- 1Thermal and Mountain Medicine Division, United States (U.S.) Army Research Institute of Environmental Medicine, Natick, MA, United States
- 2Office of the Senior Scientist, United States (U.S.) Army Research Institute of Environmental Medicine, Natick, MA, United States
The Food and Drug Administration’s (FDA) obesity drug guidance is set on the basis of body mass index (BMI), with thresholds of either BMI ≥30 or BMI ≥27 kg/m2 with weight-related comorbidities. While BMI is associated with obesity-related health outcomes, there are known limitations to use as a direct measure of body fat or metabolic health, and the American Medical Association has highlighted limitations of BMI in assessing individual obesity risks. BMI thresholds impose a barrier to treatment. In a sample from the NHANES dataset (n=6,646 men and women), 36% of individuals with metabolic syndrome (MetS) may not be eligible for obesity pharmacotherapy. This analysis provides quantifiable justification for refinement of the BMI treatment criteria with a more holistic assessment of individual obesity-related disease risk.
Introduction
It has been nearly 25 years since the Centers for Disease Control and Prevention (CDC) declared obesity an epidemic (1). Recent approvals of drugs for treatment of obesity are leading to a new age of therapeutic options. In 2015, the Food and Drug Administration (FDA) approved its first set of pharmacotherapy options for the treatment of obesity (liraglutide, phentermine/topiramate, and naltrexone/bupropion). This milestone marked a shift into an era of actual clinical treatment options for obesity. Indeed, liraglutide combined with exercise and low-calorie diet was very effective in treating metabolic syndrome (2, 3).
Existing FDA guidance for obesity treatment requires use of body mass index (BMI; kg/m2) for eligibility. Current FDA obesity drug guidance targets persons with BMI ≥30 or those BMI ≥27 with at least one accompanying weight-related comorbidity (e.g., hypertension, type 2 diabetes mellitus (T2DM), or dyslipidemia) (4). However, ten years since recognizing obesity as a disease, the American Medical Association (AMA) has now highlighted the limitations in BMI for assessing individual obesity risks, discouraging reliance on BMI to deny appropriate insurance reimbursement (5). BMI is a limited surrogate indicator of excess body fat and, at an individual level, BMI is a relatively poor predictor of adiposity or risk of metabolic disease (6).
Metabolic syndrome (MetS) is a primary obesity-related concern, with a doubling major cardiovascular disease outcomes and an increase all-cause mortality 1.5-fold, and with even higher risks among women (7). Since weight loss of 5-10% is shown to significantly improve abnormal components of MetS, the FDA uses 5% weight loss as the benchmark for obesity medications (4). However, in a systematic review of 950,000 MetS individuals, mean BMI ranged from 22-33 kg/m2 (7). Another landmark analysis of 195 countries estimated that 40% of cardiovascular deaths and 39% of high BMI-related deaths occurred among persons with BMI <30 (8). Rigid interpretation of package inserts may exclude many from obesity pharmacotherapy already at high risk for morbidity and mortality.
This analysis provides quantitative data and interpretations for improving these existing guidelines to ensure maximal benefit is allowed for those requiring treatment.
Materials and methods
Study design and sample population
A correlational analysis of data from cross sectional sampling of the US population via the National Health and Nutrition Examination Survey (NHANES) public use datasets was used for this study (9). The NHANES provides a demographically representative sampling of the US population that is collected continuously and collated into two-year datasets. The NHANES has been approved by the NCHS Research Ethics Review Board, therefore this analysis did not require a separate regulatory approval. Each participant within the NHANES study provided written informed consent prior to assessments (9).
As the FDA approved the first set of pharmacotherapy options for treatment of obesity in 2015, NHANES data was obtained from adults surveyed during 2015-2020, to evaluate for MetS prevalence and FDA obesity medication eligibility. An analysis was conducted using data from 6,646 adults (3,219 men, 3,427 women), evaluated for metabolic syndrome (MetS) prevalence and FDA obesity medication eligibility.
Demographic data was stratified by age, sex, race, and ethnicity. Using revised National Cholesterol Education Program Adult Treatment Panel III criteria (10), where MetS was defined as presence of at least 3 components: waist circumference (WC; cm) men ≥102 or women ≥88, blood pressure (BP; mmHg) systolic ≥130 or diastolic ≥85 or hypertension treatment, triglycerides (TG; mg/dL) ≥150 or dyslipidemia treatment, high-density lipoprotein cholesterol (HDL-C; mg/dL) <40 in men or <50 in women, and glucose (mg/dL) ≥100 or dysglycemia treatment. Participants with BMI ≥30 or ≥27 with hypertension, T2DM, or dyslipidemia were FDA-eligible for obesity medications (4). The consort flow diagram is presented in Supplementary Figure S1.
Statistical analyses
Statistical analyses were conducted using a combination of SAS version 9.4 (SAS Institute), SPSS version 28.01.1 (IBM Corporation), and Excel (Microsoft Corporation). Descriptive statistics are shown as mean ± standard deviation, or by number of incidences. Calculations were made for false negative (FN), false positive (FP), true positive (TP), and true negative (TN) observations. McNemar’s test was used to evaluate discordancy between criteria with statistical significance set at p <0.05 (11). Cohen’s kappa test was used to evaluate consistency between criteria with κ <0.40, between 0.40-0.75, and >0.75 denoting marginal, good, and excellent reproducibility, respectively (12). Each of these methods were chosen to describe the 2x2 data as both a contingency table (McNemar’s) and confusion matrix (Cohen’s).
Results
Data analyses were conducted on 6,646 adults (3,219 men, 3,427 women), including self-reported race/ethnicity as 34% non-Hispanic white, 26% Hispanic, 24% non-Hispanic black, 12% non-Hispanic Asian, and 5% non-Hispanic multiple. Descriptive statistics are shown in Supplementary Table S1.
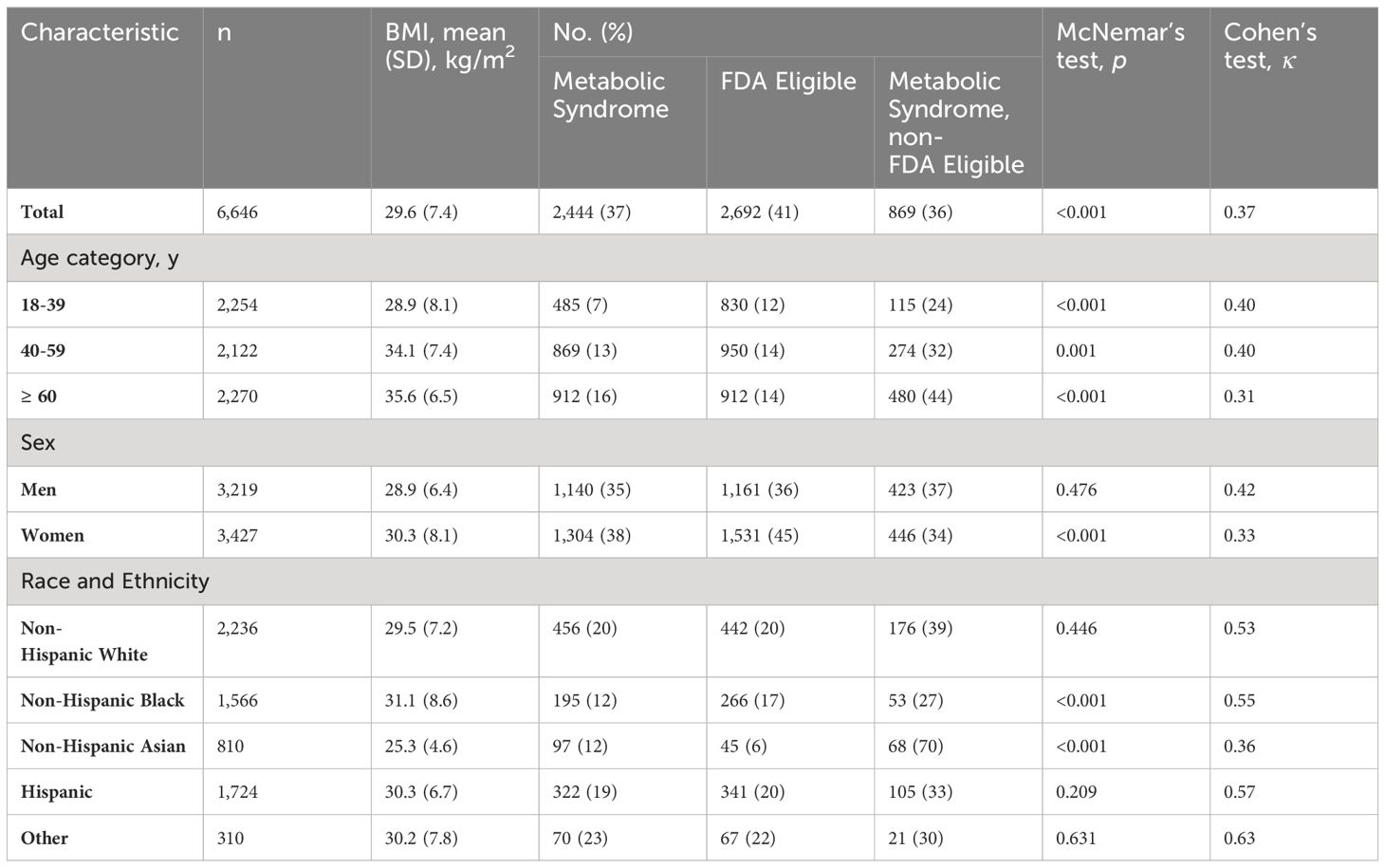
Table 1 describes the main analyses and Figure 1 shows the overall agreement by sex and age groups. Of the 6,646 sample, 37% and 41% met MetS and FDA criteria, respectively. However, of those that met the MetS criteria, 37% of men and 34% of women did not meet the FDA criteria for pharmacological treatment of obesity. Analyses of the total population showed marginal overall agreement (κ =0.37) and significant discordance (p <0.001), and approximately 36% of the total MetS individuals did not meet FDA eligibility. Analyses of by age groups (18-39, 40-59, and ≥60), and for women showed marginal agreement (0.31-0.40) and significant discordance (p <0.001). However, for men analyses showed marginal overall agreement (κ =0.33) and non-significant discordance (p =0.476); while results were mixed for race/ethnicity subgroups (non-Hispanic white κ =0.53; p = 0.446; non-Hispanic black κ =0.55; p <0.001; Non-Hispanic Asian κ =0.36; p <0.001; Hispanic κ =0.57; p = 0.209).
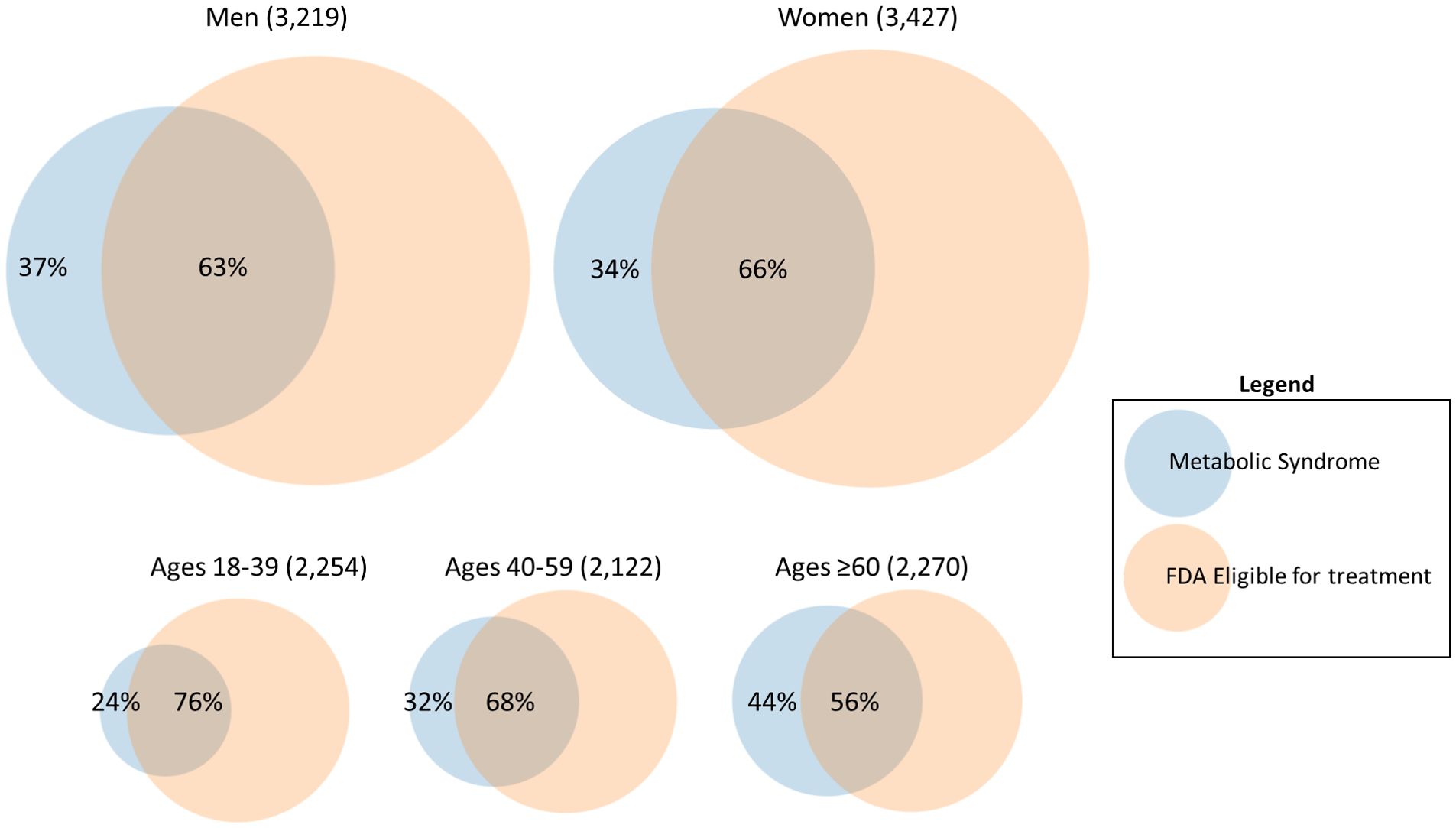
Figure 1 Overall agreement between FDA obesity medication eligibility criteria for individuals with metabolic syndrome, by sex and age groups.
Discussion
The FDA approvals of phentermine-topiramate, bupropion-naltrexone, and liraglutide for obesity by 2015 ushered in a new age of therapeutic options. Now, the AMA is encouraging a more holistic assessment of individual obesity, recognizing the heterogeneity of risk among subgroups. A previous analysis of NHANES mortality data identified normal-weight MetS participants as having the highest mortality risk (13). Recently, liraglutide combined with exercise and a low-calorie diet was highly effective in the treatment of MetS (2, 3). Yet, our analysis of NHANES data demonstrates that many people with MetS may not be eligible for obesity pharmacotherapy. Body mass index thresholds impose a barrier to treatment, where metabolic syndrome, regardless of BMI, significantly increases morbidity and mortality.
Subsets of individuals such as metabolically obese normal weight (MONW) (“skinny fat”) as well as metabolically healthy obesity (MHO) make the body size metric (i.e., BMI) a specific challenge for prescribing obesity-related drugs. Variation in the relationship between body size and metabolic disease risk across race is another disadvantage to the use of BMI metrics, affecting equity of medical care. From our analyses, groups that showed the lowest levels of agreement (i.e., people ≥60 years κ = 0.31 and non-Hispanic Asians κ = 0.36) shows areas that should be investigated further to assess individual, age, or group related differences and how they related to these classification methods. This additionally highlights potential individual differences that are lost within the classifications (i.e., non-Hispanic Asian is very broad) and the complication in using single statistical approaches for aggregate data. It should be noted that a different health risk association with BMI has been previously documented for some Asian Pacific populations compared to western cohorts, where Asians have a lower BMI health risk threshold and Pacific Islanders have a higher BMI health risk threshold (14). Further, fat redistributes from subcutaneous to visceral fat with age (15). These examples highlight the need for a different or additional metric that more closely reflects intraabdominal obesity, perhaps at least inclusion of a waist/height ratio (16).
While caveats to the application of BMI thresholds have been proposed for specific groups and populations (17, 18), other studies have highlighted important differences in relative body fat or distribution in relation to disease risk (6). For example, lower visceral adipose tissue (VAT) for non-Hispanic black populations compared to others with similar BMI and WC measures (19). Significant work has been done showing such differences across race/ethnicity groups for both WC- and BMI-based thresholds of metabolic and cardiovascular disease risk (20–22). Regardless of differences observed between race/ethnicity groups, the use of a crude surrogate metric of adiposity or central adiposity (e.g., BMI, WC) instead of direct, presumably causal, measures of total adiposity or VAT, makes strict interpretation of these criteria inadequate for access to treatment. However, a recent framework proposes use of a wider aperture for diagnosis, staging and managing obesity, and provides a more objective method of classifying obesity from a health perspective than just body size (16). Busetto et al. have suggested a framework for obesity management that goes beyond treatment of only the medical domain of metabolic disease, to include a functional/physical domain as well as a psychological domain of excess fat mass (16). This would incorporate a larger segment of the general population comprised of “apparently healthy” overweight and obese individuals.” (23).
Conclusion
Current Food and Drug Administration (FDA) guidelines using body mass index (BMI) thresholds impose a barrier to pharmacotherapy treatment that excludes a significant portion of adults with obesity-related comorbidities such as metabolic syndrome. These metabolic syndrome positive individuals, regardless of BMI, have significantly increased risk of morbidity and mortality and should be assessed in a more holistic manner.
Data availability statement
Data from this analysis is openly available to anyone under NHANES, found here: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
Ethics statement
The studies involving humans were approved by Data from cross sectional sampling of the US population via the National Health and Nutrition Examination Survey (NHANES) public use datasets was used for this study. The NHANES has been approved by the NCHS Research Ethics Review Board, therefor this analysis did not require a separate regulatory approval. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GC: Conceptualization, Writing – original draft, Writing – review & editing. AP: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. KF: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research was funded by the Military Operational Medicine Research Program, Fort Detrick, MD. Funding source had no role in study design; collection, analysis, and interpretation of data; writing of the report; and has no restrictions regarding the submission of the report for publication.
Acknowledgments
The authors are grateful to Dr. Sean Biggerstaff and Dr. Terry Rauch, Defense for Health Readiness Policy & Oversight OASD(HA), for supporting the initial research initiative on metabolic health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1444568/full#supplementary-material
References
1. Dietz WH. The response of the US Centers for Disease Control and Prevention to the obesity epidemic. Annu Rev Public Health. (2015) 36:575–96. doi: 10.1146/annurev-publhealth-031914-122415
2. Sandsdal RM, Juhl CR, Jensen SB, Lundgren JR, Janus C, Blond MB, et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: a randomized controlled trial. Cardiovasc Diabetology. (2023) 22:41. doi: 10.1186/s12933-023-01765-z
3. Lundgren JR, Janus C, Jensen SB, Juhl CR, Olsen LM, Christensen RM, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. New Engl J Med. (2021) 384:1719–30. doi: 10.1056/NEJMoa2028198
4. Colman E. Food and Drug Administration's obesity drug guidance document: a short history. Circulation. (2012) 125:2156–64. doi: 10.1161/CIRCULATIONAHA.111.028381
5. Berg S. AMA: Use of BMI alone is an imperfect clinical measure . American Medical Association. Available online at: https://www.ama-assn.org/delivering-care/public-health/ama-use-bmi-alone-imperfect-clinical-measure (Accessed June 14, 2023).
6. Potter AW, Chin GC, Looney DP, Friedl KE. Defining overweight and obesity by percent body fat instead of body mass index. J Clin Endocrinol Metab. (2024). doi: 10.1210/clinem/dgae341
7. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 56:1113–32. doi: 10.1016/j.jacc.2010.05.034
8. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
9. National Center for Health Statistics. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available at: https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm.
10. Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, Howard WJ, et al. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). J Am Med Assoc. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
11. McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. (1947) 12:153–7. doi: 10.1007/BF02295996
12. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Measurement. (1960) 20:37–46. doi: 10.1177/001316446002000104
13. Shi TH, Wang B, Natarajan S. The influence of metabolic syndrome in predicting mortality risk among US adults: importance of metabolic syndrome even in adults with normal weight. Prev Chronic Dis. (2020) 17:E36. doi: 10.5888/pcd17.200020
14. Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for Type 2 diabetes screening. Diabetes Care. (2015) 38:150–58. doi: 10.2337/dc14-2391
15. Pascot A, Lemieux S, Lemieux I, Prud'homme D, Tremblay A, Bouchard C, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. (1999) 22:1471–8. doi: 10.2337/diacare.22.9.1471
16. Busetto L, Dicker D, Frühbeck G, Halford JC, Sbraccia P, Yumuk V, et al. A new framework for the diagnosis, staging and management of obesity in adults. Nat Med. (2024) 5:1–5. doi: 10.1038/s41591-024-03095-3
17. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
18. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
19. Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. (2008) 16:600–7. doi: 10.1038/oby.2007.92
20. Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity–specific waist circumference cutoffs for identifying cardiovascular disease risk factors1–3. Am J Clin Nutr. (2005) 81:409–15. doi: 10.1093/ajcn.81.2.409
21. Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi-Sunyer X, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity. (2006) 14:727–36. doi: 10.1038/oby.2006.83
22. Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity. (2011) 19:402–8. doi: 10.1038/oby.2010.248
Keywords: body fat, body mass index, BMI, metabolic syndrome, obesity
Citation: Chin GC, Potter AW and Friedl KE (2024) Body mass index is a barrier to obesity treatment. Front. Endocrinol. 15:1444568. doi: 10.3389/fendo.2024.1444568
Received: 05 June 2024; Accepted: 19 July 2024;
Published: 01 August 2024.
Edited by:
Valeria Guglielmi, Policlinico Tor Vergata, ItalyReviewed by:
Erica Rossi, Azienda Sanitaria Locale di Brindisi, ItalyLuca Colangeli, University of Rome Tor Vergata, Italy
Copyright © 2024 Chin, Potter and Friedl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam W. Potter, YWRhbS53LnBvdHRlci5jaXZAaGVhbHRoLm1pbA==
†ORCID: Adam W. Potter, orcid.org/0000-0003-4980-8353Karl Friedlm, orcid.org/0000-0002-3134-8427
 Geoffrey C. Chin1
Geoffrey C. Chin1 Adam W. Potter
Adam W. Potter Karl E. Friedl
Karl E. Friedl