- 1Clinical Laboratory, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 2Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Aim: To explore the association between Metabolic Score for Insulin Resistance (METS-IR) and the risk of cardiovascular disease (CVD) death in patients with rheumatoid arthritis (RA).
Methods: This retrospective cohort study extracted data on 1,218 RA patients from the National Health and Nutrition Examination Survey. The weighted univariate and multivariate Cox regression model was established to explore the association between METS-IR and CVD mortality. Subgroup analysis was performed in terms of age, gender, body mass index, diabetes, and CVD. Hazard ratios (HRs) and 95% confidence levels (CIs) were presented.
Results: Increased METS-IR was associated with a significantly higher risk of CVD mortality (HR=4.59, 95%CI: 1.98-10.67), and METS-IR>2.48 was associated with higher odds of CVD mortality compared with METS-IR ≤ 2.25 (HR=3.57, 95%CI: 2.04-6.24). METS-IR was positively associated with the risk of CVD mortality (HR=3.83, 95%CI: 1.62-9.08), and METS-IR>2.48 was associated with a significantly higher risk of CVD mortality in contrast to METS-IR ≤ 2.25 (HR=3.38, 95%CI: 1.87-6.09).
Conclusion: Increased METS-IR was associated with a significantly higher risk of CVD mortality in RA patients. Clinicians could consider incorporating the METS-IR score into routine assessment of the prognosis of RA patients.
Introduction
Rheumatoid arthritis (RA) is an autoimmune joint disease related to synovial tissue proliferation, pannus formation, cartilage destruction, and systemic complications (1, 2), with a global prevalence of 460 per 100,000 people from 1980 to 2019 and a moderately increased mortality risk over the last two decades (3, 4). Individuals with RA have 1.5-2 times the rate of cardiovascular events than the general population, and cardiovascular events are the primary cause of death in people with RA (5). As evidence shows, RA patients have a 60% increased risk of cardiovascular disease (CVD) mortality compared with the general population (6–9).
Insulin resistance is an important factor affecting the occurrence and prognosis of CVD (10–12). Studies reported that insulin resistance may play a critical role in the association between RA and cardiovascular disease (13, 14). Insulin resistance significantly increases the risk of atherosclerosis and CVD in patients with RA (15–17).
The current evaluation methods for insulin resistance (IR) are diverse. The euglycemic-hyperinsulinemic clamp (EHC) is considered the gold standard, but its invasive and costly limitations make it unsuitable for large-scale clinical and epidemiological studies (18). Thus, researchers have developed several indices to assess insulin resistance using simple formulas, such as the homeostasis model assessment for IR (HOMA-IR) (19), triglyceride (TG) glucose index (20), and TG to high-density lipoprotein cholesterol ratio (HDL-C) (TG/HDL-C) (21). However, the aforementioned indexes fail to account for the impact of nutritional factors, such as body mass index (BMI), on insulin resistance. Consequently, they possess certain limitations when constructing clinical disease prediction models. Recently, a tool based on fasting blood glucose (FBG), TG, HDL-C, and BMI, namely Metabolic Score for Insulin Resistance (METS-IR), has been demonstrated to be effective in evaluating insulin resistance (22). METS-IR was illustrated to be correlated with adipokine disorder and inflammatory activity in patients with osteoarthritis (23), and the higher METS-IR in patients with diabetes was associated with the increased risk of CVD mortality (24). Therefore, we suspected that METS-IR might be associated with the CVD mortality in RA patients.
This study aimed to explore the association between METS-IR and the risk of CVD death in patients with RA, using the data of the National Health and Nutrition Examination Survey (NHANES). This association was further investigated in different age, gender, body mass index (BMI), diabetes, and CVD subpopulations.
Methods
Study population
This retrospective cohort study extracted data on patients with RA from the NHANES 1999-2018. The NHANES is a program of studies conducted to evaluate the health and nutritional status of the nationally representative population in the United States, which combines interviews and physical examinations to provide information on demographics, diets, physical examinations, laboratory tests, and questionnaire surveys (25). The Ethics Review Board of the National Center for Health Statistics (NCHS) Research approves the NHANES survey. This study was exempt from the approval of the institutional review board due to de-identified and retrospective nature of the data used. Patient records were obtained from NHANES if they 1) were ≥18 years old; 2) had RA. RA in this study was self-reported by physicians. The positive answer for the question “Doctor ever said you had arthritis?” and the “rheumatoid arthritis” answer for the question “Which type of arthritis was it” indicated the presence of RA. The above questionnaire was completed by professionally trained investigators under strict quality control (26, 27); 3) had measurement of FBG, TG and HDL-C, 4) had measurement of height and weight; and 5) had information on survival status and death causes. Patients with missing important co-variables were excluded from this study. Qualified patients were followed up until December 31, 2019.
Main and outcome variables
METS-IR was calculated as ln [(2 × FPG (mg/dL) + TG (mg/dL)) × BMI (kg/m2)]/ln [HDL-C (mg/dL)] (22). It was treated as both a continuous variable and a categorical variable. When METS-IR acted as a continuous variable, it was classified into three groups (≤2.25, 2.25-2.48, and >2.48).
The outcome in this study was CVD mortality. The follow-up was ended in December 2019. The mean follow-up time was 125.36 ± 3.99 months.
Other variables
Age (<65, ≥65 years), gender (male, female), race (non-Hispanic White, non-Hispanic Black, others), education [below high school, high school, college and above], marital status (married, never married, others), poverty income ratio (PIR; <1.0, ≥1.0), smoking (no, yes), drinking (no, yes), total energy (kcal), physical activity, duration of arthritis (years), osteoporosis (no, yes, unknown), fracture (no, yes, unknown), diabetes (no, yes), hypertension (no, yes), dyslipidemia (no, yes), CVD, chronic kidney disease (CKD; no, yes), dyslipidemia (no, yes), BMI (kg/m2), white blood cell (WBC, 1000 cells/μL), uric acid (mg/dL), antirheumatics (no, yes), nonsteroidal anti-inflammatory agents (no, yes), and glucocorticoid (no, yes) were also analyzed.
Physical activity was exhibited as energy consumption (MET × min), which was calculated by multiplying recommended metabolic equivalent (MET) by exercise time corresponding to the activity (min). Then physical activity was divided into <450 MET × min/week, ≥450 MET × min/week and unknown.
Statistical analysis
Quantitative data were reported as mean (standard error) [Mean (SE)], the independent samples t-test was used for comparison between two groups, and analysis of variance was applied for comparison among multiple groups; Enumeration data were shown as the number and percentages of cases [n (%)], and the Chi-square test was used for comparison among groups and the Mann-Whitney U rank sum test was employed for ranked data. The missing values were shown in Supplementary Table 1. The missing values concerning physical activity, osteoporosis and fracture were high, and patients without these variables data were classified as unknown group. The other variables dealt with multiple imputation method based on random forest using the miceforest package in python, and differences between pre- and post-imputation data were assessed (Supplementary Table 2). The weighted univariate Cox regression model was used to explore the factors which were significantly associated with CVD mortality in RA patients, which would be utilized as confounding variables. Then the weighted univariate and multivariate Cox regression model was established to explore the association between METS-IR and the risk of CVD mortality. Model I adjusted for age, education, marital status, physical activity, duration of arthritis, WBC, and uric acid. Model II adjusted for age, education, marital status, physical activity, duration of arthritis, WBC, uric acid, diabetes, hypertension, dyslipidemia, CVD, and CKD. Further, subgroup analysis was performed in terms of age, gender, BMI, diabetes, and CVD. Hazard ratios (HRs) and 95% confidence levels (CIs) were presented. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was applied for model statistical analysis. All statistical tests were two-sided, with P < 0.05 indicating statistically significant differences.
Results
Characteristics of the study population
A total of 2952 patients with RA who aged ≥18 years old were identified from the NHANES 1999-2018. After ruling out patients without information on FBG, TG, HDL-C, and BMI (n=1710), and without complete data on survival status (n=24), 1218 patients were included in the end. The process of study population selection is illustrated in Figure 1.
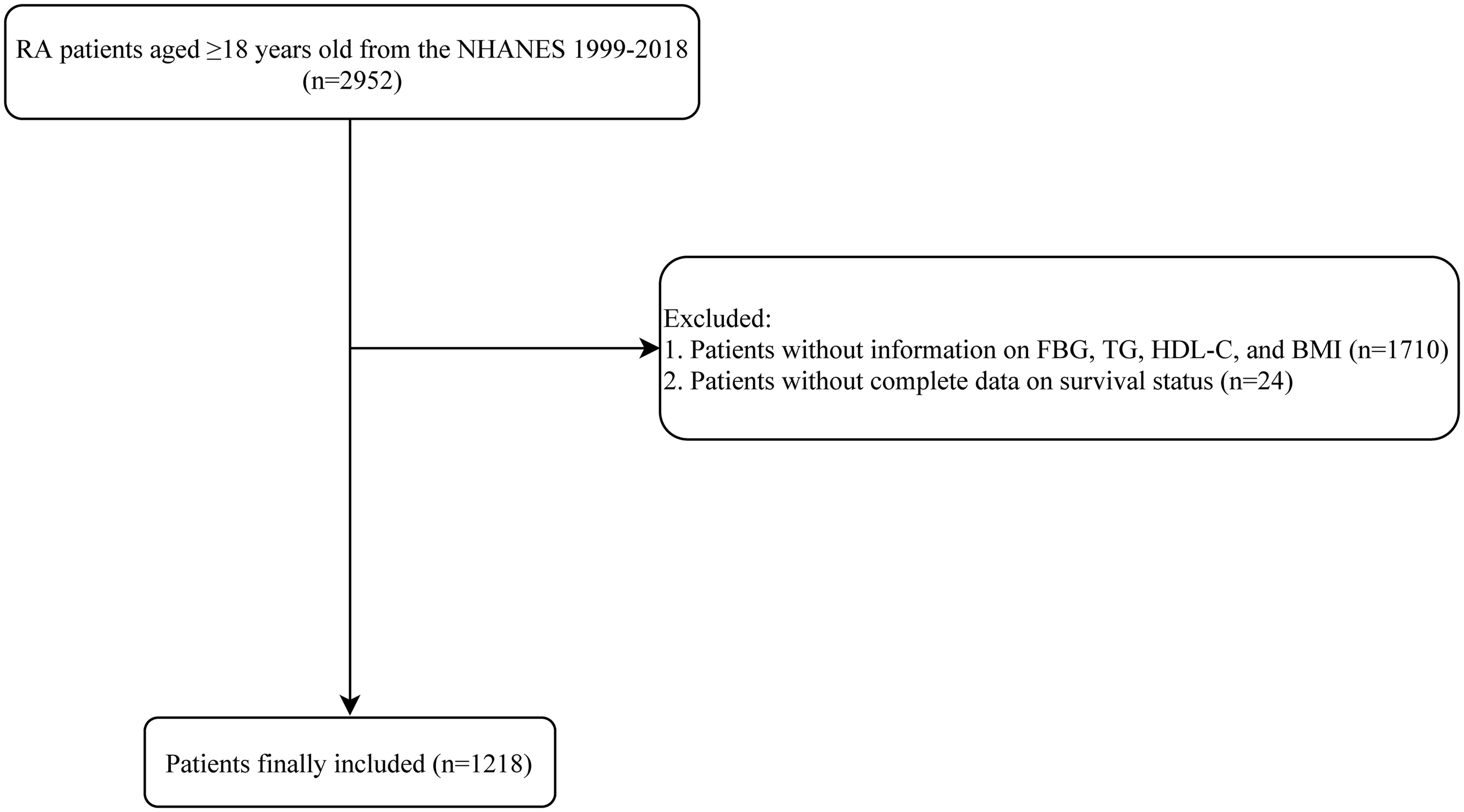
Figure 1. Process of study population selection. RA, rheumatoid arthritis; NHANES, National Health and Nutrition Examination Survey; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index.
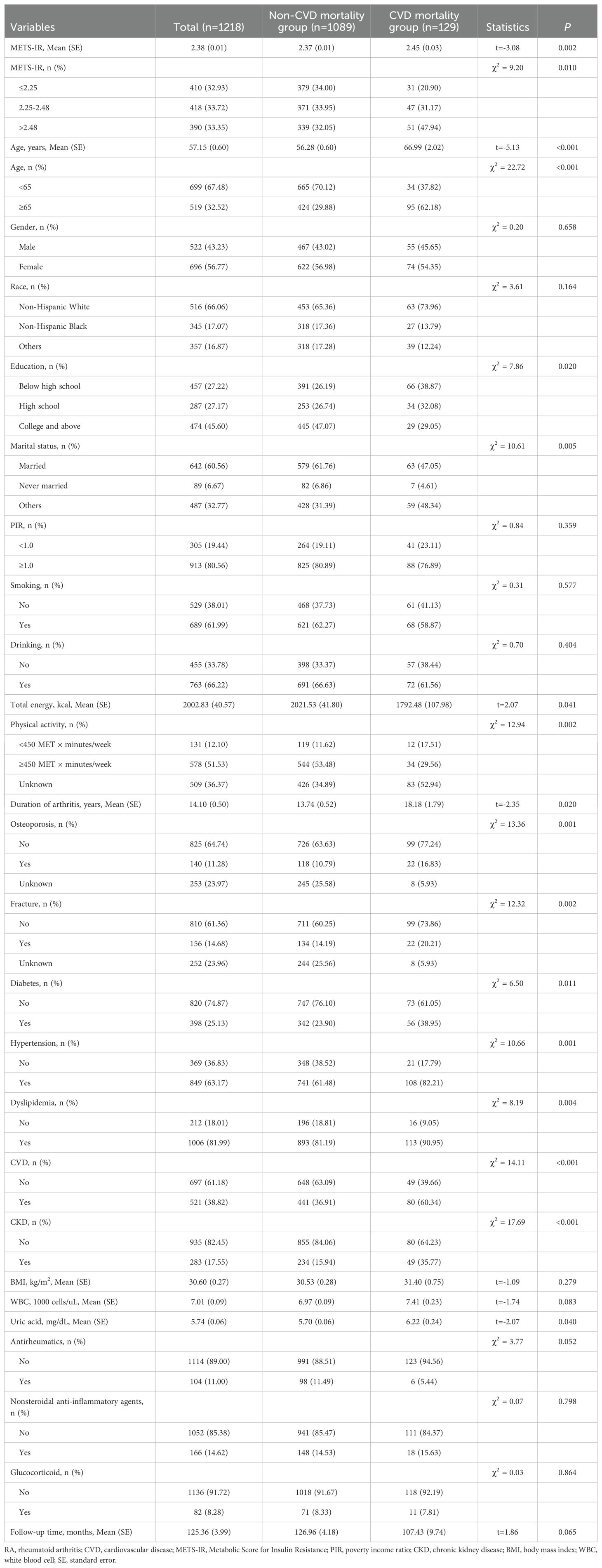
At the end of follow-up. there were 129 patients died of CVD. The average age were 57.15 years. The CVD mortality group had significantly higher METS-IR, older age, and lower education level than the non-CVD mortality group (all P<0.05). There were significant differences in marital status, total energy, physical activity, duration of arthritis, osteoporosis, fracture, diabetes, hypertension, dyslipidemia, CVD, CKD, and uric acid between the CVD mortality and non-CVD mortality groups (all P<0.05). Characteristics of the included patients with RA are demonstrated in Table 1.
Association between METS-IR and CVD mortality in RA
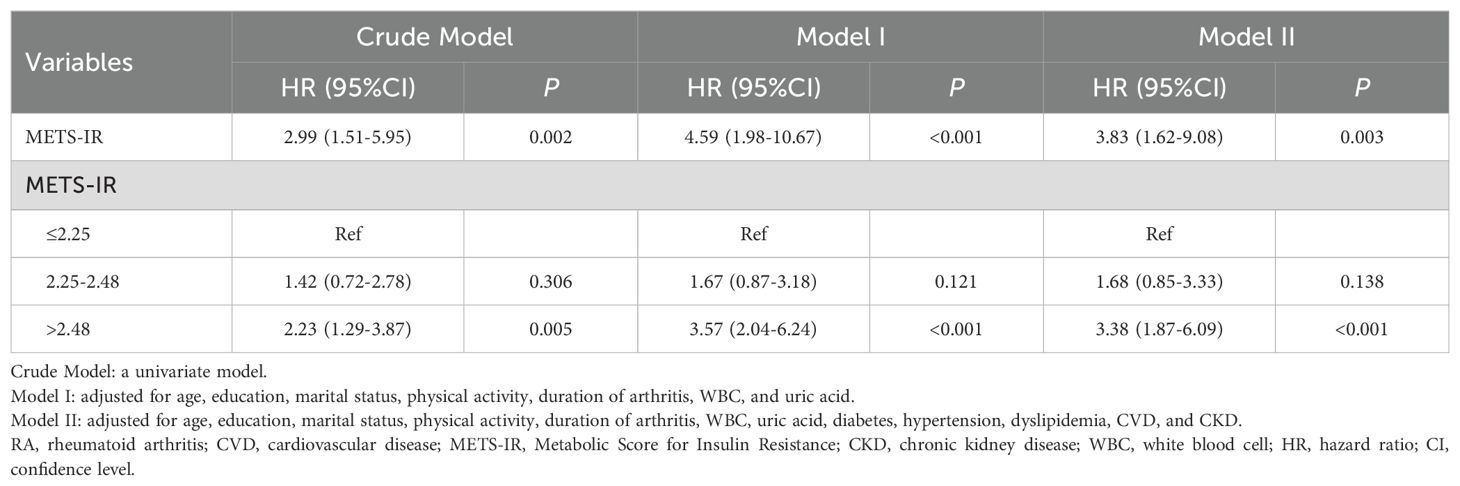
The confounding factors associated with CVD mortality in RA were screening via weighted univariate Cox regression model, which indicated that age, education, marital status, physical activity, duration of arthritis, WBC, uric acid, diabetes, hypertension, dyslipidemia, CVD, and CKD were confounding factors (Supplementary Table 3). After controlling for age, education, marital status, physical activity, duration of arthritis, WBC, and uric acid, increased METS-IR was associated with higher risk of CVD mortality (HR=4.59, 95%CI: 1.98-10.67, P<0.001), and METS-IR>2.48 was associated with a significantly greater risk of CVD mortality compared with METS-IR ≤ 2.25 (HR=3.57, 95%CI: 2.04-6.24, P<0.001). After adjusting for age, education, marital status, physical activity, duration of arthritis, WBC, uric acid, diabetes, hypertension, dyslipidemia, CVD, and CKD, METS-IR was associated with elevated risk of CVD mortality (HR=3.83, 95%CI: 1.62-9.08, P=0.003), and METS-IR>2.48 was associated with higher risk of CVD mortality in contrast to METS-IR ≤ 2.25 (HR=3.38, 95%CI: 1.87-6.09, P<0.001) (Table 2).
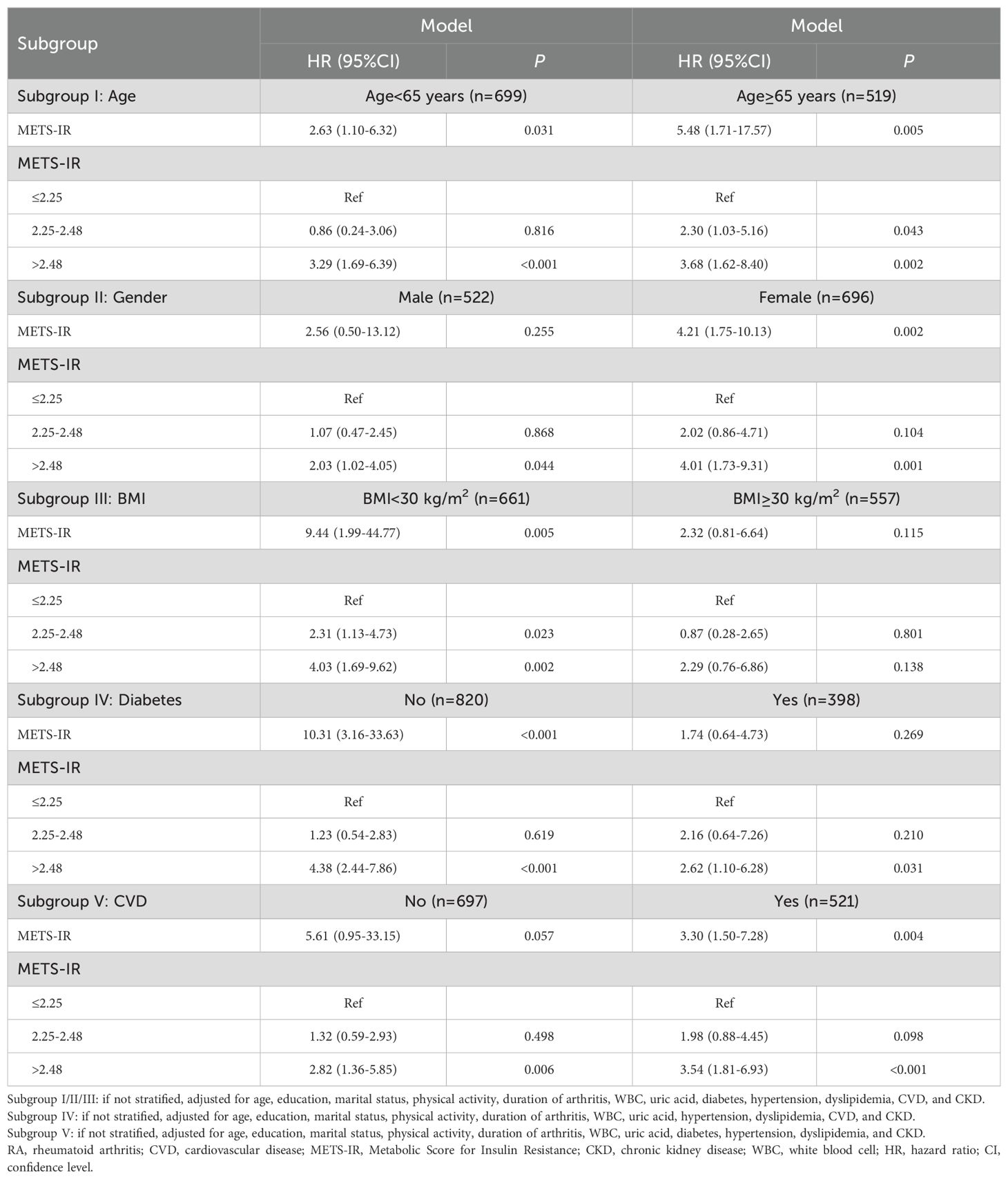
Association between METS-IR and CVD mortality in RA subpopulations
Age
In patients aged<65 years, higher METS-IR was associated with higher risk of CVD mortality (HR=2.63, 95%CI: 1.10-6.32, P=0.031); compared with METS-IR ≤ 2.25, METS-IR>2.48 was associated with higher risk of CVD mortality (HR=3.29, 95%CI: 1.69-6.39, P<0.001). For patients aged ≥65 years, METS-IR was associated with increased risk of CVD mortality (HR=5.48, 95%CI: 1.71-17.57, P=0.005), and METS-IR of 2.25-2.48 (HR=2.30, 95%CI: 1.03-5.16, P=0.043) and METS-IR>2.48 (HR=3.68, 95%CI: 1.62-8.40, P=0.002) were associated with higher risk of CVD mortality than METS-IR ≤ 2.25 (Table 3).
Gender
For male patients, compared with METS-IR ≤ 2.25, METS-IR>2.48 was associated with higher risk of CVD mortality (HR=2.03, 95%CI: 1.02-4.05, P=0.044). Regarding female patients, METS-IR was related to elevated risk of CVD mortality (HR=4.21, 95%CI: 1.75-10.13, P=0.002), and METS-IR>2.48 were associated with higher risk of CVD mortality than METS-IR ≤ 2.25 (HR=4.01, 95%CI: 1.73-9.31, P=0.001) (Table 3).
BMI
Among patients with BMI<30 kg/m2, METS-IR was correlated with increased risk of CVD mortality (HR=9.44, 95%CI: 1.99-44.77, P=0.005), and METS-IR of 2.25-2.48 (HR=2.31, 95%CI: 1.13-4.73, P=0.023) and METS-IR>2.48 (HR=4.03, 95%CI: 1.69-9.62, P=0.002) were associated with higher risk of CVD mortality than METS-IR ≤ 2.25. No significant association between METS-IR and CVD mortality was identified in patients with BMI≥30kg/m2 (all P>0.05) (Table 3).
Diabetes
In patients with diabetes, elevated METS-IR was associated with higher risk of CVD mortality (HR=10.31, 95%CI: 3.16-33.63, P<0.001), and METS-IR>2.48 was associated with increased risk of CVD mortality compared with METS-IR ≤ 2.25 (HR=4.38, 95%CI: 2.44-7.86, P<0.001). For patients without diabetes, METS-IR>2.48 was associated with higher risk of CVD mortality than METS-IR ≤ 2.25 (HR=2.62, 95%CI: 1.10-6.28, P=0.031) (Table 3).
CVD
Concerning patients with CVD, METS-IR>2.48 was associated with increased risk of CVD mortality than METS-IR ≤ 2.25 (HR=2.82, 95%CI: 1.36-5.85, P=0.006). For patients without CVD, METS-IR was related to elevated risk of CVD mortality (HR=3.30, 95%CI: 1.50-7.28, P=0.004), and METS-IR>2.48 were associated with higher risk of CVD mortality than METS-IR ≤ 2.25 (HR=3.54, 95%CI: 1.81-6.93, P<0.001) (Table 3).
Discussion
In this study, we explored the relationship between METS-IR and the risk of CVD mortality in patients with RA. It was demonstrated that increased METS-IR was associated with elevated risk of CVD mortality, and METS-IR>2.48 was associated with increased risk of CVD mortality compared with METS-IR ≤ 2.25. These findings offered the understanding of METS-IR and CVD mortality relationship, and could be considered in the clinical management of prognosis in RA patients.
In the recent studies, METS-IR was reported to be associated with elevated risk of CVD/stroke/cardiac issue in the middle-aged and elderly population (28). Wang et al. (24) illustrated that METS-IR was non-linearly associated with all-cause and CVD-related death in patients with diabetes. The correlation between METS-IR and adipokine disorder and inflammatory activity in females with knee osteoarthritis (23). METS-IR was also found to be significantly associated with all-cause and CVD mortality in the U.S. population compared to the other three alternative insulin resistance indexes (TyG index, TG/HDL-C, and HOMA-IR) (29). A cohort study reported that METS-IR was associated with elevated risk of stroke and ischemic stroke in patients with hypertension (30). Another large, prospective cohort study demonstrated that the METS-IR was independently associated with a higher risk of all-cause and CVD mortality among Chinese hypertensive population (31). Although these studies involved METS-IR, the association of METS-IR with CVD mortality has not be investigated. The present study filled this research gap and found that RA patients with increased METS-IR had a significantly higher risk of CVD mortality, and METS-IR>2.48 was associated with a significantly greater risk of CVD mortality than METS-IR ≤ 2.25. METS-IR has been shown to be the most recommended formula to evaluate insulin resistance (32), which is developed with FBG, TG, BMI, and HDL-C. FBG level is a superior predictor of mortality risk and may be applied as a simple predictive and preventative factor (33). The results indicated that higher insulin resistance was associated with a significantly increased risk of CVD mortality among patients with RA. Besides, this study clarified the specific strength of the association between excessive insulin resistance status and increased CVD mortality by setting clear METS-IR thresholds, which may provide a reliable indicator for future evaluation of the risk of CVD mortality in the future. In addition, this also added to current literature on the quantitative relationship between insulin resistance and CVD outcomes.
In patients with RA, the association between METS-IR and the risk of CVD mortality may involve several mechanisms: (1) interaction between inflammation and insulin resistance: RA itself is a systemic inflammatory disease, and chronic inflammation can lead to enhanced insulin resistance (34). Pro-inflammatory cytokines such as TNF-α, IL-6, etc., affect insulin sensitivity in adipose tissue, liver, and muscle (35), thereby increasing METS-IR. Insulin resistance is also closely related to the development and progression of atherosclerosis, thereby escalating the risk of CVDs (36); (2) shared pathophysiological mechanisms: RA patients often present with metabolic syndrome, characterized by hyperglycemia, hypertension, and abnormal lipid profiles, which are all critical risk factors for CVDs (37, 38). METS-IR takes into account both basal metabolic rate and insulin sensitivity (22). A higher METS-IR may imply that patients are concurrently facing greater metabolic abnormalities, thus augmenting the risk of CVD mortality; (3) indirect impact of drug therapy: certain medications used to treat RA, such as glucocorticoids and some disease-modifying anti-rheumatic drugs, may unfavorably affect glucose and lipid metabolism, indirectly causing increased insulin resistance and consequently elevating METS-IR, as well as the risk of cardiovascular events (39); (4) limited physical activity and lifestyle factors: due to joint pain and functional limitations, RA patients often experience restricted physical activity and reduced exercise (40), which not only affects metabolic health but may also exacerbate insulin resistance, manifesting as elevated METS-IR, further contributing to the increased risk of CVDs. Consequently, enhanced attention and management of METS-IR are crucial for improving cardiovascular outcomes in RA patients.
Furthermore, the association between METS-IR and CVD mortality differed across age, gender, BMI, diabetes, and CVD subpopulations. To be noted, among RA patients with BMI less than 30 kg/m², there was an association between METS-IR and the risk of CVD mortality, while in RA patients with BMI ≥ 30 kg/m², no such association was found between METS-IR and CVD mortality. Possible explanations include the following points. In obese RA patients with BMI of 30 kg/m² or higher, they may possess other strong risk factors for CVD, such as dyslipidemia, hypertension, and hyperglycemia (41). These factors could potentially mask the impact of METS-IR on CVD mortality risk. In other words, in an obese population, the significance of METS-IR relative to other risk factors might be relatively smaller. Additionally, RA patients with BMI ≥ 30 kg/m² may require additional medications beyond anti-inflammatory drugs, such as hypoglycemic agents or lipid-lowering drugs (42). These medications could exert different regulatory effects on insulin resistance and CVD risk, thereby influencing the relationship between METS-IR and the risk of CVD mortality risk. For another, BMI is associated with both METS-IR and CVD mortality risk (28). This may make it challenging in statistical analyses to discern the independent contribution of METS-IR to CVD mortality risk. Therefore, while METS-IR might still be related to insulin resistance and metabolic abnormalities in obese RA patients, it may cease to be a decisive predictive marker for the risk of CVD death due to the multiple risk factors and complex physiological and pathological changes brought about by obesity. Further clinical research is required to substantiate and clarify the detailed mechanisms underlying this phenomenon.
The strengths of this study included: the relationship between insulin resistance and CVD death was first explore in RA patients; the long follow-up time ensured the sample size of outcome events (CVD death); the samples were obtained through multi-stage complex sampling from the NHANES database and showed good representativeness. This work reinforces the importance of not only focusing on traditional risk factors when preventing and managing CVDs, but also emphasizing early diagnosis and intervention for insulin resistance. Based on the findings of this study, clinicians should consider incorporating the METS-IR index into routine assessment of CVD mortality risk, particularly for patients whose METS-IR exceeds certain thresholds. Some limitations should be mentioned. On the one hand, information on disease history was obtained through questionnaire surveys, which may be subject to recall bias. On the other hand, due to database constraints, glycemic and lipid indices were obtained from single-time-point measurements. Also, the data were obtained in populations in the United State, and the generalization of the results in other populations should be done with caution. Further investigation is needed to understand the potential influence of changes in METR-IS score on the CVD mortality risk for RA patients.
Conclusion
Elevated METS-IR levels was associated with CVD mortality Further, the association between METS-IR and CVD mortality varied across age, gender, BMI, diabetes, and CVD subgroups. More studies are warranted to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University for the studies involving humans. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. JG: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1444800/full#supplementary-material
References
1. Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. (2021) 10:2857. doi: 10.3390/cells10112857
2. Jang S, Kwon EJ, Lee JJ. Rheumatoid arthritis: pathogenic roles of diverse immune cells. Int J Mol Sci. (2022) 23:905. doi: 10.3390/ijms23020905
3. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol Int. (2021) 41:863–77. doi: 10.1007/s00296-020-04731-0
4. Black RJ, Lester S, Tieu J, Sinnathurai P, Barrett C, Buchbinder R, et al. Mortality estimates and excess mortality in rheumatoid arthritis. Rheumatol (Oxford). (2023) 62:3576–83. doi: 10.1093/rheumatology/kead106
5. Fragoulis GE, Panayotidis I, Nikiphorou E. Cardiovascular risk in rheumatoid arthritis and mechanistic links: from pathophysiology to treatment. Curr Vasc Pharmacol. (2020) 18:431–46. doi: 10.2174/1570161117666190619143842
6. Lee EE, Shin A, Lee J, Lee JH, Ha YJ, Lee YJ, et al. All-cause and cause-specific mortality of patients with rheumatoid arthritis in korea: A nation-wide population-based study. Joint Bone Spine. (2022) 89:105269. doi: 10.1016/j.jbspin.2021.105269
7. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. Bmj. (2018) 361:k1036. doi: 10.1136/bmj.k1036
8. van den Hoek J, Boshuizen HC, Roorda LD, Tijhuis GJ, Nurmohamed MT, van den Bos GA, et al. Mortality in patients with rheumatoid arthritis: A 15-year prospective cohort study. Rheumatol Int. (2017) 37:487–93. doi: 10.1007/s00296-016-3638-5
9. Meune C, Touzé E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: A systematic review and meta-analysis of cohort studies. Rheumatol (Oxford). (2009) 48:1309–13. doi: 10.1093/rheumatology/kep252
10. Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute st-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. (2019) 18:150. doi: 10.1186/s12933-019-0957-3
11. Zhang X, Li J, Zheng S, Luo Q, Zhou C, Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: A meta-analysis. Biosci Rep. (2017) 37:BSR20170947. doi: 10.1042/bsr20170947
12. Patel TP, Rawal K, Bagchi AK, Akolkar G, Bernardes N, Dias DDS, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. (2016) 21:11–23. doi: 10.1007/s10741-015-9515-6
13. Cai W, Tang X, Pang M. Prevalence of metabolic syndrome in patients with rheumatoid arthritis: an updated systematic review and meta-analysis. Front Med (Lausanne). (2022) 9:855141. doi: 10.3389/fmed.2022.855141
14. Gremese E, Ferraccioli G. The metabolic syndrome: the crossroads between rheumatoid arthritis and cardiovascular risk. Autoimmun Rev. (2011) 10:582–9. doi: 10.1016/j.autrev.2011.04.018
15. Wang Z, Lan T, Zhang L, Luo J, Wang J, Li L, et al. Predictive value of the tyg index and rheumatoid factor for cardiovascular disease risk in a rheumatoid arthritis population: data from a survey of 418 patients. Lipids Health Dis. (2022) 21:122. doi: 10.1186/s12944-022-01735-6
16. Guin A, Sinhamahapatra P, Misra S, Choudhury Mazumder SR, Chatterjee S, Ghosh A. Incidence and effect of insulin resistance on progression of atherosclerosis in rheumatoid arthritis patients of long disease duration. BioMed J. (2019) 42:394–402. doi: 10.1016/j.bj.2019.01.007
17. Kang Y, Park HJ, Kang MI, Lee HS, Lee SW, Lee SK, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. (2013) 15:R194. doi: 10.1186/ar4384
18. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. (1979) 237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214
19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/bf00280883
20. Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. (2021) 20:76. doi: 10.1186/s12933-021-01268-9
21. Chiang JK, Lai NS, Chang JK, Koo M. Predicting insulin resistance using the triglyceride-to-high-density lipoprotein cholesterol ratio in Taiwanese adults. Cardiovasc Diabetol. (2011) 10:93. doi: 10.1186/1475-2840-10-93
22. Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. Mets-ir, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. (2018) 178:533–44. doi: 10.1530/eje-17-0883
23. Ding L, Gao YH, Li YR, Huang YF, Wang XY, Qi X. Metabolic score for insulin resistance is correlated to adipokine disorder and inflammatory activity in female knee osteoarthritis patients in a Chinese population. Diabetes Metab Syndr Obes. (2020) 13:2109–18. doi: 10.2147/dmso.S249025
24. Wang Z, Xie J, Wang J, Feng W, Liu N, Liu Y. Association between a novel metabolic score for insulin resistance and mortality in people with diabetes. Front Cardiovasc Med. (2022) 9:895609. doi: 10.3389/fcvm.2022.895609
25. Statistics NCfH. About Nhanes (2022). Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed May 31, 2023).
26. Tian P, Xiong J, Wu W, Shi S, Chen A, Chen K, et al. Impact of the malnutrition on mortality in rheumatoid arthritis patients: A cohort study from nhanes 1999-2014. Front Nutr. (2022) 9:993061. doi: 10.3389/fnut.2022.993061
27. Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from nhanes 1999-2018. Arthritis Res Ther. (2023) 25:34. doi: 10.1186/s13075-023-03018-6
28. Qian T, Sheng X, Shen P, Fang Y, Deng Y, Zou G. Mets-ir as a predictor of cardiovascular events in the middle-aged and elderly population and mediator role of blood lipids. Front Endocrinol (Lausanne). (2023) 14:1224967. doi: 10.3389/fendo.2023.1224967
29. Duan M, Zhao X, Li S, Miao G, Bai L, Zhang Q, et al. Metabolic score for insulin resistance (METS-IR) predicts all-cause and cardiovascular mortality in the general population: evidence from NHANES 2001-2018. Cardiovasc Diabetol. (2024) 23:243. doi: 10.1186/s12933-024-02334-8
30. Cai X, Hu J, Zhu Q, Wang M, Liu S, Dang Y, et al. Relationship of the metabolic score for insulin resistance and the risk of stroke in patients with hypertension: A cohort study. Front Endocrinol. (2022) 13:1049211. doi: 10.3389/fendo.2022.1049211
31. Zhang L, Yu C, Wang T, Zhou W, Bao H, Cheng X. Association of the metabolic score for insulin resistance with cardiovascular diseases, cardiovascular and all-cause mortality in Chinese hypertensive population. Front Endocrinol. (2023) 14:1326436. doi: 10.3389/fendo.2023.1326436
32. Paublini H, López González AA, Busquets-Cortés C, Tomas-Gil P, Riutord-Sbert P, Ramírez-Manent JI. Relationship between atherogenic dyslipidaemia and lipid triad and scales that assess insulin resistance. Nutrients. (2023) 15:2105. doi: 10.3390/nu15092105
33. Kityo A, Lee SA. Association of cardiometabolic factors and insulin resistance surrogates with mortality in participants from the Korean genome and epidemiology study. Lipids Health Dis. (2023) 22:210. doi: 10.1186/s12944-023-01981-2
34. Sattar N, Kitas GD. Rheumatoid arthritis: testing the inflammation-insulin resistance link in clinical trials. Nat Rev Rheumatol. (2013) 9:702–3. doi: 10.1038/nrrheum.2013.178
35. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. BioMed Pharmacother. (2021) 137:111315. doi: 10.1016/j.biopha.2021.111315
36. Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. (2019) 40:1447–67. doi: 10.1210/er.2018-00141
37. Santos-Moreno P, Rodríguez-Vargas GS, Martínez S, Ibatá L, Rojas-Villarraga A. Metabolic abnormalities, cardiovascular disease, and metabolic syndrome in adult rheumatoid arthritis patients: current perspectives and clinical implications. Open Access Rheumatol. (2022) 14:255–67. doi: 10.2147/oarrr.S285407
38. Piya MK. Metabolic syndrome, rheumatoid and psoriatic arthritis: managing cardiovascular risk. Int J Rheum Dis. (2021) 24:1103–5. doi: 10.1111/1756-185x.14197
39. Nicolau J, Lequerré T, Bacquet H, Vittecoq O. Rheumatoid arthritis, insulin resistance, and diabetes. Joint Bone Spine. (2017) 84:411–6. doi: 10.1016/j.jbspin.2016.09.001
40. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. (2017) 4:Cd011279. doi: 10.1002/14651858.CD011279.pub3
41. Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. (2019) 92:98–107. doi: 10.1016/j.metabol.2018.10.011
Keywords: cardiovascular disease, metabolic score for insulin resistance, mortality, prognosis, rheumatoid arthritis
Citation: Zhou Y and Gao J (2024) Association between metabolic score for insulin resistance and cardiovascular disease mortality in patients with rheumatoid arthritis: evidence from the NHANES 1999-2018. Front. Endocrinol. 15:1444800. doi: 10.3389/fendo.2024.1444800
Received: 07 June 2024; Accepted: 26 August 2024;
Published: 13 September 2024.
Edited by:
Oscar Lorenzo, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), SpainReviewed by:
Azadeh Anna Nikouee, Loyola University Chicago, United StatesSadiq Umar, University of Illinois Chicago, United States
Copyright © 2024 Zhou and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Gao, YmV0aHVuZWdhb2ppZUBvdXRsb29rLmNvbQ==
 Yan Zhou1,2
Yan Zhou1,2 Jie Gao
Jie Gao