version 3.6.2 (https://www.R-project.org/). Continuous variables, such as age and BMI scores, were presented as mean ± standard deviation (SD), median, and interquartile (IQ). Chi-square test was used to assess the statistical significance of associations between the categorical parameters (sex and nationality) with obesity. Furthermore, age and BMI scores were used to test their associations with obesity using Mann-Whitney U test.
Principal component analysis (PCA) was implemented to check if there exists any hidden relationship among the samples due to covariates which could eventually lead to spurious results. PCA was conducted using the whole mtDNA variants, and the result was visualized on biplot using principal components (PC) that carry the most variation in the data. The PCA was conducted using PCAtools of the R software package.
To test for nominal association between obesity and mtDNA haplogroups, Fisher’s exact test was used. Both the odds ratio (OR) and 95% confidence intervals (C.I.) values for each haplogroup were calculated and p-value <0.05 was used as the cut-off for statistical significance. To adjust for covariates (sex, age and nationality) logistic regression was implemented using IBM® SPSS® Statistics Version 25 software. Finally, PLINK version 1.9 (31) was used to find mtDNA variants associated with obesity, and the significant cut-off value used was a two-tailed p-value <0.05.
Table 1 presents descriptive statistics for the dataset containing 1,112 Kuwaiti and Qatari individuals. Mann-Whitney U test displayed no significant difference between obese and non-obese individuals with regards to age and nationality status. However, the Chi-squared test showed a significant result for the distribution of obese and non-obese individuals with regards to gender (p-value <0.05). Both cohorts in our study, sourced from prior research (24, 25), underwent relatedness assessments based on nuclear DNA to ensure unrelated samples.
On average, the mitochondrial DNA sequence coverage for 1,112 whole exome samples was 75X. In addition, the number of mtDNA variants (SNPs and INDELs) identified was 1,850. A set of 15 mitochondrial haplogroups were identified (H, HV, J, K, L, M, N, R, T, U, W, X, B, E, and I) using HaploGrep 2 tool with average haplogroup quality scores of 93% (Table 2).
To avoid spurious results due to the presence of hidden relationships between the samples, distribution of the samples was tested using PCA based on 1,850 mitochondrial variants. The 1,112 samples clustered based on their maternal haplogroups (Figure 1A), which indicates how mtDNA is rich in ancestral information. However, as shown in Figure 1B, the samples did not cluster based on their ancestries (Kuwaiti or Qatari), or by obesity status.
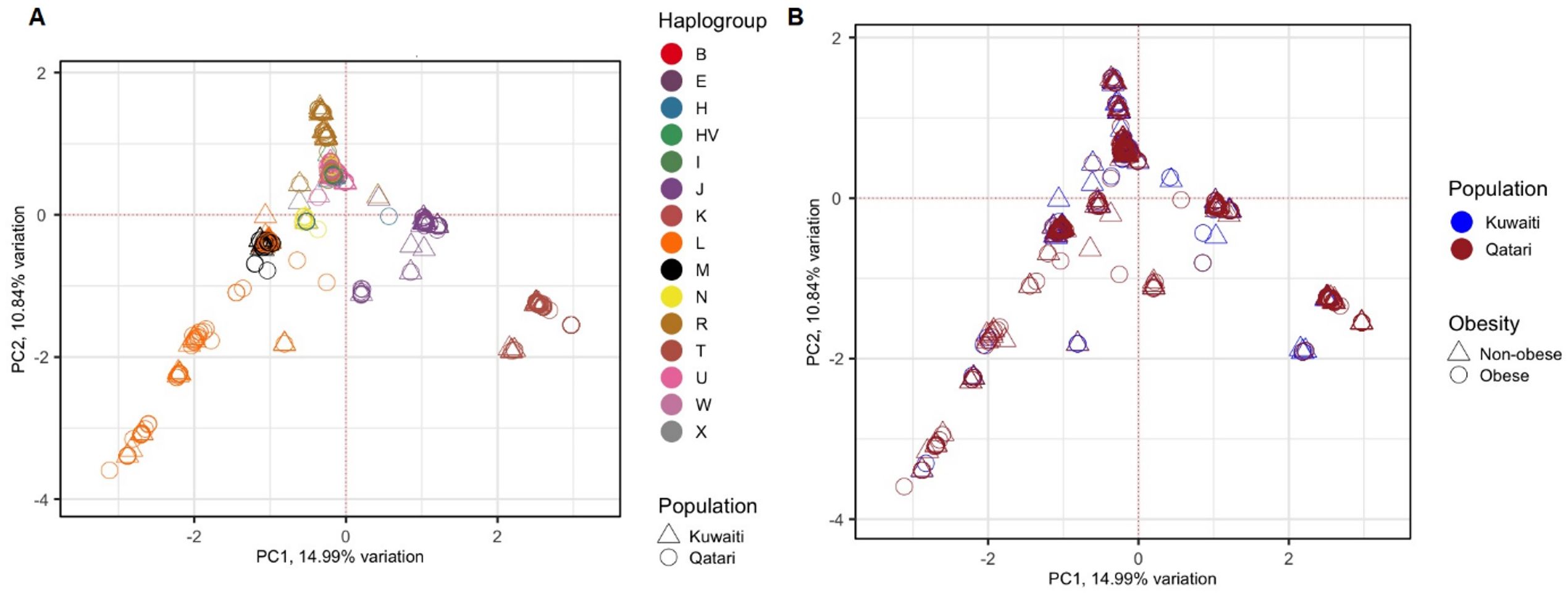
Figure 1. PCA of 1,112 Kuwaiti and Qatari samples based on their mtDNA variants. Different colours and shapes of the samples indicate different groups as shown in both plots (A, B). In both the plots, the principal components 1 (PC1) and 2 (PC2) were the first two axes of PCA and represented 14.99% and 10.84% variance, respectively. In (A), the samples were coloured based on their mitochondrial haplogroups and their ancestries. In (B), the samples were coloured based on their population ancestries and obesity status.
Frequencies of the mitochondrial haplogroups as seen in the combined and individual groups of Kuwaiti and Qatari subjects are presented in Table 2. Minor haplogroups with frequency less than 3% were grouped as “others”. The most common mitochondrial haplogroups seen within this investigation was J haplogroup, followed by U, L, R haplogroups. Results of mitochondrial haplogroup association tests (Table 2) show that individuals with R haplogroup had protective effect against developing obesity (OR = 0.69; p = 0.045). In addition, after adjusting for age and sex for individuals with R maternal haplogroup using multivariate logistic regression, the p-value remained significant (OR/95% C.I = 0.694/0.482-0.997; p = 0.048). The frequency of R haplogroup in Qatari cohort is 12.6% with the Qatari subgroups of obese and non-obese individuals having frequency values of 11.4% and 14.4%, respectively. In a similar manner, the frequency of R haplogroup in Kuwaiti cohort is 12% with the Kuwaiti subgroups of obese and non-obese individuals having frequency values of 9.2% and 15.7%, respectively. Figure 2 shows the frequency distribution of major mtDNA haplogroups across obese and non-obese groups in both Kuwaiti and Qatari populations. The R and H haplogroups are more prevalent in the non-obese groups compared to the obese groups in both populations. Moreover, no other haplogroups showed significant association trends that were consistent across both populations. Most of the individuals who carried the R haplogroup in our study belonged to the R0a subclade (Table 3).
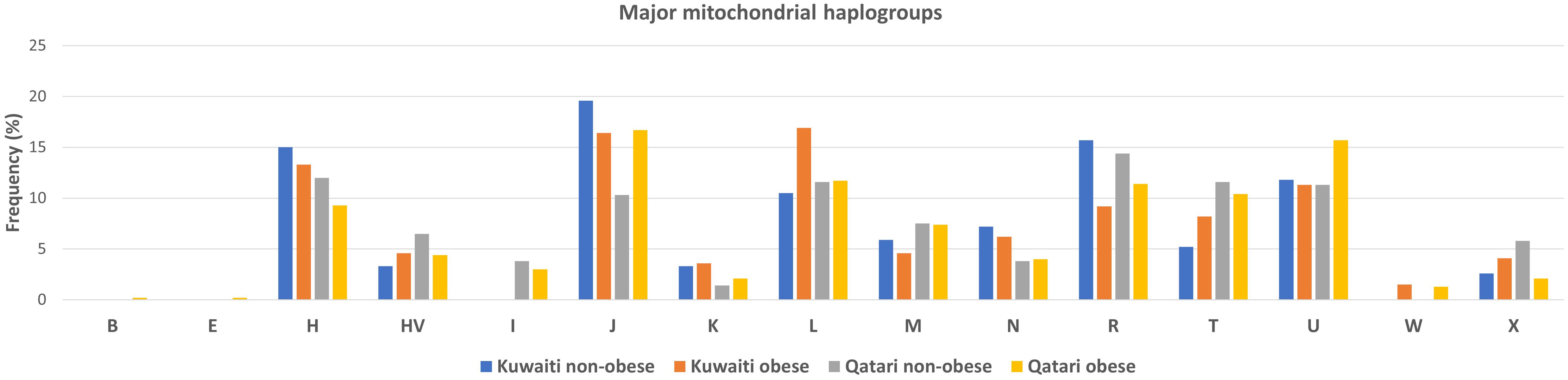
Figure 2. Frequency distribution of major mtDNA haplogroups in obese and non-obese groups for Kuwaiti and Qatari populations. The figure displays the distribution of major mtDNA haplogroups across obese and non-obese groups separately for Kuwaiti and Qatari populations, illustrating the variation in haplogroup frequencies, particularly haplogroup R, which shows a higher prevalence in non-obese groups in both populations.
Multiple logistic regression test was carried out to identify the variants associating with obesity at p-values < 0.05 (Table 4). We observed a set of 37 mtDNA variants to have nominal associations (p-value ≤ 0.05) with obesity, with models adjusted for age, sex, mitochondrial haplogroup and nationality. Table 4 shows that 17 out of 37 mtDNA variants were positively correlated with the risk of obesity, and 20 were negatively correlated with risk of obesity. All the variants exhibited variable frequencies across obese, and non-obese groups as well as R haplogroups. Conditional analysis using the top leading variants with the lowest p-value from (MT:16304T>C) (Table 4), identified 23 of the 37 variants remained significant. This suggests that these 23 variants (Table 4 footnote) have an independent effect on the phenotype with respect to the conditioned SNP. Therefore, we assume that the remaining 14 mitochondrial variants from Table 4, which lost their significance, are dependent on the top mitochondrial variant.
Furthermore, we identified DNA mitochondrial variants that were present only in obese individuals (in at least 7 individuals) but were not found in the non-obese individuals (Table 5). The identified mtDNA variants were checked against Mitomap and ClinVar databases for potential pathogenic significance relating to obesity, BMI, and body fat and were found to have no such pathogenic impact.
In our previous studies, we identified associations between specific mitochondrial haplogroups and obesity in Kuwaiti (13) and Qatari (7) populations. These studies demonstrated significant associations with obesity for different maternal haplogroups in these populations within the Arabian Gulf region. The observed variations in obesity-associated haplogroups could be due to several factors, with sample size being a primary consideration. By combining the datasets from Kuwaiti and Qatari populations, we aimed to increase the reliability of our findings and determine whether observed associations are consistent across the broader Gulf region.
An innovative aspect of our study lies in the integration of data from the Kuwaiti and Qatari cohorts. Although these are two distinct countries with their unique characteristics, they share substantial similarities in genetic and environmental factors. Combining data from these populations provides a more comprehensive analysis, enhancing statistical power and offering a broader view of the genetic landscape in the Gulf region. This approach is essential for identifying shared genetic markers and risk factors that may not be evident when analyzing each population in isolation, thereby providing a deeper understanding of obesity risk factors specific to this region.
In this study, samples from both Kuwaiti and Qatari cohorts showed a distinct clustering pattern in the PCA analysis of mitochondrial haplogroups (Figure 1). This pattern aligns with our earlier PCA analysis on DNA variants using whole exome data (23). These findings suggest that the mitochondrial DNA of Kuwaiti and Qatari populations share sufficient ethnic homogeneity, allowing us to combine the two cohorts and represent them as a unified group for Arabs in the Gulf region.
Our findings indicate that the mitochondrial haplogroup R, representing 12.41% of the total samples, is significantly more prevalent in the non-obese cohort than in the obese group (Table 2, Figure 2). The subclade R0a is observed in 80% of individuals with the maternal haplogroup R (Table 3). The frequencies of haplogroup R and subclade R0a in our study align with values previously reported in the Arabian Peninsula (32, 33). When we compared our findings on the mitochondrial haplogroup R’s association with obesity against prior studies (14, 34–36), it became evident that this significant association is distinct to Arabs in the Gulf region. We had earlier shown that haplogroup R offers a protective effect against obesity among Kuwaiti individuals (13). A pivotal mutation for the R0a clade is MT:16362T > C (37). This mutation is more prevalent in the non-obese group and is linked to a protective effect against obesity, as determined by multivariate logistic regression analyses of mitochondrial DNA variants, adjusted for age, sex, maternal haplogroup, and nationality. Furthermore, many variants with a strong negative correlation to obesity are infrequent in the R haplogroup. Our study’s most prominent mitochondrial variant was MT:16304T>C, a defining mutation for one of the H sub-haplogroups (38). It’s noteworthy that Haplogroup H was found to significantly reduce the risk of obesity in Kuwait (16). Although haplogroup H is more common in the non-obese group, its significance might be obscured due to variations in study design, such as the BMI criteria used to determine obesity and the sequencing of only the D-loop region (16) rather than the entire genome. Additionally, obesity-associated maternal haplogroups J in Qatar (7) and L in Kuwait (13) were observed at similar frequencies in both obese and non-obese groups in the combined dataset, suggesting that their effects may be influenced by unique genetic, environmental, or lifestyle factors specific to each country. In a combined analysis, these population-specific effects may become diluted, given the broader genetic and environmental landscape. In contrast, haplogroup R, previously identified as protective against obesity in the Kuwaiti study (13), remains significant in our combined analysis, suggesting a consistent effect across Gulf Arab populations. The observed effect size (OR = 0.69) represents a 31% lower risk of obesity, which is meaningful given the complex interplay of genetic and environmental factors in obesity. Even modest protective effects can have substantial public health implications at a population level. However, this association may vary with larger or more diverse samples; thus, further studies are needed to confirm its stability and explore potential modifying factors.
Given its protective association with obesity, haplogroup R has the potential to be developed as a genetic biomarker for assessing obesity risk in the Gulf region. This could support targeted prevention strategies and personalized interventions, such as customized lifestyle modifications, based on an individual’s genetic profile. Further research is needed to explore the clinical utility of haplogroup R in personalized obesity management.
Significant differences in mitochondrial DNA variants between obese and non-obese groups predominantly reside within the complex I nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit genes (MT-ND1, MT-ND2, MT-ND3, MT-ND4, MT-ND4L, MT-ND5, and MT-ND6). NADH dehydrogenase plays a critical role in cell energy production. Thus, variants in its encoding genes, especially MT-ND4:11947A>G found exclusively in obese individuals (Table 5), might sway cell energy metabolism, impacting body fat mass, BMI, and obesity risk (34, 39). The oxidative phosphorylation (OXPHOS) system, crucial for cell energy and survival, is influenced by the mitochondrial encoded cytochrome c oxidase I, II (MT-CO1 and MT-CO2) genes, previously linked to obesity (40, 41). In our study, several variants associated with obesity were identified within MT-CO1 and MT-CO2. In the mitochondrial ATP synthase membrane subunit 6 (MT-ATP6) gene, essential for OXPHOS, two exonic variants (MT:8684C>T and MT:8664A>G) were found to associate positively with obesity risk, and one exonic variant (MT:8994G>A) was unique to the obese group. Additionally, two variants (MT:750G>A and MT:153A>G) were found to positively correlate with obesity risk, and one variant (MT:1243T>C) was exclusively found in the obese group within the MT-RNR1 gene, which encodes the MOTS-C protein known for promoting metabolic homeostasis and obesity reduction (42). Lastly, three significant exonic variants and one unique to the obese group were identified in the cytochrome b (MT-CYB) gene; aberrations in this gene have been associated with exercise intolerance (43).
Most of the mitochondrial variants associated with obesity in our study are non-coding and synonymous variants. This could be explained through an epistasis mechanism. Specifically, the combined influences of mitochondrial DNA polymorphisms and nuclear DNA genetic variants might jointly affect overall metabolism, fat storage, diet, and physical health (44, 45).
Our combined analysis identified several mitochondrial DNA variants that remained significant in both the current study and previous studies on Kuwaiti and Qatari populations, including nine variants from Qatar (7) and three from Kuwait (13) with consistent directions of association with obesity. This consistency suggests these variants may play a stable role in influencing obesity risk across Gulf Arab populations. It could also be partially attributed to the changes in sample composition between studies, where the Kuwaiti sample size increased and the Qatari sample size decreased, potentially stabilizing previously observed associations. Additionally, new significant variants found only in the combined analysis may reflect the enhanced statistical power from a larger, integrated dataset, allowing for the detection of both common and population-specific genetic effects on obesity.
The current study has several limitations. First, although the associations between mitochondrial haplogroups (such as the protective effect of haplogroup R) and mitochondrial DNA variants with obesity remained significant after adjusting for covariates, none of these associations passed the Bonferroni correction for multiple testing. This raises the possibility of Type I errors, suggesting that the observed associations, while potentially indicative of genetic influences on obesity, should be interpreted with caution. The p-value for haplogroup R (p = 0.048) is close to the conventional threshold of 0.05, underscoring the need for careful interpretation as results near this threshold can be highly sensitive to variations in study design, sample size, and confounding factors. Therefore, although our findings replicate the protective effect of haplogroup R previously reported in the Kuwaiti cohort (13), further validation in larger, independent cohorts is needed to confirm these results.
Another significant limitation is the lack of comprehensive clinical data to adjust for various health-related confounders. Obesity is a complex disorder influenced by genetic, environmental, and lifestyle factors. The absence of data on lifestyle factors, such as dietary habits, physical activity, and socioeconomic status, may confound the observed genetic associations. For instance, dietary patterns and physical activity levels can significantly affect obesity risk and vary between populations, potentially influencing the observed effects of specific haplogroups. Socioeconomic status, which impacts access to healthy foods, healthcare, and health-related education, is also a crucial determinant of obesity. Including such data would allow for more nuanced insights into the genetic contributions to obesity.
Furthermore, there is no optimized statistical tool available to address the burden of multiple testing in the analysis of mitochondrial variants for complex disorders, although tools exist for rare variant association analysis in exome data (46). While our study’s sample size is comparable to those in previous studies (14, 34–36), increasing the sample size remains essential. A larger sample would help validate the protective effect of haplogroup R, increase the power to detect true associations, and reduce the risk of Type I errors associated with multiple testing. Future research should include a broader range of Arab populations to enhance the robustness and generalizability of these findings.
We aimed to ensure the data’s ethnic and geographical homogeneity to reduce the risk of population stratification. However, potential stratification issues may still exist, as evidenced by the exclusion of minor haplogroups and the need for adjustments for ethnicity. Finally, our findings have not been validated in a larger, independent cohort from the Arabian Gulf, which would provide additional insight into the genetic basis of obesity in this population. Future studies should focus on expanding the cohort size and including more diverse samples to validate these associations and explore their broader applicability.
In conclusion, we have undertaken one of the most comprehensive studies examining the association between mitochondrial haplogroups and variants with obesity in the Kuwaiti and Qatari populations, representing Arabs in the Gulf region. Our findings suggest that individuals carrying the maternal R haplogroup in this region have a decreased risk of developing obesity. Notably, while previous studies have reported associations of obesity with the maternal haplogroup J in Qataris and L haplogroup in Kuwaitis, our combined dataset did not replicate these associations despite the frequencies of these haplogroups being consistent across populations. Moreover, we identified several mitochondrial variants within genes crucial for cellular energy production that show either a protective or risk-enhancing relationship with obesity. This would not only confirm the associations observed in our study but also allow for a more nuanced understanding through sub-haplogroup analyses, potentially unveiling specific genetic factors contributing to obesity in this region.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by The institutional Ethical Review Committee at Dasman Diabetes Institute. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NA: Writing – original draft. HA: Visualization, Writing – original draft. SJ: Data curation, Writing – original draft. RN: Data curation, Methodology, Writing – original draft. FA: Writing – original draft, Writing – review & editing. TT: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Kuwait Foundation for Advancement in Sciences (KFAS).
We gratefully acknowledge the Kuwait Foundation for Advancement in Sciences for the institutional funding and Juan L. Rodriguez-Flores of Weill Cornell Medicine for providing clinical information.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1449374/full#supplementary-material
1. van der Giezen M, Tovar J, Clark CG. Mitochondrion-derived organelles in protists and fungi. Int Rev Cytology - Survey Cell Biol. (2005) 244:175–225. doi: 10.1016/S0074-7696(05)44005-X
2. Knoll N, Jarick I, Volckmar A.-L, Klingenspor M, Illig T, Grallert H, et al. Mitochondrial DNA variants in obesity. PloS One. (2014) 9:e94882. doi: 10.1371/journal.pone.0094882
3. Das M, Sauceda C, Webster NJG. Mitochondrial dysfunction in obesity and reproduction. Endocrinology. (2021) 162(1):bqaa158. doi: 10.1210/endocr/bqaa158
4. Zorzano A, Liesa M, Palacin M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. (2009) 41:1846–54. doi: 10.1016/j.biocel.2009.02.004
5. Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. (2015) 64:3135–45. doi: 10.2337/db14-1937
6. de Mello AH, Costa AB, Giustina Engel JD, Rezin GT. Mitochondrial dysfunction in obesity. Life Sci. (2018) 192:26–32. doi: 10.1016/j.lfs.2017.11.019
7. Dashti M, Alsaleh H, Rodriguez-Flores JL, Eaaswarkhanth M, Al-Mulla F, Thanaraj TA. Mitochondrial haplogroup J associated with higher risk of obesity in the Qatari population. Sci Rep. (2021) 11(1):1091. doi: 10.1038/s41598-020-80040-7
8. Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab. (2014) 99:E209–16. doi: 10.1210/jc.2013-3042
9. Bordoni L, Petracci I, Mlodzik-Czyzewska M, Malinowska AM, Szwengiel A, Sadowski M, et al. Mitochondrial DNA and epigenetics: investigating interactions with the one-carbon metabolism in obesity. Oxid Med Cell Longevity. (2022) 2022:9171684. doi: 10.1155/2022/9171684
10. Pronicka E, Piekutowska-Abramczuk D, Ciara E, Trubicka J, Rokicki D, Karkucinska-Wieckowska A, et al. New perspective in diagnostics of mitochondrial disorders: two years’ experience with whole-exome sequencing at a national paediatric centre. J Trans Med. (2016) 14:174. doi: 10.1186/s12967-016-0930-9
11. Puusepp S, Reinson K, Pajusalu S, Murumets U, Oiglane-Shlik E, Rein R, et al. Effectiveness of whole exome sequencing in unsolved patients with a clinical suspicion of a mitochondrial disorder in Estonia. Mol Genet Metab Rep. (2018) 15:80–9. doi: 10.1016/j.ymgmr.2018.03.004
12. Theunissen TEJ, Nguyen M, Kamps R, Hendrickx AT, Sallevelt SCEH, Gottschalk RWH, et al. Whole exome sequencing is the preferred strategy to identify the genetic defect in patients with a probable or possible mitochondrial cause. Front Genet. (2018) 9. doi: 10.3389/fgene.2018.00400
13. Dashti M, Alsaleh H, Eaaswarkhanth M, John SE, Nizam R, Melhem M, et al. Delineation of mitochondrial DNA variants from exome sequencing data and association of haplogroups with obesity in Kuwait. Front Genet. (2021) 12:626260. doi: 10.3389/fgene.2021.626260
14. Nardelli C, Labruna G, Liguori R, Mazzaccara C, Ferrigno M, Capobianco V, et al. Haplogroup T is an obesity risk factor: mitochondrial DNA haplotyping in a morbid obese population from southern Italy. BioMed Res Int. (2013) 2013:631082. doi: 10.1155/2013/631082
15. Chalkia D, Chang Y-C, Derbeneva O, Lvova M, Wang P, Mishmar D, et al. Mitochondrial DNA associations with East Asian metabolic syndrome. Biochim Et Biophys Acta-Bioenergetics. (2018) 1859:878–92. doi: 10.1016/j.bbabio.2018.07.002
16. Eaaswarkhanth M, Melhem M, Sharma P, Nizam R, Al Madhoun A, Chaubey G, et al. Mitochondrial DNA D-loop sequencing reveals obesity variants in an Arab population. Appl Clin Genet. (2019) 12:63–70. doi: 10.2147/tacg.s198593
17. Ludwig-Słomczyńska AH, Rehm M. Mitochondrial genome variations, mitochondrial-nuclear compatibility, and their association with metabolic diseases. Obesity. (2022) 30:1156–69. doi: 10.1002/oby.23424
18. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/s0140-6736(14)60460-8
19. Hunter-Zinck H, Musharoff S, Salit J, Al-Ali KA, Chouchane L, Gohar A, et al. Population genetic structure of the people of Qatar. Am J Hum Genet. (2010) 87:17–25. doi: 10.1016/j.ajhg.2010.05.018
20. Rodriguez-Flores JL, Fakhro K, Hackett NR, Salit J, Fuller J, Agosto-Perez F, et al. Exome sequencing identifies potential risk variants for mendelian disorders at high prevalence in Qatar. Hum Mutat. (2014) 35:105–16. doi: 10.1002/humu.22460
21. John SE, Thareja G, Hebbar P, Behbehani K, Thanaraj TA, Alsmadi O. Kuwaiti population subgroup of nomadic Bedouin ancestry-Whole genome sequence and analysis. Genomics Data. (2015) 3:116–27. doi: 10.1016/j.gdata.2014.11.016
22. Rodriguez-Flores JL, Fakhro K, Agosto-Perez F, Ramstetter MD, Arbiza L, Vincent TL, et al. Indigenous Arabs are descendants of the earliest split from ancient Eurasian populations. Genome Res. (2016) 26:151–62. doi: 10.1101/gr.191478.115
23. Dashti M, Ateyah K, Alroughani R, Al-Temaimi R. Replication analysis of variants associated with multiple sclerosis risk. Sci Rep. (2020) 10(1):7327. doi: 10.1038/s41598-020-64432-3
24. Fakhro KA, Staudt MR, Ramstetter MD, Robay A, Malek JA, Badii R, et al. The Qatar genome: a population-specific tool for precision medicine in the Middle East. Hum Genome variation. (2016) 3:16016–6. doi: 10.1038/hgv.2016.16
25. John SE, Antony D, Eaaswarkhanth M, Hebbar P, Channanath AM, Thomas D, et al. Assessment of coding region variants in Kuwaiti population: implications for medical genetics and population genomics. Sci Rep. (2018) 8:16583. doi: 10.1038/s41598-018-34815-8
26. Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv. (2013) 3:13033997. doi: 10.48550/arXiv.1303.3997
27. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. (2009) 25:2078–9. doi: 10.1093/bioinformatics/btp352
28. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. (2010) 20:1297–303. doi: 10.1101/gr.107524.110
29. Weissensteiner H, Pacher D, Kloss-Brandstaetter A, Forer L, Specht G, Bandelt H-J, et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. (2016) 44:W58–63. doi: 10.1093/nar/gkw233
30. Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, et al. mtDNA variation and analysis using mitomap and mitomaster. Curr Protoc Bioinf. (2013) 44:1.23.21–26. doi: 10.1002/0471250953.bi0123s44
31. Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. (2015) 4:7. doi: 10.1186/s13742-015-0047-8
32. Cerny V, Mulligan CJ, Fernandes V, Silva NM, Alshamali F, Non A, et al. Internal diversification of mitochondrial haplogroup R0a reveals post-last glacial maximum demographic expansions in south Arabia. Mol Biol Evol. (2011) 28:71–8. doi: 10.1093/molbev/msq178
33. Scheible M, Alenizi M, Sturk-Andreaggi K, Coble MD, Ismael S, Irwin JA. Mitochondrial DNA control region variation in a Kuwaiti population sample. Forensic Sci International-Genetics. (2011) 5:E112–3. doi: 10.1016/j.fsigen.2011.04.001
34. Yang T-L, Guo Y, Shen H, Lei S-F, Liu Y-J, Li J, et al. Genetic association study of common mitochondrial variants on body fat mass. PloS One. (2011) 6(6):e21595. doi: 10.1371/journal.pone.0021595
35. Grant SFA, Glessner JT, Bradfield JP, Zhao J, Tirone JE, Berkowitz RI, et al. Lack of relationship between mitochondrial heteroplasmy or variation and childhood obesity. Int J Obes. (2012) 36:80–3. doi: 10.1038/ijo.2011.206
36. Ebner S, Mangge H, Langhof H, Halle M, Siegrist M, Aigner E, et al. Mitochondrial haplogroup T is associated with obesity in Austrian juveniles and adults. PloS One. (2015) 10(8):e0135622. doi: 10.1371/journal.pone.0135622
37. Abu-Amero KK, Gonzalez AM, Larruga JM, Bosley TM, Cabrera VM. Eurasian and African mitochondrial DNA influences in the Saudi Arabian population. BMC Evolutionary Biol. (2007) 7. doi: 10.1186/1471-2148-7-32
38. Ennafaa H, Cabrera VM, Abu-Amero KK, Gonzalez AM, Amor MB, Bouhaha R, et al. Mitochondrial DNA haplogroup H structure in North Africa. BMC Genet. (2009) 10:8. doi: 10.1186/1471-2156-10-8
39. Flaquer A, Baumbach C, Kriebel J, Meitinger T, Peters A, Waldenberger M, et al. Mitochondrial genetic variants identified to be associated with BMI in adults. PloS One. (2014) 9:e105116. doi: 10.1371/journal.pone.0105116
40. Liu C, Yang Q, Hwang S-J, Sun F, Johnson AD, Shirihai OS, et al. Association of genetic variation in the mitochondrial genome with blood pressure and metabolic traits. Hypertension. (2012) 60:949. doi: 10.1161/HYPERTENSIONAHA.112.196519
41. Kraja AT, Liu C, Fetterman JL, Graff M, Have CT, Gu C, et al. Associations of mitochondrial and nuclear mitochondrial variants and genes with seven metabolic traits. Am J Hum Genet. (2019) 104:112–38. doi: 10.1016/j.ajhg.2018.12.001
42. Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. (2015) 21:443–54. doi: 10.1016/j.cmet.2015.02.009
43. Carossa V, Ghelli A, Tropeano CV, Valentino ML, Iommarini L, Maresca A, et al. A novel in-frame 18-bp microdeletion in MT-CYB causes a multisystem disorder with prominent exercise intolerance. Hum Mutat. (2014) 35:954–8. doi: 10.1002/humu.2014.35.issue-8
44. Dunham-Snary KJ, Sandel MW, Sammy MJ, Westbrook DG, Xiao R, McMonigle RJ, et al. Mitochondrial - nuclear genetic interaction modulates whole body metabolism, adiposity and gene expression in vivo. Ebiomedicine. (2018) 36:316–28. doi: 10.1016/j.ebiom.2018.08.036
45. Towarnicki SG, Ballard JWO. Mitotype interacts with diet to influence longevity, fitness, and mitochondrial functions in adult female drosophila. Front Genet. (2018) 9. doi: 10.3389/fgene.2018.00593
46. Lee S, Abecasis GR, Boehnke M, Lin X. Rare-variant association analysis: study designs and statistical tests. Am J Hum Genet. (2014) 95:5–23. doi: 10.1016/j.ajhg.2014.06.009
47. Schon KR, Horvath R, Wei W, Calabrese C, Tucci A, Ibañez K, et al. Use of whole genome sequencing to determine genetic basis of suspected mitochondrial disorders: cohort study. BMJ. (2021) 375:e066288. doi: 10.1136/bmj-2021-066288
48. Skuratovskaia D, Litvinova L, Vulf M, Zatolokin P, Popadin K, Mazunin I. From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index. Cells (2019) 8(5):430. doi: 10.3390/cells8050430
Keywords: obesity, mitochondrial haplogroups, mtDNA mutations, Arabs, Kuwait, Qatar
Citation: Dashti M, Ali NM, Alsaleh H, John SE, Nizam R, Al-Mulla F and Thanaraj TA (2024) Mitochondrial haplogroup R offers protection against obesity in Kuwaiti and Qatari populations. Front. Endocrinol. 15:1449374. doi: 10.3389/fendo.2024.1449374
Received: 14 June 2024; Accepted: 19 September 2024;
Published: 11 October 2024.
Edited by:
Maroof Alam, University of Michigan, United StatesReviewed by:
Nihal Medatwal, Stony Brook University, United StatesCopyright © 2024 Dashti, Ali, Alsaleh, John, Nizam, Al-Mulla and Thanaraj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahd Al-Mulla, ZmFoZC5hbG11bGxhQGRhc21hbmluc3RpdHV0ZS5vcmc=; Thangavel Alphonse Thanaraj, YWxwaG9uc2UudGhhbmdhdmVsQGRhc21hbmluc3RpdHV0ZS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.