- Department of Lab Medicine, The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Objective: Thyroid disorders are prevalently diagnosed yet face significant challenges in their accurate identification in China. Predominantly, the reference intervals (RIs) currently in use across Chinese medical facilities derive from company-provided data, lacking stringent scientific validation. This practice underscores the urgent necessity for establishing tailored RIs for thyroid-related hormones, specifically tailored to the coastal area populations. Such refined RIs are imperative for empowering clinicians with the precise tools needed for the accurate diagnosis of both overt and subclinical thyroid conditions.
Methods: This investigation analyzed the medical histories of 6021 euthyroid individuals mainly from East coastal area of China between June 2019 and December 2020. The cohort comprised residents of coastal areas, focusing on extracting insights into the regional specificity of thyroid hormone levels. A thorough examination protocol was implemented, encompassing inquiries into thyroid health history, ultrasound screenings, palpations during thyroid surgery, detections of thyroid antibodies, and reviews of medication histories. Adherence to the CLSI C28-A3 guidelines facilitated the derivation of RIs for thyroid-related hormones, subsequently juxtaposed against those provided by commercial entities.
Results: The study delineated the following gender- and age-specific RIs for Thyroid-Stimulating Hormone (TSH): for males under 50 years, 0.57-3.37; males over 50 years, 0.51-4.03; females under 50 years, 0.53-3.91; and females over 50 years, 0.63-4.31. Further analysis revealed the RIs for Free Thyroxine (FT4), Free Triiodothyronine (FT3), Total Thyroxine (TT4), and Total Triiodothyronine (TT3) amongst males and females, with notable distinctions observed between the two genders and across age brackets. These findings are in stark contrast to the standardized intervals provided by manufacturers, particularly highlighting differences in TT3 and FT3 levels between genders and a tendency for TSH levels to increase with age.
Conclusion: This research successfully establishes refined RIs for thyroid-related hormones within the Chinese coastal area populations, taking into account critical demographic factors such as gender and age. These tailored RIs are anticipated to significantly enhance the diagnostic accuracy for thyroid diseases, addressing the previously noted discrepancies with manufacturer-provided data and underscoring the importance of regionally and demographically adjusted reference intervals in clinical practice.
Introduction
The prevalence of thyroid dysfunction including primary asymptomatic thyroid disorders, in the coastal are of China, represents a significant public health challenge. This condition, often detected during routine health examinations, presents diagnostic difficulties due to the high incidence of subclinical manifestations. The Clinical and Laboratory Standards Institute (CLSI) (1, 2) underscores the complexity of accurately assessing thyroid health, acknowledging the nuanced nature of thyroid disorders and the imperative for refined diagnostic approaches.
The distribution of Thyroid-Stimulating Hormone (TSH) values among are not normally distributed, potentially as a result of preclinical thyroid infections (3). Epidemiological data suggest that 5%-15% of adults in China suffer from hypothyroidism, while 0.5%-2% of the population suffers from hyperthyroidism (4). Consequently, the evaluation of thyroid hormones has become a cornerstone in clinical practice for assessing thyroid function. Under such context, clinicians faces the critical task of delineating accurate and clinically relevant reference intervals (RIs) for thyroid-related hormones, an essential step towards improving the diagnosis and management of thyroid disorders.
To help physicians examine thyroid gland activity more precisely, the formulation of a regional typical RI for thyroid gland hormones was undertaken. The thyroid-related hormones we refer to here include TSH, FT4, FT3, TT4, and TT3. According to statistics, there are fewer than 100 hospitals in the world with regional reference intervals, and most hospitals still use Ris provided by the manufacturer. Based on existing reports, we are concerned that the Ris of thyroid-related hormones is affected by factors such as season, age, gender, different times of day, iodine levels, ethnicity, and differences in measurement methodology (5–8). Detection with different methodologies may have a greater impact on the thyroid-related hormone RIS (9). Therefore, we have more reason to establish an RIS based on the Zhejiang population and the hormone detection methodology of the second hospital affiliated with Zhejiang University.
This study adopts an indirect approach to establish robust and reliable RIs for thyroid-related hormones by leveraging a large dataset from the public health screening database of our institution. This method offers a practical, efficient, and cost-effective alternative to the traditional direct methods. Preliminary comparisons between the newly established RIs and those provided by manufacturers reveal significant discrepancies, highlighting the necessity for regionally adapted RIs in the effective clinical assessment of thyroid function within China’s coastal populations.
Materials and methods
Acquisition of information
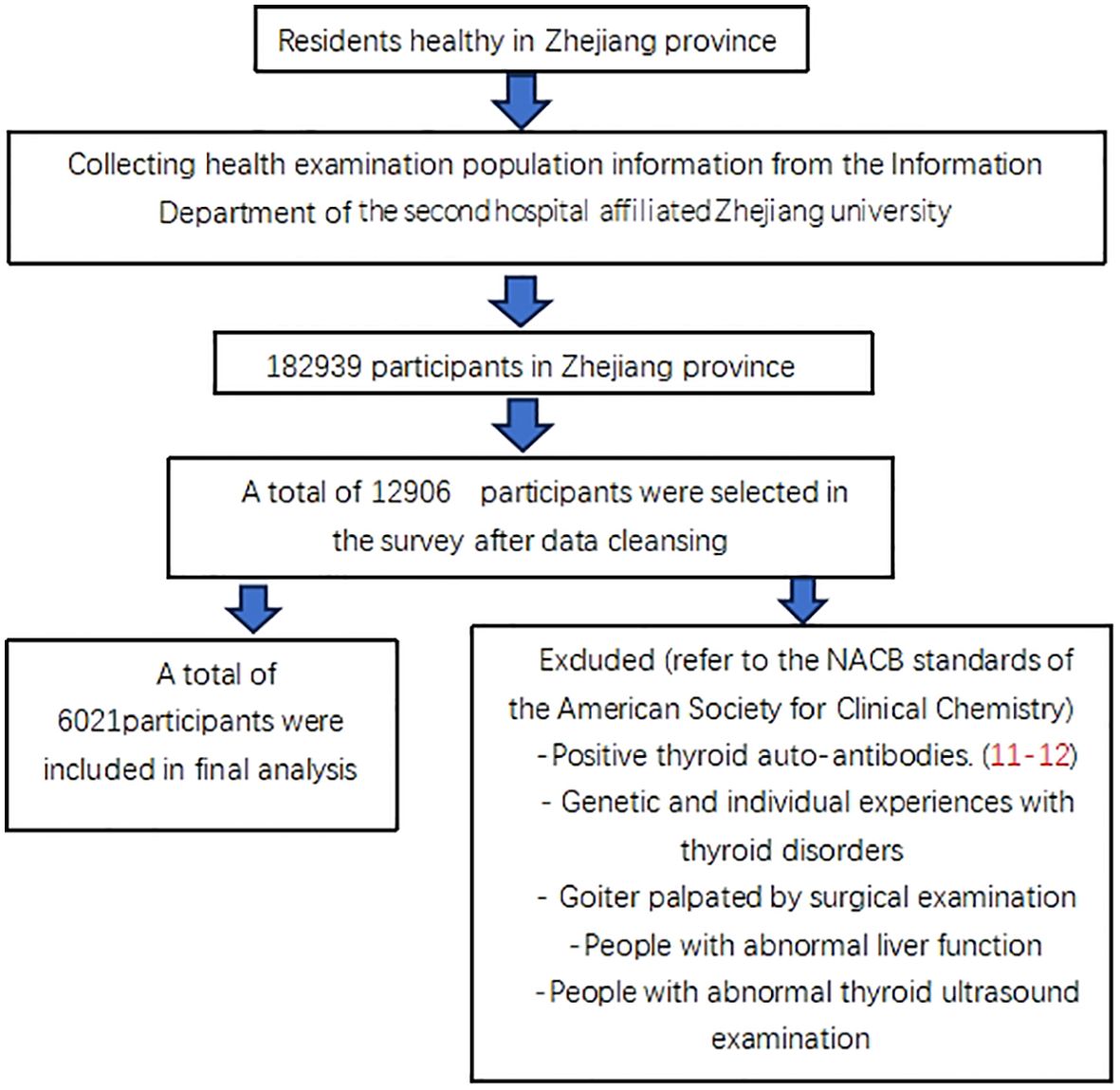
The data was taken from the Health Information System of the Second Affiliated Hospital of Zhejiang University from June to December (2019–20), pertaining to 182,939 healthy people in the Zhejiang Province of China. The information from6021 euthyroid people were ultimately included in this research (10). The additional anonymous analysis was performed, and Figure 1 illustrates the accurate criteria for selection.
The hospital health screening populace dataset was the source of all of the information. During the ten minutes of quiet relaxation, the patient’s height, body mass index, and blood pressure were measured, and their blood was obtained on an empty stomach. The Body Mass Index (BMI) is calculated by dividing body weight in kilograms by the square of height in meters.). Following collection, the blood was spun in yellow-tipped tubes using an accelerating reagent for ten minutes at 3000 revolutions per minute. Five thyroid-related hormones were examined using an Abbott immunoassay analyzer equipped with matching reagents, calibrators, and quality control. The levels of ALT, Cr, TG, TC, and Glu were measured using the 5th Olympus 5800 automated biochemical analyzer, which was equipped with quality controls, calibrators, and matching reagents.
The Ethics Review Committee at Zhejiang University’s Second Affiliated Hospital approved the current study.
The consent form is not required for this research because the findings are retrospective.
Result
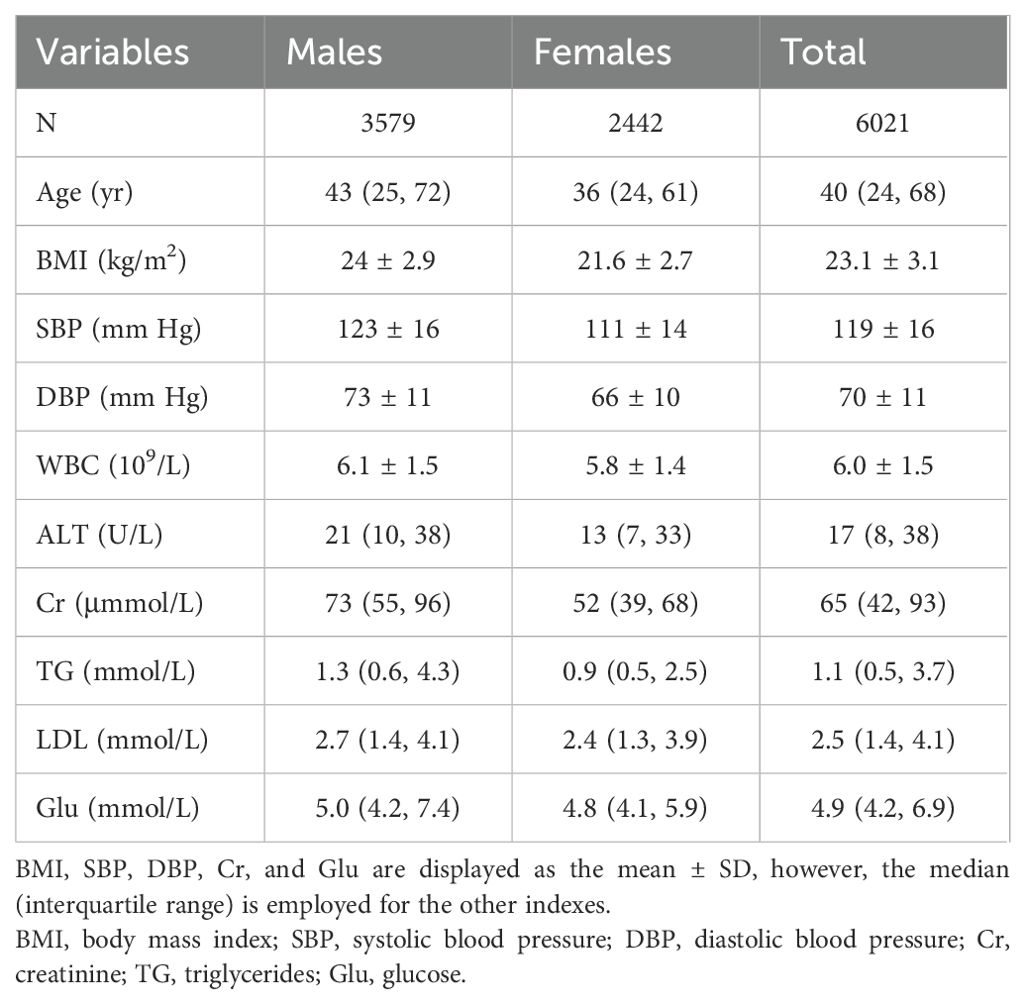
The participants’ demographic details are displayed in Table 1. The mean BMI was 23.1 and the average age was 40. While RIs for particular metrics differ for men and women, it’s important to keep in consideration that women are usually younger than men and exhibit a lower incidence of BMI, blood pressure (systolic and diastolic), ALT, Cr, white blood cell count (WBC), triglycerides (TG), low-density lipoprotein (LDL), and glucose (Glu).
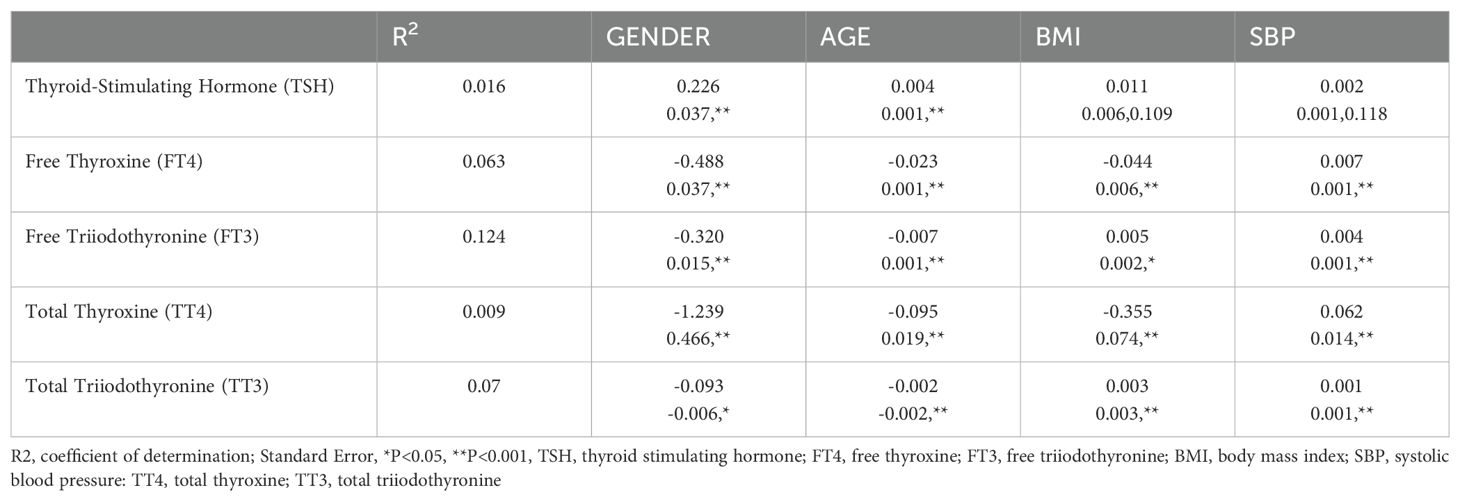
1. The findings of a multiple regression study for thyroid-related hormones are presented in Table 2. In particular, gender-specific indices are significantly greater than other metrics. The distribution variations in thyroid hormones by gender are shown in Figure 2. Specifically, it showed that blood levels of Thyroid-Stimulating Hormone (TSH) increased in females compared to males (P<0.001). However, males indicated higher amounts of Free Triiodothyronine (FT3), and Free Thyroxine (FT4) (p<0.001). Gender did not have a significant impact on overallTotal Triiodothyronine (TT3) (p>0.99)and thyroid (TT4) values (p=0.18).
2. The exact RIs for thyroid-related hormones in normal adult Chinese coastal populations are listed in Table 3. Our results highlight the significance of developing sex-specific RIs for those five hormones. There is a strong association between TSH and age (P<0.001)). To further examine, we split the patients into 5 age-based categories: Group 1, ages 18–29 years old (N = 873); Group 2: 30-39 years old (N = 2100); Group 3: 40–49 years old (N = 1569); Group 4: 50–59 years old (N = 1015); Group 5 consisted of patients who were under 60 years old (N = 464). The TSH proportions of individuals under 50 and those over 50 varied, following subgroup analysis and pooling. From these results (Figure 3), we increased the thyroid-related hormone RIs for healthy people in China’s coastal areas.

Figure 2. The levels of (1) Thyroid Stimulating Hormone (TSH), (2) Free Thyroxine (FT4), (3) Free Triiodothyronine (FT3), (4) Total Thyroxine (TT4), and (5) Total Triiodothyronine (TT3) were analyzed based on gender.
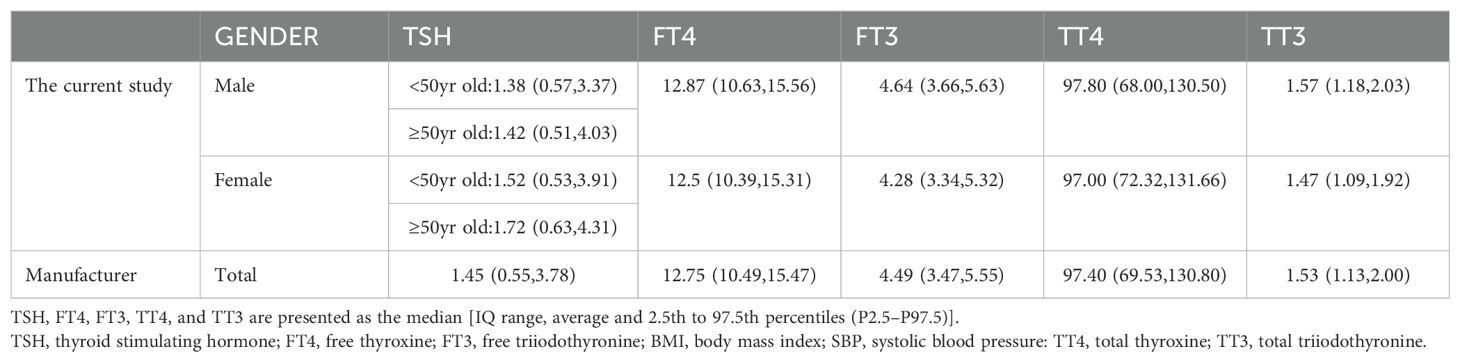
Table 3. The study’s RI for thyroid-associated hormones were contrasted with the manufacturer’s recommendations.
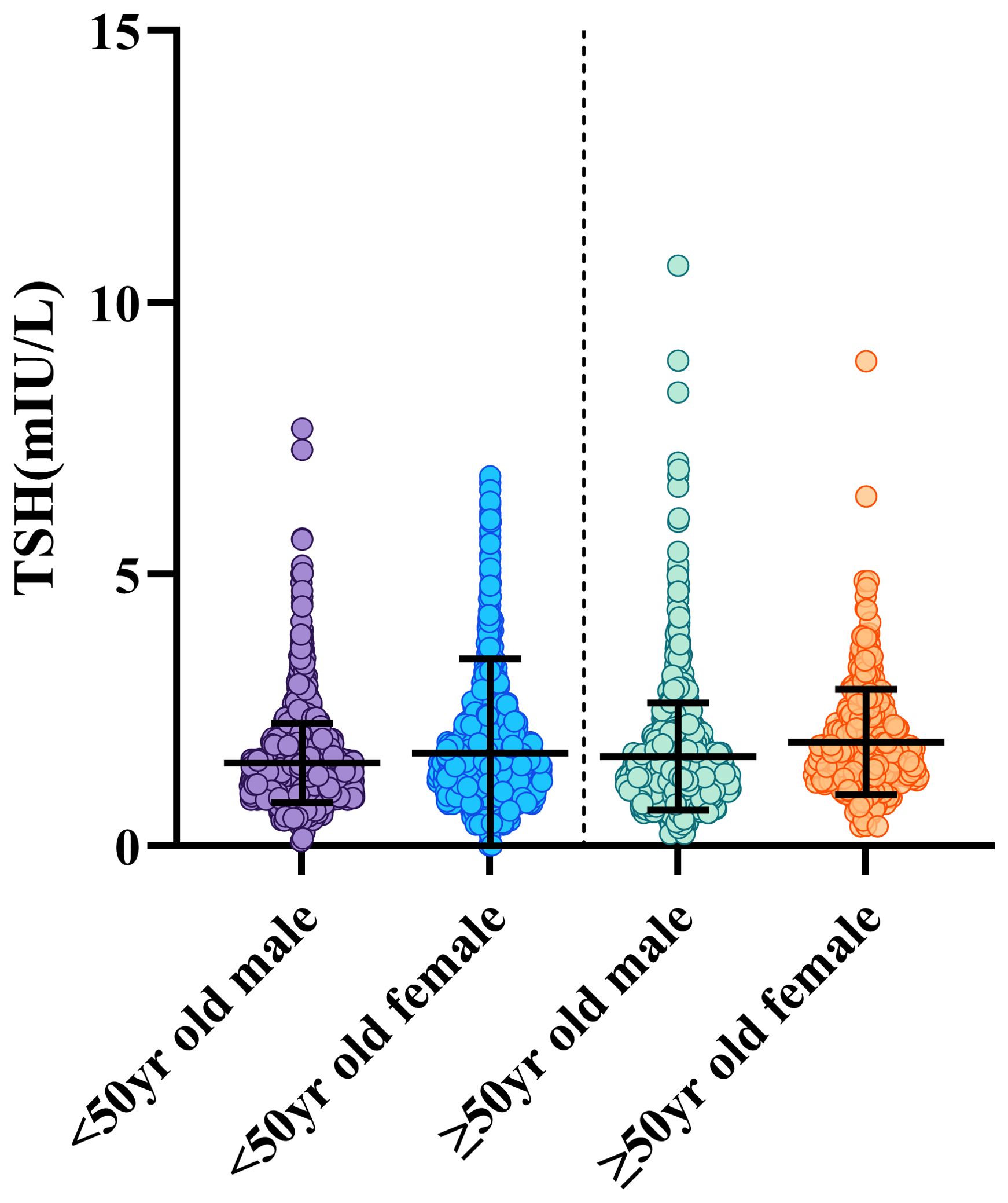
Figure 3. Participants were classified into two age groups: those over 50 and those under 50. The graphic displays the gender and age-related dynamic fluctuations in TSH. The data on men and females are displayed in different colour, including whiskers. The whiskers reach the 2.5th or 97.5th percentage, and the range across the 25th- 75th percentile. To show observed motion, averages are linked by lines and displayed as black lines inside the boxes.
Discussion
In this study, we collected data on all healthy physical examination populations from the His information system of the Second Affiliated Zhejiang University from 2019 to 2020. The study comprised 6021participants in total removing those who failed to meet the eligibility criteria. We used the American Society of Clinical Chemistry’s guidelines as our criteria for exclusion, and we excluded people who had abnormal liver function. At the same time, we reviewed the indoor quality control and interroom quality evaluation of the immunological laboratory of the laboratory department of our hospital from 2019 to 2020 and believed that these records were completely under control. To characterize the RIs of thyroid-related hormones, we analyzed information gathered from P2.5 and P97.5.
The levels of TSH, FT3, FT4, and TT4 were substantially distinct in males and females, and changes in TSH increased with age. This conclusion is consistent with existing reports (13). Furthermore, TSH in Italians adults 0-105 years old showed an unfavorable linear correlation with age (14). These research discrepancies from ours may be a consequence of using various methods or ethnic backgrounds. We also found that in this study, there were large differences in TT4 and FT3 between males and females (p<0.01). This may be related to body fat rate and estrogen levels in both males and females (13). In our study, the TSH levels of males and females aged ≥50 years were 1.42 (0.51, 4.03) and 1.72 (0.63, 4.31), respectively. In existing reports (15), the RIS of TSH in Chinese elderly individuals was 2.28 (0.35, 8.79). The variations observed among these studies possibly originate from differential exclusion standards and demographic attributes. In this study, we excluded people with abnormal liver function and used people with relatively good nutritional levels. It is worth noting that if people with abnormal liver function are not excluded, the calculated RIS is basically consistent with the reported RIS. Therefore, these existing problems need to be further analyzed in future research.
Although previous studies have established the RIs of thyroid-related hormones, this study has its own advantages: 1. The adopted indirect method is more convenient and economical and does not require volunteer recruitment or additional testing. 2. We collected data from the second hospital affiliated Zhejiang university who performed health examinations in the past two years. The samples all used the Abbott immunoassay method to avoid differences in results caused by different methodologies. 3. According to strict exclusion criteria, complete examination information, including thyroid ultrasound results and surgical palpation results, was obtained for all included individuals. Consequently, the specified RIs might offer higher accuracy and relevance for clinical labs using the same approach. 4. It is worth noting that in this study, we excluded people with abnormal liver function when selecting normal people. It is reported that patients with fatty liver, cirrhosis and other liver diseases, their thyroid function will have varying degrees of decline, thyroid related hormones will also have great changes. (16, 17) This study has many limitations. The immune system responses determined here are best suited to the Chinese coastal populace when examined with the Abbott immunoassay analyzer, due to substantial variations among immune detection methods. Several differences in thyroid-related levels of hormones are being documented among different ethnic groups, which is intriguing (18–25). The main population in Zhejiang Province is Han, and the population included in this study needs to be further subdivided in terms of ethnicity. In addition, although we can assume that Zhejiang Province is a coastal province and that the iodine intake of the resident population should be adequate, the iodine status related to thyroid disease needs to be further evaluated.
A global instance of acute hyperthyroidism is now recognized as aTSH level at the RIs’ threshold combined with Free Thyroxine (FT4) and Free Triiodothyronine (FT3) indices above the maximum value of the RIS. Moderate hyperthyroidism is indicated by TSH values above the maximum range and FT4 levels at the minimal range of the RIS. Subclinical thyroid dysfunction is indicated by TSH levels over the threshold and FT4 levels within the reference interval range. Subclinical hyperthyroidism is recognized when blood TSH levels are below the lowered threshold and FT3 and FT4 levels are within the normal range (26, 27). The establishment of a thyroid-stimulated hormone identification system in China’s coastal cities is significant, as it can help prevent those with minor spikes or declines from being misclassified. It is worth noting that the diagnosis of thyroid disease is not just about hormone detection numbers but should also be combined with clinical manifestations to diagnose and treat thyroid dysfunction (28).
In conclusion, Our study emphasizes the significance of determining thyroid-related hormonal RISs that are age-related and gender-specific in China’s coastal metropolitan population based on the methodology of the second hospital affiliated with Zhejiang University. In our postprocessing work, we need to pay more attention to laboratory external quality assessment (EQA) and indoor quality control (IQC) to make the testing work more detailed and accurate, thus reducing the deviation and coefficient of variation between different testing systems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Second Affiliated Hospital of Zhejiang University School of Medicine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the consent form is not required for this research because the findings are retrospective.
Author contributions
XH: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. XY: Writing – review & editing, Formal analysis, Data curation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med. (2016) 26:5–16. doi: 10.11613/BM.2016.001
2. Arseneau E, Balion CM. Statistical methods used in the calculation of geriatric reference intervals: a systematic review. Clin Chem Lab Med. (2016) 54:377–88. doi: 10.1515/cclm-2015-0420
3. Yamada S, Horiguchi K, Akuzawa M. The impact of age- and sex-specific reference ranges for serum TSH and FT4 on the diagnosis of subclinical thyroid dysfunction: A multicenter study from Japan. Thyroid. (2023) 33(9):1134. doi: 10.1089/thy.2022.0567
4. Ma C, Zhong J, Zou Y, Liu Z, Li H, Pang J. Establishment of Reference Intervals for Thyroid-Associated Hormones Using refineR Algorithm in Chinese Population at High-Altitude Areas. Front Endocrinol (Lausanne). (2022) 13:816970. doi: 10.3389/fendo.2022.816970
5. Andrade J, Khairy P, Dobrev D, Nattel S. The association between obesity, metabolic abnormality and thyroid function. Circ Res. (2014) 114(9):1453–68. doi: 10.1161/CIRCRESAHA.114.303211
6. National Guideline Centre (UK). Thyroid function tests: thyroid disease: assessment and management: evidence review C (2019). London, UK: National Institute for Health and Care Excellence (NICE. Available online at: https://www.nice.org.uk/guidance/ng145 (Accessed February 24, 2023).
7. Du Puy RS, Postmus I, Stott DJ, Blum MR, Poortvliet RKE, Elzen WPJD. A multicenter analysis of thyroid-stimulating hormone level in apparently healthy elderly population in China. Nan Fang Yi Ke Da Xue Xue Bao. (2023) 43(1):1–7. doi: 10.12122/j.issn.1673-4254.2023.01.01
8. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical En docrinologists and the American Thyroid Association. Thyroid. (2012) 22:1200–35. doi: 10.1089/thy.2012.0205
9. Thienpont LM, Van Uytfanghe K, De Grande LAC, Reynders D, Das B, Faix JD, et al. IFCC Committee for Standardization of Thyroid Function Tests (C-STFT). Harmonization of serum thyroidstimulating hormone measurements paves the way for the adoption of a more uniform reference interval. Clin Chem. (2017) 63:1248–60. doi: 10.1373/clinchem.2016.269456
10. Jones GRD, Haeckel R, Loh TP, Sikaris K, Streichert T, Katayev A, et al. IFCC Committee on Reference Intervals and Decision Limits. Indirect methods for reference interval determination—Review and recommendations. Clin Chem Lab Med. (2018) 57:20–9. doi: 10.1515/cclm-2018-0073
11. Drinka PJ, Nolten WE, Voeks S, Langer E. Thyroid stimulating hormone elevation without antithyroid antibody elevation in nursing home patients. J Am Geriatr Soc. (1991) 39:1000–1. doi: 10.1111/j.1532-5415.1991.tb04047.x
12. Eskelinen S, Suominen P, Vahlberg T, Löppönen M, Isoaho R, Kivelä S-L, et al. The effect of thyroid antibody positivity on reference intervals for thyroid stimulating hormone (TSH) and free thyroxine (FT4) THYROID TEST REFERENCE INTERVALS 405 Downloaded by Shanghai Jiao Tong University from www.liebertpub.com at 04/29/23. For personal use only. in an aged population. Clin Chem Lab Med. (2005) 43:1380–5. doi: 10.1515/CCLM.2005.236
13. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182
14. Clerico A, Trenti T, Aloe R, Dittadi R, Rizzardi S, Migliardi M, et al. A multicenter study for the evaluation of the reference interval for TSH in Italy (ELAS TSH Italian Study). Clin Chem Lab Med. (2018) 57(2):259–67. doi: 10.1515/cclm-2018-0541
15. Lips MA, Pijl H, van Klinken JB, de Groot GH, Janssen IM, Van Ramshorst B, et al. Roux-en-Y gastric bypass and calorie restriction induce comparable time-dependent effects on thyroid hormone function tests in obese female subjects. Eur J Endocrinol. (2013) 169:339–47. doi: 10.1530/EJE-13-0339
16. Wang K, Li H, Deng X, Yan S, Qiu Y, Cong Y. A multicenter analysis of thyroid-stimulating hormone level in apparently healthy elderly population in China. Nan Fang Yi Ke Da Xue Xue Bao. (2023) 43(1):1–7. doi: 10.12122/j.issn.1673-4254.2023.01.01
17. Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction associated steatotic liver disease (MASLD): Beyond insulin resistance. J Hepatol. (2023) 79(6):1524–41. doi: 10.1016/j.jhep.2023.08.030
18. Wan H, Yu G, Xu S, Chen X, Jiang Y, Duan H, et al. Central sensitivity to free triiodothyronine with MAFLD and its progression to Liver Fibrosis in euthyroid adults. J Clin Endocrinol Metab. (2023) 108(9):e687–97. doi: 10.1210/clinem
19. Clerico A, Trenti T, Aloe R, Dittadi R, Rizzardi S, Migliardi M, et al. A multicenter study for the evaluation of the reference interval for TSH in Italy (ELAS TSH Italian Study). Clin Chem Lab Med. (2018) 57(2):259–67. doi: 10.1515/cclm-2018-0541
20. Tozzoli R, D’Aurizio F, Metus P, Steffan A, Mazzon C, Bagnasco M. Reference intervals for thyrotropin in an area of Northern Italy: the Pordenone thyroid study (TRIPP). J Endocrinol Invest. (2018) 41:985–94. doi: 10.1007/s40618-018-0825-0
21. Yeap BB, Manning L, Chubb SA, Hankey GJ, Golledge J, Almeida OP, et al. Reference ranges for thyroid-stimulating hormone and free thyroxine in older men: results from the health in men study. J Gerontol A Biol Sci Med Sci. (2017) 72:444–9. doi: 10.1093/gerona/glw132
22. Feng Y, Bian W, Mu C, Xu Y, Wang F, Qiao W, et al. Establish and verify TSH reference intervals using optimized statistical method by analyzing laboratory-stored data. J Endocrinol Invest. (2014) 37:277–84. doi: 10.1007/s40618-013-0031-z
23. Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and genderspecific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J Clin Endocrinol Metab. (2013) 98:1147–53. doi: 10.1210/jc.2012-3191
24. Li C, Guan H, Teng X, Lai Y, Chen Y, Yu J, et al. An epidemiological study of the serum thyrotropin reference range and factors that influence serum thyrotropin levels in iodine sufficient areas of China. Endocr J. (2011) 58:995–1002. doi: 10.1507/endocrj.K11E-101
25. Yoshihara A, Noh JY, Ohye H, Sato S, Sekiya K, Kosuga Y, et al. Reference limits for serum thyrotropin in a Japanese population. Endocr J. (2011) 58:585–8. doi: 10.1507/endocrj.K11E-082
26. Sakata N, Okumura Y. Diagnosis and treatment of hypothyroidism in old people Reference intervals in the diagnosis of thyroid dysfunction: treating patients not numbers. Clin Interv Aging. (2018) 13:1219–23. doi: 10.2147/CIA.S168182
27. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF. Subclinical thyroid disease scientific review and guidelines for diagnosis and management. JAMA. (2004) 291(2):228–38. doi: 10.1001/jama.291.2.228
Keywords: 129, establishment of reference intervals, Zhejiang Province, adults with normal liver function, thyroid-related hormones, indirect method
Citation: Huang X and Yang X (2024) Establishment of reference intervals of thyroid-related hormones for adults with normal liver function in Zhejiang Province by indirect method. Front. Endocrinol. 15:1441090. doi: 10.3389/fendo.2024.1441090
Received: 30 May 2024; Accepted: 13 August 2024;
Published: 10 September 2024.
Edited by:
Joseph V. Martin, Rutgers University Camden, United StatesReviewed by:
Paul Horn, Cincinnati Children’s Hospital Medical Center, United StatesNalini Selveindran, Hospital Putrajaya, Malaysia
Copyright © 2024 Huang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xufeng Yang, MjIwMjAzOUB6anUuZWR1LmNu
 Xiying Huang
Xiying Huang Xufeng Yang*
Xufeng Yang*