- 1Division of Sports Science and Physical Education, Tsinghua University, Beijing, China
- 2School of Social Sciences, Tsinghua University, Beijing, China
- 3IDG/McGovern Institute for Brain Research, Tsinghua University, Beijing, China
- 4School of Exercise and Health, Shanghai University of Sport, Shanghai, China
- 5Shanghai Fire Research Institute of Mem, Shanghai, China
- 6Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou, China
- 7School of Medical and Health Engineering, Changzhou University, Changzhou, China
- 8Institute of Information on Traditional Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Background: The prevalence of diabetes mellitus (DM) is a significant public health concern, especially among individuals with short sleep duration. Understanding the relationship between physical exercise and DM in this population is crucial for developing effective prevention strategies. However, the presence of a potential threshold effect of exercise on DM risk remains unclear.
Methods: Using data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2007 to 2018, this population-based study investigated the association between physical exercise and DM in individuals with short sleep duration (no more than 7 hours per night). Weighted logistic regression analyses were conducted, adjusting for demographic and lifestyle factors. Additionally, a two-piecewise linear regression model was employed to identify any threshold effect of exercise on DM risk.
Results: This study included 15,092 participants identified with short sleep duration. Demographic characteristics stratified by DM status indicate higher prevalence among certain groups, such as middle-aged and older adults, males, and non-Hispanic Whites. The analysis revealed an inverse association between exercise levels and DM prevalence among the short sleep population. In the fully adjusted model, individuals engaging in sufficient exercise (> 600 MET-minutes/week) exhibited significantly reduced odds of developing DM [OR (95% CI): 0.624(0.527,0.738), p < 0.001]. Furthermore, the segmented regression model identified an inflection point at 2000 MET-minutes/week, below which a significant correlation between exercise and DM was observed.
Conclusions: This study provides evidence of a threshold effect of physical exercise on its association with DM in individuals with short sleep duration. Tailored exercise interventions targeting this population may help mitigate DM risk and improve overall health outcomes. Further research is warranted to validate these findings and explore optimal exercise thresholds for DM prevention strategies.
1 Introduction
Diabetes mellitus (DM) has become a significant public health challenge globally, with rising prevalence rates, affecting 537 million adults worldwide and impacting diverse populations across various socio-economic backgrounds (1). As an increasing concern in public health, DM not only leads to severe individual complications but also places a substantial economic burden on healthcare systems worldwide (2–5). Research has consistently highlighted the critical role of lifestyle factors in the management and prevention of this chronic condition (6–8).
Short sleep, recognized as sleep less than 7 hours per day, has been linked to several negative health outcomes (9–13), including an increased risk of developing diabetes (14–16). Lack of sleep, often resulting from lifestyle choices or occupational demands, has been associated with adverse metabolic effects that may increase the risk of diabetes. This association suggests a complex interplay between sleep, exercise, and metabolic health, which is still not fully captured in diabetes prevention strategies.
Physical exercise, as a lifestyle with several health benefits, has been identified as a crucial factor in preventing and managing numerous chronic diseases (17, 18) and has become one of the important parts of therapy strategy for diabetes (19). Numerous studies have demonstrated that regular physical activity can significantly reduce the risk of developing type 2 diabetes through various biological mechanisms such as improved insulin sensitivity, enhanced weight management, and better lipid profiles (19–23). Additionally, there is also evidence that there may be a threshold effect of exercise on cognition (24–27), inflammation (28), and aging process (29) in the short sleep participants. However, the specific impacts of exercise in populations with unique health challenges, such as those experiencing short sleep durations, are less well understood and need further exploration.
The motivation behind this study stems from the observed gap in the literature regarding the interaction between physical activity and diabetes risk among individuals with short sleep durations. While the protective effects of exercise are well-documented, the existence and nature of a potential threshold effect—where the benefits of exercise might plateau or diminish—are not well understood in this specific population (30, 31). Addressing this gap is crucial for developing tailored interventions that effectively mitigate diabetes risk among those most vulnerable due to sleep restrictions.
This study aims to explore the threshold effect of physical exercise on diabetes mellitus risk among individuals with short sleep duration, providing evidence-based guidance for this under-researched but increasingly relevant demographic. Understanding these interactions has profound implications for public health policies and diabetes prevention programs, enabling the design of personalized lifestyle recommendations for those who may not fit the typical risk profile for diabetes.
2 Materials and methods
2.1 Study population
This analysis utilized data from the NHANES, a nationally representative cross-sectional survey conducted by the Centers for Disease Control and Prevention. The survey employs a stratified multistage random sampling approach. Questionnaire data were gathered by trained interviewers at participants’ homes. Data from six NHANES cycles spanning from 2007 to 2018 were included in the analysis: 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018. All participants provided written informed consent prior to participation, and the research procedures of NHANES were approved by the Institutional Review Board (IRB) of the National Center for Health Statistics (NCHS).
Initially, 59,389 participants were enrolled, with 36,580 individuals aged over 20 years. However, 15,710 respondents either did not complete the sleep questionnaire or reported sleep durations exceeding 7 hours. Thus, 20,770 participants were eligible for further analysis. Subsequently, those lacking diagnosis information for diabetes mellitus (n = 280) were excluded. Following this, 5,398 participants without covariate data were removed, resulting in a final study cohort of 15,092 participants.
2.2 Measurement of exposure and outcome variables
Participants self-reported their sleep duration, responding to the question “How much sleep do you get (hours)?” during NHANES cycles from 2007 to 2018 (32). The National Sleep Foundation recommends that healthy adults aim for 7 to 9 hours of sleep per night. Short sleep duration, defined as no more than 7 hours per night, was consistent with prior studies (33, 34).
The Physical Activity Questionnaire (PAQ) collected data on PE during home interviews, enabling the calculation of weekly MET-minutes. Moderate and vigorous PE were assessed separately, with two minutes of moderate PE considered equivalent to one minute of vigorous PE (35–37). MET values were multiplied by weekly PE minutes to obtain MET-minutes. PE intensity was categorized as moderate (4 MET) or vigorous (8 MET). To account for cumulative PE effects, volume was measured in 100 MET-min/week units. Following WHO guidelines and previous research (38, 39), PE volume was classified into three levels: none (< 1 MET-min/week), insufficient (1 to 600 MET-min/week), and sufficient (≥ 600 MET-min/week) for analysis.
The diagnostic criteria (40–42) for diabetes mellitus (DM) include: 1) receiving a diagnosis from a doctor; 2) having a glycohemoglobin HbA1c level greater than 6.5%; 3) fasting glucose level of 7.0 mmol/l or higher; 4) random blood glucose level of 11.1 mmol/l or higher; 5) two-hour OGTT blood glucose level of 11.1 mmol/l or higher; and 6) being on diabetes medication or insulin treatment.
2.3 Covariate assessment
Demographic characteristics, including age, gender, race/ethnicity (Non-Hispanic white, non-Hispanic black, Mexican American, and other races), marital status (never married, married or living with partner, and widowed, divorced, or separated), family poverty income ratio [low income (<1), middle income [1,3), and high income (≥3)], and education level (below high school, high school, and college or above), were extracted from the demographic questionnaire, as per previous literature (43–45). Additionally, smoking status and alcohol intake status were assessed through separate questionnaires. Smoking status was categorized as never, former, and current smoking, while alcohol intake status was classified as nondrinker, moderate alcohol use, and high alcohol use, based on questionnaire responses. Detailed covariate information is available at http://www.cdc.gov/nchs/nhanes/.
Furthermore, participants’ disease histories were evaluated. Hypertension was diagnosed in participants with systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥ 90 mmHg, or those who reported taking medication for hypertension or had been informed of their hypertension status by a healthcare professional. Cardiovascular disease was defined as self-reported congestive heart failure, coronary heart disease, angina, heart attack, or stroke.
2.4 Statistical analysis
The analyses were conducted while considering the complex survey design, adhering to NHANES data usage guidelines, which included sample weights, clustering, and stratification. Survey weights from Mobile Examination Center interviews spanning twelve years of NHANES data (2007-2018) were applied to address non-response, non-coverage, and varying probabilities of selection. Initially, a crude model was employed with no covariate adjustments. Model 1 was then adjusted for age, gender, and race/ethnicity. Subsequently, Model 2 further adjusted for BMI, marital status, education, poverty income ratio, smoking status, and alcohol use status.
A weighted logistic regression model was utilized to explore the relationship between exercise and DM among individuals with short sleep duration. Stratified analyses were performed based on each covariate. To examine potential threshold effects and control for confounding variables, a two-piecewise linear regression model was constructed. The threshold level of exercise (represented by 100 * MET-minutes/week) was determined using a recurrence strategy, identifying the inflection point within a predefined interval (46–48). Comparison between the two-piecewise linear regression model and the one-line linear regression model was conducted using the log-likelihood ratio test. Furthermore, we assessed the nonlinear relationship using restricted cubic spline (RCS) analysis, employing three optimal knots. Statistical analyses were performed using software from the R Foundation (http://www.R-project.org), with significance established at a p-value of 0.05 or lower.
3 Results
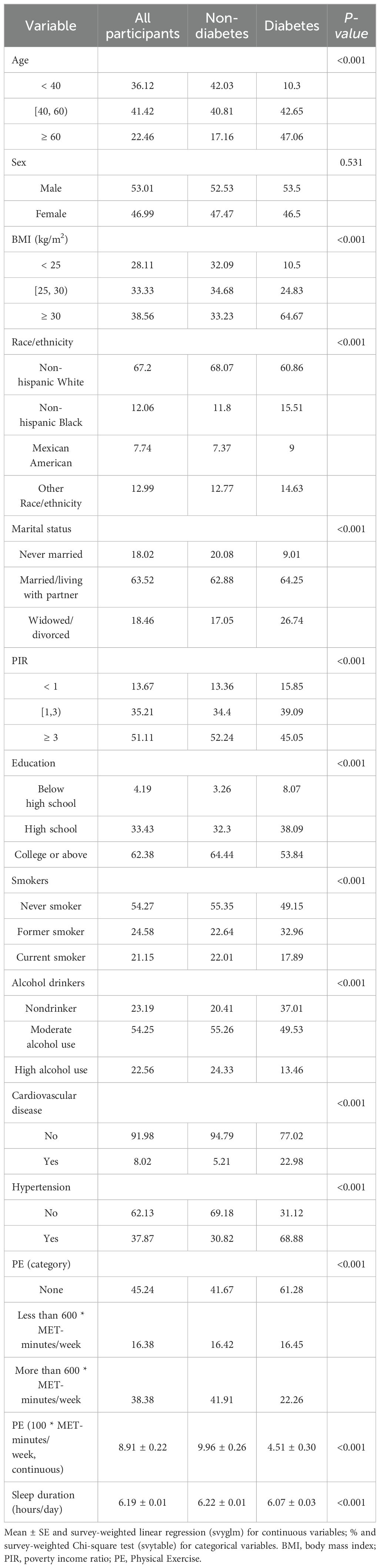
Table 1 presents the demographic characteristics of participants stratified by DM status. From the 2007-2018 NHANES dataset, a total of 15,092 participants with identified short sleep duration were included for analysis, representing a weighted population of 104,375,450 individuals. Diabetes was found to be more prevalent among middle-aged (40-60 years) and older (≥ 60 years) adults, males, and non-Hispanic Whites. Moreover, individuals with higher education levels, moderate alcohol consumption, and metabolic conditions such as overweight or hypertension showed a higher prevalence of diabetes.
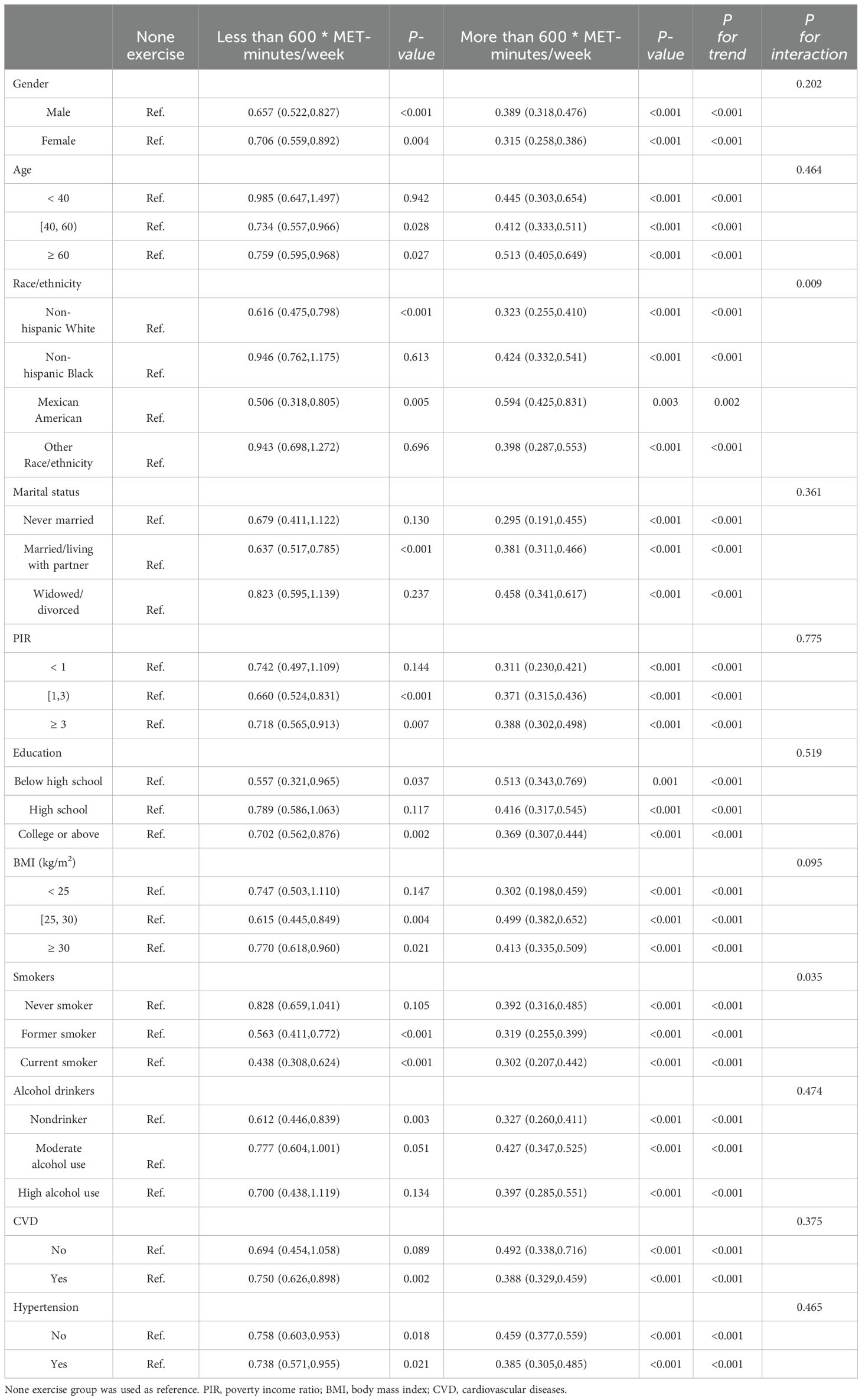
Table 2 displays the correlation between exercise and diabetes through weighted logistic regression analyses. The odds ratios (ORs) with 95% confidence intervals (CIs) represent the prevalence of DM development across exercise measured in 100 * MET-minutes per week. In the crude model, the OR was 0.960 (95% CI: 0.952, 0.969), p < 0.001, while in Model 1 and Model 2, the ORs were 0.971 (95% CI: 0.963, 0.979) and 0.983 (95% CI: 0.976, 0.991), respectively, all indicating a significant association with p < 0.001. Quantile measures consistently showed a decreasing trend in DM prevalence with increasing exercise levels, regardless of adjustment (Table 2). Specifically, individuals engaging in sufficient exercise (more than 600 MET-minutes/week) exhibited significantly decreased odds of developing DM in the Crude Model (OR = 0.361, 95% CI: 0.310, 0.422), and this association persisted after adjusting for age, sex, and race/ethnicity in Model 1 (OR = 0.465, 95% CI: 0.396, 0.544). Even after further adjustment for all confounding factors in Model 2, the association remained significant, with an OR of 0.624 (95% CI: 0.527, 0.738), p < 0.001. These results, stratified by different demographic factors, consistently demonstrated a significant association (Table 3).
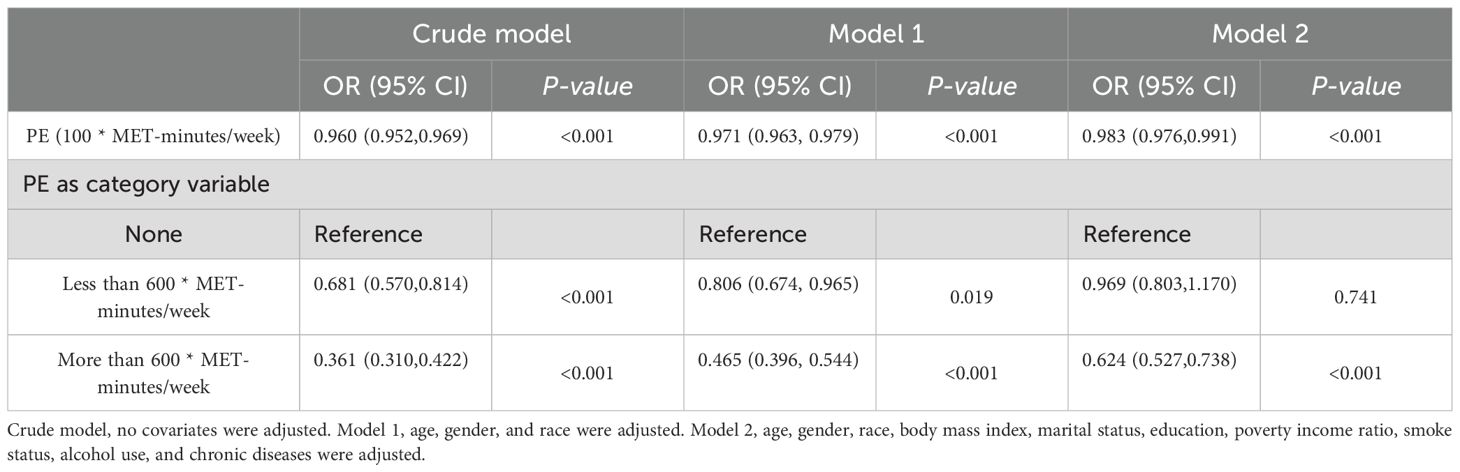
Table 2. Weighted logistic regression results for the association between exercise and DM in short sleep population.
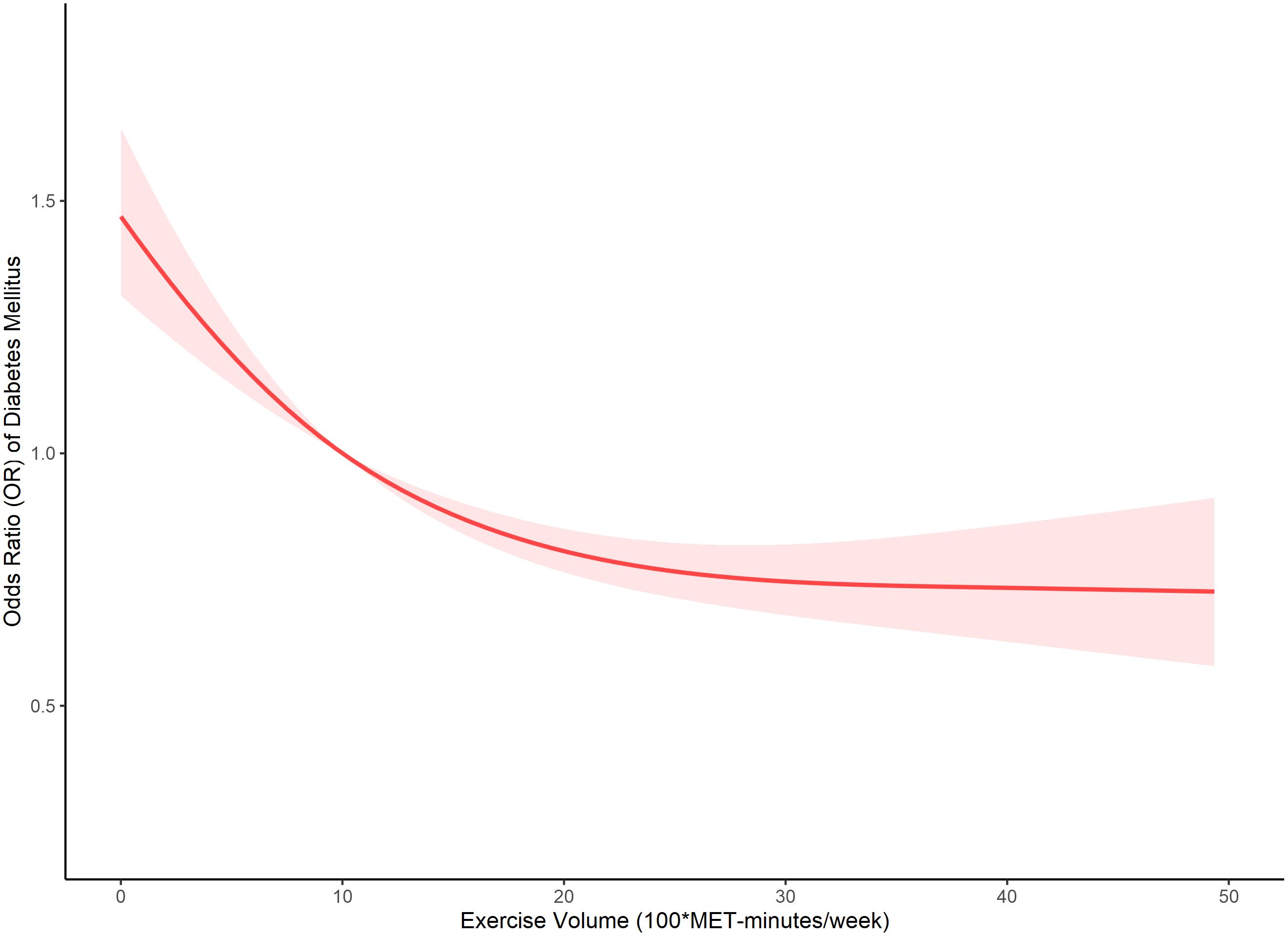
Our analysis further delved into the comparison between the single-line (non-segmented) model and the segmented regression model using a log-likelihood ratio test, which unveiled the existence of a threshold. Table 4 displays the outcomes of the two-piecewise linear regression model, pinpointing the inflection point at 2000 MET-minutes/week. Beneath this threshold, a notable correlation between exercise and DM was evident, with an odds ratio (OR) of 0.973 (95% CI: 0.964, 0.981) and a p-value below 0.001. Conversely, above this inflection point, the association lost significance, yielding an OR of 0.998 (95% CI: 0.991, 1.005) and a p-value of 0.586. Figure 1 visually represents the relationship between exercise and DM among individuals with short sleep using restricted cubic splines. Noteworthy is the observation of a threshold effect at 2000 MET-minutes/week, suggesting a saturation point beyond which the influence of exercise on DM diminishes.
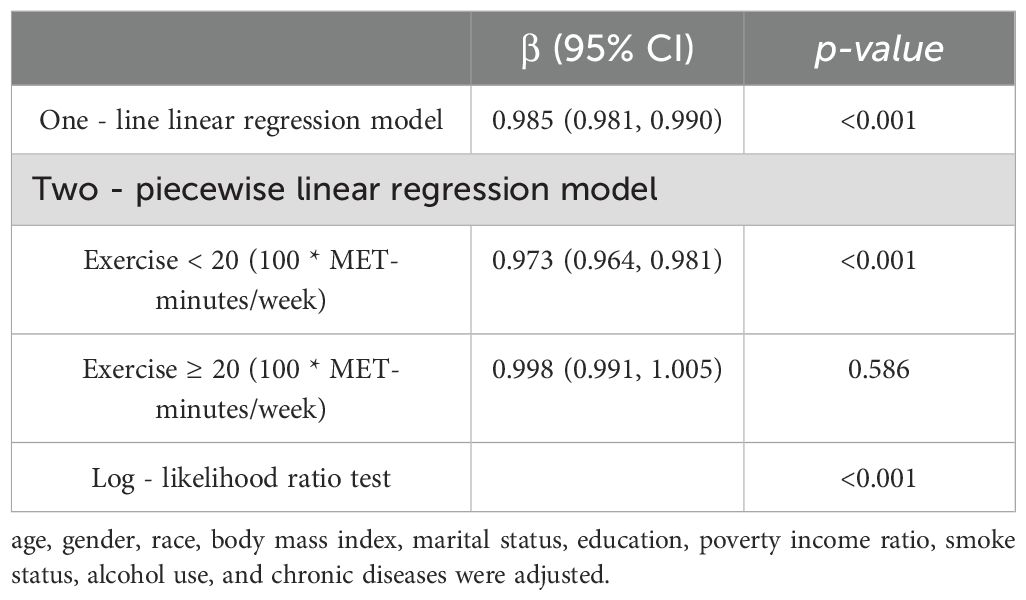
Table 4. Threshold effect analysis of relationship between exercise and DM in short sleep population.
4 Discussions
This study has identified a significant threshold effect of physical exercise on risk of diabetes among individuals with short sleep duration, suggesting that engaging in a certain level of physical activity regularly can substantially mitigate this risk. Our findings demonstrate that physical exercise exceeding 600 MET-minutes/week is associated with a decreased likelihood of developing DM, with diminishing returns observed beyond 2000 MET-minutes/week.
These results align with previous studies that have reported the beneficial effects of physical activity on glucose metabolism and insulin sensitivity (20). However, our study extends the understanding by pinpointing a specific exercise threshold, which is particularly relevant for individuals with short sleep duration. This nuanced insight contrasts with the broader generalizations often found in diabetes prevention research, where one-size-fits-all recommendations prevail (7, 49).
The biological mechanisms underpinning our findings may involve the enhanced regulation of glucose and increased insulin efficiency, which are promoted by regular physical activity (6, 50, 51). Furthermore, exercise has been shown to improve sleep quality and duration, indirectly contributing to better metabolic outcomes in populations at risk of short sleep-related metabolic disorders (52, 53).
Despite the clear benefits associated with physical exercise, our findings suggest a saturation effect at 2000 MET-minutes/week, where additional physical activity may not confer further benefits in reducing DM risk. This phenomenon may be explained by the physiological limits of exercise-induced improvements in metabolic health. Beyond a certain point, the body’s ability to further enhance glucose utilization and insulin sensitivity may plateau (54). This could be attributed to a maximal activation of biological pathways involved in metabolic regulation, after which additional exercise yields diminishing returns (54).
Moreover, excessive physical activity might lead to increased stress and fatigue (55–57), particularly in individuals with restricted sleep, potentially counteracting some of the positive effects of exercise on metabolic health. It suggests that there might be an optimal balance of exercise that maximizes health benefits without leading to overtraining or undue physical stress, especially important in populations vulnerable to sleep deprivation, as sleep quality is an essential indicator for overtraining (58).
The public health implications of these findings are substantial. By incorporating exercise thresholds into diabetes prevention programs, health policymakers can design more effective interventions that are tailored to the needs of individuals with different sleep patterns. This approach not only helps in targeting high-risk groups more effectively but also in optimizing resource allocation within public health initiatives.
Despite the strengths of this study, including a large sample size and the use of robust statistical methods, there are limitations that should be acknowledged. The cross-sectional nature of the data limits our ability to infer causality between exercise and DM risk reduction. Additionally, self-reported measures of physical activity and sleep may introduce bias. Future research should consider longitudinal designs to better establish causal relationships and utilize objective measures of physical activity and sleep to enhance the accuracy of the findings.
This study sheds light on the importance of personalized exercise prescriptions in diabetes prevention, especially among those compromised by short sleep durations. It underscores the need for further research into tailored preventive strategies that consider individual variations in lifestyle and health status.
5 Conclusion
In conclusion, our population-based study sheds light on the association between physical exercise and diabetes mellitus (DM) within the short sleep population. Our analysis, involving 15,092 participants with short sleep duration, revealed a significant inverse correlation between exercise and DM development. Specifically, engaging in sufficient exercise (> 600 MET-minutes/week) recommended by WHO was associated with decreased odds of developing DM, even after adjusting for confounding factors such as age, sex, and race/ethnicity. Notably, our comparison between single-line and segmented regression models identified a threshold effect at 2000 MET-minutes/week of exercise, beyond which the association between exercise and DM lost significance. This observation suggests a saturation point, indicating that higher exercise volumes may not confer additional benefits in reducing the risk of DM among individuals with short sleep.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YY: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Conceptualization. AA: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Conceptualization. YL: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Conceptualization. MT: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Conceptualization. WQ: Writing – review & editing, Software, Methodology, Investigation. DZ: Writing – review & editing, Software, Methodology, Investigation. YT: Writing – review & editing, Software, Methodology, Investigation. HD: Writing – review & editing, Software, Methodology, Investigation. KC: Writing – review & editing, Software, Methodology, Investigation. JL: Writing – review & editing, Supervision, Project administration, Methodology. XM: Writing – review & editing, Supervision, Project administration, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Institute of Sports Development Research of Tsinghua University (Research on John Mo’s thought and practice of Physical Education).
Acknowledgments
The authors appreciate the time and effort given by participants during the data collection phase of the National Health and Nutrition Examination Survey (NHANES) project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. International Diabetes Federation. IDF Diabetes Atlas. accessed 2024 April 23. Available online at: https://diabetesatlas.org/. (accessed April 23, 2024).
2. Liu J, Liu M, Chai Z, Li C, Wang Y, Shen M, et al. Projected rapid growth in diabetes disease burden and economic burden in China: A spatio-temporal study from 2020 to 2030. Lancet Reg Health West Pac. (2023) 33:100700. doi: 10.1016/j.lanwpc.2023.100700
3. Raman R, Vasconcelos JC, Rajalakshmi R, Prevost AT, Ramasamy K, Mohan V, et al. Prevalence of diabetic retinopathy in India stratified by known and undiagnosed diabetes, urban-rural locations, and socioeconomic indices: results from the smart India population-based cross-sectional screening study. Lancet Glob Health. (2022) 10:e1764–e73. doi: 10.1016/S2214-109X(22)00411-9
4. Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based search for diabetes in youth study. Lancet Diabetes Endocrinol. (2023) 11:242–50. doi: 10.1016/S2213-8587(23)00025-6
5. The L. Diabetes: A defining disease of the 21st century. Lancet. (2023) 401:2087. doi: 10.1016/S0140-6736(23)01296-5
6. Riddell MC, Li Z, Gal RL, Calhoun P, Jacobs PG, Clements MA, et al. Examining the acute glycemic effects of different types of structured exercise sessions in type 1 diabetes in a real-world setting: the type 1 diabetes and exercise initiative (T1dexi). Diabetes Care. (2023) 46:704–13. doi: 10.2337/dc22-1721
7. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S68–96. doi: 10.2337/dc23-S005
8. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. (2017) 15:131. doi: 10.1186/s12916-017-0901-x
9. You Y, Liu J, Li X, Wang P, Liu R, Ma X. Relationship between Accelerometer-Measured Sleep Duration and Stroop Performance: A Functional near-Infrared Spectroscopy Study among Young Adults. PeerJ. (2024) 12:e17057. doi: 10.7717/peerj.17057
10. Chow CM. Sleep and wellbeing, now and in the future. Int J Environ Res Public Health. (2020) 17(8):2883. doi: 10.3390/ijerph17082883
11. Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). (2018) 7(1):1. doi: 10.3390/healthcare7010001
12. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. (2015) 38:843–4. doi: 10.5665/sleep.4716
13. You Y, Ablitip A, Chen Y, Ding H, Chen K, Cui Y, et al. Saturation effects of the relationship between physical exercise and systemic immune inflammation index in the short-sleep population: A cross-sectional study. BMC Public Health. (2024) 24:1920. doi: 10.1186/s12889-024-19432-7
14. Noga DA, Meth E, Pacheco AP, Tan X, Cedernaes J, van Egmond LT, et al. Habitual short sleep duration, diet, and development of type 2 diabetes in adults. JAMA Netw Open. (2024) 7:e241147. doi: 10.1001/jamanetworkopen.2024.1147
15. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: A randomized, crossover study. Ann Intern Med. (2012) 157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005
16. Chaput JP, McHill AW, Cox RC, Broussard JL, Dutil C, da Costa BGG, et al. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. (2023) 19:82–97. doi: 10.1038/s41574-022-00747-7
17. You Y, Li W, Liu J, Li X, Fu Y, Ma X. Bibliometric review to explore emerging high-intensity interval training in health promotion: A new century picture. Front Public Health. (2021) 9:697633. doi: 10.3389/fpubh.2021.697633
18. You Y, Chen Y, Wang X, Wei M, Zhang Q, Cao Q. Accelerometer-measured physical activity patterns are associated with phenotypic age: isotemporal substitution effects. Heliyon. (2023) 9:e19158. doi: 10.1016/j.heliyon.2023.e19158
19. Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American college of sports medicine. Med Sci Sports Exerc. (2022) 54:353–68. doi: 10.1249/MSS.0000000000002800
20. Chen Y, Chen Z, Pan L, Ma ZM, Zhang H, Li XJ, et al. Effect of moderate and vigorous aerobic exercise on incident diabetes in adults with obesity: A 10-year follow-up of a randomized clinical trial. JAMA Intern Med. (2023) 183:272–5. doi: 10.1001/jamainternmed.2022.6291
21. Larsson Y, Persson B, Sterky G, Thoren C. Effect of exercise on blood-lipids in juvenile diabetes. Lancet. (1964) 1:350–5. doi: 10.1016/s0140-6736(64)92098-7
22. Passa P, Gauville C, Canivet J. Influence of muscular exercise on plasma level of growth hormone in diabetics with and without retinopathy. Lancet. (1974) 2:72–4. doi: 10.1016/s0140-6736(74)91635-3
23. Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. (2017) 5:377–90. doi: 10.1016/S2213-8587(17)30014-1
24. You Y, Liu J, Wang D, Fu Y, Liu R, Ma X. Cognitive performance in short sleep young adults with different physical activity levels: A cross-sectional fNIRS study. Brain Sci. (2023) 13(2):171. doi: 10.3390/brainsci13020171
25. You Y, Liu J, Yao Z, Zhang S, Chen K, Ma X. Neural mechanisms of long-term exercise intervention on cognitive performance among short-sleep young adults: A hemodynamic study. Sleep Med. (2023) 110:7–16. doi: 10.1016/j.sleep.2023.07.020
26. Bloomberg M, Brocklebank L, Hamer M, Steptoe A. Joint associations of physical activity and sleep duration with cognitive ageing: longitudinal analysis of an English cohort study. Lancet Healthy Longev. (2023) 4:e345–e53. doi: 10.1016/S2666-7568(23)00083-1
27. Williams TB, Badariotti JI, Corbett J, Miller-Dicks M, Neupert E, McMorris T, et al. The effects of sleep deprivation, acute hypoxia, and exercise on cognitive performance: A multi-experiment combined stressors study. Physiol Behav. (2024) 274:114409. doi: 10.1016/j.physbeh.2023.114409
28. You Y. Accelerometer-measured physical activity and sedentary behaviour are associated with C-reactive protein in us adults who get insufficient sleep: A threshold and isotemporal substitution effect analysis. J Sports Sci. (2024) 42(6):527–36. doi: 10.1080/02640414.2024.2348906
29. You Y, Chen Y, Liu R, Zhang Y, Wang M, Yang Z, et al. Inverted U-shaped relationship between sleep duration and phenotypic age in us adults: A population-based study. Sci Rep. (2024) 14:6247. doi: 10.1038/s41598-024-56316-7
30. Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. (2008) 31:587–91. doi: 10.1007/BF03345606
31. You Y, Wei M, Chen Y, Fu Y, Ablitip A, Liu J, et al. The association between recreational physical activity and depression in the short sleep population: A cross-sectional study. Front Neurosci. (2023) 17:1016619. doi: 10.3389/fnins.2023.1016619
32. You Y, Li J, Zhang Y, Li X, Li X, Ma X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: insights from population-based and mice studies. CNS Neurosci Ther. (2024) 30:e14783. doi: 10.1111/cns.14783
33. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. (2015) 1:40–3. doi: 10.1016/j.sleh.2014.12.010
34. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation's updated sleep duration recommendations: final report. Sleep Health. (2015) 1:233–43. doi: 10.1016/j.sleh.2015.10.004
35. You Y, Mo L, Tong J, Chen X, You Y. The role of education attainment on 24-hour movement behavior in emerging adults: evidence from a population-based study. Front Public Health. (2024) 12:1197150. doi: 10.3389/fpubh.2024.1197150
36. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and met intensities. Med Sci Sports Exerc. (2000) 32:S498–504. doi: 10.1097/00005768-200009001-00009
37. You Y, Chen Y, Yin J, Zhang Z, Zhang K, Zhou J, et al. Relationship between leisure-time physical activity and depressive symptoms under different levels of dietary inflammatory index. Front Nutr. (2022) 9:983511. doi: 10.3389/fnut.2022.983511
38. You Y, Chen Y, Fang W, Li X, Wang R, Liu J, et al. The association between sedentary behavior, exercise, and sleep disturbance: A mediation analysis of inflammatory biomarkers. Front Immunol. (2022) 13:1080782. doi: 10.3389/fimmu.2022.1080782
39. You Y, Chen Y, Zhang Y, Zhang Q, Yu Y, Cao Q. Mitigation role of physical exercise participation in the relationship between blood cadmium and sleep disturbance: A cross-sectional study. BMC Public Health. (2023) 23:1465. doi: 10.1186/s12889-023-16358-4
40. American Diabetes Association Professional Practice C. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
41. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S19–40. doi: 10.2337/dc23-S002
42. Shen R, Guo X, Zou T, Ma L. Associations of cardiovascular health assessed by life's essential 8 with diabetic retinopathy and mortality in type 2 diabetes. Prim Care Diabetes. (2023) 17:420–8. doi: 10.1016/j.pcd.2023.08.001
43. You Y, Wang R, Li J, Cao F, Zhang Y, Ma X. The role of dietary intake of live microbes in the association between leisure-time physical activity and depressive symptoms: A population-based study. Appl Physiol Nutr Metab. (2024) 49(8):1014–24. doi: 10.1139/apnm-2023-0550
44. You Y, Chen Y, Wei M, Tang M, Lu Y, Zhang Q, et al. Mediation role of recreational physical activity in the relationship between the dietary intake of live microbes and the systemic immune-inflammation index: A real-world cross-sectional study. Nutrients. (2024) 16(6):777. doi: 10.3390/nu16060777
45. You Y, Chen Y, Zhang Q, Yan N, Ning Y, Cao Q. Muscle quality index is associated with trouble sleeping: A cross-sectional population based study. BMC Public Health. (2023) 23:489. doi: 10.1186/s12889-023-15411-6
46. Wang X, Zhang J, Chen C, Lu Z, Zhang D, Li S. The association between physical activity and cognitive function in the elderly in rural areas of Northern China. Front Aging Neurosci. (2023) 15:1168892. doi: 10.3389/fnagi.2023.1168892
47. Tan MY, Mo CY, Li F, Zhao Q. The association between serum uric acid and hypertriglyceridemia: evidence from the national health and nutrition examination survey (2007-2018). Front Endocrinol (Lausanne). (2023) 14:1215521. doi: 10.3389/fendo.2023.1215521
48. You Y, Chen Y, Chen X, Wei M, Yin J, Zhang Q, et al. Threshold effects of the relationship between physical exercise and cognitive function in the short-sleep elder population. Front Aging Neurosci. (2023) 15:1214748. doi: 10.3389/fnagi.2023.1214748
49. Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd Ed. US Department of Health and Human Services. Available at: https://health.gov/paguidelines/second-edition/.
50. Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann Intern Med. (2007) 147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005
51. Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat Rev Endocrinol. (2017) 13:133–48. doi: 10.1038/nrendo.2016.162
52. Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J Physiother. (2012) 58:157–63. doi: 10.1016/S1836-9553(12)70106-6
53. Kovacevic A, Mavros Y, Heisz JJ, Fiatarone Singh MA. The effect of resistance exercise on sleep: A systematic review of randomized controlled trials. Sleep Med Rev. (2018) 39:52–68. doi: 10.1016/j.smrv.2017.07.002
54. Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, et al. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation. (2017) 136:2144–57. doi: 10.1161/CIRCULATIONAHA.117.028274
55. Alessio HM. Exercise-induced oxidative stress. Med Sci Sports Exerc. (1993) 25:218–24. doi: 10.1249/00005768-199302000-00010
56. Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. (2009) 39:389–422. doi: 10.2165/00007256-200939050-00005
57. Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. (2019) 9(6):223. doi: 10.3390/biom9060223
Keywords: diabetes mellitus, cross-sectinal study, exercise, threshold effect, short sleep
Citation: You Y, Ablitip A, Lin Y, Tang M, Qian W, Zhang D, Tong Y, Ding H, Chen K, Liu J and Ma X (2024) Threshold effect of physical exercise on its association to diabetes mellitus in short sleep population: evidence from a nationwide study. Front. Endocrinol. 15:1437452. doi: 10.3389/fendo.2024.1437452
Received: 23 May 2024; Accepted: 07 August 2024;
Published: 26 August 2024.
Edited by:
Weiwei Liu, Chongqing Medical University, ChinaReviewed by:
Xin Yu, Anhui Medical University, ChinaLiyun He, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2024 You, Ablitip, Lin, Tang, Qian, Zhang, Tong, Ding, Chen, Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianxiu Liu, bGl1amlhbnhpdUBtYWlsLnRzaW5naHVhLmVkdS5jbg==; Xindong Ma, bWF4ZEBtYWlsLnRzaW5naHVhLmVkdS5jbg==
†These authors have contributed equally to this work
 Yanwei You
Yanwei You Alimjan Ablitip1,2,3†
Alimjan Ablitip1,2,3† Yuanyuan Tong
Yuanyuan Tong Jianxiu Liu
Jianxiu Liu