- 1Department of Endocrinology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 2Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 3Peking University Fifth School of Clinical Medicine, Beijing, China
Background: Patients with osteoporosis (OP) are often associated with decreased hand grip strength and increased risk of falling. It remains unclear whether there is a genetic causal between hand grip strength and OP, falling risk.
Methods: The Mendelian randomization study was used to investigate the genetic causal effect of low hand grip strength on total body bone mineral density (BMD) at different ages, OP, and falling risk. Genes for low hand grip strength, total body BMD at different ages, OP, and falling risk were obtained from published genome-wide association studies. Inverse variance weighted, MR‐Egger, and weighted median were applied to perform the MR analysis. The Cochran’s Q test, MR‐Egger intercept test, MR-PRESSO global test, and leave-one-out analysis were used to detect the pleiotropy or heterogeneity.
Results: The results showed strong evidence that low hand grip strength was positively associated with OP (OR: 1.006, 95% CI: 1.003-1.010; P= 0.0001) and falling risk (OR: 1.069, 95% CI: 1.013-1.129; P= 0.0160), and could not directly affect the different ages of total body BMD (P> 0.05). There was no heterogeneity or horizontal pleiotropy in the sensitivity analysis (all P> 0.05).
Conclusion: The study found a positive causal relationship between low hand grip strength and higher risk of OP and falling, which should be taken into account in the development of future prevention and screening strategies for OP and falling.
1 Introduction
As the world’s population ages, osteoporosis (OP) is becoming one of the most prevalent metabolic bone diseases, increasing the risk of insufficiency fractures (1, 2). Over the last 12 years, the prevalence of OP in China has increased, affecting more than one-third of the population over the age of 50 (3). Osteoporotic fracture is the most serious consequence of OP, which has a significant financial impact on society (4). The burden of the disorder may therefore be lessened by identifying risk factors for OP, which will enable us to identify those who are at risk and create intervention techniques for prevention or early treatment (5).
Sarcopenia is a syndrome characterized by progressive and pervasive loss of skeletal muscle mass and strength (6). According to research data, the current prevalence of sarcopenia in the community is at 1-33% (7), sarcopenia affects around 50 million individuals globally, and that number is expected to rise to 200 million in the next 40 years (6). Sarcopenia can lead to mobility disorders (8), compromise life quality (9), and increase personal, social, and economic burdens (10).
Sarcopenia and OP are two disorders with similar risk factors and biological pathways (11). Bone and muscle interact closely with one another physically, chemically, and metabolically (12). OP and sarcopenia frequently coexist (13–15), and are strongly associated with frailty, falls, fractures, hospitalizations, and mortality (16–18). Current evidence also suggests that sarcopenia may be an independent predictor of low BMD and OP (13). Decreased hand grip strength is an important part of the diagnostic criteria for sarcopenia (19). Hand grip strength is the most preferable method of measuring muscle strength because it is a simple, noninvasive indicator of muscle strength and is ideal for clinical use (20). In recent years, some studies have shown that low hand grip strength could predict decreased bone mineral density (BMD) (13, 21–23), the increased prevalence of OP (24, 25), and falling risk (26, 27), but the findings are inconsistent, and the limitations of observational studies make it unclear whether these associations are confounding or causal (28). Further research at the genetic level is required in order to fully understand the significance of these associations for disease prevention and screening.
Mendelian randomization (MR) is a method based on genome-wide association study (GWAS) data, where genetic variation is used as an instrumental variable (IV) to infer the specific effect of exposure on outcome (29). Genes are randomly assigned to the offspring without being subject to confounding factors because gamete formation follows Mendelian laws (30). To the best of our knowledge, no similar MR studies have been conducted to explore the causal relationship between hand grip strength and OP and fall risk. The MR study aimed to investigate the causal effect of low hand grip strength on the total body BMD at different ages, the prevalence of OP, and falling risk.
2 Materials and methods
2.1 Data sources
Single nucleotide polymorphisms (SNPs) were used as IVs in the MR investigation to demonstrate a causal relationship between low hand grip strength and total body BMD, OP, falling risk. The summary statistics of SNPs related to low hand grip strength, total body BMD at different ages, OP, and falling risk were extracted from the GWAS database (https://gwas.mrcieu.ac.uk), which is publicly available, and the detailed information is shown in Supplementary Table S1. Since this study was based on published data, no ethical approval or informed consent was required.
2.2 Selection of genetic instruments
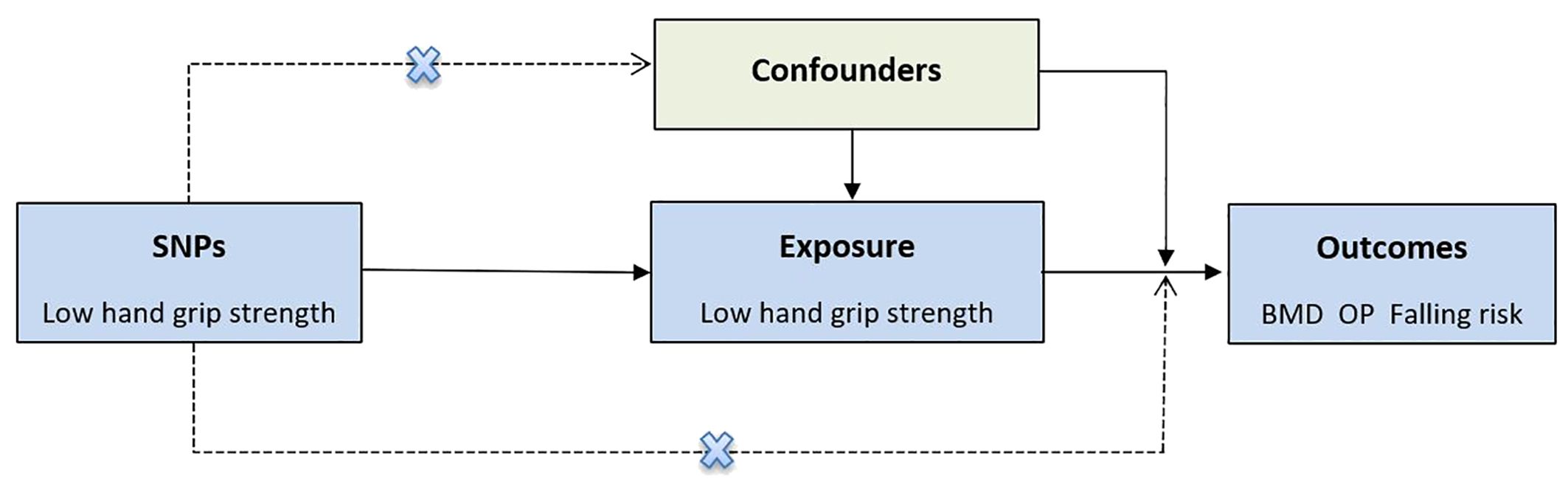
In the MR analysis, IVs must meet the three key assumptions (30, 31). First, SNPs are strongly associated with low hand grip strength. Second, SNPs shouldn’t be associated with any confounders. Third, SNPs affect the outcome only via low hand grip strength (Graphical Abstract).
With P < 5×10-8 serving as the screening condition, SNPs for low hand grip strength were selected as IVs based on published data. At the same time, we excluded SNPs that were in linkage disequilibrium status (R2 < 0.001, aggregation window = 10,000kb) to ensure independence. Finally, we calculated the R2 and F statistic to evaluate the bias of the weak IVs using the following formula: R2 = 2 × EAF × (1-EAF) × β2, F = R2 (N-K-1)/[(1-R2)], where N is the sample size, K is the number of IVs, and SNPs with F greater than 10 were further analyzed (32). Following a comprehensive screening process, the residual SNPs were employed in further investigations.
2.3 MR analysis
The random-effects inverse variance weighted (IVW) was the primary statistical method, which was used to analyze the primary causal inference of the effect of low hand grip strength on BMD, OP, and falling risk. To improve the confidence of the results, we used two additional MR methods, the weighted median and MR-Egger methods for causal association assessments. In addition, we performed a series of sensitivity analyses to assess the reliability of the MR results. The Cochran’s Q test was used to detect heterogeneity of IVs. The MR-Egger intercept test and MR-PRESSO global test were used to examine the horizontal pleiotropy, and a leave-one-out sensitivity analysis was performed to assess the stability of the MR results.
2.4 Statistical analysis
The “TwoSampleMR” and “MRPRESSO” packages of the R software (version 4.3.1) were performed to implement all statistical analyses. The MR results were represented by odds ratios (ORs) and 95% confidence intervals (CIs). P < 0.05 was statistically significant.
3 Results
3.1 Genetic variables for low hand grip strength
As shown in Supplementary Table S2, in our MR study, seventeen SNPs were chosen as IVs for low hand grip strength from published data, and the F of all SNPs was greater than 10, no bias was found for weak IVs.
3.2 The influence of genetically predicted low hand grip strength on total body BMD at different ages
According to IVW analysis, the MR results indicated low hand grip strength could not directly affect the different ages of total body BMD (BMD age 0-15: OR = 1.03, 95% CI: 0.88-1.22, p = 0.698; BMD age 15-30: OR = 0.96, 95% CI: 0.70-1.33, p = 0.808; BMD age 30-45: OR = 1.11, 95% CI: 0.92-1.33, p = 0.284; BMD age 45-60: OR = 0.89, 95% CI: 0.76-1.03, p = 0.125; BMD age over 60: OR = 1.01, 95% CI: 0.86-1.18, p = 0.916), and the results were also confirmed by the MR-Egger regression and weighted median methods (all P > 0.05), which are presented in Table 1, Figure 1 and Figure 2.
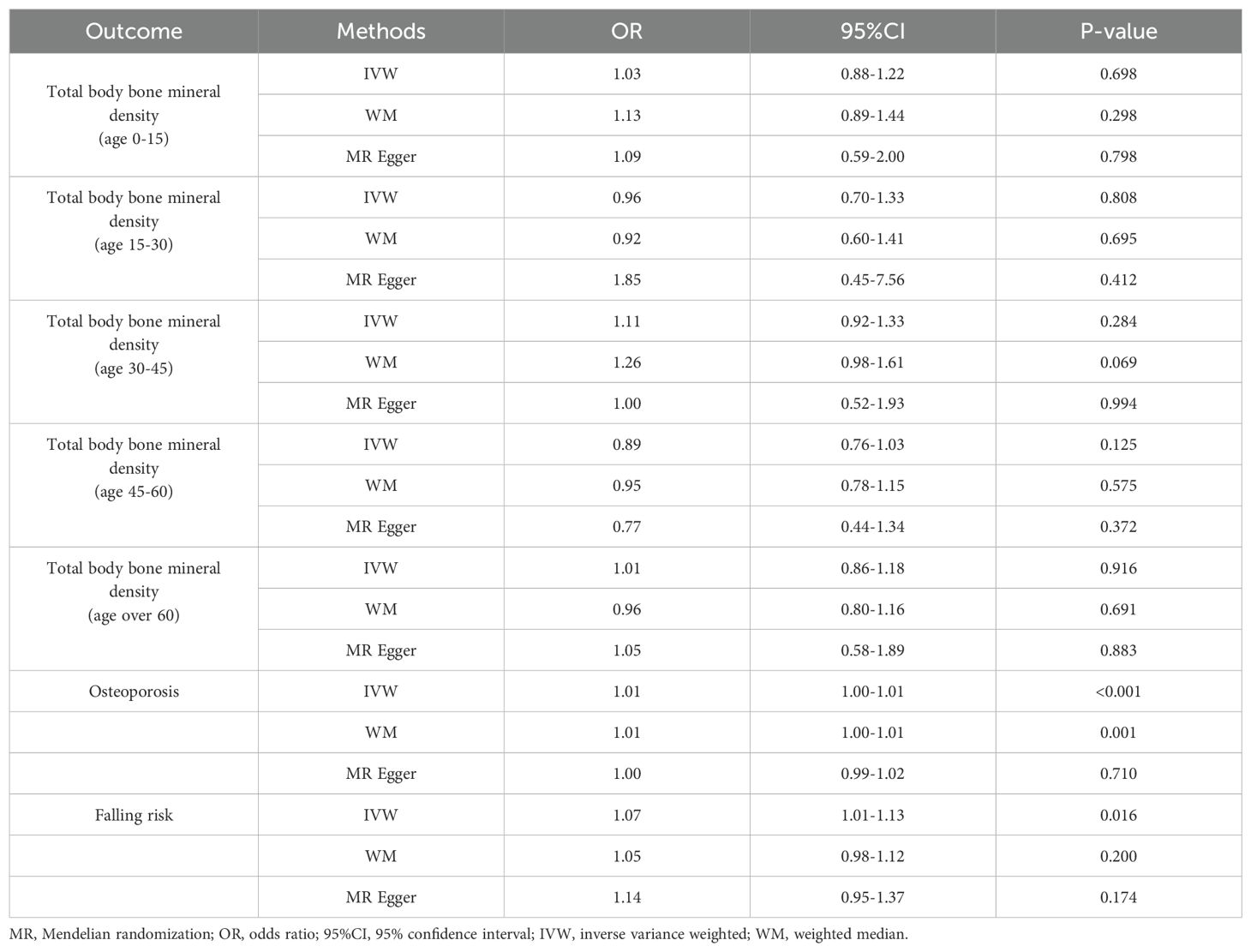
Table 1. MR estimates of the causal association between low hand grip strength, total body bone mineral density at different ages, the risk of osteoporosis, and falling.
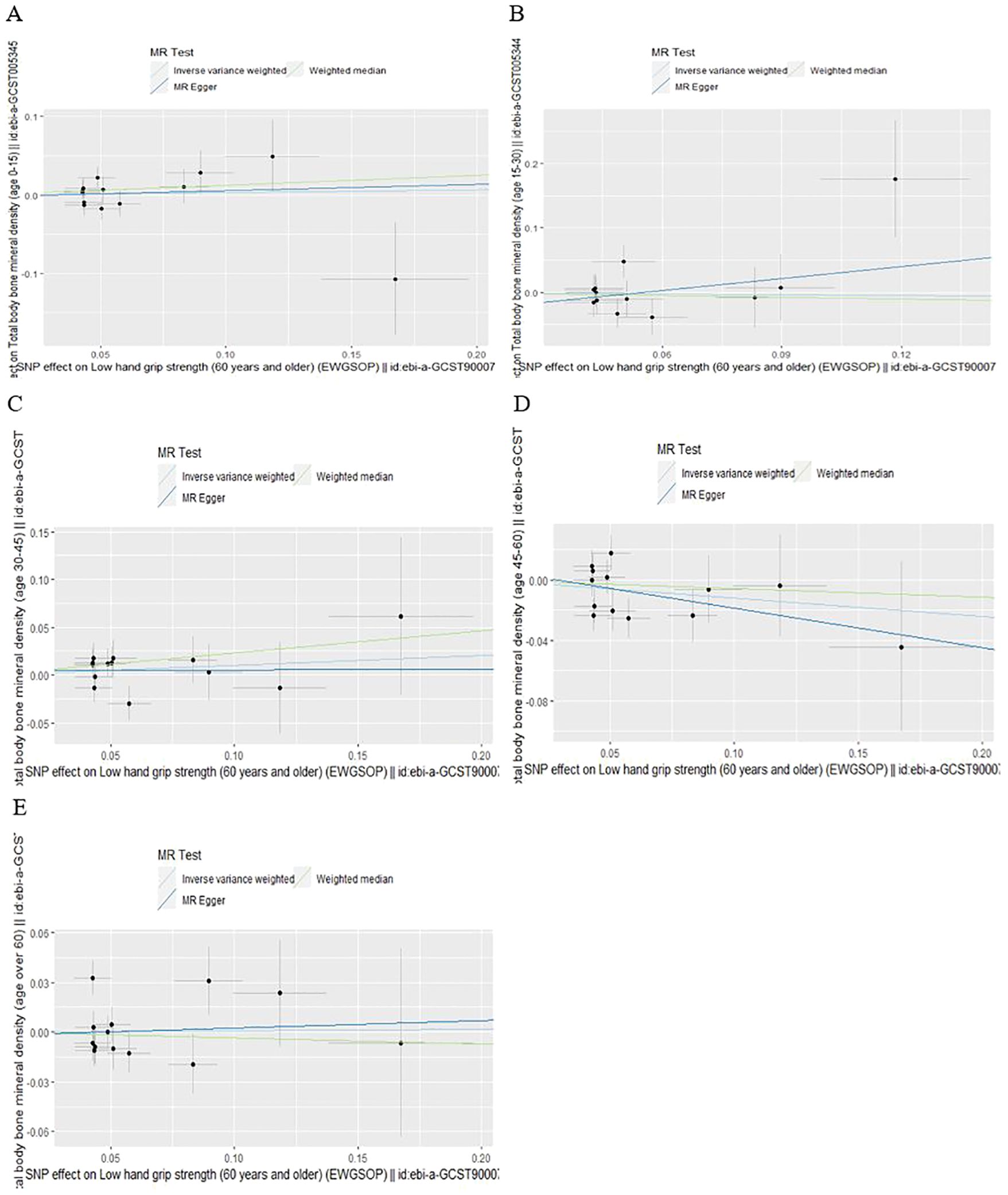
Figure 1. Scatter plot of the causal effect of low hand grip strength on different ages of total body bone mineral density (A) Age 0-15, (B) Age 15-30, (C) Age 30-45, (D) Age 45-60, (E) Age over 60.
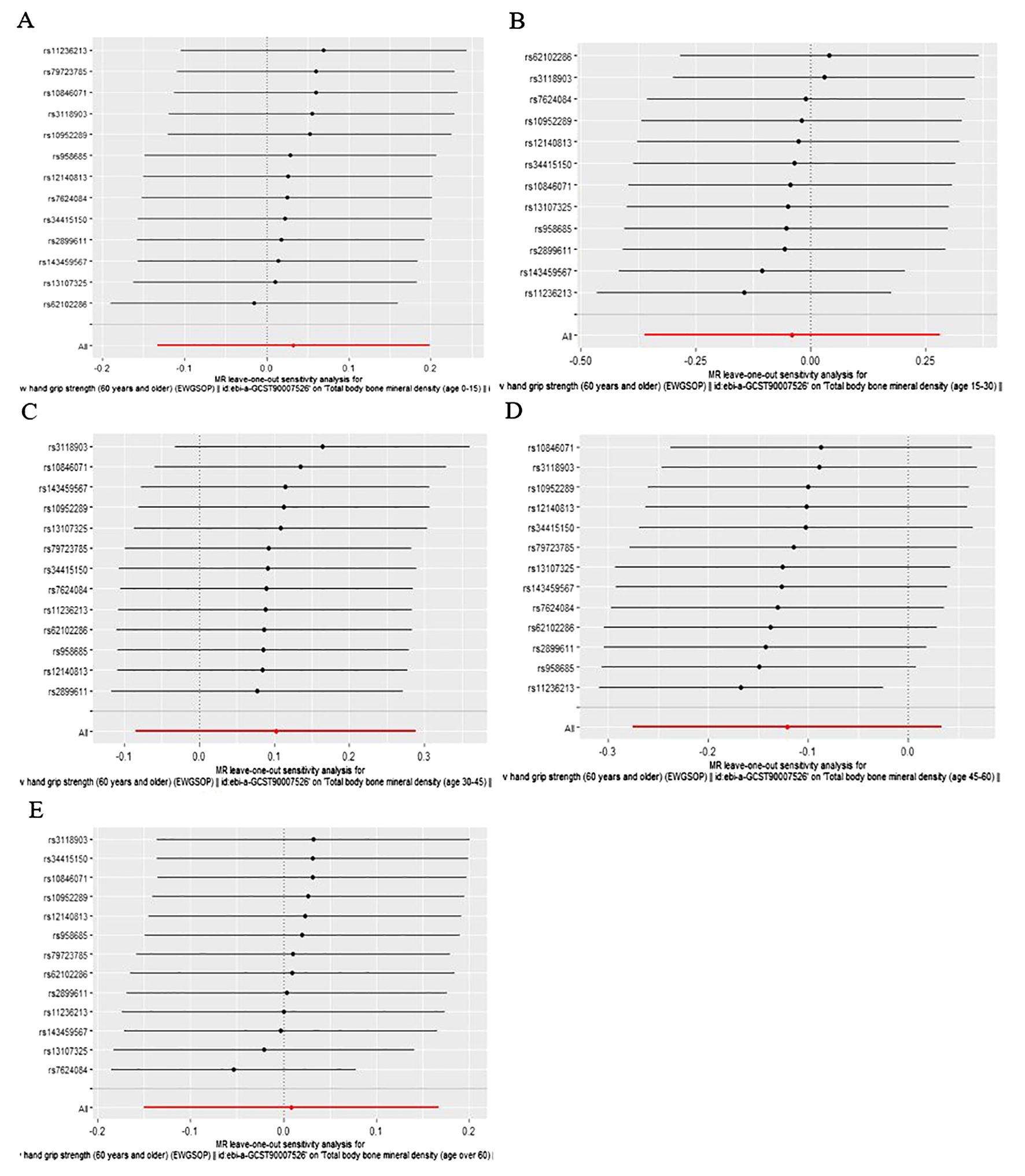
Figure 2. Leave-one-out plot of the causal effect of low hand grip strength on different ages of total body bone mineral density (A) Age 0-15, (B) Age 15-30, (C) Age 30-45, (D) Age 45-60, (E) Age over 60.
3.3 The influence of genetically predicted low hand grip strength on OP
As presented in Table 1, Figure 3, the MR results of IVW analysis indicated low hand grip strength could directly affect the OP (OR: 1.006, 95% CI: 1.003-1.010, P= 0.0001), and the results were also confirmed by the weighted median methods (OR = 1.007, 95% CI: 1.003-1.012, P= 0.421).
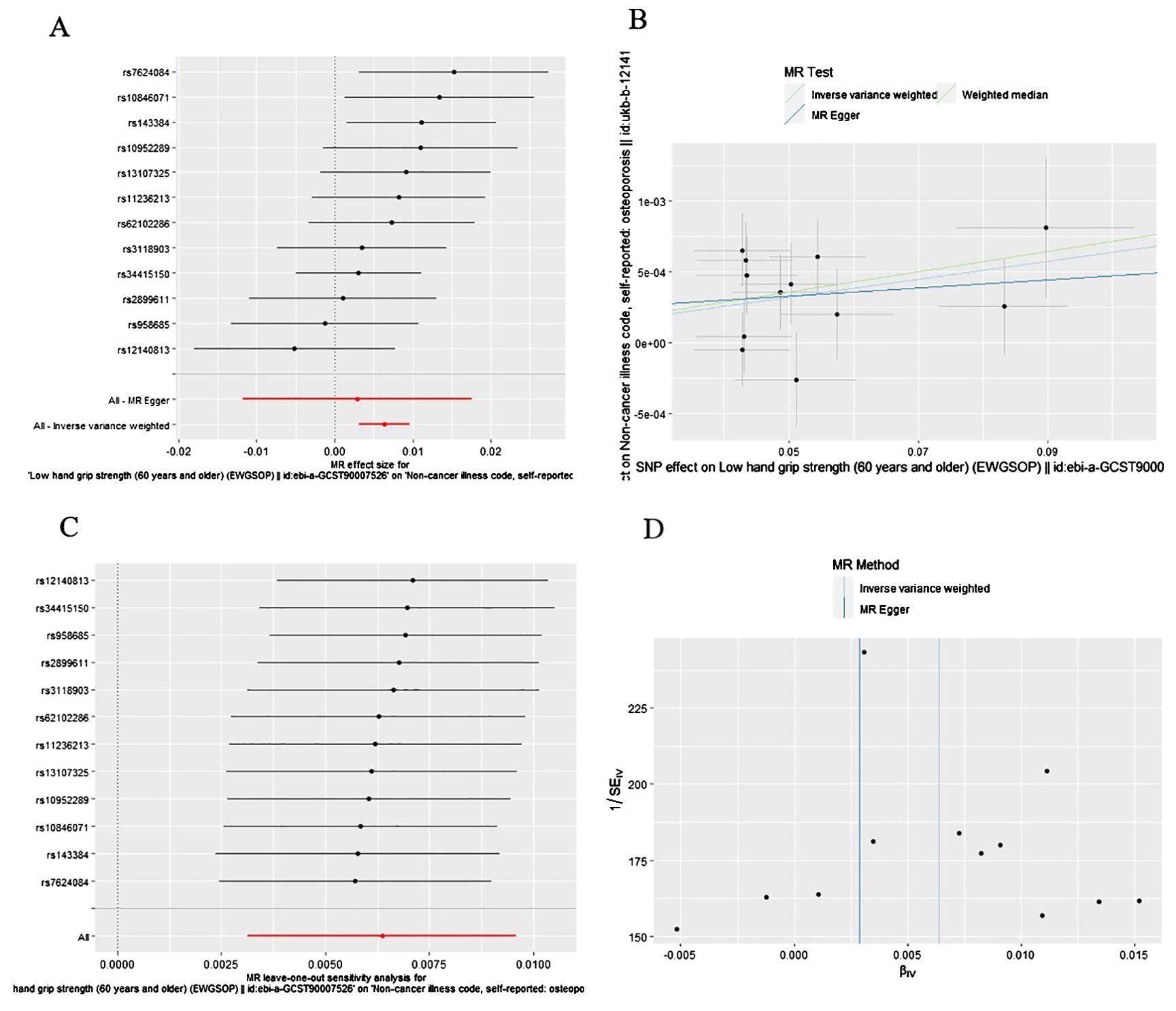
Figure 3. The causal effect of low hand grip strength on osteoporosis risk. (A) Forrest plot, (B) Scatter plot, (C) Leave-one-out plot, (D) Funnel plot.
3.4 The influence of genetically predicted low hand grip strength on falling risk
According to IVW analysis, the MR results indicated low hand grip strength could directly affect the falling risk (OR: 1.069, 95% CI: 1.013-1.129; P=0.0160), which are presented in Table 1, Figure 4.
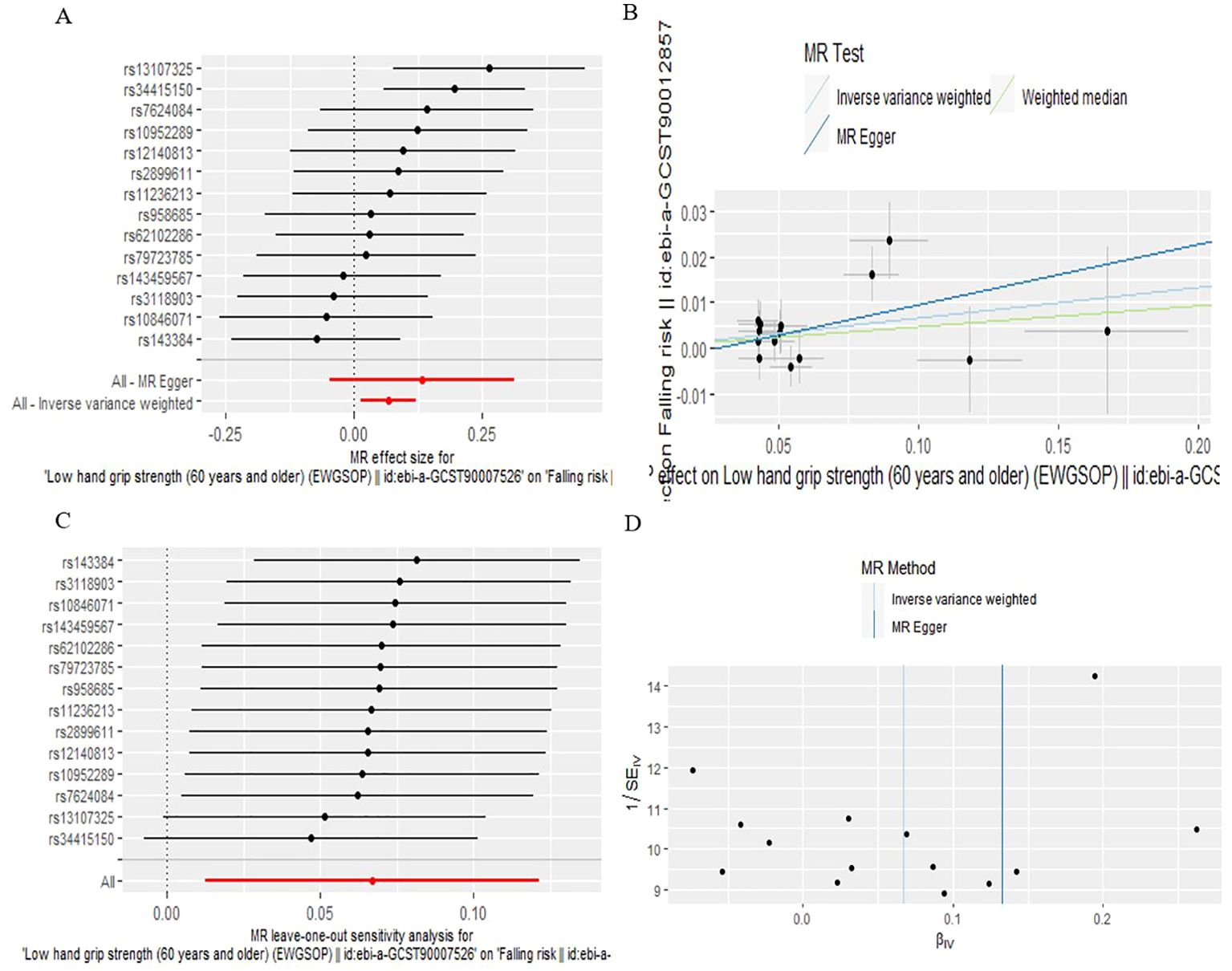
Figure 4. The causal effect of low hand grip strength on falling risk. (A) Forrest plot, (B) Scatter plot, (C) Leave-one-out plot, (D) Funnel plot.
3.5 Results of the sensitivity analysis
As is shown in Table 2, all p values for Cochran’s Q test analysis were more than 0.05, indicating no heterogeneity in the study. Also, the P values of the MR‐Egger intercept test and MR-PRESSO global test were all greater than 0.05, indicating no horizontal pleiotropy (Table 2). Additionally, each SNP was gradually removed by using the leave-one-out method, and the results were all the same as the original results, which showed the results of the study to be of heightened reliability (Figures 2–4).
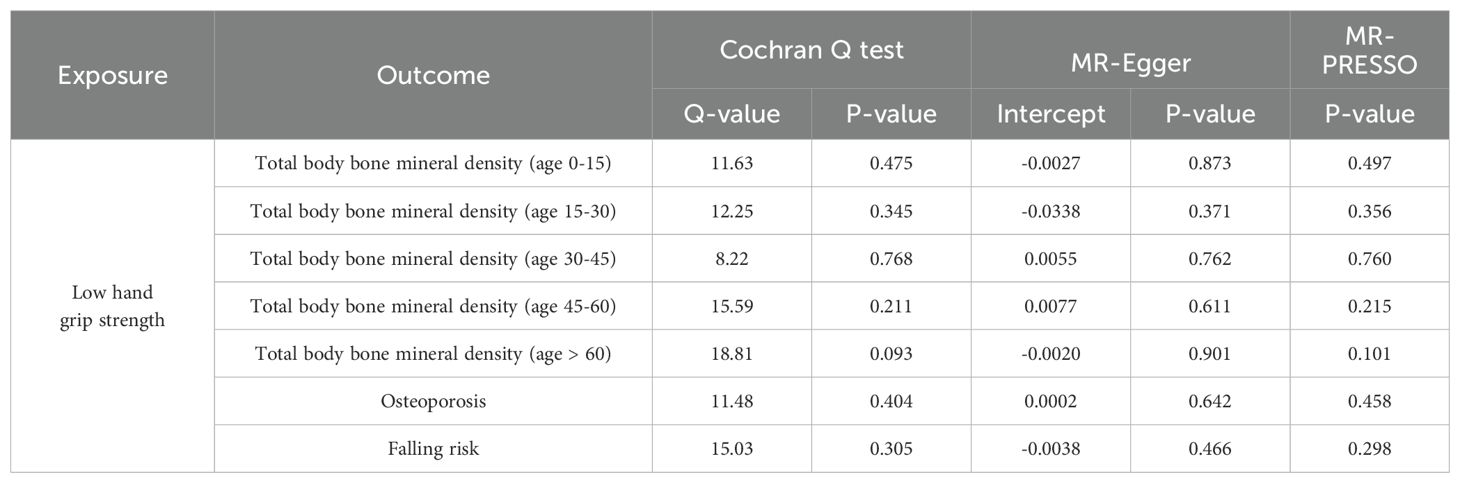
Table 2. Sensitivity analysis of the causal association between low hand grip strength, total body bone mineral density at different ages, the risk of osteoporosis, and falling.
4 Discussion
Exploring the causal relationship between hand grip strength and OP and falling risk is important for the prevention and screening of OP and falling. Previous studies are contradictory and have limitations in study design. Our study was conducted by a MR analysis method utilizing publicly available large-scale GWAS summary data, which ultimately found that genetic susceptibility to hand grip strength directly altered the risk of OP and falling. To our knowledge, this is the first MR study exploring the causal effect of hand grip strength on OP and falling risk.
In 2019, the revision of the European consensus on the definition and diagnosis of sarcopenia suggested that hand grip strength could be an initial assessment tool for sarcopenia, which could effectively help to identify cases in clinical practice (19). This study used hand grip strength as a representative indicator of sarcopenia to provide a more specific analysis of the effect of sarcopenia on BMD, OP, and fall risk at the genetic level.
Some previous studies have examined the association between hand grip strength on BMD. A retrospective analysis of 1,850 participants aged 40-80 years found that grip strength was associated with increased femoral neck and total lumbar spine BMDs in men (P < 0.001, P = 0.005), after adjusting for age, ethnicity, body mass index, use of female hormones, smoking habit, drinking habit, family history of OP, use of calcium and vitamin D supplements, physical activity, serum calcium, and phosphorus levels, with the same results obtained in both premenopausal (P = 0.040, P = 0.014) and postmenopausal women (P = 0.016, P = 0.012) (24). Besides, a study analyzed the relationship between hand grip strength and BMD in 1,427 adolescent students in Chile (750 males and 677 females, aged between 11.0 and 18.9 years) and found that grip strength was positively correlated to BMD in adolescents (25). Interestingly, some studies in recent years have found inconsistent results. A cross-sectional study analyzing the relationship between grip strength and BMD in 318 men (age range 33-92 years) and 203 women (age range 41-90 years) in China, showed that hand grip strength was not associated with BMD in men (28). In addition, a study included 234 male participants and found that there was no predictive value of hand grip strength for BMD of the lumbar spine or femoral neck (33). Besides, the study of Robert et al. (34) analysis of the Framingham Offspring study, which included 508 men and 651 women (aged 50 years and older), found that greater hand grip strength was associated with larger bone size and greater bone strength at the distal radius, which suggested that loading by muscles may not affect BMD or microarchitecture, thus the positive relation between muscle strength and bone strength may be driven primarily by bone size. Consistent with recent findings, our study found no causal relationship between low hand grip strength and total body BMD at different ages, at least at the genetic level. Although the previous MR studies found a site-specific effect of handgrip strength on lumbar spine BMD, stratified analyses by age have not been further explored (35, 36). The inconsistencies between the results of previous studies may be due to differences in the characteristics of the study population, such as race, age, gender, BMI, co-morbidities, smoking alcohol drinking status. In addition, the varying degree of standardization in the methodology and operation of the tests of grip strength and BMD in different observational studies.
In addition, recent observational studies have examined the effect of hand grip strength on OP risk. A cross-sectional descriptive study analyzing 1,168 Chinese individuals aged ≥ 60 years (mean age: 66.9 ± 6.2 years; men, n = 516; women, n = 652) found that higher grip strength was associated with a lower risk of OP (P = 0.023) (37). Besides, a retrospective study analyzed body composition data from 17,891 African American, Caucasian, and Chinese subjects and found that sarcopenia was associated with low whole-body BMDs and OP (13). Muscle weakness is one of the major predictors of falls. What’s more, recent studies have also shown that hand grip strength, a marker of sarcopenia, is associated with fall risk (27). A retrospective study that included 3,334 Swedish 70-year-olds found that patients diagnosed with sarcopenia exhibited worse BMD and were at higher risk for falls than those with suspected or no sarcopenia (P < 0.05) (38). Francesco et al.’s study (39) evaluated the relationship between sarcopenia and 2-year risk of falls in individuals aged 80 years or older, and after adjusting for confounding factors, it was found that participants with sarcopenia had a higher risk of incident falls compared with non-sarcopenic subjects (adjusted hazard ratio [HR], 3.23; 95% CI, 1.25-8.29). A cross-sectional study analyzing data from 349 patients with OP (median age 77.0 years) found that low hand grip strength is independently and positively associated with fall risk in older women with OP (26). In our study, we found a positive causal relationship between low hand grip strength and prevalence of OP and falling risk, at least at the genetic level, which was in line with the above findings. The management of sarcopenia should be regarded as a key point in the prevention and treatment of OP (40). In addition, sarcopenia also plays an important role in preventing falls in the elderly (39). It’s worth noting that in our study, low hand grip strength had no effect on total body BMD at different ages but increased the risk of OP. In addition to BMD, bone strength can be affected by bone microarchitecture, also known as bone mass, the latter of which can currently be assessed by testing trabecular bone score (TBS) for bone microarchitecture, and a recent study has also found that the low hand grip strength was positively associated with low TBS (28).
Bone and skeletal muscle are integral organs and the coupling between them is considered to be primarily mechanical (41). In addition to the direct effects of weight-bearing, physical activity is the main physiological stimulus that promotes skeletal anabolism and/or catabolism through actin production and secretion (41). However, skeletal muscle can also influence skeletal homeostasis in a non-mechanical way, i.e., through its endocrine activity, actin secreted by skeletal muscle has not only an autocrine function in regulating muscle metabolism but also a paracrine or endocrine regulatory function in distant organs and tissues such as bone and adipose tissue (41, 42). Age-related skeletal muscle decline may lead to bone loss through biomechanical stimulation and decreased growth factors, ultimately leading to the development of OP (43). As a result, patients with sarcopenia have an increased likelihood of developing OP, and some experts have suggested that the two disorders should be combined into a single disease called “osteosarcopenia” (12).
The strengths of the study include the analysis of a large sample size of GWAS data and the stratification of different ages of total body BMD. Additionally, compared to observational studies, MR methods can strengthen the evidence for causal inferences due to the strength of the MR study (44). Nevertheless, our study has some limitations. Firstly, the OP cases were from self-reported OP patients in the UK Biobank. Since disease reporting accuracy varies, there is a risk that OP cases will be misdiagnosed or underdiagnosed. Secondly, we must recognize that there are some limitations to the key assumptions of MR, as it is difficult to ensure that the exposure-outcome relationship is free of any confounders or potential pleiotropic effects. In addition, the GWAS data of the total body BMD lacked gender stratification, preventing a more detailed analysis of the causal relationship between hand grip strength and BMD in different gender subgroups. Besides, we were unable to perform stratified MR analyses based on subtypes of OP, which would have helped to improve the accuracy of the study. It is worth noting that hand grip strength is closely related to quality of life, especially among the elderly and hospitalized population (45). However, in our study, we were unable to assess the quality of life of the included participants, which is a confounding factor that needs to be considered. What’s more, the elderly population may suffer from malnutrition and malabsorption, which are related to OP and muscle strength (46), and can also affect the grip strength test results of participants, which need to be carefully considered. Furthermore, although the MR method was used in this study to assess the causal relationship, it presupposes that there is a linear relationship between exposure and outcome, otherwise, this method is not applicable, so prospective cohort data are still needed to validate this in the future.
In summary, our study provides genetic evidence to support a causal association between low hand grip strength and OP, fall risk. Hand grip strength measurement is a simple, cost-effective, and easy-to-administer assessment method for identifying people at high risk for OP and falls, which should be taken into account in the development of future prevention and screening strategies for the disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. JQ: Data curation, Investigation, Writing – original draft. ZW: Formal analysis, Writing – original draft. QP: Writing – review & editing. LG: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National High Level Hospital Clinical Research Funding (BJ-2021-200, BJ-2022-193 and BJ-2022-120), National Natural Science Foundation of China (82170848), Capital’s Funds for Health Improvement and Research (2022-1-4051) and Beijing Municipal Science and Technology Commission No Z221100007422007.
Acknowledgments
The authors acknowledge all GWAS participants and investigators for their contribution to the summary statistic data. The authors thank all investigators for sharing these data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1433805/full#supplementary-material
References
1. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet (London England). (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
2. Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. (2017) 4:46–56. doi: 10.5152/eurjrheum.2016.048
3. Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: A meta-analysis and systematic review. BMC Public Health. (2016) 16:1039. doi: 10.1186/s12889-016-3712-7
4. Lin X, Xiong D, Peng Y-Q, Sheng Z-F, Wu X-Y, Wu X-P, et al. Epidemiology and management of osteoporosis in the people's republic of China: current perspectives. Clin Interventions In Aging. (2015) 10:1017–33. doi: 10.2147/CIA.S54613
5. Petermann-Rocha F, Ferguson LD, Gray SR, Rodríguez-Gómez I, Sattar N, Siebert S, et al. Association of sarcopenia with incident osteoporosis: A prospective study of 168,682 uk biobank participants. J Cachexia Sarcopenia Muscle. (2021) 12:1179–88. doi: 10.1002/jcsm.12757
6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
7. Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the international sarcopenia initiative (Ewgsop and iwgs). Age Ageing. (2014) 43:748–59. doi: 10.1093/ageing/afu115
8. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. (2011) 12:403–9. doi: 10.1016/j.jamda.2011.04.014
9. Beaudart C, Biver E, Reginster J-Y, Rizzoli R, Rolland Y, Bautmans I, et al. Validation of the sarqol®, a specific health-related quality of life questionnaire for sarcopenia. J Cachexia Sarcopenia Muscle. (2017) 8:238–44. doi: 10.1002/jcsm.12149
10. Mijnarends DM, Luiking YC, Halfens RJG, Evers SMAA, Lenaerts ELA, Verlaan S, et al. Muscle, health and costs: A glance at their relationship. J Nutr Health Aging. (2018) 22:766–73. doi: 10.1007/s12603-018-1058-9
11. Curtis E, Litwic A, Cooper C, Dennison E. Determinants of muscle and bone aging. J Cell Physiol. (2015) 230:2618–25. doi: 10.1002/jcp.25001
12. Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporosis International: J Established as Result Cooperation Between Eur Foundation For Osteoporosis Natl Osteoporosis Foundation USA. (2017) 28:2781–90. doi: 10.1007/s00198-017-4151-8
13. He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporosis International: J Established as Result Cooperation Between Eur Foundation For Osteoporosis Natl Osteoporosis Foundation USA. (2016) 27:473–82. doi: 10.1007/s00198-015-3241-8
14. Reiss J, Iglseder B, Alzner R, Mayr-Pirker B, Pirich C, Kässmann H, et al. Sarcopenia and osteoporosis are interrelated in geriatric inpatients. Z Gerontol Geriatr. (2019) 52:688–93. doi: 10.1007/s00391-019-01553-z
15. Miyakoshi N, Kudo D, Hongo M, Kasukawa Y, Ishikawa Y, Shimada Y. Comparison of spinal alignment, muscular strength, and quality of life between women with postmenopausal osteoporosis and healthy volunteers. Osteoporosis International: J Established as Result Cooperation Between Eur Foundation For Osteoporosis Natl Osteoporosis Foundation USA. (2017) 28:3153–60. doi: 10.1007/s00198-017-4184-z
16. Sepúlveda-Loyola W, Phu S, Bani Hassan E, Brennan-Olsen SL, Zanker J, Vogrin S, et al. The joint occurrence of osteoporosis and sarcopenia (Osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. (2020) 21:220–5. doi: 10.1016/j.jamda.2019.09.005
17. Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol (Lausanne). (2019) 10:255. doi: 10.3389/fendo.2019.00255
18. Yoo JI, Kim H, Ha YC, Kwon HB, Koo KH. Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci. (2018) 33:e27. doi: 10.3346/jkms.2018.33.e27
19. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
20. Norman K, Stobäus N, Gonzalez MC, Schulzke J-D, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. (2011) 30:135–42. doi: 10.1016/j.clnu.2010.09.010
21. Li Y-Z, Zhuang H-F, Cai S-Q, Lin C-K, Wang P-W, Yan L-S, et al. Low grip strength is a strong risk factor of osteoporosis in postmenopausal women. Orthopaedic Surg. (2018) 10:17–22. doi: 10.1111/os.12360
22. Dixon WG, Lunt M, Pye SR, Reeve J, Felsenberg D, Silman AJ, et al. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatol (Oxford England). (2005) 44:642–6. doi: 10.1093/rheumatology/keh569
23. Kaya A, Ozgocmen S, Ardicoglu O, Kamanli A, Gudul H. Relationship between grip strength and hand bone mineral density in healthy adults. Arch Med Res. (2005) 36:603–6. doi: 10.1016/j.arcmed.2005.03.026
24. Luo Y, Jiang K, He M. Association between grip strength and bone mineral density in general us population of nhanes 2013-2014. Arch Osteoporosis. (2020) 15:47. doi: 10.1007/s11657-020-00719-2
25. Cossio-Bolaños M, Lee-Andruske C, de Arruda M, Luarte-Rocha C, Almonacid-Fierro A, Gómez-Campos R. Hand grip strength and maximum peak expiratory flow: determinants of bone mineral density of adolescent students. BMC Pediatr. (2018) 18:96. doi: 10.1186/s12887-018-1015-0
26. Nagai T, Okano I, Ishikawa K, Kuroda T, Oshita Y, Tsuchiya K, et al. The serum 25(Oh)D level and hand grip strength for fall risk assessment among osteoporotic elderly Japanese women. Arch Osteoporosis. (2021) 16:42. doi: 10.1007/s11657-021-00901-0
27. Kwon J-W, Lee BH, Lee S-B, Sung S, Lee C-U, Yang J-H, et al. Hand grip strength can predict clinical outcomes and risk of falls after decompression and instrumented posterolateral fusion for lumbar spinal stenosis. Spine J. (2020) 20:1960–7. doi: 10.1016/j.spinee.2020.06.022
28. Qi H, Sheng Y, Chen S, Wang S, Zhang A, Cai J, et al. Bone mineral density and trabecular bone score in chinese subjects with sarcopenia. Aging Clin Exp Res. (2019) 31:1549–56. doi: 10.1007/s40520-019-01266-8
29. Evans DM, Davey Smith G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. (2015) 16:327–50. doi: 10.1146/annurev-genom-090314-050016
30. Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. (2007) 16:309–30. doi: 10.1177/0962280206077743
31. Pierce BL, Burgess S. Efficient design for mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
32. Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in mendelian randomization: comparison of allele score and summarized data methods. Stat Med. (2016) 35:1880–906. doi: 10.1002/sim.6835
33. Aydin G, Atalar E, Keleş I, Tosun A, Zöğ G, Keleş H, et al. Predictive value of grip strength for bone mineral density in males: site specific or systemic? Rheumatol Int. (2006) 27:125–9. doi: 10.1007/s00296-006-0178-4
34. McLean RR, Samelson EJ, Lorbergs AL, Broe KE, Hannan MT, Boyd SK, et al. Higher hand grip strength is associated with greater radius bone size and strength in older men and women: the framingham osteoporosis study. JBMR Plus. (2021) 5:e10485. doi: 10.1002/jbm4.10485
35. Ma X-Y, Liu H-M, Lv W-Q, Qiu C, Xiao H-M, Deng H-W. A bi-directional mendelian randomization study of the sarcopenia-related traits and osteoporosis. Aging (Albany NY). (2022) 14:5681–98. doi: 10.18632/aging.204145
36. Song J, Liu T, Zhao J, Wang S, Dang X, Wang W. Causal associations of hand grip strength with bone mineral density and fracture risk: A mendelian randomization study. Front Endocrinol (Lausanne). (2022) 13:1020750. doi: 10.3389/fendo.2022.1020750
37. Ma Y, Fu L, Jia L, Han P, Kang L, Yu H, et al. Muscle strength rather than muscle mass is associated with osteoporosis in older chinese adults. J Formos Med Assoc. (2018) 117:101–8. doi: 10.1016/j.jfma.2017.03.004
38. Scott D, Johansson J, McMillan LB, Ebeling PR, Nordstrom P, Nordstrom A. Associations of sarcopenia and its components with bone structure and incident falls in swedish older adults. Calcified Tissue Int. (2019) 105:26–36. doi: 10.1007/s00223-019-00540-1
39. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilsirente study. Clin Nutr. (2012) 31:652–8. doi: 10.1016/j.clnu.2012.02.007
40. Giovannini S, Brau F, Forino R, Berti A, D'Ignazio F, Loreti C, et al. Sarcopenia: diagnosis and management, state of the art and contribution of ultrasound. J Clin Med. (2021) 10:5552. doi: 10.3390/jcm10235552
41. Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. (2020) 94:155–218. doi: 10.1016/bs.acc.2019.07.010
43. Laurent MR, Dubois V, Claessens F, Verschueren SMP, Vanderschueren D, Gielen E, et al. Muscle-bone interactions: from experimental models to the clinic? A critical update. Mol Cell Endocrinol. (2016) 432:14–36. doi: 10.1016/j.mce.2015.10.017
44. Li J, Zhao L, Ding X, Cui X, Qi L, Chen Y. Obstructive sleep apnea and the risk of alzheimer's disease and parkinson disease: A mendelian randomization study osa, alzheimer's disease and parkinson disease. Sleep Med. (2022) 97:55–63. doi: 10.1016/j.sleep.2022.06.004
45. Giovannini S, Brau F, Iacovelli C, Gerardino L, Bellieni A, Fusco A, et al. A snapshot of geriatric rehabilitation: one year experience. Eur Rev Med Pharmacol Sci. (2022) 26:6995–7006. doi: 10.26355/eurrev_202210_29883
Keywords: hand grip strength, osteoporosis, falling risk, single nucleotide polymorphisms, Mendelian randomization
Citation: Ma Y, Qiao J, Wang Z, Pan Q and Guo L (2024) The genetic causal effect of hand grip strength on osteoporosis and falling risk: a Mendelian randomization study. Front. Endocrinol. 15:1433805. doi: 10.3389/fendo.2024.1433805
Received: 16 May 2024; Accepted: 10 September 2024;
Published: 02 October 2024.
Edited by:
Girish Kotwal, University of Massachusetts Amherst, United StatesReviewed by:
Claudia Loreti, Agostino Gemelli University Polyclinic (IRCCS), ItalyRossella Cannarella, Cleveland Clinic, United States
Copyright © 2024 Ma, Qiao, Wang, Pan and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Guo, Z2x4MTIxOEAxNjMuY29t; Qi Pan, cGFucWk2MjFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yanhua Ma
Yanhua Ma Jingtao Qiao
Jingtao Qiao Zhenxing Wang
Zhenxing Wang Qi Pan
Qi Pan Lixin Guo
Lixin Guo