- 1Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA, United States
- 2Division of Oncology, Children’s Hospital of Philadelphia, Philadelphia, PA, United States
Objective: Levothyroxine (LT4) monotherapy is the current recommended approach for treating pediatric patients post-total thyroidectomy (TT) based on the assumption that peripheral conversion of thyroxine (T4) to triiodothyronine (T3) normalizes thyroid hormone levels. In adults, approximately 15% of post-TT patients on LT4 monotherapy have altered T4:T3 ratios with ongoing debate in regard to the clinical impact with respect to health-related quality of life (hrQOL). The ability to normalize T3 and T4 levels on LT4 monotherapy for pediatric patients’ post-TT is important but not previously described. This study reports data on T3 levels in athyreotic pediatric patients to determine if a similar cohort of patients exists on LT4 monotherapy targeting normalization of TSH (LT4 replacement) or suppression (LT4 suppression).
Methods: Thyroid function tests (TFTs) were retrospectively extracted from medical charts for patients <19 years old who underwent TT for definitive treatment of Graves’ disease (GD) or differentiated thyroid cancer (DTC) between 2010–2021. LT4 dosing was selected to normalize the TSH in GD patients (LT4 replacement) or suppress TSH in DTC patients (LT4 suppression). Pre- and post-surgical TSH, T3 and T4 levels were compared.
Results: Of 108 patients on LT4 replacement (n=53) or LT4 suppression (n=55) therapy, 94% (102/108) of patients demonstrated T3 levels in the normal range post-TT. However, the majority of patients on LT4 replacement (44/53; 83%) and LT4 suppression (31/55; 56%) displayed post-TT T3 levels in the lower half of the normal range despite 50% (22/44) and 48% (15/31) of these patients, respectively, having post-TT fT4 levels above the upper limit of the normal range.
Conclusion: A significant number of pediatric patients do not achieve similar T3 and T4:T3 levels pre- and post-TT. Future multi-center, prospective studies evaluating LT4 monotherapy in comparison to combined LT4/LT3 therapy are warranted to determine the potential clinical impact of altered T3 levels in athyreotic pediatric patients.
Introduction
The thyroid gland is responsible for secreting thyroxine (T4) and triiodothyronine (T3), hormones that influence growth, neurocognitive development and function, metabolism, and mood (1–3). Under tissue-specific regulation and expression of type 1 and type 2 deiodinases, peripheral conversion of the pro-hormone thyroxine (T4) to the active hormone triiodothyronine (T3) accounts for 70–80% of serum T3 concentrations (4, 5). For several decades, levothyroxine (LT4) monotherapy has been the standard approach to care for patients who have undergone total thyroidectomy (TT). This treatment is based on the assumption that peripheral conversion of T4 to T3 is sufficient to achieve normal serum and tissue levels and that thyroid stimulating hormone (TSH) is the most sensitive and specific marker of hypothalamic-pituitary-thyroid axis homeostasis (6, 7). While LT4 monotherapy can normalize serum TSH in athyreotic patients, it may generate low circulating levels of T3 and increased T4:T3 ratios (8). In adults, data demonstrate that even when LT4 is prescribed to target TSH and T4 levels within or above the normal range, up to 15% of patients have T3 levels at or below the lower limit of normal (LLN) (9). A current area of investigation in adults is focused on whether T3 levels on LT4 monotherapy are associated with (1) persistent symptoms, including fatigue, weight gain, depressed mood, (2) decreased health-related quality of life (hrQOL), and/or (3) treatment dissatisfaction (10, 11).
Liothyronine (LT3) combined with LT4 therapy is an alternative treatment option for hypothyroidism in athyreotic adult patients as it can be tailored to improve T3 levels and T4:T3 ratios compared to LT4 alone (12). There are mixed data in adults regarding whether combined therapy is effective at improving hrQOL, with several studies demonstrating a positive impact (13, 14) and others showing no advantage in using combination therapy to improve hrQOL compared to LT4 monotherapy (8, 15–18). While an ongoing discussion persists regarding best practices to treat post-TT hypothyroidism in adults, the applicability of LT4 monotherapy to normalize thyroid hormone levels in athyreotic children and adolescents is equally important but not previously described. This is the first study to evaluate T3 levels in athyreotic pediatric patients on LT4 monotherapy to determine whether patients achieve T3 normalization and if post-TT thyroid hormone levels are comparable to baseline, pre-operative thyroid hormone levels.
Methods
Selection criteria and cohort
A retrospective chart review was conducted of patients who underwent TT for GD or DTC between January 2010-December 2021 at the Children’s Hospital of Philadelphia. Patients were selected if they had complete TFTs, including a TSH, T3 and free T4 (free T4) prior to surgery and 12 ± 6 months post-TT. The mean treatment duration before TFT testing was 12 ± 3 months. A final cohort of 108 patients meeting the eligibility criteria were included in the analysis, 53 GD patients on LT4 replacement and 55 DTC patients on LT4 suppression post-TT. Patient demographics, medication history, surgical approach, clinical symptoms, and thyroid function tests (TFTs) were collected. TFTs were drawn at CLIA-certified labs and evaluated in accordance with insurance capitation and proximity to the patient’s home or primary care institution. LT4 was dosed to target normalization of TSH (LT4 replacement with TSH in the normal range, between 0.5–4.5 μIU/L) or suppression of TSH (LT4 suppression with TSH <0.5 μIU/L) after TT for GD or DTC, respectively, based on current clinical guidelines (19, 20). TFTs were obtained at various times throughout the day in a non-fasting state.
Statistical analysis
Continuous variables were summarized by mean ± standard deviation for parametric data and median (IQR) for nonparametric data. Categorical variables were summarized by frequency and percent. Due to the retrospective nature of this study with associated variance in laboratory assays used, fT4 and T3 values were grouped into four reference interval categories: below the limit of the normal range, in the lower half of the normal range, in the upper half of the normal range, and above the limit of the normal range. Pre- and post-TT fT4:T3 ratios [(fT4*100) ÷ T3] and TFTs were compared for both cohorts using paired t-test. The fT4:T3 ratio was adopted from Jonklaas et al. (21). P-values ≤0.05 were considered statistically significant. All analyses were performed in JMP Pro 16.
Results
Demographics
Demographics of 53 GD patients on LT4 replacement and 55 DTC patients on LT4 suppression are summarized in Table 1. Patients underwent TT at a median age 14.7 years (IQR=13.0–16.6). Female sex was predominant in both cohorts consistent with the prevalence of autoimmune thyroid disease and DTC in adolescent girls. There was no statistical difference in the mean with SD or median with IQR in the timing for pre-operative and post-operative TFTs, both approximately one month prior to surgery and one year after surgery.

Table 1. Demographic and clinical characteristics of pediatric patients on LT4 monotherapy following thyroidectomy.
TFT concentrations pre- and post-total thyroidectomy
From the total cohort, ninety four percent of patients (102/108; 94%) demonstrated post-TT T3 levels within the normal range. However, the majority of patients on LT4 replacement (44/53; 83%) or LT4 suppression (31/55; 56%) displayed post-TT T3 levels in the lower half or below the normal range despite LT4 dosing targeted to have post-TT fT4 in the upper half (GD) or above the upper limit (DTC) of the normal range (Figure 1).
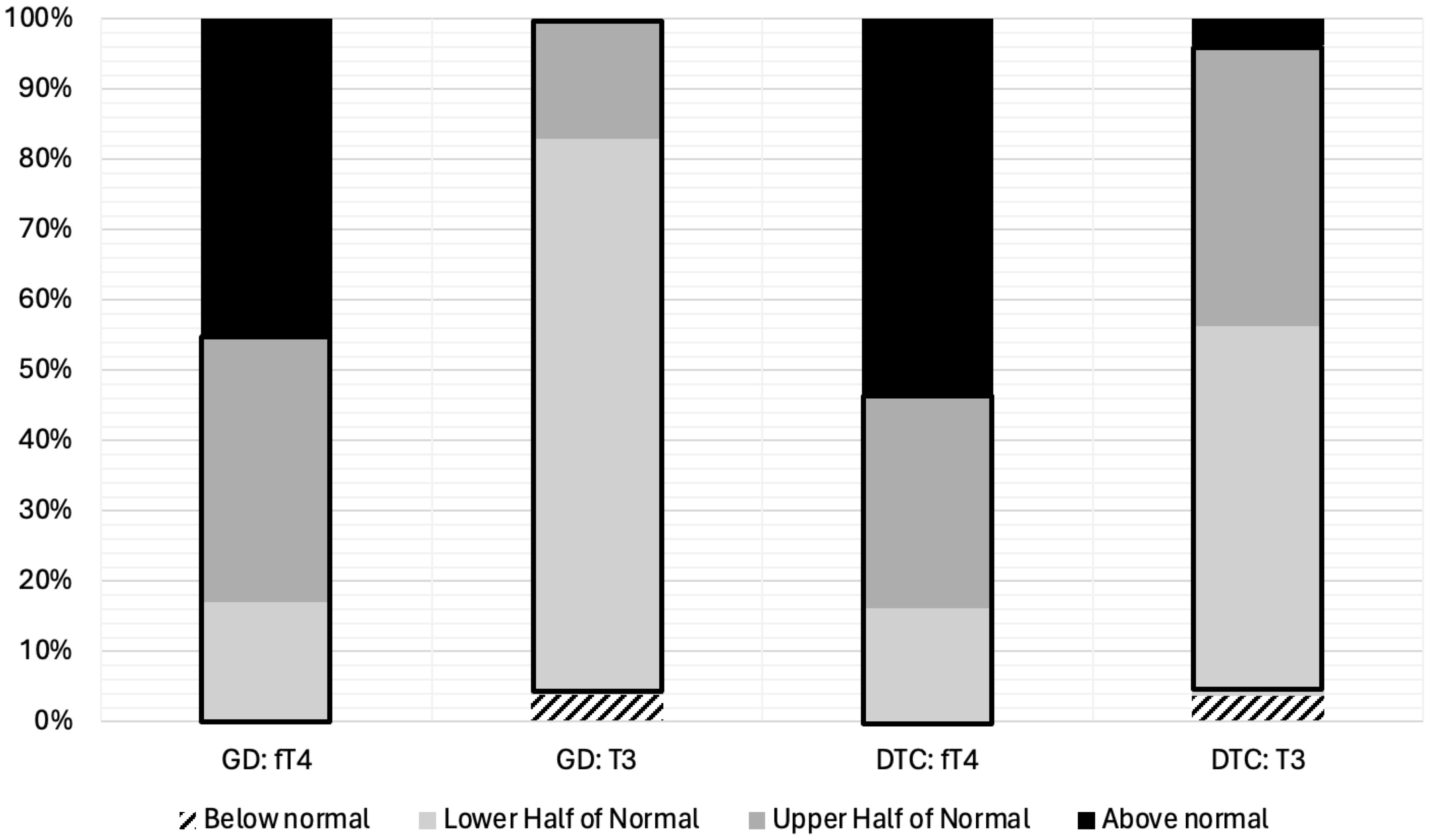
Figure 1. fT4 and T3 Levels Stratified by Quartiles for Pediatric Patients on LT4 Monotherapy Post-Thyroidectomy for Treatment of Graves’ Disease (N=53) or Differentiated Thyroid Cancer (N=55). Thyroid hormone levels have been divided into four categories; (1) below normal, (2) lower half of the normal range, (3) upper half of the normal range, and (4) above the normal range. The normal range is outlined in black. GD, Graves’ Disease; DTC, Differentiated Thyroid Cancer; fT4, free Thyroxine; T3, Triiodothyronine.
TSH, T3, and fT4 concentrations measured pre- and post-TT for GD and DTC patients are presented in Table 2 and Figure 2. In GD patients, the pre-TT T3 levels were above the normal range with a significant decrease in mean T3 concentration observed post-TT with LT4 dosed to normalize the TSH (LT4 replacement therapy, 108 ng/dL, 95% CI=103–114, 235 ng/dL, 95% CI=181–290(p<0.0001), respectively. In contrast, in DTC patients with normal pre-TT T3 and LT4 dosed TSH suppression, there was no significant decrease in mean T3 concentration pre- and post-TT, 139 ng/dL, 95% CI=127–151 compared to 129 ng/dL, 95% CI=122–136 (p=0.083), respectively. Mean TSH and fT4 concentrations were normal with no significant difference for patients with GD (Table 2). In DTC patients, with LT4 suppressive therapy, TSH was significantly lower with associated significantly higher fT4 (Table 2).
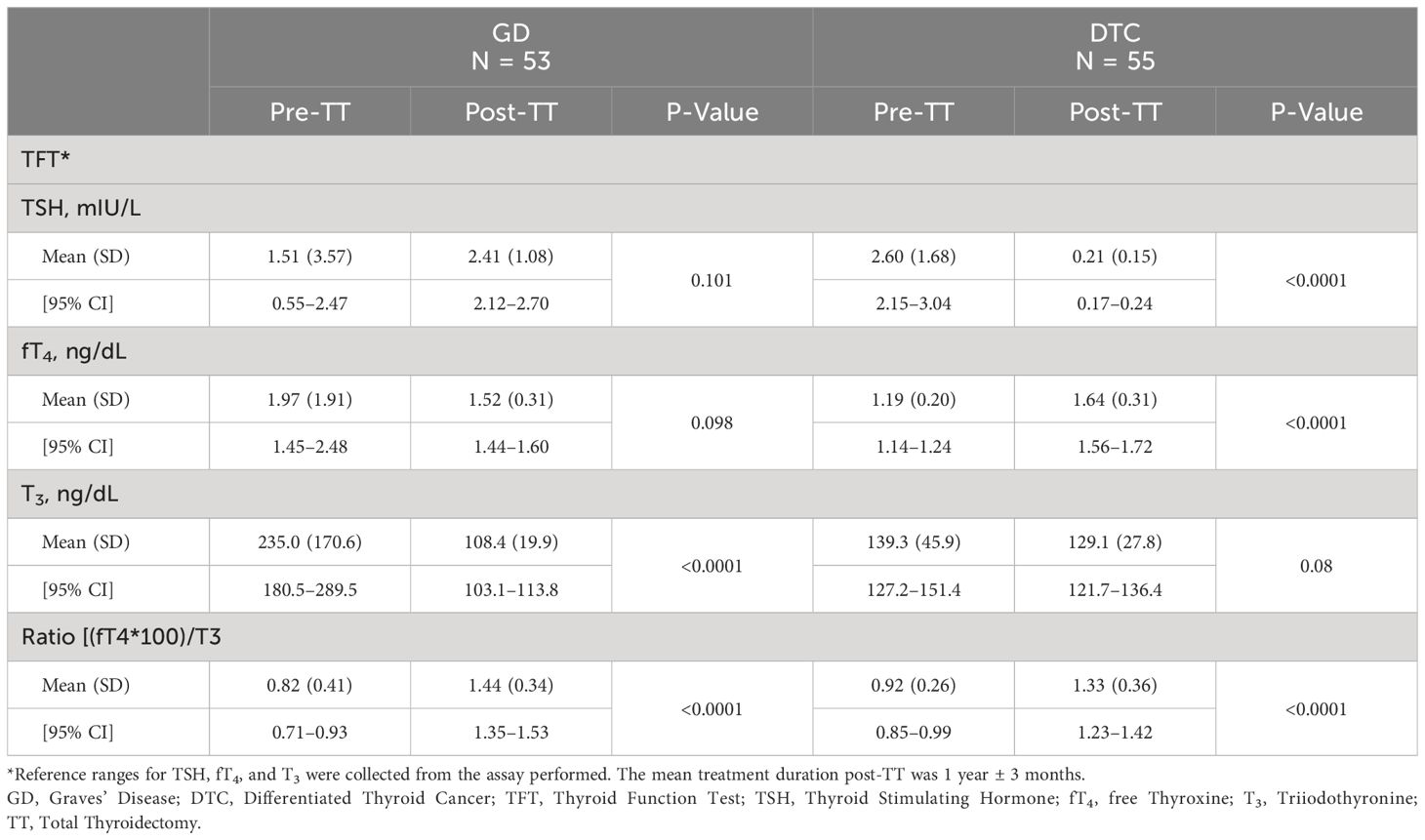
Table 2. TSH, fT4, and T3 concentrations pre- and post-thyroidectomy for pediatric patients on LT4 monotherapy.
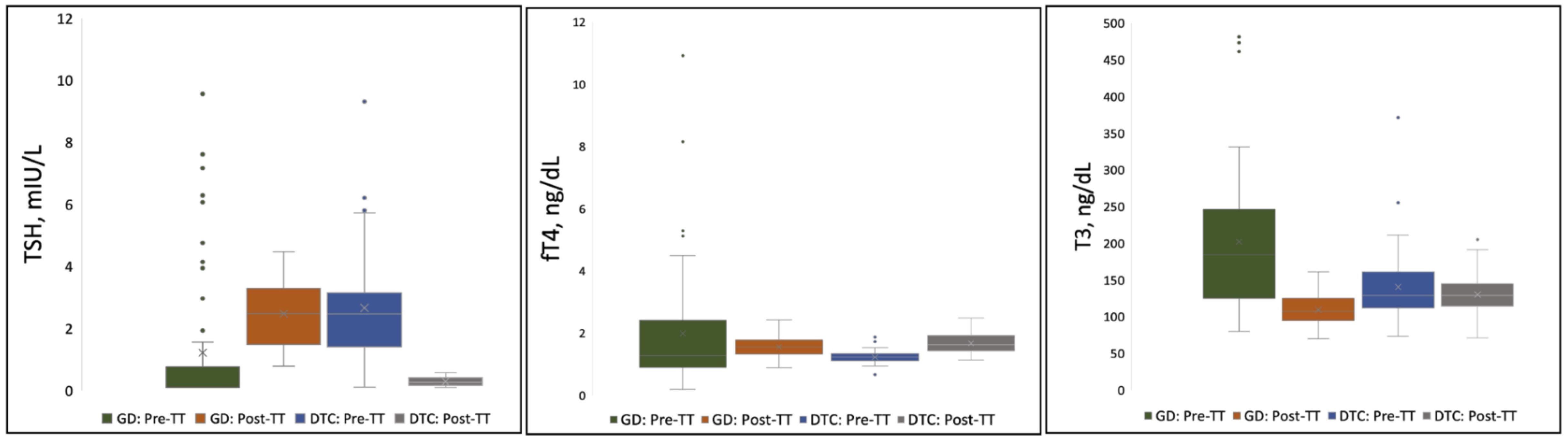
Figure 2. Distribution of TSH, fT4, and T3 Concentrations Pre- and Post-Thyroidectomy for Pediatric Patients on LT4 Monotherapy. Each shaded box represents data within the 25th-75th percentile. Lines extending from the shaded box represent data within the 5th-95th percentile. Values (point markings) above and below the 5th and 95% line represent outliers. “X”in the center of each box represents the arithmetic mean. GD, Graves’ Disease; DTC, Differentiated Thyroid Cancer; TSH, Thyroid Stimulating Hormone; fT4, free Thyroxine; T3, Triiodothyronine; TT, Total Thyroidectomy.
Mean fT4:T3 ratios for both the GD and DTC cohorts, however, were significantly higher post-TT compared to pre-TT (p<0.0001; Table 2), reflecting the majority of patients having a T3 in the lower-half or below the normal range despite high normal to elevated fT4 on LT4 replacement (GD) or suppressive (DTC) therapy. Mean pre- and post-TT fT4:T3 ratios for GD patients were 0.82 (SD=0.41; range=0.17–2.61) and 1.44 (SD=0.34; range=0.68–2.30), respectively. Mean pre- and post-TT fT4:T3 ratios for DTC patients were 0.92 (SD=0.26; range=0.37–1.53) and 1.33 (SD=0.36; range=0.66–2.59), respectively. Expectedly, post-TT T3 and fT4 levels were higher in patients on LT4 suppression compared to patients on LT4 replacement (T3 p<0.0001; fT4 p=0.046).
Discussion
We evaluated the utility of LT4 monotherapy to normalize thyroid hormone levels in a cohort of pediatric patients that underwent thyroidectomy for the treatment of GD or DTC. majority of patients (102/108; 94%) achieved T3 normalization post-TT, 69% (75/108) of patients demonstrated T3 levels in the lower half or below the normal range despite having fT4 in the upper half or above the normal range (Figure 1). The high percentage of patients with an increase in mean fT4:T3 ratio post-TT compared to fT4:T3 ratio pre-TT for both cohorts suggests that peripheral deiodination of exogenous LT4 may be insufficient in achieving similar T4 and T3 levels for some athyreotic pediatric patients. Our findings corroborate previous studies evaluating the efficacy of LT4 monotherapy to achieve normal T3 levels in the treatment of post-TT hypothyroidism in the adult population (8). In fact, our fT4:T3 ratios were comparable to those reported by Jonklaas et al. in 50 athyreotic adults (pre-TT/post-TT): 0.82/1.09 for GD patients and 0.91/1.27 for DTC patients (Table 2) (21).
While the implications of higher fT4:T3 ratios in post-TT patients treated with LT4 monotherapy is not well defined, future studies comparing LT4 monotherapy against LT4/LT3 combination therapy may be worthwhile in athyreotic pediatric patients with (1) low serum T3 concentrations and (2) who demonstrate persistent symptoms despite appropriate TSH in target on LT4 replacement or suppressive dosing. If one targets normalization of T3, T4 and TSH, there is no anticipated risk to combined LT4/LT3 therapy. The potential negative impact of combined therapy includes the additional cost to prescribing LT3 along and T3 surveillance labs and the need for multiple daily doses of LT3 secondary to the short serum half-life of current LT3 formulations. However, if patients achieve improved hrQOL, the benefit of combined therapy would be worth the additional cost and multi-daily dose schedule (22, 23). The use of combined LT4 and LT3 therapy in selected pediatric patients would be in keeping with the joint consensus statement from the American, British, and European Thyroid Associations (24) as well as other adult thyroidologists.
This study is limited by its single-center retrospective design and non-centralized laboratory assay quantification. In addition, there are no data on the clinical benefit of normalizing T3 in pediatric patients in regard to hrQOL, cardiovascular health, or, potentially, growth and development. In fact, the non-specific signs and symptoms of hypothyroidism and multiple confounding variables that impact fatigue, mood, caloric metabolism, and cardiovascular health have precluded completion of a multi-center, prospective study in the adult population secondary to the required cohort size and study cost. One would anticipate the same challenges for a potential, prospective study between LT4 monotherapy and LT4/LT3 combination therapy in pediatrics. Despite these limitations, this study is the first to evaluate T3 levels in athyreotic children and adolescents and provides valuable information that may further inform the pediatric thyroid community in on-going efforts to optimize thyroid hormone therapy management post-TT.
In an effort to optimize the evaluation of thyroid hormone replacement, future studies should also include analysis for single nucleotide polymorphisms of the deiodinase 2 gene, including Thr92Ala, that have previously been found to be associated with decreased T4 to T3 conversion in adults (25, 26), validated, patient-reported hrQOL instruments to assess patient satisfaction, and metabolomic analysis as a potential tool to more completely understand the impact of therapy on intracellular T3 levels (27, 28).
International consortia dedicated to collaborative efforts to improve patient care are critical to conducting these future studies. Accordingly, the authors have established the Child and Adolescent Thyroid Consortium, an international consortium that provides an infrastructure to conduct multi-center studies dedicated to pediatric thyroid disorders (www.thyroidcatc.org).
Conclusion
Similar to adults, a significant number of pediatric patients do not achieve similar T3 and fT4:T3 levels on LT4 monotherapy pre- and post-TT. Future multi-center, prospective studies evaluating LT4 monotherapy in comparison to LT4/LT3 combination therapy are warranted to determine the potential clinical impact of altered T3 levels in athyreotic pediatric patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Children’s Hospital of Philadelphia Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the research involves no more than minimal risk to the subjects. The risks associated with this study are minimal and involve risks to privacy and confidentiality. The research could not practicably be carried out without the waiver or alteration as there are too many patients to retrospectively consent and many patients no longer follow-up with the thyroid center. Additionally, inclusion of all patients is necessary so results of data analysis are not biased.
Author contributions
JB: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. AI: Formal analysis, Writing – review & editing. MB: Writing – review & editing. LA: Data curation, Writing – review & editing. MS: Writing – review & editing. SH: Data curation, Writing – review & editing. LS: Project administration, Writing – review & editing. SG: Project administration, Writing – review & editing. SM: Writing – review & editing. AB: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Children’s Hospital of Philadelphia’s Thyroid Center Frontier Program (GRT-000000495).
Conflict of interest
AB is a consultant for IBSA Pharm and Rare Thyroid Therapeutics/Egetis Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DTC, Differentiated Thyroid Cancer; fT4, Free Thyroxine; GD, Graves’ Disease; hrQOL, Health-Related Quality of Life; LT3, Liothyronine; LT4, Levothyroxine; LLN, Lower Limit of Normal; T3, Triiodothyronine; T4, Thyroxine; TSH, Thyroid Stimulating Hormone; TFT, Thyroid Function Test; TT, Total Thyroidectomy; ULN, Upper Limit of Normal.
References
1. Armstrong M, Asuka E, Fingeret A. Physiology, Thyroid Function. Treasure Island (FL: Statpearls (2023).
2. Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: A brief review. Neurosci Biobehav Rev. (2002) 26:45–60. doi: 10.1016/S0149-7634(01)00037-9
3. Boelaert K, Franklyn JA. Thyroid hormone in health and disease. J Endocrinol. (2005) 187:1–15. doi: 10.1677/joe.1.06131
4. Peeters RP, Visser TJ, Feingold KR, Anawalt B, Blackman MR, Boyce A. Metabolism of thyroid hormone. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al, editors. South Dartmouth, MA: Endotext [Internet] (2000).
5. Dunlap DB. Thyroid function tests. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed. Boston: Butterworth Publishers (1990).
6. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 25:716–59. doi: 10.1089/thy.2014.0460
7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
8. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PloS One. (2011) 6:e22552. doi: 10.1371/journal.pone.0022552
9. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. (2020) 105:e3090–104. doi: 10.1210/clinem/dgaa430
10. Wiersinga WM. T4+T3 combination therapy: an unsolved problem of increasing magnitude and complexity. Endocrinol Metab (Seoul). (2021) 36:938–51. doi: 10.3803/EnM.2021.501
11. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. (2018) 28:707–21. doi: 10.1089/thy.2017.0681
12. Ahluwalia R, Baldeweg SE, Boelaert K, Chatterjee K, Dayan C, Okosieme O, et al. Use of liothyronine (T3) in hypothyroidism: joint british thyroid association/society for endocrinology consensus statement. Clin Endocrinol (Oxf). (2002) 99:206–16. doi: 10.1111/cen.14935
13. Bunevicius R, Jakuboniene N, Jurkevicius R, Cernicat J, Lasas L, Prange AJ Jr. Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for graves’ Disease. Endocrine. (2002) 18:129–33. doi: 10.1385/ENDO:18:2:129
14. Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3’-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. (2009) 161:895–902. doi: 10.1530/EJE-09-0542
15. Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. (2006) 91:2592–9. doi: 10.1210/jc.2006-0448
16. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. (2003) 88:4551–5. doi: 10.1210/jc.2003-030139
17. Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: A randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. (2003) 88:4543–50. doi: 10.1210/jc.2003-030249
18. Lan H, Wen J, Mao Y, Huang H, Chen G, Lin W. Combined T4 + T3 therapy versus T4 monotherapy effect on psychological health in hypothyroidism: A systematic review and meta-analysis. Clin Endocrinol (Oxf). (2022) 97:13–25. doi: 10.1111/cen.14742
19. Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European society for paediatric endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. (2014) 99:363–84. doi: 10.1210/jc.2013-1891
20. Bauer AJ, Wassner AJ. Thyroid hormone therapy in congenital hypothyroidism and pediatric hypothyroidism. Endocrine. (2019) 66:51–62. doi: 10.1007/s12020-019-02024-6
21. Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. (2008) 299:769–77. doi: 10.1001/jama.299.7.769
22. Casula S, Ettleson MD, Bianco AC. Are we restoring thyroid hormone signaling in levothyroxine-treated patients with residual symptoms of hypothyroidism? Endocr Pract. (2023) 29:581–8. doi: 10.1016/j.eprac.2023.04.003
23. Biondi B, Celi FS, McAninch EA. Critical approach to hypothyroid patients with persistent symptoms. J Clin Endocrinol Metab. (2023) 108:2708–16. doi: 10.1210/clinem/dgad224
24. Jonklaas J, Bianco AC, Cappola AR, Celi FS, Fliers E, Heuer H, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: A consensus document. Eur Thyroid J. (2021) 10:10–38. doi: 10.1159/000512970
25. Castagna MG, Dentice M, Cantara S, Ambrosio R, Maino F, Porcelli T, et al. Dio2 thr92ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. (2017) 102:1623–30. doi: 10.1210/jc.2016-2587
26. Penna GC, Salas-Lucia F, Ribeiro MO, Bianco AC. Gene polymorphisms and thyroid hormone signaling: implication for the treatment of hypothyroidism. Endocrine. (2024) 84:309–19. doi: 10.1007/s12020-023-03528-y
27. Benabdelkamel H, Jaber MA, Dahabiyeh LA, Masood A, Almalki RH, Musambil M, et al. Metabolomic profile of patients on levothyroxine treatment for hypothyroidism. Eur Thyroid J. (2023) 12. doi: 10.1530/ETJ-23-0062
Keywords: combination therapy, T4 monotherapy, levothyroxine, hypothyroidism, pediatrics, liothyronine
Citation: Baran J, Isaza A, Bojarsky M, Alzoebie L, Song M, Halada S, Sisko L, Gonzales S, Mostoufi-Moab S and Bauer AJ (2024) Triiodothyronine levels in athyreotic pediatric patients during levothyroxine therapy. Front. Endocrinol. 15:1443394. doi: 10.3389/fendo.2024.1443394
Received: 04 June 2024; Accepted: 22 July 2024;
Published: 14 August 2024.
Edited by:
Malgorzata Gabriela Wasniewska, University of Messina, ItalyReviewed by:
Kaitlyn Liu, University of California, Los Angeles, United StatesGiorgio Radetti, Ospedale di Bolzano, Italy
Copyright © 2024 Baran, Isaza, Bojarsky, Alzoebie, Song, Halada, Sisko, Gonzales, Mostoufi-Moab and Bauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew J. Bauer, YmF1ZXJhQGNob3AuZWR1
 Julia Baran
Julia Baran Amber Isaza
Amber Isaza Mya Bojarsky
Mya Bojarsky Lama Alzoebie1
Lama Alzoebie1 Minkeun Song
Minkeun Song Andrew J. Bauer
Andrew J. Bauer