- 1Department of Endocrinology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Cardiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
Background: Insulin resistance (IR) is closely correlated with a deficiency or decrease of testosterone levels in males. Cardiometabolic index (CMI) is correlated with various diseases correlated with IR. The primary objective of this study is to explore the correlation between CMI and testosterone levels in male adults.
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) during the period from 2013 to 2020 were analyzed through a cross-sectional design. CMI was calculated by multiplying waist-to-height ratio (WHtR) with the triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-C).
Results: A total of 5012 subjects were included in the final analysis. After controlling confounding variables, multiple linear regression analysis indicated an independent negative correlation between CMI and testosterone levels (β= -6.40, 95% CI: -8.95, -3.86, P<0.001) through the. In addition, a negative non-linear correlation was also found between CMI and testosterone (P<0.05), with CMI’s inflection point as 0.73. Subgroup analyses indicated a more significant negative correlation among those with normal weight and the elderly (p< 0.05 for all interactions). The area under the ROC curve (AUC) of CMI (AUC =0.724, 95% CI: 0.709–0.740) was the largest compared with those of TG/HDL and WHtR.
Conclusion: Elevated CMI is significantly and negatively correlated with testosterone in male adults.
Introduction
As a crucial male sex hormone produced by Leydig cells, testosterone plays a vital role in various physiological processes in males, such as cardiovascular health, sexual function, bone density, metabolism and cognitive function (1–3). The deficiency or decrease of serum testosterone levels in males can lead to the dysfunction in various organs. And low testosterone level may also contribute to or exacerbate metabolic conditions such as osteoporosis and depression, known as male hypogonadism (4) or testosterone deficiency syndrome (5–8), alongside diminished erectile dysfunction and libido. Testosterone deficiency is a prevalent disorder affecting approximately 7% of males aged over 50 years old, with its prevalence rising with age. It is projected that the prevalence of this condition will escalate with the increasing average lifespan in the coming decades (9). The prevalence of testosterone deficiency has emerged as a growing concern global.
IR is recognized as a key contributing factor in the pathogenesis of cardiometabolic diseases, with hypogonadism being frequently observed in individuals with metabolic comorbidities such as diabetes mellitus and obesity (10). Multiple studies have underscored the strong correlation between testosterone deficiency and IR (10–14). Souteiro et al. found that IR is the predominant risk factor for low testosterone levels in males with obesity, and some individuals experiencing testosterone deficiency demonstrating a higher IR compared to those with mild diabetes mellitus (11). Furthermore, a deficiency or decrease in testosterone has been correlated with the onset of metabolic disorders, such as elevated visceral lipids and IR (15). Additionally, the reciprocal correlation between hypogonadism and metabolic disorders has been confirmed in instances of late-onset and functional hypogonadism (16). Consequently, the investigation on the correlation between IR and male testosterone has become a prominent field for studying.
The intricacy, protracted process, and restricted utility of conventional IR assessments, such as HOMA-IR, have necessitated the exploration of alternative methodologies. The scholar works have been extensively examined the correlation between dyslipidemia, obesity and IR (17–19). Specifically, WHtR and TG/HDL-C has emerged as a valuable indicator for effectively evaluating IR. Derived from the multiplication of WHtR by TG/HDL-C, CMI was first introduced by Ichiro Wakabayashi in 2015 as a mean of evaluating the susceptibility to cardiometabolic disorders, T2DM, and IR (20–22). Currently, there are few studies exploring the potential correlation between CMI and testosterone.
Therefore, this study aims to explore the correlation between CMI and testosterone in male adults through a nationally representative sample on American adults, as well as assessing the predictive utility of CMI in detecting testosterone deficiency.
Methods
Subjects
For a cross-sectional study, data were obtained from NHANES, a research program sponsored by the Centers for Disease Control and Prevention (CDC) to assess the health conditions of American citizens during the period from 2013 to 2020. Due to the global outbreak of COVID-19, NHANES has been suspended from 2020 and beyond. Additionally, the samples were highly representative of the national population of US as NHANES applied a stratified multi-stage sampling method. All the data adopted from NHANES could be obtained at https://www.cdc.gov/nchs/nhanes/.
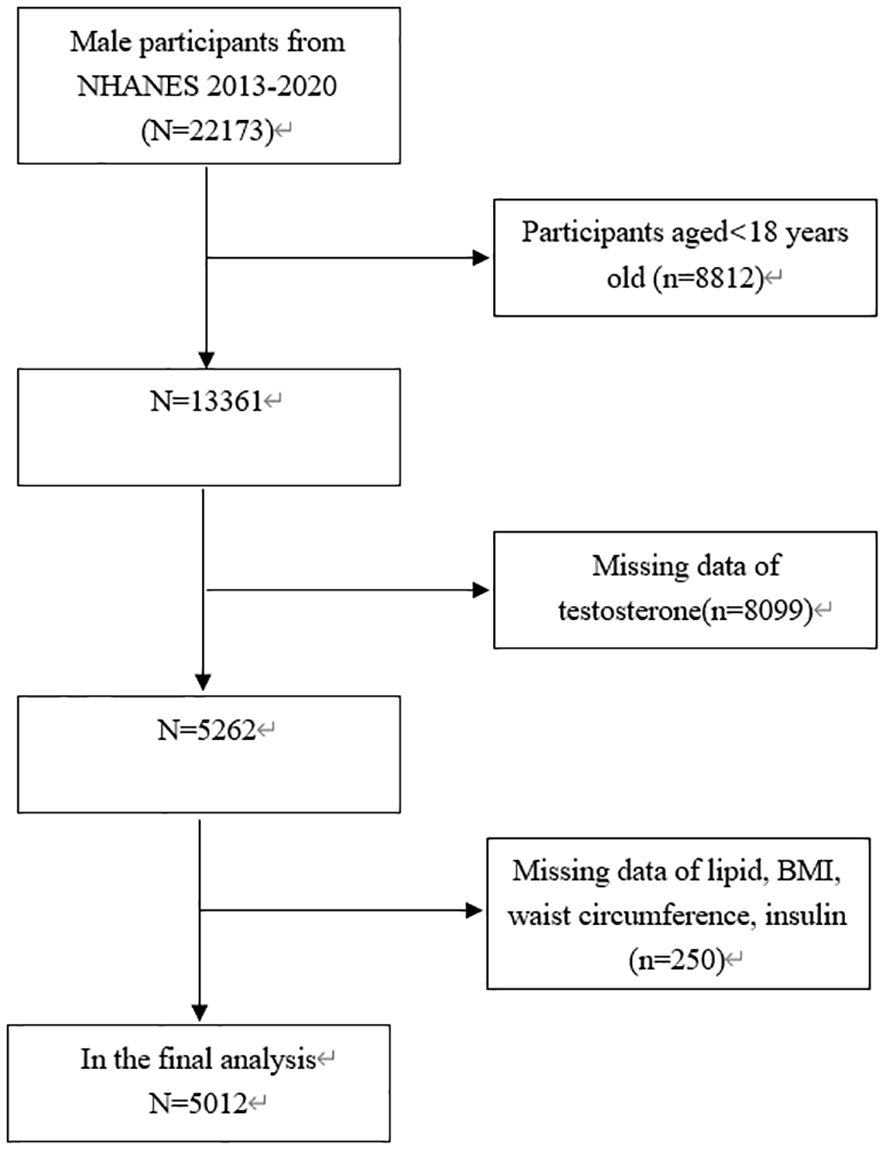
This study focused exclusively on males aged 18 years old and above (n =22173). In addition, 17161 subjects were eliminated due to missing data on testosterone (n = 8099) or BMI, lipid, waist circumference and insulin data (n = 250). Consequently, 5012 subjects aged from 18 to 80 years old were involved in the final analysis (Figure 1).
NHANES was approved from NCHS Ethics Review Board for its implementation, and written informed consent has been received from all subjects (23).
Anthropometric measurements
Demographic and lifestyle information was collected via household interview questionnaires administered by highly trained medical personnel. Anthropometric indices and biochemical parameters were acquired through medical examinations and subsequent laboratory analyses conducted at the Mobile Examination Centre (MEC). Data collected at enrollment included the information on history of diabetes mellitus, education level, physical activities, race, height, weight, blood pressure and waist circumference (WC), dietary intake factors, encompassing energy and fat. All the participants completed 24-hour dietary recalls on the mean consumption rates derived from these two recalls, obesity as BMI≥30kg/m2, and normal weight or overweight as BMI<30kg/m2.
TC, FINS, HbA1c, LDL-C, ALT, UA, TG, estradiol (E2), AST, creatinine, albumin, sex hormone binding globulin (SHBG), HDL-C, testosterone and FPG were collected with blood samples. Less than 3% of values were missed in total. Multiple imputation was performed for missing values. Detailed measurement methodology and data acquisition for each variable can be accessed at www.cdc.gov/nchs/nhanes. As defined in the American Urological Association Guidelines, testosterone deficiency is characterized as total testosterone levels below 300 ng/dL (24).
IR was assessed with HOMA-IR formula, calculated by multiplying FPG in mmol/L by FINS in IU/L, and then dividing by 22.5 (25). The formula for calculating CMI was expressed as [WC in cm/height in cm] *[TG in mmol/L/HDL-C in mmol/L] (20).
Statistical analysis
Subjects were categorized into quartiles based on CMI levels (Q1: ≤0.373; Q2: 0.373-0.699; Q3: 0.699-1.347; Q4: ≥1.347). The normality of continuous variables were assessed by presenting them as either median and interquartile range or mean ± SD in this study. Disparities among quartile groups were evaluated through Kruskal-Wallis H test for continuous variables and chi-square tests for categorical variables. The correlation between CMI and metabolic risk factors was examined through Spearman’s correlation analysis. And the correlation between CMI and testosterone was explored through a regression model analysis, with β values and 95% CI as indicators. Meanwhile, three models were utilized in the study: Model I without any adjustment, Model II with adjustments for race and age, and Model III with adjustments for DBP, age, SHBG, race, SBP, diabetes mellitus, E2, HOMA-IR, AST, moderate physical activities, HbA1c, serum uric, ALT, serum creatinine, albumin, education level, total energy and total fat intake. Subgroup analyses were conducted to stratify patients according to diabetes mellitus status, age, education level, race, moderate physical activities and BMI. Additionally, generalized additive models and smooth curve fitting were utilized to explore potential nonlinear correlation between CMI and testosterone. The diagnostic utility of CMI, TG/HDL-C, and WHtR in detecting testosterone deficiency was evaluated through ROC curve analysis. Statistical analyses were performed with EmpowerStats software and R, with statistical significance at p< 0.05.
Results
Baseline characteristics of subjects
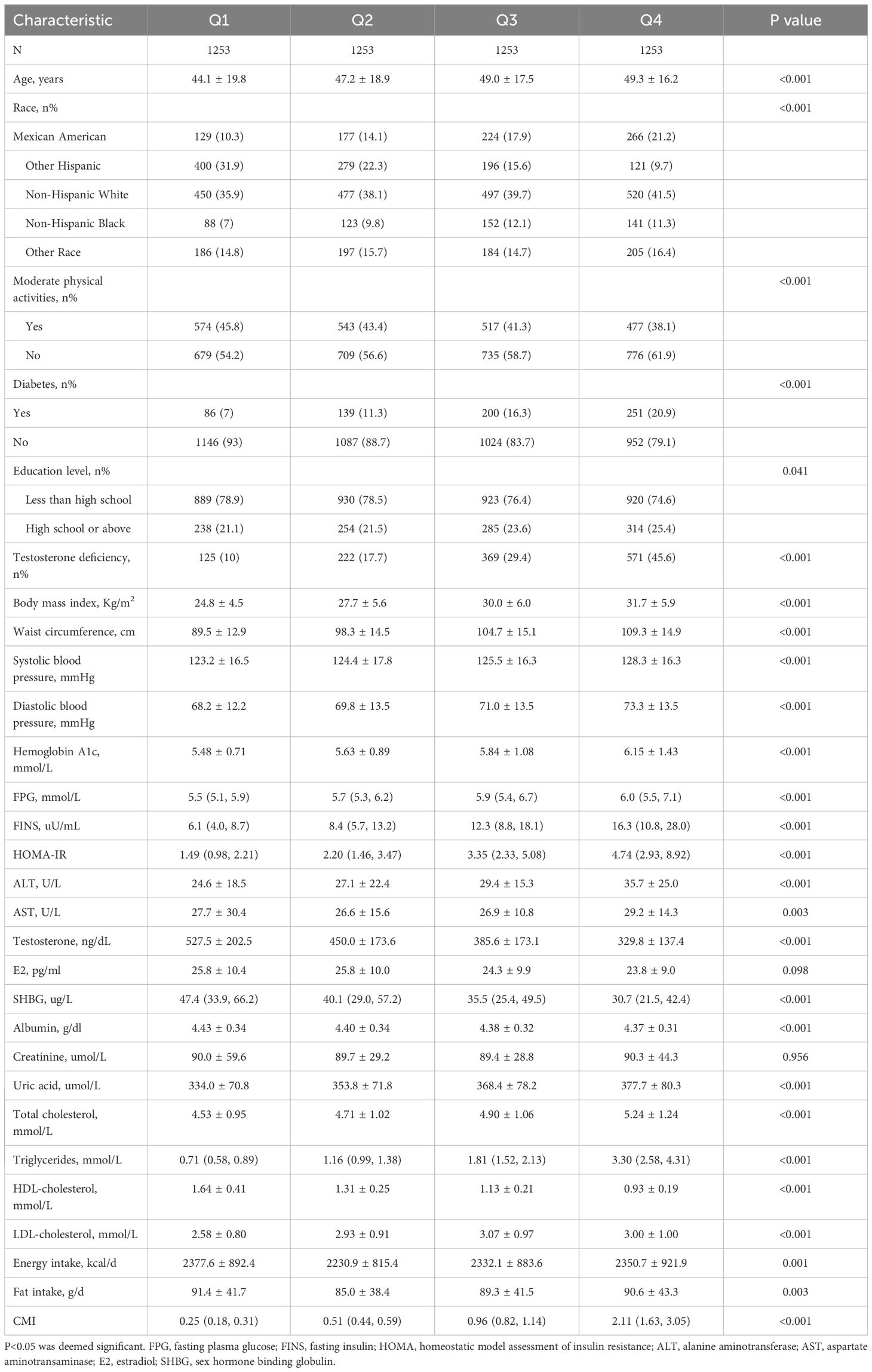
A total of 5012 subjects ranging from 18 to 80 years old were included in this study, with a prevalence of testosterone deficiency at 25.7%. The demographic characteristics of the subjects stratified by CMI quartiles are presented in Table 1. Subjects in the highest CMI quartile exhibited a higher prevalence of diabetes mellitus, testosterone deficiency and elevated AST, weight, uric acid, BMI, TG, waist circumference, ALT, hemoglobin A1c, TC, LDL-C, total energy and total fat intake, compared to those in the lowest quartile. Conversely, subjects in the highest quartile had lower HDL-C, SHBG and moderate physical activities (p<0.01) (Table 1).
Correlation between CMI and clinical and biochemical parameters
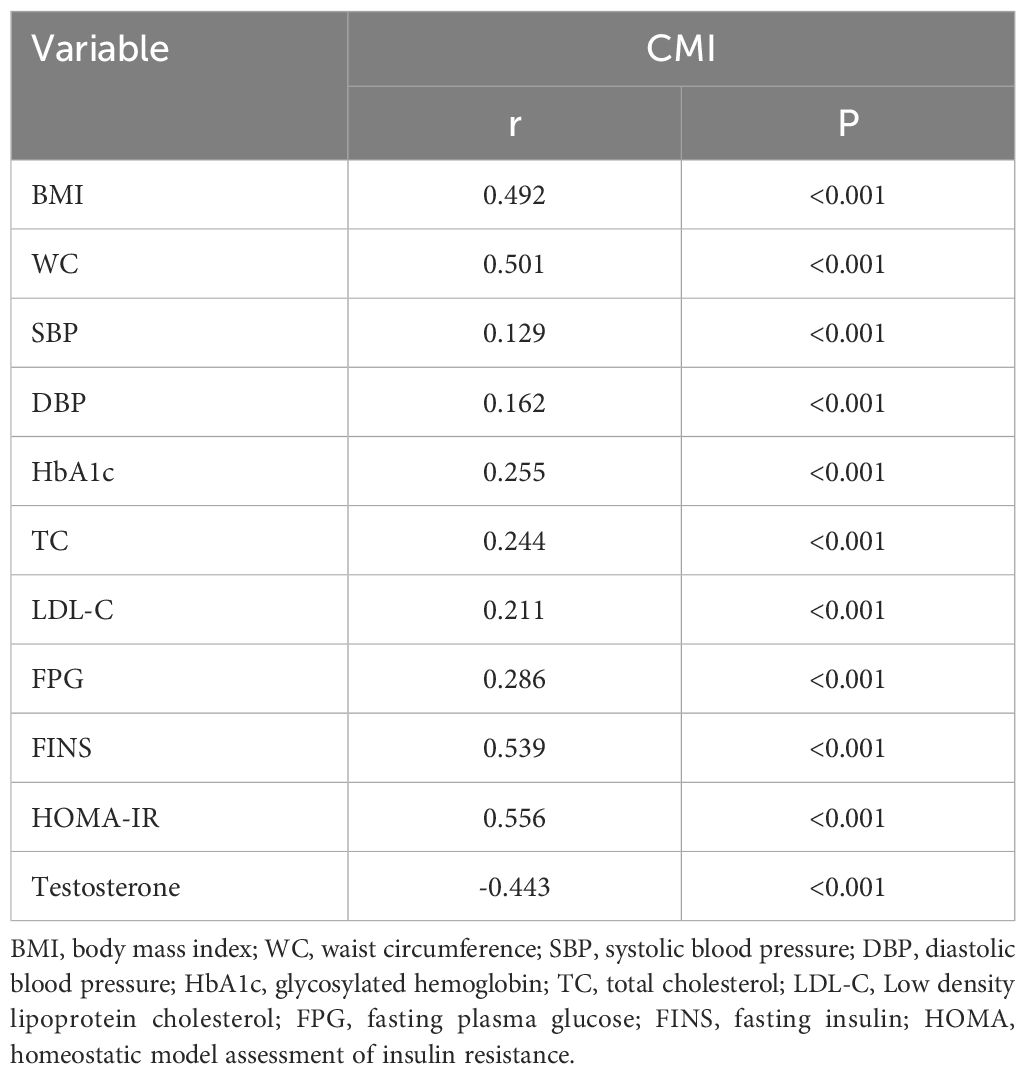
According to the Spearman correlation analysis, CMI was positively correlated with DBP, BMI, HbA1c, WC, SBP, FINS, FPG, TC, HOMA-IR and testosterone, as indicated in Table 2 (all p<0.001).
Linear correlation between CMI and testosterone
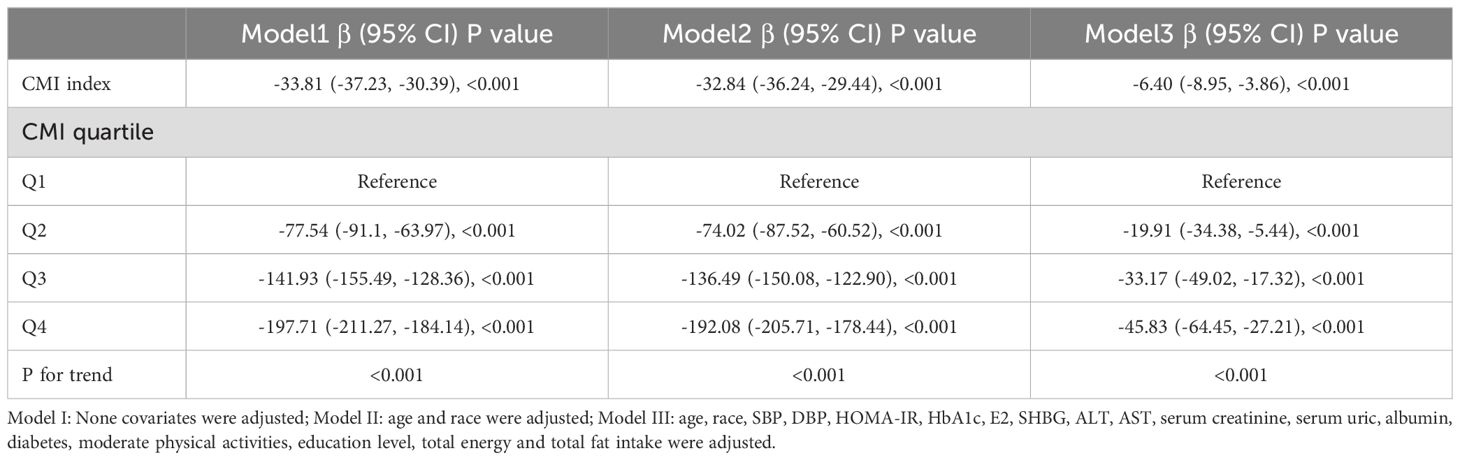
β values and 95% CI for CMI and testosterone in various models are shown in Table 3. The findings suggest a significant and independent negative correlation between CMI and testosterone across different adjusted models (Model I, β= -33.81, 95% CI: -37.23, -30.39; Model II, β= -32.84, 95% CI: -36.24, -29.44; Model III, β= -6.40, 95% CI: -8.95, -3.86; all p < 0.01). Specifically, testosterone levels in Model III were significantly lower in the Q2, Q3, and Q4 groups compared to that in the Q1 group after adjusting for confounding variables.
Subgroup analysis
To assess the impact of subgroups on the correlation between CMI and testosterone levels, subgroup analyses were conducted on race, age, diabetes mellitus, BMI, education level and moderate physical activities. As shown in Table 4, the negative correlation between CMI and testosterone levels was significantly affected by age and BMI (p < 0.05 for all interactions). The correlation between CMI and testosterone was stronger in subjects with normal weight and those aged 60 years old or more.
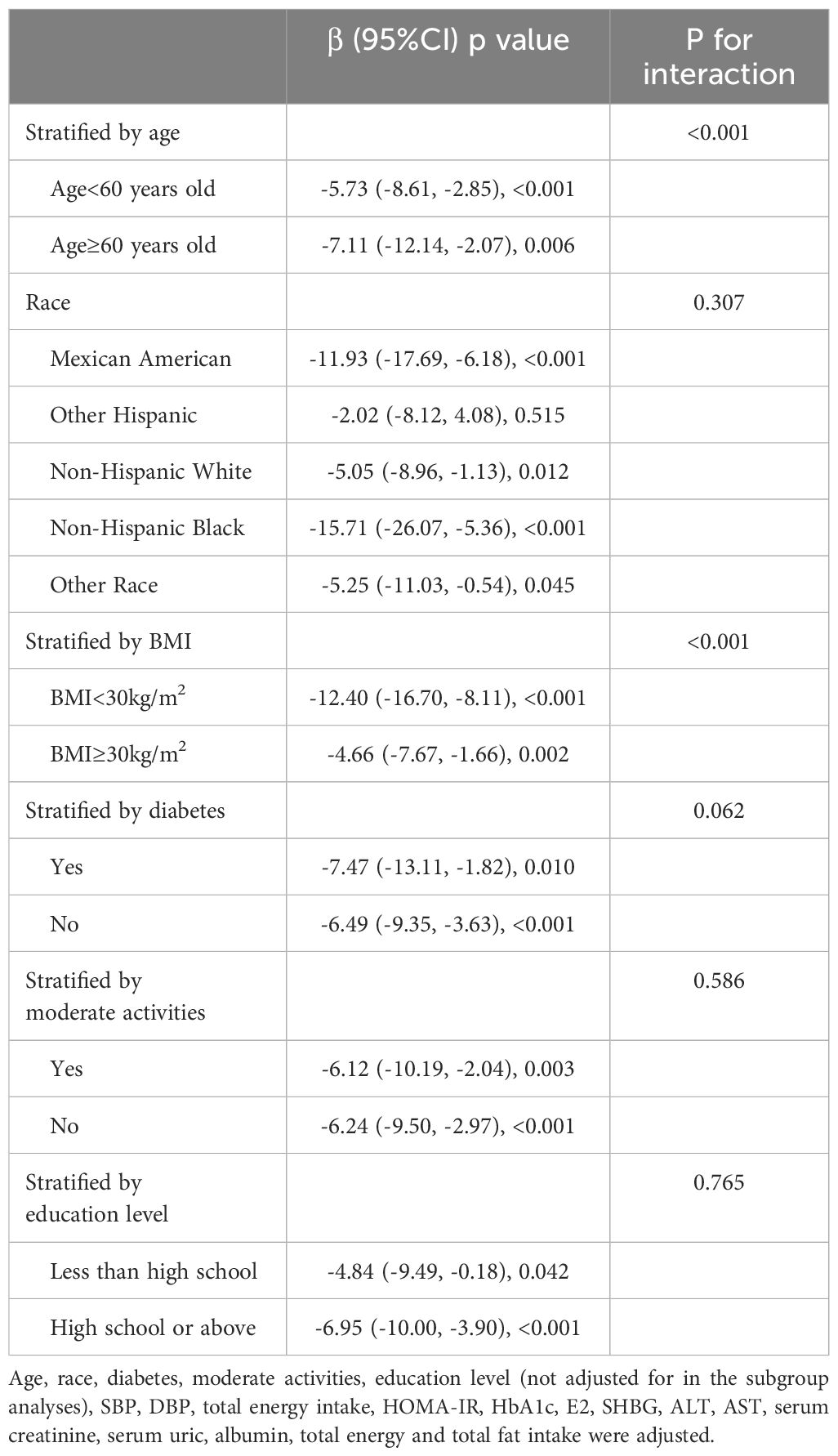
Table 4. Association between CMI and testosterone stratified by age, race, BMI, diabetes, moderate activities and Education level.
Non-linearity and threshold effect analysis between CMI and testosterone
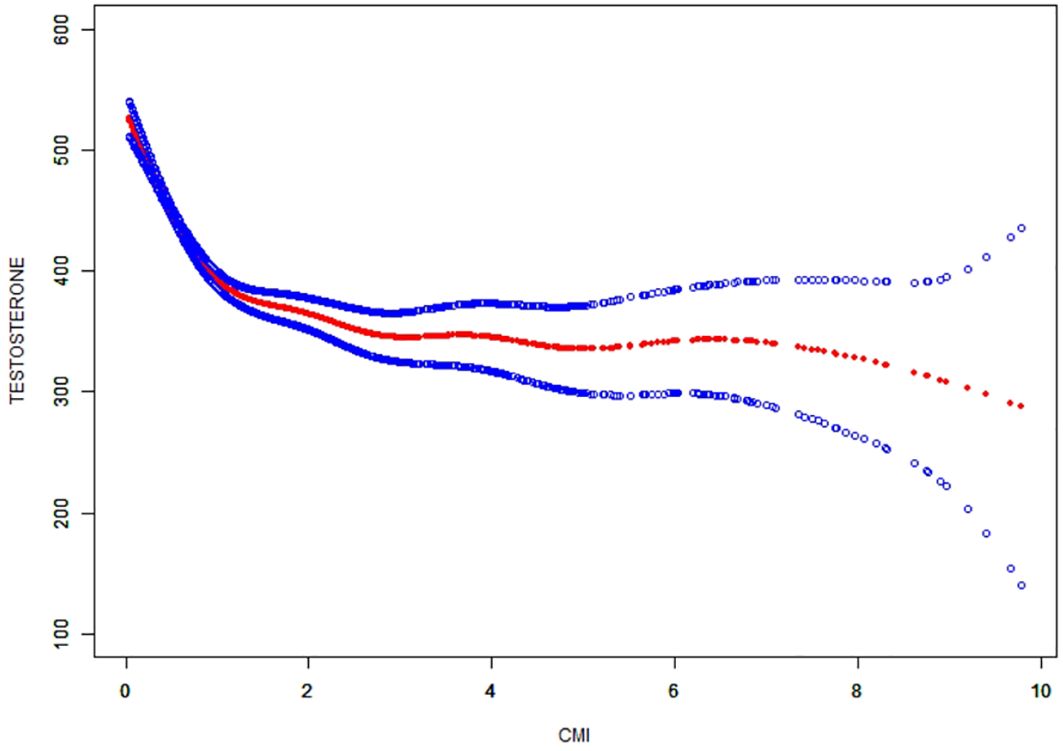
The non-linear correlation and saturation effect between CMI and testosterone have been illustrated with smooth curve fitting techniques as depicted in Figures 2, 3. In the subjects, a negative correlation was observed in the non-linear correlation, with inflection points at 0.73 (as shown in Table 5). Below the threshold of 0.73, there was a significant effect value of -209.16, while the value dropped to -14.82 when the measurement value of CMI exceeded 0.73.
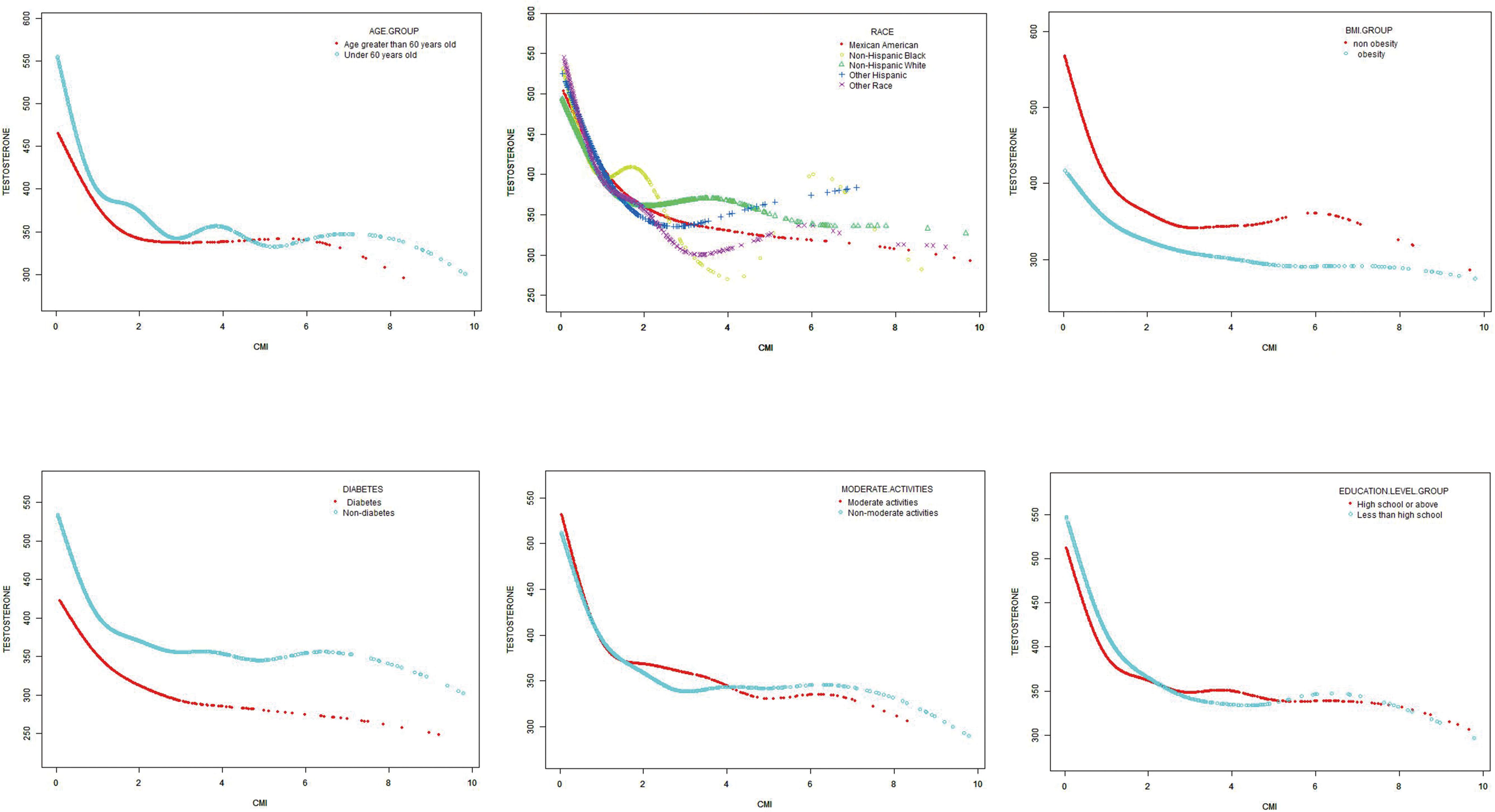
Figure 3. Subgroups analysis for the association between CMI and prevalence of testosterone by age, race, BMI, diabetes, moderate physical activities and education level.
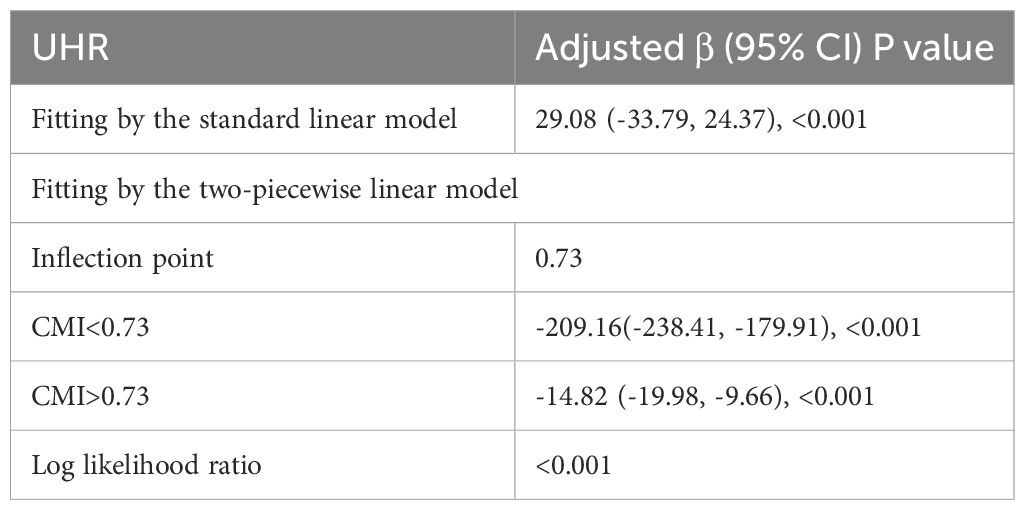
Table 5. Threshold effect analysis of CMI on testosterone using the two-piecewise linear regression model.
The predictive value of CMI for testosterone deficiency
ROC of CMI, TG/HDL and WHtR to diagnose testosterone deficiency is shown in Figure 4. AUC for CMI in the ROC analysis was found to be 0.724 (95% CI: 0.709–0.740), significantly higher than that for WHtR and TG/HDL (P < 0.001), with a sensitivity of 60.1%, a specificity of 71.3%, and a cutoff of 0.239.
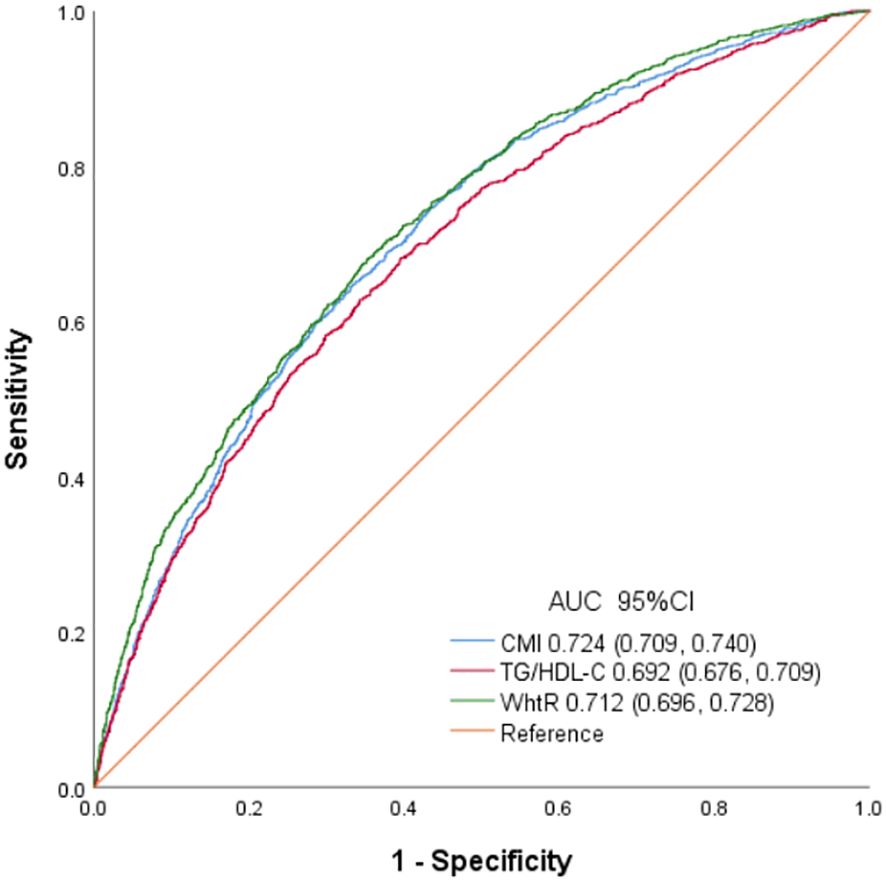
Figure 4. Receiver operating characteristic curves of CMI, TG/HDL and WhtR to identify testosterone deficiency.
Discussion
The results showed that high CMI in American male adults is negatively correlated with serum testosterone levels. Subgroup analyses and interaction assessments showed a stronger correlation in the subjects with normal weight and those aged over 60 years old. In addition, the findings revealed a non-linear correlation between CMI and testosterone levels, with a notable inflection of CMI at 0.73. Furthermore, CMI had a superior diagnostic accuracy for testosterone deficiency compared to TG/HDL-C and WHtR alone.
CMI serves as an innovative clinical marker integrating the measurements of WHtR and TG/HDL-C. It was first discovered by Wakabayashi et al. in 2015 that CMI is of great significance in evaluating diabetes mellitus (20). Further research was conducted based on this discovery, revealing a significant correlation between CMI and IR related diseases such as kidney disease, cardiovascular disease, stroke and hypertension (26–29). suggesting that CMI may serve as a valuable indicator for metabolic diseases. However, there is a lack of data on the correlation between testosterone and CMI. This study is the first to effectively establish the correlation between CMI and testosterone in American male adults, utilizing a large sample size.
The potential risk factors linked to a deficiency or decrease in testosterone levels include advanced aging, obesity, hyperlipidemia and diabetes mellitus. IR plays a critical role in metabolic disorders, which is considered as a significant independent predictor of testosterone deficiency or decrease. Recent studies have confirmed a reciprocal correlation between IR and testosterone deficiency. IR can lower testosterone levels, leading to further development of obesity and IR (16). Tsai et al. conducted a study examining testosterone levels in 221 middle-aged males without diabetes mellitus. Multivariate analysis revealed a significant negative correlation between testosterone levels and C-peptide, insulin, and HOMA-IR (30). Additionally, a separate study involving 2361 Chinese males aged 20-73 years old identified a negative correlation between total testosterone levels and Mets (31). Similar findings were found in a study specifically focused on males in Taiwan (32). According to this study, there was a significant positive correlation between CMI and HOMA-IR, which is the key driving factor for testosterone decrease.
The distribution of body fat accumulation has a significant impact on the occurrence of IR and testosterone deficiency (5, 33–35). Previous studies have consistently demonstrated a strong correlation between traditional obesity indicators, such as WC and WHtR, and decreased testosterone levels (34, 35). TG/HDL-C has been extensively researched as a reliable and straightforward indicator of IR and testosterone deficiency in relation to T2DM (9, 36–38). Combining TG/HDL-C and WHtR, CMI can comprehensively evaluate dyslipidemia and obesity, making it a valuable tool for identifying testosterone deficiency. The findings as illustrated in Figure 4 demonstrated that CMI is superior to TG/HDL and WHtR in terms of AUC, indicating its superior ability to detect testosterone deficiency. These results suggested that CMI may be suitable for evaluating testosterone deficiency in clinical settings.
Previous studies have reported that CMI is correlated with various diseases related to IR (20, 25, 39). Ichiro Wakabayashi et al. conducted a cohort survey of 10,196 individuals underwent a health assessment, revealing a significant positive correlation between elevated CMI values and the presence of hyperglycemia and the increased risk of diabetes mellitus (20). Additionally, Zou et al. determined that CMI was a reliable predictor of NAFLD within the general Japanese population (39). Furthermore, Luo et al. identified a positive correlation between high CMI values and the development of cardiovascular disease in patients with hypertension and obstructive sleep apnea (40). Another study by Xu et al. demonstrated a significant positive correlation between CMI and IR in patients with T2DM (25). The findings align with the conclusions drawn from the aforementioned studies. A significant correlation was observed between CMI and various factors, including BMI, HbA1c, WC, SBP, TC, DBP, HOMAI-IR, suggesting a potential value for CMI in future clinical applications.
The influence of established risk factors on the correlation between CMI and testosterone levels was further explored through stratified analyses. Studies have shown that the correlation is more obvious among subjects with normal weight and the elderly. It is well known that aging has been viewed as a risk factor for the development of hypogonadism (41, 42). Individuals with normal BMI and high body fat have a higher correlation with metabolic syndrome and IR (43). It is important to note that testosterone decrease is often overlooked in the subjects, emphasizing the importance of considering CMI as a factor for identifying testosterone decrease, particularly in this population.
The specific mechanism by which CMI leads to a decrease in male testosterone is still unclear. However, abnormal lipid metabolism and obesity may explain these results in CMI patients. Firstly, CMI functions as an indicator of abdominal obesity, which can directly influence testosterone levels. An increase in obesity is correlated with elevated levels of leptin, a hormone that stimulates the release of luteinizing hormone (LH) via hypothalamic gonadotropin-releasing hormone (GnRH) neurons. However, hypothalamus may develop resistance to leptin’s effects, leading to diminished feedback stimulation of testosterone production (44, 45). Furthermore, adipocytes prevalent in individuals with obesity exhibit elevated expression of aromatase, which are capable of producing inflammatory cytokines. Aromatase facilitates the conversion of testosterone into estradiol, consequently reducing circulating androgen levels. In addition, the increased estrogen levels and inflammatory mediators secreted by adipocytes initiate a negative feedback mechanism on the hypothalamic-pituitary (HP) axis. This feedback inhibits the secretion of gonadotropin-releasing hormone (GnRH) and subsequent release of luteinizing hormone (LH), ultimately resulting in diminished testosterone levels (46).
One of the strengths of this study lies in the thorough characterization of subjects based on a large population, and the use of subgroup analysis to evaluate changes in CMI and testosterone levels in different populations, thereby enhancing the credibility of the findings. Nevertheless, certain limitations still exist. Firstly, this trial only collected data from American male adults, which ignores the impact of CMI levels on testosterone levels in female adults and non-American populations, which is worth further exploration in the future. Secondly, the absence of gonadotropin data in NHANES database limits the ability to pinpoint the exact type of hypogonadism. Thirdly, the cross-sectional nature of this study precludes the establishment of causality, future research should consider using more longitudinal designs to deepen understanding of the research question. Fourthly, lack of data on testicular volume prevents its inclusion as a covariate in the study.
Conclusion
In conclusion, CMI has an independent and negative correlation with testosterone levels in American male adults. Furthermore, CMI have higher AUC values of testosterone deficiency than WHtR and TG/HDL-C in American male adults. CMI provides a methodology for monitoring serum testosterone levels and recommends that patients experiencing testosterone deficiency address obesity and lipid levels. These findings hold substantial implications for clinical decision-making and preventive strategies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES, www.cdc.gov/nchs/NHANES/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JX: Writing – original draft, Conceptualization. Y-CL: Writing – review & editing, Software, Investigation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Key Program of Natural Science Foundation of Zhejiang Province (LY22H020009 to LY-C).
Acknowledgments
We would like to thank the NHANES database for providing the data source for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Corona G, Rastrelli G, Vignozzi L, Maggi M. Androgens and male sexual function. Best Pract Res Clin Endocrinol Metab. (2022) 36:101615. doi: 10.1016/j.beem.2022.101615
2. Di Lodovico E, Facondo P, Delbarba A, Pezzaioli LC, Maffezzoni F, Cappelli C, et al. Testosterone, hypogonadism, and heart failure. Circ Heart Fail. (2022) 15:e008755. doi: 10.1161/CIRCHEARTFAILURE.121.008755
3. Shigehara K, Izumi K, Kadono Y, Mizokami A. Testosterone and bone health in men: A narrative review. J Clin Med. (2021) 10:530–42. doi: 10.3390/jcm10030530
4. Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, et al. Association of Triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. (2020) 19:33. doi: 10.1186/s12944-020-01213-x
5. Wang K, Gong M, Xie S, Zhang M, Zheng H, Zhao X, et al. Nomogram prediction for the 3-year risk of type 2 diabetes in healthy mainland China residents. EPMA J. (2019) 10:227–37. doi: 10.1007/s13167-019-00181-2
6. Gagliano-Juca T, Alvarez M, Basaria S. The medicalization of testosterone: reinventing the elixir of life. Rev Endocr Metab Disord. (2022) 23:1275–84. doi: 10.1007/s11154-022-09751-8
7. Wittert G, Grossmann M. Obesity, type 2 diabetes, and testosterone in ageing men. Rev Endocr Metab Disord. (2022) 23:1233–42. doi: 10.1007/s11154-022-09746-5
8. Grandys M, Majerczak J, Zapart-Bukowska J, Duda K, Kulpa JK, Zoladz JA. Lowered serum testosterone concentration is associated with enhanced inflammation and worsened lipid profile in men. Front Endocrinol (Lausanne). (2021) 12:735638. doi: 10.3389/fendo.2021.735638
9. Lim TK, Lee HS, Lee YJ. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 diabetes in community dwelling adults: A longitudinal 12-year analysis of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. (2020) 163:108150. doi: 10.1016/j.diabres.2020.108150
10. Li C, Xu J. Negative correlation between metabolic score for insulin resistance index and testosterone in male adults. Diabetol Metab Syndr. (2024) 16:113. doi: 10.1186/s13098-024-01353-5
11. Souteiro P, Belo S, Oliveira SC, Neves JS, Magalhaes D, Pedro J, et al. Insulin resistance and sex hormone-binding globulin are independently correlated with low free testosterone levels in obese males. Andrologia. (2018) 50:e13035. doi: 10.1111/and.2018.50.issue-7
12. Krysiak R, Kowalcze K, Okopien B. Hypothalamic-pituitary-gonadal axis and sexual functioning in metformin-treated men after discontinuation of testosterone replacement therapy: A pilot study. J Clin Pharm Ther. (2021) 46:1764–75. doi: 10.1111/jcpt.13528
13. Wu S, Wu Y, Fang L, Zhao J, Cai Y, Xia W. A negative association between triglyceride glucose-body mass index and testosterone in adult males: a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1187212. doi: 10.3389/fendo.2023.1187212
14. Zitzmann M, deficiency T. insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. (2009) 5:673–81. doi: 10.1038/nrendo.2009.212
15. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. (2006) 91:843–50. doi: 10.1210/jc.2005-1326
16. Pivonello R, Menafra D, Riccio E, Garifalos F, Mazzella M, de Angelis C, et al. Metabolic disorders and male hypogonadotropic hypogonadism. Front Endocrinol (Lausanne). (2019) 10:345. doi: 10.3389/fendo.2019.00345
17. Yang S, Xu J. Elevated small dense low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio is associated with an increased risk of metabolic dysfunction associated fatty liver disease in Chinese patients with type 2 diabetes mellitus. J Diabetes Investig. (2024) 15:634–42. doi: 10.1111/jdi.14148
18. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. (2019) 92:82–97. doi: 10.1016/j.metabol.2018.11.014
19. Rezaei M, Taheri M, Irandoust K, Bragazzi NL. The effects of a period of aerobic exercises on sexual hormones and appetite in obese women with polycystic ovary syndrome. Int J Body Mind Culture. (2024) 15:2345–5802.
20. Wakabayashi I, Daimon T. The “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. (2015) 438:274–8. doi: 10.1016/j.cca.2014.08.042
21. Qiu Y, Yi Q, Li S, Sun W, Ren Z, Shen Y, et al. Transition of cardiometabolic status and the risk of type 2 diabetes mellitus among middle-aged and older Chinese: A national cohort study. J Diabetes Investig. (2022) 13:1426–37. doi: 10.1111/jdi.13805
22. Acosta-Garcia E, Concepcion-Paez M. Cardiometabolic index as a predictor of cardiovascular risk factors in adolescents. Rev Salud Publica (Bogota). (2018) 20:340–5. doi: 10.15446/rsap.V20n3.61259
23. Zhou X, Xu J. Association between serum uric acid-to-high-density lipoprotein cholesterol ratio and insulin resistance in an American population: A population-based analysis. J Diabetes Investig. (2024) 15:762–71. doi: 10.1111/jdi.14170
24. Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. (2018) 200:423–32. doi: 10.1016/j.juro.2018.03.115
25. Wu L, Xu J. Relationship between cardiometabolic index and insulin resistance in patients with type 2 diabetes. Diabetes Metab Syndr Obes. (2024) 17:305–15. doi: 10.2147/DMSO.S449374
26. Dursun M, Besiroglu H, Otunctemur A, Ozbek E. Association between cardiometabolic index and erectile dysfunction: A new index for predicting cardiovascular disease. Kaohsiung J Med Sci. (2016) 32:620–3. doi: 10.1016/j.kjms.2016.10.003
27. Wang H, Chen Y, Guo X, Chang Y, Sun Y. Usefulness of cardiometabolic index for the estimation of ischemic stroke risk among general population in rural China. Postgrad Med. (2017) 129:834–41. doi: 10.1080/00325481.2017.1375714
28. Wang H, Chen Y, Sun G, Jia P, Qian H, Sun Y. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med. (2018) 130:325–33. doi: 10.1080/00325481.2018.1444901
29. Wang HY, Shi WR, Yi X, Wang SZ, Luan SY, Sun YX. Value of reduced glomerular filtration rate assessment with cardiometabolic index: insights from a population-based Chinese cohort. BMC Nephrol. (2018) 19:294. doi: 10.1186/s12882-018-1098-8
30. Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. (2004) 27:861–8. doi: 10.2337/diacare.27.4.861
31. Zhang J, Huang X, Liao M, Gao Y, Tan A, Yang X, et al. Both total testosterone and sex hormone-binding globulin are independent risk factors for metabolic syndrome: results from Fangchenggang Area Male Health and Examination Survey in China. Diabetes Metab Res Rev. (2013) 29:391–7. doi: 10.1002/dmrr.v29.5
32. Liao CH, Huang CY, Li HY, Yu HJ, Chiang HS, Liu CK. Testosterone and sex hormone-binding globulin have significant association with metabolic syndrome in Taiwanese men. Aging Male. (2012) 15:1–6. doi: 10.3109/13685538.2011.597462
33. Golubnitschaja O, Costigliola V. and Epma, General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. (2012) 3:14. doi: 10.1186/1878-5085-3-14
34. Hsu PS, Hung CL, Tu SK, Chen HH, Yang DH, Liao CC. Waist circumference is more closely associated with hypogonadism than is hyperglycemia, independent of BMI in middle-aged men. J Diabetes Res. (2021) 2021:1347588. doi: 10.1155/2021/1347588
35. Allan CA, Peverill RE, Strauss BJ, Forbes EA, McLachlan RI. Waist-to-height ratio as a predictor of serum testosterone in ageing men with symptoms of androgen deficiency. Asian J Androl. (2011) 13:424–31. doi: 10.1038/aja.2011.13
36. Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. (2012) 13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x
37. Zheng D, Li H, Ai F, Sun F, Singh M, Cao X, et al. Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of type 2 diabetes mellitus among Chinese elderly: the Beijing Longitudinal Study of Aging. BMJ Open Diabetes Res Care. (2020) 8:1–9. doi: 10.1136/bmjdrc-2019-000811
38. Chung TH, Kwon YJ, Lee YJ. High triglyceride to HDL cholesterol ratio is associated with low testosterone and sex hormone-binding globulin levels in Middle-aged and elderly men. Aging Male. (2020) 23:93–7. doi: 10.1080/13685538.2018.1501015
39. Zou J, Xiong H, Zhang H, Hu C, Lu S, Zou Y. Association between the cardiometabolic index and non-alcoholic fatty liver disease: insights from a general population. BMC Gastroenterol. (2022) 22:20. doi: 10.1186/s12876-022-02099-y
40. Cai X, Hu J, Wen W, Wang J, Wang M, Liu S, et al. Associations of the cardiometabolic index with the risk of cardiovascular disease in patients with hypertension and obstructive sleep apnea: results of a longitudinal cohort study. Oxid Med Cell Longev. (2022) 2022:4914791. doi: 10.1155/2022/4914791
41. Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED Jr., et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. (2010) 7:1598–607. doi: 10.1111/j.1743-6109.2010.01778.x
42. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. (2000) 163:460–3. doi: 10.1016/S0022-5347(05)67900-1
43. Madeira FB, Silva AA, Veloso HF, Goldani MZ, Kac G, Cardoso VC, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PloS One. (2013) 8:e60673. doi: 10.1371/journal.pone.0060673
44. Lima TFN, Nackeeran S, Rakitina E, Lima GFN, Arora H, Kargi AY, et al. Association of leptin with total and free testosterone: results from the national health and nutrition examination surveys. Androg Clin Res Ther. (2020) 1:94–100. doi: 10.1089/andro.2020.0007
45. Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obes (Silver Spring). (2015) 23:713–9. doi: 10.1002/oby.21033
Keywords: cardiometabolic index, testosterone deficiency, insulin resistance, diabetes mellitus, obesity
Citation: Xu J and Li Y-C (2024) Negative correlation between cardiometabolic index and testosterone in male adults. Front. Endocrinol. 15:1447230. doi: 10.3389/fendo.2024.1447230
Received: 11 June 2024; Accepted: 26 November 2024;
Published: 11 December 2024.
Edited by:
Senthilkumar Palaniappan, Karpagam Academy of Higher Education, IndiaReviewed by:
Palanisamy Sivanandy, International Medical University, MalaysiaKhadijeh Irandoust, Imam Khomeini International University, Iran
Copyright © 2024 Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue-Chun Li, bGl5dWVjaHVuMTk4MEBzaW5hLmNvbQ==
 Jing Xu
Jing Xu Yue-Chun Li
Yue-Chun Li