- 1Department of Respiratory and Critical Care Medicine, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Structural Heart Disease, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Background: Although descriptive studies have found an association between thyroid dysfunction (TD) and alopecia areata (AA), however, the causal relationship between TD and AA remains unclear. The purpose of this study is to investigate the causal relationship between the two and the specific directions.
Methods: We performed large-scale, two-sample Mendelian randomization (MR) analyses to examine whether there was an association between TD (such as Graves’ disease (GD), Hashimoto’s thyroiditis (HT), thyroid cancer (TC), thyroid stimulating hormone (TSH), thyrotropin-releasing hormone (TRH), etc.) and AA. Genome-wide association study (GWAS) summary statistics for TD and AA were from the IEU OpenGwas project. The inverse variance-weighted (IVW) method was used as the primary analysis method to evaluate the causality between TD and AA, supplemented by the weighted median, MR-Egger, simple mode and weighted mode. In addition, sensitivity analyses were performed to assess the reliability of the study results.
Results: Our study found that single nucleotide polymorphisms (SNPs) in HT (IVW OR = 1.396, 95% CI 1.030-1.892, P=0.031) and hypothyroidism (IVW OR = 1.431, 95% CI 1.138-1.799, P=0.002) significantly increased the risk of AA. Reverse MR analysis indicated that genetic susceptibility to AA (β=-0.029, 95%CI=-0.051 to -0.007, P=0.009) may be a risk for TRH. Positive MR analysis observed no statistically significant causal relationship between other TD and AA (IVW P>0.05). Reverse MR analysis also showed no statistically significant association between AA and other TD (IVW P>0.05) other than TRH. Furthermore, additional sensitivity analyses were performed, including a leave-one-out test, a heterogeneity test, and a pleiotropy test to assess the robustness of the results.
Conclusions: This study provides a very comprehensive analysis of the causal relationship between TD and AA, providing convincing genetic evidence to support the causal relationship between TD and alopecia areata. It reveals some causes of AA patients, which is of great significance for the management and treatment of AA patients.
1 Introduction
Alopecia areata (AA) is a common autoimmune disease characterized by non-scarring alopecia. The global prevalence of the disease is about 2% (1, 2), and the prevalence in China is 0.27% (3). The symptoms and signs of AA vary depending on the severity of the condition, from patchy hair loss to diffuse hair involvement on the scalp or the whole body (4, 5). Most AA patients experience unpredictable relapses and remissions (6). The disease not only reduces the quality of life of patients, but also may lead to emotional disorders such as depression and anxiety (7, 8).Further associations between AA and certain inflammatory, metabolic and autoimmune diseases have been observed, increasing the probability of developing them (9, 10); however, causality remains to be established and the exact pathogenesis of AA is still to be discovered. Consequently, comprehending the potential pathogenesis of AA is of utmost importance in order to facilitate the development of efficacious therapeutic approaches and enhance prognosis.
Non-scarring alopecia is a complex process involving genetic predisposition, environmental triggers, impaired hair growth, and inflammatory and immune factors, which may lead to its pathological mechanism (11). The exact pathogenesis of AA has not been completely established; however, a study has shown that alopecia areata is a T cell-mediated autoimmune state due to the collapse of immune privilege in hair follicles (12). Among them, the abnormal thyroid hormone (TH) level and antithyroid autoantibodies have been frequently reported in AA patients, and screening tests for thyroid dysfunction are sometimes recommended for patients with AA (13–16). The effects of thyroid hormone on hair growth have been the subject of special research in recent years, and previous studies have provided strong evidence linking TH to hair loss (17). One comparative study found that the hypothyroidism group (34%) had a higher proportion of patients with severe hair loss compared with the normal thyroid function group (18%) and the hyperthyroidism group (20%) (18). Observational studies have found a significant increase in the incidence of thyroid disease in patients with AA (19). A meta-analysis of patients with AA found that the prevalence of GD, hypothyroidism, TD, and HT in AA patients was 1.4%, 2.3%, 13.3%, and 2.9%, respectively (20). Extensive observational studies have revealed the link between AA and thyroid disease. However, the causal relationship between the two is not yet clear. Furthermore, observational studies may mask the real causal relationship due to reverse causality, selection bias, and confounding factors. Consequently, it is crucial to investigate the correlation between AA and TD in order to gain insights into the fundamental mechanisms of these diseases and improve their therapeutic approaches and quality of life.
Priority of the double-blind randomized controlled trial (RCT) may be compromised due to inherent disadvantages, including challenging ethical approval, substantial time, human and financial investments (21). Mendelian randomization (MR) analysis, a strategy for investigating causation between different traits, is widely used to explore the casual correlation between an exposure and an outcome (22). By including exposure-associated genetic variants of interest as instrumental variables(IVs), MR can avoid unmeasured confounding factors in observational studies and examine the causal relationship between potentially modifiable risk factors and health outcomes (23). Therefore, a two-sample MR analysis was performed in our study, and SNPs strongly correlated with exposure were collected as IVs to assess the causal relationship between AA and TD. This investigation was based on twelve extensive GWAS summary statistics, predominantly focusing on the European population’s data pertaining to AA and TD, including GD, HT, hyperthyroidism, hypothyroidism, thyroid cancer (TC), TSH, thyrotropin-releasing hormone (TRH), thyroxine-binding globulin (TBG), thyroid hormone receptor alpha (THRα), thyroid peroxidase (TP) and thyroglobulin (TG). In addition, in order to test the reliability of the study results, we performed various sensitivity analyses, including heterogeneity test, pleiotropy test, leave-one-out test, and reverse MR analysis.
2 Methods
2.1 STROBE-MR (strengthening the reporting of observational studies in epidemiology using mendelian randomisation) checklist
This study was guided by the STROBE-MR guidelines. This article adheres to the STROBE-MR checklist for reporting. (Supplementary Table S5).
2.2 Source of data and study design
The analysis was conducted using publicly accessible summary-level data from GWAS that specifically examined traits of interest, primarily in individuals of European ancestry, encompassing both males and females. A total of 12 datasets on AA and TD traits were collected. Original data for alopecia areata came from the FinnGen study (accessed through https://www.finngen.fi/en/access_results).The FinnGen study was designed to collect and analyze genomic information from more than 500,000 participants from the Finnish Biobank and combine it with information from national healthcare registries (24). AITD (Autoimmune thyroid disease) dataset includes both HT (n=395640) and GD (n=458620). The GWAS dataset associated with GD and HT was derived from the UK Biobank Project (25). Hyperthyroidism dataset (n= 460499) and Hypothyroidism dataset (n=410141) were also derived from the British Biobank project. Data on genetic variants associated with thyroid cancer were obtained by Deutsches the Krebsforschungszentrum (DKFZ) through GWAS (26) and included 1080 European participants, including 649 in the case group and 431 in the control group. TSH, TRH, THRα, TP and TG dataset all contained 3301 individuals in European population (27). TBG dataset was obtained from GWAS through the human blood plasma proteome (28).
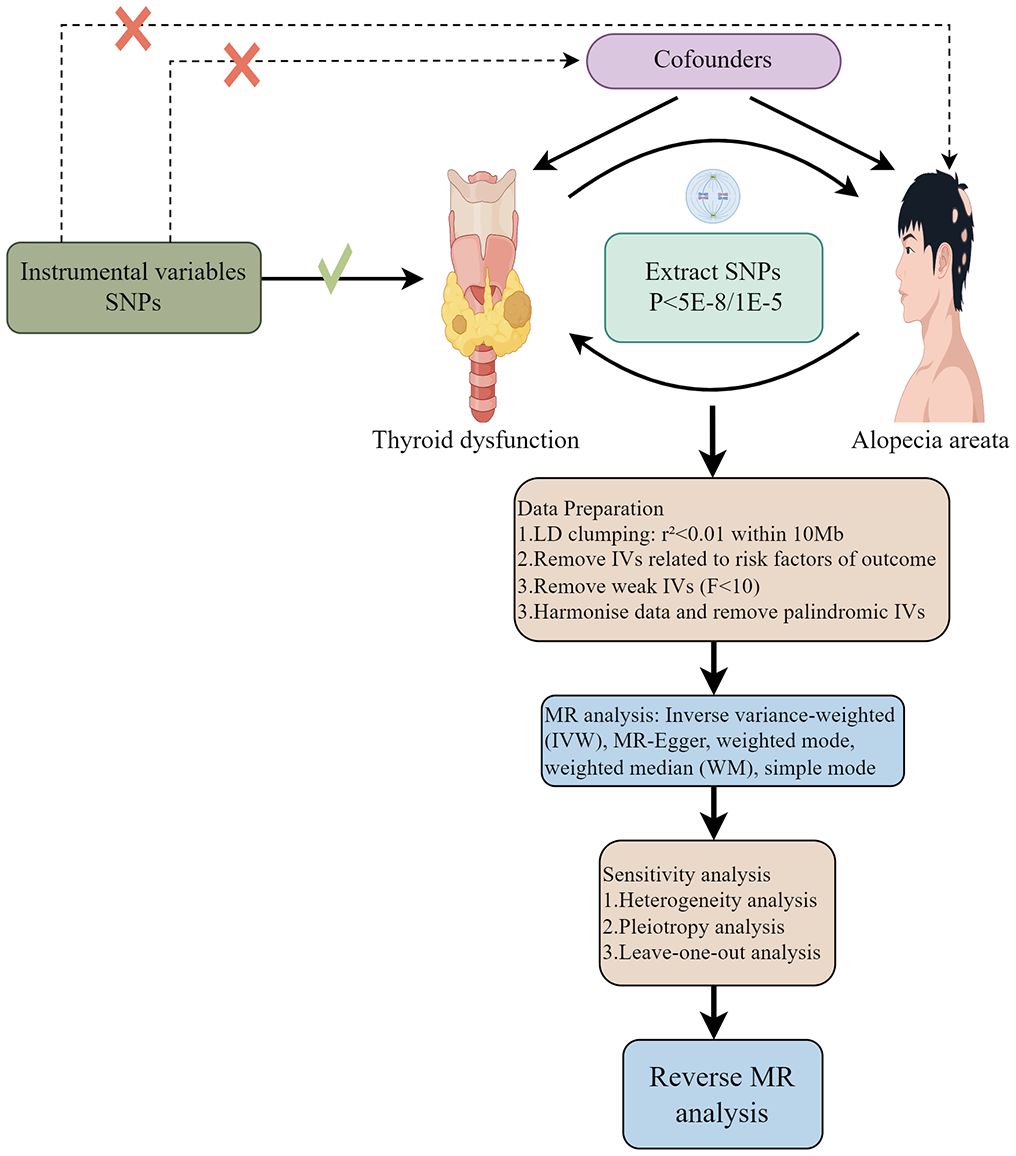
The flowchart of two-sample MR analyses in this study is shown in Figure 1. The exposed and outcome populations were Europeans, avoiding the bias caused by population stratification.
Ethical approval is not sought as the datasets in this study are publicly available.
2.3 Variants selection criterion
Single nucleotide polymorphisms (SNPs) were selected as instrumental variables (IVs). In order to make these SNPs show higher statistical power in genetic variation analysis, a series of quality control procedures were implemented.
The legitimate IVs should meet the following assumptions: (1) IVs (genetic variation) are closely related to TD (exposure). (2) genetic variants do not share common causes (potential confounders) with alopecia areata-related indicators (outcomes). (3) genetic variation affects alopecia areata-related indicators (outcomes) only through its effect on thyroid dysfunction-related traits (exposure). In forward MR analysis, P value significance threshold of the SNP was set to 5×10-8 (29, 30). In the event that the number of filtered SNPs was insufficient, it was feasible to modify the threshold to P<1×10-5 (31). Next, linkage disequilibrium (LD) pruning was conducted to remove linked SNPs (R2<0.001, kb=10000).
No proxy SNP was used in this MR analysis. Nevertheless, it remains possible that some pleiotropic SNPs, which are difficult to detect, might still exist. To further evaluate whether each SNP is associated with established risk factors of AA, including genetic factors (32), mental stress (Such as anxiety, depression, insomnia, etc.) (33) and intestinal dysbiosis (34), we utilized the LDlink website (https://ldlink.nih.gov/?tab=ldtrait) (35) to scrutinize exposure-related instrumental variables (IVs). If we identify any SNPs are significantly linked to the aforementioned confounding factors (P< 1 × 10− 8), we will exclude these SNPs and re-conduct the MR analysis. This crucial step aims to ensure the robustness and reliability of our analysis results. IVs intensity was measured by calculating the F-statistic (36). SNPs with F-statistics smaller than 10 were removed (30). In reverse MR analyses, SNPs with P<1×10-5 were considered significant. The following procedures was similar to the above one.
2.4 Statistical analyses
Exposure and outcome datasets were harmonized to ensure consistency between exposure and outcome alleles (37).To investigate the causal effect between TD and AA, the inverse variance weighting (IVW) method was used as the main method, and MR Egger, weighted median, simple mode and weighted mode were used as important supplements. Additionally, reverse-direction MR was conducted to evaluate the potential reverse causal association of TD on AA.
We used several sensitivity analyses to examine and correct causal estimates. Firstly, heterogeneity was assessed by Cohran Q test (38), When the P-value of the heterogeneity test was lower than 0.05, it indicated heterogeneity. Then, Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) technique was used to detect and remove heterogeneous SNPs (39). Besides, we also used MR-Egger intercept and funnel plots to assess horizontal pleiotropy. Sensitivity analysis was carried out based on the leave-one-out method.
All statistical analyses were conducted using the “Two-Sample MR” (version 0.5.9) packages in R version 4.0.3. Statistical significance was defined as a P-value less than 0.05.
3 Results
In summary, according to the specified selection criteria, the datasets included in this study were all published in the most recent time and in European populations, as detailed in Supplementary Table S1.
3.1 The causal effect of TD on AA via forward MR
To conduct a comprehensive assessment of the association between TD and the likelihood of AA development, MR analyses were employed to validate associations with a statistically significant P-value of less than 0.05. After removing palindromic SNPs and outliers, we obtained 26, 12, 65,11,258,22,26,16,20, and 23 SNPs for GD, HT, hypothyroidism, hyperthyroidism, TC, TSH, TRH, THRα, TP and TG, respectively. Supplementary Table S2 contains SNP information for all instrumental variables in forward and reverse MR analyses. We observed a significant causal relationship between TD and AA using the IVW method (HT and AA, IVW OR = 1.396, 95% CI 1.030-1.892, P=0.031; Hypothyroidism and AA, IVW OR = 1.431, 95% CI 1.138-1.799, P=0.002) (Figure 2A) (Supplementary Table S3). MR-Egger and Weight median were estimated in the same direction as the IVW method (HT and AA, P=0.157, P=0.084; hypothyroidism and AA, P = 0.006, P=0.03), despite not significant (Figure 2A) (Supplementary Table S3). The reliability of the MR analysis results can be further substantiated by employing the Leave-one-out method of sensitivity analyses to ascertain the impact of individual genetic variants on the overall outcomes (Figures 3C, 4C). The absence of significant horizontal pleiotropy was indicated by the MR-pleiotropy test and MR-Egger regression (HT for AA, P=0.428; Hypothyroidism for AA, P=0.129) (Figures 3B, 4B) (Supplementary Table S4). The Cochran’s Q test and MR-PRESSO global test suggested no evidence of heterogeneity (HT on AA, Cochran’s Q=11.13, P=0.432, P =0.49; Hypothyroidism on AA, Cochran’s Q=64, P=0.339, P=0.354) (Figures 3A, 4A) (Supplementary Table S4). In addition, the summary data from IVW analysis showed no evidence of a causal relationship between GD (P=0.398), Hyperthyroidism (P=0.974), TC (P=0.669), TSH (P=0.847), TRH (P=0.386), THRα (P=0.942), TP (P=0.828), TG (P=0.460), and AA (Supplementary Table S3). In addition, the results of funnel plot, leave-one-out analysis and forest plot of forward MR analysis in this study were presented in Supplementary Figures S1, S2. It is worth mentioning that according to our screening criteria, SNPs strongly associated with TBG were not screened out, so we did not establish a causal relationship between TBG and AA.
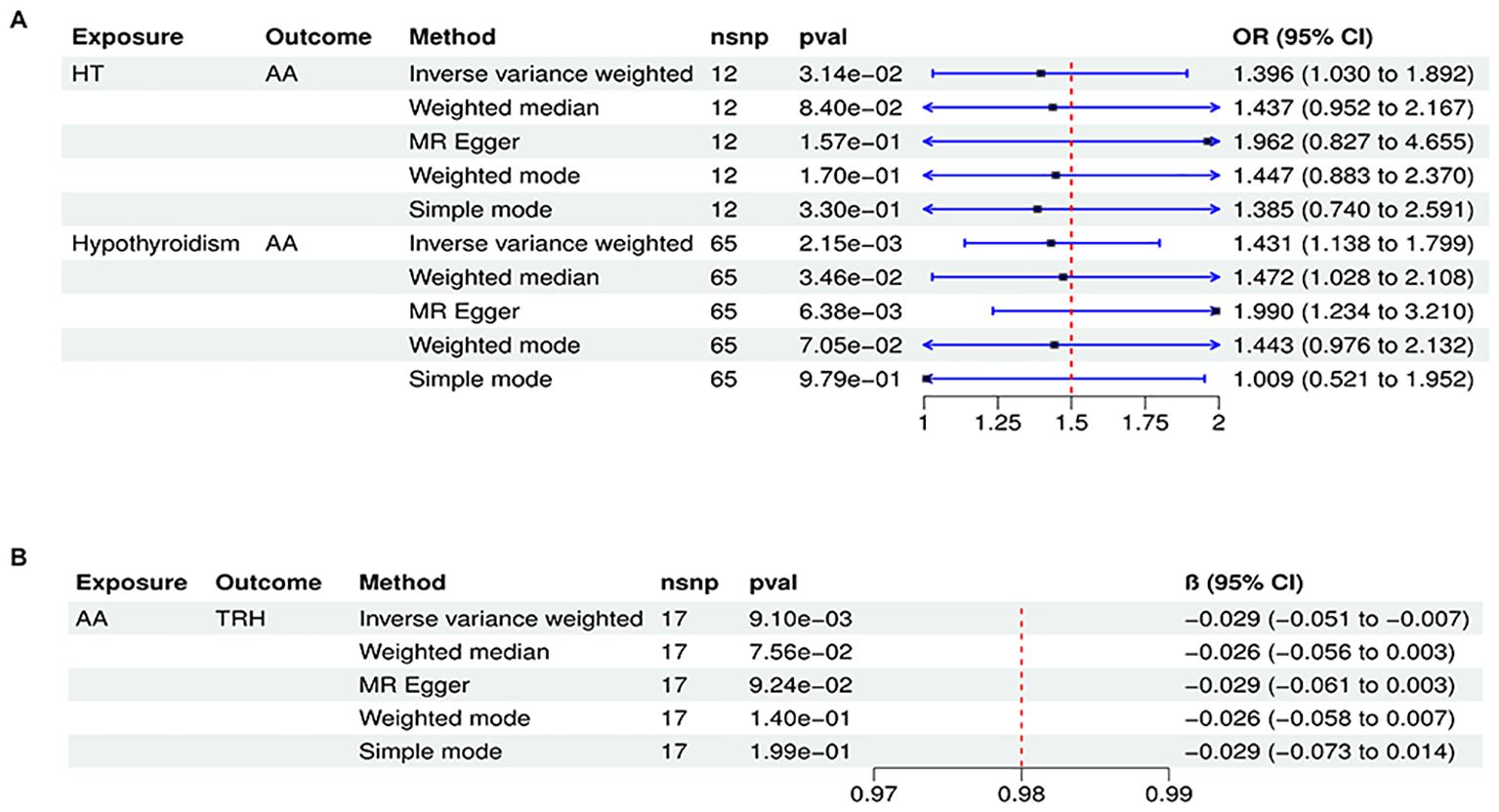
Figure 2. Significant causal plot between TD and AA in MR analysis. (A) Significant MR analysis of the causal effects of HT/Hypothyroidism on AA. (B) Significant MR analysis of the causal effects of AA on TRH.
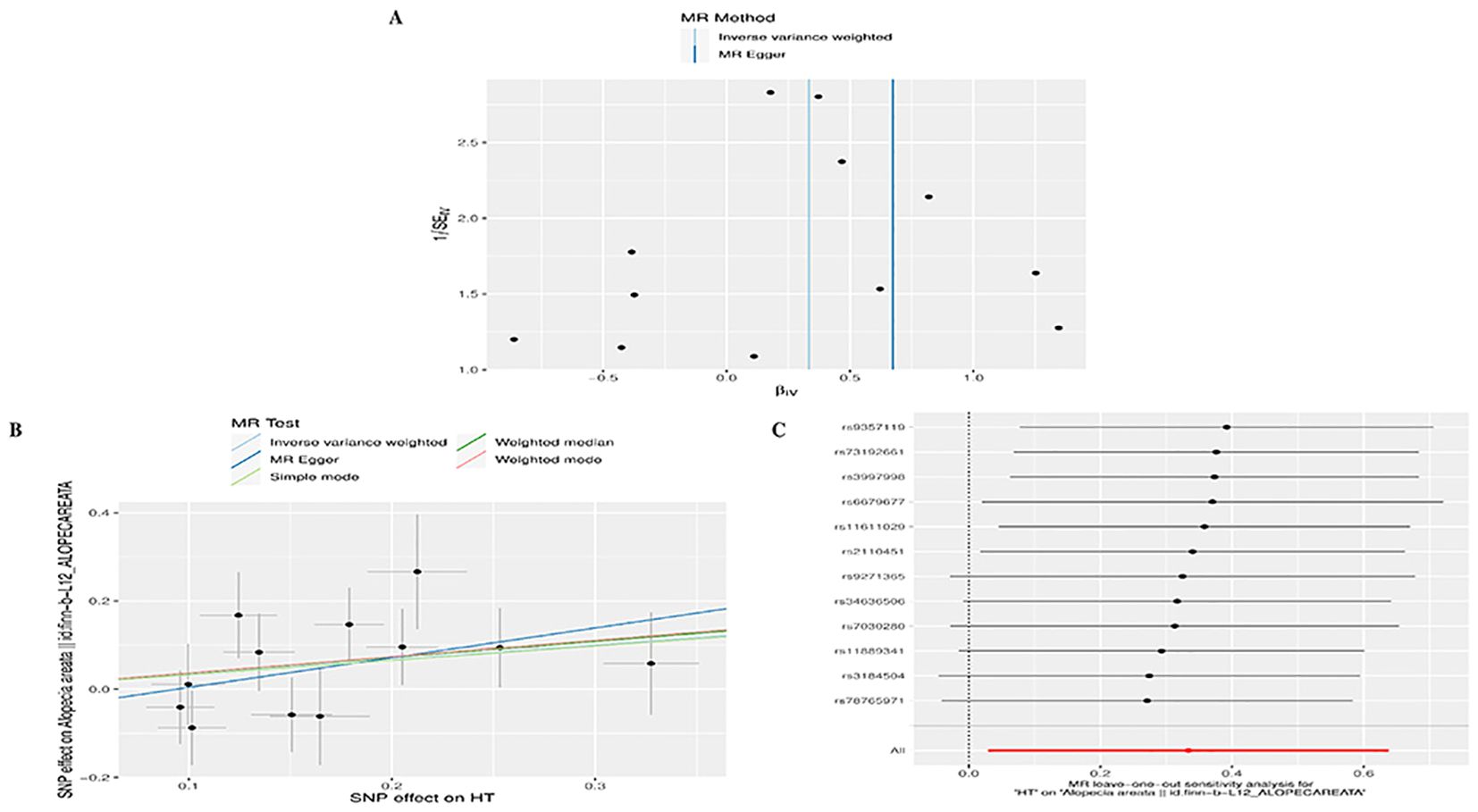
Figure 3. Plots of MR estimates of the causal relationship between HT and AA. (A) Leave-one-out sensitivity analysis of the association of HT on AA. (B) Scatter plot of the association of HT on AA. (C) Funnel plot of the association of HT on AA.
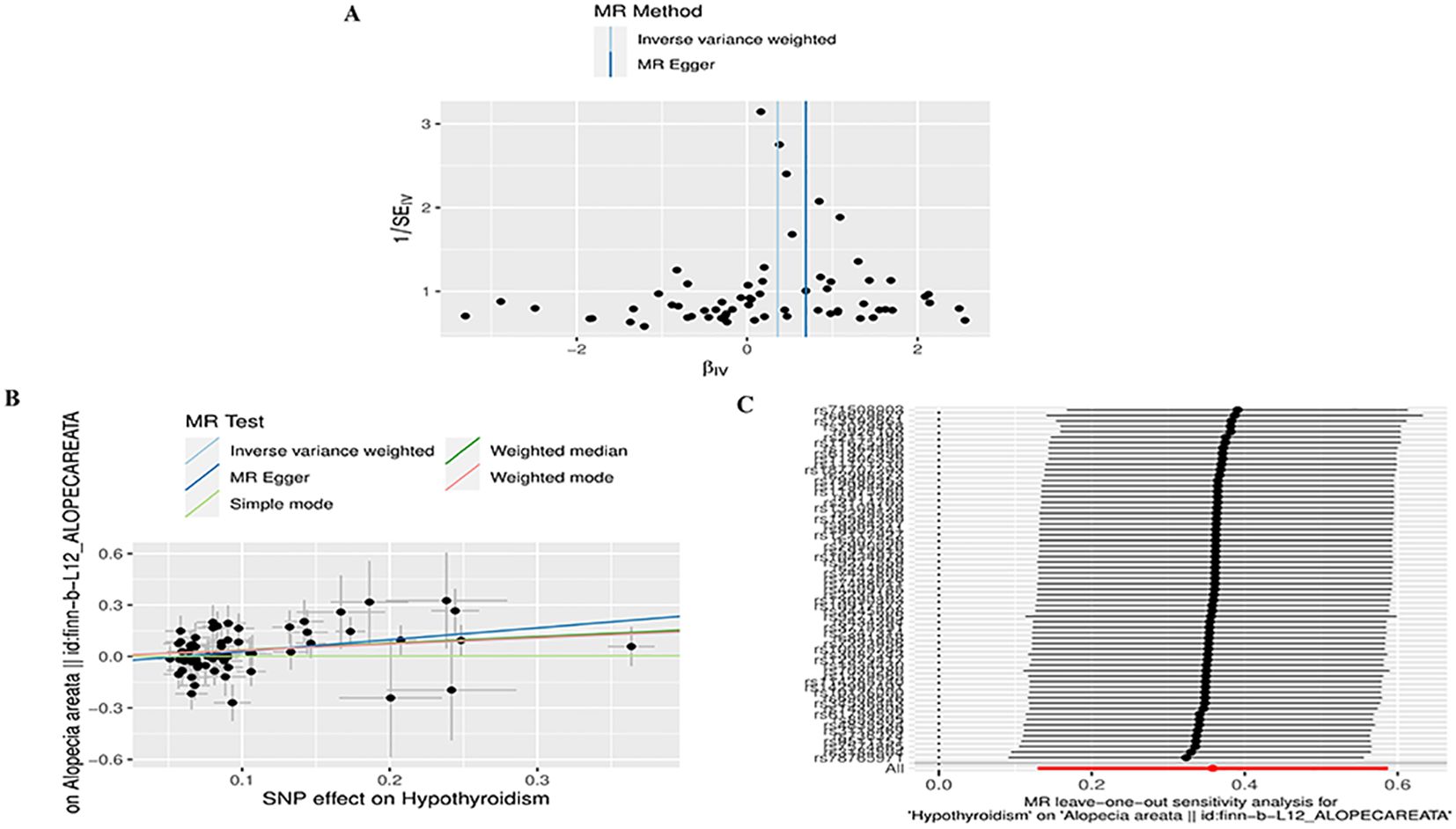
Figure 4. Plots of MR estimates of the causal relationship between Hypothyroidism and AA. (A) Funnel plot of the association of Hypothyroidism on AA. (B) Scatter plot of the association of Hypothyroidism on AA. (C) Leave-one-out sensitivity analysis of the association of Hypothyroidism on AA.
3.2 The causal effect of AA on TD via reverse MR
In reverse MR analysis, we considered AA as an exposure and TD as outcome. As a result of SNP filtering, 18, 18, 18,18,5,17,17,17,17,17 and 4 SNPs were included for GD, HT, hypothyroidism, hyperthyroidism, TC, TSH, TRH, THRα, TP, TG and TBG, respectively, in final analysis (Supplementary Table S2). According to Figure 2B, IVW findings suggested that the existence of AA was linked to a reduction in TRH levels (AA on TRH, β=-0.029, 95%CI=-0.051 to -0.007, P=0.009). The Leave-one-out method was used to assess robustness of these results (Figure 5C). MR pleiotropy trials and MR Egger regression were performed to assess horizontal pleiotropy, and there was no evidence of horizontal pleiotropy (AA for TRH, P=0.998) (Figure 5B) (Supplementary Table S4). The Cochran’s Q test and MR-PRESSO global test suggested no evidence of heterogeneity (AA on TRH, Cochran’s Q=7.47, P=0.963, Global Test’P =0.969) (Figure 5A) (Supplementary Table S4). MR analysis showed that there had no causal association between AA and GD, HT, Hypothyroidism, Hyperthyroidism, TC, TSH, TBG, THRα, TP, TG and TBG (Supplementary Table S3). Leave-one-out, scatter plot, and funnel plots of the results of the reverse MR analysis are shown in Supplementary Figures S3-S5, respectively.
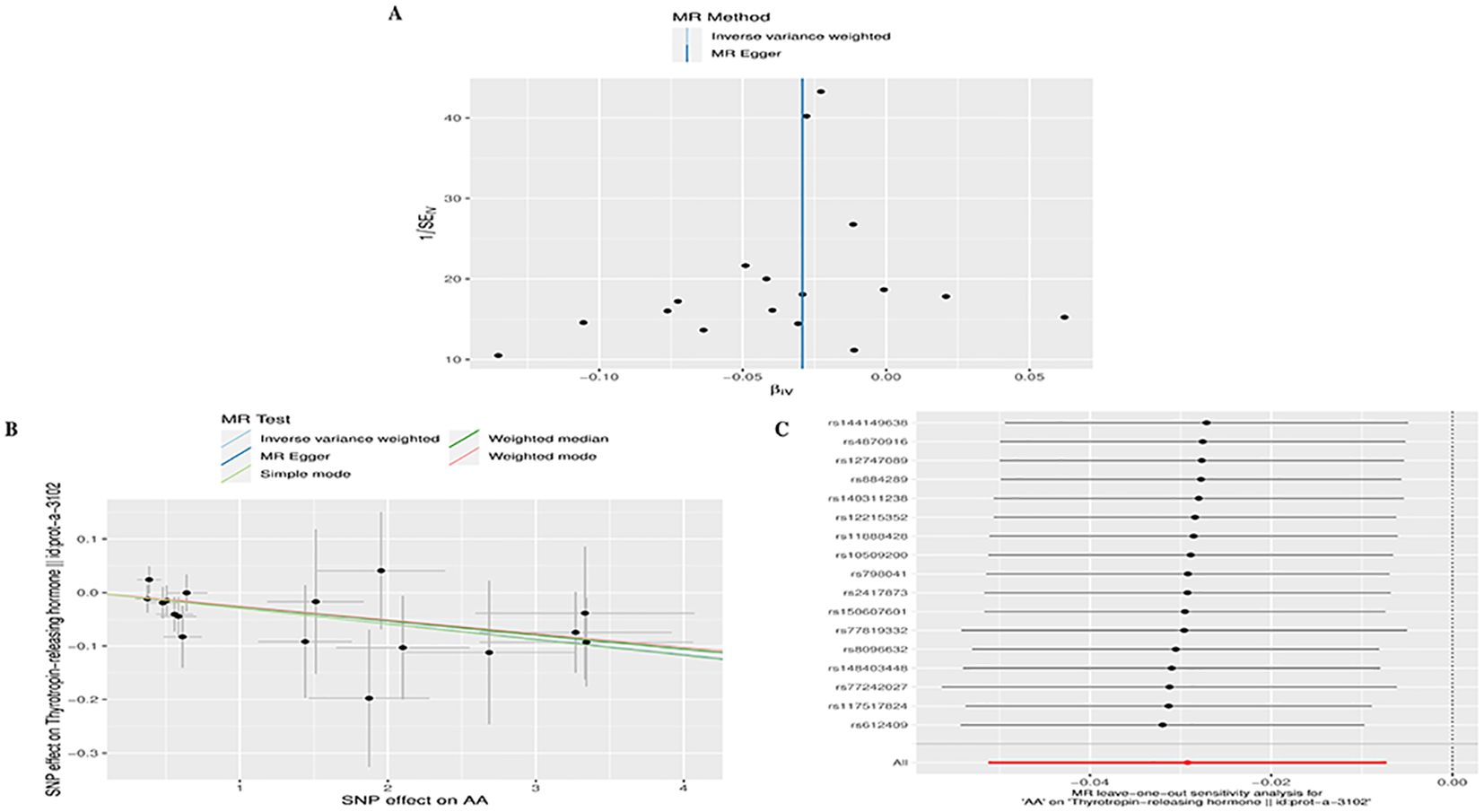
Figure 5. Plots of MR estimates of the causal relationship between AA and TRH. (A) Leave-one-out sensitivity analysis of the association of AA on TRH. (B) Scatter plot of the association of AA on TRH. (C) Funnel plot of the association of AA on TRH.
3.3 Confounding analysis
Through forward and reverse MR analysis, our study found that HT and hypothyroidism had causal effects on AA. To ensure that the instrumental variables (IVs) associated with hypothyroidism and HT were independent of confounding factors, we examined the autonomy of our selected SNPs from common risk factors for AA. Specifically, we assessed their associations with recognized risk factors such as genetic factors, mental stress and intestinal dysbiosis. In hypothyroidism, we found that rs12593201, rs434294 and rs9271365 were associated with AA risk factors such as depression, insomnia and sleep disorders. No suspicious SNP was identified in HT. Re-analysis with exclusion of three SNPs demonstrated that our estimates remain significant: hypoglycemia: (IVW OR = 1.431, 95% CI 1.138-1.799, P=0.002).
4 Discussion
Our two-sample MR study provides novel evidence for a causal relationship between thyroid dysfunction and AA. Our study found that HT and hypothyroidism have a causal effect on AA, suggesting that HT and hypothyroidism themselves may play a causal role in the pathogenesis of AA. The results of two recently published MR studies on AA and hypothyroidism are consistent with our findings (18, 40), but they only explored the causal relationship between hypothyroidism and AA, the sample size of the hypothyroidism GWAS data was not the most up-to-date and comprehensive, and it did not cover other thyroid disease and relevant measures of thyroid function. However, there was no causal relationship between GD, hyperthyroidism, TC, TSH, TRH, TBG, THRα, TP, TG, and AA. In addition, reverse MR analysis showed that genetic susceptibility to AA may affect the risk of TRH. A series of sensitive analyses support these findings.
Autoimmune thyroid disease (AITD) is the most common organ-specific autoimmune disease (41). The AITD spectrum includes Graves-Basedow disease (GBD) and Hashimoto’s thyroiditis (HT) as well as a wide range of clinical manifestations, ranging from Hashimoto’s overt or subclinical hypothyroidism, euthyroidism, to Graves’ subclinical or overt hyperthyroidism (42). According to our search, the first case of hypothyroidism with AA was reported in 1960. Alopecia areata (AA) is often associated with other autoimmune diseases, such as thyroid disease (43), asthma, allergic rhinitis (44), and other skin diseases (45) with autoimmune causes. Thyroid dysfunctions resulting from the autoimmune mechanism are the leading disorders reported in the literature related to AA patients (15, 46). Several descriptive studies have investigated the association between AA and thyroid dysfunction in the form of prevalence, comorbidities, and clinical significance (13, 16, 43, 47–49). Recently, a new systematic review and meta-analysis of AA-related medical comorbidities showed that Hashimoto ‘s thyroiditis (OR 4.31,95% CI 2.51-7.40) was one of the comorbidities with a higher odds ratio in AA patients compared with healthy controls (50).
Previous studies have shown that hypothyroidism is associated with an increased risk of AA (51, 52). A retrospective study of 78 newly diagnosed alopecia areata who presented to community dermatology clinics between 2007 and 2011 found that 13 cases of AA (16.6%) had hypothyroidism (53). A prospective study of women in the United States from 2002 to 2014 found that a history of hypothyroidism was associated with an increased risk of AA (54). A study comparing the clinical patterns of 89 patients with alopecia areata found that approximately 25% of patients with AA had comorbid thyroid lesions, the most common of which was hypothyroidism (n=20) (55).
A meta-analysis by Kinoshita-Ise et al. (56) found that compared with healthy controls, positive anti-thyroid peroxidase antibody (TPO-Ab) (OR = 3.58; 95% CI 1.96–6.53) and anti-thyroglobulin antibody (TG-Ab) (OR = 4.44; 95% CI 1.54–12.75) were more common in AA patients. Moreover, the risk of both or either of TPO-Ab and TG-Ab being positive in the same AA patient was OR = 2.32 (95% CI 1.08–4.98) and OR = 6.34 (95% CI 2.24–17.93), respectively. In addition, the study also found that the proportion of TSH (thyroid stimulating hormone) receptor antibody (TR-Ab) positive patients in AA patients was higher (OR = 60.90; 95% CI 34.61-107.18).
Previous studies have observed a correlation between thyroid disease and AA, but the causal and biological links between the two are unclear. AA shares an autoimmune background with autoimmune thyroid disease, either sporadic or autoimmune polyglandular syndrome (52). The cause of AA is unknown; however, genetic susceptibility, different types of autoimmunity, and, probably, stress are regarded as contributors (43, 57). In HT, autoimmune processes lead to apoptosis and destruction of thyroid follicles, and subsequent hypothyroidism. Thyroid hormones are essential for the growth and maintenance of hair follicles (58, 59). In hypothyroid patients, the epidermis is thin, and they frequently develop alopecia (60), showing that thyroid hormone signaling can regulate both skin proliferation and hair growth (61, 62). HT is currently the leading cause of primary hypothyroidism, both in adolescents and adults. It is a T-cell-mediated autoimmune disorder characterized by thyroid lymphocytic infiltration (63). T lymphocytes cause hair destruction in AA, hence T lymphocytes and associated cytokines that infiltrate the areas around the hair follicles play a major part in the disease’s etiology. Cytotoxic CD8+NKG2D+ T lymphocytes are the primary immunocytes that infiltrate the surroundings of HFs and are held to be the key cells that drive the disease pathogenesis NKG2D is an activating receptor expressed on CD8+ T cells and NK cells which recognize NKG2D ligands, like ULBP3/6 and MICA, and then upregulate MHC expression, which is crucial in mediating HP-IP collapse (64). HLA (Human Leucocytes Antigens) represents another link between AA and autoimmune thyroid disease. Some data showed that HLA-DQB1*03 is connected to both AA and antibodies-induced hypothyroidism. A genetic association study of HLA genes also found that the DRB1*15: 01 DQB1*06: 02 haplotype frequency was considerably greater in TR-Ab-positive individuals diagnosed with AA versus controls (65). Although the etiopathogenesis of AA has yet to be fully elucidated, its current understanding includes the genetic factors and various environmental triggers, whose interaction influences the autoreactive cytotoxic T-lymphocyte activation and increased secretion of interferon (IFN)-γ in a predisposed individual. Type 1 inflammatory response leads to the loss of the hair follicle immune privilege, overexpression of MHC class I, and consequent autoimmune assault on hair follicles (66). In summary, previous studies support the causal relationship between HT and hypothyroidism and alopecia areata observed in our current study. Of course, not all patients with HT and hypothyroidism will have alopecia areata. This may be due to individual differences, disease severity, treatment measures, and other factors. In clinical practice, we recommend routine screening of thyroid function in patients with alopecia areata, and further identify prevention and treatment methods.
Our results have certain significance for the diagnosis and management of TD and AA patients. First, our study is the largest and most comprehensive MR study of TD and AA to date, assessing the causal relationship between TD and AA and minimizing potential confounders. Secondly, our findings provide new insights into the occurrence of AA in TD patients, provide guidance for the treatment of AA patients, and help improve the quality of life of AA patients. Consistent monitoring of thyroid-related hormones and prompt diagnosis and treatment of TD can provide valuable insights for the management of AA and lifestyle interventions. In addition, when treating patients with AA, it is important to pay attention to the effect that the drug may have on thyroid function. Furthermore, future research should focus on establishing the pathogenesis between TD and AA, and explore novel effective biomarkers that affect the pathogenesis of AA.
Our studies also have some limitations. Firstly, due to the limited data available for GWAS in alopecia areata, this study failed to perform a confirmatory analysis. Secondly, our research population was all European, and the conclusions of the study may not be applicable to other ethnic groups. Finally, although our study provides genetic evidence for causality, additional research is needed to further elucidate the underlying mechanisms.
5 Conclusion
In conclusion, our study suggests that HT and hypothyroidism can lead to AA. Based on the results of our study, we recommend thyroid function and related antibody tests for the clinical treatment of patients with AA. Prompt treatment of HT and hypothyroidism may reduce the incidence of AA. In addition, the identification of a potential causal relationship between HT and hypothyroidism and AA provides a new avenue for studying the origin and progression of AA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MC: Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. LG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. WL: Conceptualization, Writing – original draft. QS: Conceptualization, Validation, Writing – original draft. HG: Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Figure 1 is drawn by Figdraw (https://www.figdraw.com/#/paint_my_collect). Thanks for the drawing platform of “Figuredraw”. In addition, we would like to thank the participants and researchers from the public GWAS dataset (https://gwas.mrcieu.ac.uk/datasets/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1440941/full#supplementary-material
Supplementary Figure 1 | Funnel plot, Scatter plot, Leave-one-out sensitivity analysis of the association of GD (A–C), hyperthyroidism (D–F), TC (G–I), TSH (J–L) on AA. GD, Graves’ disease; TC, Thyroid cancer; TSH, Thyroid Stimulating Hormone; AA, alopecia areata.
Supplementary Figure 2 | Funnel plot, Scatter plot, Leave-one-out sensitivity analysis of the association of TRH (A–C), THRα (D–F), TP (G–I), TG (J–L) on AA. TRH, Thyrotropin-releasing hormone; THRα, Thyroid hormone receptor alpha; TP, Thyroid peroxidase; TG, Thyroglobulin; AA, alopecia areata.
Supplementary Figure 3 | Funnel plot of the association of AA on GD (A), HT (B), hypothyroidism(C), hyperthyroidism (D), TC (E), TSH (F), THRα(G), TP (H), TG(I), TBG(J). AA, alopecia areata; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; TC, Thyroid cancer; TSH, Thyroid Stimulating Hormone; THRα, Thyroid hormone receptor alpha; TP, Thyroid peroxidase; TG, Thyroglobulin; TBG, Thyroxine-Binding Globulin.
Supplementary Figure 4 | Scatter plot of the association of AA on GD (A), HT (B), hypothyroidism (C), hyperthyroidism (D), TC (E), TSH (F), THRα (G), TP (H), TG (I), TBG (J). AA, alopecia areata; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; TC, Thyroid cancer; TSH, Thyroid Stimulating Hormone; THRα, Thyroid hormone receptor alpha; TP, Thyroid peroxidase; TG, Thyroglobulin; TBG, Thyroxine-Binding Globulin.
Supplementary Figure 5 | Leave-one-out sensitivity analysis of the association of AA on GD (A), HT (B), hypothyroidism (C), hyperthyroidism (D), TC (E), TSH (F), THRα (G), TP (H), TG(I), TBG(J). AA, alopecia areata; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; TC, Thyroid cancer; TSH, Thyroid Stimulating Hormone; THRα, Thyroid hormone receptor alpha; TP, Thyroid peroxidase; TG, Thyroglobulin; TBG, Thyroxine-Binding Globulin.
Abbreviations
TD, thyroid dysfunction; AA, alopecia areata; TH, thyroid hormone; HT, Hashimoto’s thyroiditis; GD, Graves’ disease; TC, Thyroid cancer; TSH, Thyroid Stimulating Hormone; TRH, Thyrotropin-releasing hormone; THRα, Thyroid hormone receptor alpha; TP, Thyroid peroxidase; TG, Thyroglobulin; TBG, Thyroxine-Binding Globulin; GWAS, genome-wide association study; SNPs, single-nucleotide polymorphisms; IVW, inverse-variance weighted.
References
1. Lee HH, Gwillim E, Patel KR, Hua T, Rastogi S, Ibler E, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. J Am Acad Dermatol. (2020) 82:675–82. doi: 10.1016/j.jaad.2019.08.032
2. Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. (2018) 78:1–12. doi: 10.1016/j.jaad.2017.04.1141
3. Rossi A, Muscianese M, Piraccini BM, Starace M, Carlesimo M, Mandel VD, et al. Italian Guidelines in diagnosis and treatment of alopecia areata. G Ital Dermatol Venereol. (2019) 154:609–23. doi: 10.23736/S0392-0488.19.06458-7
4. Meah N, Wall D, York K, Bhoyrul B, Bokhari L, Asz-Sigall D, et al. The Alopecia Areata Consensus of Experts (ACE) study part II: Results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J Am Acad Dermatol. (2021) 84:1594–601. doi: 10.1016/j.jaad.2020.09.028
5. King BA, Mesinkovska NA, Craiglow B, Kindred C, Ko J, McMichael A, et al. Development of the alopecia areata scale for clinical use: Results of an academic-industry collaborative effort. J Am Acad Dermatol. (2022) 86:359–64. doi: 10.1016/j.jaad.2021.08.043
6. Lee S, Kim BJ, Lee YB, Lee W-S. Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: A systematic review and meta-analysis. JAMA Dermatol. (2018) 154:1145–51. doi: 10.1001/jamadermatol.2018.2312
7. Okhovat J-P, Marks DH, Manatis-Lornell A, Hagigeorges D, Locascio JJ, Senna MM. Association between alopecia areata, anxiety, and depression: A systematic review and meta-analysis. J Am Acad Dermatol. (2023) 88:1040–50. doi: 10.1016/j.jaad.2019.05.086
8. Toussi A, Barton VR, Le ST, Agbai ON, Kiuru M. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: A systematic review. J Am Acad Dermatol. (2021) 85:162–75. doi: 10.1016/j.jaad.2020.06.047
9. Conic RRZ, Chu S, Tamashunas NL, Damiani G, Bergfeld W. Prevalence of cardiac and metabolic diseases among patients with alopecia areata. J Eur Acad Dermatol Venereol. (2021) 35:e128–9. doi: 10.1111/jdv.16864
10. Park S-M, Oh Y-J, Lew B-L, Sim W-Y. The association among thyroid dysfunction, thyroid autoimmunity, and clinical features of alopecia areata: A retrospective study. J Am Acad Dermatol. (2019) 81:602–5. doi: 10.1016/j.jaad.2018.04.051
11. Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. (2021) 105:599–610. doi: 10.1016/j.mcna.2021.04.002
12. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. (2018) 179:1033–48. doi: 10.1111/bjd.16808
13. Bin Saif GA. Severe subtype of alopecia areata is highly associated with thyroid autoimmunity. Saudi Med J. (2016) 37:656–61. doi: 10.15537/smj.2016.6.13777
14. Díaz-Angulo S, López-Hoyos M, Muñoz-Cacho P, López-Escobar M, González-López MA. High prevalence of thyroid autoimmunity in patients with alopecia areata and vitiligo: a controlled study. Australas J Dermatol. (2015) 56:142–3. doi: 10.1111/ajd.12321
15. Bakry OA, Basha MA, El Shafiee MK, Shehata WA. Thyroid disorders associated with alopecia areata in Egyptian patients. Indian J Dermatol. (2014) 59:49–55. doi: 10.4103/0019-5154.123494
16. Noso S, Park C, Babaya N, Hiromine Y, Harada T, Ito H, et al. Organ specificity in autoimmune diseases: thyroid and islet autoimmunity in alopecia areata. J Clin Endocrinol Metab. (2015) 100:1976–83. doi: 10.1210/jc.2014-3985
17. Grymowicz M, Rudnicka E, Podfigurna A, Napierala P, Smolarczyk R, Smolarczyk K, et al. Hormonal effects on hair follicles. Int J Mol Sci. (2020) 21:5342. doi: 10.3390/ijms21155342
18. Bin Dayel S, Hussein RS, Atia T, Abahussein O, Al Yahya RS, Elsayed SH. Is thyroid dysfunction a common cause of telogen effluvium?: A retrospective study. Med (Baltimore). (2024) 103:e36803. doi: 10.1097/MD.0000000000036803
19. Xin C, Sun X, Lu L, Yang R, Shan L, Wang Y. Increased incidence of thyroid disease in patients with alopecia areata: A systematic review and meta-analysis. Dermatology. (2019) 236:251–4. doi: 10.1159/000502025
20. Lee S, Lee YB, Kim BJ, Lee W-S. Screening of thyroid function and autoantibodies in patients with alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. (2019) 80:1410–1413.e4. doi: 10.1016/j.jaad.2018.10.066
21. Bhide A, Shah PS, Acharya G. A simplified guide to randomized controlled trials. Acta Obstet Gynecol Scand. (2018) 97:380–7. doi: 10.1111/aogs.13309
22. Raj D, Pooja F, Chhabria P, Kalpana F, Lohana S, Lal K, et al. Frequency of subclinical hypothyroidism in women with polycystic ovary syndrome. Cureus. (2021) 13:e17722. doi: 10.7759/cureus.17722
23. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
24. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
25. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
26. Köhler A, Chen B, Gemignani F, Elisei R, Romei C, Figlioli G, et al. Genome-wide association study on differentiated thyroid cancer. J Clin Endocrinol Metab. (2013) 98:E1674–1681. doi: 10.1210/jc.2013-1941
27. Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature. (2018) 558:73–9. doi: 10.1038/s41586-018-0175-2
28. Suhre K, Arnold M, Bhagwat AM, Cotton RJ, Engelke R, Raffler J, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. (2017) 8:14357. doi: 10.1038/ncomms14357
29. Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346
30. Zoccali C, Testa A, Spoto B, Tripepi G, Mallamaci F. Mendelian randomization: a new approach to studying epidemiology in ESRD. Am J Kidney Dis. (2006) 47:332–41. doi: 10.1053/j.ajkd.2005.10.027
31. Zhang W, Lang R. Genetic link between primary sclerosing cholangitis and thyroid dysfunction: a bidirectional two-sample Mendelian randomization study. Front Immunol. (2023) 14:1276459. doi: 10.3389/fimmu.2023.1276459
32. Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. (2017) 3:17011. doi: 10.1038/nrdp.2017.11
33. Brajac I, Tkalcic M, Dragojević DM, Gruber F. Roles of stress, stress perception and trait-anxiety in the onset and course of alopecia areata. J Dermatol. (2003) 30:871–8. doi: 10.1111/j.1346-8138.2003.tb00341.x
34. Hayashi A, Mikami Y, Miyamoto K, Kamada N, Sato T, Mizuno S, et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Rep. (2017) 20:1513–24. doi: 10.1016/j.celrep.2017.07.057
35. Lin S-H, Brown DW, Machiela MJ. LDtrait: an online tool for identifying published phenotype associations in linkage disequilibrium. Cancer Res. (2020) 80:3443–6. doi: 10.1158/0008-5472.CAN-20-0985
36. Zhang Q, Mu Y, Jiang X, Zhao Y, Wang Q, Shen Z. Causal relationship between thyroid dysfunction and gastric cancer: a two-sample Mendelian randomization study. Front Endocrinol (Lausanne). (2024) 15:1335149. doi: 10.3389/fendo.2024.1335149
37. Zuber V, Grinberg NF, Gill D, Manipur I, Slob EAW, Patel A, et al. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am J Hum Genet. (2022) 109:767–82. doi: 10.1016/j.ajhg.2022.04.001
38. Sleiman PMA, Grant SFA. Mendelian randomization in the era of genomewide association studies. Clin Chem. (2010) 56:723–8. doi: 10.1373/clinchem.2009.141564
39. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
40. Zheng X-Y, Ma Y-P, Zhang B, Chen Y-X, Tang L, Tai X-H, et al. Mendelian randomization study highlights hypothyroidism as a causal determinant of alopecia areata. Front Endocrinol (Lausanne). (2024) 14:1309620. doi: 10.3389/fendo.2023.1309620
41. Vargas-Uricoechea H. Molecular mechanisms in autoimmune thyroid disease. Cells. (2023) 12:918. doi: 10.3390/cells12060918
42. Vargas-Uricoechea H, Nogueira JP, Pinzón-Fernández MV, Schwarzstein D. The usefulness of thyroid antibodies in the diagnostic approach to autoimmune thyroid disease. Antibodies (Basel). (2023) 12:48. doi: 10.3390/antib12030048
43. Han TY, Lee JH, Noh TK, Choi MW, Yun J-S, Lee KH, et al. Alopecia areata and overt thyroid diseases: A nationwide population-based study. J Dermatol. (2018) 45:1411–7. doi: 10.1111/1346-8138.14648
44. Ghaffari J, Rokni GR, Kazeminejad A, Abedi H. Association among thyroid dysfunction, asthma, allergic rhinitis and eczema in children with alopecia areata. Open Access Maced J Med Sci. (2017) 5:305–9. doi: 10.3889/oamjms.2017.050
45. Thomas EA, Kadyan RS. Alopecia areata and autoimmunity: A clinical study. Indian J Dermatol. (2008) 53:70–4. doi: 10.4103/0019-5154.41650
46. Puavilai S, Puavilai G, Charuwichitratana S, Sakuntabhai A, Sriprachya-Anunt S. Prevalence of thyroid diseases in patients with alopecia areata. Int J Dermatol. (1994) 33:632–3. doi: 10.1111/j.1365-4362.1994.tb02921.x
47. Vañó-Galván S, Egeberg A, Piraccini BM, Marwaha S, Reed C, Johansson E, et al. Characteristics and management of patients with alopecia areata and selected comorbid conditions: results from a survey in five european countries. Dermatol Ther (Heidelb). (2024) 14:1027–37. doi: 10.1007/s13555-024-01133-z
48. Chanprapaph K, Mahasaksiri T, Kositkuljorn C, Leerunyakul K, Suchonwanit P. Prevalence and risk factors associated with the occurrence of autoimmune diseases in patients with alopecia areata. J Inflammation Res. (2021) 14:4881–91. doi: 10.2147/JIR.S331579
49. Saylam Kurtipek G, Cihan FG, Erayman Demirbaş Ş, Ataseven A. The frequency of autoimmune thyroid disease in alopecia areata and vitiligo patients. BioMed Res Int. (2015) 2015:435947. doi: 10.1155/2015/435947
50. Ly S, Manjaly P, Kamal K, Shields A, Wafae B, Afzal N, et al. Comorbid conditions associated with alopecia areata: A systematic review and meta-analysis. Am J Clin Dermatol. (2023) 24:875–93. doi: 10.1007/s40257-023-00805-4
51. Dai Y-X, Tai Y-H, Chang Y-T, Chen T-J, Chen M-H. Bidirectional association between alopecia areata and thyroid diseases: a nationwide population-based cohort study. Arch Dermatol Res. (2021) 313:339–46. doi: 10.1007/s00403-020-02109-7
52. Popa A, Carsote M, Cretoiu D, Dumitrascu MC, Nistor C-E, Sandru F. Study of the thyroid profile of patients with alopecia. J Clin Med. (2023) 12:1115. doi: 10.3390/jcm12031115
53. Lyakhovitsky A, Shemer A, Amichai B. Increased prevalence of thyroid disorders in patients with new onset alopecia areata. Australas J Dermatol. (2015) 56:103–6. doi: 10.1111/ajd.12178
54. Moseley IH, Thompson JM, George EA, Ragi SD, Kang JH, Reginato AM, et al. Immune-mediated diseases and subsequent risk of alopecia areata in a prospective study of US women. Arch Dermatol Res. (2023) 315:807–13. doi: 10.1007/s00403-022-02444-x
55. Celorio W, Cifuentes L, Cantor E, Wagner A. Thyroid function and thyroid antibodies in patients with alopecia areata: a comparison of clinical patterns. Bras Dermatol. (2023) 98:523–5. doi: 10.1016/j.abd.2022.10.007
56. Kinoshita-Ise M, Martinez-Cabriales SA, Alhusayen R. Chronological association between alopecia areata and autoimmune thyroid diseases: A systematic review and meta-analysis. J Dermatol. (2019) 46:702–9. doi: 10.1111/1346-8138.14940
57. Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. (2006) 5:64–9. doi: 10.1016/j.autrev.2005.07.001
58. Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. (2013) 1830:3908–16. doi: 10.1016/j.bbagen.2012.03.012
59. Zhu P, Deng W, Yu J, Yang S. Thyroid hormone receptor agonist promotes hair growth in mice. Clin Cosmet Investig Dermatol. (2022) 15:319–30. doi: 10.2147/CCID.S354219
60. Hussein RS, Atia T, Bin Dayel S. Impact of thyroid dysfunction on hair disorders. Cureus. (2023) 15:e43266. doi: 10.7759/cureus.43266
61. Guran T, Bircan R, Turan S, Bereket A. Alopecia: association with resistance to thyroid hormones. J Pediatr Endocrinol Metab. (2009) 22:1075–81. doi: 10.1515/JPEM.2009.22.11.1075
62. Billoni N, Buan B, Gautier B, Gaillard O, Mahé YF, Bernard BA. Thyroid hormone receptor beta1 is expressed in the human hair follicle. Br J Dermatol. (2000) 142:645–52. doi: 10.1046/j.1365-2133.2000.03408.x
63. Durá-Travé T, Gallinas-Victoriano F. Autoimmune thyroiditis and vitamin D. Int J Mol Sci. (2024) 25:3154. doi: 10.3390/ijms25063154
64. Suchonwanit P, Kositkuljorn C, Pomsoong C. Alopecia areata: an autoimmune disease of multiple players. Immunotargets Ther. (2021) 10:299–312. doi: 10.2147/ITT.S266409
65. Xin C, Sun X, Lu L, Yang R, Shan L, Wang Y. Increased incidence of thyroid disease in patients with alopecia areata: A systematic review and meta-analysis. Dermatology. (2020) 236:251–4. doi: 10.1159/000502025
Keywords: alopecia areata, causal relationship, Hashimoto’s thyroiditis, thyroid dysfunction, two-sample Mendelian randomization
Citation: Gao L, Li W, Song Q, Gao H and Chen M (2024) The genetic link between thyroid dysfunction and alopecia areata: a bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 15:1440941. doi: 10.3389/fendo.2024.1440941
Received: 30 May 2024; Accepted: 30 July 2024;
Published: 14 August 2024.
Edited by:
Poupak Fallahi, University of Pisa, ItalyReviewed by:
Georgia Damoraki, National and Kapodistrian University of Athens, GreeceGeorge Grant, Independent Researcher, Aberdeen, United Kingdom
Copyright © 2024 Gao, Li, Song, Gao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingwei Chen, Y2hlbm1pbmd3ZWlAeGp0dWZoLmVkdS5jbg==
 Le Gao
Le Gao Wenrui Li
Wenrui Li Qiang Song
Qiang Song Hengxing Gao
Hengxing Gao Mingwei Chen
Mingwei Chen