- 1Department of Endocrinology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Key Laboratory for Molecular Diagnosis of Hubei Province, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Hubei Regenerative Medicine Clinical Research Center, Wuhan, China
- 4Wuhan Diabetes Clinical Research Center, Wuhan, China
Objective: This study explored the utility of NLR (neutrophil-to-lymphocyte ratio) as a marker to predict Lower Extremity Peripheral Artery Disease (PAD) in the Chinese population, as well as to assess its consistency and diagnostic value with digital subtraction angiography.
Methods: Patients were distributed into three groups according to the angiography in lower limb arterial: group L1, plaque with no stenosis; group L2, plaque with luminal stenosis and group L3, total vascular occlusion. Changes in the neutrophil-to-lymphocyte ratio were documented and compared among groups.
Results: Compared to group L1, NLR was significantly increased in L2 (1.76 vs 2.35, p=0.037) and L3 (1.76 vs 3.60, p<0.001), with a gradual decrease in ABI (Ankle-Brachial Index, 1.11 vs 1.02 vs 0.94, p<0.001). Those older patients with higher prevalence of hypertension (p=0.002), obesity (p=0.032), or reduced high-density lipoprotein cholesterol (p=0.020) were more likely to develop PAD; higher glycosylated hemoglobin (p=0.045), low-density lipoprotein cholesterol (p=0.006), and systolic blood pressure (p<0.001) levels led to a greater tendency to suffer stenosis or even occlusion; the probability of severe stenosis (>70%) increased to 2.075 times for every 1 increase in NLR, while it was 46.8% for every 0.1 increase in ABI. The optimal NLR cut-off value to predict severe stenosis in PAD was 2.73. Receiver operating characteristic curve analysis of the inflammatory biomarkers and severe stenosis prediction displayed an area under the curve of 0.81.
Conclusion: NLR could serve as a new noninvasive and accurate marker in predicting PAD.
1 Introduction
Diabetes mellitus (DM) is a disease characterized by the poor control of blood glucose, the prevalence is predicted to be 12.2% by 2045 (1). Patients with DM are at high risk for various cardiovascular diseases (CVD), e.g., coronary artery disease (CAD), stroke, and peripheral artery disease (PAD), which are the leading causes of DM-related mortality and morbidity. PAD is characterized by atherosclerotic stenosis or obstruction of the limb arteries, it can occur in the upper limbs, but more often in the lower limbs (2). From the epidemiological perspective, PAD occurs in patients with DM at a two- to four-fold higher incidence than in whom without DM (3), and the disease progression is more severe in the diabetic population (4). PAD affects 20–28% of the diabetic population, and up to 50% of patients with diabetic foot disease (DFD) (2). In China, the occurrence of peripheral artery disease in type 2 diabetic cases is reported to be 55.3-65% in different studies (5, 6). As claimed by the China DIA-LEAD epidemiological investigations, the currency of type 2 diabetes mellitus combined with lower extremity PAD was 21.2% in patients over 50 years old (7). What is more, PAD can result in a threat to the quality of life and survival period in diabetic individuals (8–10). In the United States, 4.6% of diabetic patients develop lower extremity chronic ulcers (at least 6 weeks) or amputations below the knee (of at least one toe) (11). So, early PAD diagnosis in diabetic patients is particularly important for preventing complications, such as ulcers or gangrene, and for reducing major adverse limb events, adverse cardiovascular events, and mortality. However, PAD diagnosis in diabetic patients is often made difficult by the characteristics of the diseases. Characteristic PAD symptoms are frequently absent in diabetic patients. Often, patients do not report claudication due to a lack of physical activity, and do not refer pain due to concomitant neuropathy (12). This implies that PAD diagnosis is made already at the most advanced stages of the disease, often when patients already present DFD. Currently, diagnosis of lower extremity arterial stenosis is dependent on lower extremity arteriography (Digital Subtraction Angiography, DSA) as an assessment (13), its great advantage is that it is diagnostic and can be interventional at the same time (14). However, due to its high technical difficulty, invasiveness, high cost, and possible postoperative complications such as hematoma, bruising, and even contrast nephropathy, the widespread implementation of DSA has been limited; meanwhile, the traditional Doppler ultrasound diagnosis, although convenient and economical, is still difficult to accurately screen patients with lower extremity occlusion at an early stage (15), it is operator-dependent particularly in the case of calcification or structures close to bone or gas-filled cavities (16). ABI is currently a valid measurement for the diagnosis of PAD, but it is usually higher than actual in diabetic foot ulcers because of its association with vascular calcification and impaired elasticity (17). Therefore, there is an urgent need for a screening tool that can accurately and early detect diabetic lower limb vascular lesions but is also convenient, cost-effective, and easy to perform widely for early detection and intervention.
Studies have shown that many risk factors such as hyperglycemia, hyperlipidemia, and hypertension can lead to atherosclerosis. From a pathophysiological perspective, various inflammatory cytokines including chemokines, adipokines, adhesion molecules, and cytokines may contribute to the development of peripheral artery disease in the lower limbs in diabetes. In the early stages, oxidative stress accompanied by inflammatory responses occurs after the elevation of blood glucose, massive neutrophil infiltration mediates non-specific inflammatory response (18, 19), causing vascular endothelial damage, followed by a buildup of plaque in the inner arterial wall (20), the accumulation of neutrophil protease further drives plaque instability, even plaque hemorrhage and plaque detachment (21). At the same time, damaged vascular endothelial cells express chemokines and adhesion molecules can recruit lymphocytes to infiltrate the endothelium (22): lymphocytes is an immune system regulation pathway, by reducing the number of CD8+ T lymphocytes to inhibit the anti-inflammatory environment, maintaining low chronic inflammation, long-term inflammation leads to the proliferation of vascular smooth muscle, microangiogenesis, subsequent arterial lesions and plaque formation (23). To make matters worse, decreased neutrophil apoptosis and the increase of lymphocyte apoptosis lead to abnormal cell ratio mediating insulin resistance and insulin secretion dysfunction, accelerating the production of more active oxidants, leading to permeability and dysfunction of vascular endothelial cells, decreased nitric oxide and capillary expansion, eventually reducing microcirculation to peripheral tissues (24), followed by atherosclerotic plaques and varying degrees of stenosis even up to occlusion (25), which we called PAD.
Several studies have shown that PAD is associated with pro-inflammatory cytokines, such as interleukin-6, interleukin-1, and tumor necrosis factor (26, 27). However, the detection of the inflammatory markers mentioned above is not used in common work because of the payments and technical hardships. The blood-routine test is a sensitive and cost-effective detection that can be done easily in the laboratory, the neutrophil-to-lymphocyte ratio (NLR) is currently a novel predictor of inflammation (28), it has been monitored in other diseases such as coronary heart disease (29), tumors (30, 31), thyroid disease (32). Studies have shown that NLR is a risk factor for elevated blood glucose, positively correlates with HbA1c levels in patients with T2DM (33, 34), and is involved in the development of diabetes-related complications including diabetic nephropathy (35) or diabetic retinopathy (36), but the role and function of NLR in diabetes mellitus combined with PAD has not been studied. Based on the description of the changes and roles of neutrophils and lymphocytes in atherosclerosis in the paragraph above, therefore, this study proposed to assess the change of NLR in diabetic PAD and whether or not NLR can serve as a better predictor.
2 Materials and methods
2.1 Study design and participants
Between January 2021 and June 2023, 194 diabetic patients with PAD (age 18-75 years) were enrolled. Inclusion criteria: (1) All patients were diagnosed type 2 diabetes mellitus according to the 2021 American Diabetes Association (ADA) criteria (37); (2) All patients were accompanied by different degrees of distal lower limb ischemia symptoms, manifested as intermittent limp and rest pain, or some patients were asymptomatic, manifested only by low skin temperature of the extremities, and diminished or absent dorsal pedis artery or posterior tibial artery pulsations found during physical examination (The Leriche Fontaine and Rutherford classifications are based on clinical symptomatology, Table 1.); (3) All patients underwent lower extremity arteriography and had varying degrees of vascular plaque or stenosis. Exclusion criteria: (1) ketosis, diabetic nonketotic hyperosmolar coma, acute cardiovascular and cerebrovascular disease, and other states of stress; (2) acute infections in the combined lower limbs or elsewhere; (3) special types of diabetes mellitus; (4) acute or chronic renal insufficiency (eGFR <60ml/min/1.73m2) or coagulation disorders; and (5) hepatitis, cirrhosis, tuberculosis, tumors, or other immune-deficiency diseases, or those who were receiving hormone therapy.
The study was a retrospective analysis, data were obtained from patient records file regarding age, sex, duration of diabetes, height, weight, blood pressure, smoking history, Body Mass Index (BMI) =weight/(height squared) (international unit kg/m2) and other necessary information.
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and the research protocol was priorly approved by the Ethics Committee of Wuhan Central Hospital (Ethical No. WHZXKYL2023-186). Written informed consent was obtained from all the patient to process their personal data for scientific research purpose.
2.2 Laboratory measurements
Blood samples (fasted for more than 8 hours) were drawn from all patients on the day after admission. A routine blood test was measured using mindary BC-6800 (flow cytometric analysis), assessment of NLR dividing the absolute neutrophil count on the absolute lymphocyte count, lipid profile was quantified using Beckman Coulter AU5800 (enzyme electrode method), and HbA1c was measured using D-10 Glycated Hemoglobin Meter (microcolumn method), serum CRP (C-reactive protein) has been determined using turbidimetry (Beckman Coulter, Brea, USA). ABI was evaluated by VBP-9 atherosclerosis detector.
2.3 Digital subtraction angiography
DSA was carried out by Advantx LCV digital subtraction angiography equipment of GE, and the right femoral artery was punctured by using a 5F-6F catheter, which was sent to the bifurcation of the abdominal aorta under fluoroscopy, and the lower limb vascular visualization was observed. Patients were sorted into three groups: group L1, plaque with no stenosis; group L2, plaque with luminal stenosis (L2a: stenosis<70%; L2b: stenosis at 70%-99%) and group L3, total vascular occlusion in lower limb arterial.
2.4 Statistical analysis
Statistical Package for Social Science (SPSS, version 27.0; SPSS, Inc., Chicago, USA) was applied for statistical calculations. The difference among groups was settled with ANOVAs for normally distributed data or Kruskal-Wallis tests for skewed data, chi-square test was used for counting data; Spearman’s test was used to detect the correlation; logistic regression was used to analyze the risk factors for PAD; the optimum cutoff level was analyzed using the receiver operating characteristics (ROC) curve, and the area under the curve (AUC) was calculated. p<0.05 was considered statistically significant.
3 Results
3.1 Demographic and clinical characteristics
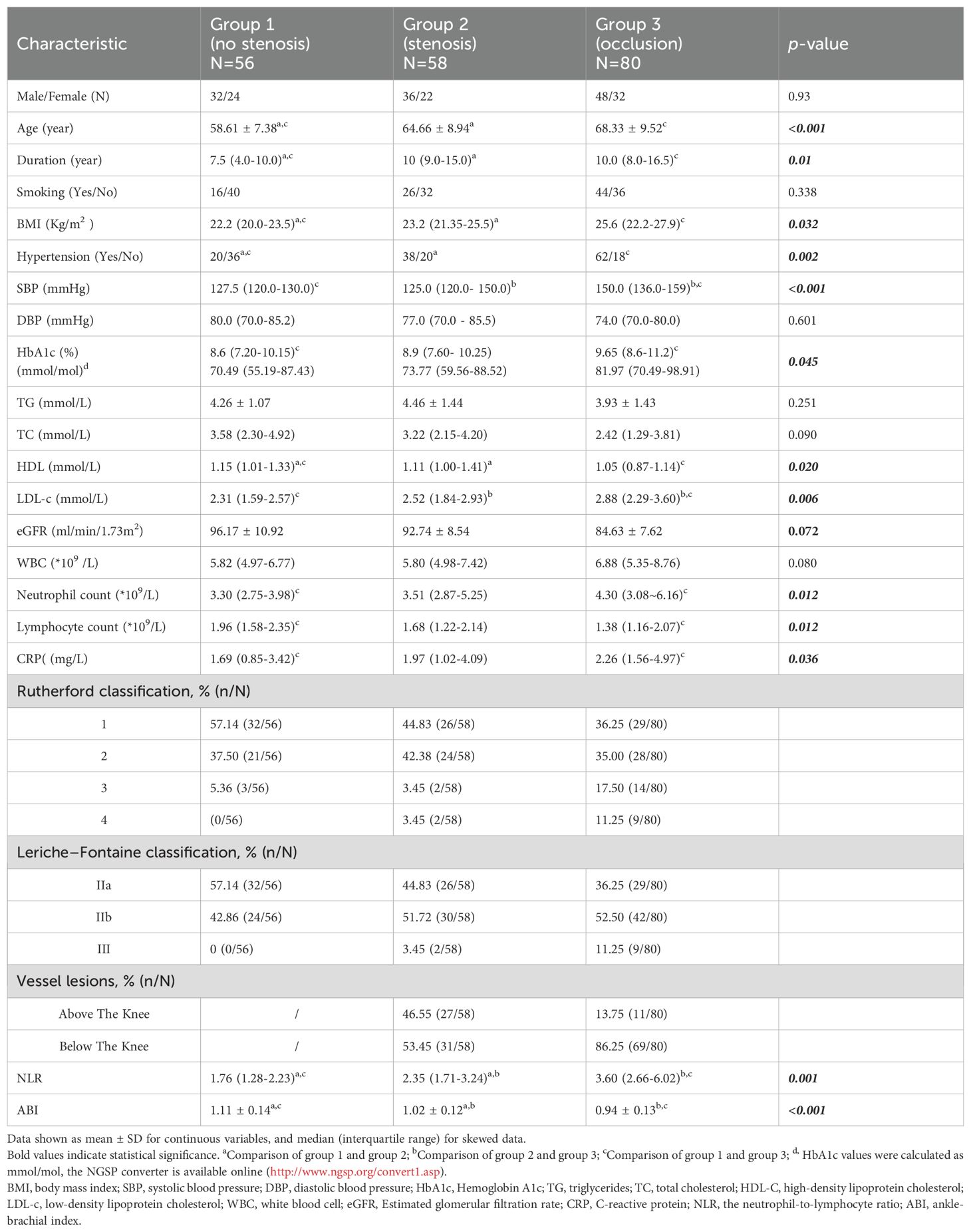
Patients were classified into group L1 (N=56, 28.9%), group L2 (N=58, 29.9%) and group L3 (N=80, 41.2%). The demographic statistics and clinical characteristics of the patients are presented in Table 1. No pronounced change was noted among groups regarding sex, smoking, eGFR, TC, and TG. As revealed, the age (58.61 ± 7.38 vs 64.66 ± 8.94 vs 68.33 ± 9.52, p<0.001), disease duration (7.5 vs 10.0 vs 10.0, p=0.01), BMI (22.2 vs 23.2 vs 25.6, p=0.032), and prevalence of hypertension (35.7% vs 65.5% vs 77.5%, p=0.002) were considerably higher in group L2 and group L3 than in group L1, systolic blood pressure was significantly higher in group L3 (127.5 vs 125.0 vs 150.0, p<0.001), but this change was not related to diastolic blood pressure (80.0 vs 77.0 vs 74.0, p=0.601); there was no notable difference in the WBC counts, while the neutrophil counts was higher in group L3 (3.30 vs 3.51 vs 4.30, p=0.012) and the lymphocyte counts was lower compared with group L1 (1.96 vs 1.68 vs 1.38, p=0.012); CRP was significantly higher in the group L3 than in group L1, but there was no significant difference between them and group L2 (1.69 vs 1.97 vs 2.26, p=0.036), HbA1c gradually increased in three groups (8.6 vs 8.9 vs 9.65, p=0.045); in the analysis of lipid profiles, there was a progressive increase in LDL-c (2.31 vs 2.52 vs 2.88, p=0.006) accompanied by a mild HDL decline (1.15 vs 1.11 vs 1.05, p=0.020), and this change was particularly significant in L3. In addition, with increasing stenosis confirmed by angiography, there was a progressive decrease in ABI (1.11 vs 1.02 vs 0.94, p<0.001) accompanied by an increase in NLR (1.76 vs 2.35 vs 3.60, p=0.001). Graphical representations of NLR distribution are shown by box plot in Figure 1.
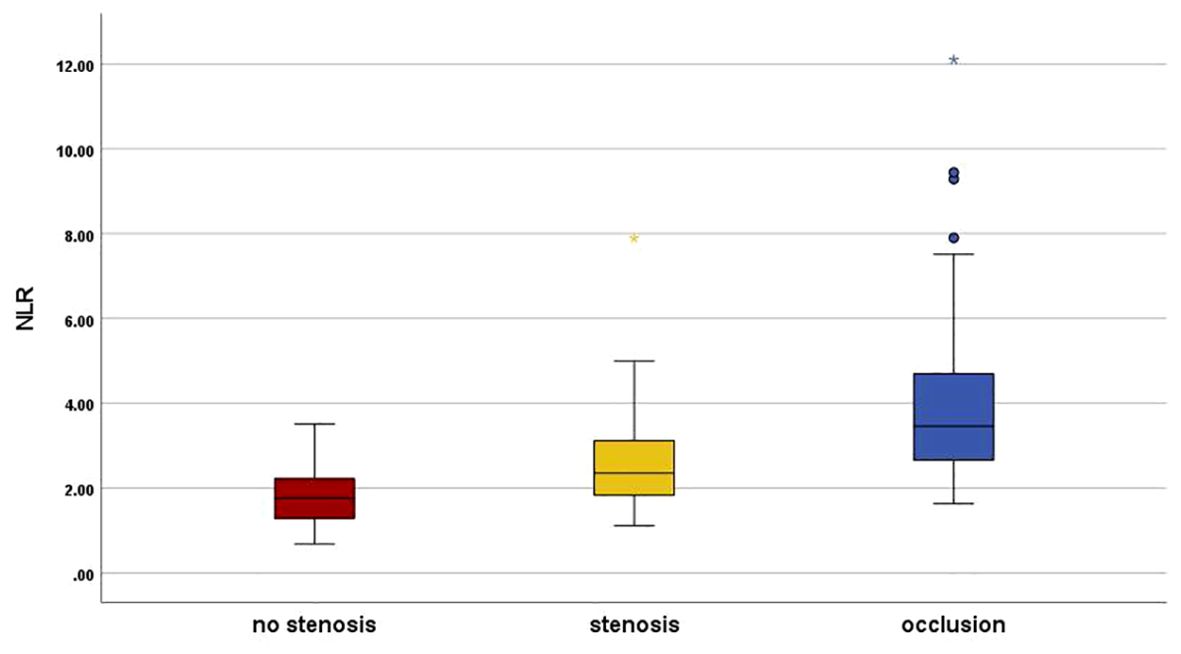
Figure 1. Neutrophil–to–lymphocyte ratio distribution in the study groups. Graphical representations of NLR distribution are shown by box plot. Group L1 (Red), plaque with no stenosis; group L2 (Yellow), plaque with luminal stenosis<99% and group L3 (Blue), total vascular occlusion in lower limb arterial. NLR, the neutrophil-to-lymphocyte ratio. *There was a statistically significant difference in post hoc analysis between the three groups
3.2 Multivariable analysis on risk factors for PAD
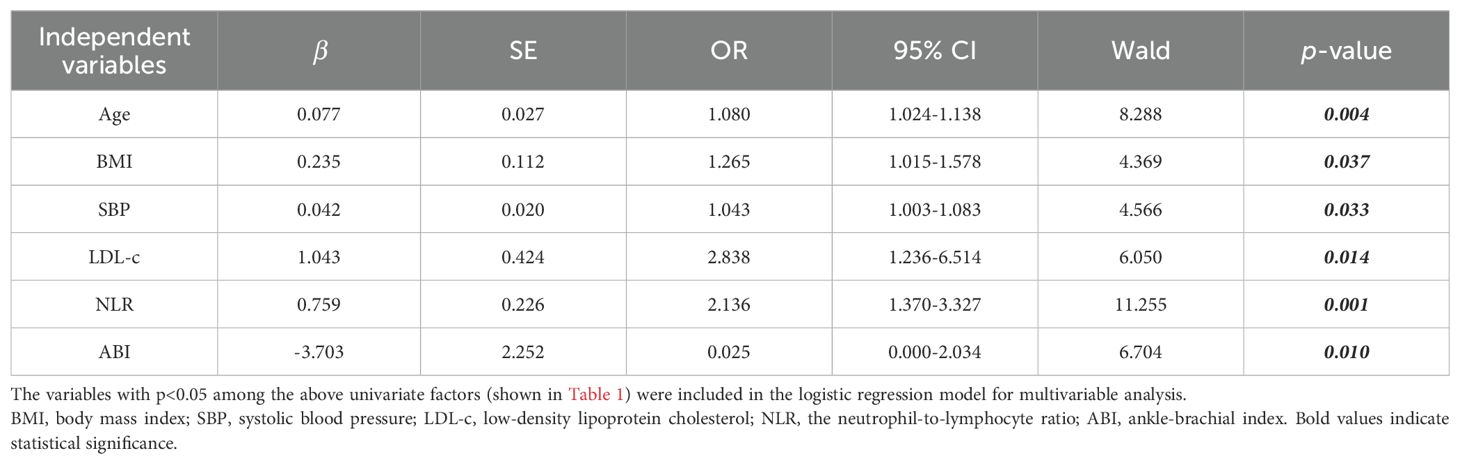
The variables with p<0.05 among the above univariate factors (shown in Table 1) were included in the logistic regression model for multivariable analysis, we found that NLR, ABI, age, BMI, systolic blood pressure and LDL-c were independent influences on PAD (p<0.05, Table 2). Remarkably, ABI values were negatively correlated with the severity of PAD stenosis (OR=0.025, p=0.01), whereas all the other factors were facilitators (OR>1); compared with the mild changes in Neutrophil count and Lymphocyte count (p>0.05), the elevation of NLR was more pronounced (OR=2.136, p=0.001). Lastly, the disease duration, HbA1c, CRP and high-density lipoprotein cholesterol did not have any independent effect on the onset of PAD (p>0.05).
3.3 Correlation analysis on NLR and ABI in different groups
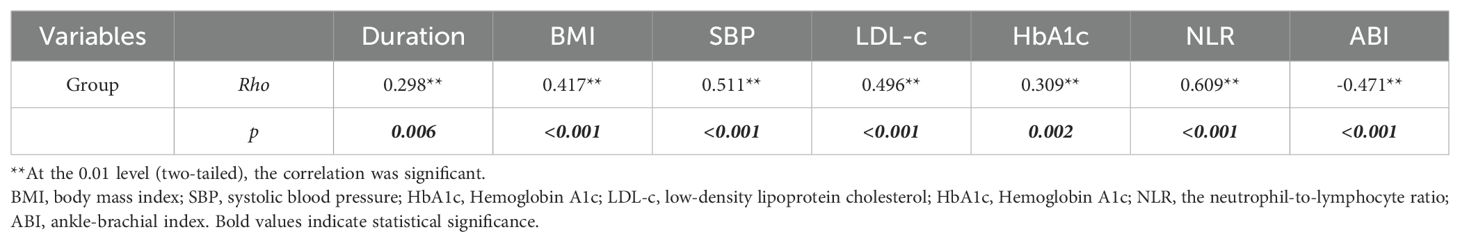
Spearman’s correlation analysis showed a negative correlation between ABI and the group, while the relationship between the NLR and group was positive and more relevant (Rho= -0.471, p<0.001; Rho=0.609, p<0.001, respectively. Shown in Table 3).
3.4 Predictive role of elevated NLR and decreased ABI in severe PAD
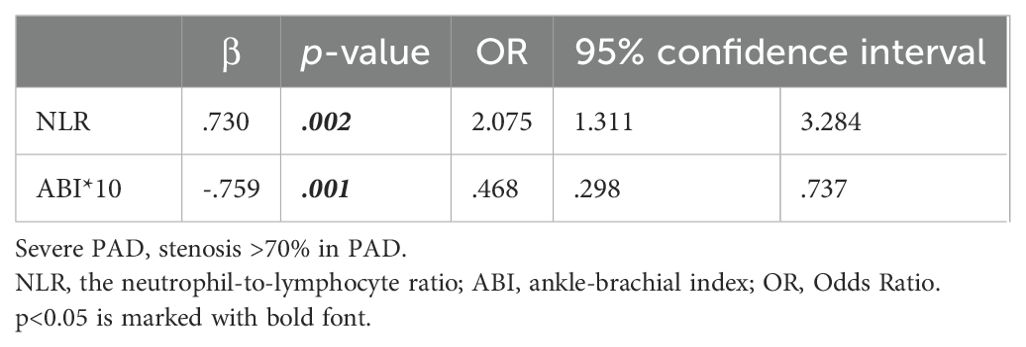
Binary logistic regression analysis with the occurrence of severe stenosis (>70%) showed that the probability increased to 2.075 times for every 1 increase in NLR, while 46.8% for every 0.1 increase in the ABI (Table 4, Figure 2). Finally, to assess the NLR prediction performance compared with CRP, we constructed an ROC curve and calculated the AUC, showing that AUC of NLR was better than CRP at predicting severe stenosis(0.81 vs 0.69), meanwhile the NLR optimal cutoff value of 2.73 with a sensitivity of 82.7% and a specificity of 75.6%, whereas the cutoff point of 1.58 was taken to have a sensitivity of 100% and a specificity of 9.8% (Figure 3).
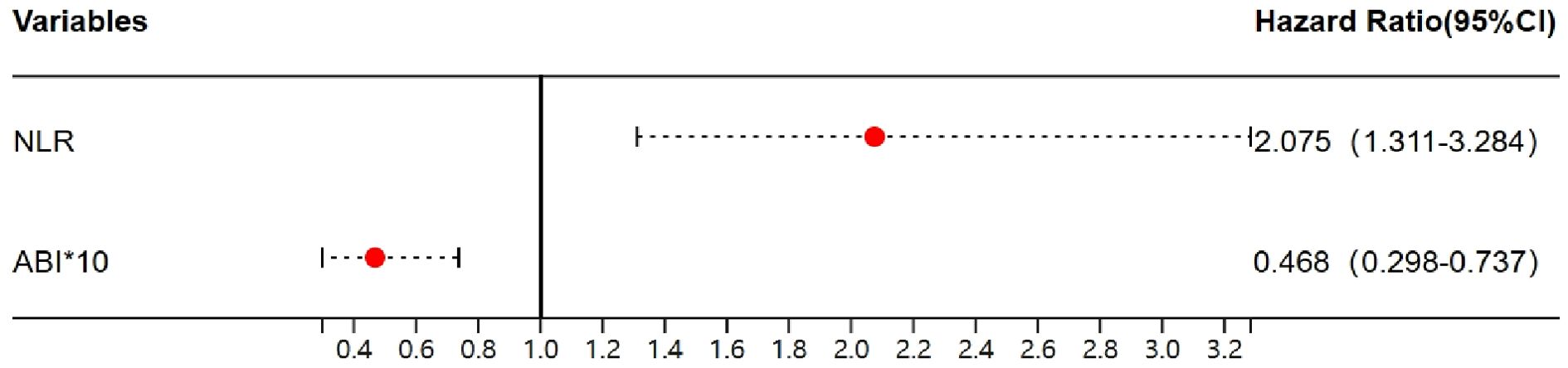
Figure 2. The forest plot of NLR and ABI in severe PAD. NLR, the neutrophil-to-lymphocyte ratio; ABI, ankle-brachial index.
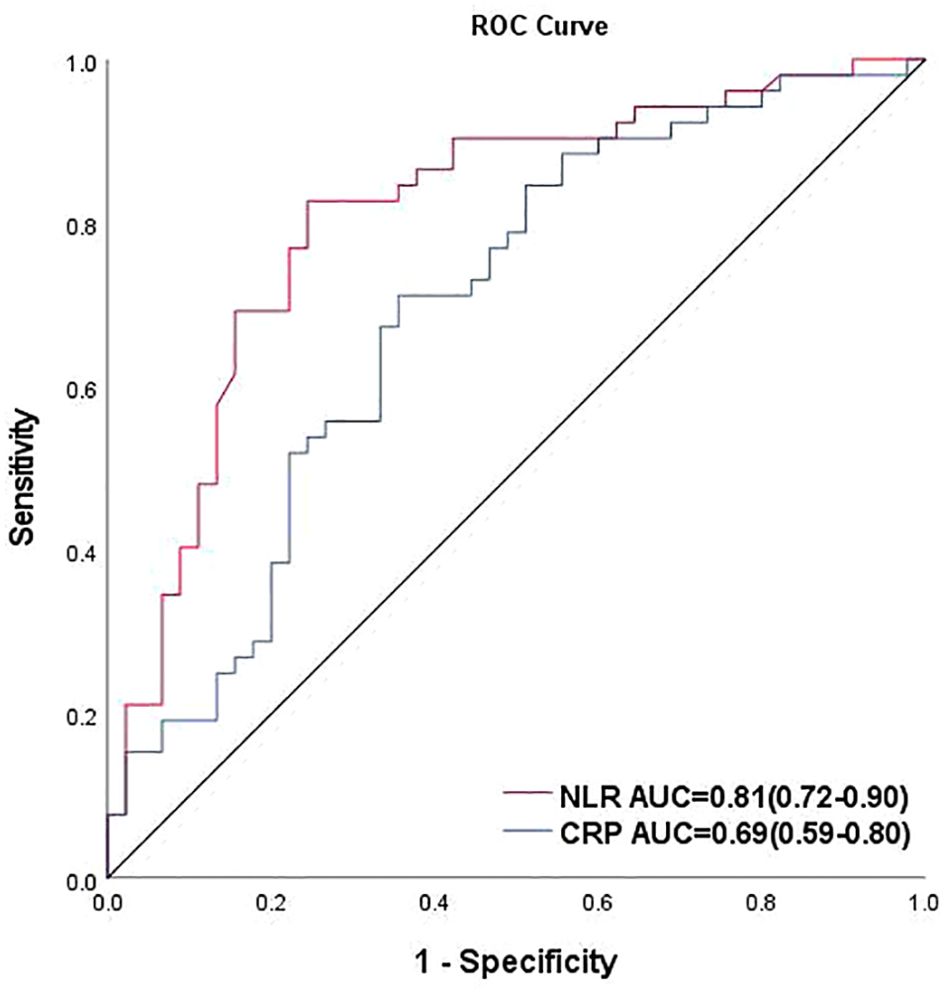
Figure 3. Receiver operating characteristic curve of NLR and CRP for predicting severe PAD. AUC, area under the curve; cutoff value: the maximum value of the Jordon index also corresponds to the optimal diagnostic threshold of the method.
4 Discussion
Diabetic peripheral vascular disease is one of the most common complications in diabetic patients, which can lead to disability and amputation due to atherosclerotic occlusion of the lower limbs (4, 38), inflammatory factors and inflammatory cells play an important role in this, such as neutrophils and lymphocytes.
NLR is a novel marker of systemic inflammatory response that stably responds to the inflammatory state of the body. NLR intermixes the predicted risk of two leukocyte subtypes into a single factor, which has a stronger predictive value, and can minimize the effects of physiological conditions (dehydration, sample error) compared to changes in absolutes count or lymphocytes alone. Previous studies have indicated that NLR is elevated in patients with coronary arteriopathy, but the differences in absolute neutrophil and lymphocyte counts are not statistically significant, suggesting that NLR has its unique sensitivity in response to arteriopathy (39). In diabetic retinopathy and nephropathy, NLR also has an important role in predicting the severity of complications in patients (35, 36, 40, 41). In addition, NLR can predict the prognosis of heart failure (29, 42), cerebral infarction (43), pancreatitis (44, 45), and other acute and chronic inflammation-related diseases, and it is closely related to the severity of these diseases, which can be used as an evaluation index of clinical effect, thus becoming a point of special interest in recent years.
At present, no study has been found on the correlation between diabetic PAD and NLR, so this paper mainly investigates the relationship between the development of lower limb arteriopathy in diabetes mellitus and the change of NLR, results suggest that: NLR ratio was critically higher in the stenosis or occlusion group compared with the no-stenosis group, it is positively correlated with the degree of stenosis, accompanied by the progressive decline of the ABI, this conclusion is consistent with the findings of a meta-analysis demonstrated that high NLR values increased the risk of coronary artery disease (CAD) 1.62-fold and the risk of stroke 3.86-fold (46), Onofrei V also found that NLR was higher in patients with severe obstruction in PAD without diabetes (47), these studies confirm the important role of NLR in promoting the development of various types of peripheral arterial disease. Patients presenting with arterial stenosis and occlusion showed older age, longer disease duration, bigger body mass index, and higher prevalence of hypertension than those in the group without stenosis, as well as being at high risk for needing early intervention, showed the same trend as characteristics of the population with other vascular diseases (48–50), suggests that these influences may be common risk factors for various cardiovascular diseases. HbA1c was found to be progressively elevated with the aggravation of stenosis, confirming that poor glycemic control was a determinant of the severity of PAD, this finding was consistent with the studies by other researchers (33, 34, 51). In the lipid profile, LDL-c increased significantly in severe PAD while HDL tended to decrease gradually, a finding consistent with previous studies in other vascular diseases (52, 53). Surprisingly, TG and TC were not independent risk factors for the development of PAD, Bertrand C found the similar result, they described the independent associations between HDL-cholesterol, total cholesterol/HDL-cholesterol ratio or non-HDL-cholesterol and the prevalence of major PAD in people with type 2 diabetes, neither TG nor TC (54); more importantly, the fluctuations in TG were greater compared to changes in LDL and HDL therefore the differences between groups were smaller, a similar study by Gillian M Keating was also reported (55); another possible reason cannot be excluded is due to the fact that patients with higher blood glucose have higher use of lipid-regulating drugs (56), some participants were on statin therapy leaving some uncertainty during follow-up that may possibly bias our results. However, Kuo-Cheng Chang found no significant correlation between peripheral neuropathy in type 2 diabetes mellitus and lipid levels and statin usage (57). Lastly, the role of smoking does not seem to be as important as expected, which is not consistent with most other studies (58–60), however, similar findings were reported in the study by Al-Momany A on type 2 diabetic nephropathy and smoking (61), it’s not the only case, O’Donnell TFX (62) found that black patients were younger, less likely to smoke, but more likely to have diabetes, limb-threatening ischemia. As to our study, the specific reason for this may be related to the differences in the distribution of smokers by gender and the fact that some ex-smokers are currently quitting.
In order to accurately screen patients who truly need timely angiography and intervention, and to avoid the waste of medical resources caused by non-essential examination, we set diabetes mellitus combined with “severe stenosis” of the lower extremity vessels (>70%) as the critical intervention point to explore the cutoff value of NLR. Binary Logistic regression analysis showed an important role of imbalance in the ratio between neutrophils and lymphocytes in lower extremity arterial stenosis as a positive predictor. Previous studies have shown that the development of atherosclerotic disease is positively associated with the inflammatory factors CRP and IL-6 (63–65), which revealed the role of traditional biomarkers in risk assessment and highlights the strong association between inflammation and CVD. In our study, we compared the ROC curves of CRP and NLR, found that CRP (AUC: 0.69, 95%CI: 0.59-0.80) was worse than NLR (AUC: 0.81, 95%CI: 0.72-0.90) at predicting severe PAD. Coincidentally, Hoes LLF et al. recently published a study showing that NLR is the inflammatory marker that is more strongly related to CVD risk than CRP in patients with T2D (66), while Huang L also found that the diagnostic value of NLR was better than CRP in patients with the anti-synthetase sydrome (67). The Edinburgh Artery Study compared IL-6 with the major inflammatory marker CRP and found that IL-6 was an earlier predictor of worsening ABI values at 12 years of follow-up (68). In predicting coronary artery disease (69), the mean AUC for IL-6 and CRP were 0.74 (95% CI: 0.57-0.84) and 0.60 (95% CI: 0.44-0.74), respectively. In our study, elevated NLR predicted the onset and severity of PAD, which is consistent with the role of traditional inflammatory factors, what’ more, it can be hypothesized that NLR has a higher predictive power than traditional factors.
By ROC curve analysis, it was found that NLR plays a high predictive role in determining whether the stenosis of PAD is severe or worse, Arbel et al. (70) demonstrated that an NLR value above three is associated with a relative risk of 2.45 regarding the existence of sub-occlusive coronary lesions. In our study, taking the cutoff point of 1.58, it has 100% sensitivity and 9.8% specificity, while the optimal cutoff point of 2.73 appeared to have the maximum Youden index and the highest prediction efficiency. That is to say, if the NLR is less than 1.58, the lower extremity arterial ultrasound examination combined with ABI examination can be firstly used to evaluate the lower extremity vascular condition, there is no need to prefer digital imaging, while if the NLR is more than 2.73, DSA imaging or even interventional treatment is strongly recommended.
Currently, there are studies on NLR in diabetes mellitus combined with other peripheral vascular diseases such as CVD, stroke, NLR in patients with PAD without diabetes mellitus, but there are no studies focusing on the changes in NLR in diabetes mellitus combined with PAD, so this study fills this gap and find a more cost-effective way of screening patients with diabetes mellitus with PAD from a novel perspective. However, this research has some imperfections. Firstly, the research was only carried out in one single center, and the sample size was small. Secondly, due to the retrospective design of the study, many factors could not be involved. Lastly, as neutrophil and lymphocyte numbers may alter over time, it is a changing marker. Hence, further investigations are still required with longer follow-up, while larger samples are need to confirm their effectiveness as probable risk factors for PAD.
5 Conclusion
In our study, we demonstrated the predictive value of the NLR and its importance in the evaluation of patients with severe obstruction of the PAD. Indeed, NLR is derived from blood routine, simple and easy to obtain and perform in patients with suspected PAD, the use of ABI in combination with NLR may help to better identify patients with severe lower extremity arterial stenosis earlier, more conveniently, and noninvasively. This change in NLR makes it possible to become a new indicator for assessing the severity of lower-extremity peripheral arterial disease.
It is hoped that, in the future, there will be greater interest in and further studies on this topic to provide stronger recommendations to clinicians for an earlier diagnosis of the disease.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Wuhan Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Writing – original draft, Methodology, Conceptualization. MW: Resources, Investigation, Formal analysis, Data curation, Writing – original draft. TJ: Writing – review & editing, Visualization, Supervision, Conceptualization. XJ: Writing – review & editing, Resources, Investigation, Formal analysis, Data curation. FY: Writing – original draft, Visualization, Project administration, Resources, Formal analysis. ZW: Writing – review & editing, Supervision, Conceptualization. XZ: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research Projects of Wuhan Municipal Health Commission (Grant number WX20B32).
Acknowledgments
The authors thank all the participants of this study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119. Erratum in: Diabetes Res Clin Pract. 2023 Oct;204:110945. doi: 10.1016/j.diabres.2023.110945.
2. Stoberock K, Kaschwich M, Nicolay SS, Mahmoud N, Heidemann F, Rieß HC, et al. The interrelationship between diabetes mellitus and peripheral arterial disease. Vasa. (2021) 50:323–30. doi: 10.1024/0301-1526/a000925
3. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. (2004) 110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0
4. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486. Erratum in: Eur Heart J. 2020 Dec 1;41(45):4317. doi: 10.1093/eurheartj/ehz828.
5. You M, Liu Y, Wang B, Li L, Zhang H, He H, et al. Asprosin induces vascular endothelial-to-mesenchymal transition in diabetic lower extremity peripheral artery disease. Cardiovasc Diabetol. (2022) 21:25–39. doi: 10.1186/s12933-022-01457-0
6. Liu Y, Wang Y, Xu L, Yang J, Zhao Y, Qiao J, et al. Relationship between dietary patterns and diabetic microvascular complications in patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. (2023) 27:8780–94. doi: 10.26355/eurrev_202309_33800
7. Zhang X, Ran X, Xu Z, Cheng Z, Shen F, Yu Y, et al. Epidemiological characteristics of lower extremity arterial disease in Chinese diabetes patients at high risk: a prospective, multicenter, cross-sectional study. J Diabetes Complications. (2018) 32:150–6. doi: 10.1016/j.jdiacomp.2017.10.003
8. Gornik HL, Aronow HD, Goodney PP, Arya S, Brewster LP, Byrd L, et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the management of lower extremity peripheral artery disease: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2024) 149:e1313–410. doi: 10.1161/CIR.0000000000001251
9. Li Q, Birmpili P, Atkins E, Johal AS, Waton S, Williams R, et al. Illness trajectories after revascularization in patients with peripheral artery disease: A unified approach to understanding the risk of major amputation and death. Circulation. (2024) 150:261–71. doi: 10.1161/CIRCULATIONAHA.123.067687
10. Beckman J, Creager M, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. (2002) 287:2570–81. doi: 10.1001/jama.287.19.2570
11. Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Colagiuri S, Hamet P, et al. Presentations of major peripheral arterial disease and risk of major outcomes in patients with type 2 diabetes: results from the ADVANCE-ON study. Cardiovasc Diabetol. (2016) 15:129. doi: 10.1186/s12933-016-0446-x
12. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. (2019) 5:42. doi: 10.1038/s41572-019-0097-9
13. Met R, Bipat S, Legemate D, Reekers J, Koelemay M. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. (2009) 301:415–24. doi: 10.1001/jama.301.4.415
14. Cheng TW, Doros G, Jones DW, Vazirani A, Malikova MA. Evaluation of computerized tomography utilization in comparison to digital subtraction angiography in patients with peripheral arterial disease. Ann Vasc Surg. (2024) S0890-5096:00165–1. doi: 10.1016/j.avsg.2024.03.001
15. Träger AP, Günther JS, Raming R, Paulus LP, Lang W, Meyer A, et al. Hybrid ultrasound and single wavelength optoacoustic imaging reveals muscle degeneration in peripheral artery disease. Photoacoustics. (2023) 35:100579. doi: 10.1016/j.pacs.2023.100579
16. Ghirardini F, Martini R. Current opinion on diagnosis of peripheral artery disease in diabetic patients. Medicina (Kaunas). (2024) 60:1179. doi: 10.3390/medicina60071179
17. Deng W, Dong X, Zhang Y, Jiang Y, Lu D, Wu Q, et al. Transcutaneous oxygen pressure (TcPO2): a novel diagnostic tool for peripheral neuropathy in type 2 diabetes patients. Diabetes Res Clin Pract. (2014) 105:336–43. doi: 10.1016/j.diabres.2014.05.012
18. Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. (2012) 110:875–88. doi: 10.1161/CIRCRESAHA.111.257535
19. Hartwig H, Silvestre Roig C, Daemen M, Lutgens E, Soehnlein O. Neutrophils in atherosclerosis. A Brief overview. Hamostaseologie. (2015) 35:121–7. doi: 10.5482/HAMO-14-09-0040
20. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
21. Cao Y, Chen M, Jiao X, Li S, Wang D, Zhan Y, et al. Neutrophil extracellular traps mediate the crosstalk between plaque microenvironment and unstable carotid plaque formation. Exp Mol Med. (2024) [Ahead of print] doi: 10.1038/s12276-024-01281-4.
22. Maga P, Mikolajczyk TP, Partyka L, Siedlinski M, Maga M, Krzanowski M, et al. Involvement of CD8+ T cell subsets in early response to vascular injury in patients with peripheral artery disease in vivo. Clin Immunol. (2018) 194:26–33. doi: 10.1016/j.clim.2018.06.006
23. Chistiakov DA, Grechko AV, Myasoedova VA, Melnichenko AA, Orekhov AN. The role of monocytosis and neutrophilia in atherosclerosis. J Cell Mol Med. (2018) 22:1366–82. doi: 10.1111/jcmm.13462
24. Lou M, Luo P, Tang R, Peng Y, Yu S, Huang W, et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord. (2015) 15:9. doi: 10.1186/s12902-015-0002-9
25. Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. (2022) 185:1630–45. doi: 10.1016/j.cell.2022.04.004
26. Akbari M, Hassan-Zadeh V. IL-6 signaling pathways and the development of type 2 diabetes. Inflammopharmacology. (2018) 26:685–98. doi: 10.1007/s10787-018-0458-0
27. Yuan Y, Haas A, Williams G, Taylor H, Seely E, Adler G. Association between life’s simple 7 and biomarkers of cardiovascular disease: aldosterone, interleukin-6, C-reactive protein. J Am Heart Assoc. (2023) 12:e028718. doi: 10.1161/JAHA.122.028718
28. Ulu S, Dogan M, Ahsen A, Altug A, Demir K, Acartürk G, et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. (2013) 15:942–7. doi: 10.1089/dia.2013.0097
29. García-Escobar A, Vera-Vera S, Tébar-Márquez D, Rivero-Santana B, Jurado-Román A, Jiménez-Valero S, et al. Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: New insights for a potential predictor of anti-cytokine therapy responsiveness. Microvasc Res. (2023) 150:104598. doi: 10.1016/j.mvr.2023.104598
30. Li S, Wang D, Wei R, Yu G, Wang X, Jiang Z. Predictive value of the neutrophil-lymphocyte ratio for tumor regression grade and prognosis of local advanced rectal cancer patients undergoing neoadjuvant chemoradiotherapy. Technol Cancer Res Treat. (2023) 22:15330338231202611. doi: 10.1177/15330338231202611
31. Ouyang H, Xiao B, Huang Y, Wang Z. Baseline and early changes in the neutrophil-lymphocyte ratio (NLR) predict survival outcomes in advanced colorectal cancer patients treated with immunotherapy. Int Immunopharmacol. (2023) 123:110703. doi: 10.1016/j.intimp.2023.110703
32. Deng Y, Zhang J, Zou G, Li S, Gong Z, Yue G, et al. Peripheral blood inflammatory markers can predict benign and Malignant thyroid nodules. Int J Endocrinol. (2022) 2022:2319660. doi: 10.1155/2022/2319660
33. Adane T, Melku M, Worku Y, Fasil A, Aynalem M, Kelem A, et al. The association between neutrophil-to-lymphocyte ratio and glycemic control in type 2 diabetes mellitus: A systematic review and meta-analysis. J Diabetes Res. (2023) 2023:3117396. doi: 10.1155/2023/3117396
34. Low Wang CC, Blomster JI, Heizer G, Berger JS, Baumgartner I, Fowkes FGR, et al. EUCLID trial executive committee and investigators. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. J Am Coll Cardiol. (2018) 72:3274–84. doi: 10.1016/j.jacc.2018.09.078. Erratum in: J Am Coll Cardiol. 2019 Jul 16;74(2):264-269. doi: 10.1016/j.jacc.2019.06.001.
35. Azab B, Daoud J, Naeem F, Nasr R, Ross J, Ghimire P, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study). Ren Fail. (2012) 34:571–6. doi: 10.3109/0886022X.2012.668741
36. Rajendrakumar A, Hapca S, Nair A, Huang Y, Chourasia M, Kwan R, et al. Competing risks analysis for neutrophil to lymphocyte ratio as a predictor of diabetic retinopathy incidence in the Scottish population. BMC Med. (2023) 21:304. doi: 10.1186/s12916-023-02976-7
37. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-ad09
38. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. (2021) 128:1818–32. doi: 10.1161/CIRCRESAHA.121.318535
39. Shi L, Li Y, Xu X, Cheng Y, Meng B, Xu J, et al. Brown adipose tissue-derived Nrg4 alleviates endothelial inflammation and atherosclerosis in male mice. Nat Metab. (2022) 4:1573–90. doi: 10.1038/s42255-022-00726-2
40. Tutan D, Doğan M. Evaluation of neutrophil/lymphocyte ratio, low-density lipoprotein/albumin ratio, and red cell distribution width/albumin ratio in the estimation of proteinuria in uncontrolled diabetic patients. Cureus. (2023) 15:e44497. doi: 10.7759/cureus.44497
41. Jaaban M, Zetoune AB, Hesenow S, Hessenow R. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as novel risk markers for diabetic nephropathy in patients with type 2 diabetes. Heliyon. (2021) 7:e07564. doi: 10.1016/j.heliyon.2021.e07564
42. Wang X, Chen X, Wang Y, Peng S, Pi J, Yue J, et al. The association of lipoprotein(a) and neutrophil-to-lymphocyte ratio combination with atherosclerotic cardiovascular disease in Chinese patients. Int J Gen Med. (2023) 16:2805–17. doi: 10.2147/IJGM.S410840
43. Ferro D, Matias M, Neto J, Dias R, Moreira G, Petersen N, et al. Neutrophil-to-lymphocyte ratio predicts cerebral edema and clinical worsening early after reperfusion therapy in stroke. Stroke. (2021) 52:859–67. doi: 10.1161/STROKEAHA.120.032130
44. Suppiah A, Malde D, Arab T, Hamed M, Allgar V, Smith A, et al. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. (2013) 17:675–81. doi: 10.1007/s11605-012-2121-1
45. Forsmark C, Vege S, Wilcox C. Acute pancreatitis. N Engl J Med. (2016) 375:1972–81. doi: 10.1056/NEJMra1505202
46. Angkananard T, Anothaisintawee T, McEvoy M, Attia J, Thakkinstian A. Neutrophil lymphocyte ratio and cardiovascular disease risk: A systematic review and meta-analysis. BioMed Res Int. (2018) 2018:2703518. doi: 10.1155/2018/2703518
47. Onofrei V, Crișan A, Adam CA, Marcu DTM, Haba MȘC, Tribus LC, et al. The role played by novel inflammatory markers in assessment of peripheral artery disease. Medicina (Kaunas). (2023) 59:1557. doi: 10.3390/medicina59091557
48. Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol. (2013) 61:1736–43. doi: 10.1016/j.jacc.2013.01.054
49. Gong H, Ren Y, Li Z, Zha P, Bista R, Li Y, et al. Clinical characteristics and risk factors of lower extremity amputation in the diabetic inpatients with foot ulcers. Front Endocrinol (Lausanne). (2023) 14:1144806. doi: 10.3389/fendo.2023.1144806
50. Heffron SP, Dwivedi A, Rockman CB, Xia Y, Guo Y, Zhong J, et al. Body mass index and peripheral artery disease. Atherosclerosis. (2020) 292:31–6. doi: 10.1016/j.atherosclerosis.2019.10.017
51. Beaney AJ, Nunney I, Gooday C, Dhatariya K. Factors determining the risk of diabetes foot amputations–A retrospective analysis of a tertiary diabetes foot care service. Diabetes Res Clin Pract. (2016) 114:69–74. doi: 10.1016/j.diabres.2016.02.001
52. Raja V, Aguiar C, Alsayed N, Chibber Y, ElBadawi H, Ezhov M, et al. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis. (2023) 383:117312. doi: 10.1016/j.atherosclerosis.2023.117312
53. Wadström B, Pedersen K, Wulff A, Nordestgaard B. Elevated remnant cholesterol and atherosclerotic cardiovascular disease in diabetes: a population-based prospective cohort study. Diabetologia. (2023) 66:2238–49. doi: 10.1007/s00125-023-06016-0
54. Bertrand C, Saulnier PJ, Potier L, Croyal M, Blanchard V, Gand E, et al. Plasma concentrations of lipoproteins and risk of lower-limb peripheral artery disease in people with type 2 diabetes: the SURDIAGENE study. Diabetologia. (2021) 64:668–80. doi: 10.1007/s00125-020-05326-x
55. Keating G, Croom K. Fenofibrate: a review of its use in primary dyslipidemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs. (2007) 67:121–53. doi: 10.2165/00003495-200767010-00013
56. Yuan S, Song C, He J, Zhang R, Bian X, Song W, et al. Trends in cardiovascular risk factors control among US adults by glycemic statuses, 2007-2018. Eur J Prev Cardiol. (2023) 30:1513–23. doi: 10.1093/eurjpc/zwad080
57. Chang K, Pai Y, Lin C, Lee I, Chang M. The association between hyperlipidemia, lipid-lowering drugs and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus. PloS One. (2023) 18:e0287373. doi: 10.1371/journal.pone.0287373
58. Ambrose J, Barua R. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. (2004) 43:1731–7. doi: 10.1016/j.jacc.2003.12.047
59. Guan W, Zheng X, Chung K, Zhong N. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. (2016) 388:1939–51. doi: 10.1016/S0140-6736(16)31597-5
60. Tan C, Glantz S. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. (2012) 126:2177–83. doi: 10.1161/CIRCULATIONAHA.112.121301
61. Al-Momany A, Almomani E, Almomani H, Al-Azzam S, Qablan A. Evaluating kidney function and the associated risk factors among patients with type 2 diabetes mellitus: a cross-sectional study at a tertiary hospital in Jordan. BMJ Open. (2023) 13:e073536. doi: 10.1136/bmjopen-2023-073536
62. O’Donnell TFX, Powell C, Deery SE, Darling JD, Hughes K, Giles KA, et al. Regional variation in racial disparities among patients with peripheral artery disease. J Vasc Surg. (2018) 68:519–26. doi: 10.1016/j.jvs.2017.10.090
63. Jia X, Buckley L, Sun C, Al Rifai M, Yu B, Nambi V, et al. Association of interleukin-6 and interleukin-18 with cardiovascular disease in older adults: Atherosclerosis Risk in Communities study. Eur J Prev Cardiol. (2023) 30:1731–40. doi: 10.1093/eurjpc/zwad197
64. Schulz S, Rehm S, Schlitt A, Lierath M, Lüdike H, Hofmann B, et al. C-reactive protein level and the genetic variant rs1130864 in the CRP gene as prognostic factors for 10-year cardiovascular outcome. Cells. (2023) 12:1775. doi: 10.3390/cells12131775
65. Georgakis MK, Malik R, Richardson TG, Howson JMM, Anderson CD, Burgess S, et al. Associations of genetically predicted IL-6 signaling with cardiovascular disease risk across population subgroups. BMC Med. (2022) 20:245. doi: 10.1186/s12916-022-02446-6
66. Hoes LLF, Riksen NP, Geleijnse JM, de Groot MCH, T van der Schouw Y, Visseren FLJ, et al. Relationship of neutrophil-to-lymphocyte ratio, in addition to C-reactive protein, with cardiovascular events in patients with type 2 diabetes. Diabetes Res Clin Pract. (2024) 213:111727. doi: 10.1016/j.diabres.2024.111727
67. Huang L, Li X, Zhou W, Zhu H, Lao Y, Huang X, et al. The clinical value of the neutrophil-to-lymphocyte ratio, the C-reactive protein-to-albumin ratio, the systemic inflammatory index, and the systemic inflammatory response index in patients with the anti-synthetase syndrome. J Inflammation Res. (2024) 17:3617–28. doi: 10.2147/JIR.S460610
68. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. (2005) 112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085
69. Wainstein M, Mossmann M, Araujo G, Gonçalves S, Gravina G, Sangalli M, et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate-risk overweight patients referred for coronary angiography. Diabetol Metab Syndr. (2017) 9:67. doi: 10.1186/s13098-017-0266-5
Keywords: type 2 diabetes mellitus, lower extremity peripheral artery disease, neutrophil-to-lymphocyte ratio, angiography, stenosis
Citation: Li L, Wang M, Jia T, Jiang X, Yang F, Wang Z and Zhang X (2024) Neutrophil-to-lymphocyte ratio in type 2 diabetes patients combined with Lower Extremity Peripheral Artery Disease. Front. Endocrinol. 15:1434580. doi: 10.3389/fendo.2024.1434580
Received: 18 May 2024; Accepted: 16 August 2024;
Published: 30 August 2024.
Edited by:
Ying Xin, Jilin University, ChinaReviewed by:
Guanghua Zhai, Nanjing Medical University, ChinaSayaka Funabashi, Kyorin University Hospital, Japan
Susmita Sinha, Khulna City Medical College and Hospital, Bangladesh
Alexandr Ceasovschih, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright © 2024 Li, Wang, Jia, Jiang, Yang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuyan Zhang, MTM1NDUwMjEzMjJAMTYzLmNvbQ==; Zhongjing Wang, ZG9jdG9yd2FuZ3pqQDE2My5jb20=
†These authors have contributed equally to this work
 Li Li
Li Li Mengjie Wang1
Mengjie Wang1 Xuyan Zhang
Xuyan Zhang